Abstract
During development, embryos of the pulmonate gastropod, Helisoma trivolvis, undergo a rotation behaviour due to the co-ordinated beating of three bands of ciliated epithelial cells. This behaviour is in part mediated by the neurotransmitter serotonin (5-HT) released from a pair of identified embryonic neurons. Using time-lapse videomicroscopy to measure ciliary beat frequency (CBF) in response to pharmacological manipulations, we determined whether protein kinase C (PKC) is involved in mediating 5-HT-stimulated ciliary beating.
Diacylglycerol (DAG) analogues sn-1,2-dioctanoyl glycerol (DiC8; 100 μM) and 1-oleoyl-2-acetyl-sn-glycerol (OAG; 100 μM), partially mimicked the 5-HT-induced increase in CBF. In contrast, application of OAG in the absence of extracellular Ca2+ did not result in an increase in CBF.
5-HT-stimulated CBF was effectively blocked by PKC inhibitors bisindolylmaleimide (10 and 100 nM) and calphostin C (10 nM). In addition, bisindolylmaleimide (100 nM) inhibited DiC8-induced increases in CBF. At a higher concentration (200 nM), bisindolylmaleimide did not significantly reduce 5-HT-stimulated cilio-excitation.
Two different phorbol esters, phorbol 12-myristate 13-acetate (TPA; 0.1, 10 or 1000 nM) and phorbol 12β, 13α-dibenzoate (PDBn; 10 μM) did not alter basal CBF. TPA (1 μM) did not alter 5-HT-stimulated CBF. Likewise, the synthetic form of phosphatidylserine, N-(6-phenylhexyl)-5-chloro-1-naphthalenesulphonamide (SC-9; 10 μM), did not increase CBF, whereas a strong increase in CBF was observed upon exposure to 5-HT.
The results suggest that a DAG-dependent, phorbol ester-insensitive isoform of PKC mediates 5-HT-stimulated CBF in ciliated epithelial cells from embryos of Helisoma trivolvis.
Numerous cellular signalling pathways have been implicated in the control of ciliary activity. Calcium and cyclic AMP are the most commonly observed regulators of ciliary beat frequency (CBF) and direction. Cyclic AMP is involved in the cilio-excitatory response of the lateral gill of Mytilus edulis (Murakami, 1983, 1987), the branchial basket of the ascidian Ciona intestinalis (Bergles & Tamm, 1992), human nasal epithelium (Di Benedetto et al. 1990), rabbit tracheal epithelium (Tamaoki et al. 1989; Lansley et al. 1992) and the forward swimming behaviour of Paramecium (Bonini et al. 1986). Similarly, Ca2+ is another common regulator of CBF. Excitatory effects of Ca2+ are observed in the abfrontal gill cilia of Mytilus edulis (Stommel, 1984a, b), the macrocilia of Beroë cucumis (Tamm, 1988), human (Di Benedetto et al. 1991), rabbit (Lansley et al. 1992) and sheep (Salathe & Bookman, 1995) respiratory tract cilia, as well as the ciliated epithelium of embryos of the pulmonate gastropod Helisoma trivolvis (Christopher et al. 1996). In addition to cilio-excitatory roles, increased intracellular Ca2+ concentrations cause ciliary arrest in the veliger of Calliostoma ligatum (Arkett et al. 1987) and ciliary reversal in both Paramecium caudatum (Nakaoka et al. 1984) and Mnemiopsis leidyi (Nakamura & Tamm, 1985). Cilio-inhibitory effects of Ca2+ are also observed in the lateral gill of Mytilus edulis (Paparo & Murphy, 1975), the gill of Aequipecten irridians (Stommel et al. 1982) and the branchial basket of Ciona intestinalis (Bergles & Tamm, 1992).
In addition to cAMP and Ca2+, several other pathways have been shown to modulate CBF, including nitric oxide (Jain et al. 1993; Sisson, 1995), arachidonic acid metabolites (Weisman et al. 1990; Chiyotani et al. 1992), calmodulin (Stommel & Stephens, 1985), cyclic GMP (Geary et al. 1995) and a Ca2+-calmodulin-regulated guanylate cyclase (Schultz et al. 1983). Recently, diacylglycerol (DAG)-protein kinase C (PKC) pathways have also been implicated in the control of ciliary activity. For example, in rabbit tracheal epithelial cells, activation of PKC pathways causes cilio-inhibition (Kobayashi et al. 1988). Further, a decrease in ciliary activity in isolated ovine tracheal ciliated cells is mediated by PKC-dependent phosphorylation of a 37 kDa protein located in the membrane matrix fraction of the cell (Salathe et al. 1993). ATP-dependent cilio-excitation in frog oesophageal tissue also occurs through a PKC pathway (Levin et al. 1997). Taken together, these results suggest that PKC may be a widespread regulator of ciliary activity in many systems, as has been shown for cAMP and Ca2+.
During development, embryos of the pulmonate gastropod, Helisoma trivolvis, undergo a rotation behaviour due to the co-ordinated beating of three bands of ciliated epithelium (Diefenbach et al. 1991). This behaviour is in part modulated by the release of the neurotransmitter serotonin (5-HT) from a pair of early embryonic neurons, known as embryonic neurons C1 (ENC1). Cell culture studies have demonstrated that 5-HT directly influences ciliary activity, and thus behaviour, via an influx of extracellular calcium through voltage-gated calcium channels (Christopher et al. 1996). Furthermore, the effect of 5-HT on ciliary activity is mediated by a 5-HT receptor with a novel pharmacological profile (Goldberg et al. 1994) and does not appear to involve the cAMP second messenger system (Christopher et al. 1996). In the present study, we test the hypothesis that activation of PKC is a signal transduction component in this cilio-excitatory response.
PKC, a member of the serine-threonine family of kinases, is a ubiquitous signalling messenger known to phosphorylate a vast number of cellular substrates (Nishizuka, 1984; Newton, 1997), including Ca2+ channels (DeRiemer et al. 1985). Typically, activation of PKC is mediated by DAG that is formed from the hydrolysis of membrane phosphoinositols by phospholipase C (PLC) (Sanders-Bush et al. 1990). To date, 11 isoforms of PKC have been identified in mammalian systems and several of these, in addition to other isoforms, have been found in lower vertebrates, invertebrates and yeast (reviewed by Geiges et al. 1997).
In both vertebrate and invertebrate systems, evidence that 5-HT induces cellular responses through the activation of PKC is vast (Taussig et al. 1989; Kruger et al. 1991; Hill-Venning & Cottrell, 1992). For instance, 5-HT-induced contraction of guinea-pig tracheal muscle is mediated by a PKC-dependent pathway (Watts et al. 1994). In many molluscan systems, the effects of 5-HT on cation channels occur through a PKC-dependent pathway. 5-HT, through activation of PKC, results in a decrease in the S-like potassium current in the motor neuron B15 of Aplysia californica (Taussig et al. 1989). Likewise, 5-HT activation of the M neurones of the buccal ganglia in Helix aspersa involves a slow Ca2+-dependent depolarizing response that is mediated by a PKC-dependent pathway (Hill-Venning & Cottrell, 1992). 5-HT-induced facilitation of depressed sensory-to-motor synapses in Aplysia is also mediated by PKC activation (Sossin & Schwartz, 1992). These findings, taken together with the above-mentioned studies on PKC involvement in ciliary activity, prompted the hypothesis that 5-HT-induced cilio-excitation in embryos of Helisoma trivolvis is mediated by a PKC-dependent pathway.
In this study, the role of PKC in 5-HT-induced cilio-excitation was examined by using time-lapse videomicroscopy to measure CBF in cultured embryonic ciliated cells from Helisoma trivolvis. Taken together, our pharmacological experiments with 5-HT, DAG analogues, PKC inhibitors and PKC activators suggest that a PKC-dependent pathway underlies 5-HT-induced cilio-excitation. Furthermore, the PKC isoform involved in this response appears to be both DAG dependent and phorbol ester insensitive.
METHODS
Embryos were obtained from an inbred, laboratory-reared colony of Helisoma trivolvis. Snails were housed in filtered, flow-through glass aquaria (45 l), with an oyster shell substratum, maintained at 25°C on a 12 h-12 h light-dark cycle. Their diet consisted of Trout Ration (NU-WAY; United Feeds, Calgary, AB, Canada) and lettuce. Egg masses containing approximately 25 sibling embryos were collected from Petri dishes placed at the bottom of the aquaria and transferred to artificial pond water (0.025% Instant Ocean, Aquarium Systems, Mentor, OH, USA). Embryos were viewed through a dissecting microscope and their development staged (McKenney & Goldberg, 1989; Diefenbach et al. 1998). All embryos used in this study were of embryonic stage E25-30, which represents completion of 25-30% of intracapsular development.
