Abstract
The effect of membrane potential on [Ca2+]i in rat megakaryocytes was studied using simultaneous whole-cell patch clamp and fura-2 fluorescence recordings.
Depolarization from −75 to 0 mV had no effect on [Ca2+]i in unstimulated cells, but evoked one or more spikes of Ca2+ increase (peak increase: 714 ± 95 nM) during activation of metabotropic purinoceptors by 1 μM ADP.
The depolarization-evoked Ca2+ increase was present in Ca2+-free medium and also following removal of Na+. Thus depolarization mobilizes Ca2+ from an intracellular store without a requirement for altered Na+-Ca2+ exchange activity.
Intracellular dialysis with heparin blocked the depolarization-evoked Ca2+ increase, indicating a role for functional IP3 receptors.
Under current clamp, ADP caused the membrane potential to fluctuate between −43 ± 1 and −76 ± 1 mV. Under voltage clamp, depolarization from −75 to −45 mV evoked a transient [Ca2+]i increase (398 ± 91 nM) during exposure to ADP.
We conclude that during stimulation of metabotropic purinoceptors, membrane depolarization over the physiological range can stimulate Ca2+ release from intracellular stores in the rat megakaryocyte, a non-excitable cell type. This may represent an important mechanism by which electrogenic influences can control patterns of [Ca2+]i increase.
Non-excitable cell types are characterized by an inability to generate all-or-none action potentials in response to depolarizing stimuli due to a lack of voltage-gated Na+ or Ca2+ channels (Rink & Jacob, 1989; Fewtrell, 1993; Clapham, 1995; Berridge, 1997). Consequently, membrane potential changes are proposed to influence [Ca2+]i responses mainly by altering the driving force for Ca2+ entry through ligand-gated or second messenger-operated channels. Indeed, several reports have shown that depolarization results in a decrease in [Ca2+]i and hyperpolarization causes an increase of [Ca2+]i during activation of mast cells, lymphocytes and related cell lines (Penner et al. 1988; Lewis & Cahalan, 1989; Demaurex et al. 1992).
Previous electrophysiological recordings from rat bone marrow-derived megakaryocytes have failed to detect voltage-dependent inward currents, which implies that this is a non-excitable cell type (Uneyama et al. 1993a; Somasundaram & Mahaut-Smith, 1994; Hussain & Mahaut-Smith, 1998). We now report that, during stimulation of metabotropic purinoceptors, membrane depolarization evokes an increase in [Ca2+]i, primarily due to release of Ca2+ from intracellular stores. Brief reports of this work have appeared elsewhere in abstract form (Mahaut-Smith et al. 1998a,b).
METHODS
Male Wistar rats (150-300 g) were killed by CO2 asphyxiation or cervical dislocation. Megakaryocytes were isolated from femoral and tibial marrow as previously described (Hussain & Mahaut-Smith, 1998) with the exception that the marrow extract was not filtered through nylon mesh. The standard external saline contained (mM): 145 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 Hepes, 10 D-glucose, titrated to pH 7.35 with NaOH. For Ca2+-free saline, CaCl2 was replaced by an equal concentration of MgCl2; 0.5 mM EGTA was also included in some experiments. The standard pipette saline contained (mM): 150 KCl, 2 MgCl2, 0.1 EGTA, 0.05 Na2GTP, 0.05 K5fura-2, 10 Hepes, adjusted to pH 7.2 with KOH. Membrane potentials have not been corrected for the small offset (approximately -3 mV) that results from the liquid-liquid junction potential between internal and external salines. ADP was applied either from a nearby puffer pipette under the control of a PLI-100 picolitre injector (Medical Systems, Greenvale, NY, USA) or by bath perfusion. K5fura-2 was obtained from Molecular Probes and heparin (average molecular weight 6000) from Sigma.
