Abstract
The paranodal Schwann cell region is of major importance for the function of a myelinated axon. In the present study we searched for a possible ionotropic effect of extracellular ATP in this Schwann cell compartment.
Whole-cell patch-clamp recordings from cultured rat Schwann cells revealed that ATP and 2′–3′-O-(4-benzoylbenzoyl)-adenosine 5′-triphosphate (BzATP) induced a non-specific cation current. The effect of ATP was much enhanced in a Ca2+- and Mg2+-free solution. ADP, UTP and α,β-methylene adenosine 5′-triphosphate (α,β-meATP) had no effect.
Confocal Ca2+ imaging of myelinating Schwann cells in isolated rat spinal roots showed a BzATP-induced rise in the free intracellular Ca2+ concentration in the paranodal Schwann cell cytoplasm whereas α,β-meATP and 2-(methylthio)-adenosine 5′-triphosphate were without effect. In contrast to the known metabotropic effect of UTP on these Schwann cell regions, the BzATP-induced Ca2+ signal was not transient, was unaffected by depletion of intracellular Ca2+ stores and dependent on the presence of extracellular Ca2+.
These results suggest that an ionotropic ATP receptor with electrophysiological and pharmacological characteristics of the P2X7 subtype of nucleotide receptors is functionally active in myelinating Schwann cells of peripheral nerves. Such a receptor might contribute to Schwann cell reactions in nerve injury or neuropathy.
The paranodal Schwann cell region is of major functional importance for the physiology and/or pathophysiology of peripheral nerves. Although receptors for neuroligands in this area of the Schwann cell membrane might contribute to hitherto unknown interactions of Schwann cells and axons or might be a key element for the responses of Schwann cells following axonal injury or in the pathogenesis of neuropathies, knowledge of such receptors is still fragmentary. At present, there is experimental evidence for the presence of metabotropic ATP receptors. Such P2Y receptors have been found in the paranodal and other cell regions of Schwann cells in situ (Lyons et al. 1995; Robitaille, 1995; Green et al. 1997; Mayer et al. 1997, 1998), as well as in Schwann cells in culture (Lyons et al. 1994; Berti-Mattera et al. 1996; Ansselin et al. 1997; Jeftinija & Jeftinija, 1998).
In addition to P2Y receptors, ATP and/or related nucleotides act at ionotropic (P2X) receptors (for review, see North & Barnard, 1997; Burnstock, 1998; Ralevic & Burnstock, 1998). Up to now, only one study has indicated the presence of an ionotropic ATP receptor on Schwann cells. Amédée & Despeyroux (1995) observed an ATP-induced inward current in cultured mouse Schwann cells. However, the concentration of ATP necessary to induce this effect (Kd of 8.4 mM) was about 1000-fold higher than that required to activate intracellular Ca2+ transients by P2Y nucleotide receptors and the authors did not classify the underlying receptor. Furthermore, the possibility of an ionotropic effect of ATP in Schwann cells in intact peripheral nerve tissue was not tested. Recently, several important advances have been made in the area of ionotropic P2X nucleotide receptors. In particular, the mRNA and the protein of the P2X7 receptor have been isolated from a brain cDNA library (Collo et al. 1997). The P2X7 nucleotide receptor, in contrast to the other P2X subtypes, is activated only by high (>0.3 mM; North & Barnard, 1997) concentrations of ATP and, therefore, might underlie the ATP-induced current observed in Schwann cells by Amédée & Despeyroux (1995). The active ligand is suggested to be the tetrabasic acid ATP4−, which is present as approximately 1% of the relatively high concentration of ATP (Ralevic & Burnstock, 1998). Thus, reducing the extracellular divalent cation concentration increases agonist potency. Furthermore, it is known that BzATP is a potent agonist at P2X7 nucleotide receptors (Wiley et al. 1994). Interestingly, some of the cellular responses described following activation of P2X7 receptors are apoptosis (Chow et al. 1997; Ferrari et al. 1997a) and cytokine release (Ferrari et al. 1997b). These phenomena are well known in Schwann cells but there is little understanding of the underlying mechanism. Using BzATP as a pharmacological tool we have now re-investigated the ATP-induced membrane current in cultured Schwann cells. In addition, we have used confocal calcium imaging to explore the possible presence of P2X7 receptors in intact peripheral nerve preparations. The data indicate that P2X7 receptors are in fact functionally active in the paranodal Schwann cell region of myelinated nerve fibres.