5-HT (creatine sulphate complex; Sigma) was dissolved in Helisoma saline (HS; (mM): 51.3 NaCl, 1.7 KCl, 4.1 CaCl2, 1.5 MgCl2, 5.0 Hepes; pH 7.3-7.35), whereas bisindolylmaleimide (Calbiochem; catalogue no. 203291), calphostin C (Calbiochem), sn-1,2-dioctanoyl glycerol (DiC8; Calbiochem), 1-oleoyl-2-acetyl-sn-glycerol (OAG; Calbiochem), phorbol 12-myristate 13-acetate (TPA; Research Biochemicals International), phorbol 12β, 13α-dibenzoate (PDBn; Sigma) and N-(6-phenylhexyl)-5-chloro-1-naphthalenesulphonamide (SC-9; Calbiochem) were dissolved in dimethyl sulphoxide (DMSO; BDH Chemicals). Experiments with calphostin C were carried out under normal overhead fluorescence illumination, in combination with transmitted microscope light, in view of the light-dependent activation properties of this compound (Bruns et al. 1991). DiC8, OAG and SC-9 solutions were mixed with 2 μl HS immediately prior to addition to the culture dishes. Concentrated drug and vehicle control solutions were added following the protocol of Christopher et al. (1996). Concentrated solutions were added to culture dishes in volumes ranging from 2 to 40 μl in order to produce the desired final concentration upon dilution. The maximum concentration of DMSO used was 0.1%. The dishes were gently agitated for a minimum of 30 s to ensure sufficient mixing.
Isolation and culture of ciliated cells
Embryonic ciliated cells were cultured as described previously (Goldberg et al. 1994; Christopher et al. 1996). Intact egg masses were washed with 35% ethanol for 2 min, followed by three washes in antibiotic-containing Helisoma saline (AHS: HS + 150 μg ml−1 gentamicin; Sigma). Embryos were isolated from their egg capsules, followed by a 30-45 min incubation in 0.2% trypsin in AHS. Embryos were transferred to 0.1% trypsin inhibitor in AHS for 15 min, followed by a 10 min incubation in Helisoma defined medium (HDM: 50% Liebovitz-15 (Gibco), 40.0 mM NaCl, 1.7 mM KCl, 4.1 mM CaCl2, 1.5 mM MgCl2, 5.0 mM Hepes, 50 μg ml−1 gentamicin, 0.015% L-glutamine (Sigma); pH 7.3-7.35). Mechanical dissociation was performed by repeatedly passing the embryos through a 63 μm nylon mesh (Small Parts Inc., Miami, FL, USA). The resultant cell suspension was plated in 0.5 ml droplets onto poly-l-lysine-coated (hydrobromide; molecular weight, 4000–15000; 1 mg ml−1; Sigma) plastic 35 mm tissue culture dishes (Falcon 3001) that were inscribed with circles to contain the droplet. The dishes were covered and kept at room temperature (20-22°C) for 20-24 h to allow for cell adhesion. On the following day, the culture medium was supplemented with 1.5 ml HDM or replaced with 2 ml Helisoma saline containing 5 mM dextrose (HSD) or Ca2+-depleted defined medium (50% Liebovitz-15, 40.0 mM NaCl, 1.7 mM KCl, 5.6 mM MgCl2, 5.0 mM Hepes, 1.0 mM EGTA, 50 μg ml−1 gentamicin, 0.015% L-glutamine; pH 7.3-7.35). Since HSD causes a transient (< 45 min) reduction in ciliary activity (Christopher, 1997), cells were incubated in HSD for a minimum of 60 min prior to use. After 60 min of incubation, a normal response to 5-HT application was always observed (Christopher, 1997). The cultured cells were used for experiments on this same day.
Experimental protocol and data analysis
Ciliated cells were identified in culture and time-lapse videomicroscopy was performed using the protocol of Christopher et al. (1996). In each culture plate, three to four ciliated cells were identified, mapped out on an x-y array, and recorded under phase contrast optics on videotape for later analysis. Drug or vehicle solutions were added (see above) and the three to four cells were again videotaped at specific time points post-addition. Each culture plate was used for a single treatment group. CBF was determined in an off-line analysis. The playback speed was slowed to 1/24 of normal and the number of ciliary beats in a 1 min interval was counted visually. Data were expressed as a percentage of the pre-addition CBF. The cilia examined in this study behaved as a homogeneous population in terms of basal activity, as determined through an analysis of the distribution of basal CBF in cultured ciliated cells (Christopher, 1997). At least three separate experiments were performed for each treatment. For each experiment, a matched vehicle control group was included; in each case, the vehicle solution had no effect on CBF. For experiments examining the combined effects of drugs, the second drug was added in the presence of the first drug; the cells were not washed with medium between drug applications. Statistical significance was determined by analysis of variance (ANOVA) followed by Fisher's protected least significant difference (PLSD) test. In each graph, error bars represent the standard error of the mean (s.e.m.).
RESULTS
Effects of DAG analogues on CBF
Classical activation of PKC is via DAG produced by metabolism of membrane phosphoinositols (Sanders-Bush et al. 1990). In many systems, DAG analogues, such as OAG (Lapetina et al. 1985; Hill-Venning & Cottrell, 1992) and DiC8 (Lapetina et al. 1985; Kobayashi et al. 1988), are potent activators of PKC. Addition of the membrane-permeant DAG analogue OAG (100 μM) caused a transient increase in CBF (Fig. 1). Within 1 min, CBF rose to 114% of pre-addition levels. However, this effect was lost by 10 min post-addition of OAG (Fig. 1). In contrast, 5-HT (100 μM) induced a sustained increase in CBF to levels ranging between 120 and 150% of basal values (Fig. 1; Goldberg et al. 1994; Christopher et al. 1996). DMSO (0.1%), the vehicle for OAG, had no effect on CBF.
Figure 1. Effect of OAG, a membrane-permeant DAG analogue, on CBF.
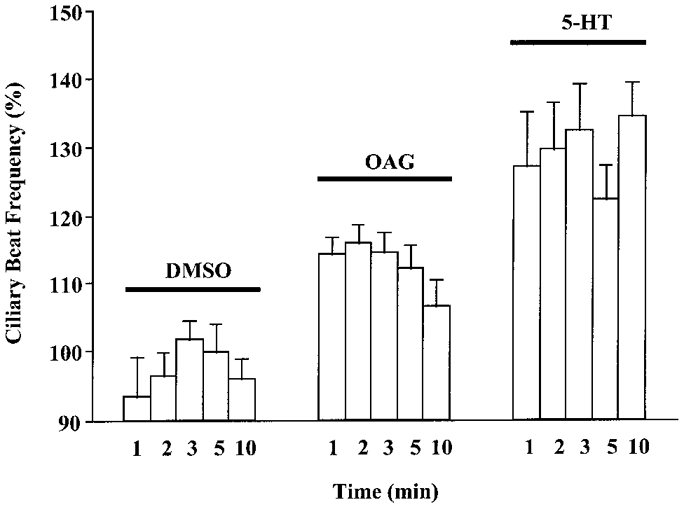
OAG (100 μM) significantly increased CBF within 1 min of addition (P < 0.05). By 10 min post-addition, CBF levels had returned to baseline values. Addition of the vehicle control DMSO (0.1%) did not affect CBF, whereas addition of 5-HT (100 μM) produced an immediate, sustained increase in CBF. Within each treatment, CBF values are statistically similar. Between matched time points, all responses are significantly different (P < 0.05) with the following exceptions: at 5 min, the OAG value is not significantly different from either the DMSO or 5-HT values; at 10 min, the OAG value is not significantly different from the DMSO value. Basal CBF values were: DMSO, 5.4 ± 0.3 beats s−1 (n = 6 cells); OAG, 6.5 ± 0.5 beats s−1 (n = 15 cells); 5-HT, 5.7 ± 0.3 beats s−1 (n = 7 cells).
Previous experiments have suggested that 5-HT-stimulated cilio-excitation is mediated by an influx of extracellular Ca2+, as the 5-HT response was abolished in Ca2+-depleted medium (Christopher et al. 1996). Likewise, after cells were placed in a Ca2+-depleted medium for 10 min, application of OAG (100 μM) did not produce an increase in CBF (Fig. 2).
Figure 2. Effect of OAG on CBF in a Ca2+-depleted medium (- Ca2+).
