Abstract
The metabolic processes that occur around the tendon during mechanical loading and exercise are undescribed in man. These processes are important for understanding the development of overuse inflammation and injury.
A microdialysis technique was used to determine interstitial concentrations of glycerol, glucose, lactate, prostaglandin E2 (PGE2) and thromboxane B2 (TXB2) as well as to calculate tissue substrate balance in the peritendinous region of the human Achilles tendon. Recovery of 48–62% (range) at rest and 70–77% during exercise were obtained for glycerol, glucose and PGE2.
Six young healthy humans were studied at rest, during 30 min of intermittent static plantar flexion of the ankle at a workload corresponding to individual body weight, and during 60 min of recovery. Microdialysis was performed in both legs with simultaneous determination of blood flow by 133Xe washout in the same area, and blood sampling from the radial artery.
With exercise, the net release of lactate as well as of glycerol from the peritendinous space of the Achilles tendon increased 2-fold (P < 0.05). Furthermore a 100% increase in interstitial concentration of PGE2 and TXB2 was found, but it was only significant for TXB2(P < 0.05). As peritendinous blood flow increased 2- to 3-fold during intermittent static contractions, this indicates also that the output of these substances from the tissue increased during exercise.
This study indicates that both lipid and carbohydrate metabolism as well as inflammatory activity is accelerated in the peritendinous region of the human Achilles tendon with dynamic loading.
Connective tissue is influenced by the level of physical activity, and inflammation in and especially around human tendon represents a major problem in relation to strenuous mechanical loading within sports (James et al. 1978; Lysholm & Wiklander, 1987; Kvist, 1991; Jarvinen, 1992). In spite of a high incidence of this phenomenon, little is known about the metabolic and inflammatory processes that accompany intense loading upon this tissue, ultimately leading to inflammation and degeneration of, for example, the Achilles tendon (Davidsson & Salo, 1969; Clancy et al. 1976). Thus the mechanism by which the human tendon and its surroundings detect and convert mechanical load into a biological adaptation in vivo is largely undescribed. It has been demonstrated that connective tissue of a tendon has a low basal metabolic rate (Peacock, 1959). This has been suggested to contribute to a high tolerance towards low oxygen tension (Williams, 1986) and resistance towards tissue injury associated with prolonged mechanical loading. However, it is at present unknown to what extent metabolism and concentration of inflammatory mediators change in and around tendons during physical activity.
The use of the microdialysis technique allows for in vivo determination of metabolic changes in a variety of human tissues (Delgado et al. 1972; Ungerstedt & Pycock, 1974). By the placement of a thin dialysis tube in the tissue and pumping of a fluid resembling the interstitial fluid through that tube, it is possible to detect changes in interstitial concentration of specific metabolites and mediators, and with this technique it has been demonstrated that metabolic parameters change going from rest to physical exercise in both skeletal muscle (Rosdahl et al. 1993) and subcutaneous adipose tissue (Arner & Bolinder, 1991). Furthermore, with microdialysis, release of inflammatory mediators in response to substance P infusion has been determined in skin (Petersen et al. 1994). In the present study, microdialysis in combination with the 133Xe washout blood flow technique was used to determine in vivo metabolic and inflammatory concentration changes in, as well as to calculate substrate output from, the peritendinous area just ventral to the human Achilles tendon at rest and during physical loading.
METHODS
Subjects
Six healthy volunteers ((2 women, 4 men); median age, 27 years (range, 23–31 years); median body weight, 78 kg (range, 66–85 kg)) were included in this study after obtaining written and oral acceptance. The study was approved by The Ethical Committee of Copenhagen (KF; 01–065/98). All but one volunteer were involved in recreational endurance sports (mean training, 4 h week−1), but none had any previous history of Achilles tendon symptoms or injuries. None of the subjects took any medication and all were non-smokers.
Experimental protocol
The subjects were told not to perform any kind of exercise 24 h prior to the experiment, except for ordinary daily working activities (students or sedentary office jobs). All experiments were started at 09.00 h. During the experiment the subjects rested supine with the ankle joints in a relaxed neutral position (70–80 deg) at a room temperature of 25°C. After positioning of the microdialysis and arterial catheters and injection of 133Xe for blood flow measurements (described in detail below) the subjects rested for at least an additional 30 min before starting the experiment. This procedure ensured a time delay of at least 60 min from insertion of the last microdialysis catheter to the first measurement and thus minimized the tissue response to the insertion trauma (Fig. 1). The experiment was initiated by a resting period of 90 min during which blood samples and microdialysis samples were collected for obtaining basal values (see below). The resting period was followed by an exercise period of 30 min. During exercise the subjects were seated in a specially constructed apparatus (Fig. 2), with the trunk perpendicular to the seat and the knees extended. The extension of the knees ensured that the torque moment registered was generated by the calf muscle triceps surae only and that activity in the extensor muscles of the thigh was excluded. Both feet were positioned on a vertical sheet with the axis of the sheet aligned with the axis of the flexion in the ankle joint. The torque moment developed by m. triceps surae of the two legs in plantar direction was registered by a precalibrated (range, 0–2000 N) strain gauge (lever arm, 28 cm) and it was amplified by a custom-built instrumental AC amplifier and displayed on-line for visual feedback. The subjects were told to generate a plantar flexor torque by which the force at the strain gauge corresponded to their respective body weights, simulating the workload of the triceps muscles during normal walking. Intermittent contractions were performed continuously for 1.5 s (time to peak torque, 0.7 s) followed by a resting period of 1.5 s, for a total of 30 min. A metronome with a frequency of 40 Hz was used to control the length of the working/resting periods during exercise. The defined torque was generated with high precision by the subjects and controlled by the test personel. The study was terminated by an additional recovery period of 60 min of rest.