METHODS
Preparation of Schwann cell cultures
Schwann cells were obtained from sciatic nerves of 2-day-old Wistar rats of both sexes that were killed by decapitation after anaesthesia with pentobarbital (0.1 mg g−1, i.p.). All animal experiments were performed in accordance with national guidelines. The nerves were dissociated enzymatically by 0.25% collagenase type I and 0.25% trypsin and mechanically by trituration. The suspension obtained was pelleted by centrifugation (1000 r.p.m., 10 min) and the pellet resuspended in medium. The cells were plated on uncoated Falcon culture dishes (35 mm, Becton Dickinson Labware, Oxnard, CA, USA) with a medium containing Dulbecco's modified Eagle's medium (DMEM), 10% fetal calf serum (FCS) and 500 U ml−1 penicillin-streptomycin (Sigma). Contaminating fibroblasts were removed by application of 10 μM cytosine arabinoside for 3 days. After that, medium was changed to proliferation medium containing DMEM, 10% FCS, 0.5% forskolin, 0.5 mM 3-isobutyl-1-methylxanthine, 2.5 μg ml−1 insulin, 5 ng ml−1 rhGGF2 (Cambridge Neuroscience, Cambridge, MA, USA), and 500 U ml−1 penicillin- streptomycin. Cultured cells were used for experiments 8-14 days after preparation.
Electrophysiology
Membrane currents were studied using the patch-clamp technique in the whole-cell configuration. Cells were maintained in culture dishes on the stage of an inverted microscope (Axiovert 35, Zeiss, Jena, Germany) at room temperature. Patch pipettes were pulled (DMZ puller, Zeitz, Augsburg, Germany) from borosilicate glass tubes (GC150F-10, Clark Electromedical Instruments, Pangbourne, UK). Electrode tip resistance was 6.2 ± 0.2 MΩ (n = 40) and the series resistance of 17.8 ± 1.7 MΩ was compensated to 74.4 ± 0.9% (n = 27). The mean membrane capacitance of the cultured Schwann cells was determined as 29.8 ± 3.6 pF (n = 27).
The standard bath solution contained (mM): 140 NaCl, 3.0 KCl, 1.2 MgCl2, 2.2 CaCl2, 10 Hepes, 10 glucose; pH adjusted to 7.4 with NaOH. In some of the experiments, a Ca2+- and Mg2+-free standard bath solution was used. Patch pipettes were filled with the following solution (mM): 140 potassium gluconate, 3 MgCl2, 5 EGTA, 2 Na2ATP, 2 Na2GTP, 5 Hepes; pH adjusted to 7.25 with KOH. Drugs were applied by a rapid application (Y-tube) method (Nakagawa et al. 1991). Recordings were made with an Axopatch 200 amplifier (Axon Instruments). pCLAMP 6 software (Axon Instruments) was used for the generation of the different pulse protocols and the acquisition and analysis of data.
Preparation of isolated rat spinal roots
Wistar rats (200-400 g) were anaesthetized by i.p. injection (1.5 g kg−1) and later killed by intracardiac injection of urethane (1.5 g kg−1). Spinal roots were removed and split mechanically into two or three fibre bundles. After preparation, the nerves were loaded with the fluorescent dyes using the membrane-permeant AM esters. Stock solutions of 0.9 mM Calcium Green-1 AM and 1 mM Fura Red AM were prepared by dissolving 50 μg of each in 20 μl dimethylsulphoxide (DMSO) plus 20 μl Pluronic F-127 20%. The isolated fascicles were incubated for 90 min at 37°C in 3 ml Hepes-buffered saline to which 20 μl of each stock solution had been added (final concentration 6 μM Calcium Green-1 and 7 μM Fura Red). After incubation, the nerves were washed thoroughly and stored at 4-8°C until use.
