Abstract
We used intracellular microelectrodes to record the membrane potential (Vm) of intact murine colonic smooth muscle. Electrical activity consisted of spike complexes separated by quiescent periods (Vm≈−60 mV). The spike complexes consisted of about a dozen action potentials of approximately 30 mV amplitude. Tetraethylammonium (TEA, 1–10 mM) had little effect on the quiescent periods but increased the amplitude of the action potential spikes. 4-Aminopyridine (4-AP, ⋧ 5 mM) caused continuous spiking.
Voltage clamp of isolated myocytes identified delayed rectifier K+ currents that activated rapidly (time to half-maximum current, 11.5 ms at 0 mV) and inactivated in two phases (τf = 96 ms, τs = 1.5 s at 0 mV). The half-activation voltage of the permeability was −27 mV, with significant activation at −50 mV.
TEA (10 mM) reduced the outward current at potentials positive to 0 mV. 4-AP (5 mM) reduced the early current but increased outward current at later times (100–500 ms) consistent with block of resting channels relieved by depolarization. 4-AP inhibited outward current at potentials negative to −20 mV, potentials where TEA had no effect.
Qualitative PCR amplification of mRNA identified transcripts encoding delayed rectifier K+ channel subunits Kv1.6, Kv4.1, Kv4.2, Kv4.3 and the Kvβ1.1 subunit in murine colon myocytes. mRNA encoding Kv 1.4 was not detected.
We find that TEA-sensitive delayed rectifier currents are important determinants of action potential amplitude but not rhythmicity. Delayed rectifier currents sensitive to 4-AP are important determinants of rhythmicity but not action potential amplitude.
Potassium channels control contraction of gastrointestinal smooth muscles by setting resting potential and influencing slow wave and action potential configuration (Du et al. 1994; Huizinga et al. 1985; Sanders, 1992). Members of several K+ channel families are expressed by smooth muscles, including Ca2+-activated K+ channels (Carl et al. 1991), ATP-sensitive K+ channels (Bonev & Nelson, 1993), inward rectifier K+ channels (Knot et al. 1996; Quayle et al. 1993), and delayed rectifier K+ channels (Carl, 1995; Koh et al. 1996). A given smooth muscle myocyte can express several families of K+ channels and several members of a single family of channels. For example, intestinal myocytes express three or more kinds of delayed rectifier currents (Carl, 1995) and a similar number of Kv channels (Hart et al. 1993). This complexity is also found for other K+ channels and appears to be necessary for the control of smooth muscle function.
The delayed rectifier K+ channels are of particular importance in the regulation of colonic smooth muscle electrical activity because they provide outward currents over the voltage range in which these tissues operate. Members of this family have been identified in vascular (Hume & Leblanc, 1989; Clapp & Gurney, 1991; Gelband & Hume, 1992; Smirnov & Aaronson, 1992) as well as gastrointestinal (Thornbury et al. 1992a, b; Carl, 1995; Koh et al. 1996) smooth muscles. Delayed rectifiers influence resting potential (Ishikawa et al. 1997), action potential threshold (Vogalis et al. 1993), and waveforms of slow waves (Thornbury et al. 1992a, b). The delayed rectifier channels from canine colonic smooth muscle have been studied extensively. Voltage-clamp experiments have characterized the voltage sensitivity, kinetics and pharmacological sensitivities of three components of delayed rectifier current (Carl, 1995). Molecular studies have documented expression of mRNA encoding Kv1.2, Kv1.5 and Kv2.2 channels (Hart et al. 1993; Overturf et al. 1994; Schmalz et al. 1998). In addition, this work has related Kv1.2-Kv1.5 heteromers and Kv2.2 channels with the IdK(f) and IdK(s) components of current in canine colonic myocytes described by Carl (1995). This work has provided information about how different components of delayed rectifier current determine whether the electrical activity of colonic muscle will be slow waves, action potentials, or a combination of these behaviours.
In the present study, we used physiological and molecular techniques to determine the expression and function of delayed rectifier channels in murine colonic muscles. The roles of specific components of delayed rectifier current in spontaneous electrical activity were investigated, and we attempted to relate specific molecular entities expressed by colonic muscles to components of the delayed rectifier current. These studies are particularly relevant because the electrical activities of murine and human colonic muscles are similar (Lomax et al. 1996; Rae et al. 1998). Therefore, information about the K+ channels that regulate electrical activity in murine colon may provide a model of human colonic electrophysiology. The development of new murine gene knock-outs makes the mouse the ideal animal preparation to test hypotheses regarding the regulation of electrical activity. Little is known about the mechanisms of colonic electrical activity in either the mouse or the human. In particular, the origins of the slow depolarizations and rhythmic spike complexes are not understood. There is also no explanation for the organization of spike complexes into periodic bursts. It has been proposed that neuronal input generates the rhythmic activation of spike complexes (Bywater et al. 1989), but rhythmic activity can be observed in the presence of neuronal blocking drugs (Lomax et al. 1996). This suggests that non-neuronal pacemakers are sufficient to generate spontaneous rhythmicity and spike complexes in colonic muscles. Knowledge of the types of K+ channels expressed and their properties will be useful in understanding the responses of colonic muscles to pacemaker and neural inputs.
Accordingly, we have begun physiological and molecular studies of the function of the murine colon with attention in this work to the delayed rectifier K+ channels. We find different actions of two K+ channel blockers on the electrical activity of the intact colon that we relate to actions on two components of delayed rectifier K+ currents. Furthermore, the kinetics of 4-AP action on murine colonic delayed rectifier K+ currents match those described for cloned Kv4 channels and we find evidence for mRNA encoding these channels in murine colonic tissue.
METHODS
Methods for intracellular microelectrode recording
BALB/c mice (20-30 days old, Harlan Sprague-Dawley; Indianapolis, IN, USA) were anaesthetized by chloroform inhalation and decapitated following cervical dislocation. Proximal colons, 1 cm from the ileocaecal sphincter, were removed and opened along the mesenteric border. The contents of the colon were removed by washing with a bicarbonate buffer solution (see below) and tissues were pinned down onto a Sylgard base with the mucosa facing upwards. The mucosa was removed and the muscularis tunica was cut into 10 mm × 6 mm pieces before being pinned to the base of a recording chamber using tungsten pins. The use and treatment of animals was approved by the Institutional Animal Use and Care Committee at the University of Nevada.
Muscles were maintained at 37.5 ± 0.5°C in a bicarbonate buffer solution containing (mM): 120.4 NaCl, 5.9 KCl, 15.5 NaHCO3, 11.5 glucose, 1.2 MgCl2, 1.2 NaH2PO4 and 2.5 CaCl2. The solution was bubbled with 97% O2 and 3% CO2 and had a pH of 7.3-7.4. Circular muscle cells were impaled with glass microelectrodes filled with 3 M KCl with resistances between 50 and 80 MΩ. Transmembrane potential was measured with a high-input impedance amplifier (WPI S-7071, Sarasota, FL, USA). Tissues were equilibrated for approximately 1 h before recording.
L-NAME, tetraethylammonium chloride (TEA) and 4-aminopyridine (4-AP) were obtained from Sigma. Test compounds were made up in stock solutions of 10−1 to 10−3 M before being diluted to the stated concentrations in buffer.
Preparation of isolated myocytes
Colonic smooth muscle cells were prepared from colons removed from BALB/c mice as described above. Colons were cut open along the longitudinal axis, pinned out in a Sylgard-lined dish, and washed with Ca2+-free solution containing (mM): 125 NaCl, 5.36 KCl, 15.5 NaOH, 0.336 Na2HPO4, 0.44 KH2PO4, 10 glucose, 2.9 sucrose and 11 Hepes, adjusted to pH 7.4 with tris (hydroxymethyl)aminomethane (Tris). Mucosa and submucosa were removed. Most experiments were conducted using myocytes isolated from bulk smooth muscle. In some experiments, the circular muscle and longitudinal muscle layers were isolated using fine-tipped forceps in order to study cells from an identified smooth muscle layer. Pieces of muscle were incubated in a Ca2+-free solution supplemented with 4 mg ml−1 fatty acid-free bovine serum albumin, 2 mg ml−1 papain, 1 mg ml−1 collagenase and 1 mM dithiothreitol. Tissue was incubated at 37°C in enzyme solution for 8-12 min and then washed with Ca2+-free solution. Tissue pieces were gently agitated to create a cell suspension. Dispersed cells were stored at 4°C in Ca2+-free solution supplemented with minimum essential medium for suspension culture (S-MEM; Sigma) plus 0.5 mM CaCl2, 0.5 mM MgCl2, 4.17 mM NaHCO3 and 10 mM Hepes. Experiments were done at room temperature within 6 h of dispersing cells.