Figure 1. The tissue reaction upon insertion of the microdialysis catheter was measured as interstitial concentration of prostaglandin E2 (PGE2) and thromboxane B2 (TXB2).
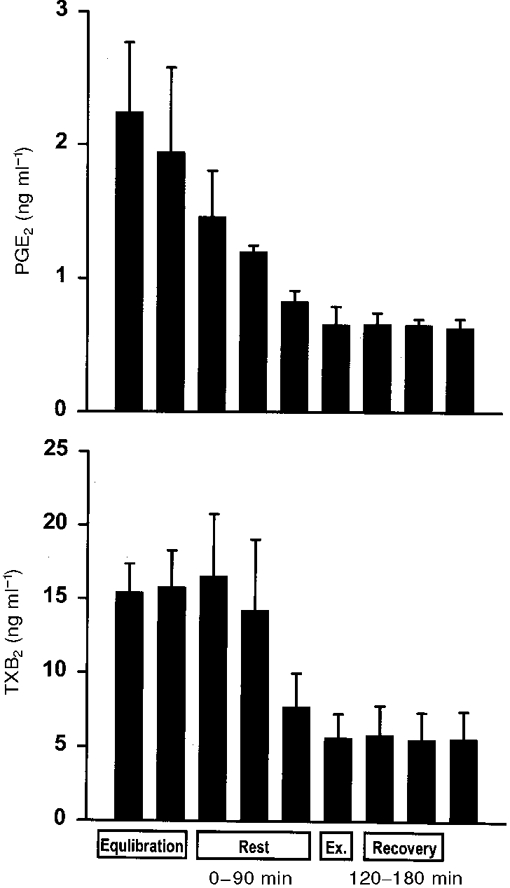
Four subjects were rested for 4.5 h after insertion of a microdialysis catheter just ventral to the Achilles tendon as described in the text. The interstitial concentration of PGE2 and TXB2 was determined in 30 min periods with the first period starting immediately after insertion of the catheter. For comparison, the time coupling between the sampling periods in this resting control study and the sampling periods in the metabolic study is indicated in the text as rest (0–90 min), exercise (Ex.; 90 to 120 min) and recovery (120–180 min).
Figure 2. A schematic drawing of the experimental set-up.
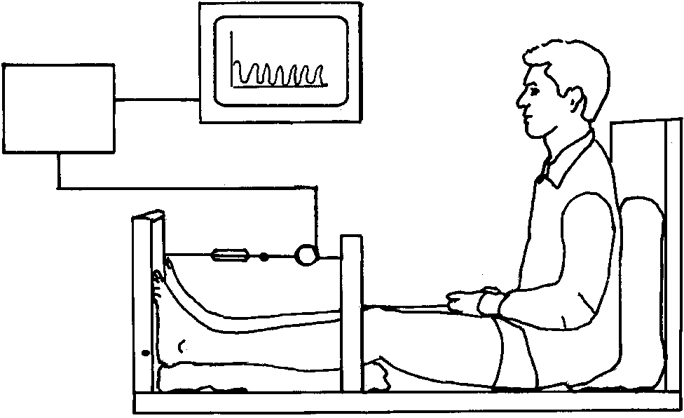
The subject is seated with the trunk perpendicular to the seat, the knee extended and both feet positioned on the vertical sheet aligned with the axis of flexion in the ankle joint. The torque moment developed in plantar direction is registered by a strain gauge. The torque is amplified by a custom-built instrumental AC amplifier and displayed on-line to the subject.
Catheterization
During local analgesia, an arterial catheter (Ohmeda, Swindon, UK) was inserted percutaneously into the radial artery of the non-dominant arm for blood sampling. The catheter was kept patent by regular flushing with isotonic sodium chloride containing heparin (10 U ml−1). Arterial blood samples were drawn every 5 min at both rest and during exercise. The samples were deproteinized by mixing with an equal volume of 0.6 M perchloric acid, centrifuged at 2000 g for 10 min, and the supernatant stored at −20°C for subsequent analysis.
Microdialysis
Microdialysis was performed in principle as described by Lönnroth et al. (1987). Microdialysis catheters (CMA 60; CMA/Microdialysis AB; 20 kDa molecular cut-off, 0.5 mm outer diameter; 30 mm in length) were placed, under ultrasound guidance, from the medial side in the peritendinous space 0.5 cm ventrally to the Achilles tendon with the active part of the membrane covering the upper medial area from 3 to 6 cm proximal to the Achilles tendon insertion on the calcaneus bone. One dialysis catheter was placed ventrally to both Achilles tendons in each individual. The dialysis catheters were perfused, via a high-precision syringe pump (CMA 100; Carnegie Medicine, Solna, Sweden), at a rate of 1 μl min−1 with a Ringer-acetate solution (Pharmacia & Upjohn, Copenhagen, Denmark) containing 3 mM glucose and 1 mM lactate. The in vivo recovery of glucose and glycerol was determined by the internal reference method (Scheller & Kolb, 1991) by addition of 11 nM D-[3-3H]-glucose (specific activity, 6475 GBq mmol−1; NEN, Boston, USA) and 5 nM [14C(U)]-glycerol (specific activity, 7400 GBq mmol−1; NEN, Boston, USA) to the perfusion solution. The recovery of [14C(U)]-glycerol was used for calculation of interstitial lactate concentration, as glycerol and lactate are expected to have identical recovery due to equal size and molecular weight.