Confocal Ca2+ imaging
The method used for confocal Ca2+ imaging has been described before (Mayer et al. 1997). In brief, the optics consist of an upright microscope (Olympus BX50WI) in combination with a Bio-Rad MRC-1024 confocal imaging system. The spinal root fascicles were fastened in an organ bath and confocal images were collected at 530 nm (emission of Calcium Green-1) and 660 nm (emission of Fura Red) after excitation at 488 nm at intervals of 5-10 s. An elevation in the free intracellular Ca2+ concentration ([Ca2+]i) increases Calcium Green emission at 530 nm (F530), but decreases Fura Red emission at 660 nm (F660). Therefore, the mean grey values F530 and F660 of selected regions of interest were used to calculate the emission ratio: R’= F530/F660. Ratio values are always given normalized R = R′/R0, i.e. divided by a reference value R0 obtained immediately before drug administration.
Chemicals
The organ bath was continuously perfused with a bathing solution containing (mM): 118 NaCl, 3.0 KCl, 1.5 CaCl2, 1.0 MgCl2, 5 D-glucose, 25 NaHCO3, and 1.2 NaH2PO4; gassed with carbogen (95% O2-5% CO2). ADP, ATP, 2′-3′-O-(4-benzoylbenzoyl)-adenosine 5′-triphosphate (BzATP), α,β-methylene adenosine 5′-triphosphate (α,β-meATP), 2-(methylthio)-adenosine 5′-triphosphate (2-MeSATP), and uridine 5′-triphosphate (UTP) were purchased from Sigma. Calcium Green-1 AM, Fura Red AM amd Pluronic F-127 were from Molecular Probes.
Statistics
All results are expressed as means ±s.e.m. with n being the sample size.
RESULTS
Electrophysiological observations on cultured Schwann cells
After the whole-cell configuration was achieved, slow voltage ramps (-140 to 80 mV in 2 s) were applied to cultured rat Schwann cells in the control bathing solution and during addition of ATP and BzATP. Both agonists produced an increase in membrane conductance. In the case of ATP (1 mM; Fig. 1A), this effect was more prominent at negative membrane potentials, whereas BzATP (150 μM; Fig. 1B) enhanced the membrane conductance over the entire voltage range tested. The reversal potential for the BzATP-induced current in a Cl−-free bathing solution was -1.7 ± 2.9 mV (n = 5) which indicates an increase in a non-specific cation conductance. The kinetics of the current produced by ATP and BzATP were measured at a constant holding potential (Fig. 1C and D) and showed a rapid onset and no desensitization (Fig. 1D). Quantitatively, the inward current at a membrane potential of -80 mV was analysed in 15 Schwann cells responsive to ATP and/or BzATP (total number of cells tested: n = 52). ATP (1 mM) induced a current of -2.46 ± 0.5 pA pF−1 (n = 6) that was enhanced in a Ca2+- and Mg2+-free solution to -7.25 ± 1.7 pA pF−1 (n = 11; ATP 0.3 mM). BzATP (150 μM) was more potent than ATP; however, the BzATP-induced current (-12.69 ± 4.2 pA pF−1; n = 9) did not show clear augmentation in a Ca2+- and Mg2+-free solution. We also tested the effects of ADP, UTP (1 mM each), and of α,β-meATP (0.4 mM); these agonists at different types of P2 receptors did not change the membrane conductance of cultured rat Schwann cells (n = 3-6).
Figure 1. ATP and BzATP induce membrane currents in cultured rat Schwann cells.