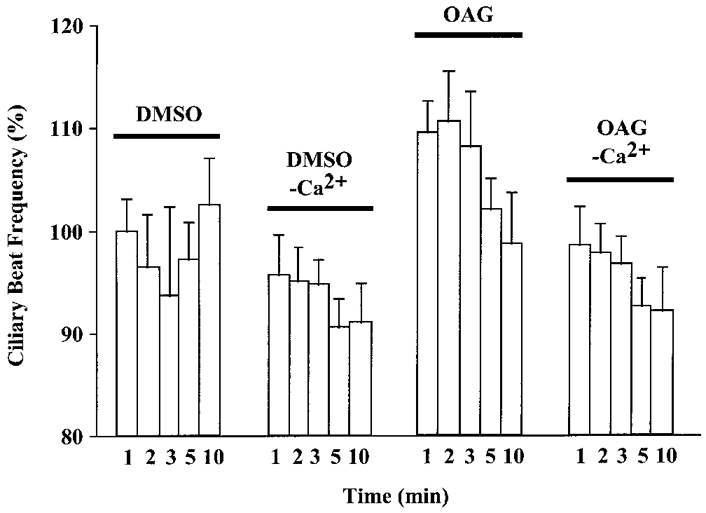
Ciliated cells bathed in a Ca2+-depleted medium beat at a normal frequency compared with control cells (DMSO (0.1%), vehicle control). The addition of 100 μM OAG in a Ca2+-depleted medium caused no change in CBF, whereas OAG induced a significant increase in CBF (P < 0.05) at 2 and 3 min post-addition when cells were bathed in a normal, Ca2+-containing medium. Basal CBF values were: DMSO, 6.4 ± 0.6 beats s−1 (n = 5 cells); DMSO - Ca2+, 6.5 ± 0.3 beats s−1 (n = 6 cells); OAG, 6.8 ± 0.7 beats s−1 (n = 8 cells); OAG - Ca2+, 7.3 ± 0.6 beats s−1 (n = 10 cells).
DiC8 (100 μM), another DAG analogue, also increased CBF in a similar manner to OAG (Fig. 3). Again, the effect of DiC8 was transient; the increase in CBF persisted for 5 min post-addition, but was lost by 10 min post-addition (Fig. 3). These results suggest that a DAG-mediated signalling pathway may underlie 5-HT-stimulated increases in CBF.
Figure 3. Effect of DiC8, a DAG analogue, on CBF.
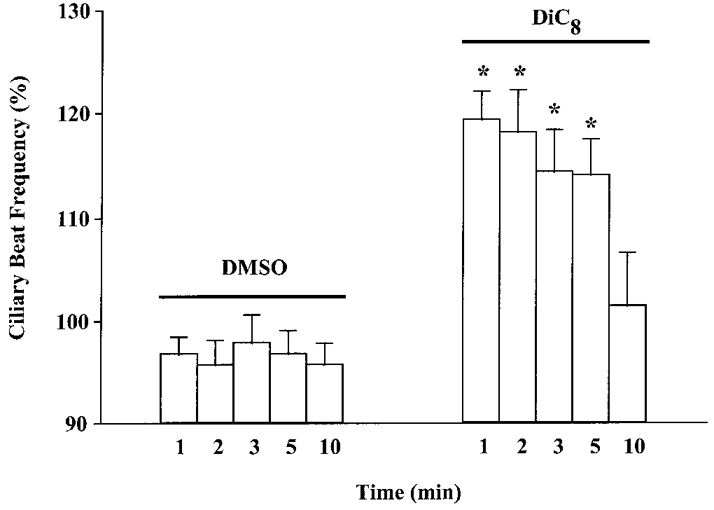
DiC8 (100 μM) induced a transient increase in CBF, whereas DMSO (0.1%) did not affect CBF at all time points examined. Asterisks denote statistically significant differences compared with the matching time point between treatment groups (P < 0.05). Basal CBF values were: DMSO, 5.4 ± 0.2 beats s−1 (n = 20 cells); DiC8, 5.2 ± 0.3 beats s−1 (n = 12 cells).
Effects of protein kinase C inhibitors on 5-HT-stimulated CBF
PKC inhibitors were utilized to further examine PKC involvement in mediating 5-HT-induced increases in CBF. Bisindolylmaleimide inhibits PKC activity in guinea-pig tracheal muscle (Watts et al. 1994) and rat cerebellar granule cells (Pizzi et al. 1996). Bisindolylmaleimide acts through competition with the ATP binding site within the catalytic domain of PKC (Toullec et al. 1991). Pre-incubation of the ciliated cells with bisindolylmaleimide (10, 100 or 200 nM) or the vehicle control DMSO (0.01%) for 10 min did not alter ciliary beating (Fig. 4). Subsequent to control vehicle application, 5-HT (100 μM) induced a characteristic increase in CBF when measured 10 min post-addition. However, bisindolylmaleimide (10 and 100 nM) significantly inhibited the 5-HT-stimulated increase in CBF, whereas a concentration of bisindolylmaleimide of 200 nM had no effect (Fig. 4). These results suggest that a PKC pathway mediates the cilio-excitatory effect of 5-HT.
Figure 4. Effect of bisindolylmaleimide, a specific PKC inhibitor, on 5-HT-stimulated CBF.
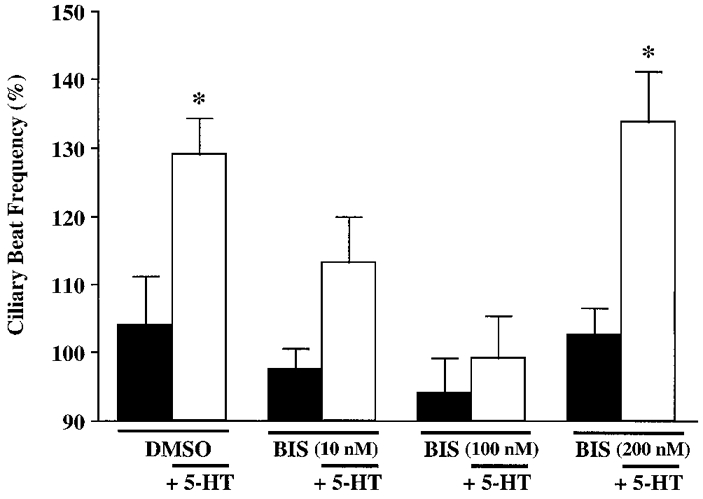
DMSO (0.01%, vehicle control) or bisindolylmaleimide (BIS; 10, 100 and 200 nM), added 10 min prior to determination of CBF, did not affect basal CBF. A characteristic increase in CBF in response to 5-HT (100 μM) was observed in cells pretreated with DMSO. Prior exposure to 10 and 100 nM bisindolylmaleimide significantly reduced 5-HT-induced ciliary activity. In contrast, 200 nM bisindolylmaleimide did not affect 5-HT-induced cilio-excitation. All pre-5-HT treatments are statistically similar (P > 0.05). Asterisks denote a statistically significant difference between pre- and post-addition of 5-HT (P < 0.05). Basal CBF values were: DMSO, 6.3 ± 0.8 beats s−1 (n = 10 cells); bisindolylmaleimide (10 nM), 5.4 ± 0.3 beats s−1 (n = 9 cells); bisindolylmaleimide (100 nM), 5.6 ± 0.4 beats s−1 (n = 10 cells); bisindolylmaleimide (200 nM), 5.8 ± 0.2 beats s−1 (n = 12 cells).
The specificity of bisindolylmaleimide inhibition was examined by pre-incubation of ciliated cells with bisindolylmaleimide for 10 min, followed by addition of DiC8 (100 μM). Bisindolylmaleimide (100 nM) significantly inhibited the DiC8-induced increases in CBF (Fig. 5), suggesting that bisindolylmaleimide acted specifically on a DAG-PKC pathway at the concentrations tested.
Figure 5. Effect of bisindolylmaleimide on the cilio-excitatory action of DiC8.