To obtain the recovery of PGE2 and TXB2in vivo, a separate study with a similar protocol to that above has been performed in six subjects, matching the present ones with respect to sex, age and weight. Two microdialysis probes, one in each leg, were positioned as above, and perfused with a Ringer-acetate solution containing 5 nM [15-3H(N)]-prostaglandin E2 (specific activity, 3.7 GBq mmol−1; NEN, Boston, USA). The concentrations of PGE2 and TXB2 in the dialysate were found to be at the same level as in the present study during both rest and exercise, and based on this the mean recoveries found in the additional six subjects were used for calculations of interstitial concentration of PGE2 and TXB2 in the present study.
During pilot studies the authors found that dialysate volume decreased dramatically from the expected 10 μl (10 min)−1 to approximately 0.5 μl (10 min)−1 during exercise. In separate studies we found that the interstitial pressure in the region studied decreased to less than −40 mmHg during exercise (authors’ unpublished results). By adding 0.1 g ml−1 of Dextran (71 kDa; D-1537, Sigma) to the perfusion liquid, giving a total osmotic pressure for the perfusion liquid of 27 mmHg and thereby aiming at counteracting the low interstitial pressure, the volume of the dialysate equalled the perfusate, as controlled by weighing of the dialysate volume (authors’ unpublished results). After flushing the system, sampling of the dialysate was done every 10 min, with a delay of 3 min due to a void volume from the probe to the sample collector of 3 μl, providing dialysis samples of 10 μl each. The samples were immediately frozen to −70°C until analyses were done 1–2 weeks later.
Blood flow
Blood flow was measured in the region in which dialysis was performed by the local 133Xe washout method. The 133Xe dissolved in sterile isotonic saline solution to a concentration of ∼10 MBq ml−1, was transferred anaerobically into a syringe, from which 0.1 ml was injected directly into the tissue ventral to the Achilles tendon. Great care was taken not to inject any gas bubble. The injection was made with a fine needle (outer diameter, 0.4 mm) from the medial side at a depth of 1–2 cm. The depot was placed 5 cm proximal to the upper medial portion of the Achilles tendon insertion on the calcaneus. The needle was withdrawn from the tissue half a minute after the injection had been given to ensure that no leak appeared. The 133Xe washout was measured via portable silicon iodide scintillation detectors (Mediscint, Oakfield Instruments Ltd, Oxon, UK) strapped to the skin above the 133Xe depots. The detectors were connected to a multichannel analyser system (Oakfield Instruments, Oxford, UK), and counts were collected in 30 s periods starting at least 30 min after the injection. The initial counting-rate was ≡ 1.5 × 103 s−1, and blood flow was calculated from the monoexponential washout curves assuming a tissue-blood partition coefficient λ of 10 (Lassen et al. 1964; Simonsen et al. 1992). To ensure that the xenon depot was placed in the tissue in which microdialysis was performed, aliquots of dialysate were counted in a well counter, and 133Xe was found in the dialysate.
Calculations
The interstitial concentrations (Ci) were calculated using the internal reference calibration method (Scheller & Kolb, 1991). The relative recovery (RR) was calculated for each microcatheter as (Cp - Cd)/Cp, where Cp is disintegrations per minute (d.p.m.) in the perfusate and Cd is d.p.m. in the dialysate. It is assumed that RR from interstitial fluid to perfusate of unlabelled metabolite equals relative loss from perfusate to interstitial fluid of labelled metabolite.
Venous concentrations of glucose, lactate and glycerol were calculated (Cv,calc) based on the principle described by Intaglietta & Johnson (1978). The calculation is based on Fick's law of diffusions for thin membranes: J =−PS (C1 - C2), where J is the substrate flux, P is the membrane permeability of the substrate, S the membrane surface area, and C1 and C2 the concentrations on the two sides of the membrane with C1 being higher than C2. If this equation is integrated over the entire length of the capillary the following expression is obtained:
giving:
for the tissue uptake situation or:
for the tissue output situation, Cv,calc being calculated venous plasma water concentration, Ca arterial plasma water concentration, Ci intercellular water concentration, Q plasma water flow, and PS the permeability surface area product in ml (100 g tissue)−1 (min)−1. By calculating plasma flow as (1 - haematocrit) × blood flow, where the haematocrit was measured in the arterial blood, blood water flow was calculated by multiplying plasma flow by 0.9 (Paaske & Sejrsen, 1989). The PS product was set to 3 ml (100 g tissue)−1 (min)−1 for glycerol and lactate and 2 ml (100 g tissue)−1 (min)−1 for glucose, since values in this range have been found for molecules of similar sizes (Linde et al. 1974). The PS product was assumed to be constant within the range of blood flow variations registered (Paaske & Sejrsen, 1989).
The tissue substrate net uptake or net release was calculated as the product of the difference between Cv,calc and Ca and the blood water flow in which the metabolites was distributed.
Analytical methods
Metabolites
Glucose, lactate and glycerol concentrations in the arterial plasma were determined by a Monarch Plus 750 (Instrumentation Laboratory, Lexington, MA, USA). The corresponding concentrations of glucose, lactate and glycerol in the dialysates were determined by a CMA600 Microdialysis Analyser (CMA/Microdialysis AB, Solna, Sweden).
Inflammatory mediators
Two of the products of the arachidonic acid metabolic pathway, prostaglandin E2 (PGE2) and thromboxane B2 (TXB2; molecular mass, 350–375 Da), are both well suited to be examined by the microdialysis technique. However, to obtain sufficient material for analysis the samples from pre-exercise (n = 9), exercise (n = 3) and post-exercise (n = 6), respectively, were pooled in samples for each leg separately in each subject. The samples from the right legs were used for determination of PGE2 and from the left legs for determination of TXB2. PGE2 was analysed using a commercially available PGE2 radioimmunoassay kit (NEK-020, Du Pont, Boston, MA, USA). Samples or standards, together with 125I-PGE2 as the tracer, were incubated with rabbit anti-PGE2 antibodies overnight at 4°C. The samples were precipitated using polyethylene glycol, centrifuged, decanted and radioactivity in the pellet was determined in a gamma counter. The sensitivity of the assay is 5 pg ml−1. TXB2 was determined using a radioimmunoassay (NEK-024, Du Pont, Bad Homburg, Germany). The sensitivity of the assay is 8 pg ml−1, and the inter- and intra-assay coefficients of variation are 2 and 4 %, respectively.