Voltage-clamp methods
The whole-cell patch-clamp technique was used to record membrane currents from dissociated murine colonic smooth muscle cells. Currents were amplified with a List EPC-7 (List Electronic, Darmstadt, Germany) or Axopatch 1A (Axon Instruments). Data were filtered at 1 kHz and stored on videotape or digitized on-line using pCLAMP software (Axon Instruments). Pipette resistances were 1-4 MΩ. In most experiments, the uncompensated series resistance was between 2 and 4 MΩ, and voltage errors during the largest currents approached 12 mV. Voltage errors were much smaller during steps to negative potentials. In the experiments shown in Figs 4 and 5, average series resistance was 2.8 ± 1 MΩ, and we compensated for at least 70% of the series resistance.
Figure 4. Determination of the reversal potential of delayed rectifier current.
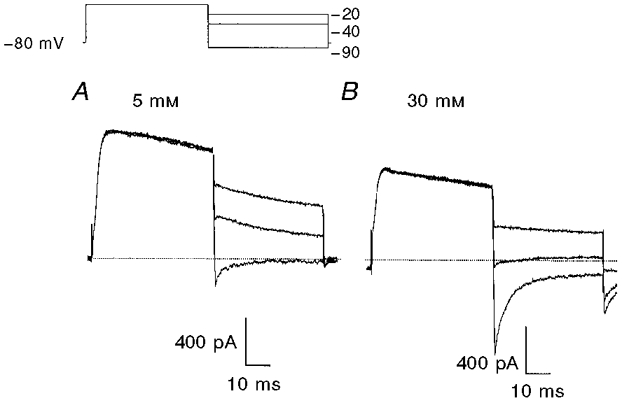
Membrane potential was stepped from -80 to 0 mV followed by test steps to potentials ranging from -15 to -90 mV in 5 mV increments. The inset shows conditioning and three selected test potentials. A, currents recorded during superfusion with a solution containing 5 mM K+. B, currents recorded during superfusion with a solution containing 30 mM K+. The dotted lines mark zero current.
Figure 5. Voltage dependence of inactivation and activation of delayed rectifier K+ currents.
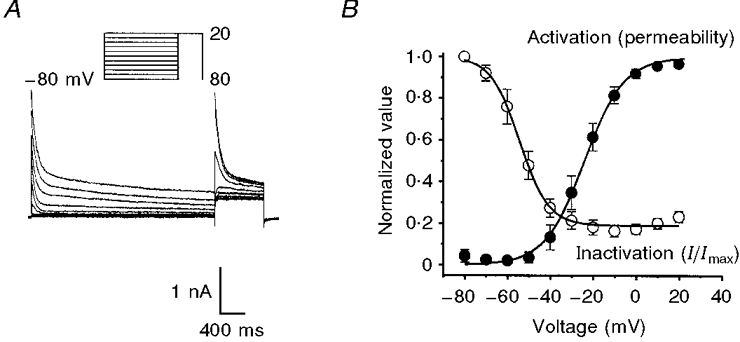
A, membrane potential was stepped to conditioning potentials between -80 and +20 mV for 3 s and then stepped to a test potential of +20 mV. B, the voltage dependence of inactivation of the delayed rectifier K+ current is shown as a plot of normalized peak outward current during the test step as a function of the conditioning potential (○). The voltage-dependent activation of the delayed rectifier K+ permeability is shown in B as a plot of normalized peak conductance as a function of step potential (•). n = 10 cells.
Smooth muscle myocytes were bathed in a Ca2+-free solution containing (mM): 5 KCl, 135 NaCl, 2 MnCl2, 10 glucose, 1.2 MgCl2, and 10 Hepes adjusted to pH 7.4 with Tris. In most experiments, the pipette solution contained (mM): 110 potassium gluconate, 20 KCl, 5 MgCl2, 2.7 K2ATP, 0.1 Na2GTP, 2.5 creatine phosphate disodium, 5 Hepes and 1 EGTA, adjusted to pH 7.2 with Tris. In order to reduce the junction potential in experiments determining the reversal potential of the delayed rectifier current (Fig. 4), we replaced potassium gluconate with KCl. 4-Aminopyridine (4-AP, Sigma) or tetraethylammonium (TEA, Sigma) were added as described in the Results section. Experiments were performed at room temperature (22°C).
We calculated (Barry, 1994) and measured (Ng & Barry, 1995) a junction potential of -11 to -13 mV between our bath and potassium gluconate electrode solutions, and a potential of -4 mV between bath and KCl electrode solutions. The voltages listed in our protocols and figures are not corrected for these offsets. We have corrected the values of reversal potential, half-activation and half-inactivation.
Isolation of RNA from murine circular smooth muscle cells and RT-PCR methods
Murine colonic circular smooth muscle cells were isolated as described above. Cells were allowed to settle to the bottom of a recording chamber on an inverted microscope for 5 min. Individual myocytes were selected using the criteria used during voltage-clamp experiments (elongated, spindle-shaped cells, 20-500 μm long, 5-60 μm in diameter) and aspirated into large bore pipettes (tip diameters larger than 10 μm). After 10-60 smooth muscle cells were obtained, the contents of the pipette were expelled into an RNase-free 0.5 ml tube, snap-frozen in liquid nitrogen, and stored at -70°C.
Total RNA was prepared from colonic myocytes using SNAP Total RNA isolation kits (Invitrogen, San Diego, CA, USA) as per the manufacturer's instructions, including the use of polyinosinic acid (20 μg) as an RNA carrier. Total RNA was also isolated from mouse brain using this method. First strand cDNA was prepared from the RNA preparations using the Superscript II Reverse Transcriptase kit (Gibco/BRL, Gaithersburg, MD, USA); 500 μg μl−1 of oligo-dT primers and random oligonucleotides were used together to reverse transcribe the RNA sample. The cDNA reverse transcription product was amplified with channel-specific primers by PCR. PCR was performed in a 50 μl reaction containing 60 mM Tris-HCl (pH 8.5), 1.5 mM MgCl2, 15 mM (NH4)2 SO4, 10% DMSO, 1 mM deoxynucleotide triphosphate (dNTP) mix, 10 μM of each primer, cDNA and 1.0 unit of Taq polymerase (Promega). The PCR cycling parameters were denaturation at 94°C for 3 min, followed by 3 cycles (94°C for 1 min, 60°C for 2 min, 72°C for 3 min), 3 cycles (94°C for 1 min, 57°C for 2 min, 72°C for 3 min), 3 cycles (94°C for 1 min, 54°C for 2 min, 72°C for 3 min), 3 cycles (94°C for 1 min, 51°C for 2 min, 72°C for 3 min), 3 cycles (94°C for 1 min, 48°C for 2 min, 72°C for 3 min), 25 cycles (94°C for 1 min, 50°C for 2 min, 72°C for 3 min), and a final extension step at 72°C for 7 min. Temperature cycling was performed in an Applied Biosystems thermal cycler (Perkin Elmer, Norwalk, CT, USA). The amplified products (8 μl) were separated by electrophoresis on a 2% agarose/1 × TAE (Tris, acetic acid, EDTA) gel, and the DNA bands were visualized by ethidium bromide staining.
The following PCR primers were used (numbers in parentheses are the gene bank accession numbers): Kv1.4 (L02751) nucleotides (nt) 1320-1344 and 1943-1962, Kv1.6 (X77622) nt 981-1001 and 1441-1463, Kv4.1 (M64226) nt 1630-1649 and nt 1934-1953, Kv4.2 (M59980) nt 330-349 and 862-881, Kv4.3 (AB003587) nt 96-113 and 492-509, and Kvβ1.1 (X00662) nt 514-534 and 1127-1147. In addition, PCR primers for β-actin (V01217) nt 2383-2402 and 3071-3091 were used to confirm the integrity of the RNA as well as to detect genomic DNA contamination.
Statistical analyses
Data are reported as means ±s.e.m.n is the number of animals that contributed RNA for PCR, or from which recordings were made of the electrical activity of intact tissue. In describing the voltage-clamp results, n is the number of cells tested. Statistical significance was evaluated by Student's t test or ANOVA, as appropriate. P values less than 0.05 were considered significant.