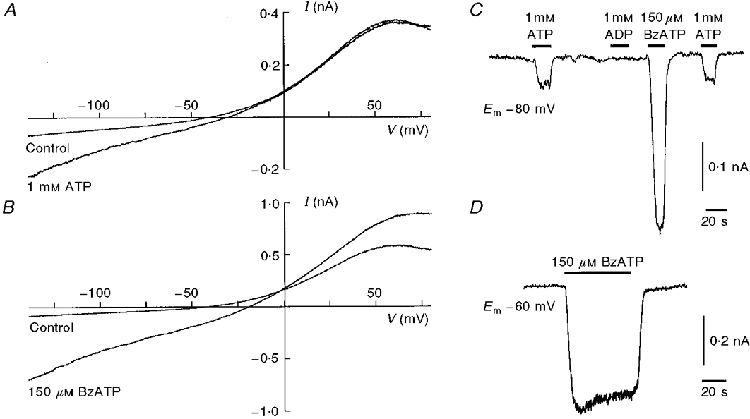
The illustrated examples from four different Schwann cells are representative for the effects of ATP and BzATP. A and B, membrane currents induced by slow voltage ramps from -140 to 80 mV in 2 s were recorded from two different cultured rat Schwann cells before and during bath application of ATP (1 mM) and BzATP (150 μM). Note the different scaling of the current axes. C and D, the membranes of these Schwann cells were voltage clamped at a constant holding potential (Em) and changes in the holding current were recorded during bath application of different P2 nucleotide receptor agonists.
Confocal calcium imaging of Schwann cells in situ
Bath application of BzATP produced a rise in [Ca2+]i in the paranodal Schwann cell region of myelinated nerve fibres in isolated rat spinal roots (Fig. 2). Quantitatively, 150 μM BzATP produced a mean change in the emission ratio of Calcium Green and Fura Red of 46.5 ± 6.6% in 27 paranodes responsive to BzATP. Eleven paranodes (28%) were considered non-responsive, i.e. the changes in the ratio were less than 5%. In contrast, α,β-meATP (0.3 mM; n = 11) and 2-MeSATP (0.1 mM; n = 7), both agonists tested at various P2X receptors, did not induce a rise in [Ca2+]i in the paranodal cytoplasm of BzATP-sensitive Schwann cells.
Figure 2. BzATP induces a rise in [Ca2+]i in the paranodal Schwann cell cytoplasm.
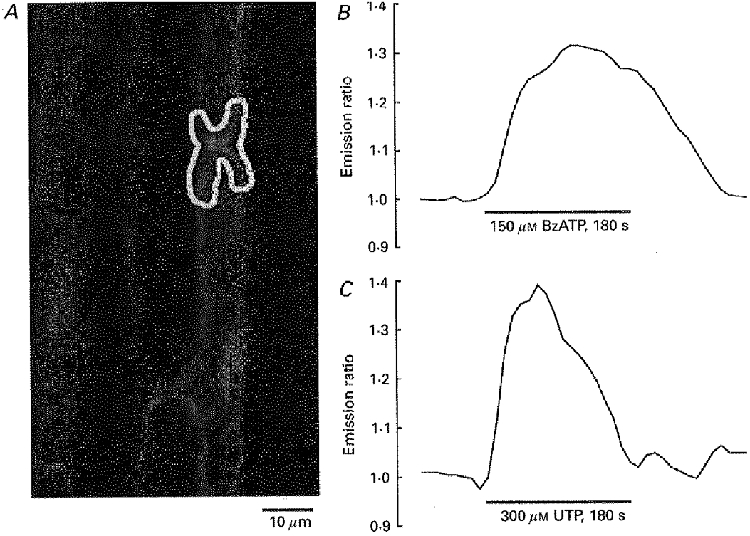
A, confocal image of myelinated nerve fibres within an isolated rat spinal root stained with the Ca2+-sensitive dyes Calcium Green-1 and Fura Red (excitation wavelength 488 nm; emission wavelengths 530 and 660 nm, respectively). B and C, the mean grey value of the paranodal Schwann cell area (selected region of interest indicated in A) and its normalized ratio was measured during bath application of BzATP (150 μM; B) and UTP (300 μM; C). Note the maintained rise in [Ca2+]i during application of BzATP whereas UTP induced a transient rise in [Ca2+]i only.