Statistics
Relative recovery (RR) and interstitial (Ci) and venous (Cv) concentrations were calculated for each sample separately and used for calculating the tissue output/uptake (Fig. 4). RR (Table 1), Ci (Fig. 3, Table 3) and Ca (Table 2) are given as pooled data. All data are presented as means ±s.e.m. Wilcoxon's non-parametric ranking sum test for paired data was used to detect significant differences between rest and exercise and between rest and recovery periods. P < 0.05 (two tailed testing) was considered significant.
Figure 4. Tissue uptake of glucose and output of lactate and glycerol from the peritendinous space of the human Achilles tendon.
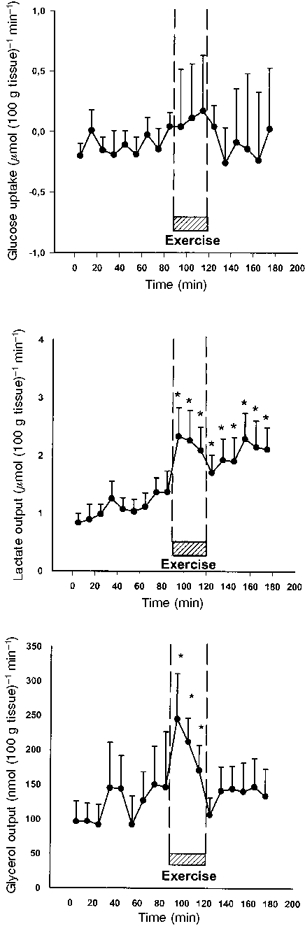
Measurements were taken during rest, intermittent exercise and subsequent recovery every 10 min, using blood concentrations determined at 5 min intervals in the radial artery and interstitial concentrations measured by microdialysis every 10 min. No changes in tissue glucose uptake could be determined during the 30 min of intermittent static exercise with m. triceps surae (P > 0.05). Tissue output of lactate was found to rise significantly (*) as a result of the intermittent static muscles contraction during the exercising period compared with rest values (P < 0.05). The output of lactate from the tissue continued to be significantly (*) increased throughout the recovery period compared with rest (P < 0.05). The tissue output of glycerol was found to be significantly (*) increased during exercise (P < 0.05). The output of glycerol returned to basal levels during the subsequent recovery period (P > 0.05).
Table 1. Glucose, glycerol and prostaglandin E2 recovery determined by internal reference calibration.
| Rest(%) | Exercise(%) | Recovery(%) | |
|---|---|---|---|
| Glucose | 48 ± 5 | 70 ± 4* | 56 ± 5 |
| Glycerol | 55 ± 5 | 76 ± 5* | 62 ± 6 |
| Prostaglandin E2 | 59 ± 4 | 77 ± 4* | 55 ± 6 |
For all three substances recovery was found to increase significantly during exercise
P < 0.05. Values returned to the resting levels during the recovery phase (P > 0.05). Values are means ± s.e.m.
Figure 3. Interstitial concentrations of glucose, lactate and glycerol in the peritendinous space of the human Achilles tendon during rest, intermittent exercise and subsequent recovery.
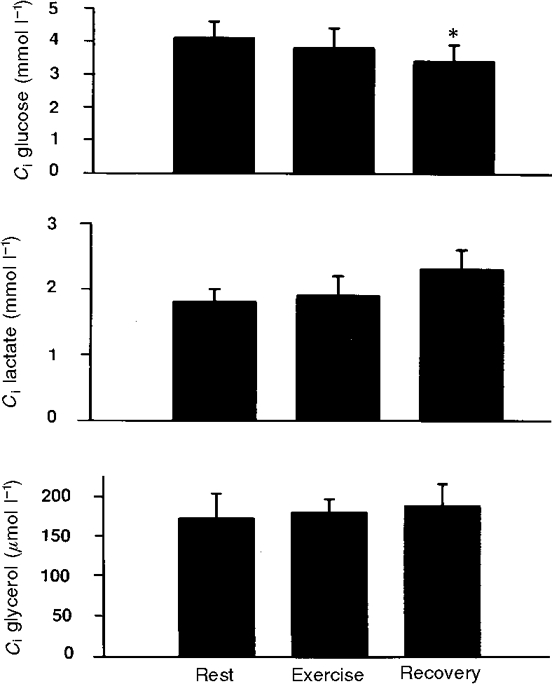
No significant differences were determined (P > 0.05) apart from glucose which decreased significantly during recovery (*P < 0.05).
Table 3. Interstitial concentrations (Ci) of prostaglandin E2 and thromboxane B2 in the peritendinous space of the human Achilles tendon during rest and intermittent static exercise of m. triceps surae.
| Rest | Exercise | Recovery | |
|---|---|---|---|
| PGE2(Ci(ng ml−1) | 0.6 ± 0.3 | 1.4 ± 0.6 | 1.3 ± 0.4 |
| TXB2(Ci(ng ml−1) | 4.8 ± 1.3 | 8.1 ± 1.4* | 5.9 ± 2.5 |
Thromboxane B2 rose significantly
as a response to exercise (P < 0.05) and returned to resting level during the following recovery period (P > 0.05).