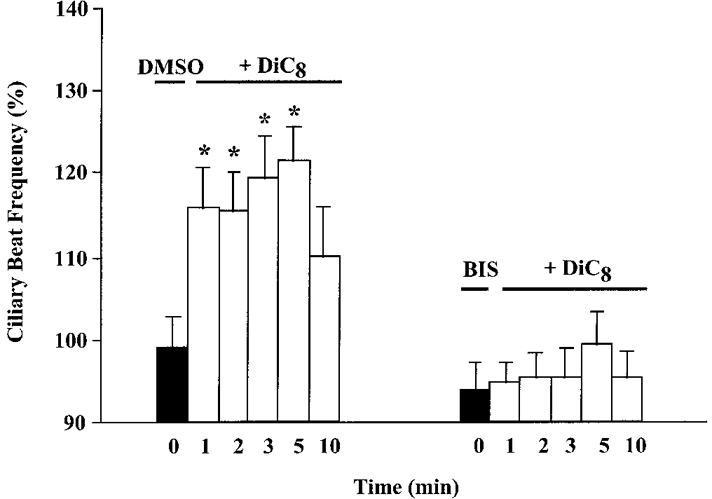
Exposure of ciliated cells to DMSO (0.01%) or bisindolylmaleimide (100 nM) for 10 min did not affect basal CBF. DiC8 added following DMSO exposure significantly increased CBF for up to 5 min. The cilio-excitatory responses of DiC8 were significantly inhibited by pre-exposure to bisindolylmaleimide at all time points examined. Asterisks denote statistically significant differences from the control response (DMSO) at 0 min (P < 0.05). Matched time points between treatment groups following exposure to DiC8 are significantly different (P < 0.05). Basal CBF values were: DMSO, 6.7 ± 0.6 beats s−1 (n = 6 cells); bisindolylmaleimide, 7.0 ± 0.6 beats s−1 (n = 10 cells).
Another specific PKC inhibitor, calphostin C, was used to confirm a role for PKC in mediating the effects of 5-HT. Calphostin C inhibits PKC activity through competition with the DAG/phorbol ester binding site within the regulatory domain (Kobayashi et al. 1989). Ciliated cells pre-incubated for 10 min with calphostin C (10 nM) or DMSO (0.1%) did not demonstrate an alteration in CBF. However, following exposure to calphostin C, addition of 5-HT (100 μM) did not cause the significant increase in CBF that was observed under control conditions (Fig. 6). These results, together with the results from experiments using bisindolylmaleimide, suggest that a PKC pathway is involved in 5-HT-stimulated cilio-excitation.
Figure 6. Effect of calphostin C on 5-HT-induced cilio-excitation.
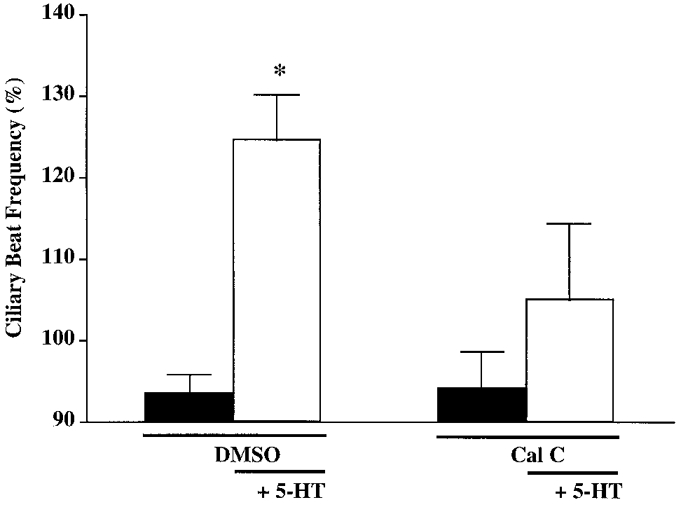
Ciliated cells were exposed to DMSO (0.1%, vehicle control) or calphostin C (Cal C; 10 nM) for 10 min in the presence of direct light, followed by 5-HT challenge (100 μM). Addition of DMSO or calphostin C did not affect basal CBF. Cilio-excitation was observed upon 5-HT addition following DMSO pretreatment, but was prevented by pretreatment with calphostin C. Asterisk denotes significance compared with the vehicle control and with the matched time point between separate treatments (P < 0.05). Basal CBF values were: DMSO, 5.4 ± 0.3 beats s−1 (n = 8 cells); calphostin C, 6.0 ± 0.6 beats s−1 (n = 13 cells).
Effects of phorbol esters on CBF
As a role for PKC was implicated by the above findings, the effects of phorbol esters on CBF were investigated. Phorbol esters are commonly used in numerous systems to activate PKC (DeRiemer et al. 1985; Hill-Venning & Cottrell, 1992; Geiges et al. 1997). Whereas a characteristic increase in CBF was observed upon addition of 5-HT (100 μM), TPA did not stimulate CBF at all three concentrations tested (0.1, 10 and 1000 nM; Table 1). These findings were contrary to the effect observed upon addition of either DAG analogue. As the cilio-excitatory effects of both DiC8 and OAG were transient, the actions of TPA at earlier time points were examined. At 1, 2, 3, 5 and 10 min post-addition, TPA (1 μM) or DMSO (0.1%) did not alter CBF. In contrast, cilio-excitation upon 5-HT (100 μM) addition was observed at all time points examined (Fig. 7A).
Table 1.
Effect of TPA, an activator of PKC, on CBF
| Treatment | Concentration | n | CBF*(% basal) | Basal CBF(beats s−1) |
|---|---|---|---|---|
| DMSO | 0.1% | 16 | 96.3 ± 2.5 | 7.2 ± 0.4 |
| 5-HT | 100 μM | 8 | 119.1 ± 5.6† | 6.6 ± 0.5 |
| TPA | 1 μM | 22 | 95.0 ± 3.0 | 7.2 ± 0.4 |
| 10 nM | 10 | 94.2 ± 4.4 | 7.9 ± 0.4 | |
| 0.1 nM | 8 | 100.3 ± 2.1 | 7.7 ± 0.5 |
Ciliated cells were exposed to each pharmacological agent for 10 min, followed by determination of CBF.
Statistically significant difference compared with control (DMSO), P < 0.05. n, number of cells.
Figure 7. Effects of two phorbol esters, activators of PKC, on CBF.
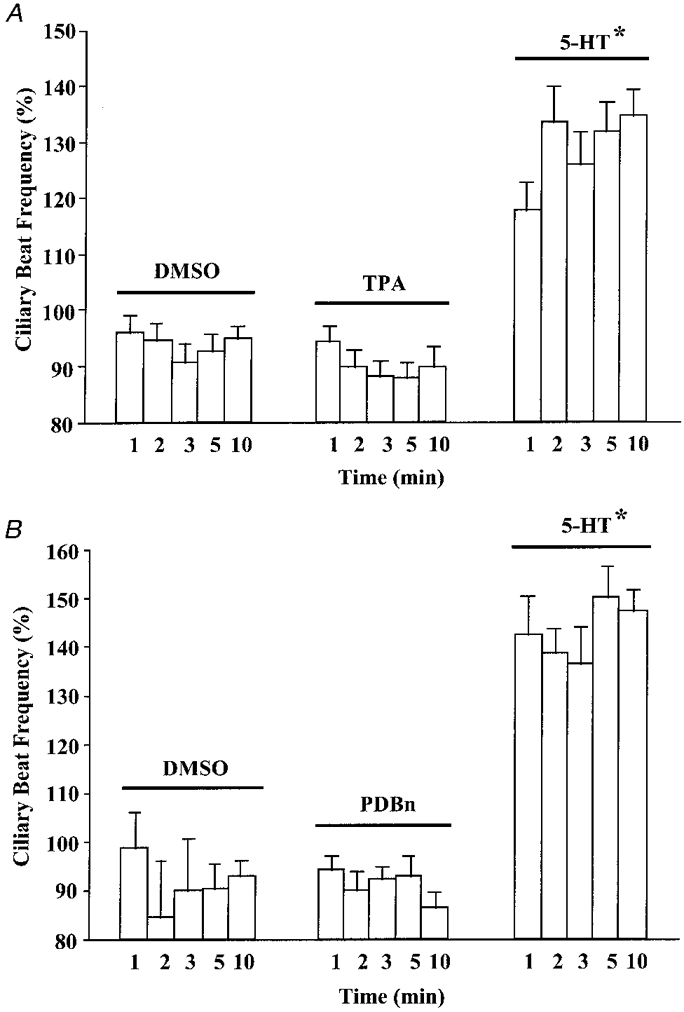
Each pharmacological agent was added, followed by determination of CBF at 1, 2, 3, 5 and 10 min post- addition. A, at all time points, DMSO (vehicle control; 0.1%) and TPA (1 μM) did not increase CBF. In contrast, a characteristic increase in CBF was observed upon 5-HT (100 μM) exposure at all time points examined. Basal CBF values were: DMSO, 5.4 ± 0.4 beats s−1 (n = 10 cells); TPA, 5.6 ± 0.1 beats s−1 (n = 13 cells); 5-HT, 5.5 ± 0.6 beats s−1 (n = 6 cells). B, at all time points, DMSO (vehicle control; 0.1%) and PDBn (10 μM) did not increase CBF. Addition of 5-HT (100 μM) resulted in a characteristic increase in CBF. Basal CBF values were DMSO, 4.9 ± 0.5 beats s−1 (n = 4 cells); PDBn, 4.8 ± 0.2 beats s−1 (n = 8 cells); 5-HT, 5.0 ± 0.5 beats s−1 (n = 5 cells). In A and B, asterisks denote significant difference between all matched time points in the separate treatment groups (P < 0.05).