RESULTS
Electrical activity of intact murine colon
The spontaneous electrical (and mechanical) activity of intact murine colonic smooth muscle is described in detail elsewhere (Bywater et al. 1989; Lomax et al. 1996) and shown in Figs 1 and 2. Briefly, the cycle of electrical activity is composed of periodic ‘spike complexes’. Each spike complex consists of a burst of action potentials that are initiated from approximately -50 mV and have amplitudes of about 30 mV. Intervals between the spike complexes lasted several seconds, during which membrane potential was close to -60 mV. This activity is quantified in the control column of Table 1. Although the pacemaker mechanism responsible for the rhythmic development of the spike complexes is unknown, data presented in Fig. 1A-C suggest that delayed rectifier currents play an important role. In all preparations tested, the intervals between the spike complexes decreased during exposure to 5 mM or higher 4-AP, until the quiescent periods between spike complexes were abolished and continuous spike activity was recorded (Fig. 1B). The only other action of 4-AP in these experiments was a slight depolarization of the membrane potential between the action potential spikes at the highest concentration tested (10 mM, Table 1). These observations suggest that 4-AP-sensitive membrane currents participate in the regulation of the quiescent period between spike complexes.
Figure 1. Effect of 4-AP on the electrical activity of intact murine colonic smooth muscle.
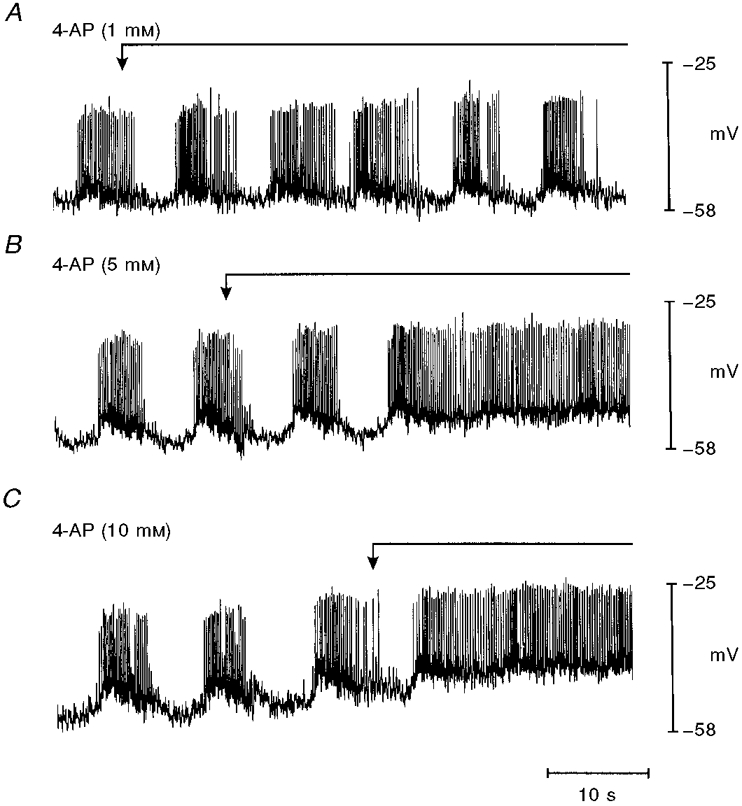
The traces are sequential records of membrane potential from a single preparation exposed to increasing concentrations of 4-AP (washed out between runs). The arrows and continuous lines above the traces mark application of 4-AP. A, 1 mM 4-AP had no effect on the spike complexes compared with control conditions. B and C, at concentrations of 5 and 10 mM, 4-AP caused a membrane depolarization and continuous spiking of muscle preparations.
Figure 2. Effect of TEA on the electrical activity of intact murine colonic smooth muscle.
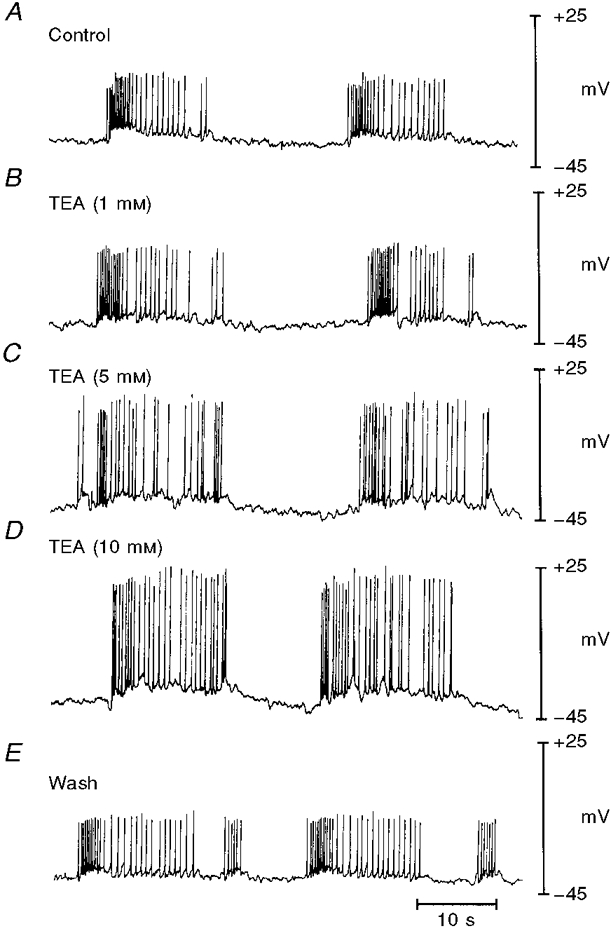
The traces are sequential records of membrane potential from a single preparation exposed to increasing concentrations of TEA (washed out between runs) in the presence of 100 μM L-NAME, as described in the text. A and B, control and activity in 1 mM TEA recorded from the same cell. C, D and E are from different impalements and show electrical activity in 5 and 10 mM TEA and following wash-out of the compound.
Table 1.
Characterization of the electrical activity of intact murine colonic smooth muscle in control conditions and in the presence of increasing concentrations of 4-AP
| 4-AP(mM) | ||||
|---|---|---|---|---|
| Parameter | Control | 1 | 5 | 10 |
| MMP(mV)† | −58 ± 3 | −63 ± 4 | § | § |
| Spike frequency(spikes s−1) | 1.4 ± 0.2 | 1.1 ± 0.3 | 1.9 ± 0.2 | 2.7 ± 0.6 |
| Spike amplitude(mV) | 33 ± 3 | 30 ± 8 | 28 ± 1 | 26 ± 2 |
| MMP-SC(mV) | −50 ± 2 | −55 ± 5 | −54 ± 2 | −47 ± 2* |
Values are means ±s.e.m. (n = 5).
P < 0.05 compared with control.
MMP is the maximum membrane polarization between spike complexes. Spike frequency was determined by counting the number of spikes over a 30 s trace and calculating the average number of spikes per second. Spike amplitude is the difference between the peak of the spike and the maximum membrane polarization between spikes. MMP-SC is the maximum membrane polarization between spikes.
At ≥ 5 mM 4-AP, the tissues spiked continuously.
We also investigated the effect of TEA on the electrical activity of the intact tissue. In preliminary experiments, we found that exposure to TEA caused a hyperpolarization of the smooth muscle. This effect is inconsistent with inhibition of smooth muscle K+ conductance, and is likely to result from the release of inhibitory neurotransmitters from enteric neurons. This suggestion is supported by observations that TEA relaxed murine colon in mechanical studies and that this relaxation was inhibited by L-NAME (data not shown). Accordingly, all experiments examining the effects of TEA on intact colonic preparations were conducted in the presence of 100 μM L-NAME. Comparison of the parameters listed in Table 2 with those in Table 1 shows that L-NAME caused modest but significant reductions in maximum polarization between spike complexes (MMP) and the maximum membrane polarization between spikes (MMP-SC) and increased the spike amplitude. However, the overall pattern of slow waves and action potentials was retained and the effects of TEA on this activity are shown in Fig. 2 and summarized in Table 2. TEA had no significant effect on the MMP, the spike frequency, or the MMP-SC. In contrast, TEA significantly increased the spike amplitude. These findings suggest that although TEA-sensitive conductances are relatively unimportant in determining the rate of generation of spike complexes, activation of a TEA-sensitive conductance limits the amplitude of action potentials within the spike complexes.
Table 2.
Characterization of the electrical activity of intact murine colonic smooth muscle in control conditions (solutions containing L-NAME as described in the text), and in the presence of increasing concentrations of TEA
| TEA(mM) | ||||
|---|---|---|---|---|
| Parameter | Control | 1 | 5 | 10 |
| MMP(mV) | −42 ± 2† | −38 ± 1 | −43 ± 2 | −42 ± 2 |
| Spike frequency(spikes s−1) | 1.2 ± 0.3 | 1.2 ± 0.2 | 1.5 ± 0.9 | 1.6 ± 0.1 |
| Spike amplitude(mV) | 45 ± 3† | 51 ± 4 | 58 ± 4* | 64 ± 3* |
| MMP-SC(mV) | −36 ± 2† | −35 ± 2 | −35 ± 3 | −36 ± 3 |
Values are means ±s.e.m. (n = 4).