Table 2. Blood flow determined by 133Xe washout in the peritendinous space.
| Rest | Exercise | Recovery | |
|---|---|---|---|
| Blood flow(ml(100 g tissue)−1(min−1) | 2.3 ± 0.6 | 6.1 ± 1.2* | 2.0 ± 0.3 |
| Glucose(Ca(mmol l−1) | 5.2 ± 0.1 | 4.6 ± 0.2* | 5.0 ± 0.1 |
| Lactate(Ca(mmol l−1) | 0.6 ± 0.0 | 0.7 ± 0.1 | 0.6 ± 0.1 |
| Glycerol(Ca(μmol l−1) | 61.8 ± 8.8 | 77.5 ± 12.7* | 87.0 ± 7.5** |
Blood flow was found to increase significantly
from rest to intermittent static exercise (P < 0.05), and to return to basal level during recovery (P > 0.05). These findings are in line with previous studies (Langberg et al. 1998). Concentrations of arterial plasma (Ca) glucose, lactate and glycerol were measured from samples drawn from the radial artery. Arterial glucose concentration was found to decrease significantly (*) during intermittent isometric contractions of the m. triceps surae compared with rest values (P < 0.05). No changes were found in the concentrations of lactate during the experiment (P > 0.05). Arterial glycerol concentration increased significantly (*)as a response to intermittent static exercise (P < 0.05), and continued to increase throughout the recovery period
compared with rest levels (P < 0.05).
RESULTS
Recovery of glucose, glycerol and prostaglandin E2
The relative recovery (RR) of glucose, glycerol and prostaglandin E2 determined by internal reference calibration is shown in Table 1. For all three substances the relative recovery was higher during exercise than at rest (P < 0.05).
Tissue blood flow and metabolism
Blood flow in the peritendinous area was found to increase 2- to 3-fold with exercise (P < 0.05) and returned to basal level in the recovery period (Table 2). The arterial plasma glucose concentrations (Ca) were found to decrease significantly during exercise (P < 0.05) and to be restored during the recovery phase (Table 2), whereas the interstitial glucose concentration (Ci) was maintained during exercise but decreased significantly in the recovery period (P < 0.05) (Fig. 3). The decrease in glucose Ca during exercise and in Ci during recovery did not result in any significant change in calculated glucose net uptake, which was found to be unchanged during exercise compared with resting values (P > 0.05) (Fig. 4). Both arterial plasma lactate concentration (Ca) (Table 2) and interstitial lactate concentration (Ci) (Fig. 3) were found to be stable throughout the whole experiment, with no significant changes during exercise (P > 0.05). The calculated net release of lactate from the tissue ventrally to the Achilles tendon increased significantly as a result of the exercise (P < 0.05) (Fig. 4), and the net release continued to be significantly increased during the recovery period compared with the concentration determined prior to exercise (P < 0.05) (Fig. 4). The arterial plasma glycerol concentration (Ca) was found to increase significantly during exercise (P > 0.05) (Table 2), and the concentration remained significantly increased compared with resting level during recovery (P < 0.05) (Table 2). Interstitial glycerol concentration (Ci) was stable throughout the whole experiment (P > 0.05) (Fig. 3). The calculated glycerol net release from the tissue increased significantly during exercise (P < 0.05) (Fig. 4), and returned to resting level during the recovery phase (P > 0.05).
Inflammatory mediators
No significant difference in the calculated interstitial concentration of prostaglandin E2 was found in the tissue as a result of exercise (P > 0.05) although a more than 100 % increase was demonstrated (Table 3). In addition the interstitial concentration of thromboxane B2was found to increase significantly during exercise (P < 0.05) and returned to a level not significantly different from the basal resting level during recovery (P > 0.05) (Table 3). As no arterial concentration of neither PGE2 nor TXB2 were measured, it was not possible to calculate venous concentrations (Cv,calc) of the inflammatory mediators. Thus no net release of prostaglandin E2 and thromboxane B2 could be calculated.
DISCUSSION
In the present study the microdialysis technique was used to determine indicators of metabolic and inflammatory activity in the peritendinous space of the human Achilles tendon. A major finding is the demonstration of an increased net release of lactate and glycerol from the tissue during 30 min of intermittent static exercise. In addition to these metabolic changes, indices of an inflammatory response to exercise appeared as both interstitial concentrations of PGE2 and TXB2 were doubled during exercise together with a 3-fold increase in the peritendinous region blood flow.
To our knowledge metabolic and inflammatory parameters have not previously been determined in the peritendinous region of exercising humans. Ultrasound guidance ensured a positioning of the microdialysis catheters in close relation with the epitenon and thus relevant for the area affected by overuse injury in relation to physical training and mechanical overloading (Snook, 1972; Clancy et al. 1976). Histological examination of that area indicates dominance of connective tissue together with infiltration of adipose tissue (Chaletzkaja, 1934; Jozsa et al. 1984a,b). In support of this, the interstitial glycerol concentration at rest in the peritendinous space was 70 % of the values obtained in abdominal periumbilical subcutaneous adipose tissue (Hagstrom-Toft et al. 1997; Hickner et al. 1997; Stallknecht et al. 1997), and 200 % of the values measured in the medial gastrocnemius muscle (Hagstrom-Toft et al. 1997), and a significant tissue release of glycerol was demonstrated (Fig. 4). Furthermore, during the first 10 min of exercise, glycerol release was increased indicating lipolytic activity during intermittent static calf muscle exercise, and was found to be of a similar magnitude as demonstrated earlier in adipose tissue during bicycling exercise (Arner et al. 1990; Wennlund et al. 1994; Hellstrom et al. 1996; Ranneries et al. 1998). However, the release of glycerol was found to decrease after the first initial increase in spite of maintenance of the workload throughout the entire exercising period. This decrease in lipolytic activity over the exercising period could indicate an initial stimulation followed by a subsequent local downregulation of lipolysis during prolonged exercise and support the view that the role of lipid in the peritendinous space is not metabolic.
