Abstract
Immunocytochemistry with polyclonal antibodies directed against specific fragments of intracellular loops of α2A- and α2C-adrenergic receptors (α2A-AR, α2C-AR) was used to explore the possibility that expression of these receptors in dorsal root ganglion (DRG) neurones of rat alters as a result of peripheral nerve injury or localized inflammation.
Small numbers of neurones with positive α2A-AR immunoreactivity (α2A-AR-IR) were detected in DRG from normal animals or contralateral to nerve lesions. In contrast, after complete or partial sciatic nerve transection the numbers of ipsilateral L4 and L5 DRG somata expressing α2A-AR-IR sharply increased (>5-fold). There was no discernible change in the number of DRG neurones exhibiting α2A-AR-IR innervating a region in association with localized chemically induced inflammation.
After nerve injury, double labelling with Fluoro-Gold, a marker of retrograde transport from transected fibres, or by immunoreactivity for c-jun protein, an indicator of injury and regeneration, suggested that many of the neurones expressing α2A-AR-IR were uninjured by the sciatic lesions.
In general the largest proportionate increase in numbers of neurones labelled by α2A-AR-IR after nerve lesions appeared in the medium-large diameter range (31–40 μm), a group principally composed of cell bodies of low threshold mechanoreceptors. The number of small diameter DRG neurones labelled by α2A-AR-IR, a category likely to include somata of nociceptors, also increased but proportionately less.
Relatively few DRG neurones exhibited α2C-AR-IR; this population did not appear to change after either nerve lesions or inflammation.
These observations are considered in relation to effects of nerve injury on excitation of primary afferent neurones by sympathetic activity or adrenergic agents, sympathetically related neuropathy and reports of sprouting of sympathetic fibres in DRG.
After nerve injury some primary afferent neurones, particularly nociceptors, develop a novel excitatory response to sympathetic efferent activity or exogenously applied adrenergic agonists (Sato & Perl, 1991; Bossut & Perl, 1995; O'Halloran & Perl, 1997; Abdulla & Smith, 1997). Similarly, afferent fibres terminating in a neuroma formed after transection of a peripheral nerve become responsive to adrenergic agents (Wall & Gutnick, 1974). A partial loss of sympathetic innervation also induces an adrenergically produced excitation of cutaneous nociceptors (Bossut et al. 1996). The sympathetic adrenergic excitation takes place at the peripheral terminals of nociceptors or the cell body of dorsal root ganglion (DRG) neurones and is reversibly blocked by α2-adrenergic antagonists. Excitation of nociceptors by adrenergic agents and sympathetic stimuli has also been reported to be associated with inflammation (Hu & Zhu, 1989; Sato et al. 1993). It is possible that denervation and inflammation serve as stimuli for alterations in expression of adrenergic receptors by DRG neurones. Increased catecholaminergic innervation of DRG after peripheral nerve injury (McLachlan et al. 1993) also is possibly related to altered expression of adrenergic receptors.
Causalgia and related dystrophies are syndromes in which injury to regional innervation leads to a spontaneous burning pain accompanied by allodynia (pain resulting from non-noxious stimuli) and hyperalgesia in a partially denervated area (Nathan, 1947; Janig, 1992). These symptoms are often relieved or modified, at least temporarily, by sympathectomy (see review by Gybels & Sweet, 1989). Several proposals have been put forth to explain the relationship of the sympathetic system to pain after nerve injury or denervation, including the possibility that primary afferent neurones develop novel adrenergic receptors (Nathan, 1947; Devor & Janig, 1981; Sato & Perl, 1991; Perl, 1994).
Two α2-adrenergic receptor subtypes (A and C) have wide distribution in the central nervous system (Nicholas et al. 1993; Scheinin et al. 1994; Rosin et al. 1996; Talley et al. 1996; Gold et al. 1997). Our experiments were directed at determining whether the number and spectrum of primary afferent neurones expressing these adrenergic receptors alters as a consequence of either injury to a mixed somatosensory nerve or an artificially induced inflammation. These observations have been reported in a preliminary fashion (Birder & Perl, 1996).
METHODS
A total of 42 adult Wistar rats (150-250 g) of both sexes were used; eight served as unoperated controls. All procedures on living rats were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of North Carolina, Chapel Hill.
Peripheral nerve lesions
Twenty animals were deeply anaesthetized by a combination of ketamine (10 mg kg−1i.p.) and xylazine (10 mg kg−1i.p.). Anaesthesia was established as being adequate for surgery by periodically testing for the absence of a withdrawal reflex to a strong pinch of a hindpaw and absence of an eye blink reflex to contact with the cornea. Additional anaesthetic was given to maintain the areflexic state until surgery was completed. Usual surgical aseptic precautions were employed to expose the sciatic nerve of one leg through an incision over the sciatic notch and to either partially (n = 7) or totally (n = 13) transect it using fine sharp scissors. The aim of the partial transection was to divide the same one-half of the nerve each time; however, variability in exact exposure and orientation of the sciatic nerve made it probable that the partial lesions varied from animal to animal. In five animals, after total transection of the sciatic nerve, the central cut end was dipped into Fluoro-Gold (3% w/v), a retrogradely transported substance taken up by cut/damaged nerve fibres of all sizes (Schmued & Fallon, 1986; Baranowski et al. 1992), to identify DRG somata with transected peripheral fibres. Eighteen of the 20 animals were studied 7-14 days after the sciatic lesions; the survival period for two animals was 72 days. We made no systematic attempt to test for hyperalgesia, although the lesioned animals’ behaviour was unremarkable during their survival with no visible signs of distress other than relatively minor favouring of the affected limb. The rare animal exhibiting autotomy after complete sciatic transection was killed immediately by an overdose of pentobarbital anaesthetic and was not included in the analyses.
Induced inflammation
Twelve other animals anaesthetized with ketamine and xylazine to areflexia had 0.1 ml of one of the following injected subcutaneously into the plantar part of one hindpaw through a 30 gauge needle: carrageenan (4 mg in 0.9% NaCl), formalin (4% in 0.9% NaCl) or Freund's complete adjuvant. These agents cause inflammation and hyperalgesia, which, in some cases, can persist for up to 2 weeks (Iadarola et al. 1988). After these injections the animals developed localized oedema and guarded the affected limb; otherwise their behaviour was unremarkable. Subcutaneous plantar injections of 0.1 ml of Fluoro-Gold (3% w/v) were made in two additional anaesthetized rats to identify DRG neurones with fibres innervating the foot. Eight of this group of 14 animals, including two injected with Fluoro-Gold, survived for 1-5 days; the remaining six were killed 2 weeks after the plantar injections.