Different isoforms of PKC can have different sensitivities to phorbol ester activation (Gibson & Logan, 1992; Geiges et al. 1997). Therefore, phorbol dibenzoate (PDBn), another active phorbol ester, was used to verify the lack of cilio-excitation by TPA. Similar to the effects of TPA, PDBn (10 μM) did not cause an increase in CBF (Fig. 7B). Taken together, the effects of TPA and PDBn on CBF suggest that 5-HT-stimulated ciliary activity is not mimicked by application of phorbol esters.
In both ovine and rabbit tracheal ciliary systems, activation of PKC results in a significant decrease in CBF (Kobayashi et al. 1988; Salathe et al. 1993). While TPA and PDBn did not alter CBF significantly from basal levels nor mimic the 5-HT-induced cilio-excitation, phorbol esters may have inhibitory actions on 5-HT-stimulated ciliary activity. Thus, an examination of the effect of TPA on 5-HT-stimulated CBF was performed. Ciliated cells were incubated with TPA (1 μM) or DMSO (0.1%) for 10 min, followed by addition of 5-HT (100 μM). In both cases, a robust increase in CBF was observed (Fig. 8), suggesting that pre-incubation with TPA does not alter the effects of 5-HT. In addition, co-addition of TPA (1 μM) and 5-HT (100 μM) resulted in an increase in CBF (132 ± 4%) that was similar to that observed with 5-HT alone (128 ± 5%). These results strongly suggest that phorbol esters neither mimic nor inhibit 5-HT-stimulated increases in CBF.
Figure 8. Effect of TPA on 5-HT-stimulated CBF.
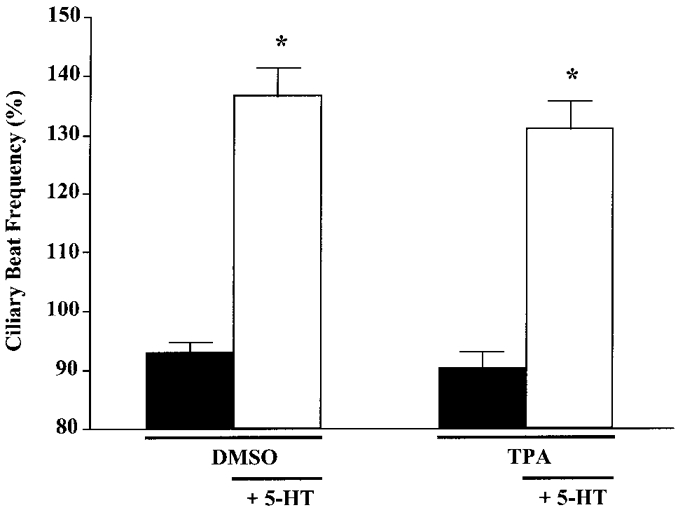
Ciliated cells were exposed to TPA (1 μM) or DMSO (0.1%) for 10 min, followed by addition of 5-HT (100 μM) for 10 min. Addition of TPA or DMSO did not affect basal or 5-HT-stimulated CBF. Asterisks denote significant differences compared with pre-5-HT values (P < 0.05). Basal CBF values were: DMSO, 5.5 ± 0.2 beats s−1 (n = 15 cells); TPA, 5.0 ± 0.3 beats s−1 (n = 13 cells).
A strong dependence on phosphatidylserine is a common feature of several members of the PKC family (Nishizuka, 1992). The synthetic form of phosphatidylserine, SC-9, is an agent capable of activating Ca2+-dependent isoforms of PKC (Nishino et al. 1986; Bargon et al. 1992). CBF was unchanged in the presence of SC-9 (10 μM) or DMSO (0.1%; vehicle control) for up to 10 min following addition (Fig. 9). However, a robust 5-HT-stimulated increase in CBF was observed at all time points examined (100 μM 5-HT; Fig. 9). These results suggest that a Ca2+-insensitive isoform of PKC may mediate 5-HT-induced increases in CBF.
Figure 9. Effect of SC-9, an activator of Ca2+/phosphatidylserine-dependent PKC, on CBF.
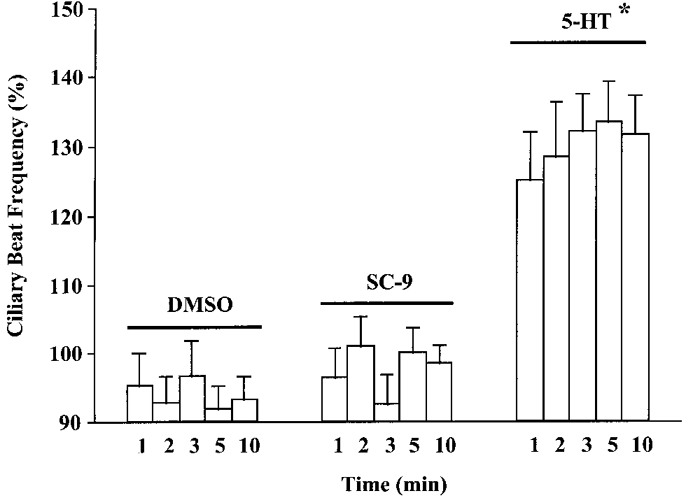
CBF was determined 1, 2, 3, 5 and 10 min post-addition of DMSO (vehicle control; 0.1%), SC-9 (10 μM) or 5-HT (100 μM). SC-9 did not mimic the 5-HT-induced cilio-excitation. The asterisk denotes a significant difference between matched time points between the treatment groups (P < 0.05). There was no difference between time points within individual treatment groups. Basal CBF values were: DMSO, 5.5 ± 0.6 beats s−1 (n = 8 cells); SC-9, 5.2 ± 0.6 beats s−1 (n = 9 cells); 5-HT, 6.1 ± 0.4 beats s−1 (n = 12 cells).
DISCUSSION
We have previously shown that 5-HT-stimulated cilio-excitation in ciliated cells of Helisoma trivolvis embryos is mediated by a novel 5-HT receptor (Goldberg et al. 1994). Although the receptor subtype mediating 5-HT stimulation of whole embryo rotation behaviour is distinct from all other 5-HT receptors known in vertebrates and invertebrates, it is most closely related to the 5-HT2/1C receptor subtype. This receptor subtype classically works through a G-protein-mediated pathway involving formation of inositol 1,4,5-trisphosphate (IP3) and DAG, and activation of PKC to increase intracellular Ca2+ (Sanders-Bush et al. 1990). Interestingly, 5-HT-stimulated ciliary activity requires the influx of extracellular Ca2+ through voltage-gated Ca2+ channels (Christopher et al. 1996). The main objective of the present study was to further explore the signal transduction pathway activated by 5-HT to enhance CBF. In many molluscan systems, the actions of 5-HT are mediated by PKC-dependent pathways (Taussig et al. 1989; Hill-Venning & Cottrell, 1992; Sossin & Schwartz, 1992). For instance Apl I, one of two forms of PKC that have been identified and cloned in nervous tissue of Aplysia californica, underlies 5-HT-stimulated synaptic facilitation (Sossin et al. 1993). PKC has also been shown to enhance Ca2+ channel activity in bag cell neurons of Aplysia californica (DeRiemer et al. 1985). In addition, a cilio-excitatory role for PKC has recently been demonstrated for ATP-stimulated ciliary activity in frog oesophageal tissue (Levin et al. 1997). Likewise, the results presented herein strongly suggest that a PKC-dependent pathway mediates 5-HT cilio-excitation. However, since the PKC isoform involved in this response is activated by DAG analogues, but not by phorbol esters, it may be distinct from all PKC subtypes studied previously (see below).
Comparative analysis of PKC isoenzymes
Eleven isoforms of PKC have been identified in mammalian tissues (Geiges et al. 1997). These isoforms have been grouped into three categories, based on structure and activation profiles: ‘conventional’ Ca2+-dependent PKC subtypes (α, β1, β2 and γ), ‘novel’ or ‘non-conventional’ Ca2+-independent PKC subtypes (δ, -ε, -η, -μ and θ), and ‘atypical’ Ca2+-independent and DAG- or phorbol ester-unresponsive PKC subtypes (ζ and λ) (Geiges et al. 1997). Conventional isoforms of PKC are activated by phorbol esters or DAG in the presence of Ca2+ (Geiges et al. 1997). Novel PKC isoforms are also activated by phorbol esters or DAG, but Ca2+ is not required for activation. In contrast, atypical PKC isoforms do not bind and are not activated by phorbol esters (McGlynn et al. 1992; Geiges et al. 1997) or DAG (Nakanishi & Exton, 1992). In addition, the atypical isoform, PKCζ, displays kinase activity that is independent of phosphatidylserine, a phospholipid that is usually required for activation of several PKC subtypes (McGlynn et al. 1992).