P < 0.05 compared with control values in Table 1.
P < 0.05 compared with control values in this table.
Delayed rectifier K+ currents in isolated myocytes
The whole-cell configuration of the patch-clamp technique was used on isolated colonic myocytes to identify the currents responsible for the effects observed in intact tissues. Under the conditions of our recordings (no added Ca2+ and 2 mM Mn2+ in the superfusate, 1 mM EGTA in the pipettes), currents through Ca2+-activated K+ channels are minimized. Indeed, we found no charybdotoxin- or iberiotoxin-sensitive currents in voltage-clamped colonic myocytes (three each) at potentials negative to +20 mV (data not shown). As shown below, membrane currents under these conditions are carried by K+, and we refer to them as delayed rectifier K+ currents. Typical responses to 500 and 5000 ms depolarizations to potentials between -70 and +20 mV are shown in Fig. 3A and B. Depolarizations to potentials positive to -50 mV activated non-linear, time-dependent outward currents that peaked within 50 ms before decreasing in amplitude. We characterized the rising phase of the current by measuring the time to half-maximum current (t½) at test potentials that elicited currents large enough for quantitative analysis. t½ decreased from 21.8 ± 4.9 to 11.5 ± 4.1 ms and to 2.9 ± 0.7 ms (n = 6) as the test potential was changed from -40 to 0 mV and to +20 mV.
Figure 3. Voltage dependence and inactivation time constants of delayed rectifier K+ currents.
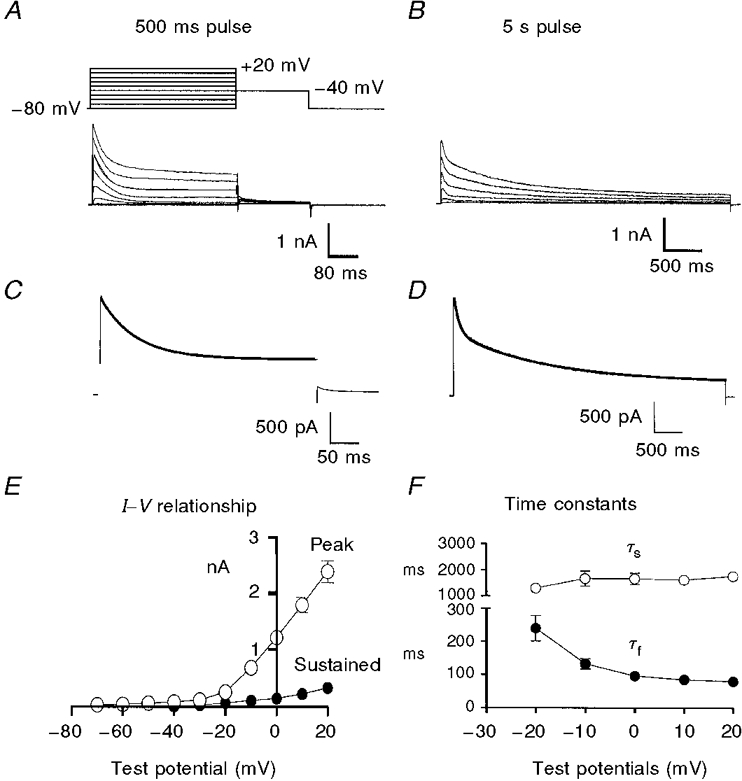
Membrane potential was stepped from -80 to +20 mV in 10 mV increments for 500 ms (A) or 5 s (B). C and D, membrane currents recorded at 0 mV from this experiment with double time constant fits superimposed (thick lines). E, plot of peak (○) and sustained (•) currents measured during 5 s depolarizations (n = 9 cells). F, plot of the fast (τf, •) and slow (τs, ○) time constants describing inactivation of the current during the 5 s steps as a function of step potential (n = 9 cells). Error bars denote s.e.m.; where error bars cannot be seen, they are smaller than the symbol.
After peaking, the outward current declined in two phases (Fig. 3B) that were well described by the sum of two exponentials (Fig. 3C and D). The voltage dependence of the rate of decline of the outward currents is summarized in Fig. 3F, where the averaged fast and slow time constants (τf and τs) from nine cells are plotted as a function of test potential. τf decreased as test potentials were increased from -20 to 0 mV. Negative to -20 mV, the magnitude and rate of decline of current were too small to be measured accurately in this protocol. At positive potentials, τf was insensitive to voltage. τs was relatively insensitive to potential over the range of voltages tested. The amplitude of the decay of outward current is illustrated in Fig. 3E. The peak outward current and the current remaining at the end of the 5000 ms step (i.e. the sustained current) are plotted as a function of step potential (n = 9). The outward current declined within 5 s to less than 15% of its peak value at potentials between -20 and +20 mV.
We established the ionic nature of the outward current by measuring the K+ sensitivity of the reversal potential of currents activated by 50 ms depolarizations using test steps to potentials ranging from -15 to -90 mV. Sample currents are shown in Fig. 4. We determined the reversal potential of the current as the test potential at which the ionic current changed from a declining outward current to a declining inward current. When extracellular K+ was raised from 5 to 30 mM the reversal potential shifted from -86 ± 1 mV (n = 7) to -46 ± 1 mV (n = 5) after correction for junction potential. These values are close to those predicted for a pure K+ current (-87 and -40 mV), and we conclude that the currents recorded under these conditions are mediated by K+-selective channels.
The voltage dependence of the inactivation is illustrated in Fig. 5. We examined the voltage dependence of inactivation of the delayed rectifier K+ current using the protocol shown in the inset of Fig. 5A. Membrane potential was held at conditioning potentials ranging from -80 to +20 mV in 10 mV increments for 3 s followed by test steps to +20 mV. Currents from one myocyte studied with this protocol are shown in Fig. 5A, and the data from ten myocytes are summarized in Fig. 5B, where the normalized peak currents elicited by the test steps are plotted (○). Conditioning potentials positive to -10 mV reduced the test current to about 20% of the maximum current. The half-inactivation potential, determined from a Boltzmann function fitted to the data, was -58 ± 1 mV (n = 10) after correction for junction potential. The kinetics of inactivation described earlier (Fig. 3) and the voltage dependence of inactivation seen here imply that changes in the membrane potential between -70 and -40 mV will greatly influence the availability of delayed rectifier current.
Figure 5B also shows the voltage dependence of activation of the delayed rectifier obtained by converting the peak currents elicited by test steps into permeabilities using the Goldman-Hodgkin-Katz current equation. The resulting permeabilities were normalized and plotted as a function of test potential (Fig. 5B, •). There was significant permeability activated at potentials positive to -50 mV and the half-activation of the Boltzmann function fitted to the data was -27 ± 0.4 mV (n = 10) after correction for junction potential. We note that there was activation and incomplete inactivation of the delayed rectifier at potentials positive to -50 mV, consistent with a sustained current flow (i.e. window current) in this potential range.
The ability of the delayed rectifier currents to influence membrane potential will also depend on how the conductance recovers from inactivation. We investigated this by determining the rate of recovery from inactivation following depolarizations to 0 mV of 1 or 10 s duration, as shown in Fig. 6A and B. Note that the 1 s conditioning pulse is approximately ten times τf and less than τs, whereas the 10 s pulse is approximately five times τs. The conditioning pulses were followed by increasing recovery intervals at -80 mV and 200 ms test steps to 0 mV. The test currents were normalized by dividing by the peak current during the conditioning pulse. The results from four cells are plotted in Fig. 6C. The degree of inactivation was greater following the longer conditioning pulse. The time course of recovery from inactivation was well described by a single time constant of 55 ms following the 1 s conditioning pulse, and 130 ms following the 10 s pulse (Fig. 6C). These results show that the delayed rectifier channels recover from inactivation relatively rapidly upon repolarization.
Figure 6. The recovery from inactivation of delayed rectifier K+ current.