Immunohistochemistry
Terminally, the animals were deeply anaesthetized to areflexia with the ketamine-xylazine mixture and killed by intracardiac perfusion with ice-cold 0.9% NaCl followed by a zinc formalin fixative (Anatech, Inc., Battle Creek, MI, USA), a solution of formaldehyde and zinc sulphate used at a working concentration of 4% formaldehyde. Good perfusion fixation proved essential for consistent immunocytochemical staining and low background. The DRGs contributing to the innervated region were removed, postfixed for 1-2 h in the zinc formalin fixative and then cryoprotected in 25% sucrose. Serial frozen (10 μm) sections were mounted on slides (superfrost). Non-specific binding was blocked by 3% normal goat serum.
The primary polyclonal antibody against the presumed rat α2A-adrenergic receptor (α2A-AR) was developed by immunizing rabbits with a recombinant fusion protein prepared from the cloned rat gene; the protein consisted of glutathione-S-transferase (GST) and a 47 amino acid fragment from the third intracellular loop (Rosin et al. 1993). The targeted fragment of the receptor differed substantially from equivalent regions of other α2-AR subtypes. Receptor selectivity of the affinity purified antisera was established by Western blot analysis of COS cells (a transformed fibroblast-like cell line derived from African green monkey kidney) expressing only the α2A receptor subtype. It was established that this α2A-AR antibody lacked cross-reactivity with other α2-receptor subtypes and recognized native receptor as well as receptor in fixed tissue (Rosin et al. 1993). The polyclonal antibody against the α2C-AR was similarly produced using a recombinant fusion protein consisting of GST and a 70 amino acid fragment of the 3rd intracellular loop and characterized in the same fashion (Rosin et al. 1996).
DRG sections were first incubated with affinity purified α2A-AR or α2C-AR antibodies (2.5 μg ml−1 for 48 h) which had been preabsorbed for 2-3 h with a 5- to 10-fold excess (w/w) of GST (Rosin et al. 1993) and then, after rinsing, with a cyanine fluorescent probe (cy-2 or cy-3 conjugated anti-rabbit IgG).
The c-jun marker for DRG neuronal injury (Jenkins et al. 1993) was compared with α2-AR labelling using a cocktail for the initial incubation containing both the α2A-AR primary antibody and a c-jun antibody (mouse monoclonal). After rinsing, these sections were further incubated in a second cocktail containing cyanine fluorescent probes (cy-2 conjugated anti-rabbit IgG, cy-3 conjugated anti-mouse IgG).
Data analysis
The processed DRG sections were examined by epifluorescence and transmitted light microscopy; images were captured using a high resolution video camera (Optronics DE1-470T) and an MS DOS computer fitted with an image analysis program (Image-Pro Plus, v. 2.0 or 3.0, Media Cybernetics, Silver Spring, MD, USA). The diameter of labelled neurones was determined by the imaging program from a tracing of the perimeter of each counted profile containing a nucleus to give a mean diameter for each cell. The number of labelled neurones in a ganglion was estimated from counts at moderate to high magnification (× 250-630) of positively stained profiles containing nuclei in 10 sequential sections separated by 100 μm. All neurones in each counted section were examined. All cell counts (either α2A-AR-IR or α2C-AR-IR) are presented as the mean number of positive neurones per 10 sections ± standard deviation (s.d.) from each ganglion. For the size distributions shown in Fig. 7, the values reported are the percentage of α2A- or α2C-AR-IR neurones counted. Student's t test was used to estimate the probability that differences between means of the experimental groups could have occurred by chance.
Figure 7. Diameter distribution of neurones displaying c-jun-IR after nerve injury.
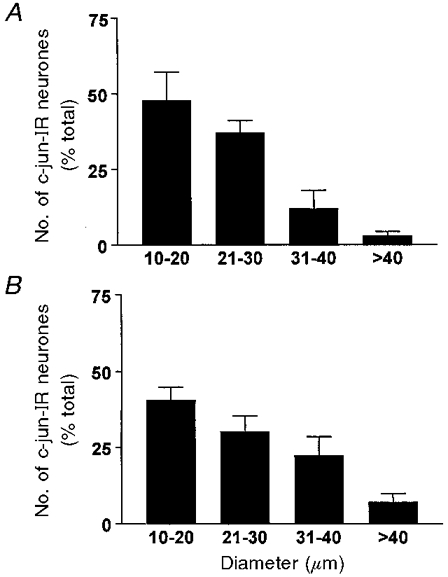
Note that the ordinate is the proportion (percentage) of the total counted in L4 ganglia (±s.d.). A, 788 counted positive neurones from 2 animals following complete ipsilateral sciatic transection. B, 658 counted positive neurones from 2 animals after partial ipsilateral sciatic transection.
Materials
Chemicals were obtained as follows: Fluoro-Gold from Fluorochrome, Inc., Eaglewood, CO, USA; cyanine conjugated anti-rabbit IgG from Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA or Rockland, Inc., Gilbertsville, PA, USA; normal goat serum from Vector Laboratories, Inc., Burlingame, CA, USA. Other chemical compounds were obtained from Sigma. Slides (superfrost) used to mount the tissue sections were obtained from Fisher Scientific, Pittsburgh, PA, USA.
RESULTS
α2A-IR and α2C-IR expression after partial sciatic nerve lesions
Examples of the α2A-IR-like reaction product in DRG neurones are shown in Fig. 1A and C. As described by Rosin et al. (1993), the punctate bodies varied in both size and number (mean 12, range 8-16, for each positive neuronal profile) and often circled the soma. The α2C reaction product differed; it appeared as a more typical diffuse label distributed throughout the cytoplasm (Fig. 1E). Neither the punctate stained bodies nor the diffuse label was present when the primary antibodies were preabsorbed with the respective α2A or α2C fusion protein (5- to 10-fold excess; Fig. 1B, D and F) or if the primary antisera were omitted.
Figure 1. Epifluorescence image of sections from L4 dorsal root ganglia depicting α2-AR-IR.