The evidence presented in this study strongly implicates the involvement of PKC in mediating 5-HT-stimulated CBF. However, based on the pharmacological profile, the PKC isoform involved cannot be placed into any of the three main PKC categories. The lack of cilio-excitation by phorbol esters or the synthetic form of phosphatidylserine (SC-9), in addition to the cilio-excitation observed by DAG analogues (DiC8 and OAG), suggests that the PKC isoform involved in cilio-excitation is functionally distinct from previously characterized subtypes.
In nervous tissue of Aplysia californica, two isoforms of PKC, referred to as Apl I and Apl II, have been identified and cloned (Kruger et al. 1991; Sossin et al. 1993). Apl I shares carboxyl-terminal sequence homology with the vertebrate isoform β1 and contains a C-2 region, suggesting similarity with the conventional subtypes of PKC isoforms (Sossin et al. 1993). Functionally, activation of Apl I in Aplysia sensory neurons mediates the 5-HT-induced facilitation of depressed sensory-to-motor synapses (Sossin & Schwartz, 1992). The second isoform of PKC found in Aplysia, Apl II, can be closely aligned with the vertebrate isoform ε, a member of the novel subtype. Apl II, unlike Apl I, is not activated by application of phosphatidylserine in the presence of Ca2+ (Sossin et al. 1993). Whereas the present study suggests that the Helisoma PKC is also insensitive to phosphatidylserine, the inability of phorbol esters to stimulate PKC activity is not characteristic of either Aplysia subtype. Thus, the Helisoma ciliary PKC appears to be a distinct isoform with a pharmacological activation profile not observed in any other system studied to date.
Transient activation of CBF by DAG analogues
The classical method of PKC activation is through DAG, formed by the metabolism of membrane phosphoinositols via the action of PLC (Sanders-Bush et al. 1990). In addition to phosphatidylinositol metabolism, hydrolysis of phosphatidylcholine by phospholipase D (PLD) produces phosphatidic acid, which also leads to DAG formation through phosphatidic acid phosphomonoesterase activity (Nishizuka, 1992). In molluscan plasma membranes, the main phospholipids are phosphatidylcholine and phosphatidylethanolamine (Stephens, 1983), thus the action of PLD to produce DAG and subsequent activation of PKC is likely. In addition, findings from several systems suggest that phosphatidylinositol hydrolysis is not always required for activation of PKC. Proliferation of the FDCP-Mix 1 stem cell line by interleukin 3 is mediated by PKC activation, but phosphatidylinositol hydrolysis was not observed (Whetton et al. 1988). Similarly, 5-HT-stimulated contraction of guinea-pig tracheal muscle is via a PKC pathway. However, phosphoinositide hydrolysis was once again not evident (Watts et al. 1994). Thus, the mechanism underlying DAG formation in 5-HT-stimulated ciliated cells may be a classical PLC pathway or alternatively, a PLD pathway. Attempts to distinguish between the two pathways have yet to be undertaken.
Regardless of which phospholipase pathway is involved, 5-HT-induced cilio-excitation was only partially mimicked by application of the DAG analogues, DiC8 and OAG. Application of either of these agents resulted in a transient cilio-excitation. Since 5-HT causes a sustained increase in CBF, a DAG-dependent pathway may only partially mediate 5-HT-induced cilio-excitation. At the concentrations examined and using the extracellular application procedure employed, DAG analogues may produce short-term activation of the PKC cascade, thus resulting in only a transient excitatory effect. Metabolism of the DAG analogues by DAG lipase or DAG kinase may contribute to this transient effect. On the other hand, only partial activation of CBF by DAG analogues would occur if 5-HT receptor binding activates a PKC pathway and an additional signalling pathway. This second pathway could either modulate or act downstream of DAG-PKC to produce a sustained increase in CBF.
Modulatory effects of PKC pathways have been demonstrated in several systems. In several cell types, including neutrophils, fibroblasts, hepatocytes and mast cells, phosphatidylinositol-dependent DAG activation of PKC modulates subsequent activation of PKC by DAG formed through phosphatidylcholine metabolism (reviewed by Nishizuka, 1992). Likewise, cis-fatty acids, such as arachidonic, oleic, linoleic, linolenic and docosahexaenoic, formed by hydrolysis of phosphatidylcholine via non-specific phospholipase A2, have been demonstrated to be potentiators of DAG-stimulated PKC activation (Shinomura et al. 1991; Nishizuka, 1992). If such potentiating events are activated by 5-HT receptor binding, addition of DAG analogues alone would surely be unable to fully mimic 5-HT-induced cilio-excitation.
PKC inhibitors block the 5-HT response
5-HT-induced cilio-excitation was examined with two specific PKC inhibitors, bisindolylmaleimide and calphostin C. When added at concentrations expected to produce specific activity (Kobayashi et al. 1989; Toullec et al. 1991), both compounds significantly reduced cilio-excitation by 5-HT. However, when bisindolylmaleimide was tested at a higher concentration (200 nM), it did not abrogate the 5-HT response as it did at lower concentrations. The specificity of bisindolylmaleimide at lower concentrations (100 nM) on PKC inhibition was demonstrated by its effective inhibition of DiC8-induced cilio-stimulation. At higher concentrations, bisindolylmaleimide has been shown to exert non-specific effects on a variety of signal transduction pathways (Toullec et al. 1991). It is possible, therefore, that the paradoxical effect of bisindolylmaleimide at 200 nM may be due to its action on additional targets. In any event, as both PKC inhibitors were most effective in blocking the 5-HT response when applied at lower concentrations, where confounding secondary effects are not expected, PKC is strongly implicated in mediating 5-HT-induced cilio-excitation.
Interactions between Ca2+ and PKC
We have previously demonstrated that 5-HT-stimulated CBF is dependent on an influx of extracellular Ca2+ (Christopher et al. 1996). The results presented here suggest that PKC pathways also underlie cilio-excitation, although the specific interactions between Ca2+ influx and PKC activation are unknown. That the OAG-induced increase in CBF was also dependent on the presence of extracellular Ca2+ provides some clues as to the mechanism of PKC action. The most likely possibility is that activation of PKC modulates the activity of voltage-dependent ion channels, as has been demonstrated in other systems (DeRiemer et al. 1985; Doerner & Alger, 1992; Levin et al. 1997), leading to an influx of Ca2+ ions. Ca2+, in concert with calmodulin, may then act within the soma or cilium to alter CBF. To lend further support for this role of PKC in 5-HT-stimulated ciliary activity, measurements of intracellular Ca2+ concentration are required to confirm that OAG actually leads to an influx of Ca2+.
The present results do not rule out other possible interactions between Ca2+ and PKC. For example, Ca2+ may be required for the activation of PKC, as is the case for conventional PKC isoforms (Geiges et al. 1997). PKC activity would then result in phosphorylation of intrasomal or intraciliary proteins to increase CBF. Furthermore, Ca2+ and PKC may act synergistically on either one or more sites in the ciliary apparatus. A similar synergistic model has been proposed for the effects of Ca2+ and cAMP underlying the switching mechanism of the ciliary beat cycle (Satir, 1985). Finally, 5-HT may also activate a number of other signalling pathways, which would act in concert with Ca2+ and PKC to regulate ciliary activity. Second messengers such as arachidonic acid and its metabolites (Weisman et al. 1990; Chiyotani et al. 1992), cyclic GMP (Geary et al. 1995) and nitric oxide (Jain et al. 1993; Sisson, 1995) have been implicated in the regulation of ciliary activity in other systems. In fact, a recent histochemical examination of nitric oxide synthase activity in early embryos of Helisoma trivolvis suggests the presence of this enzyme in the cilio-excitatory ENC1 neurons and in the dorsolateral ciliated cells (Cole et al. 1998). Studies are now underway to test whether nitric oxide is involved in the complex regulation of ciliary beating in Helisoma embryos.
Acknowledgments
This research was supported by the Natural Sciences and Engineering Research Council of Canada operating grants 122303 (J. I. G.) and 121399 (J. P. C.).
