Abstract
The patch-clamp technique in the whole-cell configuration was used to measure the effects of intracellular adenine nucleotides on activity of the volume-sensitive anion channel in single, isolated rat pancreatic β-cells.
In the absence of intracellular nucleotides, swelling of cells with a hypertonic pipette solution failed to activate the conductance. Addition of ATP over the range 2–10 mM maintaining the same degree of hypertonicity caused a progressive activation of the conductance. An increase in ATP produced a similar activation of the conductance in non-swollen cells, albeit with reduced current amplitudes.
Activation of the conductance was also observed in the presence of ATPγS, adenylyl imidophosphate (AMP-PNP), ADP, diadenosine tetraphosphate and GTPγS. Neither ADP nor GDPβS inhibited activation of the conductance by ATP.
It is concluded that activity of the β-cell volume-sensitive anion channel can be modulated by changes in intracellular concentrations of ATP within the physiological concentration range by a mechanism that does not require nucleotide hydrolysis. Activity of the channel does not appear to be modulated by a G protein-coupled mechanism.
The stimulation of insulin secretion by glucose requires metabolism of the sugar in the pancreatic β-cell and involves the generation of electrical activity that results from depolarization of the β-cell membrane. This depolarization is thought to result, at least in part, from the closure of KATP channels, which is itself mediated by an increase in ATP/ADP ratio resulting from glucose metabolism (see Ashcroft & Rorsman, 1989, for review).
An outwardly rectifying volume-sensitive anion-selective channel has recently been described in insulin-secreting cells (Best et al. 1995, 1996b; Kinard & Satin, 1995). Activation of this channel by cell swelling generates an inward current leading to depolarization and stimulated insulin release (Best et al. 1996a). Thus activation of this channel could also play a role in the modulation of β-cell electrical and secretory activity by glucose (Best, 1997). It is, however, unclear how activation of the anion channel is brought about. One possibility is that small changes in cell volume under normal physiological conditions could regulate the channel. For example, we have recently shown that glucose causes an increase in β-cell volume sufficient to activate the channel (Miley et al. 1997).
Although the volume-sensitive anion channel in the pancreatic β-cell appears to be distinct from that in other cell types, at least on the basis of the unusual halide selectivity sequence Br > Cl > I, activity of the β-cell channel is, in common with other volume-sensitive anion channels, ATP dependent (Kinard & Satin, 1995; Best et al. 1996b). Thus in view of the changes in ATP and ADP levels thought to occur in glucose-stimulated β-cells (Detimary et al. 1996), an additional possibility is that activity of the channel could be modulated by changes in intracellular nucleotide concentrations. The present study demonstrates that the volume-sensitive anion channel in rat pancreatic β-cells is progressively activated by ATP over a range of concentrations encompassing those that would be expected to occur under physiological conditions. Activation of the channel can also be evoked by a range of other adenine and guanine nucleotides.
METHODS
Islet and β-cell preparation
Sprague-Dawley rats (300-400 g) of either sex were killed by stunning and cervical dislocation. Pancreatic islets were isolated by collagenase digestion (Lacy & Kostianovsky, 1965) and dispersed into single cells and small clusters by a 5-10 min incubation in a Ca2+-free medium containing (mM): 135 NaCl, 5 KCl, 1 MgSO4, 10 Hepes-NaOH and 1 EGTA; pH 7.4. The cells were then resuspended in Hepes-buffered RPMI medium (Gibco), plated into 30 mm diameter polystyrene dishes and cultured for 3-10 days in humidified air at 37°C. Single β-cells, identified by their size and characteristic granular appearance, were used in all cases in order to avoid contaminating currents from adjacent electrically coupled cells.