Conventional whole-cell patch clamp recordings were carried out using an Axopatch 200A amplifier with CV202 headstage (Axon Instruments) in either voltage clamp or slow current clamp mode. pCLAMP 6.0 (Axon Instruments) was used to generate voltage step protocols. Patch pipettes were pulled from borosilicate glass tubing (Clark Electromedical Instruments, UK). Cell capacitance and series resistance (Rs) were in the range 80-408 pF and 2.5-12.5 MΩ, respectively and up to 75%Rs compensation was achieved. A Cairn Spectrophotometer System (Cairn Research Ltd, UK) coupled to a Nikon Diaphot TMD inverted microscope (Nikon, Japan) was used to measure fura-2 fluorescence as previously described (Mahaut-Smith, 1998). Briefly, light from a 75 W xenon arc lamp passed through a spinning filter wheel containing bandpass interference filters to provide alternating 340 and 380 nm excitation. A ×40 1.3 NA Nikon fluor lens focused excitation light onto the cells within a saline-filled chamber. Fluorescence emission from a rectangular area slightly larger than the patch-clamped cell was collected by a photomuliplier tube and sampled at 60 Hz, together with the electrophysiological signals, using Cairn fluorescence software. An emission bandwidth of 480-600 nm was achieved using a 600 nm dichroic mirror together with a 480 nm long pass filter. Data were further averaged to give a final acquisition rate of 15 or 30 Hz and exported for analysis within Microcal Origin (Microcal Software Inc., Northampton, MA, USA). For presentation, 7 point smoothing was applied using a Savitzky-Golay filter within Origin; measurements of [Ca2+]i and latency were from unsmoothed traces. The 340 nm/380 nm ratio under Ca2+-free conditions (Rmin) and that when Fura-2 was saturated with Ca2+ (Rmax), were obtained extracellularly since it was difficult to clamp [Ca2+]i at high levels in the megakaryocyte. A calibration kit (Molecular Probes) was used to derive a Kd for fura-2 (258 nM). After application of a viscosity correction factor (0.85) to Rmin and Rmax (Poenie, 1990), background-corrected 340 nm/380 nm values were converted to [Ca2+]i as described by Grynkiewicz et al. (1985). All recordings were made at the ambient temperature (20-25°C) and statistical values are expressed as the means ±s.e.m.
RESULTS
In unstimulated rat megakaryocytes under whole-cell patch clamp, depolarization to 0 mV (Fig. 1A; n= 46) or -45 mV (not shown; n= 10) from a holding potential of -75 mV had no effect on [Ca2+]i, consistent with a lack of voltage-dependent Ca2+ influx in this cell type. This finding is in agreement with previous electrophysiological studies of rat megakaryocytes which failed to detect voltage-dependent inward currents under K+ current-free conditions (Somasundaram & Mahaut-Smith, 1994, 1995; Hussain & Mahaut-Smith, 1998). Application of 1 μM ADP with the cell clamped at -75 mV evoked a large, transient increase in [Ca2+]i, followed by a sustained plateau level of raised [Ca2+]i (Fig. 1A). In other cells (Fig. 1B), [Ca2+]i oscillated for a variable duration prior to settling at a raised plateau level. In contrast to the unstimulated condition, depolarization to 0 mV during the plateau phase evoked an increase of [Ca2+]i (Fig. 1A and B). This response was observed in all cells tested, although the pattern varied, from a single transient elevation of [Ca2+]i (Fig. 1B) to multiple [Ca2+]i spikes (Fig. 1A). The amplitude of the [Ca2+]i increase showed considerable variation between cells; in 13 cells the peak increase during the initial spike ranged from 322 to 1648 nM (714 ± 95 nM). A clear delay was observed from depolarization to the first detectable increase in [Ca2+]i; in 13 cells this latency ranged from 0.5 to 1.4 s (0.8 ± 0.1 s). The other ionic events activated during cell depolarization in the megakaryocyte include a large outward current (Fig. 1A) due to activation of a voltage-dependent K+ conductance (Kapural, 1995; Romero & Sullivan, 1997), which was largely unaffected during the plateau phase of the Ca2+ increase evoked by ADP. As the voltage-dependent K+ conductance decayed, a smaller Ca2+-dependent K+ current (Uneyama et al. 1993a) was seen to be activated by the voltage-dependent Ca2+ spikes of larger amplitude. The induction of the depolarization-dependent Ca2+ increase by ADP was completely reversible, as shown in Fig. 1.
Figure 1. Depolarization-evoked [Ca2+]i increases during exposure to ADP.