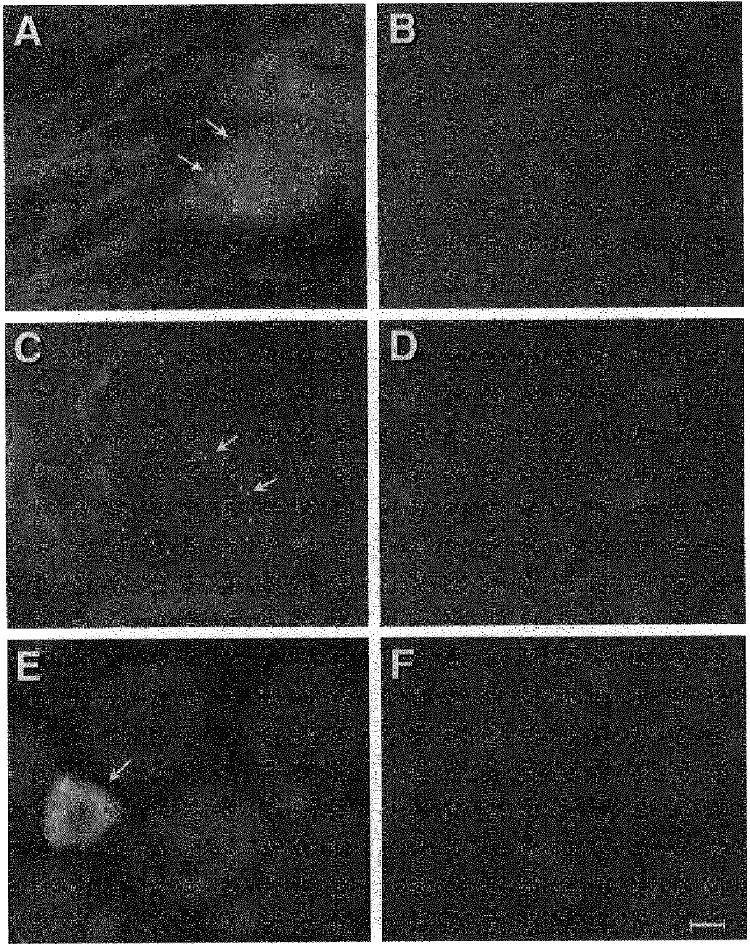
A and C, sections depicting α2A-AR-IR after partial sciatic nerve transection (A) and complete sciatic nerve transection (C). B and D, adjacent sections to A and C showing block of reaction product by addition of α2A-AR fusion protein. Arrows indicate positive reaction product. E, epifluorescence image of a section from L4 dorsal root ganglion depicting α2C-AR-IR (complete sciatic transection 14 days previously). Identical images were found in DRG of normal animals. F, block of reaction in a section adjacent to E by addition of α2C-AR fusion protein. Arrow indicates neurone with positive reaction product. Calibration bar, 10 μm for each panel.
As Table 1, Fig. 2 and Fig. 3 indicate, in unoperated animals few DRG neurones (< 25 neurones per 10 sections) exhibited either α2A or α2C immunoreactivity (IR). Seven to 14 days after either partial or complete transection of the sciatic nerve, the number of neurones in the ipsilateral L4 and L5 DRG exhibiting α2A-AR-IR was sharply increased. Most of the observations were from animals surviving 14 days after the sciatic lesion; however, there was considerable variation between animals at all time points. No significant differences were detected between animals killed at 7 days and those surviving up to 14 days. Therefore, data were pooled for the animals studied 7-14 days after the sciatic transections.
Table 1.
Average number of ipsilateral or contralateral neurones in DRG expressing α2A-AR (L4, L5) or α2C-AR (L4 only) in unoperated animals and animals with partial or complete sciatic transection or inflammation
| Normal | Partial | Complete | Inflammation | |
|---|---|---|---|---|
| α2A-like-IR | ||||
| Ipsilateral L4 | 19.2 ± 5.8 | 135.2 ± 46.5 | 194.3 ± 57.3 | 12.8 ± 3.4 |
| Contralateral L4 | 20.3 ± 5.7 | 21.4 ± 8.7 | 27.7 ± 9.4 | 16.1 ± 7.4 |
| α2A-like-IR | ||||
| Ipsilateral L5 | 16.3 ± 1.0 | 238.3 ± 65.1 | 125.5 ± 52.3 | — |
| Contralateral L5 | 15.2 ± 1.1 | 34.4 ± 14.7 | 21.4 ± 9.2 | — |
| α2C-like-IR | ||||
| Ipsilateral L4 | 17.4 ± 5.4 | 13.6 ± 5.7 | 16.2 ± 7.6 | 17.2 ± 5.1 |
| Contralateral L4 | 17.1 ± 5.2 | 11.8 ± 3.2 | 18.4 ± 5.4 | 19.5 ± 5.4 |
Normal, unoperated animals (n = 8); Partial, 7–14 days after partial sciatic transection (n = 7); Complete, complete sciatic transaction (n = 11); Inflammation, 1–14 days after injection of Freund's adjuvant (n = 4). Data are means ± S.D. The range of the number of positive cells in 10 sections in different animals was as follows. α2A-AR-IR normal: ipsilateral (IL), L4, 12.8–27.1; contralateral (CL), L4, 11.4–24.7; IL, L5, 14.5–17.5; CL, L5, 14.0–16.5. Partial sciatic transection: IL, L5, 82.1–184.2; CL, L5, 12.2–32.3; IL, L5, 118.5–315.0; CL, L5, 20.1–61.2. Complete sciatic transection: IL, L5, 71.5–257.0; CL, L5, 8.8–35.4; IL, L5, 31.2–195.6; CL, L5, 10.2–35.6. Inflammation: IL, L4, 8.7–17.4; CL, L4, 8.1–26.8. For α2C-AR-IR, as follows. Normal: IL, L4, 11.8–23.2; CL, L4, 12.5–24.1. Partial sciatic transection: IL, L4, 4.2–20.5; CL, L4, 3.5–25.3. Complete sciatic transection: IL, L4, 8.5–28.2; CL, L4, 9.2–29.1. Inflammation: IL, L4, 10.3–22.4; CL, L4, 10.7–25.2.
Figure 2. Number of L4 DRG neurones expressing α2A.

AR-IR in 8 unoperated animals (Control) or after injection into the ipsilateral hindpaw of the inflammatory agents Freund's complete adjuvant (n = 4), carrageenan (Carrag., n = 4) and formalin (n = 4). Data are means per 10 sections ±s.d.
Figure 3. Number of L4 DRG neurones expressing α2C-AR-IR in unoperated animals (n = 8) or after partial (n = 7) or complete transection (n = 11) of the sciatic nerve.