Patch-clamp experiments
Cells were superfused at a rate of 4 ml min−1 with a medium consisting of (mM): 135 NaCl, 5 KCl, 1 NaH2PO4, 1 MgSO4, 1.2 CaCl2, 4 glucose and 10 Hepes-NaOH (pH 7.4, 285 mosmol l−1). Whole-cell current recordings from single β-cells were measured using a List EP-7 amplifier (List Electronic, Darmstadt, Germany) essentially as described previously (Best et al. 1996b). Cells were held at -30 mV and subjected to 500 ms pulses from -100 to +100 mV in 20 mV increments using pCLAMP software (Axon Instruments). Recordings were filtered at 3 kHz, sampled at 1 kHz and stored on computer disk. Current amplitudes were measured 20 ms after the start of the voltage pulse. Whole-cell capacitance, calculated by ‘nulling’ the capacitance transients, was 10-15 pF. Two basic intracellular (pipette) solutions were used. A hypertonic solution (305 mosmol l−1) designed to induce cell swelling consisted of (mM): 30 CsCl, 1 MgSO4, 0.5 EGTA, 220 mannitol and 10 Hepes-NaOH (pH 7.2). A hypotonic solution (265 mosmol l−1), similar in composition to the above but containing 180 mM mannitol, was used in experiments where cell swelling was avoided. In both cases, the addition of nucleotides was made by substitution of mannitol in order to maintain osmolarity of the solution. The latter was measured by freezing-point depression. Data are presented as means ±s.e.m. and statistical significance was ascribed by a two-tailed Student's unpaired t test.
Materials
ATP and ADP were supplied by Calbiochem, ATPγS, GTPγS and GDPβS by Boehringer and diadenosine tetraphosphate and adenylyl imidophosphate (AMP-PNP) by the Sigma Chemical Company.
RESULTS
Figure 1 shows the effects of increasing concentrations of Mg2+-ATP on activity of the outwardly rectifying anion conductance in rat β-cells swollen by a hypertonic pipette solution. A progressive increase in the amplitude of both inward and outward currents was observed with increasing concentrations of ATP in the range 2-10 mM whilst maintaining the osmolarity of the pipette solution at 305 mosmol l−1. No significant activation of the conductance in rat β-cells was seen with a low concentration (0.2 mM) of ATP (not shown). Figure 1Ab also shows a typical current-voltage plot for a cell dialysed with 2 mM ATP. The reversal potential of the conductance in this cell was -31 mV. The deviation of this value from the calculated ECl of -40 mV probably reflects the significant cation permeability of the channel (Best et al. 1996b). In order to examine the influence of added Mg2+ on activity of the conductance, control experiments were performed with MgSO4 added to the pipette solution. With a pipette solution containing 10 mM MgSO4 and no ATP, the current densities in response to voltage pulses of +100 and -100 mV were 10.9 ± 0.7 and -11.5 ± 2.0 pA pF−1 (n = 6), respectively. In the presence of 2 mM ATP, addition of 10 mM MgSO4 to the pipette solution markedly inhibited the outward currents, the corresponding current densities being 12.6 ± 1.0 and -16.3 ± 3.4 pA pF−1 (n = 6).
Figure 1. Effect of ATP concentration on the volume-sensitive anion conductance in swollen rat β-cells.
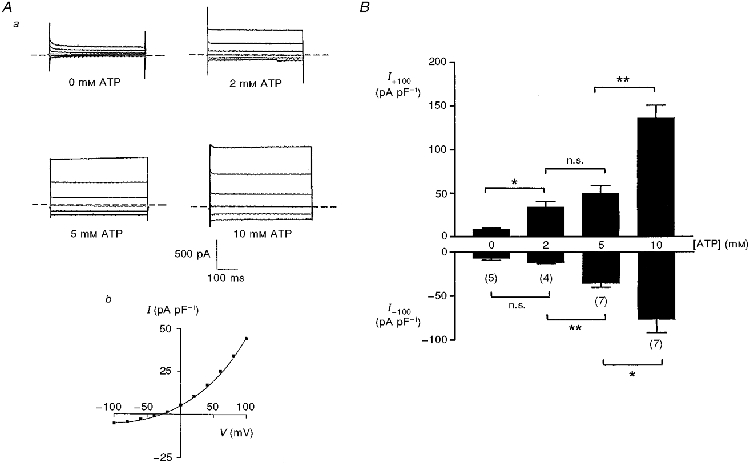
Aa, typical current profiles with different concentrations of intracellular Mg2+-ATP. Cells were held at -30 mV and subjected to 500 ms voltage pulses from -100 to +100 mV. The pipette solutions were maintained at constant hypertonicity (305 mosmol l−1) in order to induce cell swelling. Ab, current-voltage (I-V) plot for a typical cell dialysed with a pipette solution containing 2 mM ATP. The reversal potential for this cell was -31 mV. B, outward (I+100) and inward (I-100) current densities in response to voltage pulses of +100 and -100 mV, respectively: effects of increasing concentrations of Mg2+-ATP. All values represent maximum currents attained in each cell. The values in parentheses represent the number of cells for each treatment. *P < 0.05, **P < 0.01 between the groups as shown; n.s., P > 0.05.