Interstitial glucose concentration in the peritendinous area was lower at rest compared with arterial values (Fig. 3 and Table 2). Despite this no tissue uptake could be demonstrated. The difference between interstitial and arterial values were in accordance with findings in skeletal muscle (Muller et al. 1995) and adipose tissue (Stallknecht et al. 1997). During exercise no change in the interstitial concentration of glucose was demonstrated despite a drop in plasma glucose (Fig. 3 and Table 2). This indicates that the peritendinous area does not contribute quantitatively to glucose disposal and that the major cause for the decline in plasma glucose is an increased glucose uptake in the contracting skeletal muscle, in the present case m. triceps surae (Richter, 1996).
For lactate the interstitial concentration was found to be higher than the corresponding arterial concentration, and lactate was released from the tissue during rest. Studies of microdialysis performed in the subcutaneous adipose tissue (Simonsen et al. 1994) and in the gastrocnemius muscle (Hagstrom-Toft et al. 1997) support the present findings. The higher interstitial concentration determined in the present study resulted in a net release of lactate to the blood stream during resting conditions. Exercise resulted in increased lactate release from the peritendinous area (Fig. 4) indicating increased anaerobic glycolytic metabolism despite a 3-fold increase in blood flow. The fact that no increase in arterial lactate concentration could be determined, indicates that overall lactate production was modest. Alternatively the exercise-induced increase in lactate clearance of both the liver and muscles could be sufficient to maintain arterial lactate concentration at basal level (Stanley et al. 1986; MacRae et al. 1992). This is a very likely explanation since the decreasing glucose concentration will stimulate gluconeogenesis.
Inflammatory mediators prostaglandin E2 and thromboxane B2 were determined in the peritendinous tissue and their concentrations doubled, although significance was obtained only for thromboxane B2 (Table 3). Lack of determination of arterial plasma concentrations of prostaglandin E2 and thromboxane B2 excluded the possibility of calculating an accurate tissue release for these substances, but the fact that blood flow rose 3-fold during muscle contraction strongly indicates that the release of these mediators increased with exercise. This supports the view of an acute activation of inflammatory activity in peritendinous tissue with mechanical loading. This could be of importance for regulation of blood flow during exercise as PGE2 and TXB2 possess vasodilatory and vasoconstrictory capabilities, respectively (Kadowitz, 1972; Conway & Hatton, 1975; Kadowitz & Hyman, 1980).
Inflammatory mediators have previously been measured in response to mechanical loading of human bone, where interstitial concentrations of PGE2 were reported to increase (Thorsen et al. 1996). In that study, however, no measurement of recovery for PGE2 was performed either at rest or during mechanical loading, thus making it difficult to detect true quantitative changes in inflammatory mediators with exercise (Thorsen et al. 1996). In the present study, recovery was determined and found to increase with exercise. This indicates that any attempt to calculate the interstitial tissue concentration without taking recovery into account would overestimate changes occurring with onset of contraction in the peritendinous tissue. The finding of a high recovery both at rest and during exercise for PGE2 supports the notion that the interstitial concentration of inflammatory mediators can be measured in a reliable way and that changes in concentration together with increased tissue flow represents an increased release of inflammatory mediators from the peritendinous space of the Achilles tendon in humans with exercise (Table 3). In line with this, in vitro experiments using an osteoblast cell line from mice have demonstrated that mechanical strain increased PGE2 release 2-fold (Murray & Rushton, 1990). Furthermore, in a fibroblast cell line from human finger flexor tendons PGE2 release increased with graded mechanical loading, and addition of indomethacin completely inhibited the exercise-induced increase in PGE2 (Almekinders et al. 1993). In the present study subjects did not subjectively experience any pain due to the positioning of the microdialysis catheters either at rest or during exercise. Despite this it cannot be excluded that local tissue reactions due to the placement of the catheters could influence the measurements. On the other hand, the inflammatory reaction, expressed as interstitial concentrations of PGE2 and TXB2, was measured for over 4 h following the insertion of the microdialysis catheters, and it was found that the concentration of both mediators decreased over time (Fig. 1). This indicates that the reaction to the insertion trauma was short lasting and that an increase in concentration during the experiment is due to the intervention, rather than to a late response to the insertion trauma. In addition, isometric contractions were used during the exercising period giving as little movement of the contracting tissue in relation to the catheter as possible, and thus reducing the trauma to a minimum. Furthermore recent studies in human muscle have indicated that induction of muscle pain by saline injection does not change interstitial PGE2 concentration (Graven-Nielsen et al. 1997). Based on this, it is likely that the increased tissue release of inflammatory mediators represents inflammatory activity due to exercise rather than due to pain or local irritation by the catheter (Fig. 1).
In conclusion, metabolism of both lipid and carbohydrate increases in the peritendinous region of the human Achilles tendon with isometric intermittent loading of the plantar flexor muscles. Furthermore indicators of inflammatory activity are produced in response to exercise.
Acknowledgments
We thank Inge Rasmussen for skilled technical assistance. This study was supported by the Team Denmark Research Council, the Danish Sports Science Foundation, the Novo Nordisk Foundation and the Danish National Research Foundation (504–14).