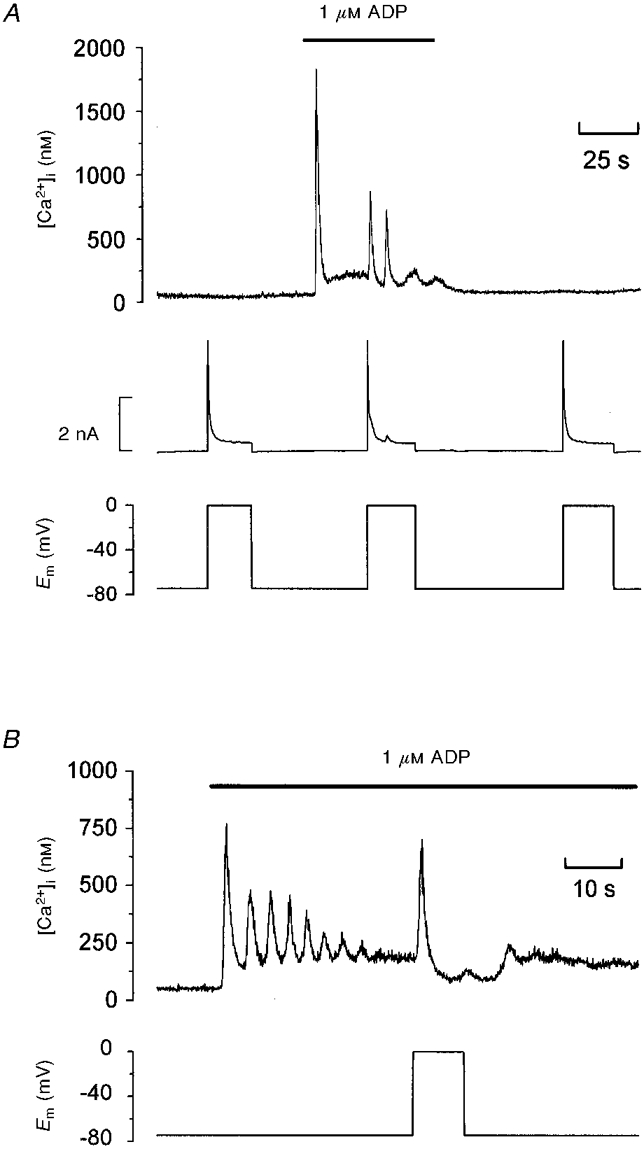
A and B, simultaneous recordings of [Ca2+]i and membrane potential (Em) under whole-cell voltage clamp; the whole-cell current is also shown in A (middle trace). The bars indicate extracellular application of 1 μM ADP. A and B are from two different megakaryocytes representing the range of [Ca2+]i responses to ADP and depolarization.
To assess whether the voltage-dependent Ca2+ increase observed during stimulation by ADP was the result of Ca2+ influx or release from intracellular Ca2+ stores, the experiment was repeated in Ca2+-free saline. As shown in Fig. 2, depolarization from -75 to 0 mV in Ca2+-free (EGTA) saline still evoked a large, transient increase in [Ca2+]i. This effect was seen in nine other cells in Ca2+-free, EGTA-containing saline, and in eight cells perfused with nominally Ca2+-free saline. The [Ca2+]i increase evoked by a depolarization from -75 to 0 mV in the absence of external Ca2+ showed a wide variation in amplitude; in 13 cells the peak increase ranged from 127 to 1031 nM (478 ± 78 nM). The delay from depolarization to the first detectable [Ca2+]i increase was 1.2 ± 0.2 s (n= 13; range 0.6-2.5 s). These data are consistent with a major role for Ca2+ release from an intracellular storage site in the depolarization-evoked increase of [Ca2+]i. Alternatively, voltage modulation of the electrogenic Na+-Ca2+ exchanger may underlie the response. However, in Na+-free (Na+ replaced by N-methyl-D-glucamine (NMDG)) external saline, depolarization in the presence of ADP was still able to evoke an increase in [Ca2+]i (n= 9; data not shown). Taken in concert, these data indicate that, in the presence of ADP, depolarization is able to stimulate Ca2+ release from internal stores without a requirement for Na+-Ca2+ exchange activity or Ca2+ influx.
Figure 2. Depolarization-evoked [Ca2+]i increase induced by ADP in Ca2+-free saline.
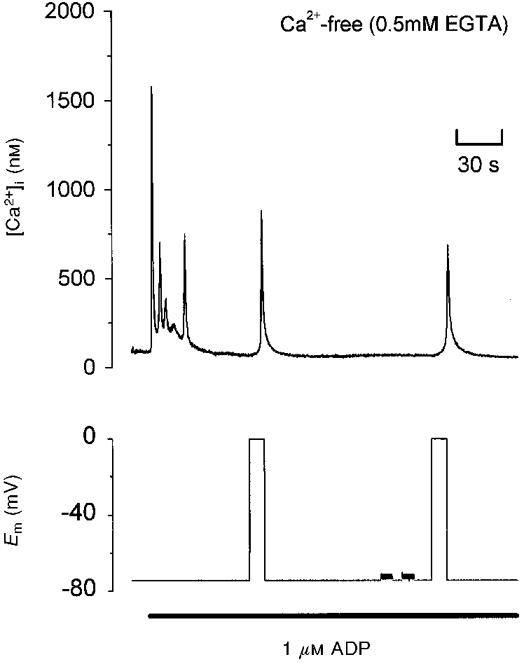
Simultaneous recording of [Ca2+]i and membrane potential in Ca2+-free saline with 0.5 mM EGTA. The cell was clamped at either -75 or 0 mV, except for two brief periods prior to the second step to 0 mV, when compensation for whole-cell capacitance and series resistance were checked using a 5 mV pulse at line frequency. The bar indicates application of 1 μM ADP.
