Abstract
Patch-clamp recordings were made from rat ventromedial hypothalamic neurones in slices of brain tissue in vitro. In cell-attached recordings, removal of extracellular glucose or metabolic inhibition with sodium azide reduced the firing rate of a subpopulation of cells through the activation of a 65 pS channel that was blocked by the sulphonylureas tolbutamide and glibenclamide.
In whole-cell patch-clamp recordings, in the absence of ATP in the electrode solution, glucose-receptive neurones gradually hyperpolarized due to the induction of an outward current at -60 mV. This outward current and the resultant hyperpolarization were blocked by the sulphonylureas tolbutamide and glibenclamide.
In recordings where the electrode solution contained 4 mM ATP, this outward current was not observed. Under these conditions, 500 μM diazoxide was found to induce an outward current that was blocked by tolbutamide.
In cell-attached recordings diazoxide and the active fragment of leptin (leptin 22–56) reduced the firing rate of glucose-receptive neurones by the activation of a channel with similar properties to that induced by removal of extracellular glucose.
Reverse transcription followed by the polymerase chain reaction using cytoplasm from single glucose-receptive neurones demonstrated the expression of the ATP-sensitive potassium (KATP) channel subunits Kir6.1 and SUR1 but not Kir6.2 or SUR2.
It is concluded that glucose-receptive neurones within the rat ventromedial hypothalamus exhibit a KATP channel current with pharmacological and molecular properties similar to those reported in other tissues.
It is well established that the ventromedial hypothalamus (VMH) is an important centre involved in the monitoring of glucose status and the regulation of feeding (Anand et al. 1964; Dunn-Meynell et al. 1997). The ability of the VMH to respond to extracellular glucose levels is thought to arise due to the presence of glucose-receptive neurones within this nucleus (Ono et al. 1982). These neurones respond to changes in extracellular glucose as a result of ATP-sensitive potassium (KATP) channel expression (Ashford et al. 1990a).
This group of potassium channels has been well characterized in peripheral tissues and the channels have been found in many types of excitable cell where they act to link cell excitability with metabolic status (Ashcroft & Ashcroft, 1990). In addition to their regulation by intracellular ATP, various pharmacological agents modulate KATP channel activity. For example, potassium channel openers such as diazoxide potentiate channel activity (Kozlowski et al. 1989) whilst agents including the antidiabetic sulphonylureas (Sturgess et al. 1985) and imidazoline compounds (Dunne, 1991) inhibit channel activity.
In contrast to most forms of KATP channel, the VMH KATP channel is reported to display several features which distinguish it from other types of KATP channel. For example, this channel is reported to exhibit a large unitary conductance (150 pS under symmetrical conditions; Ashford et al. 1990a; but see Routh et al. 1997) and has been shown to be less sensitive to ATP in excised inside-out patches (half-maximal inhibitory concentration of 3 mM compared with 12-20 μM in pancreatic β cells).
Importantly, the pharmacological properties of this channel are poorly understood and this factor has greatly hindered its more extensive investigation. Although it has been reported that this channel can be inhibited by the sulphonylurea tolbutamide in cell-attached patch recordings, this sensitivity is lost following patch excision indicating a novel form of coupling between the channel and its associated sulphonylurea receptor (Ashford et al. 1990b). Furthermore, it has been reported that although the channel itself is insensitive to glibenclamide, this agent can block the inhibitory effects of tolbutamide.
In view of the complex nature with which this channel interacts with the sulphonylureas, the present study was initiated to examine the properties of this channel in more detail. In contrast to previous studies, our findings suggest that glucose-receptive neurones in the VMH display KATP channel characteristics similar to those found in the pancreatic β cell.
METHODS
Preparation
Male Sprague-Dawley rats (14-28 days old) were killed by cervical dislocation, their brains were removed and 300 μm coronal slices containing the VMH were prepared in ice-cold physiological saline using a vibratome. A Zeiss Axioskop microscope (Carl Zeiss Ltd, Welwyn Garden City, UK) fitted with a × 64 water-immersion objective lens was used to view slices (see Stuart et al. 1993).
Recording and analysis
Recording electrodes were pulled from borosilicate glass capillaries and when filled with electrolyte had resistances of 8-12 MΩ for isolated patch experiments, and 3-6 MΩ for whole-cell recording. Electrophysiological signals were detected using an Axopatch-1D patch-clamp amplifier (Axon Instruments Inc.) and were recorded onto digital audio tape. Following formation of the whole-cell configuration, series resistance was partially compensated using the amplifier and cellular conductance was continually monitored via the injection of hyperpolarizing current (current-clamp mode; -50 pA in amplitude, 300 ms in duration at 0.1 Hz) or voltage (voltage-clamp mode; -10 mV in amplitude, 300 ms duration at 0.1 Hz). Membrane signals were filtered at 1 kHz for whole-cell experiments and at 500 Hz for single channel recordings. These data were digitized at 5 kHz through a Digidata 1200B A/D converter using pCLAMP 6.0.3 software (Axon Instruments Inc.). In voltage-clamp recordings, neurones were clamped at -60 mV and current-voltage relationships were examined using a voltage ramp protocol between -140 and -60 mV (20 mV s−1).
To assess whether a particular procedure led to a significant change in the magnitude of the current under study, data were subjected to Student's t test. All data in the text and figures are presented as mean values ±s.e.m. unless otherwise stated.
Solutions and drugs
The physiological saline contained (mM): 125.0 NaCl, 25.0 NaHCO3, 10.0 glucose, 2.5 KCl, 1.25 NaH2PO4, 2.0 CaCl2 and 1.0 MgCl2, and was bubbled with a 95% O2-5% CO2 gas mixture. In some experiments, glucose was removed from the physiological saline and was replaced with 10 mM sucrose to maintain osmolarity. The intracellular (pipette) solution was adjusted to pH 7.4 using KOH in all experiments. For whole-cell recordings this solution comprised (mM): 120.0 potassium gluconate, 10.0 NaCl, 2.0 MgCl2, 0.5 K2EGTA, 10.0 Hepes, 4.0 Na2ATP and 0.1 Na2GTP. In some experiments, Na2ATP was removed from this solution. For subsequent epifluorescence examination of cell morphology, 1 mg ml−1 Lucifer Yellow was added to the pipette solution used to dialyse some cells. Slices were fixed with 4% paraformaldehyde before being cleared in DMSO and viewed whole mount (Grace & Llinas, 1985). In cell-attached and outside-out patch experiments, the electrode solution comprised (mM): 120.0 potassium gluconate, 0.5 K2EGTA, 0.5 MgCl2 and 10.0 Hepes.
Throughout the course of an experiment, slices were continuously perfused at a rate of 3-4 ml min−1 with physiological saline by means of a gravity feed system. Tolbutamide and other drugs used in this study were applied by changing the solution which superfused the slice to one which contained the drug. In voltage-clamp recordings, 1 μM TTX, 50 μM D-2-amino-5-phosphovalerate (D-APV) and 1 μM 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(f) quinoxalone (NBQX) were usually added to the physiological saline. All experiments were conducted at 32-35°C.
All drugs were obtained from Sigma except NBQX and D-APV which were from Tocris Cookson (Bristol, UK) and the active fragment of leptin (leptin 22-56 (Powis et al. 1998)) which was from Bachem (Saffron Walden, UK).
Single cell RT-PCR
Analysis of gene expression in single neurones was achieved as described previously (Lee et al. 1998). Briefly, at least 40% of the somatic cytoplasm from single neurones was aspirated into the recording electrode which was subsequently withdrawn from the cell forming an outside-out patch. The contents of the electrode were forced into a microtube and the RNA reverse transcribed using an anchored oligo-dT primer and 200 U of Moloney murine leukaemia virus (MMLV) reverse transcriptase (BRL) according to the manufacturer's recommendations. After 60 min at 37°C the cDNA was stored frozen at -20°C prior to processing. After amplification of the cDNA using Taq polymerase (Dixon et al. 1998), the expression of specific genes was measured using primers designed to amplify products of between 120 and 250 base pairs in length, close to the 3′ ends of the mRNA transcripts.