References
- Almekinders LC, Banes AJ, Ballenger CA. Effects of repetitive motion on human fibroblasts. Medicine and Science in Sports and Exercise. 1993;25:603–607. [PubMed] [Google Scholar]
- Arner P, Bolinder J. Microdialysis of adipose tissue. Journal of Internal Medicine. 1991;230:381–386. doi: 10.1111/j.1365-2796.1991.tb00461.x. [DOI] [PubMed] [Google Scholar]
- Arner P, Kriegholm E, Engfeldt P, Bolinder J. Adrenergic regulation of lipolysis in situ at rest and during exercise. Journal of Clinical Investigation. 1990;85:893–898. doi: 10.1172/JCI114516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaletzkaja F. Über die Lipoidablagerung (Lipoidose) und die Anhäufung von Eiweissmassen in den Sehnen. Virchows Archiv. 1934;292:84–95. [Google Scholar]
- Clancy WG, Jr, Neidhart D, Brand RL. Achilles tendonitis in runners: a report of five cases. American Journal of Sports Medicine. 1976;4:46–57. doi: 10.1177/036354657600400202. [DOI] [PubMed] [Google Scholar]
- Conway J, Hatton R. Effects of prostaglandins E1, E2, A1, and A2 on the resistance and capacitance vessels in the hind limb of the dog. Cardiovascular Research. 1975;9:229–235. doi: 10.1093/cvr/9.2.229. [DOI] [PubMed] [Google Scholar]
- Davidsson L, Salo M. Pathogenesis of subcutaneous tendon ruptures. Acta Chirugica Scandinavica. 1969;135:209–215. [PubMed] [Google Scholar]
- Delgado JM, Defeudis FV, Roth RH, Ryugo DK, Mitruka BM. Dialytrode for long term intracerebral perfusion in awake monkeys. Archives of International Pharmacodynamic Therapy. 1972;198:9–21. [PubMed] [Google Scholar]
- Graven-Nielsen T, McArdle A, Phoenix J, Arendt-Nielsen L, Jensen TS, Jackson MJ, Edwards RH. In vivo model of muscle pain: quantification of intramuscular chemical, electrical, and pressure changes associated with saline-induced muscle pain in humans. Pain. 1997;69:137–143. doi: 10.1016/s0304-3959(96)03270-8. 10.1016/S0304-3959(96)03270-8. [DOI] [PubMed] [Google Scholar]
- Hagstrom-Toft E, Enoksson S, Moberg E, Bolinder J, Arner P. Absolute concentrations of glycerol and lactate in human skeletal muscle, adipose tissue, and blood. American Journal of Physiology. 1997;273:E584–592. doi: 10.1152/ajpendo.1997.273.3.E584. [DOI] [PubMed] [Google Scholar]
- Hellstrom L, Blaak E, Hagstrom-Toft E. Gender differences in adrenergic regulation of lipid mobilization during exercise. International Journal of Sports Medicine. 1996;17:439–447. doi: 10.1055/s-2007-972875. [DOI] [PubMed] [Google Scholar]
- Hickner RC, Fisher JS, Kohrt WM. Regional differences in interstitial glycerol concentration in subcutaneous adipose tissue of women. American Journal of Physiology. 1997;273:E1033–1038. doi: 10.1152/ajpendo.1997.273.5.E1033. [DOI] [PubMed] [Google Scholar]
- Intaglietta M, Johnson PC. Principles of capillary exchange. In: Johnson PC, editor. Peripheral Circulation. New York: Wiley Press; 1978. pp. 141–166. [Google Scholar]
- James SL, Bates BT, Osternig LR. Injuries to runners. American Journal of Sports Medicine. 1978;6:40–50. doi: 10.1177/036354657800600202. [DOI] [PubMed] [Google Scholar]
- Jarvinen M. Epidemiology of tendon injuries in sports. Clinical Sports Medicine. 1992;11:493–504. [PubMed] [Google Scholar]
- Jozsa L, Reffy A, Balint JB. Polarization and electron microscopic studies on the collagen of intact and ruptured human tendons. Acta Histochemistry. 1984a;74:209–215. doi: 10.1016/S0065-1281(84)80011-2. [DOI] [PubMed] [Google Scholar]
- Jozsa L, Reffy A, Balint JB. The pathogenesis of tendolipomatosis; an electron microscopical study. International Orthopedics. 1984b;7:251–255. doi: 10.1007/BF00266836. [DOI] [PubMed] [Google Scholar]
- Kadowitz PJ. Effect of prostaglandins E 1, E 2 and A 2 on vascular resistance and responses to noradrenaline, nerve stimulation and angiotensin in the dog hindlimb. British Journal of Pharmacology. 1972;46:395–400. doi: 10.1111/j.1476-5381.1972.tb08136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowitz PJ, Hyman AL. Comparative effects of thromboxane B2 on the canine and feline pulmonary vascular bed. Journal of Pharmacology and Experimental Therapy. 1980;213:300–305. [PubMed] [Google Scholar]
- Kvist M. Achilles tendon injuries in athletes. Annales Chirurgiae et Gynaecologiae. 1991;80:188–201. [PubMed] [Google Scholar]
- Langberg H, Bülow J, Kjær M. Blood flow in the peritendinous space of the human Achilles tendon during exercise. Acta Physiologica Scandinavica. 1998;163:149–153. doi: 10.1046/j.1365-201X.1998.00361.x. 10.1046/j.1365-201X.1998.00361.x. [DOI] [PubMed] [Google Scholar]
- Lassen NA, Lindbjerg J, Munck O. Measurement of blood-flow through skeletal muscle by intramuscular injection of Xenon-133. Lancet. 1964;i:686–689. doi: 10.1016/s0140-6736(64)91518-1. 10.1016/S0140-6736(64)91518-1. [DOI] [PubMed] [Google Scholar]
- Linde B, Chisolm G, Rosell S. The influence of sympathetic activity and histamine on the blood-tissue exchange of solutes in canine adipose tissue. Acta Physiologica Scandinavica. 1974;92:145–155. doi: 10.1111/j.1748-1716.1974.tb05731.x. [DOI] [PubMed] [Google Scholar]