Data are shown as means per 10 sections ±s.d. No change of α2C-AR expression was noted after injection of identical inflammatory agents as in Fig. 2.
Figure 4A and B and Table 1 show that after a partial transection of the sciatic nerve (n = 7) significantly more (>6-fold, P < 0.05) ipsilateral L4 and L5 DRG neurones demonstrated α2A-AR-IR than in normal or equivalent contralateral ganglia. Complete transection of the sciatic nerve resulted in similar marked increases in the number of neurones demonstrating α2A-positive IR in L4 and L5 ganglia of the lesioned side relative to normal ganglia (Fig. 5A and B and Table 1). Some ganglia contralateral to the sciatic lesions had apparent small increases in numbers of α2A-AR-IR neurones (Table 1) compared with normal animals but the variation from animal to animal was large enough for these differences to have occurred by chance (P > 0.05). To estimate the proportion of neurones in a ganglion expressing α2A-AR-IR, 500 L4 neurones were counted in 10 sections spaced by 100 μm. After a partial sciatic transection, 14.8% of this count exhibited α2A-AR-IR. After complete sciatic transection in another animal, a somewhat larger proportion, 21.1% of 500 neurones, showed α2A-AR-IR. Considerable variation occurred from animal to animal as is documented by the range of values in the legend to Table 1, although even the smallest number of labelled neurones in the experimental group was substantially greater than was found in normal ganglia. Few α2A-labelled neurones (<20 neurones per 10 sections) were found in adjacent segments (L1-L3, L6) after sciatic lesions. Seventy-two days after complete sciatic nerve transection (n = 2), the number of α2A-AR-IR neurones in ipsilateral L4 and L5 DRG did not significantly differ from findings in unoperated controls (<20 cells per 10 sections, P > 0.05).
Figure 4. Diameter distribution of DRG neurones expressing α2A-AR-IR after partial transection of the sciatic nerve 7-14 days previously.

Data are means per 10 sections ±s.d. (n = 7). Filled bars, ipsilateral to lesion; hatched bars, contralateral to lesion. A, L4 DRG; B, L5 DRG. See Methods for additional details.
Figure 5. Diameter distribution of DRG neurones expressing α2A-AR-IR after complete transection of the sciatic nerve 7-14 days previously.
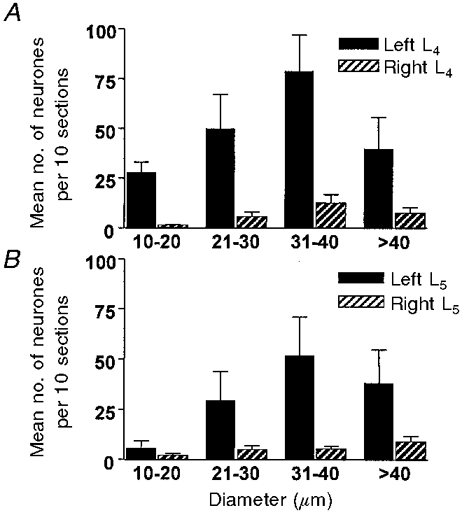
Data are means per 10 sections ±s.d. (n = 11 animals). Filled bars, ipsilateral to the lesion; hatched bars, contralateral. A, L4 DRG; B, L5 DRG.
In contrast to the α2A observations, after partial or complete sciatic transection no increase appeared in the number of ipsilateral neurones labelled by α2C-AR-IR (Fig. 3 and Table 1).
Only limited and scattered α2A-AR-IR appeared in the control spinal dorsal horn. No obvious increase in α2A-AR-IR was noted with the antibody used in the ipsilateral L4/L5 spinal dorsal horn after nerve injury. Spinal cord α2C-AR-IR also did not increase after either complete or partial sciatic transections.
After the sciatic nerve lesions, all diameter ranges of ipsilateral L4 DRG neurones expressing α2A-ARs increased in number, although the largest proportionate increase usually occurred in the medium-large diameter category (31-40 μm). After partial transection, 41.5% of labelled L4 neurones were in the 31-40 μm category, with a mean diameter of 34 μm. Following complete sciatic transection 40.3% of the labelled L4 cells were in the 31-40 μm range (mean diameter of 36 μm). This distribution contrasts with the diameter spectra of neuronal somata of comparable DRG from normal rat in which notably smaller cells predominate (Harper & Lawson, 1985; Tandrup, 1993). On the other hand, in mean values from L5 ganglia after partial lesions, the greatest number of α2A-AR-IR appeared in the 21-30 μm group, although the size distribution of labelled neurones remained skewed toward the larger diameters relative to that of the DRG population as a whole (Fig. 4). Counting only neurones in histological sections with nuclei theoretically creates a bias in favour of large neurones (Harper & Lawson, 1985; Tandrup, 1993); however, this shift should account for only a small part of these distributions.
α2A-AR-IR and evidence of injury
An indication of which DRG neurones in the L4/L5 ganglia had divided peripheral fibres after complete sciatic transections was gained from experiments with Fluoro-Gold (FG). Seven to 14 days after complete sciatic nerve transection, the number of DRG neurones that were labelled by both α2A-AR-IR and FG (72 neurones per 10 sections) was significantly greater (P < 0.05) than the number of α2A-AR-IR-positive cells in control or contralateral ganglia (Fig. 6A); however, more neurones showed only α2A-AR-IR than those that were double-labelled (P < 0.05, Fig. 6A). About 25% of FG-containing neurones were α2A-AR-IR positive. For both FG-positive and FG-negative subsets most of the α2A-AR-IR-positive neurones were of the medium- large diameter category (31-40 μm).
Figure 6. Diameter distribution of DRG neurones expressing α2A-AR-IR in relation to markers for transection or injury of peripheral fibres.

A, mean number (±s.d.) of α2A-AR-IR neurones in 10 sections of the ipsilateral L4 DRG from 5 animals after complete sciatic nerve transection 7-14 days previously. Filled bars, α2A-AR-IR only; hatched bars, α2A-AR-IR and retrogradely transported Fluoro-Gold (FG). B, mean number (±s.d.) of α2A-AR-IR neurones in 10 sections of the ipsilateral L4 DRG from 6 animals after partial transection of the ipsilateral sciatic nerve. Filled bars, α2A-AR-IR only; hatched bars, α2A-AR-IR and c-jun-IR. See Methods and Results for additional details.