References
- Arkett SA, Mackie GO, Singla CL. Neuronal control of ciliary locomotion in a gastropod veliger (Calliostoma) Biological Bulletin. 1987;173:513–526. doi: 10.2307/1541697. [DOI] [PubMed] [Google Scholar]
- Bargon J, Trapnell BC, Yoshimura K, Dalemans W, Pavirani A, Lecocq J, Crystal RG. Expression of the cystic fibrosis transmembrane conductance regulator gene can be regulated by protein kinase C. Journal of Biological Chemistry. 1992;267:16056–16060. [PubMed] [Google Scholar]
- Bergles D, Tamm S. Control of cilia in the branchial basket of Ciona intestinalis. Biological Bulletin. 1992;182:382–390. doi: 10.2307/1542257. [DOI] [PubMed] [Google Scholar]
- Bonini NM, Gustin MC, Nelson DL. Regulation of ciliary motility by membrane potential in Paramecium: a role for cyclic AMP. Cell Motility and the Cytoskeleton. 1986;6:256–272. doi: 10.1002/cm.970060303. [DOI] [PubMed] [Google Scholar]
- Bruns RF, Miller D, Merriman RL, Howbert JJ, Heath WF, Kobayashi E, Takahashi I, Tamaoki T, Nakano H. Inhibition of protein kinase C by calphostin C is light-dependent. Biochemical and Biophysical Research Communications. 1991;176:288–293. doi: 10.1016/0006-291x(91)90922-t. [DOI] [PubMed] [Google Scholar]
- Chiyotani A, Tamaoki J, Sakai N, Isono K, Kondo M, Kondo K. Thromboxane A2 mimetic U46619 stimulates ciliary motility of rabbit tracheal epithelial cells. Prostaglandins. 1992;43:111–120. doi: 10.1016/0090-6980(92)90080-d. [DOI] [PubMed] [Google Scholar]
- Christopher KJ. Edmonton, Alberta, Canada: University of Alberta; 1997. Stimulation of ciliary activity by serotonin in isolated ciliated cells from Helisoma trivolvis embryos: Involvement of cyclic AMP, Ca2+, and protein kinase C. MSc thesis. [Google Scholar]
- Christopher KJ, Chang JP, Goldberg JI. Stimulation of ciliary beat frequency by serotonin is mediated by a Ca2+ influx in ciliated cells of Helisoma trivolvis embryos. Journal of Experimental Biology. 1996;199:1105–1113. doi: 10.1242/jeb.199.5.1105. [DOI] [PubMed] [Google Scholar]
- Cole AG, Young KG, Robbins SJ, Crowe AM, Goldberg JI. Expression and function of nitric oxide in embryos of the pond snail, Helisoma trivolvis. Bulletin of the Canadian Society of Zoologists. 1998;29:50. Abstract. [Google Scholar]
- DeRiemer SA, Strong JA, Albert KA, Greengard P, Kaczmarek LK. Enhancement of calcium current in Aplysia neurones by phorbol ester and protein kinase C. Nature. 1985;313:313–316. doi: 10.1038/313313a0. [DOI] [PubMed] [Google Scholar]
- van Benedetto G, Magnus CJ, Gray PTA, Mehta A. Calcium regulation of ciliary beat frequency in human respiratory epithelium in vitro. The Journal of Physiology. 1991;439:103–113. doi: 10.1113/jphysiol.1991.sp018659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Benedetto G, Manara-Shediac FS, Mehta A. The effect of dibutyryl-cyclic AMP on the ciliary activity of human respiratory epithelium in vitro. The Journal of Physiology. 1990;422:36P. [Google Scholar]
- Diefenbach TJ, Koehncke NK, Goldberg JI. Characterization and development of rotational behaviour in Helisoma embryos: Role of endogenous serotonin. Journal of Neurobiology. 1991;22:922–934. doi: 10.1002/neu.480220905. [DOI] [PubMed] [Google Scholar]
- Diefenbach TJ, Koss R, Goldberg JI. Early development of an identified serotonergic neuron in Helisoma trivolvis embryos: Serotonin expression, de-expression and uptake. Journal of Neurobiology. 1998;34:361–376. doi: 10.1002/(sici)1097-4695(199803)34:4<361::aid-neu6>3.0.co;2-4. 10.1002/(SICI)1097-4695(199803)34:4<361::AID-NEU6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Doerner D, Alger BE. Evidence for hippocampal calcium channel regulation by PKC based on comparison of diacylglycerols and phorbol esters. Brain Research. 1992;597:30–40. doi: 10.1016/0006-8993(92)91502-6. 10.1016/0006-8993(92)91502-6. [DOI] [PubMed] [Google Scholar]
- Geary CA, Davis CW, Paradiso AM, Boucher RC. Role of CNP in human airways: cGMP-mediated stimulation of ciliary beat frequency. American Journal of Physiology. 1995;268:L1021–1028. doi: 10.1152/ajplung.1995.268.6.L1021. [DOI] [PubMed] [Google Scholar]
- Geiges D, Meyer T, Marte B, Vanek M, Weissgerber G, Stabel S, Pfeilschifter J, Fabbro D, Huwiler A. Activation of protein kinase C subtypes α, γ, δ, ε, ζ, and η by tumour-promoting and nontumour-promoting agents. Biochemical Pharmacology. 1997;53:865–875. doi: 10.1016/s0006-2952(96)00885-4. 10.1016/S0006-2952(96)00885-4. [DOI] [PubMed] [Google Scholar]
- Gibson IC, Logan SD. The actions of phorbol esters upon isolated calcium currents of Helix aspersa neurones. Comparative Biochemistry and Physiology. 1992;C 102:297–303. doi: 10.1016/0742-8413(92)90115-n. [DOI] [PubMed] [Google Scholar]
- Goldberg JI, Koehncke NK, Christopher KJ, Neumann C, Diefenbach TJ. Pharmacological characterization of a serotonin receptor involved in an early embryonic behavior of Helisoma trivolvis. Journal of Neurobiology. 1994;25:1545–1557. doi: 10.1002/neu.480251207. [DOI] [PubMed] [Google Scholar]
- Hill-Venning C, Cottrell GA. Modulation of voltage-dependent calcium current in Helix aspersa buccal neurones by serotonin and protein kinase C activators. Experimental Physiology. 1992;77:891–901. doi: 10.1113/expphysiol.1992.sp003656. [DOI] [PubMed] [Google Scholar]
- Jain B, Rubinstein I, Robbins RA, Leise KL, Sisson JH. Modulation of airway epithelial cell ciliary beat frequency by nitric oxide. Biochemical and Biophysical Research Communications. 1993;191:83–88. doi: 10.1006/bbrc.1993.1187. 10.1006/bbrc.1993.1187. [DOI] [PubMed] [Google Scholar]
- Kobayashi E, Nakano H, Morimoto M, Tamaoki T. Calphostin C (UNC-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochemical and Biophysical Research Communications. 1989;159:548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- Kobayashi E, Tamaoki J, Sakai N, Chiyotani A, Takizawa T. Inhibition of ciliary activity by phorbol esters in rabbit tracheal epithelial cells. Lung. 1988;167:277–284. doi: 10.1007/BF02714957. [DOI] [PubMed] [Google Scholar]
- Kruger KE, Sossin WS, Sacktor TC, Bergold PJ, Beushausen S, Schwartz JH. Cloning and characterization of Ca2+-dependent and Ca2+-independent PKCs expressed in Aplysia sensory cells. Journal of Neuroscience. 1991;11:2303–2313. doi: 10.1523/JNEUROSCI.11-08-02303.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansley AB, Sanderson MJ, Dirksen ER. Control of the beat cycle of respiratory tract cilia by Ca2+ and cAMP. American Journal of Physiology. 1992;263:L232–242. doi: 10.1152/ajplung.1992.263.2.L232. [DOI] [PubMed] [Google Scholar]
- Lapetina EG, Reep B, Ganong BR, Bell RM. Exogenous sn-1,2-diacylglycerols containing saturated fatty acids function as bioregulators of protein kinase C in human platelets. Journal of Biological Chemistry. 1985;260:1358–1361. [PubMed] [Google Scholar]
- Levin R, Braiman A, Priel Z. Protein kinase C induced calcium influx and sustained enhancement of ciliary beating by extracellular calcium. Cell Calcium. 1997;21:103–113. doi: 10.1016/s0143-4160(97)90034-8. 10.1016/S0143-4160(97)90034-8. [DOI] [PubMed] [Google Scholar]
- McGlynn E, Liebetanz J, Reutener S, Wood J, Lydon NB, Hofstetter H, Vanek M, Meyer T, Fabbro D. Expression and partial characterization of rat protein kinase C-δ and protein kinase C-ζ in insect cells using recombinant baculovirus. Journal of Cellular Biochemistry. 1992;49:239–250. doi: 10.1002/jcb.240490306. [DOI] [PubMed] [Google Scholar]