Previous studies in rat megakaryocytes have shown that dialysis of the cytoplasm with heparin, a blocker of IP3 receptors, inhibits the ADP-evoked [Ca2+]i increase (Somasundaram & Mahaut-Smith, 1995), consistent with the coupling of this class of purinoceptor to phospholipase C (PLC) and the generation of IP3. In our experiments, heparin also blocked the depolarization-evoked increase in [Ca2+]i induced during exposure to ADP (Fig. 3; n= 6). Heparin with an average molecular weight of 6000 was used in these experiments, and therefore several minutes of dialysis were required to observe complete block. In Fig. 3, two sections of the [Ca2+]i and membrane potential recording are shown, starting at 53 and 228 s after transition to the whole-cell mode with 10 mg ml−1 of heparin in the pipette. The first trace shows that this cell displayed a single transient [Ca2+]i increase in response to both ADP and depolarization. After a further 175 s, the [Ca2+]i response to the second application of ADP was greatly reduced and the response to depolarization was completely blocked. The loss of response was not the result of receptor desensitization or time-dependent run-down since in the absence of heparin, [Ca2+]i responses to ADP and depolarization could be obtained at least twice in the same cell (n= 19) and depolarization could evoke an [Ca2+]i increase more than 8 min after transition to the whole-cell mode (n= 12). The ability of heparin to block the voltage-dependent Ca2+ increase is consistent with a requirement for functional IP3 receptors to observe the response.
Figure 3. Heparin blocks the [Ca2+]i increase evoked by ADP and depolarization.
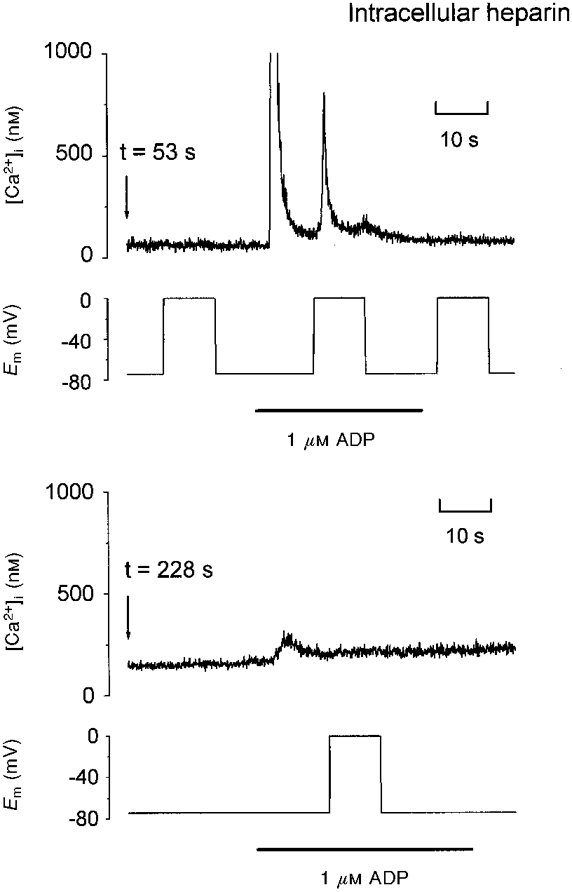
Two sections of simultaneous [Ca2+]i and membrane voltage recordings from a single voltage clamp experiment are shown, starting at 53 and 228 s after transition to the whole-cell configuration. The [Ca2+]i axis range was limited in order to clearly illustrate the depolarization-evoked response. The pipette contained 10 mg ml−1 heparin. The bars indicate application of 1 μM ADP.
The physiological relevance of the depolarization-evoked Ca2+ increase is dependent upon the megakaryocyte undergoing membrane potential changes that are capable of activating the response. Figure 4A is an example of a current clamp recording during exposure to 1 μM ADP. Prior to stimulation, the cell resting potential was steady at -41 mV; the mean value in 13 cells was -43 ± 1 mV. In response to ADP, the cell showed either a single transient hyperpolarization or oscillations of membrane potential (Fig. 4A) consistent with previous observations that a Ca2+-gated K+ channel is the major conductance activated during agonist-evoked Ca2+ elevations in the rat megakaryocyte (Uneyama et al. 1993a). In a total of 13 cells, the most negative potential reached during a hyperpolarization measured -76 ± 1 mV. Therefore, following each membrane hyperpolarization to approximately -75 mV, the cell is subjected to a depolarization of approximately 30 mV. In the experiment of Fig. 4B, the cell was initially exposed to ADP under current clamp and then voltage clamped at -75 mV. Three separate potential steps to 0 mV each evoked an [Ca2+]i transient, demonstrating the repeatable nature of the depolarization-evoked Ca2+ increase. Importantly, a step to -45 mV also elicited a rise in [Ca2+]i. Thus, membrane potential changes over the physiological range are also capable of inducing the response. In six cells exposed to 1 μM ADP, the peak [Ca2+]i increase evoked by a step from -75 to -45 mV was 398 ± 91 nM.