Transection of a peripheral nerve results in a massive increase in the number of DRG neurones expressing the c-jun proto-oncogene from the very low level present in the absence of injury (Jenkins & Hunt, 1991). The c-jun expression may be due to alterations in growth or neurotropic factors (Jenkins et al. 1993). The increase in c-jun labelling continues until regeneration of the nerve is complete (Herdegen et al. 1993). We used immunoreactivity to detect the presence of c-jun protein and thereby to identify neurones with peripheral fibres putatively transected or otherwise injured by the nerve lesion. The proportion of neurones in an L4 DRG exhibiting c-jun-IR after the complete sciatic nerve transections (36%) was roughly comparable to those marked by FG in similar experiments (28%; difference P > 0.05). The number of neurones in the ipsilateral L4 DRG expressing the α2A-AR-IR alone or in combination with c-jun-IR increased significantly relative to the contralateral side (P < 0.05); however, as with the FG marker, the profiles with only α2A-AR-IR (presumably uninjured) were more numerous (Fig. 6B). As depicted in Fig. 7, after partial or complete sciatic transection, the c-jun protein appeared in more small diameter than large diameter DRG neurones (44% in the smallest diameter category, 10-20 μm), as might be anticipated from the relative proportion of small neurones in DRG. The distribution of injury marked cells in Fig. 7 contrasts with the size distribution of the largest category staining with both labels (e.g. Fig. 6), those of medium-large diameter (31-40 μm). Nonetheless, some smaller diameter neurones (21-30 μm) had both α2A-AR-IR and c-jun-IR. It is noteworthy that not all FG or c-jun-labelled neurones showed α2A-AR-IR. While there is no certainty that either the retrograde and the c-jun tags label all and only injured neurones, the similarity of results with the two techniques suggests that after sciatic lesions more uninjured neurones than injured ones develop α2A-AR-IR.
Effects of local inflammation
The three agents injected into the plantar footpad to induce local inflammation - carrageenan, formalin and Freund's adjuvant - produced variable degrees of oedema and guarding of the hindlimb. Formalin and Freund's adjuvant caused the most marked local reactions. After induction of the local inflammation, the number of neurones labelled by either the α2A or the α2C antibodies in the L4/L5 ganglia did not significantly differ from controls (P > 0.05) either ipsilaterally or contralaterally to the injected paw (Table 1 and Fig. 2).
DISCUSSION
Following partial and complete transection of the sciatic nerve, the antibody we used against the α2A adrenergic receptor fragmant labels a sharply increased number of DRG neurones. The increase is confined to ipsilateral ganglia supplying large numbers of afferent fibres to the injured nerve (L4-L5). The proportion of α2A-AR-IR neurones in ipsilateral ganglia adjacent to L4 and L5 remains comparable to that of control animals or contralateral ganglia. On the other hand, we noted no increase in the number of neurones expressing α2C-AR-IR after the sciatic injuries, an indication that the α2A-AR-IR changes were specific. Increases in α2A-AR-IR also did not appear in the inflammatory models.
There are considerable data favouring the concept that immunoreactivity to the antibody we employed marks an α2A-type of adrenergic receptor. The antibody has been extensively characterized in other studies and shown to label selectively neurones established to express the α2A-type of adrenergic receptor (Rosin et al. 1993; Talley et al. 1996). This characterization includes in situ hybridization studies demonstrating the presence of α2A-AR-like mRNA (Zeng & Lynch, 1991; Nicholas et al. 1993). However, other immunocytochemical studies analysing the presence of α2-adrenergic receptors in DRG neurones have reported varying results, including some differing quantitatively from ours (e.g. Roberts & Connick, 1997; Gold et al. 1997). We can only speculate about the basis of these differences. In part, it could reflect differences in experimental protocols (e.g. Gold et al. 1997). These could represent different experimental procedures, varying immunocytochemical methods, cross-reactivity with other proteins and/or problems in interpretation due to high levels of background reactivity. From their published illustration, the latter could contribute to the larger number of α2A- and α2C-immunoreactive DRG neurones reported by Gold et al. (1997). We took special care to keep the background in our analyses very low. Our judgements were based on the presence of the punctate reaction product, at least partially distributed around profiles containing a nucleus. These criteria made for ready identification of positive labelling. In our experiments, the appearance of the reaction product and α2A-AR-IR staining of neurones was regularly blocked by preabsorbance with the fusion protein or omission of the primary antibody.
Uninjured as well as injured neurones participate in the increase in α2A-AR-IR, as evidenced by the absence of labels for transection/injury (presence of Fluoro-Gold and c-jun expression). Admittedly, the completeness of marking of injured neurones can be questioned. The FG marker was used only for complete transection and in this situation, it has been argued to label retrogradely most, if not all, neurones whose fibres are transected (Baranowski et al. 1992). Similarly, the c-jun protein or mRNA is reported to appear in many, if not all, injured and regrowing neurones after axotomy (Jenkins et al. 1993; Herdegren et al. 1993). While it is possible that some neurones with injured fibres were unmarked by either the FG or the c-jun technique, it is notable that substantial numbers of neurones with α2A-AR-IR but lacking the other marker appeared in both variations of the double-labelling experiments. Moreover, the distribution of sizes of neurones labelled for the c-jun protein compares well with the established distribution of neuronal size in normal ganglia but differs distinctly from the size distribution of the DRG neurones exhibiting the α2A-IR after the partial or complete transections (cf. Fig 4, Fig. 5, Fig. 6 and Fig. 7). Therefore, the conclusion that substantial numbers of uninjured neurones develop increased α2A-immunoreactivity after nerve injury seems tenable.
The proportion of neurones in a DRG exhibiting the increased α2A-AR-IR presence after sciatic transection is considerable. We estimate it to be about 15% or more. That many neurones changing phenotype after nerve injury have the capability to produce significant changes in afferent signalling and sensory function. It is important to note that not every injured neurone exhibited α2A-AR-IR, implying a form of selectivity in the process.
A particularly puzzling part of our data is that the number of L5 DRG neurones in seven animals showing the α2A-AR-IR after partial sciatic lesions was greater than either the mean for L4 or L5 after complete transection. It is possible that this apparent anomaly is the result of the large proportion of apparently uninjured neurones that develop the immunoreactivity. Whatever combination of factors links the lesion of the nerve to the change in adrenergic receptor expression, the partial lesions in our sample may have selected more of those triggering the change in the L5 distribution. A possibility is the relative proportion of sympathetic efferent fibres divided by the lesion (Bossut et al. 1996). Perhaps interuption of both sets of fibres, primary sensory and sympathetic postganglionic, combines to influence the increased α2A-AR-IR. In any case, the number of peripheral fibres divided does not appear to be directly related to the number of DRG neurones showing increased appearance of α2A-AR-IR.