The BzATP-induced Ca2+ signal differed in three aspects from the Ca2+ transient produced by bath application of UTP. Previously, UTP has been found to activate metabotropic P2Y2 receptors in myelinated Schwann cells (Mayer et al. 1997, 1998). First, the effect of BzATP on [Ca2+]i was not transient. This is illustrated in Fig. 2 which shows one of three experiments in which BzATP and UTP were both applied for 3 min. The effect of UTP on [Ca2+]i was transient, i.e. the Ca2+ signal returned to the baseline level despite the continuing presence of the receptor agonist. In contrast, the rise in [Ca2+]i produced by BzATP was maintained: it remained elevated until the end of the application time.
The second difference between BzATP and UTP was the source of Ca2+ for the rise in [Ca2+]i. As illustrated in Fig. 3, the Ca2+ signal produced by BzATP was completely absent in a Ca2+-free bath solution (n = 7), whereas the UTP-induced Ca2+ transient was only slightly reduced under these circumstances (to 80 ± 12% of control; n = 3). Finally, the effects of BzATP and UTP differed in their dependence on intracellular Ca2+ stores. Cyclopiazonic acid (CPA, 5 μM), an inhibitor of the Ca2+-ATPase of the sarco/endoplasmic reticulum, was added to the bathing solution 5 min before and during the application of the two P2 nucleotide receptor agonists (Fig. 4). CPA blocked the effect of UTP (n = 4) whereas the BzATP-induced Ca2+ signal was not much changed (to 95 ± 18% of control; n = 12).
Figure 3. BzATP induces a transmembrane Ca2+ influx.
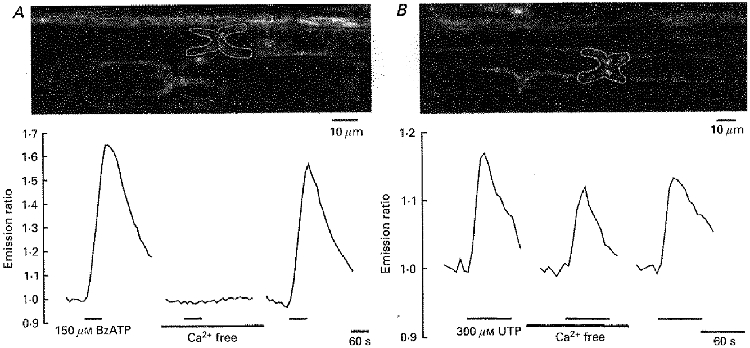
A, the white outline in the upper panel shows a paranodal Schwann cell region within an isolated rat spinal root stained with the Ca2+-sensitive dyes Calcium Green-1 and Fura Red. Changes in [Ca2+]i were measured during application of BzATP (150 μM) into the standard and into a Ca2+-free bathing solution. B, a similar experimental protocol was applied to a different rat spinal root. In this case, changes in [Ca2+]i were measured during application of UTP (300 μM) into the standard and into a Ca2+-free bathing solution. Note that only the BzATP-induced intracellular Ca2+ transient was completely and reversibly blocked during removal of extracellular Ca2+.
Figure 4. BzATP does not release Ca2+ from intracellular stores.
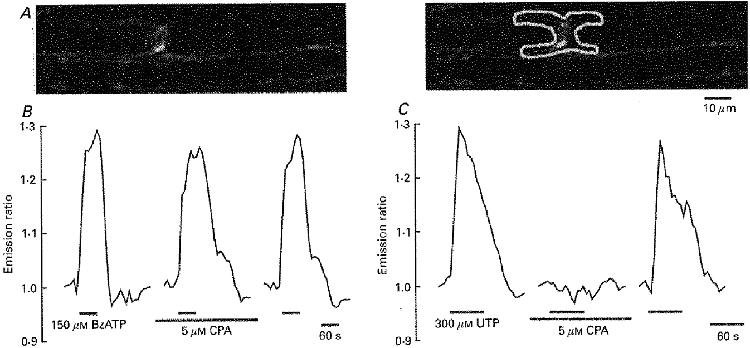
A, confocal image of two myelinated nerve fibres within an isolated rat spinal root stained with the Ca2+-sensitive dyes Calcium Green-1 and Fura Red. The mean grey value of the paranodal Schwann cell area (selected region of interest indicated in the right panel) and its ratio was used for the analysis. B and C, changes in [Ca2+]i were measured during application of BzATP (150 μM) and UTP (300 μM) into the standard bathing solution and after depletion of intracellular Ca2+ stores with cyclopiazonic acid (CPA, 5 μM for 5 min). Note that only the UTP-induced intracellular Ca2+ transient was completely and reversibly blocked by CPA.