Figure 4. Depolarization over the physiological range evokes an [Ca2+]i increase.
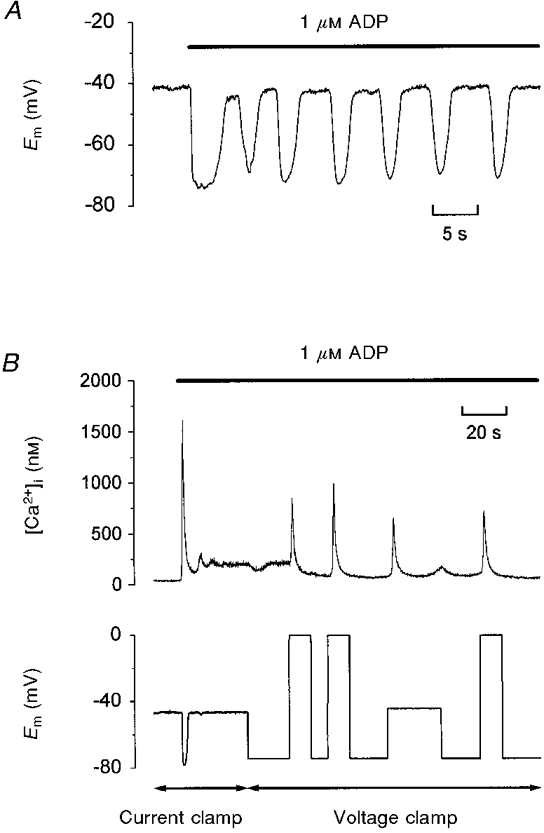
A, whole-cell current clamp recording of membrane potential during application of 1 μM ADP (bar). B, simultaneous recording of [Ca2+]i and membrane potential during exposure to 1 μM ADP (bar) under whole-cell current clamp or voltage clamp as indicated by the double-ended arrows below. During voltage clamp, the cell was held at -75, -45 or 0 mV.
DISCUSSION
Previous studies in non-excitable cells have concluded that depolarization leads to a reduction in [Ca2+]i during activation of Ca2+ signalling pathways primarily due to a decrease in the electrical driving force for Ca2+ entry (Penner et al. 1988; Lewis & Cahalan, 1989; Demaurex et al. 1992). This is consistent with the fact that the Ca2+-permeable channels in these cells are not gated by depolarization, in contrast to nerve, muscle and other excitable cells (Fasolato et al. 1994; Clapham, 1995). In the rat megakarycoyte, a cell type lacking voltage-dependent Ca2+ channels (Uneyama et al. 1993a; Somasundaram & Mahaut-Smith, 1994, 1995), we now present evidence that depolarization during stimulation of metabotropic purinoceptors evokes a large increase in [Ca2+]i. The response was observed in Ca2+-free and Na+-free salines, and therefore involves Ca2+ release from internal stores and does not require Na+-Ca2+ exchange activity. Although the peak increase in [Ca2+]i upon depolarization to 0 mV was smaller in Ca2+-free saline (478 ± 78 nM) than in 1 mM Ca2+ saline (714 ± 95 nM), it was not possible to accurately assess the contribution of Ca2+ influx to the response due to the large variation in amplitude in both the presence and absence of external Ca2+; furthermore, the stores will lose a significant amount of their Ca2+ content in Ca2+-free salines (Uneyama et al. 1993b). The delay from depolarization to intial [Ca2+]i increase was slightly longer in Ca2+-free saline (1.2 ± 0.2 s) than in the presence of external Ca2+ (0.8 ± 0.1 s); however, this small difference may also reflect reduced store and cytosolic Ca2+ levels rather than the timing of contribution of Ca2+ influx. Although store-dependent Ca2+ entry (Somasundaram & Mahaut-Smith, 1994) will be activated during the Ca2+ response evoked by depolarization, this influx will be secondary to the release mechanism. In addition, at depolarized potentials, Ca2+ influx will be small due to the reduced inward driving force for Ca2+ and inwardly rectifying properties of the underlying conductance (Somasundaram & Mahaut-Smith, 1994). Therefore, the depolarization-evoked Ca2+ increase is mainly the result of Ca2+ release from intracellular stores rather than Ca2+ influx.