The primers used were as follows. Kir6.1 (accession number D42145): forward primer (bases 1292-1311), GAGTGAACTGTCGCACCAGA; reverse primer (bases 1539-1520), CGATCACCAGAACTCAGCAA. Kir6.2 (accession number X97041): forward primer (bases 787-804), TCCAACAGCCCGCTCTAC; reverse primer (bases 954-937), GATGGGGACAAAACGCTG. Sulphonylurea receptor 1 (SUR1; accession number L40624): forward primer (bases 4824-4842), TGAAGCAACTGCCTCCATC; reverse primer (bases 5005-4987), GAAGCTTTTCCGGCTTGTC. Sulphonylurea receptor 2 (SUR2; accession number D83598): forward primer (bases 4853-4872), ACCTGCTCCAGCACAAGAAT; reverse primer (bases 4997-4976), TCTCTTCATCACAATGACCAGG. α-Tubulin (accession number V01226): forward primer (bases 300-318), CACTGGTACGTGGGTGAGG; reverse primer (bases 471-450): TTTGACATGATACAGGGACTGC. Cytochrome oxidase (accession number L48209): forward primer (bases 96-116), ATCACCATTGGGCTCACTTC; reverse primer (bases 281-264), ATCCCAGGGTAAGCCAGC. Synaptotagmin 1 (accession number X52772): forward primer (bases 4022-4042), AGGGGCTTTCCTATCTAAGGG; reverse primer (bases 4223-4204), GTTGGCAGTGTTGCAAGAGA. Leptin receptor (ObRb; accession number D84551): forward primer (bases 3301-3320), CCATTCCCAGCTCACTGTCT; reverse primer (bases 3437-3418), GAACAGGATTGAAACTGGGG. These PCR reactions were run for 45 cycles of 92°C (denaturing, 2.5 min), 55°C (annealing, 1.5 min) and 72°C (extension, 1 min), followed by a final extension of 10 min at 72°C. The PCR products were separated on 2.5% agarose gels and the product sizes were as predicted from the sequences. Confirmation of the sensitivity and specificity of these PCR reactions was achieved as described previously (Lee et al. 1998; Dixon et al. 1998). The nature of the Kir6.1 and SUR1 products was confirmed by sequencing of the amplified PCR products on an ABI 310 sequencer (Applied Biosystems, Warrington, UK).
RESULTS
Identification of glucose-receptive neurones in the VMH
Initial identification of glucose-receptive neurones within the VMH was achieved using cell-attached patch recordings. In preliminary studies 12 out of 37 neurones within the VMH were identified as glucose receptive by virtue of the reduction in firing rate that was observed in response to removal of extracellular glucose (Fig. 1). Within 5 min of glucose removal, the firing rate of these neurones had reduced from 3.2± 0.9 to 1.5 ± 0.5 Hz (n = 9 observations). This reduction in firing rate continued until complete cessation of action potential firing was achieved 15-30 min after glucose removal. In each case, this decrease in firing rate was associated with the appearance of a single channel current 3.8±0.7 pA in amplitude (at 0 mV pipette potential) with a channel open probability of 0.3±0.1 (Fig. 2A; n = 8). The current was observed to reverse polarity at a pipette potential between 60 and 80 mV and exhibited a unitary conductance of 65.4±5.6 pS (as judged over the linear part of the I-V relationship; Fig. 2C, n = 8). The activity of this channel was unaffected by membrane voltage (Fig. 2B) but was completely and reversibly inhibited by bath application of 200 μM tolbutamide (Fig. 2A; n = 7) or the re-addition of 10 mM glucose to the bath solution (n = 6).
Figure 1. Cell-attached recording illustrating the effect of aglycaemia on the firing properties of a glucose-receptive neurone.
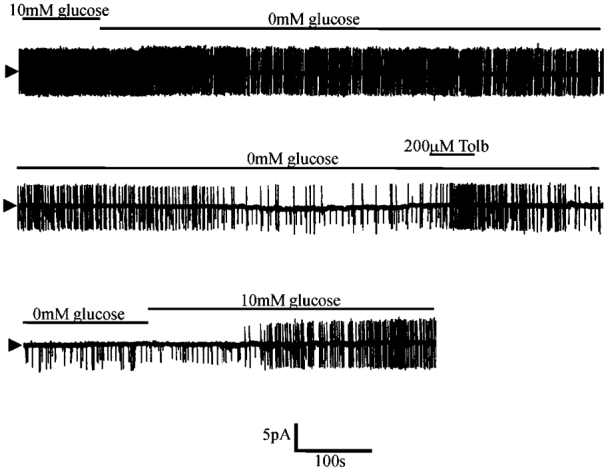
Note that within 2 min of the onset of glucose removal a change in firing rate is observed, gradually resulting in complete cessation of firing after a further 15-20 min. This process was associated with the activation of a 3.3 pA channel at this pipette potential (0 mV). Tolb, tolbutamide.
Figure 2. Single channel characteristics of the tolbutamide-sensitive channel activated by aglycaemia.
A, cell-attached recording made at a pipette potential of 0 mV. Removal of glucose from the bath led to the activation of two 3.7 pA channels which were inhibited by tolbutamide. Arrowheads mark closed state. B, cell-attached recording of the aglycaemia-activated channel at different pipette potentials. C, current-voltage relationship revealed a unitary conductance of 65.4 ± 5.6 pS in the linear part of the plot (denoted by the line). Each point is the mean of between three and five observations. D, subsequent formation of an outside-out patch held at 0 mV under quasi-physiological ionic conditions (the electrode contained 120.0 mM potassium gluconate, 0.5 mM K2EGTA, 0.5 mM MgCl2 and 10.0 mM Hepes whilst physiological saline was in the bath) resulted in the rapid loss of channel activity.
To examine the properties of this channel in more detail, further single channel analysis was attempted after the formation of outside-out patches. Unfortunately, as shown in Fig. 2D, following patch excision there was a rapid loss of channel activity (run-down, n = 5) which precluded further study.
Due to the long time interval between removal of extracellular glucose and channel activation, in further experiments 1 mM NaN3 was used in order to inhibit VMH neurones metabolically. In these experiments 8 out of 25 neurones were found to respond with a reduction in action potential firing rate and the opening of a channel with characteristics indistinguishable from those observed above (Fig. 3). As found previously, the activity of this channel was completely inhibited by 200 μM tolbutamide (Fig. 3A). Furthermore, in four separate experiments, this channel was found to be inhibited by the application of 100 or 200 nM glibenclamide to the bath solution. However, unlike tolbutamide, the effects of this second generation sulphonylurea were not reversed on continued washing (Fig. 3B).
Figure 3. The effect of metabolic inhibition with NaN3 on the electrical characteristics of glucose-receptive neurones.