DISCUSSION
The action of ATP at P2X7 receptors differs in three important respects from those at other P2X receptors (Evans et al. 1998). First, the effective concentrations of ATP are 30- to 100-fold higher. Second, removal of extracellular Mg2+ and Ca2+ potentiates the inward current. Third, BzATP is more potent than ATP. The ATP-induced current observed in the present study showed all these characteristics and, therefore, indicates the presence of P2X7 receptors on cultured rat Schwann cells. This view is supported by the lack of effect of α,β-meATP, an agonist on P2X1 and P2X3 receptors (Evans et al. 1998). An ATP-induced, P2X7-like non-specific cation current has also been found in other glial cells such as mouse microglia (Haas et al. 1996; Chessell et al. 1997) and rat astrocytes (Ballerini et al. 1996). Moreover, the previously described ATP-induced current in cultured mouse Schwann cells (Amédée & Despeyroux, 1995) seems to be due to activation of a P2X7-like nucleotide receptor although the effects of BzATP and low divalent cations were not tested in their study. So far, the classification as P2X7 is based on electrophysiological and pharmacological criteria. To our knowledge, the presence of mRNA and/or of the P2X7 protein itself has not been reported for Schwann cells.
It is well known that observations on cultured Schwann cells do not necessarily resemble the situation of these cells in an intact peripheral nerve tissue. For example, the cultures used in the present study were prepared from postnatal (P2) rats and it is, therefore, unclear whether P2X7 receptors are active in an intact adult peripheral nerve. Schwann cells in situ can be analysed with a combination of ion-sensitive fluorescent dyes and confocal microscopy. An important advantage of this method is that Ca2+-sensitive dyes are taken up preferentially into Schwann cells but not into axons of myelinated nerve fibres (Lev-Ram & Ellisman, 1995; Lyons et al. 1995). In the present study, we observed a BzATP-induced intracellular Ca2+ signal in paranodal Schwann cell regions. However, in contrast to the previously described metabotropic effects of ATP analogues (Mayer et al. 1997), BzATP induced a rise in [Ca2+]i which was entirely dependent on the presence of extracellular Ca2+ and was not mediated by Ca2+ release from intracellular stores. This finding is similar to the BzATP-induced influx of extracellular Ca2+ observed in cultures of astrocytes (Ballerini et al. 1996) and microglia (Inoue et al. 1998). It is highly plausible, therefore, that the non-specific cation current seen during the exposure of cultured Schwann cells to BzATP is also the mechanism underlying the rise in [Ca2+]i produced by BzATP in Schwann cells in situ. To our knowledge, this is the first description of an ionotropic ATP receptor in Schwann cells of an intact peripheral nerve. At present, we have concentrated our analysis on the paranodal Schwann cell membrane because this structure is of major importance for the function of a myelinated axon. However, P2X receptors might be present on the other Schwann cell membrane regions as well.
Although the possible function of an ionotropic ATP receptor on the paranodal Schwann cell membrane was not addressed in the present study, the following considerations may be relevant. It is not very likely that action potentials in axons release sufficient ATP to activate the P2X7 receptor. In fact, in a previous study on the isolated rat vagus nerve, we did not find intracellular Ca2+ transients in Schwann cells during trains of axonal action potentials (Wächtler et al. 1998). Nevertheless, it is possible that high concentrations of extracellular ATP in combination with low concentrations of extracellular divalent cations are reached following axonal damage. In this situation, the reactions of the Schwann cells might be due to stimulation of P2X7 receptors. Cellular responses attributed to P2X7 receptors are apoptosis (Chow et al. 1997; Ferrari et al. 1997a), cytokine release (Ferrari et al. 1997b), and the formation of a permeabilizing, cytotoxic membrane pore (Surprenant et al. 1996). Therefore, the data obtained in the present study also suggest a possible new mechanism in the pathophysiology of nerve injury or neuropathy.