At present, we cannot identify the precise site of the voltage sensor that transduces a depolarization of the plasma membrane into stimulation of Ca2+ release from intracellular stores, although it clearly requires prior activation of plasma membrane purinoceptors and functional IP3 receptors. The ADP receptor that stimulates Ca2+ mobilization in the megakaryocyte has not been well characterized, but several pieces of evidence suggest that it is a G-protein-coupled receptor linked to phospholipase Cβ. Firstly, heparin, a blocker of IP3 receptors (Bezprozvanny & Ehrlich, 1995), prevents the [Ca2+]i increase activated by ADP (Somasundaram & Mahaut-Smith, 1995; this study). Secondly, intracellular dialysis with IP3 or GTPγS evokes intracellular Ca2+ oscillations similar to those activated by ADP (Uneyama et al. 1993b,Hussain & Mahaut-Smith, 1998). Thirdly, megakaryocytes lack Ca2+-induced Ca2+ release via ryanodine receptors (Uneyama et al. 1993b). Depolarization may affect one or more stages in the signalling pathway, including the purinoceptor itself, its G-protein or phospholipase C. Several of the molecules required for, or consumed within, the purinoceptor-evoked pathway are highly polar, including ADP, GTP and phospha-tidylinositol 4,5-bisphosphate. Therefore, depolarization may affect their binding and consequently the efficacy of IP3 generation. Stimulation of IP3 formation would result in increased Ca2+ release and hence adequately explain the depolarization-evoked response. Evidence that IP3 formation can be increased by depolarization has been provided in skeletal muscle (Vergara et al. 1985). In addition, hyperpolarization has been suggested to reduce IP3 generation during agonist activation of rabbit mesenteric artery (Itoh et al. 1992). An alternative explanation for the depolarization-evoked Ca2+ release in the megakarocyte is that a form of configurational coupling develops between the plasma membrane and intracellular stores during IP3-dependent Ca2+ release which allows voltage changes to control internal Ca2+ release. This type of coupling allows action potentials in skeletal muscle to gate Ca2+ release through ryanodine receptors in skeletal muscle (Schneider & Chandler, 1973). A configurational coupling mechanism has also been proposed to account for activation of a Ca2+ influx pathway by depletion of intracellular Ca2+ stores (Irvine, 1990). However, depolarization during thapsigargin-evoked store depletion resulted in either a decrease in [Ca2+]i or a slowing of the Ca2+ increase, as expected from a reduction in driving force for Ca2+ entry (M. P. Mahaut-Smith & M. J. Mason unpublished data). Therefore a lowering of store Ca2+ content, which triggers Ca2+ influx in megakaryocytes and other non-excitable cells (Putney, 1986; Fasolato et al. 1994; Somasundaram & Mahaut-Smith, 1994), is not by itself the signal that induces voltage-dependent Ca2+ release. This does not rule out the possibility that during stimulation by ADP, Ca2+ is sequestered into another storage site which is directly sensitive to the plasma membrane voltage. A depolarization-evoked Ca2+ release similar to that which we describe here has been reported in one excitable tissue, coronary artery smooth muscle, during stimulation of metabotropic cholinergic receptors (Ganitkevich & Isenberg, 1993). Interestingly, Marty & Tan (1989) reported that muscarinic cholinergic receptor-evoked Ca2+ release in rat lacrimal acinar cells is voltage dependent, but, in contrast to coronary artery smooth muscle, depolarization leads to a decrease in the amplitude and increase in the delay of the initial ACh-induced [Ca2+]i increase. The effect of voltage was not observed at saturating levels of the agonist response in both acinar cells and coronary artery smooth muscle (Marty & Tan, 1989; Ganitkevich & Isenberg, 1996). On the basis of this result and arguments that neither the G-protein nor PLC can sense the voltage field, Marty & Tan (1989) concluded that the receptor protein is the site of the voltage dependence. However, this interpretation is difficult to reconcile with the fact that depolarization has the opposite effect on the magnitude and kinetics of the ACh-evoked Ca2+ increase in acinar versus smooth muscle cells (Marty & Tan, 1989; Ganitkevich & Isenberg, 1996). In addition, in smooth muscle cells (Ganitkevich & Isenberg 1993), depolarization-evoked Ca2+ release was induced by intracellular GTPγS, suggesting that a voltage-dependent site exists downstream of the receptor. Clearly, further studies are required to determine the mechanism(s) underlying the agonist-dependent depolarization-evoked Ca2+ increase in both the rat megakaryocyte and coronary artery smooth muscle.