- McKenney K, Goldberg JI. Helisoma embryogenesis: Morphological, behavioural and neural development. Society for Neuroscience Abstracts. 1989;15:1016. [Google Scholar]
- Murakami A. Control of cilia beat frequency in Mytilus. Journal of Submicroscopic Cytology. 1983;15:313–316. [Google Scholar]
- Murakami A. Control of ciliary beat frequency in the gill of Mytilus-I. Activation of the lateral cilia by cyclic AMP. Comparative Biochemistry and Physiology. 1987;C 86:273–279. doi: 10.1016/0742-8413(87)90079-x. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Tamm SL. Calcium control of ciliary reversal in ionophore-treated and ATP-reactivated comb plates of ctenophores. Journal of Cell Biology. 1985;100:1447–1454. doi: 10.1083/jcb.100.5.1447. 10.1083/jcb.100.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi H, Exton JH. Purification and characterization of the ζ isoform of protein kinase C from bovine kidney. Journal of Biological Chemistry. 1992;267:16347–16354. [PubMed] [Google Scholar]
- Nakaoka Y, Tanaka H, Oosawa F. Ca2+-dependent regulation of beat frequency of cilia in Paramecium. Journal of Cell Science. 1984;65:223–231. doi: 10.1242/jcs.65.1.223. [DOI] [PubMed] [Google Scholar]
- Newton AC. Regulation of protein kinase C. Current Opinion in Cell Biology. 1997;9:161–167. doi: 10.1016/s0955-0674(97)80058-0. 10.1016/S0955-0674(97)80058-0. [DOI] [PubMed] [Google Scholar]
- Nishino H, Kitagawa K, Iwashima A, Ito M, Tanaka T, Hidaka H. N-(6-Phenylhexyl)-5-chloro-1-naphthalenesulfonamide is one of a new class of activators for Ca2+-activated, phospholipid-dependent protein kinase. Biochimica et Biophysica Acta. 1986;889:236–239. doi: 10.1016/0167-4889(86)90109-6. 10.1016/0167-4889(86)90109-6. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984;308:693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Paparo AA, Murphy JA. The effect of Ca on the rate of beating of lateral cilia in Mytilus edulis - I. A response to perfusion with 5-HT, DA, BOL, and PBZ. Comparative Biochemistry and Physiology. 1975;C 50:9–14. [PubMed] [Google Scholar]
- Pizzi M, Galli P, Consolandi O, Arrighi V, Memo M, Spano PF. Metabotropic and ionotropic transducers of glutamate signal inversely control cytoplasmic Ca2+ concentration and excitotoxicity in cultured cerebellar granule cells: Pivotal role of protein kinase C. Molecular Pharmacology. 1996;49:586–594. [PubMed] [Google Scholar]
- Salathe M, Bookman RJ. Coupling of [Ca2+]i and ciliary beating in cultured tracheal epithelial cells. Journal of Cell Science. 1995;108:431–440. doi: 10.1242/jcs.108.2.431. [DOI] [PubMed] [Google Scholar]
- Salathe M, Pratt MM, Wanner A. Protein kinase C-dependent phosphorylation of a ciliary membrane protein and inhibition of ciliary beating. Journal of Cell Science. 1993;106:1211–1220. doi: 10.1242/jcs.106.4.1211. [DOI] [PubMed] [Google Scholar]
- Sanders-Bush E, Tsutsumi M, Burris KD. Serotonin receptors and phosphatidylinositol turnover. Annals of the New York Academy of Sciences. 1990;600:224–235. doi: 10.1111/j.1749-6632.1990.tb16885.x. [DOI] [PubMed] [Google Scholar]
- Satir P. Switching mechanisms in the control of ciliary motility. In: Satir BH, editor. Modern Cell Biology. New York: Alan R. Liss, Inc.; 1985. pp. 1–46. [Google Scholar]
- Schultz JE, Schönefeld U, Klumpp S. Calcium/calmodulin-regulated guanylate cyclase and calcium-permeability in the ciliary membrane from Tetrahymena. European Journal of Biochemistry. 1983;137:89–94. doi: 10.1111/j.1432-1033.1983.tb07799.x. [DOI] [PubMed] [Google Scholar]
- Shinomura T, Asaoka Y, Oka M, Yoshida K, Nishizuka Y. Synergistic action of diacylglycerol and unsaturated fatty acid for protein kinase C activation: Its possible implications. Proceedings of the National Academy of Sciences of the USA. 1991;88:5149–5153. doi: 10.1073/pnas.88.12.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson JH. Ethanol stimulates apparent nitric oxide-dependent ciliary beat frequency in bovine airway epithelial cells. American Journal of Physiology. 1995;268:L596–600. doi: 10.1152/ajplung.1995.268.4.L596. [DOI] [PubMed] [Google Scholar]
- Sossin WS, Diaz-Arrastia R, Schwartz JH. Characterization of two isoforms of protein kinase C in the nervous system of Aplysia californica. Journal of Biological Chemistry. 1993;268:5763–5768. [PubMed] [Google Scholar]
- Sossin WS, Schwartz JH. Selective activation of Ca2+-activated PKCs in Aplysia neurons by 5-HT. Journal of Neuroscience. 1992;12:1160–1168. doi: 10.1523/JNEUROSCI.12-04-01160.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens RE. Reconstitution of ciliary membranes containing tubulin. Journal of Cell Biology. 1983;96:68–75. doi: 10.1083/jcb.96.1.68. 10.1083/jcb.96.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stommel EW. Calcium regenerative potentials in Mytilus edulis gill abfrontal ciliated epithelial cells. Journal of Comparative Physiology A. 1984a;155:445–456. [Google Scholar]
- Stommel EW. Calcium activation of mussel gill abfrontal cilia. Journal of Comparative Physiology A. 1984b;155:457–469. [Google Scholar]
- Stommel EW, Stephens RE. Cyclic AMP and calcium in the differential control of Mytilus gill cilia. Journal of Comparative Physiology A. 1985;157:451–459. doi: 10.1007/BF00615145. [DOI] [PubMed] [Google Scholar]
- Stommel EW, Stephens RE, Masure HR, Head JF. Specific localization of scallop gill epithelial calmodulin in cilia. Journal of Cell Biology. 1982;92:622–628. doi: 10.1083/jcb.92.3.622. 10.1083/jcb.92.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoki J, Kondo M, Takizawa T. Effect of cAMP on ciliary function in rabbit tracheal epithelial cells. Journal of Applied Physiology. 1989;66:1035–1039. doi: 10.1152/jappl.1989.66.3.1035. [DOI] [PubMed] [Google Scholar]
- Tamm SL. Calcium activation of macrocilia in the ctenophore Beroë. Journal of Comparative Physiology A. 1988;163:23–31. doi: 10.1007/BF00611993. [DOI] [PubMed] [Google Scholar]
- Taussig R, Sweet-Cordero A, Scheller RH. Modulation of ionic currents in Aplysia motor neuron B15 by serotonin, neuropeptides, and second messengers. Journal of Neuroscience. 1989;9:3218–3229. doi: 10.1523/JNEUROSCI.09-09-03218.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, Duhamel L, Charon D, Kirilovsky J. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. Journal of Biological Chemistry. 1991;266:15771–15781. [PubMed] [Google Scholar]
- Watts SW, Cox DA, Johnson BG, Schoepp DD, Cohen ML. Contractile serotonin-2A receptor signal transduction in guinea pig trachea: Importance of protein kinase C and extracellular and intracellular calcium but not phosphoinositide hydrolysis. Journal of Pharmacology and Experimental Therapeutics. 1994;271:832–844. [PubMed] [Google Scholar]
- Weisman Z, Fink A, Alon A, Poliak Z, Tabachnik E, Priscu L, Bentwich Z. Leukotriene C4 decreases the activity of respiratory cilia in vitro. Clinical and Experimental Allergy. 1990;20:389–393. doi: 10.1111/j.1365-2222.1990.tb02799.x. [DOI] [PubMed] [Google Scholar]
- Whetton AD, Monk PN, Consalvey SD, Huang SJ, Dexter TM, Downes CP. Interleukin 3 stimulates proliferation via protein kinase C activation without increasing inositol lipid turnover. Proceedings of the National Academy of Sciences of the USA. 1988;85:3284–3288. doi: 10.1073/pnas.85.10.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]