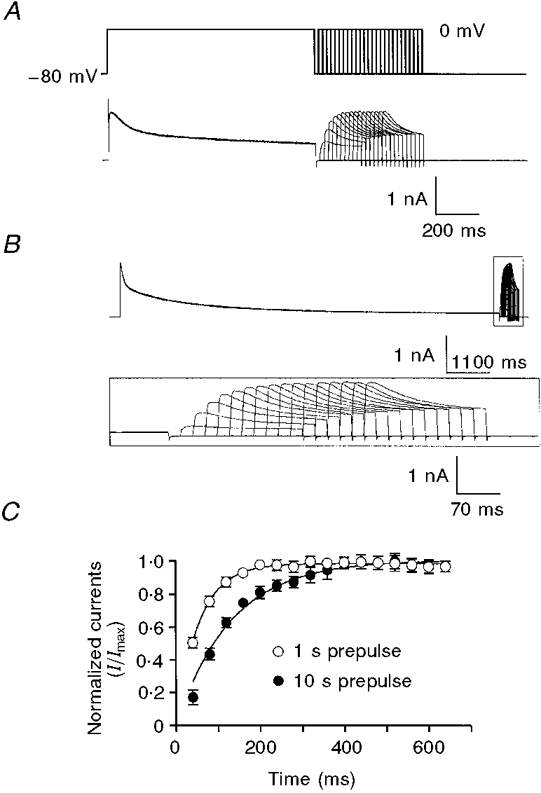
Membrane currents elicited as membrane potentials were stepped from -80 to 0 mV for 1 s (A) or 10 s (B) followed by variable recovery periods at -80 mV and test steps to 0 mV for 200 ms. The inset in B shows test currents (box in upper trace) at an expanded time scale. C, recovery of the delayed rectifier K+ current obtained by normalizing the peak current during the test step and plotting as a function of the recovery interval. ○, recovery after a 1 s conditioning step. •, recovery after a 10 s conditioning step. n = 4 cells. The continuous lines are curve fits to the equation It/Imax = 1 - (1 - I0/Imax) exp (-t/τ), where It is the amplitude of the current after t ms of recovery, Imax is the amplitude of the fully recovered current, I0 is the (extrapolated) current at the end of the conditioning step, and t and τ are time and the time constant in milliseconds.
Inhibition of delayed rectifier currents by TEA and 4-AP
In order to understand the contributions of delayed rectifier currents to the electrical activity of intact smooth muscle, we characterized the inhibition of K+ currents in isolated myocytes by TEA and 4-AP. These experiments were designed to provide information on the components of delayed rectifier current for comparison with studies applying these compounds to intact preparations (Figs 1 and 2).
TEA (10 mM) reduced the amplitude of the delayed rectifier current elicited by steps to potentials positive to -10 mV (Fig. 7A and B). The difference currents in Fig. 7C show that TEA reduced the outward current by an amount roughly equal to the amplitude of the sustained current at the end of the pulse. Thus the delayed rectifier current in the presence of TEA inactivates nearly completely (Fig. 7B). In contrast, the time course of the outward current was not changed by TEA: t½ for the activation of the current and the time constants of inactivation of the current were not significantly different from control. At 0 mV, t½ of activation was 11.5 ± 4.1 ms in control and 9.4 ± 3.3 ms in TEA (n = 6). Similarly, τf was 96 ± 10 ms in control and 78 ± 8 ms in TEA (n = 7). The voltage dependence of the inhibition of the peak delayed rectifier current by TEA is summarized in the current-voltage relationships plotted in Fig. 7D. TEA had no resolvable effect on membrane currents at negative potentials, but significantly reduced currents at positive potentials.
Figure 7. Inhibition of delayed rectifier K+ currents by 10 mM TEA.
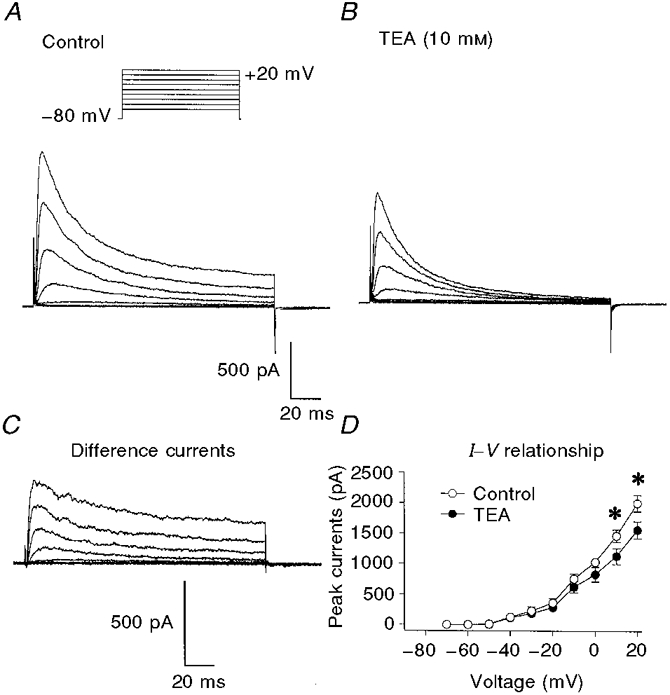
Membrane potential was stepped from -80 mV to test potentials between -60 and +50 mV in 10 mV increments for 200 ms. A, control. B, in the presence of 10 mM TEA. C, difference currents obtained by subtracting currents obtained in the presence of TEA from control currents. D, average peak current-voltage relationships in control (○) and TEA (•). n = 6 cells. * Significant difference between the currents in control and TEA. The currents in TEA at -10 mV are not significantly different from control (P = 0.066).
Inhibition of delayed rectifier current by 4-AP was strikingly different from the effects of TEA (Fig. 8A and B). 4-AP (5 mM) nearly abolished the early current following a step depolarization at potentials positive to -50 mV. At potentials positive to -30 mV, outward current increased slowly until it was larger than the control current after about 100 ms. Thus at these potentials, delayed rectifier current activates slowly in the presence of 4-AP: t½ for activation at 0 mV increased from 11.5 ± 4.1 ms in control to 189 ± 38 ms (n = 6) in the presence of 4-AP. 4-AP-difference currents (Fig. 8C) rise rapidly to a peak before declining slightly below baseline. At test potentials negative to -20 mV, the prolonged activation and increased outward currents were not detected. The voltage dependence of the inhibition of the peak delayed rectifier currents by 4-AP is shown in Fig. 8D. Comparison of Figs 7D and 8D illustrates the different effects of TEA and 4-AP on currents at negative potentials: 4-AP significantly reduced the early outward currents in the voltage range -40 to -20 mV. Lastly, 4-AP slowed the fast component of inactivation of the outward current (τf at 0 mV increased from 96 ± 10 ms in control to 210 ± 40 ms in 4-AP, n = 9 and 6, respectively), but had no effect on τs (data not shown). Although we interpret the action of TEA as the simple inhibition of a component of delayed rectifier current, several observations suggest that this is not the case with 4-AP. Firstly, the early current in the presence of 4-AP is smaller than the sustained control current, suggesting that 4-AP blocks part of the non-inactivating current. In addition, 4-AP significantly prolonged the time to peak current and increased the outward current after about 100 ms. These findings are consistent with the idea that 4-AP causes a strong block of resting K+ channels and the block is reduced by depolarization (see Discussion).
Figure 8. Effect of 5 mM 4-AP on delayed rectifier K+ current.
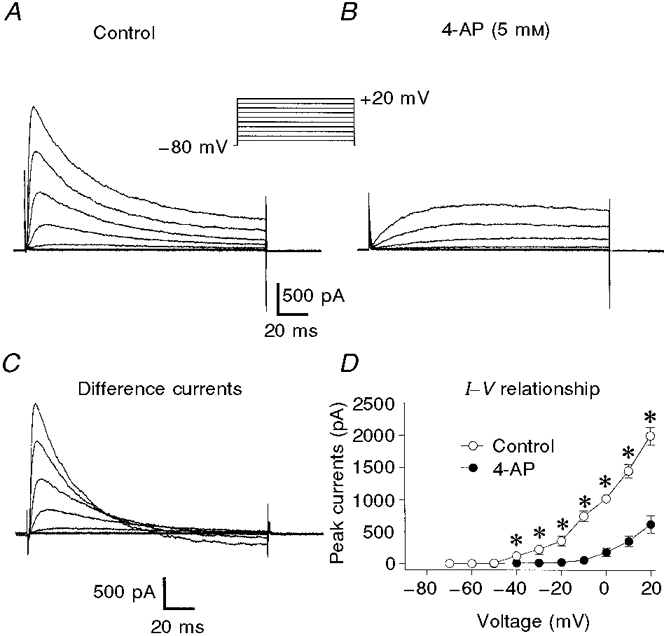
Membrane potential was stepped from -80 mV to test potentials between -70 and +20 mV in 10 mV increments for 200 ms. A, control. B, in the presence of 5 mM 4-AP. C, difference currents obtained by subtracting currents obtained in the presence of 4-AP from control currents. D, average current-voltage relationships in control (○) and 4-AP (•). n = 6 cells. * Significant difference between the currents in control and 4-AP.