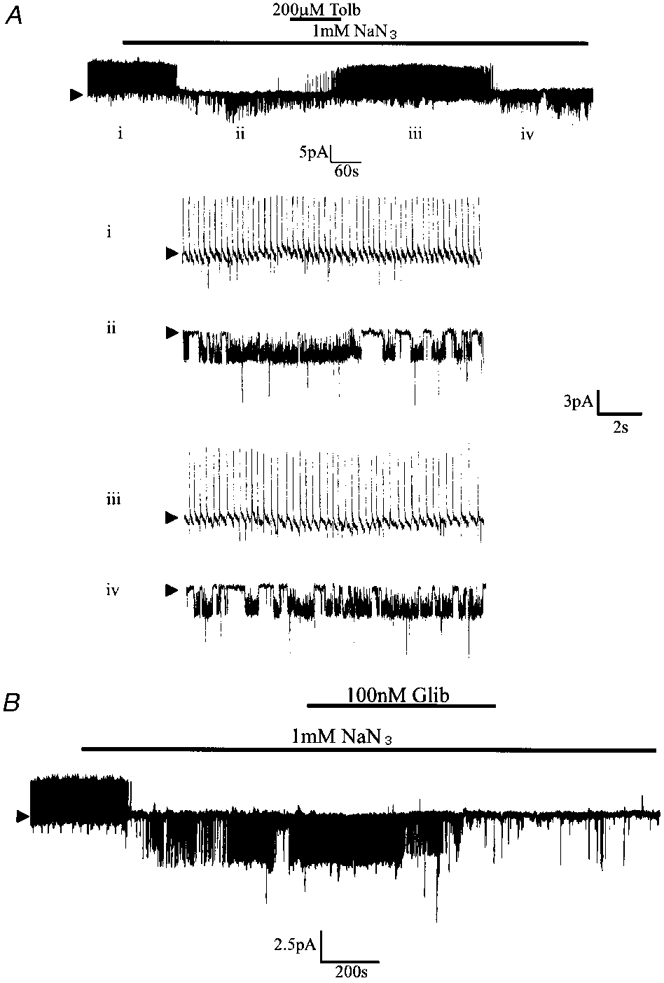
A, cell-attached recording made at a pipette potential of 0 mV. Bath application of NaN3 induced the activity of a tolbutamide-sensitive 3.5 pA channel. The numerals refer to the traces shown on an expanded time scale in the lower part of the figure. B, cell-attached recording at 0 mV illustrating the effect of 100 nM glibenclamide (Glib) on the NaN3-activated channel.
In contrast to these findings, in control experiments performed on seven VMH neurones whose activity was not altered by removal of extracellular glucose or metabolic inhibition with NaN3, neither tolbutamide nor glibenclamide was found to affect action potential firing rate or background channel activity (not shown).
Identification of KATP channel activity
When VMH neurones were dialysed with an ATP-free solution and glucose was removed from the physiological saline, 86 out of 234 neurones underwent a gradual hyperpolarization with cessation of action potential firing and an associated decrease in apparent input resistance. The initial resting membrane potential of these neurones was -49.4±3.2 mV (n = 42) and the input resistance was 395 ± 43.2 MΩ (n = 25). At this membrane potential, these cells were found to fire spontaneous action potentials at a rate of 1-4 Hz. The amplitude of these action potentials was 72.5 ± 2.9 mV (n = 27) whilst the amplitude of the associated after-hyperpolarization was 17.3 ± 1.2 mV (n = 27). Morphological analysis of Lucifer Yellow-filled cells revealed that these neurones were usually multipolar with three or more primary dendrites extending from the soma (n = 8/12). Furthermore, this analysis revealed these cells to be located towards the upper and lateral borders of the VMH.
Within approximately 10 min of membrane breakthrough, these neurones hyperpolarized from their initial resting membrane potential to -71.6 ± 5.2 mV (n = 42). This hyperpolarization was associated with a marked reduction in apparent input resistance (to 123.5 ± 12.3 MΩ; n = 42, Fig. 4A).
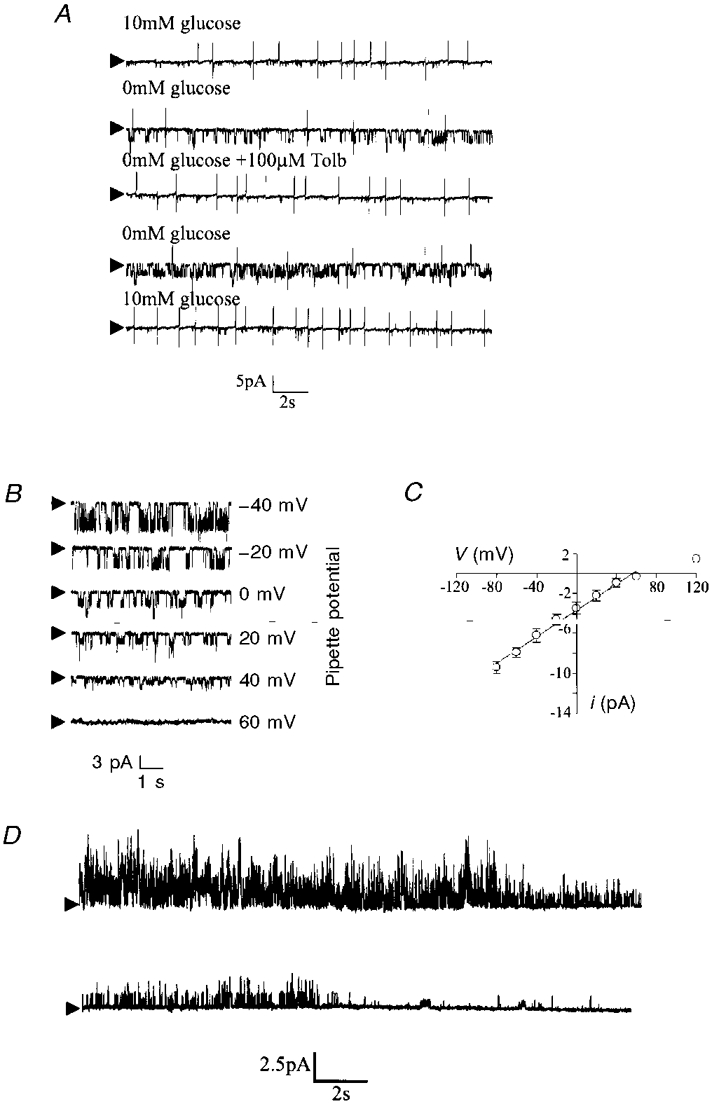
Figure 4. The effect of dialysis with an ATP-free electrode solution.
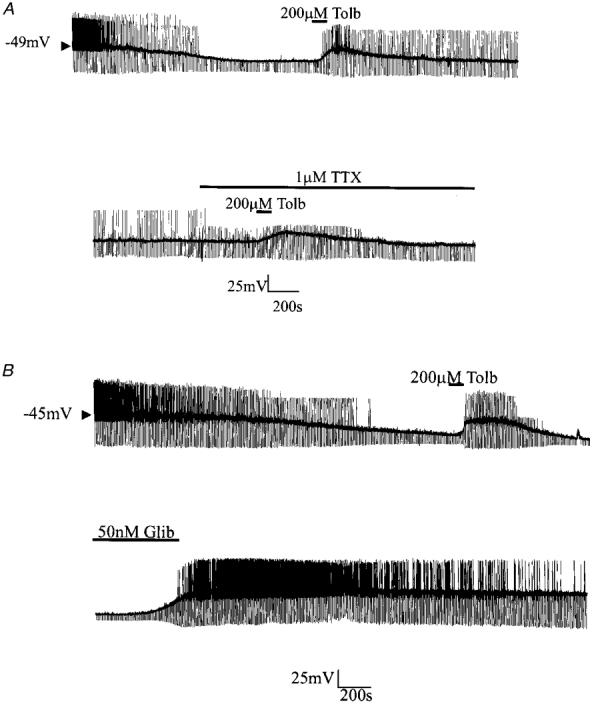
A, continuous whole-cell current-clamp recording demonstrating the effect of tolbutamide on the hyperpolarization resulting from dialysis with an ATP-free electrode solution. The effects of tolbutamide were retained in the presence of TTX. B, continuous whole-cell current-clamp recording illustrating the effect of glibenclamide on membrane properties.