Acknowledgments
We would like to thank Franz Rucker for service on the confocal imaging system, Christa Müller for technical assistance, and Dr John Davis for critical reading of the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft (SFB 391/A1).
References
- Amédée T, Despeyroux S. ATP activates cationic and anionic conductances in Schwann cells cultured from dorsal root ganglia of the mouse. Proceedings of the Royal Society B. 1995;259:277–284. doi: 10.1098/rspb.1995.0041. [DOI] [PubMed] [Google Scholar]
- Ansselin AD, Davey DF, Allen DG. Extracellular ATP increases intracellular calcium in cultured adult Schwann cells. Neuroscience. 1997;76:947–955. doi: 10.1016/s0306-4522(96)00370-3. 10.1016/S0306-4522(96)00370-3. [DOI] [PubMed] [Google Scholar]
- Ballerini P, Rathbone MP, Di Iorio P, Renzetti A, Giuliani P, D'Alimonte I, Trubiani O, Caciagli F, Ciccarelli R. Rat astroglial P2Z (P2X7) receptors regulate intracellular calcium and purine release. NeuroReport. 1996;7:2533–2537. doi: 10.1097/00001756-199611040-00026. [DOI] [PubMed] [Google Scholar]
- Berti-Mattera LN, Wilkins PL, Madhun Z, Suchovsky D. P2-purinergic receptors regulate phospholipase C and adenylate cyclase activities in immortalized Schwann cells. Biochemical Journal. 1996;314:555–561. doi: 10.1042/bj3140555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. History of extracellular nucleotides and their receptors. In: Turner JT, Weisman GA, Fedan JS, editors. The P2 Nucleotide Receptors. Totowa, NJ, USA: Humana Press; 1998. pp. 3–40. [Google Scholar]
- Chessell IP, Michel AD, Humphrey PP. Properties of the pore-forming P2X7 purinoceptor in mouse NTW8 microglial cells. British Journal of Pharmacology. 1997;121:1429–1437. doi: 10.1038/sj.bjp.0701278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow SC, Kass GE, Orrenius S. Purines and their roles in apoptosis. Neuropharmacology. 1997;36:1149–1156. doi: 10.1016/s0028-3908(97)00123-8. [DOI] [PubMed] [Google Scholar]
- Collo G, Neidhart S, Kawashima E, Kosco VM, North RA, Buell G. Tissue distribution of the P2X7 receptor. Neuropharmacology. 1997;36:1277–1283. doi: 10.1016/s0028-3908(97)00140-8. [DOI] [PubMed] [Google Scholar]
- Evans RJ, Surprenant A, North RA. P2X receptors. In: Turner JT, Weisman GA, Fedan JS, editors. The P2 Nucleotide Receptors. Totowa, NJ, USA: Humana Press; 1998. pp. 43–61. [Google Scholar]
- Ferrari D, Chiozzi P, Falzoni S, Dal SM, Collo G, Buell G, Di VF. ATP-mediated cytotoxicity in microglial cells. Neuropharmacology. 1997a;36:1295–1301. doi: 10.1016/s0028-3908(97)00137-8. 10.1016/S0028-3908(97)00137-8. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Chiozzi P, Falzoni S, Hanau S, Di VF. Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. Journal of Experimental Medicine. 1997b;185:579–582. doi: 10.1084/jem.185.3.579. 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AC, Dowdall MJ, Richardson CM. ATP acting on P2Y receptors triggers calcium mobilization in Schwann cells at the neuroelectrocyte junction in skate. Neuroscience. 1997;80:635–651. doi: 10.1016/s0306-4522(97)00117-6. 10.1016/S0306-4522(97)00117-6. [DOI] [PubMed] [Google Scholar]
- Haas S, Brockhaus J, Verkhratsky A, Kettenmann H. ATP-induced membrane currents in ameboid microglia acutely isolated from mouse brain slices. Neuroscience. 1996;75:257–261. doi: 10.1016/0306-4522(96)00270-9. 10.1016/0306-4522(96)00270-9. [DOI] [PubMed] [Google Scholar]