At present, the importance of the ADP-induced, depolarization-dependent Ca2+ release in the megakaryocyte remains speculative. The mechanism can be repetitively stimulated and is active over the range of potentials evoked by ADP under current clamp (approximately -75 to -45 mV), thus it may be involved in controlling the patterns of [Ca2+]i increase during oscillations. The present study and previous patch clamp investigations of rat megakaryocytes (Uneyama et al. 1993a,b; Hussain & Mahaut-Smith, 1998) show that [Ca2+]i oscillations can occur during clamp of the membrane voltage to a single potential, even during maximum possible correction for voltage errors across the series resistance (Hussain & Mahaut-Smith, 1998; this study). Therefore, voltage-dependent Ca2+ release is not a requirement for purinoceptor-evoked [Ca2+]i oscillations in the megakarycoyte, but may instead represent a means by which cells can control temporal or spatial patterns of [Ca2+]i increase. Our data provide evidence that the depolarization-evoked Ca2+ increase is larger when the membrane is stepped from -75 to 0 mV (714 ± 95 nM) compared with a step from -75 to -45 mV (398 ± 91 nM). Therefore this phenomenon may play an important role in Ca2+ signalling if present in cells that display larger changes in membrane potential. For example, certain non-excitable cells such as rat basophilic leukaemia cells show rapid fluctuations in membrane potential between two zero current states within the current-voltage relationship, approximately -80 and -25 mV (Mason et al. 1997). Finally, this mechanism could be extremely important in excitable cells that exhibit an IP3-dependent Ca2+ release since larger voltage changes will be frequently experienced. Further experiments are required to investigate how widespread voltage control of Ca2+ release is during stimulation of metabotropic receptors in excitable and non-excitable tissue.
In conclusion, we have shown that stimulation of metabotropic ADP receptors in a non-excitable cell, the rat megakarycoyte, leads to an ability of membrane depolarization to evoke [Ca2+]i increases due predominantly to release of intracellularly stored Ca2+. Depolarization was able to stimulate Ca2+ mobilization in a repetitive manner and was effective over the physiological potential range. This mechanism may have important consequences for control of Ca2+ signalling by stimuli that invoke an electrogenic influence.
Acknowledgments
This work was supported by grants from the British Heart Foundation (BHF) (PG 94151 and 95005). M.P.M.-S. holds a BHF Basic Science Lectureship (BS 10). We thank Jon Holdich for technical assistance and Neil Hardingham for participation in preliminary current clamp experiments.
References
- Berridge MJ. Elementary and global aspects of calcium signalling. The Journal of Physiology. 1997;499:290–306. doi: 10.1113/jphysiol.1997.sp021927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I, Ehrlich BE. The inositol 1,4,5-trisphosphate (InsP3) receptor. Journal of Membrane Biology. 1995;145:205–216. doi: 10.1007/BF00232713. [DOI] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- Demaurex N, Schlegel W, Varnai P, Mayr G, Lew DP, Krause KH. Regulation of Ca2+ influx in myeloid cells. Role of plasma membrane potential, inositol phosphates, cytosolic free [Ca2+], and filling state of intracellular Ca2+ stores. Journal of Clinical Investigation. 1992;90:830–839. doi: 10.1172/JCI115958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasolato C, Innocenti B, Pozzan T. Receptor-activated Ca2+ influx: how many mechanisms for how many channels? Trends in Pharmacological Sciences. 1994;15:77–83. doi: 10.1016/0165-6147(94)90282-8. 10.1016/0165-6147(94)90282-8. [DOI] [PubMed] [Google Scholar]
- Fewtrell C. Ca2+ oscillations in non-excitable cells. Annual Review of Physiology. 1993;55:427–454. doi: 10.1146/annurev.ph.55.030193.002235. 10.1146/annurev.ph.55.030193.002235. [DOI] [PubMed] [Google Scholar]
- Ganitkevich VY, Isenberg G. Membrane potential modulates inositol 1,4,5-trisphosphate-mediated Ca2+ transients in guinea-pig coronary myocytes. The Journal of Physiology. 1993;470:35–44. doi: 10.1113/jphysiol.1993.sp019845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VY, Isenberg G. Effect of membrane potential on the initiation of acetylcholine-induced Ca2+ transients in isolated guinea pig coronary myocytes. Circulation Research. 1996;78:717–723. doi: 10.1161/01.res.78.4.717. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hussain JF, Mahaut-Smith MP. ADP and inositol trisphosphate evoke oscillations of a monovalent cation conductance in rat megakaryocytes. The Journal of Physiology. 1998;511:791–801. doi: 10.1111/j.1469-7793.1998.791bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine RF. Quantal’ Ca2+ release and the control of Ca2+ entry by inositol phosphates - a possible mechanism. FEBS Letters. 1990;263:5–9. doi: 10.1016/0014-5793(90)80692-c. 10.1016/0014-5793(90)80692-C. [DOI] [PubMed] [Google Scholar]