- Lönnroth P, Jansson PA, Smith U. A microdialysis method allowing characterization of intercellular water space in humans. American Journal of Physiology. 1987;253:E228–231. doi: 10.1152/ajpendo.1987.253.2.E228. [DOI] [PubMed] [Google Scholar]
- Lysholm J, Wiklander J. Injuries in runners. American Journal of Sports Medicine. 1987;15:168–171. doi: 10.1177/036354658701500213. [DOI] [PubMed] [Google Scholar]
- MacRae HS, Dennis SC, Bosch AN, Noakes TD. Effects of training on lactate production and removal during progressive exercise in humans. Journal of Applied Physiology. 1992;72:1649–1656. doi: 10.1152/jappl.1992.72.5.1649. [DOI] [PubMed] [Google Scholar]
- Muller M, Schmid R, Nieszpaur-Los M, Fassolt A, Lonnroth P, Fasching P, Eichler HG. Key metabolite kinetics in human skeletal muscle during ischaemia and reperfusion: measurement by microdialysis. European Journal of Clinival Investigation. 1995;25:601–607. doi: 10.1111/j.1365-2362.1995.tb01752.x. [DOI] [PubMed] [Google Scholar]
- Murray DW, Rushton N. The effect of strain on bone cell prostaglandin E2 release: a new experimental method. Calcified Tissue International. 1990;47:35–39. doi: 10.1007/BF02555863. [DOI] [PubMed] [Google Scholar]
- Paaske WP, Sejrsen P. Permeability of continuous capillaries. Danish Medical Bulletin. 1989;36:570–590. [PubMed] [Google Scholar]
- Peacock EE. A study of the circulation in normal tendons and healing grafts. Annals of Surgery. 1959;149:415–428. doi: 10.1097/00000658-195903000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen LJ, Poulsen LK, Soendergaard J, Skov PS. The use of cutaneous microdialysis to measure substance P-induced histamine release in intact human skin in vivo. Journal of Allergy and Clinical Immunology. 1994;94:773–783. doi: 10.1016/0091-6749(94)90186-4. [DOI] [PubMed] [Google Scholar]
- Ranneries C, Bulow J, Buemann B, Christensen NJ, Madsen J, Astrup A. Fat metabolism in formerly obese women. American Journal of Physiology. 1998;274:E155–161. doi: 10.1152/ajpendo.1998.274.1.E155. [DOI] [PubMed] [Google Scholar]
- Richter EA. Glucose utilization. In: Rowell LB, Shepherd JT, editors. Exercise: Regulation and Integration of Multiple Systems. Oxford: Oxford University Press; 1996. pp. 912–952. [Google Scholar]
- Rosdahl H, Ungerstedt U, Jorfeldt L, Henriksson J. Interstitial glucose and lactate balance in human skeletal muscle and adipose tissue studied by microdialysis. The Journal of Physiology. 1993;471:637–657. doi: 10.1113/jphysiol.1993.sp019920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller D, Kolb J. The internal reference technique in microdialysis: a practical approach to monitoring dialysis efficiency and to calculating tissue concentration from dialysate samples. Journal of Neuroscience Methods. 1991;40:31–38. doi: 10.1016/0165-0270(91)90114-f. 10.1016/0165-0270(91)90114-F. [DOI] [PubMed] [Google Scholar]
- Simonsen L, Bulow J, Madsen J. Adipose tissue metabolism in humans determined by vein catheterization and microdialysis techniques. American Journal of Physiology. 1994;266:E357–365. doi: 10.1152/ajpendo.1994.266.3.E357. [DOI] [PubMed] [Google Scholar]
- Simonsen L, Bulow J, Madsen J, Christensen NJ. Thermogenic response to epinephrine in the forearm and abdominal subcutaneous adipose tissue. American Journal of Physiology. 1992;263:E850–855. doi: 10.1152/ajpendo.1992.263.5.E850. [DOI] [PubMed] [Google Scholar]
- Snook GA. Achilles tendon tenosynovitis in long-distance runners. Medicine and Science in Sports. 1972;4:155–158. [PubMed] [Google Scholar]
- Stallknecht B, Bulow J, Frandsen E, Galbo H. Desensitization of human adipose tissue to adrenaline stimulation studied by microdialysis. The Journal of Physiology. 1997;500:271–282. doi: 10.1113/jphysiol.1997.sp022017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley WC, Gertz EW, Wisneski JA, Neese RA, Morris DL, Brooks GA. Lactate extraction during net lactate release in legs of humans during exercise. Journal of Applied Physiology. 1986;60:1116–1120. doi: 10.1152/jappl.1986.60.4.1116. [DOI] [PubMed] [Google Scholar]
- Thorsen K, Kristoffersson AO, Lerner UH, Lorentzon RP. In situ microdialysis in bone tissue. Stimulation of prostaglandin E2 release by weight-bearing mechanical loading. Journal of Clinical Investigation. 1996;98:2446–2449. doi: 10.1172/JCI119061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerstedt U, Pycock C. Functional correlates of dopamine neurotransmission. Bulletin der Schweizerischen Akademische der Medizinischen Wissenshaften. 1974;30:44–55. [PubMed] [Google Scholar]
- Wennlund A, Wahrenberg H, Hagstrom-Toft E, Bolinder J, Arner P. Lipolytic and cardiac responses to various forms of stress in humans. International Journal of Sports Medicine. 1994;15:408–413. doi: 10.1055/s-2007-1021079. [DOI] [PubMed] [Google Scholar]
- Williams JGP. Achilles tendon lesions in sports. Sports Medicine. 1986;3:114–135. doi: 10.2165/00007256-198603020-00003. [DOI] [PubMed] [Google Scholar]