Expression of candidate genes for the 4-AP-sensitive, rapidly inactivating component of delayed rectifier current in mouse proximal colon
We used RT-PCR to test for the presence of mRNA transcripts encoding voltage-gated K+ channel subunits that contribute to the formation of K+ channels with gating properties and pharmacological properties similar to those we found in the native smooth muscle cells. Kv1.4 and the Kv4 family are 4-AP-sensitive, rapidly inactivating delayed rectifier K+ channels. Expression of Kvβ1.1 confers rapid inactivation to Kv1 subunit family members when expressed together in heterologous systems (Sheng et al. 1994). Kv1.6, on the other hand, acts as a dominant negative influence on rapid inactivation when expressed as a heterotetramer with Kv1 channels that have rapidly inactivating amino-terminal components (Roeper et al. 1998). Its expression in mouse proximal colon myocytes would suggest that Kv1 channels were not contributing to this current.
Total RNA was prepared from isolated mouse proximal colon circular smooth muscle cells and from mouse brain as a positive control to ensure Kv channel primer specificity. PCR products were separated by electrophoresis on 2% agarose/TAE gels and visualized by ethidium bromide staining. As shown in Fig. 9, this approach detected mRNA encoding Kv1.6, Kv4.1, Kv4.2, Kv4.3 and Kvβ1.1 in murine colonic smooth muscle myocytes. We did not find a transcript for Kv1.4 in any of three circular smooth muscle cell preparations tested (Fig. 9), although a transcript for Kv1.4 was detected in RNA prepared from colonic tissue (not shown). β-Actin-specific primers amplified only the intron-less amplification product from all RNA samples, indicating that these preparations were free of genomic DNA contamination. In addition, all Kv channel primers detected specific amplification products from mouse brain derived RNA, demonstrating their specificity.
Figure 9. mRNA expression of Kv1, Kv4 and Kvβ subunits in murine proximal colon circular smooth muscle cells.
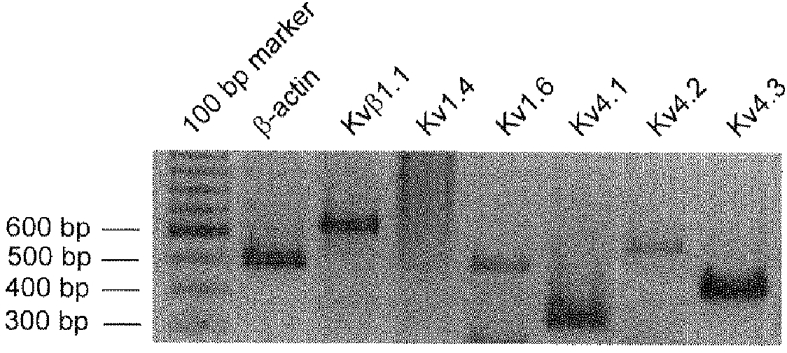
Two per cent agarose/TAE gel showing results of PCR amplifications using probes specific for β-actin (lane 2), Kvβ1.1 (lane 3), Kv1.4 (lane 4), Kv1.6 (lane 5), Kv4.1 (lane 6), Kv4.2 (lane 7) and Kv4.3 (lane 8). Lane 1 shows the molecular weight marker used to indicate the size of the PCR fragments. RT-PCR demonstrated the presence of Kvβ1.1 (633 bp), Kv1.6 (483 bp), Kv4.1 (323 bp), Kv4.2 (551 bp) and Kv4.3 (413 bp) in murine proximal colon circular smooth muscle cells. Kv1.4 (640 bp) was not detected in any smooth muscle cell preparations. β-Actin (498 bp) was used as an internal control to test for DNA contamination in the RNA preparations (see Methods). The primer used to detect mRNA in lane 5 amplifies a 483 bp PCR product that has been sequenced and identified as Kv1.6. It also amplifies a smaller mRNA non-specifically, which can be seen at the bottom of the figure.
DISCUSSION
K+ currents in murine colonic smooth muscle myocytes
We find that under conditions designed to minimize Ca2+ and Ca2+-activated currents, the outward currents of murine colonic myocytes are dominated by components of delayed rectifier K+ current. Although this result is qualitatively similar to the description of delayed rectifier current in canine colonic smooth muscle cells reported by Carl (1995), there are important differences. First, the current density of the delayed rectifier currents in murine colonic smooth muscle is four to six times larger than in canine cells: although membrane capacitance of the murine cells is about half that of canine cells (45.6 ± 5 pF, n = 12 for mouse vs. 100 pF for canine (Langton et al. 1989)), murine currents are two to three times larger than those of canine cells (cf. Carl, 1995). Second, the murine delayed rectifier currents have a fast component of inactivation (τf in the range 70-220 ms) that is not seen in canine cells. These observations imply the presence of greater numbers and different kinds of delayed rectifier channels in the murine preparation.
The delayed rectifier currents in canine and murine myocytes also differ in the dynamics of 4-AP block (described below). The block of current in canine myocytes is increased by depolarization, allowing a straightforward identification of a rapidly activating 4-AP-sensitive current component, IdK(f) (Carl, 1995). In contrast, we find that block in murine myocytes is relieved by depolarization, precluding such an interpretation of 4-AP difference currents.
The voltage range of the inactivation of the delayed rectifier current in murine colonic myocytes (half-inactivation near -58 mV following 3 s conditioning pulses) coincides with the voltage range of the slow waves in the intact preparations. We point out that the delayed rectifier conductance is nearly fully available at the maximum membrane polarization between spike complexes (MMP in Table 1). Furthermore, at the maximum membrane potential between spikes (MMP-SC, the initiation potential for the spikes) approximately half of the delayed rectifier is available for activation. Thus the inactivation properties of the delayed rectifier conductances are appropriate for these currents to play an important role in the control of the electrical activity of murine colonic smooth muscle.
Small but significant delayed rectifier currents were activated at potentials between -50 and -40 mV in murine colonic myocytes. These currents are not clearly shown at the gains used in Fig. 3, but can be seen at higher gain (cf. Figs 7 and 8). Furthermore, an increase in K+ permeability at these potentials is apparent in Fig. 5B. Activation of outward current in this potential range will inhibit action potential generation.
Pharmacology of the delayed rectifier K+ currents
The reduction of delayed rectifier K+ current by TEA is consistent with a simple block of a non-inactivating component of outward current positive to -10 mV. In contrast, the action of 4-AP is complex. 4-AP strongly reduced the current early in a depolarization. At potentials positive to -20 mV, where there is significant rapid inactivation, outward currents in the presence of this compound increased slowly and exceeded control currents after about 100 ms. At more negative test potentials (-40 and -30 mV), the effect of 4-AP was limited to the inhibition of outward current.
The slowed activation of delayed rectifier and increased current at late times in the presence of 4-AP are consistent with models where 4-AP blocks resting K+ channels, but the block is relieved during depolarization and channel activation (Yeh et al. 1976; Remillard & Leblanc, 1996; Tseng et al. 1996). Increased late outward current in the presence of 4-AP can be explained by these models if the channels must first unblock and open before they can undergo normal inactivation. In this scheme, the slower increase of the outward current in the presence of 4-AP reflects, in part, the unblocking process. Similar observations and interpretations have been made in studies of 4-AP effects on K+ currents in axons (Yeh et al. 1976), smooth muscle myocytes (Vogalis et al. 1993; Remillard & Leblanc, 1996), cardiac myocytes (Castle & Slawsky, 1992), and in studies of Kv1.4 and Kv4.2 channels expressed in Xenopus oocytes (Rasmusson et al. 1995; Tseng et al. 1996).
Other studies of 4-AP actions on delayed rectifiers find little resting block but increased block upon depolarization and channel activation (see references in Tseng et al. 1996). This result was obtained in studies of Kv1.2 and Kv1.5 (Russell et al. 1994) as well as Kv2.1 and Kv3.1 (Kirsch & Drewe, 1993) channels expressed in Xenopus oocytes. Interestingly, Carl (1995) observed this effect in canine colonic smooth muscle myocytes where delayed rectifier currents are attributed to expression of Kv1.2, Kv1.5 and Kv2.2 channels (Hart et al. 1993; Overturf et al. 1994; Schmalz et al. 1998). Thus the relief of 4-AP block of delayed rectifier K+ current by depolarization seen in murine colonic myocytes is unlike the effect of 4-AP on Kv1.2, Kv1.5, Kv2.1 and Kv3.1, but resembles the effect of 4-AP on Kv1.4 and Kv4.2.