It is also unclear how the increase in adrenergic receptor expression after nerve lesions relates to reports of inflammation-induced appearance of adrenergic excitation of nociceptive afferent units. The absence of an increase in the number of DRG neurones exhibiting α2A-AR-IR (or α2C-AR-IR) in association with inflammation emphasizes differences in the factors mediating its actions and those of nerve injury.
A number of functional changes take place in primary afferent neurones after a peripheral nerve injury including the appearance of abnormal spontaneous activity (Wall & Gutnick, 1974; Devor et al. 1994) and adrenergic excitation of nociceptors (Sato & Perl, 1991; Bossut & Perl, 1995; O'Halloran & Perl, 1997). It seems reasonable to hypothesize that the increased number of DRG somata expressing an α2-adrenergic receptor marker is related to the induction of adrenergic excitation of these afferent neurones at their peripheral terminals (Sato & Perl, 1991; O'Halloran & Perl, 1997; Abdulla & Smith, 1997). This latter is consistent with the apparent peripheral origin of some symptoms in human cases of sympathetically related neuralgias (Nathan, 1947; Wallin et al. 1976; Torebjork et al. 1995). A moderate increase was detected in the number of small diameter neurones with α2A-AR-IR. This size range of somata is likely to be associated with C fibre receptors and therefore with nociceptors (Harper & Lawson, 1985). However, the greatest increase in α2A-AR-IR appeared in the medium-large diameter group of DRG neurones, a category primarily related to myelinated fibre, low threshold mechanoreceptors (Harper & Lawson, 1985). Some types of low threshold mechanoreceptors, muscle spindles, cutaneous slowly adapting type I and Pacinian corpuscles in the normal animal manifest enhanced activity after sympathetic stimulation (e.g. Hunt, 1960; Akoev, 1981; Roberts et al. 1985; Barasi & Lynn, 1986). Our observations suggest that the number of the latter types of sensory neurones with the potential for excitement by adrenergic agents markedly increases after partial denervation and that this increase takes place in both injured and uninjured neurones. The cellular mechanisms by which activation of an α2-adrenergic receptor leads to excitation of a neurone is not well understood. Abdulla & Smith (1997) have provided evidence indicating that after sciatic transection, nor-adrenaline acting through an α2-adrenergic receptor suppresses N-type Ca2+ channel current in dissociated DRG neurones and possibly thereby reduces Ca2+-mediated K+ conductances. The latter could be expected to increase excitability of neurones. In their work noradrenaline actions were particularly evident in the smaller DRG somata. The α2-AR-mediated effect was not found for DRG neurones dissociated from normal animals.
The development of sympathetic adrenergic excitation of nociceptors conceptually is readily linked to neuropathic pain (Sato & Perl, 1991; O'Halloran & Perl, 1997). On the other hand, a connection between a possible sympathetic excitatory action on low threshold mechanoreceptors and the symptom of pain requires a more involved explanation (e.g. Roberts, 1986). At this stage, the relationship of the increased α2A-AR presence to neuropathic states is a matter of conjecture (Devor, 1983; Sato & Perl, 1991; Perl, 1994).
Removal or loss of the peripheral sympathetic innervation has been reported to be associated with increased adrenergic receptor binding by some DRG somata (Nishiyama et al. 1993) as well as by cells in other tissues (Davies et al. 1982). The present increased expression of α2-AR-IR corresponds in time (1-3 weeks) to expected expression of adrenergic denervation supersensitivity (Cannon & Rosenblueth, 1949). The suggestion has been made that sympathetically associated neuropathies may be related to mechanisms underlying sympathetic denervation supersensitivity (Perl, 1994). In this concept the presence of sympathetic innervation and mediators would suppress expression of the α2-adrenergic receptors by DRG neurones.
The augmented α2A-adrenergic receptor-like expression we observed may be a factor in the increase reported in adrenergic connections from sympathetic postganglionic fibres to dorsal root ganglia after peripheral nerve injury (McLachlan et al. 1993; Chung et al. 1996). The ingrowth of fibres staining for sympathetic markers is described by McLachlan et al. (1993) to surround medium and large DRG neurones at a time (2-3 months) when our observations on two animals showed the α2A-AR-IR no longer to be elevated. Although there may be a causal relationship between these two phenomena, the nature of that linkage and the significance of the increased sympathetic innervation remains unclear.
The large number of apparently uninjured neurones developing the α2A-receptor expression suggests a possible basis could be alteration in the environment of DRG neurones. The increased adrenergic receptor expression conceivably could be triggered by processes related to growth of transected fibres or expansion of the peripheral terminal field (of uninjured fibres) following division of other peripheral nerve fibres, afferent or sympathetic. Peripheral nerve injury has been shown to result in a number of changes in DRG neurones including induction of immediate-early genes (e.g. c-jun) and trophic factors (Jenkins et al. 1993; Zhou et al. 1996). It is possible that whatever factors (e.g. leukemia inhibitory factor, Thompson & Majithia, 1998) and processes stimulate DRG neurones with transected fibres to respond with growth may spread to adjacent uninjured neurones and cause them to undergo similar alterations.
Acknowledgments
This study was supported by grants NS10321 and NS14899 from the NINDS of the NIH. The authors gratefully acknowledge Drs D. Rosin and K. R. Lynch (USPHS grant no. DA07216; K. R. L.) for their generous gift of antibodies directed against the α2-adrenoreceptors. We thank K. McNaughton and C. Connor for their expert technical assistance and S. Derr for her substantial help in preparation of this manuscript.