In cells dialysed with a slightly hypotonic pipette solution to prevent cell swelling, ATP caused a similar concentration-dependent activation of channel activity (Fig. 2). Note, however, that under non-swollen conditions, the amplitudes of the volume-sensitive currents were, in general, less than the corresponding values in swollen cells. This effect of cell swelling was statistically significant in the presence of 2 mM (P < 0.02) and 10 mM (P < 0.05) ATP. The amplitude of the outward current measured at +100 mV showed linear correlation with concentration of ATP, the correlation coefficients being 0.96 and 0.95 in swollen and non-swollen cells, respectively.
Figure 2. Effect of ATP concentration on the volume-sensitive anion conductance in non-swollen rat β-cells.
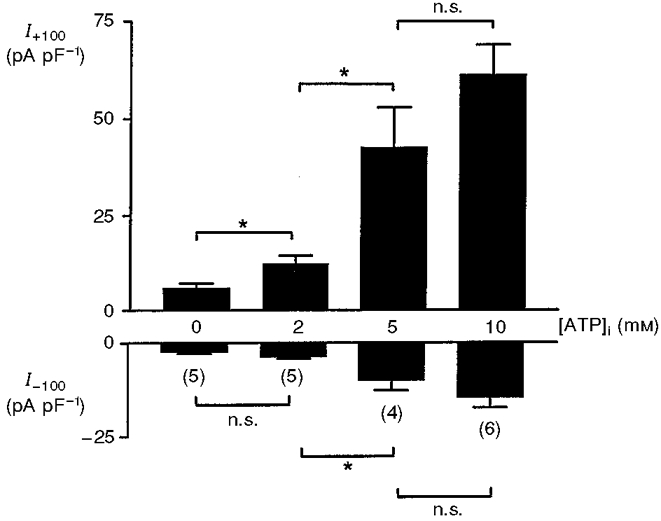
Outward (I+100) and inward (I-100) current densities in response to voltage pulses of +100 and -100 mV, respectively: effects of increasing concentrations of Mg2+-ATP. The pipette solutions were maintained slightly hypotonic (265 mosmol l−1) in order to prevent cell swelling. All values represent maximum currents attained in each cell. The values in parentheses represent the number of cells for each treatment. *P < 0.05, between the groups as shown; n.s., P > 0.05.
Since ATP and ADP have been shown to have opposite effects on activity of the KATP channel (Kakei et al. 1986), we investigated the effects of ADP on activity of the volume-sensitive anion channel. As shown in Fig. 3, 2 mM ADP supported activation of the channel to the same extent as an equivalent concentration of ATP. ADP also failed to antagonize the stimulatory effect of ATP on channel activity (Fig. 3). In order to assess whether the activation produced by ATP involved hydrolysis of the nucleotide, the non-hydrolysable analogues ATPγS and AMP-PNP were also used. As shown in Fig. 3, both of these analogues at a concentration of 2 mM were effective in activating the conductance. Figure 3 also shows that channel activation could be induced by diadenosine tetraphosphate, levels of which have been reported to be sensitive to glucose in the pancreatic β-cell (Ripoll et al. 1996). The stimulatory effects of ATPγS, AMP-PNP and diadenosine tetraphosphate on both outward and inward currents were significantly greater than an equivalent concentration of ATP (P < 0.05 or less).
Figure 3. Activation of volume-sensitive anion conductance by adenosine nucleotides.