Bath application of the sulphonylurea tolbutamide at this point rapidly (within 2 min) depolarized neurones (by 19.5 ± 3.9 mV; n = 21) with a concomitant increase in apparent input resistance (to 281.9 ± 11.1 MΩ; n = 21) and return of action potential firing (Fig. 4A). These effects of tolbutamide were reversible on washout. The ability of tolbutamide to depolarize these cells and to increase input resistance persisted after treatment of the slice with 1 μM TTX (control, 18.1 ± 4.5 mV depolarization; 1 μM TTX, 18.2 ± 4.1 mV depolarization; n = 5).
In contrast to previous findings (Ashford et al. 1990b) all neurones found to respond in this manner were also sensitive to the second generation sulphonylurea glibenclamide (n = 15; Fig. 4B). Bath application of 50-100 nM glibenclamide induced a slow (taking 3-5 min) membrane depolarization 22.3 ± 5.2 mV (n = 15) in magnitude that was associated with a concomitant increase in apparent input resistance (from 111.9± 12.1 MΩ (n = 21) to 312.9± 14.2 MΩ (n = 15)). Glibenclamide was found to exert similar effects in the presence of 1 μM TTX (n = 5, not shown).
To determine the nature of the sulphonylurea-sensitive current(s) responsible for these effects, neurones were voltage clamped at -60 mV. At this potential, an outward current of 122.3 ± 25.3 pA (n = 28) was observed to develop over time when ATP was omitted from the electrode solution (Fig. 5A). The development of this outward current exhibited a similar time course to the hyperpolarization seen in current-clamp recordings and was associated with an increase in cellular conductance (from 4.5 ± 0.5 to 9.2 ± 0.7 nS as assessed by 10 mV hyperpolarizing step commands (n = 5) from -60 mV). Using depolarizing ramps from -140 to -60 mV this time-dependent outward current was found to exhibit a reversal potential of -98.5 ± 3.9 mV (n = 12) under the present ionic conditions (Fig. 5B).
Figure 5. The effect of glibenclamide on the time-dependent outward current at a holding potential of -60 mV.
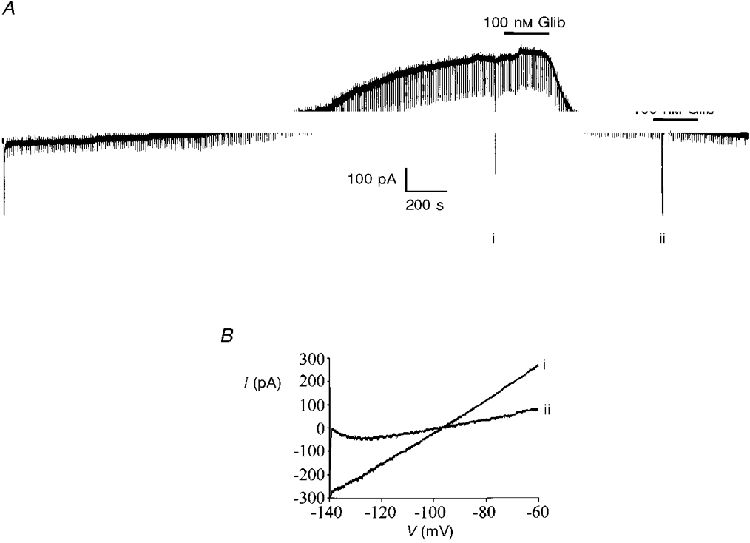
A, the large deflection at the start of the recording indicates formation of the whole-cell configuration. The regular downward pulses are current responses to -10 mV steps whilst the larger labelled deflections are the current responses to voltage ramps from -140 to -60 mV as depicted in B. B, expanded traces of the current responses to the voltage ramps shown in A. The numerals beside these currents correspond to those shown in A.
Once this time-dependent current had reached its peak magnitude, bath application of either tolbutamide (200 μM) or glibenclamide (100 nM) was found to inhibit this current (Fig. 5A). However, although the inhibitory effects of tolbutamide were reversible on washout, the inhibition produced by glibenclamide was not reversed during the time course of these experiments. The ability of both tolbutamide and glibenclamide to inhibit this current was unaffected by pre-application of the glutamate receptor antagonists D-APV (50 μM) and NBQX (1 μM), or by the use of a Ca2+-free artificial cerebrospinal fluid (ACSF) containing 10 mM MgCl2 in conjunction with D-APV and NBQX, thus discounting any possible presynaptic effects (Fig. 5A).
In a small number of experiments neurones identified as glucose receptive using cell-attached patch recordings were subsequently re-patched using whole-cell electrodes containing a pipette solution devoid of ATP. In each case, these neurones gradually underwent a hyperpolarization that was indistinguishable from that described above and which was readily reversed by application of 200 μM tolbutamide (n = 4, not shown). Furthermore, three cells which were characterized as non-glucose receptive using cell-attached recording criteria did not undergo such a sequence of events when susequently re-patched using ATP-free whole-cell electrodes.
Pharmacology of the KATP channel current
Since previous studies report that the KATP channel current present in VMH neurones differs from that found in the periphery, we examined the effects of several potassium channel inhibitors on the sulphonylurea-sensitive current that develops when glucose-receptive neurones are held at -60 mV and ATP is removed from the electrode solution. (see Fig. 6).
Figure 6. Pharmacology of the sulphonylurea-sensitive current.
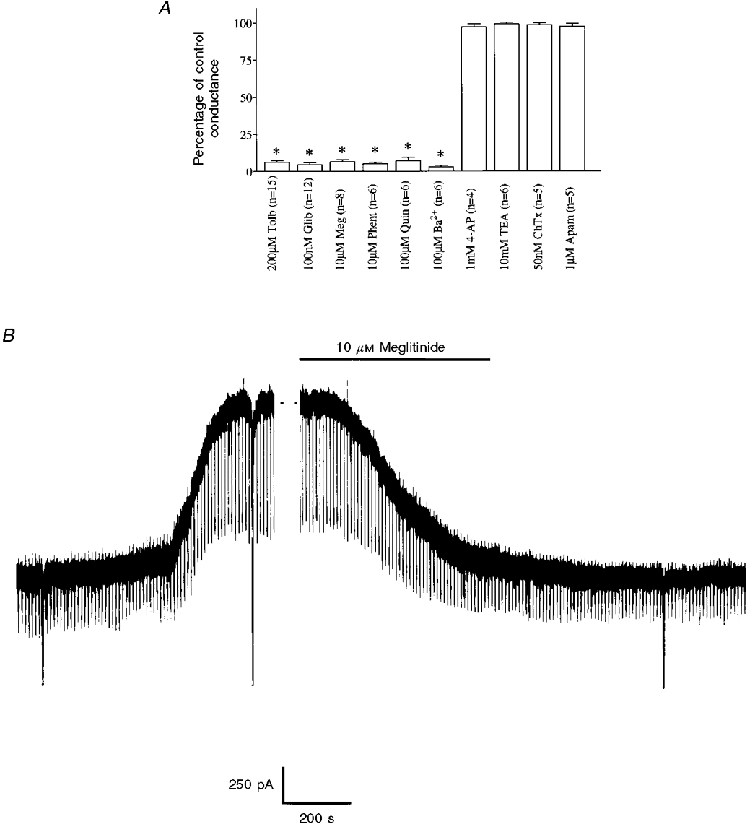
A, mean data illustrating the pharmacology of the sulphonylurea-sensitive current at -60 mV. Control conductance was measured as the difference between five consecutive -10 mV voltage steps immediately following membrane breakthrough and after complete activation of the KATP channel current. Each value is the mean of the number of observations indicated in parentheses. Error bars indicate s.e.m.* Significantly less than control, P < 0.05. Meg, meglitinide; Phent, phentolamine; Quin, quinine; ChTX, charybdotoxin; Apam, apamin. B, the effect of meglitinide in a cell voltage clamped at -60 mV. The large vertical deflections are current responses to voltage ramps whilst the regular pulses are current responses to -10 mV voltage steps.