- Itoh T, Seki N, Suzuki S, Ito S, Kajikuri J, Kuriyama H. Membrane hyperpolarization inhibits agonist-induced synthesis of inositol 1,4,5-trisphosphate in rabbit mesenteric artery. The Journal of Physiology. 1992;451:307–328. doi: 10.1113/jphysiol.1992.sp019166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapural L. Characterization of the voltage-gated K+ current in rat megakaryocytes. Croatian Medical Journal. 1995;36:157–161. [Google Scholar]
- Lewis RS, Cahalan MD. Mitogen-induced oscillations of cytosolic Ca2+ and transmembrane Ca2+ current in human leukemic T cells. Cell Regulation. 1989;1:99–112. doi: 10.1091/mbc.1.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaut-Smith MP. An infra-red light-transmitting aperture controller for use in single cell fluorescence photometry. Journal of Microscopy. 1998;191:60–66. doi: 10.1046/j.1365-2818.1998.00349.x. 10.1046/j.1365-2818.1998.00349.x. [DOI] [PubMed] [Google Scholar]
- Mahaut-Smith MP, Hardingham NR, Hussain JF, Mason MJ. Voltage-dependent [Ca2+]i changes during purinoceptor activation in the rat megakaryocyte. The Journal of Physiology. 1998a;506.P:69P. [Google Scholar]
- Mahaut-Smith MP, Hussain JF, Mason MJ. Depolarization-induced Ca2+ release from intracellular stores in a nonexcitable cell: Experiments in rat megakaryocytes. Journal of General Physiology. 1998b;112:35a. [Google Scholar]
- Marty A, Tan YP. The initiation of calcium release following muscarinic stimulation in rat lacrimal glands. The Journal of Physiology. 1989;419:665–687. doi: 10.1113/jphysiol.1989.sp017892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MJ, Bambha KM, Limberis J, Schofield GG. Interactions between the anomalous rectifier and capacitative Ca2+ entry currents generate oscillations in membrane potential and [Ca2+]i in RBL-1 cells. Biophysical Journal. 1997;72:A295. [Google Scholar]
- Penner R, Matthews G, Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988;334:499–504. doi: 10.1038/334499a0. 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- Poenie M. Alteration of intracellular fura-2 fluorescence by viscosity: A simple correction. Cell Calcium. 1990;11:85–91. doi: 10.1016/0143-4160(90)90062-y. 10.1016/0143-4160(90)90062-Y. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Rink TJ, Jacob R. Calcium oscillations in non-excitable cells. Trends in Neurosciences. 1989;12:43–46. doi: 10.1016/0166-2236(89)90133-1. 10.1016/0166-2236(89)90133-1. [DOI] [PubMed] [Google Scholar]
- Romero E, Sullivan R. Complexity of the outward K+ current of the rat megakaryocyte. American Journal of Physiology. 1997;272:C1525–1531. doi: 10.1152/ajpcell.1997.272.5.C1525. [DOI] [PubMed] [Google Scholar]
- Schneider MF, Chandler WK. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973;242:244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Somasundaram B, Mahaut-Smith MP. Three cation influx currents activated by purinergic receptor stimulation in rat megakaryocytes. The Journal of Physiology. 1994;480:225–231. doi: 10.1113/jphysiol.1994.sp020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasundaram B, Mahaut-Smith MP. A novel monovalent cation channel activated by inositol trisphosphate in the plasma membrane of rat megakaryocytes. Journal of Biological Chemistry. 1995;270:16638–16644. doi: 10.1074/jbc.270.28.16638. 10.1074/jbc.270.28.16638. [DOI] [PubMed] [Google Scholar]
- Uneyama C, Uneyama H, Akaike N. Cytoplasmic Ca2+ oscillations in rat megakaryocytes evoked by a novel type of purinoceptor. The Journal of Physiology. 1993a;470:731–749. doi: 10.1113/jphysiol.1993.sp019885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uneyama H, Uneyama C, Akaike N. Intracellular mechanisms of cytoplasmic Ca2+ oscillation in rat megakaryocyte. Journal of Biological Chemistry. 1993b;268:168–174. [PubMed] [Google Scholar]
- Vergara J, Tsien RY, Delay M. Inositol 1,4,5-trisphosphate: a possible chemical link in excitation-contraction coupling in muscle. Proceedings of the National Academy of Sciences of the USA. 1985;82:6352–6356. doi: 10.1073/pnas.82.18.6352. [DOI] [PMC free article] [PubMed] [Google Scholar]