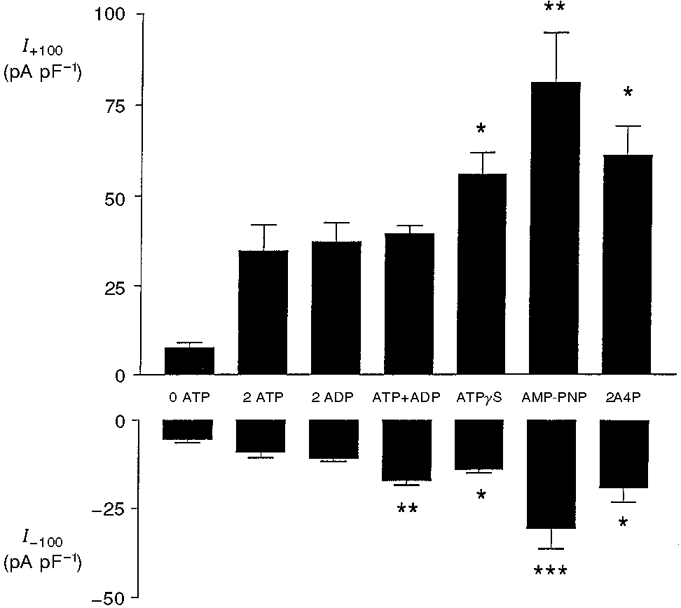
Outward (I+100) and inward (I-100) current densities in response to voltage pulses of +100 and -100 mV, respectively: effects of various adenine nucleotides (all 2 mM; 2A4P, diadenosine tetraphosphate). The pipette solutions were maintained at constant hypertonicity (305 mosmol l−1) in order to induce cell swelling. All values represent maximum currents attained in each cell. Each bar represents the mean ±s.e.m. of 5-7 separate determinations. The asterisks denote significant differences from 2 ATP (column 2): *P < 0.05, **P < 0.02, ***P < 0.01.
It has been reported that a volume-sensitive anion channel in bovine chromaffin cells can be activated and inactivated, respectively, by the non-hydrolysable guanine nucleotides GTPγS and GDPβS (Doroshenko et al. 1991; Voets et al. 1998). Furthermore, changes in guanine nucleotide concentrations have been demonstrated in glucose-stimulated β-cells (Detimary et al. 1996). The final series of experiments therefore examined the effects of guanine nucleotides on activity of the volume-sensitive anion channel. In the presence of 2 mM ATP, the non-hydrolysable GTP analogue GTPγS (0.1 mM) failed to cause further activation of the conductance, whether in swollen cells (Fig. 4) or non-swollen cells (not shown). The inclusion of GDPβS (2 mM) in the pipette solution failed to inhibit the conductance, but caused a significant (P < 0.05) increase in amplitude of the outward current (Fig. 4). In swollen cells, the substitution of 2 mM ATP by 2 mM GTPγS resulted in activation of the conductance, the amplitude of the outward current being significantly (P < 0.05) greater than with the equivalent concentration of ATP (Fig. 4).
Figure 4. Effect of GTPγS and GDPβS on the volume-sensitive anion conductance in rat β-cells.
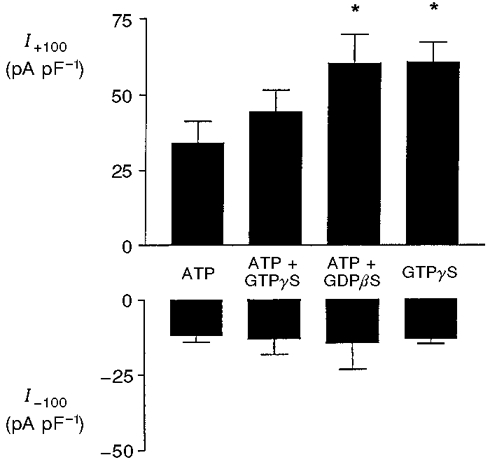
Outward (I+100) and inward (I-100) current densities in response to voltage pulses of +100 and -100 mV, respectively: effects of 2 mM ATP, 2 mM ATP + 0.1 mM GTPγS, 2 mM ATP + 2 mM GDPβS and 2 mM GTPγS alone. The pipette solutions were maintained at constant hypertonicity (305 mosmol l−1) in order to induce cell swelling. All values represent maximum currents attained in each cell. Each bar represents the mean ±s.e.m. of 4-5 separate determinations. *P < 0.05 compared with 2 mM ATP alone (column 1).