Under these conditions this current was completely and reversibly inhibited by application of the non-specific potassium channel inhibitor BaCl2 (100 μM). Similarly, this conductance was sensitive to bath application of quinine (100 μM) although neither 10 mM TEA+ nor 1 mM 4-aminopyridine (4-AP) was found to exert any significant effect on this current (Fig. 6A).
In addition to tolbutamide and the second generation sulphonylurea glibenclamide, the non-sulphonylurea structural component of glibenclamide, HB699 or meglitinide (Lee et al. 1994a), also inhibited this current in a poorly reversible manner (Fig. 6B). Similarly, the imidazoline compound phentolamine (Fig. 6A; Dunne, 1991) inhibited this current in a manner that was not readily reversible on continued washing. In contrast to the inhibition observed with the above compounds the Ca2+-activated potassium channel inhibitors charybdotoxin and apamin were both without effect on this current in these cells (Fig. 6A).
Potassium channel openers
In cell-attached recordings the effect of 500 μM diazoxide was tested in seven glucose-receptive neurones. This potassium channel opener reduced the rate of action potential firing via the activation of a tolbutamide-sensitive channel with properties indistinguishable from those observed above. In contrast, 500 μM diazoxide was without significant effect in five cells that were insensitive to glucose. These effects of diazoxide on glucose-receptive neurones were mimicked by bath application of the physiologically active fragment of leptin (leptin 22-56, 50 nM; n = 3; Fig. 7). However, in contrast the effects of this peptide took longer to develop and were not readily reversed.
Figure 7. The effect diazoxide and the active fragment of leptin on the electrical characteristics of a glucose-receptive neurone.
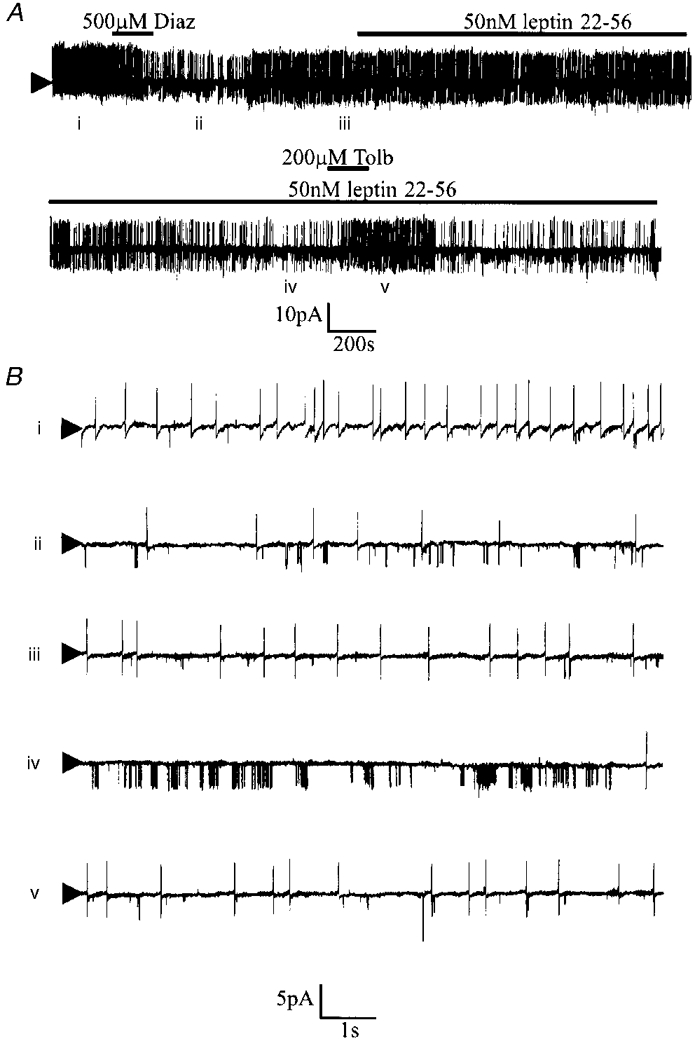
A, cell-attached recording made at a pipette potential of 0 mV. Bath application of diazoxide or leptin 22-56 induced the activity of a tolbutamide-sensitive 3.5 pA channel. The numerals refer to the traces shown on an expanded time scale in B.
In whole-cell recordings in the presence of 4 mM Na2ATP, no sulphonylurea-sensitive hyperpolarization was observed to develop with time in any of the VMH neurones tested (n = 35). Under these conditions, bath application of 500 μM diazoxide hyperpolarized 12 of the neurones tested (by 14.1 ± 3.9 mV; n = 12, Fig. 8) in a manner that was unaffected by treatment of the slice with 1 μM TTX (n = 5) but was blocked by co-application of tolbutamide (200 μM, n = 3, not shown).
Figure 8. The effect of diazoxide on VMH glucose-receptive neurones.
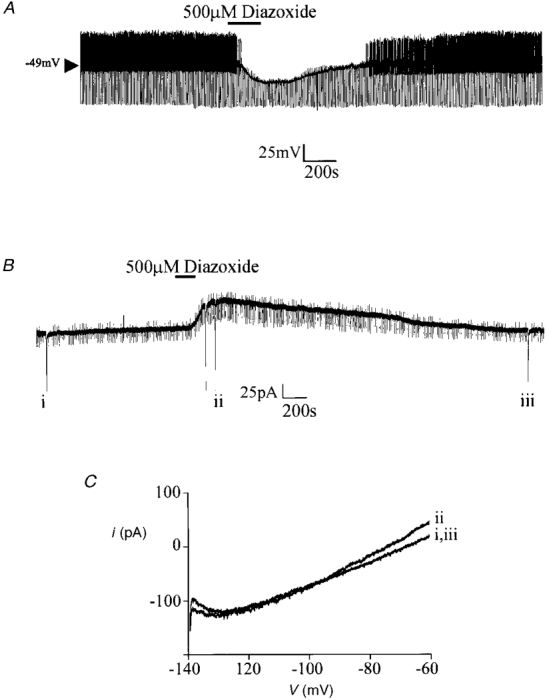
A, in the presence of 4 mM ATP in the electrode, diazoxide induces a membrane hyperpolarization with an associated decrease in input resistance. B, in a neurone voltage clamped at -60 mV, diazoxide induces an outward current. The regular downward pulses are current responses to -10 mV steps whilst the large deflections are the current responses to voltage ramps from -140 to -60 mV as depicted in C. C, expanded traces of the current responses to the voltage ramps shown in B. The numerals beside these currents correspond to those shown in B.
Similarly, with 4 mM Na2ATP in the electrode solution, no time-dependent outward current was observed in neurones voltage clamped at -60 mV. However, bath application of diazoxide induced an outward current, 89.3 ± 32.4 pA in magnitude (n = 9, Fig. 8B), which was associated with an increase in cellular conductance (from 4.2 ± 0.6 to 8.9 ± 0.5 nS as assessed by 10 mV hyperpolarizing step commands; n = 5) and had a reversal potential of -99.2 ± 4.4 mV with 2.5 mM K+ present in the extracellular solution (n = 5, not shown). In contrast, when diazoxide was applied concomitantly with 200 μM tolbutamide, this outward current was not seen to develop (n = 3, not shown). The effects of diazoxide were not mimicked by the potassium channel openers pinacidil (500 μM, n = 4) or levcromakalim (500 μM, n = 4).