References
- Abdulla FA, Smith PA. Ectopic α2 adrenoceptors couple to N-type Ca2+ channels in axotomized rat sensory neurons. Journal of Neuroscience. 1997;17:1633–1641. doi: 10.1523/JNEUROSCI.17-05-01633.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akoev GN. Catecholamine, acetylcholine and excitability of mechanoreceptors. Progress in Neurobiology. 1981;15:269–294. doi: 10.1016/0301-0082(80)90007-6. 10.1016/0301-0082(80)90007-6. [DOI] [PubMed] [Google Scholar]
- Baranowski AP, Anand U, McMahon SB. Retrograde labelling of dorsal root ganglion cells in the rat: a quantitative and morphological comparison of Fluoro-Gold with horseradish peroxidase labelling. Neuroscience Letters. 1992;141:53–56. doi: 10.1016/0304-3940(92)90332-2. [DOI] [PubMed] [Google Scholar]
- Barasi S, Lynn B. Effects of sympathetic stimulation on mechanoreceptive and nociceptive afferent units from the rabbit pinna. Brain Research. 1986;378:21–27. doi: 10.1016/0006-8993(86)90282-9. [DOI] [PubMed] [Google Scholar]
- Birder LA, Perl ER. Upregulation of the α2A adrenergic receptor subtype after peripheral nerve injury. Society for Neuroscience Abstracts. 1996;22:1803. [Google Scholar]
- Bossut DF, Perl ER. Effects of nerve injury on sympathetic excitation of Aδ mechanical nociceptors. Journal of Neurophysiology. 1995;73:1721–1723. doi: 10.1152/jn.1995.73.4.1721. [DOI] [PubMed] [Google Scholar]
- Bossut DF, Shea VK, Perl ER. Sympathectomy induces adrenergic excitability of cutaneous C-fiber nociceptors. Journal of Neurophysiology. 1996;75:514–517. doi: 10.1152/jn.1996.75.1.514. [DOI] [PubMed] [Google Scholar]
- Cannon WB, Rosenblueth A. The Supersensitivity of Denervated Structures. New York: Macmillan; 1949. [Google Scholar]
- Chung K, Lee BH, Yoon YW, Chung JM. Sympathetic sprouting in the dorsal root ganglia of the injured peripheral nerve in a rat neuropathic pain model. Journal of Comparative Neurology. 1996;376:241–252. doi: 10.1002/(SICI)1096-9861(19961209)376:2<241::AID-CNE6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Davies B, Sudera D, Sagnella G, Marchiesi-Saviotti E, Mathias C, Bannister R, Sever P. Increased numbers of alpha receptors in sympathetic denervation supersensitivity in man. Journal of Clinical Investigation. 1982;69:779–784. doi: 10.1172/JCI110516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor M. Nerve pathophysiology and mechanisms of pain in causalgia. Journal of the Autonomic Nervous System. 1983;7:371–384. doi: 10.1016/0165-1838(83)90090-5. 10.1016/0165-1838(83)90090-5. [DOI] [PubMed] [Google Scholar]
- Devor M, Janig W. Activation of myelinated afferents ending in a neuroma but stimulation of the sympathetic supply in the rat. Neuroscience Letters. 1981;24:43–47. doi: 10.1016/0304-3940(81)90356-6. 10.1016/0304-3940(81)90356-6. [DOI] [PubMed] [Google Scholar]
- Devor M, Janig W, Michaelis M. Modulation of activity in dorsal root ganglion neurons by sympathetic activation in nerve-injured rats. Journal of Neurophysiology. 1994;71:38–47. doi: 10.1152/jn.1994.71.1.38. [DOI] [PubMed] [Google Scholar]
- Gold MS, Dastmalchi S, Levine JD. α2-Adrenergic receptor subtypes in rat dorsal root and superior cervical ganglion neurons. Pain. 1997;69:179–190. doi: 10.1016/s0304-3959(96)03218-6. 10.1016/S0304-3959(96)03218-6. [DOI] [PubMed] [Google Scholar]
- Gybels JM, Sweet WH. Neurosurgical treatment of persistent pain. Physiological and pathological mechanism of human pain. Pain and Headache. 1989;11:1–402. [PubMed] [Google Scholar]
- Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurons. The Journal of Physiology. 1985;359:31–46. doi: 10.1113/jphysiol.1985.sp015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdegen T, Fiallos-Estrada CE, Schmid W, Bravo R, Zimmermann M. The transcription factors c-jun, Jun d and CREB but not FOS and Krox 24 are differentially regulated in axotomized neurons following transection of the rat sciatic nerve. Molecular Brain Research. 1993;14:155–163. doi: 10.1016/0169-328x(92)90170-g. 10.1016/0169-328X(92)90170-G. [DOI] [PubMed] [Google Scholar]
- Hu S, Zhu J. Sympathetic facilitation of sustained discharges of polymodal nociceptors. Pain. 1989;38:85–90. doi: 10.1016/0304-3959(89)90077-8. 10.1016/0304-3959(89)90077-8. [DOI] [PubMed] [Google Scholar]
- Hunt CC. The effect of sympathetic stimulation on mammalian muscle spindles. The Journal of Physiology. 1960;151:332–341. doi: 10.1113/jphysiol.1960.sp006441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadarola MJ, Ruda MA, Cohen LV, Flores CM, Naranjo JR. Enhanced dynorphin gene expression in spinal cord dorsal horn neurons during peripheral inflammation: behavioral, neuropeptide, immunocytochemical and mRNA studies. In: Dubner R, Gebhart GF, Bond MR, editors. Proceedings of the Vth World Congress on Pain. Amsterdam: Elsevier; 1988. pp. 61–71. [Google Scholar]
- Janig W. Pathological mechanisms operating in reflex sympathetic dystrophy. In: Sicuteri F, editor. Advances in Pain and Therapy. New York: Raven Press; 1992. pp. 111–127. [Google Scholar]
- Jenkins R, Hunt SP. Long-term increase in the levels of c-jun mRNA and Jun protein-like immunoreactivity in motor and sensory neurons following axon damage. Neuroscience Letters. 1991;129:107–110. doi: 10.1016/0304-3940(91)90731-8. 10.1016/0304-3940(91)90731-8. [DOI] [PubMed] [Google Scholar]
- Jenkins R, McMahon SB, Bond AB, Hunt SP. Expression of c-jun as a response to dorsal root and peripheral nerve section in damaged and adjacent intact primary sensory neurons in the rat. European Journal of Neuroscience. 1993;5:751–759. doi: 10.1111/j.1460-9568.1993.tb00539.x. [DOI] [PubMed] [Google Scholar]
- McLachlan EM, Janig W, Devor M, Michaelis M. Peripheral nerve injury triggers noradrenergic sprouting within dorsal root ganglia. Nature. 1993;363:543–546. doi: 10.1038/363543a0. 10.1038/363543a0. [DOI] [PubMed] [Google Scholar]