- Inoue K, Nakajima K, Morimoto T, Kikuchi Y, Koizumi S, Illes P, Kohsaka S. ATP stimulation of Ca2+-dependent plasminogen release from cultured microglia. British Journal of Pharmacology. 1998;123:1304–1310. doi: 10.1038/sj.bjp.0701732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeftinija SD, Jeftinija KV. ATP stimulates release of excitatory amino acids from cultured Schwann cells. Neuroscience. 1998;82:927–934. doi: 10.1016/s0306-4522(97)00310-2. 10.1016/S0306-4522(97)00310-2. [DOI] [PubMed] [Google Scholar]
- Lev-Ram V, Ellisman MH. Axonal activation-induced calcium transients in myelinating Schwann cells, sources, and mechanisms. Journal of Neuroscience. 1995;15:2628–2637. doi: 10.1523/JNEUROSCI.15-04-02628.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons SA, Morell P, McCarthy KD. Schwann cells exhibit P2Y purinergic receptors that regulate intracellular calcium and are up-regulated by cyclic AMP analogues. Journal of Neurochemistry. 1994;63:552–560. doi: 10.1046/j.1471-4159.1994.63020552.x. [DOI] [PubMed] [Google Scholar]
- Lyons SA, Morell P, McCarthy KD. Schwann cell ATP-mediated calcium increases in vitro and in situ are dependent on contact with neurons. Glia. 1995;13:27–38. doi: 10.1002/glia.440130104. [DOI] [PubMed] [Google Scholar]
- Mayer C, Quasthoff S, Grafe P. Differences in the sensitivity to purinergic stimulation of myelinating and non-myelinating Schwann cells in peripheral human and rat nerve. Glia. 1998;23:374–382. doi: 10.1002/(sici)1098-1136(199808)23:4<374::aid-glia9>3.0.co;2-2. 10.1002/(SICI)1098-1136(199808)23:4<374::AID-GLIA9>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Mayer C, Wächtler J, Kamleiter M, Grafe P. Intracellular calcium transients mediated by P2 receptors in the paranodal Schwann cell region of myelinated rat spinal root axons. Neuroscience Letters. 1997;224:49–52. doi: 10.1016/s0304-3940(97)13457-7. 10.1016/S0304-3940(97)13457-7. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Komune S, Uemura T, Akaike N. Excitatory amino acid response in isolated spiral ganglion cells of guinea pig cochlea. Journal of Neurophysiology. 1991;65:715–723. doi: 10.1152/jn.1991.65.3.715. [DOI] [PubMed] [Google Scholar]
- North RA, Barnard EA. Nucleotide receptors. Current Opinion in Neurobiology. 1997;7:346–357. doi: 10.1016/s0959-4388(97)80062-1. 10.1016/S0959-4388(97)80062-1. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacological Reviews. 1998;50:413–492. [PubMed] [Google Scholar]
- Robitaille R. Purinergic receptors and their activation by endogenous purines at perisynaptic glial cells of the frog neuromuscular junction. Journal of Neuroscience. 1995;15:7121–7131. doi: 10.1523/JNEUROSCI.15-11-07121.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- Wächtler J, Mayer C, Grafe P. Activity-dependent intracellular Ca2+ transients in unmyelinated nerve fibres of the isolated adult rat vagus nerve. Pflügers Archiv. 1998;435:678–686. doi: 10.1007/s004240050569. [DOI] [PubMed] [Google Scholar]
- Wiley JS, Chen JR, Snook MB, Jamieson GP. The P2Z-purinoceptor of human lymphocytes: actions of nucleotide agonists and irreversible inhibition by oxidized ATP. British Journal of Pharmacology. 1994;112:946–950. doi: 10.1111/j.1476-5381.1994.tb13172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