Single cell RT-PCR studies
The results presented so far suggest that the channel responsible for conferring glucose sensitivity upon these VMH neurones has a similar sulphonylurea and diazoxide sensitivity to the KATP channel found in pancreatic β cells (Ashcroft & Ashcroft, 1990). Recent studies have shown that these channels are formed by the molecular interaction between an inwardly rectifying K+ channel subunit (Kir 6.1 or Kir 6.2; Inagaki et al. 1995b; Sakura et al. 1995) and a high affinity receptor for the sulphonylureas (SUR1 or SUR2; Inagaki et al. 1995a). To examine whether the KATP channel present in these glucose-receptive neurones was formed from the same subunits the cytoplasm of six cells identified as glucose responsive under whole-cell recording conditions was harvested and subjected to reverse transcription. The resulting cDNA was subsquently amplified and subjected to PCR analysis using primer oligonucleotides specific for the housekeeper genes synaptotagmin 1, cytochrome oxidase and α-tubulin together with the known KATP channel subunits (Kir6.1, Kir6.2, SUR1 and SUR2) and the long form of the leptin receptor (Ob-Rb). In each glucose-receptive neurone tested, mRNA for the three housekeeping genes and the leptin receptor was detected. Importantly, the KATP channel subunits Kir6.1 and SUR1 were also detected whilst neither Kir6.2 nor SUR2 mRNA was found (Fig. 9Ba).
Figure 9. Molecular identity of the KATP channel complex.
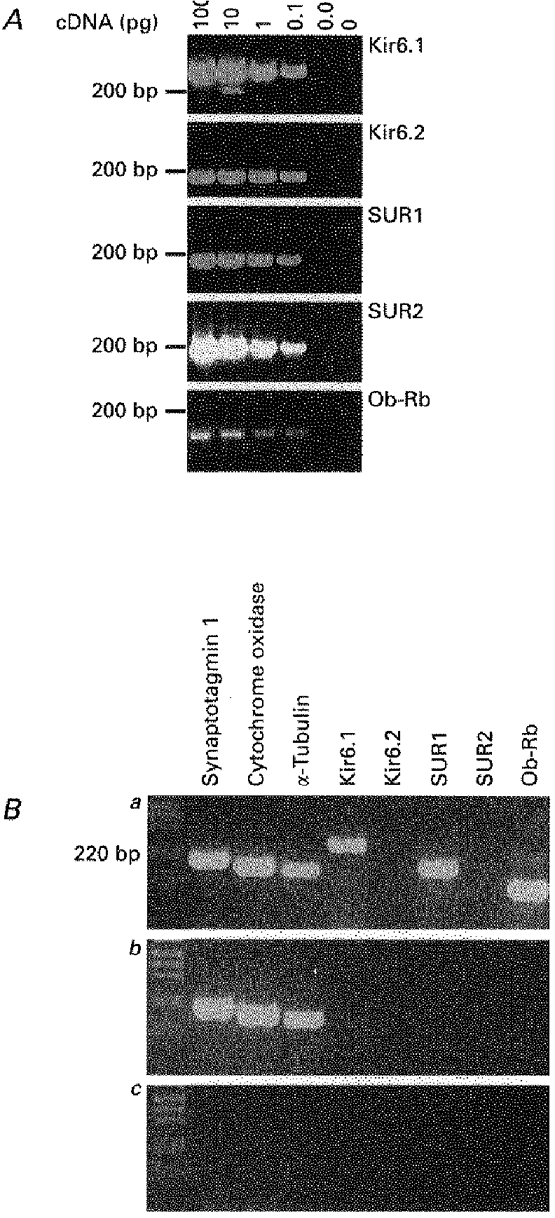
A, 2.5% agarose gel showing the sensitivity of the Kir6.1, Kir6.2, SUR1, SUR2 and leptin receptor primer pairs. Ba, using the cytoplasmic contents harvested from a single glucose-receptive VMH neurone, the expression of the KATP channel subunits Kir6.1 and SUR1 together with the housekeeping genes synaptotagmin 1, cytochrome oxidase and α-tubulin, and the leptin receptor gene Ob-Rb were detectable. Bb, using the cytoplasmic contents harvested from a single non-glucose-receptive VMH neurone only the housekeeping genes synaptotagmin 1, cytochrome oxidase and α-tubulin were detectable. Bc, under the same conditions, replacement of cytoplasmic contents with the same volume of ACSF from the bath solution failed to yield any products.
In a parallel control experiment, the cytoplasmic contents of five non-glucose-receptive VMH neurones were harvested and after reverse transcription the cDNA was subjected to PCR analysis using the same primer oligonucleotides as used on the glucose-receptive neurones. As observed in the glucose-receptive neurones, the housekeeping genes synaptotagmin 1, cytochrome oxidase and α-tubulin were detected. However although one of the five cells was found to express mRNA for SUR2, none of the five cells was found to contain mRNA for the leptin receptor or the KATP channel subunits present in the glucose-receptive neurones (Fig. 9Bb).
To confirm that this pattern of KATP channel expression was indeed representative of the glucose-receptive neurones under study, the cytosolic contents of four neurones identified as glucose responsive using cell-attached recordings were also harvested and examined for KATP channel expression. In this instance the KATP channel subunits Kir6.1 and SUR1 were again detected whilst neither Kir6.2 nor SUR2 mRNA was found (Fig. 10).
Figure 10. Glucose-receptive neurones in the VMH express KATP channels composed of Kir6.1 and SUR1 subunits.
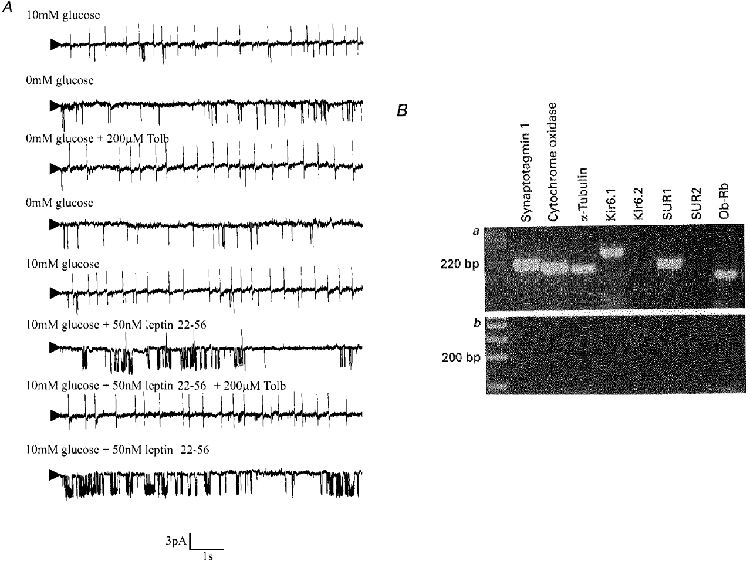
A, cell-attached recording made at a pipette potential of 0 mV. Removal of glucose from the bath led to the reversible activation of a 3.7 pA channel which was inhibited by tolbutamide. Leptin 22-56 mimicked the effect of glucose removal in a non-reversible manner. Ba, using the cytoplasmic contents harvested from the same glucose-receptive VMH neurone, the expression of the KATP channel subunits Kir6.1 and SUR1 together with the housekeeping genes synaptotagmin 1, cytochrome oxidase and α-tubulin, and the leptin receptor gene Ob-Rb were detectable. Bb, in a parallel control experiment, replacement of cytoplasmic contents with the same volume of ACSF from the bath solution failed to yield any PCR products.