Lastly, we note the other difference between the actions of TEA and 4-AP; namely, 4-AP but not TEA inhibited K+ currents at potentials between -40 and -20 mV.
Influences of the TEA- and 4-AP-sensitive currents on the rhythmicity of murine colon
One aim of the present work was to correlate the membrane currents of isolated colonic myocytes with the electrical activity of the intact tissue. In order to do this, we must establish that the same kinds of cells were studied by the various techniques we used and that results obtained under different conditions are comparable. We have considered specifically the possibility that cells from the circular and longitudinal smooth muscle layers contributed differently in our protocols. Although the different properties of these muscle layers in canine colon are well documented (Thornbury et al. 1992a, b), less information is available concerning these layers in the murine colon. Intact preparations of mouse colon are much thinner than canine preparations, making it difficult to impale selectively a particular layer. Furthermore, based on the relative thickness of the two layers and the orientation of our preparations, we expect that most or all of our tissue recordings came from the circular smooth muscle layer. Similarly, we expect that most of our voltage-clamp studies of myocytes from bulk smooth muscle preparations were done on circular myocytes because of the relative thickness of this layer. Certainly, we did not detect two populations of cells in these experiments. However, we also studied myocytes from preparations where we separated the two layers and found no obvious differences, particularly with regard to the effects of TEA and 4-AP (data not shown). Accordingly, we conclude that the results reported here characterize the properties of the circular smooth muscle layer of the murine colon and that the data from the intact tissue recordings, the voltage-clamp studies, and the molecular studies can be compared directly.
A second issue is the different temperatures used in the intact tissue experiments and patch-clamp experiments. We have been unable to perform voltage-clamp experiments at 37°C because the isolated murine colonic myocytes go into contracture at this temperature. In a series of experiments done at 32°C, we found that the peak delayed rectifier currents were about 20% larger and inactivated more rapidly than control. Similar observations have been made in canine colonic smooth muscle (Thornbury et al. 1992a, b). The actions of 4-AP and TEA were independent of temperature. In particular, 4-AP but not TEA reduced the delayed rectifier current at negative potentials. Thus we conclude that the pharmacological responses in the whole tissue and patch-clamp experiments are directly comparable.
Although our voltage-clamp experiments were done under conditions that prevented contribution by Ca2+-activated K+ channels, we cannot rule out the possibility that these channels contribute to the response of the intact tissue to TEA. Nevertheless, our results provide strong evidence that delayed rectifier K+ channels play an important role in the electrical activity of murine colonic smooth muscle.
TEA and 4-AP had different actions on the electrical activity of intact murine colon. TEA had no significant effect on the development of slow waves, but increased the amplitude of the action potentials in the spike complexes. In contrast, 4-AP had no significant effect on the spike depolarizations but abolished the quiescent periods between the spike complexes, i.e. TEA affected events at potentials positive to -10 mV, whereas 4-AP affected events at potentials negative to -30 mV.
The voltage dependence and time dependence of inactivation of the 4-AP-sensitive current in our experiments are similar to those seen in K+ currents in neurons (Connor & Stevens, 1971) and in other smooth muscle cells (Clapp & Gurney, 1991; Beech & Bolton, 1989; Hume & Leblanc, 1989; Vogalis et al. 1993) and classifies it as an ‘IA-like’ current (Hume & Leblanc, 1989; Vogalis et al. 1993). Interestingly, our data implicating this delayed rectifier component in the control of the frequency of spike complexes are consistent with the function of IA-like currents proposed in the studies cited above (see also Hille, 1992).
The origins of the rhythmic slow waves and spike complexes of murine colonic smooth muscle are unclear. Bywater et al. (1989) proposed that the slow waves are initiated by neuronal activity because they are sensitive to hexamethonium, tetrodotoxin and atropine. However, Lomax et al. (1996) obtained data suggesting that slow waves and spike complexes can be generated in the absence of neuronal activity. Although our results do not address this issue directly, the ability of 4-AP to abolish rhythmic slow waves and to cause continuous spiking demonstrates the importance of smooth muscle K+ conductance in determining the electrical activity of this tissue.
Molecular identification of the 4-AP-sensitive, rapidly inactivating component of delayed rectifier K+ currents of murine colonic smooth muscle
We determined the transcriptional expression of genes encoding 4-AP-sensitive, rapidly inactivating K+ channels. Based on properties found in heterologous expression systems, Kv1.4 and the Kv4 family of channels have these properties (Rettig et al. 1992; Po et al. 1993). However, the Kvβ1.1 subunit confers rapid inactivation on other Kv1 gene family members. Finally, Kv1.6 has a dominant negative influence on rapid inactivation when expressed as heterotetramers with those Kv1 channels that show N-type inactivation. Thus co-expression of Kv1.6 results in slowly inactivating currents (Roeper et al. 1998). We found that murine proximal colonic smooth muscle cells express transcripts encoding Kv1.6, Kv4.1, Kv4.2 and Kv4.3 channels, as well as a transcript encoding the Kvβ1.1 subunit. The lack of Kv1.4 expression in circular myocytes implies that this channel does not contribute to the delayed rectifier current. Expression of Kvβ1.1 suggests that Kv1/β channels may contribute to a rapidly inactivating current, although expression of Kv1.6 is expected to limit the inactivation of such channels. In our view, the most likely candidates to underlie the 4-AP-sensitive, rapidly inactivating delayed rectifier component are the Kv4 family members expressed in murine colonic myocytes. Transgenic knock-out animals would provide conclusive evidence for this assignment.
Summary
We characterized the effects of K+ channel blockers on the electrical activity of murine colonic smooth muscle and on the delayed rectifier K+ currents of isolated smooth muscle myocytes. The results of this work suggest that pharmacologically distinct current components are important in specific aspects of colonic rhythmicity. Of particular interest is the identification of a small, but important component of delayed rectifier current between -40 and -20 mV that is essential for the quiescent periods that separate spike complexes.
Acknowledgments
This project was supported by NIDDK with DK 41315. We are very grateful to V. M. Jackson for excellent technical assistance in electrophysiological experiments.