- Nathan PW. On the pathogenesis of Causalgia in peripheral nerve injuries. Brain. 1947;70:145–170. doi: 10.1093/brain/70.2.145. [DOI] [PubMed] [Google Scholar]
- Nicholas AP, Pieribone VA, Hokfelt T. Distribution of messenger RNAs for alpha-2 adrenergic receptor subtypes in rat brain: An in situ hybridization study. Journal of Comparative Neurology. 1993;328:575–594. doi: 10.1002/cne.903280409. [DOI] [PubMed] [Google Scholar]
- Nishiyama K, Brighton BW, Bossut DF, Perl ER. Peripheral nerve injury enhances α2 adrenergic receptor expression by some DRG neurons. Society for Neuroscience Abstracts. 1993;19:499. [Google Scholar]
- O'Halloran KD, Perl ER. Effects of partial nerve injury on the responses of C-fiber polymodal nociceptors to adrenergic agonists. Brain Research. 1997;759:233–240. doi: 10.1016/s0006-8993(97)00261-8. 10.1016/S0006-8993(97)00261-8. [DOI] [PubMed] [Google Scholar]
- Perl ER. A reevaluation of mechanisms leading to sympathetically related pain. In: Fields HL, Liebeskind JC, editors. Progress in Pain Research and Management. Seattle: IASP Press; 1994. pp. 129–150. [Google Scholar]
- Roberts WJ. A hypothesis on the physiological basis for causalgia and related pains. Pain. 1986;24:297–311. doi: 10.1016/0304-3959(86)90116-8. 10.1016/0304-3959(86)90116-8. [DOI] [PubMed] [Google Scholar]
- Roberts B, Connick J. The distribution of α2A and α2C adrenoceptors in rat dorsal root ganglion. Society for Neuroscience Abstracts. 1997;23:1539. [Google Scholar]
- Roberts WJ, Elardo SM, King KA. Sympathetically induced changes in the responses of slowly adapting type I receptors in cat skin. Somatosensory Research. 1985;2:223–236. doi: 10.3109/07367228509144565. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Talley EM, Lee A, Stornetta RL, Gaylinn BD, Guyenet PG, Lynch KR. Distribution of α2C-adrenergic receptor-like immunoreactivity in the rat central nervous system. Journal of Comparative Neurology. 1996;372:135–165. doi: 10.1002/(SICI)1096-9861(19960812)372:1<135::AID-CNE9>3.0.CO;2-4. 10.1002/(SICI)1096-9861(19960812)372:1>135::AID-CNE9>3.3.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Zeng D, Stornetta RL, Norton FR, Riley T, Okusa MD, Guyenet PG, Lynch KR. Immunohistochemical location of α2A adrenergic receptors in catecholaminergic and other brainstem neurons in the rat. Neuroscience. 1993;56:139–155. doi: 10.1016/0306-4522(93)90569-2. 10.1016/0306-4522(93)90569-2. [DOI] [PubMed] [Google Scholar]
- Sato J, Perl ER. Adrenergic excitation of cutaneous pain receptors induced by peripheral nerve injury. Science. 1991;251:1608–1610. doi: 10.1126/science.2011742. [DOI] [PubMed] [Google Scholar]
- Sato J, Suzuki S, Iseki T, Kumazawa T. Adrenergic excitation of cutaneous nociceptors in chronically inflamed rats. Neuroscience Letters. 1993;164:225–228. doi: 10.1016/0304-3940(93)90897-t. 10.1016/0304-3940(93)90897-T. [DOI] [PubMed] [Google Scholar]
- Scheinin M, Lomasney JW, Hayden-Hixson DM, Schambra UB, Caron MG, Lefkowitz RJ, Fremeau RT. Distribution of α2A-adrenergic receptor subtype gene expression in rat brain. Molecular Brain Research. 1994;21:133–149. doi: 10.1016/0169-328x(94)90386-7. 10.1016/0169-328X(94)90386-7. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Fallon JH. Fluoro-gold: a new fluorescent retrograde axonal tracer with numerous unique properties. Brain Research. 1986;377:147–154. doi: 10.1016/0006-8993(86)91199-6. 10.1016/0006-8993(86)91199-6. [DOI] [PubMed] [Google Scholar]
- Talley EM, Rosin DL, Lee A, Guyenet PG, Lynch KR. Distribution of α2A-adrenergic receptor-like immunoreactivity in the rat central nervous system. Journal of Comparative Neurology. 1996;372:111–134. doi: 10.1002/(SICI)1096-9861(19960812)372:1<111::AID-CNE8>3.0.CO;2-6. 10.1002/(SICI)1096-9861(19960812)372:1<111::AID-CNE8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Tandrup T. A method of unbiased and efficient estimation of number and mean volume of specified neuron subtypes in rat dorsal root ganglion. Journal of Comparative Neurology. 1993;329:269–276. doi: 10.1002/cne.903290208. [DOI] [PubMed] [Google Scholar]
- Thompson SWN, Majithia AA. Leukemia inhibitory factor induces sympathetic sprouting in intact dorsal root ganglia in the adult rat in vivo. The Journal of Physiology. 1998;506:809–816. doi: 10.1111/j.1469-7793.1998.809bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torebjork E, Wahren L, Wallin G, Hallin R, Koltzenburg M. Noradrenaline-evoked pain in neuralgia. Pain. 1995;63:11–20. doi: 10.1016/0304-3959(95)00140-N. 10.1016/0304-3959(95)00140-N. [DOI] [PubMed] [Google Scholar]
- Wall PD, Gutnick M. Ongoing activity in peripheral nerves: The physiology and pharmacology of impulses originating from a neuroma. Experimental Neurology. 1974;43:580–593. doi: 10.1016/0014-4886(74)90197-6. 10.1016/0014-4886(74)90197-6. [DOI] [PubMed] [Google Scholar]
- Wallin G, Torebjork E, Hallin R. Preliminary observations on the pathophysiology of hyperalgesia in the causalgic pain syndrome. In: Zotterman Y, editor. Sensory Functions of the Skin in Primates, Wenner-Gren Center International Symposium Series. Vol. 27. Oxford: Pergamon Press; 1976. pp. 489–502. [Google Scholar]
- Zeng D, Lynch KR. Distribution of α2-adrenergic receptor mRNAs in the rat CNS. Molecular Brain Research. 1991;10:219–225. doi: 10.1016/0169-328x(91)90064-5. 10.1016/0169-328X(91)90064-5. [DOI] [PubMed] [Google Scholar]
- Zhou X-F, Rush RA, McLachlan EM. Differential expression of the p75 nerve growth factor receptor in glia and neurons of the rat dorsal root ganglia after peripheral nerve transection. Journal of Neuroscience. 1996;16:2901–2911. doi: 10.1523/JNEUROSCI.16-09-02901.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]