DISCUSSION
Identification of glucose-receptive neurones in the rat VMH
Using cell-attached patch recordings, a subset of VMH neurones has been identified whose firing rates are regulated by the concentration of extracellular glucose. This ability to respond to extracellular glucose was found to be due to the presence of a sulphonylurea-sensitive channel with characteristics similar to those found in the pancreatic β cell. In accordance with these findings, whole-cell recordings, performed with an electrode solution containing no ATP, hyperpolarized a similar proportion of VMH neurones through the activation of a sulphonylurea-sensitive outward current possessing a reversal potential close to the calculated equilibrium potential for potassium ions (approximately -100 mV). Since this current was not observed when ATP was present in the electrode solution it is likely that the outward sulphonylurea-sensitive current observed in this study is also ATP sensitive. In a further series of experiments we were able to exploit the ability to identify visually and hence re-patch individual neurones to confirm that those cells identified as glucose responsive in cell-attached recordings were synonymous with those hyperpolarized by intracellular dialysis with an ATP-free solution.
Examination of the resting membrane properties of these neurones immediately following formation of the whole-cell configuration revealed characteristics similar to those previously associated with glucose-receptive neurones in the guinea-pig VMH using sharp electrodes (Minami et al. 1986) and those of rat type 3 VMH neurones previously described by Priestley (1992).
Properties of the KATP channel current
Under voltage-clamp conditions, this KATP channel current was sensitive to the sulphonylureas tolbutamide and glibenclamide and to the non-sulphonylurea moiety of glibenclamide, meglitinide. The ability of these agents to inhibit the KATP channel current profoundly at nanomolar and low micromolar concentrations together with their irreversibility is similar to that seen for both the native and cloned pancreatic β cell KATP channel complexes (Kir6.2 and SUR1; Lee et al. 1994a; Gribble et al. 1997). Furthermore, this current also displays a similar sensitivity to the imidazoline KATP channel inhibitor phentolamine, and to the potassium channel opener diazoxide (Dunne, 1991). On the basis of these findings, it would appear that the KATP channel complex present in these neurones is pharmacologically similar to the complex present in the β cell.
In agreement with this suggestion, in cell-attached patch recordings, the unitary properties of the sulphonylurea-sensitive channel were virtually indistinguishable from those reported in the pancreatic β cell. Furthermore, single cell RT-PCR analysis demonstrated the co-expression of Kir6.1 and SUR1 subunits in these neurones. Although this combination of KATP channel subunits has not been observed in the periphery, we have recently found that the same combination comprises the KATP channel in rat striatal cholinergic interneurones (Lee et al. 1998). Interestingly the unitary conductance of the two channels appears to differ in the two sets of neurones raising the possibilty that other as yet unknown factors also contribute to the properties of the native KATP channel complex.
It is noteworthy that the unitary conductance of the Kir6.1 subunit has been demonstrated to differ when expressed with different ABC transporter molecules. Thus when expressed alone, this subunit exhibits a unitary conductance of 70 pS (Inagaki et al. 1995b) compared with 33 or 52 pS when expressed with SUR2B or cystic fibrosis transmembrane conductance regulator (CFTR), respectively, (Yamada et al. 1997; Ishida-Takahashi et al. 1998). Although these discrepancies may be related to differences in experimental protocol it is also feasible that ABC transporter molecules together with other as yet unknown factors can affect the conductance properties of this KATP channel subunit in a presently poorly understood manner.
Comparison with previous studies
These results differ considerably from previous studies performed upon glucose-receptive neurones in this nucleus. In these former studies, the channel was found to exhibit a much larger unitary conductance (150 pS) than that reported here and was seemingly unaffected by glibenclamide. In addition, this channel was found to be insensitive to the potassium channel opener diazoxide (Sellars et al. 1992). At present we are unable to provide a definitive answer for these discrepancies, although a number of potential reasons exist.
One possibility is that the former and present studies were performed on the same KATP channel complex but that the different experimental conditions employed produced an alteration in the channel properties. With regard to this it is noteworthy that the results obtained previously were from enzymatically dispersed neurones from the VMH area. It is now well established that enzymes such as trypsin when applied to the intracellular surface of the KATP channel complex alter both the ATP and sulphonylurea sensitivity of these molecules (Lee et al. 1994b). However, this procedure has not previously been shown to alter the unitary conductance of such channels and we consider that it is unlikely for these proteases to have gained access to the intracellular surface of neurones that have remained viable for electrophysiological analysis. Additionally, a second group has used a similar enzymatic dispersal procedure and obtained a glibenclamide sensitivity more akin to that obtained in the present study (Routh et al. 1997). These authors suggest that the difference in unitary conductance may be due to the channel adopting a lower subconductance state in their studies. While this remains feasible, this suggestion cannot account for the different pharmacological properties found in this study.
Another possibilty for these discrepancies is that more than one type of glucose-receptive neurone exists within the VMH. However, if this were the case, it is hard to reconcile why this second type of glucose-receptive neurone was not identified in at least some of the recordings performed in the present study. Since previous studies have been performed on acutely dispersed neurones obtained from hypothalamic slices containing the VMH (and also arcuate nucleus; M. L. J. Ashford, personal communication) the precise location of this second class of glucose-receptive neurone is not entirely known. It is therefore plausible that glucose-receptive neurones in the VMH are composed of classical KATP channel subunits whilst a second class of glucose-receptive neurone exists in hypothalamic nuclei bordering the VMH containing a different type of KATP channel. Furthermore, since former studies have been performed primarily on excised patches, the rapid rate of run-down exhibited by the present channel would immediately bias results in favour of the non-classical KATP channel which does not run down.
This suggestion is supported by the results of Routh et al. (1997) which were obtained using punch dissections of the VMH. Using this procedure, a channel with characteristics similar to those found presently was reported. In these studies, the authors report that the channel was sensitive to micromolar concentrations of glibenclamide although it is unclear whether lower concentrations were tested. The glibenclamide-sensitive channel was also found to exhibit a rapid loss of activity on patch excision due to run-down but this process was reversed by phosphorylating conditions.
Physiological significance
It is now well established that the VMH is an important satiety centre in the regulation of food intake. Lesions of this nucleus are known to cause hyperphagia whilst electrical stimulation suppresses food intake (Anand et al. 1964). Furthermore, various neuromodulators of food intake are known to exert their effects at least partly via the VMH (Morley & Levine, 1985). It is also well established that a subset of VMH neurones are able to modify their electrical activity in response to changes in the extracellular concentration of glucose (Ono et al. 1982). Recent studies have demonstrated that the ability of these neurones to react to changes in blood glucose may be mediated by KATP channel activity (Ashford et al. 1990a).
In the present series of experiments we have confirmed these observations. However, the nature of the KATP channel conductance observed in the present study contrasts significantly with that obtained previously in the rat. In the present study we demonstrate that the pharmacological and molecular biological properties of this channel are essentially the same as those found for the KATP channel complex in tissues such as the pancreatic β cell and mouse VMH (Rowe et al. 1998). Since we have been unable to distinguish a second class of glucose-receptive neurone in this study, the exact location and molecular make-up of these cells remain to be elucidated.
In future studies it will be important to examine the physiological and pathophysiological modulation of this channel complex with a view to elucidating its potential for therapeutic intervention. In relation to this it is interesting to note that both our studies and those of other groups (Spanswick et al. 1997) indicate a close and apparently exclusive relationship between the peptide hormone leptin and glucose-receptive neurones in this area of the CNS. These findings support the suggestion that these neurones perform an important role in the regulation of food intake and indicate that it may be possible to modulate the activity of these neurones therapeutically.