DISCUSSION
An increase in intracellular ATP concentration is thought to be an important physiological modulator of β-cell electrical and secretory activity during stimulation by nutrients, by regulating activity of the KATP channel (Ashcroft & Rorsman, 1989). The results of the present study suggest that changes in intracellular ATP concentration could also affect activity of the volume-sensitive anion channel. It has previously been demonstrated that swelling-induced activation of this channel requires ATP (Kinard & Satin, 1995; Best et al. 1996b). However, the present study demonstrates that a progressive increase in channel activity was apparent over a range of ATP concentrations (2-10 mM) that encompasses the expected levels of the nucleotide in an intact β-cell. Due to the fact that channel activation was still not apparently maximal at a high concentration (10 mM) of ATP, we have not formally estimated the half-maximally effective concentration of the nucleotide. However, this value would appear to be at least an order of magnitude higher than that of 0.3 mM, which has been reported to cause half-maximal inhibition of the KATP channel in whole-cell recordings from β-cells (Panten et al. 1989). The finding that addition of MgSO4 to the intracellular solution failed to activate the conductance indicates that the stimulatory action of ATP-Mg2+ was not the result of increased [Mg2+]. Indeed, in the presence of ATP, addition of a high concentration of Mg2+ was found to reduce the amplitude of the outward currents markedly. This observation is consistent with previous reports of inhibition of volume-sensitive anion channels by Mg2+ (Anderson et al. 1995).
The finding that ADP antagonizes the inhibition by ATP of KATP channel activity has led to the suggestion that the ATP/ADP ratio may be of greater importance than absolute concentration of ATP in the regulation of this channel (Kakei et al. 1986). However, in the case of the volume-sensitive anion channel, it appears unlikely that the channel is modulated by the ATP/ADP ratio, since both nucleotides at a concentration of 2 mM were found to cause channel activation to a similar extent, whilst ADP failed to reverse activation of the channel by ATP.
The activation of anion channel activity in swollen β-cells by ATP could also be demonstrated in non-swollen cells. This implies that the channel could be active under non-stimulated conditions, a suggestion consistent with the observation that channel inhibition by 4, 4′-dithiocyanatostilbene-2, 2′-disulphonic acid leads to hyperpolarization of the cell in the presence of a sub-stimulatory concentration of glucose (Best et al. 1996a). Furthermore, this finding suggests that changes in intracellular ATP concentration within the physiological range could be a primary signal in channel activation in the absence of cell swelling, or could modulate channel activation induced by swelling or some additional mechanism.
The ability of raised concentrations of ATP to potentiate activity of the volume-sensitive anion channel did not depend upon hydrolysis of the nucleotide. This is consistent with findings in some, but not all, cell types (see Okada, 1998, for review) and suggests that activation of the β-cell channel by ATP is not the result of phosphorylation of the channel protein. Thus it is possible that the channel may have a nucleotide binding site, which can bind a range of nucleotides and modulate channel activity by an allosteric mechanism. The finding that channel activation was also observed in the presence of diadenosine tetraphosphate and GTPγS suggests that this putative binding site is non-selective and discriminates poorly between adenine and guanidine nucleotides. Our findings that channel activity could not be activated by a low concentration of GTPγS, nor inhibited by GDPβS, suggest that unlike the volume-sensitive anion channel in bovine chromaffin and endothelial cells (Doroshenko et al. 1991; Voets et al. 1998), the anion channel in pancreatic β-cells is not modulated by a G protein-coupled mechanism.
In conclusion, this study demonstrates that activity of the volume-sensitive anion channel in rat pancreatic β-cells can be modulated in a concentration-dependent manner by intracellular ATP within the expected physiological range. This effect does not require ATP hydrolysis and was observed with other adenine and guanine nucleotides, and could thus represent activation of the channel via an allosteric nucleotide binding site. Changes in intracellular [ATP] could therefore be a physiological modulator of channel activity in the pancreatic β-cell.