References
- Barry PH. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. Journal of Neuroscience Methods. 1994;51:107–116. doi: 10.1016/0165-0270(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Beech DJ, Bolton TB. Properties of the cromakalim-induced potassium conductance in smooth muscle cells isolated from the rabbit portal vein. British Journal of Pharmacology. 1989;98:851–864. doi: 10.1111/j.1476-5381.1989.tb14614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonev AD, Nelson MT. ATP-sensitive potassium channels in smooth muscle cells from guinea pig urinary bladder. American Journal of Physiology. 1993;264:C1190–1200. doi: 10.1152/ajpcell.1993.264.5.C1190. [DOI] [PubMed] [Google Scholar]
- Bywater RAR, Small RC, Taylor GS. Neurogenic slow depolarizations and rapid oscillations in the membrane potential of circular muscle of mouse colon. The Journal of Physiology. 1989;413:505–519. doi: 10.1113/jphysiol.1989.sp017666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carl A. Multiple components of delayed rectifier K+ current in canine colonic smooth muscle. The Journal of Physiology. 1995;484:339–353. doi: 10.1113/jphysiol.1995.sp020669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carl A, Kenyon JL, Uemura D, Fusetani N, Sanders KM. Regulation of Ca2+-activated K+ channels by protein kinase A and phosphatase inhibitors. American Journal of Physiology. 1991;261:C387–392. doi: 10.1152/ajpcell.1991.261.2.C387. [DOI] [PubMed] [Google Scholar]
- Castle NA, Slawsky MT. Characterization of 4-aminopyridine block of the transient outward K+ current in adult rat ventricular myocytes. Journal of Pharmacology and Experimental Therapeutics. 1992;264:1450–1459. [PubMed] [Google Scholar]
- Clapp LH, Gurney AM. Outward currents in rabbit pulmonary artery cells dissociated with a new technique. Experimental Physiology. 1991;76:677–693. doi: 10.1113/expphysiol.1991.sp003535. [DOI] [PubMed] [Google Scholar]
- Connor JA, Stevens CF. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. The Journal of Physiology. 1971;213:21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C, Carl A, Smith TK, Sanders KM, Keef KD. Mechanism of cyclic AMP-induced hyperpolarization in canine colon. Journal of Pharmacology and Experimental Therapeutics. 1994;268:208–215. [PubMed] [Google Scholar]
- Gelband CH, Hume JR. Ionic currents in single smooth muscle cells of the canine renal artery. Circulation Research. 1992;71:745–758. doi: 10.1161/01.res.71.4.745. [DOI] [PubMed] [Google Scholar]
- Hart PJ, Overturf KE, Russell SN, Carl A, Hume JR, Sanders KM, Horowitz B. Cloning and expression of a Kv1.2 class delayed rectifier K+ channel from canine colonic smooth muscle. Proceedings of the National Academy of Sciences of the USA. 1993;90:9659–9663. doi: 10.1073/pnas.90.20.9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA, USA: Sinauer Associates; 1992. Potassium channels and chloride channels; pp. 115–139. [Google Scholar]
- Huizinga JD, Stern H, Chow E, Eiamant NE, El-Sharkawy TY. Electrophysiological control of motility in the human colon. Gastroenterology. 1985;88:500–511. doi: 10.1016/0016-5085(85)90513-x. [DOI] [PubMed] [Google Scholar]
- Hume JR, Leblanc N. Macroscopic K+ currents in single smooth muscle cells of the rabbit portal vein. The Journal of Physiology. 1989;413:49–73. doi: 10.1113/jphysiol.1989.sp017641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Eckman DM, Keef KD. Characterization of delayed rectifier K+ currents in rabbit coronary artery cells near resting membrane potential. Canadian The Journal of Physiology and Pharmacology. 1997;75:1116–1122. 10.1139/cjpp-75-9-1116. [PubMed] [Google Scholar]
- Kirsch GE, Drewe JA. Gating-dependent mechanism of 4-aminopyridine block in two related potassium channels. Journal of General Physiology. 1993;102:797–816. doi: 10.1085/jgp.102.5.797. 10.1085/jgp.102.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knot HJ, Zimmermann PA, Nelson MT. Extracellular K+-induced hyperpolarizations and dilatations of rat coronary and cerebral arteries involve inward rectifier K+ channels. The Journal of Physiology. 1996;492:419–430. doi: 10.1113/jphysiol.1996.sp021318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SD, Sanders KM, Carl A. Regulation of smooth muscle delayed rectifier K+ channels by protein kinase A. Pflügers Archiv. 1996;432:401–412. doi: 10.1007/s004240050151. [DOI] [PubMed] [Google Scholar]
- Langton PD, Burke EP, Sanders KM. Participation of Ca currents in colonic electrical activity. American Journal of Physiology. 1989;257:C451–460. doi: 10.1152/ajpcell.1989.257.3.C451. [DOI] [PubMed] [Google Scholar]
- Lomax A, Harney SC, Jackson VM, Ward SM. Interaction of inhibitory transmitters regulate electrical and mechanical activity in the murine colon. Gastroenterology. 1996;110:A709. Abstract. [Google Scholar]
- Ng B, Barry PH. The measurement of ionic conductivities and mobilities of certain less common organic ions needed for junction potential corrections in electrophysiology. Journal of Neuroscience Methods. 1995;56:37–41. doi: 10.1016/0165-0270(94)00087-w. [DOI] [PubMed] [Google Scholar]
- Overturf KE, Russell SN, Carl A, Vogalis F, Hart PJ, Hume JR, Sanders KM, Horowitz B. Cloning and characterization of a Kv1.5 delayed rectifier K+ channel from vascular and visceral smooth muscles. American Journal of Physiology. 1994;267:C1231–1238. doi: 10.1152/ajpcell.1994.267.5.C1231. [DOI] [PubMed] [Google Scholar]
- Po S, Roberds S, Snyders DJ, Tamkun MM, Bennett PB. Heteromultimeric assembly of human potassium channels. Molecular basis of a transient outward current? Circulation Research. 1993;72:1326–1336. doi: 10.1161/01.res.72.6.1326. [DOI] [PubMed] [Google Scholar]
- Quayle JM, McCarron JG, Brayden JE, Nelson MT. Inward rectifier K+ currents in smooth muscle cells from rat resistance-sized cerebral arteries. American Journal of Physiology. 1993;265:C1363–1370. doi: 10.1152/ajpcell.1993.265.5.C1363. [DOI] [PubMed] [Google Scholar]
- Rae MG, Fleming N, McGregor DB, Sanders KM, Keef KD. Control of motility patterns in the human colonic circular muscle layer by pacemaker activity. The Journal of Physiology. 1998;510:309–320. doi: 10.1111/j.1469-7793.1998.309bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson RL, Zhang Y, Campbell DL, Comer MB, Castellano RC, Strauss HC. Bi-stable block by 4-aminopyridine of a transient K+ channel (Kv1.4) cloned from ferret ventricle and expressed in Xenopus oocytes. The Journal of Physiology. 1995;485:59–71. doi: 10.1113/jphysiol.1995.sp020712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remillard CV, Leblanc N. Mechanism of inhibition of delayed rectifier K+ current by 4-aminopyridine in rabbit coronary myocytes. The Journal of Physiology. 1996;491:383–400. doi: 10.1113/jphysiol.1996.sp021223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig J, Wunder F, Stocker M, Lichtinghagen R, Mastiaux F, Beckh S, Kues W, Pedarzani P, Schröter KH, Ruppersberg JP, Veh R, Pongs O. Characterization of a Shaw-related potassium channel family in rat brain. EMBO Journal. 1992;11:2473–2486. doi: 10.1002/j.1460-2075.1992.tb05312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeper J, Sewing S, Zhang Y, Sommer T, Wanner SG, Pongs O. NIP domain prevents N-type inactivation in voltage-gated potassium channels. Nature. 1998;391:390–393. doi: 10.1038/34916. [DOI] [PubMed] [Google Scholar]
- Russell SN, Publicover NG, Hart PJ, Carl A, Hume JR, Sanders KM, Horowitz B. Block by 4-aminopyridine of a Kv1.2 delayed rectifier K+ current expressed in Xenopus oocytes. The Journal of Physiology. 1994;481:571–584. doi: 10.1113/jphysiol.1994.sp020464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM. Ionic mechanisms of electrical rhythmicity in gastrointestinal smooth muscles. Annual Review of Physiology. 1992;54:439–453. doi: 10.1146/annurev.ph.54.030192.002255. [DOI] [PubMed] [Google Scholar]
- Schmalz F, Kinsella J, Koh SD, Vogalis F, Schneider A, Flynn ER, Kenyon JL, Horowitz B. Molecular identification of a component of delayed rectifier current in gastrointestinal smooth muscles. American Journal of Physiology. 1998;274:G901–911. doi: 10.1152/ajpgi.1998.274.5.G901. [DOI] [PubMed] [Google Scholar]
- Sheng Z-H, Rettig J, Takahashi M, Catterall WA. Identification of a syntaxin-binding site on N-type calcium channels. Neuron. 1994;13:1303–1313. doi: 10.1016/0896-6273(94)90417-0. [DOI] [PubMed] [Google Scholar]
- Smirnov SV, Aaronson PI. Ca2+-activated and voltage-gated K+ currents in smooth muscle cells isolated from human mesenteric arteries. The Journal of Physiology. 1992;457:431–454. doi: 10.1113/jphysiol.1992.sp019386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornbury KD, Ward SM, Sanders KM. Participation of fast-activating, voltage-dependent K currents in electrical slow waves of colonic circular muscle. American Journal of Physiology. 1992a;263:C226–236. doi: 10.1152/ajpcell.1992.263.1.C226. [DOI] [PubMed] [Google Scholar]
- Thornbury KD, Ward SM, Sanders KM. Outward currents in longitudinal colonic muscle cells contribute to spiking electrical behavior. American Journal of Physiology. 1992b;263:C237–245. doi: 10.1152/ajpcell.1992.263.1.C237. [DOI] [PubMed] [Google Scholar]
- Tseng G-N, Jiang M, Yao J-A. Reverse use dependence of Kv4.2 blockade by 4-aminopyridine. Journal of Pharmacology and Experimental Therapeutics. 1996;279:865–876. [PubMed] [Google Scholar]
- Vogalis F, Lang RJ, Bywater RAR, Taylor GS. Voltage-gated ionic currents in smooth muscle cells of guinea pig proximal colon. American Journal of Physiology. 1993;264:C527–563. doi: 10.1152/ajpcell.1993.264.3.C527. [DOI] [PubMed] [Google Scholar]
- Yeh JZ, Oxford GS, Wu CH, Narahashi T. Dynamics of aminopyridine block of potassium channels in squid axon membrane. Journal of General Physiology. 1976;68:519–535. doi: 10.1085/jgp.68.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]