References
- Anand BK, Chhina GS, Sharma KN, Dua S, Singh B. Activity of single neurons in the hypothalamic feeding centres: effects of glucose. American Journal of Physiology. 1964;207:1146–1154. doi: 10.1152/ajplegacy.1964.207.5.1146. [DOI] [PubMed] [Google Scholar]
- Ashcroft SJH, Ashcroft FM. Properties and functions of ATP-sensitive K+ channels. Cellular Signalling. 1990;2:197–214. doi: 10.1016/0898-6568(90)90048-f. 10.1016/0898-6568(90)90048-F. [DOI] [PubMed] [Google Scholar]
- Ashford MLJ, Boden PR, Treherne JM. Glucose-induced excitation of hypothalamic neurones is mediated by ATP-sensitive K+ channels. Pflügers Archiv. 1990a;415:479–483. doi: 10.1007/BF00373626. [DOI] [PubMed] [Google Scholar]
- Ashford MLJ, Boden PR, Treherne JM. Tolbutamide excites rat glucoreceptive ventromedial hypothalamic neurones by indirect inhibition of ATP-K+ channels. British Journal of Pharmacology. 1990b;101:531–540. doi: 10.1111/j.1476-5381.1990.tb14116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon AK, Richardson PJ, Lee K, Carter N, Freeman TC. Expression profiling of single cells using 3 prime end amplification (TPEA) PCR. Nucleic Acids Research. 1998;26:4426–4431. doi: 10.1093/nar/26.19.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne MJ. Block of ATP-regulated potassium channels by phentolamine and other adrenoceptor antagonists. British Journal of Pharmacology. 1991;103:1847–1850. doi: 10.1111/j.1476-5381.1991.tb12340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn-Meynell AA, Govek E, Levin BE. Intracarotid glucose infusions selectively increase FOS-like immunoreactivity in paraventricular and ventromedial nucleus neurons. Brain Research. 1997;748:100–106. doi: 10.1016/s0006-8993(96)01280-2. [DOI] [PubMed] [Google Scholar]
- Grace AA, Llinas R. Morphological artifacts induced in intracellularly stained neurons by dehydration; circumvention using rapid dimethyl sulfoxide clearing. Neuroscience. 1985;16:461–475. doi: 10.1016/0306-4522(85)90018-1. [DOI] [PubMed] [Google Scholar]
- Gribble FM, Ashfield R, Ämmälä C, Ashcroft FM. Properties of cloned ATP-sensitive K+ currents expressed in Xenopus oocytes. The Journal of Physiology. 1997;498:87–98. doi: 10.1113/jphysiol.1997.sp021843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JP, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: An inward rectifier subunit plus the sulfonylurea receptor. Science. 1995a;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Tsuura Y, Namba N, Masuda K, Gonoi T, Horie M, Mizuta M, Seino S. Cloning and functional characterization of a novel ATP-sensitive potassium channel ubiquitously expressed in rat tissues including pancreatic islets, pituitary, skeletal muscle and heart. Journal of Biological Chemistry. 1995b;270:5691–5694. doi: 10.1074/jbc.270.11.5691. [DOI] [PubMed] [Google Scholar]
- Ishida-Takahashi A, Otani H, Takahashi C, Washizuka T, Tsuji K, Noda K, Horie M, Sasayama S. Cystic fibrosis transmembrane conductance regulator mediates sulphonylurea block of the inwardly rectifying K+ channel Kir6.1. The Journal of Physiology. 1998;508:23–30. doi: 10.1111/j.1469-7793.1998.023br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski RZ, Hales CN, Ashford MLJ. Dual effects of diazoxide on ATP-K+ currents recorded from an insulin-secreting cell line. British Journal of Pharmacology. 1989;97:1039–1050. doi: 10.1111/j.1476-5381.1989.tb12560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Dixon AK, Freeman TC, Richardson PJ. Identification of a KATP channel current in rat striatal cholinergic interneurones. The Journal of Physiology. 1998;510:441–453. doi: 10.1111/j.1469-7793.1998.441bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Ozanne SE, Hales CN, Ashford MLJ. Mg2+ dependent inhibition of KATP channels by sulphonylureas in CRI-G1 insulin secreting cells. British Journal of Pharmacology. 1994a;111:632–640. doi: 10.1111/j.1476-5381.1994.tb14783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Ozanne SE, Rowe ICM, Hales CN, Ashford MLJ. The effects of trypsin on ATP-sensitive potassium channel properties and sulfonylurea receptors in the CRI-G1 insulin-secreting cell line. Molecular Pharmacology. 1994b;45:176–185. [PubMed] [Google Scholar]
- Minami T, Oomura Y, Sugimora M. Electrophysiological properties and glucose responsiveness of guinea-pig ventromedial hypothalamic neurones in vitro. The Journal of Physiology. 1986;380:127–143. doi: 10.1113/jphysiol.1986.sp016276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JE, Levine AS. The pharmacology of eating behavior. Annual Review of Pharmacology and Toxicology. 1985;25:127–146. doi: 10.1146/annurev.pa.25.040185.001015. [DOI] [PubMed] [Google Scholar]
- Ono T, Nishino H, Fukuda M, Sasaki K, Muramoto K-I, Oomura Y. Glucoresponsive neurons in rat ventromedial hypothalamic tissue slices in vitro. Brain Research. 1982;232:494–499. doi: 10.1016/0006-8993(82)90295-5. [DOI] [PubMed] [Google Scholar]
- Powis JE, Bains JS, Ferguson AV. Leptin depolarises rat hypothalamic paraventricular nucleus neurons. American Journal of Physiology. 1998;274:R1468–1472. doi: 10.1152/ajpregu.1998.274.5.R1468. [DOI] [PubMed] [Google Scholar]
- Priestley T. The effect of baclofen and somatostatin on neuronal activity in the rat ventromedial hypothalamic nucleus in vitro. Neuropharmacology. 1992;31:103–109. doi: 10.1016/0028-3908(92)90018-k. [DOI] [PubMed] [Google Scholar]
- Routh VH, McArdle JJ, Levin BE. Phosphorylation modulates the activity of the ATP-sensitive K+ channel in the ventromedial hypothalamic nucleus. Brain Research. 1997;778:107–119. doi: 10.1016/s0006-8993(97)01043-3. [DOI] [PubMed] [Google Scholar]
- Rowe ICM, Spanswick D, Ashford MLJ. The characterization of potassium channels present in glucose- and sulphonylurea-sensitive hypothalamic neurones in isolation and in brain slices of the mf1 mouse. The Journal of Physiology. 1998;506.P:152P. [Google Scholar]
- Sakura H, Ämmälä C, Smith PA, Gribble FM, Ashcroft FM. Cloning and functional expression of the cDNA encoding a novel ATP-sensitive potassium channel expressed in pancreatic β cells, brain, heart and skeletal muscle. FEBS Letters. 1995;377:338–344. doi: 10.1016/0014-5793(95)01369-5. [DOI] [PubMed] [Google Scholar]
- Sellars AJ, Boden PR, Ashford MLJ. Lack of effect of potassium channel openers on ATP-modulated potassium channels recorded from rat ventromedial hypothalamic neurones. British Journal of Pharmacology. 1992;107:1068–1074. doi: 10.1111/j.1476-5381.1992.tb13408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford MLJ. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature. 1997;390:521–525. doi: 10.1038/37379. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Dodt H-U, Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflügers Archiv. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Sturgess NC, Hales CN, Cook DL, Ashford MLJ. The sulphonylurea receptor may be an ATP-sensitive K+ channel. Lancet. 1985;ii:474–475. doi: 10.1016/s0140-6736(85)90403-9. [DOI] [PubMed] [Google Scholar]
- Yamada M, Isomoto S, Matsumoto S, Kondo C, Shindo T, Horio Y, Kurachi Y. Sulphonylurea receptor 2B and Kir6.1 form a sulphonylurea-sensitive but ATP-insensitive K+ channel. The Journal of Physiology. 1997;499:715–720. doi: 10.1113/jphysiol.1997.sp021963. [DOI] [PMC free article] [PubMed] [Google Scholar]