Acknowledgments
This work was supported by the British Diabetic Association and the NHS R&D Executive North-West.
References
- Anderson JW, Jirsch JD, Fedida D. Cation regulation of anion current activated by cell swelling in two types of human epithelial cancer cells. The Journal of Physiology. 1995;483:549–557. doi: 10.1113/jphysiol.1995.sp020605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic β-cell. Progress in Biophysics and Molecular Biology. 1989;54:87–143. doi: 10.1016/0079-6107(89)90013-8. 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Best L. Glucose and α-ketoisocaproate induce transient inward currents in rat pancreatic β-cells. Diabetologia. 1997;40:1–6. doi: 10.1007/s001250050635. [DOI] [PubMed] [Google Scholar]
- Best L, Miley HE, Yates AP. Activation of an anion conductance and β-cell depolarization during hypotonically induced insulin release. Experimental Physiology. 1996a;81:927–933. doi: 10.1113/expphysiol.1996.sp003993. [DOI] [PubMed] [Google Scholar]
- Best L, Sheader EA, Brown PD. A volume-activated anion conductance in insulin-secreting cells. The Journal of Physiology. 1995;489.P:64P. doi: 10.1007/BF02207273. [DOI] [PubMed] [Google Scholar]
- Best L, Sheader EA, Brown PD. A volume-activated anion conductance in insulin-secreting cells. Pflügers Archiv. 1996b;431:363–370. doi: 10.1007/BF02207273. [DOI] [PubMed] [Google Scholar]
- Detimary P, Van Den Berghe G, Henquin JC. Concentration dependence and time course of the effects of glucose on adenine and guanine nucleotides in mouse pancreatic islets. Journal of Biological Chemistry. 1996;271:20559–20565. doi: 10.1074/jbc.271.34.20559. [DOI] [PubMed] [Google Scholar]
- Doroshenko PA, Penner R, Neher E. Novel chloride conductance in the membrane of bovine chromaffin cells activated by intracellular GTPγS. The Journal of Physiology. 1991;436:711–724. doi: 10.1113/jphysiol.1991.sp018575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakei M, Kelly RP, Ashcroft FM. The ATP-sensitivity of K+ channels in rat pancreatic β-cells is modulated by ADP. FEBS Letters. 1986;208:63–66. doi: 10.1016/0014-5793(86)81533-2. [DOI] [PubMed] [Google Scholar]
- Kinard TA, Satin LS. An ATP-sensitive Cl− channel current that is activated by cell swelling, cAMP and glyburide in insulin-secreting cells. Diabetes. 1995;44:1461–1466. doi: 10.2337/diab.44.12.1461. [DOI] [PubMed] [Google Scholar]
- Lacy PE, Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967;16:35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Miley HE, Sheader EA, Brown PD, Best L. Glucose-induced swelling in rat pancreatic β-cells. The Journal of Physiology. 1997;504:191–198. doi: 10.1111/j.1469-7793.1997.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y. Volume expansion-sensing outward-rectifier Cl− channel: fresh start to the molecular identity and volume sensor. American Journal of Physiology. 1998;273:C755–789. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- Panten U, Heipel C, Lins S, Zunkler BJ. Activity of ATP-sensitive K+ channels in β-cells is regulated by ATP utilization in the vicinity of the plasma membrane. Diabetologia. 1989;32:527A. [Google Scholar]
- Ripoll C, Martin F, Manuel Rovira J, Pintor J, Miras-Portugal MT, Soria B. Diadenosine polyphosphates. A novel class of glucose-induced intracellular messengers in the pancreatic β-cell. Diabetes. 1996;45:1431–1434. doi: 10.2337/diab.45.10.1431. [DOI] [PubMed] [Google Scholar]
- Voets T, Manolopoulos V, Eggermont J, Ellory C, Droogmans G, Nilius B. Regulation of a swelling-activated chloride current in bovine endothelium by protein tyrosine phosphorylation and G proteins. The Journal of Physiology. 1998;506:341–352. doi: 10.1111/j.1469-7793.1998.341bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


