Abstract
Voltage-dependent calcium channels (VDCCs) show a highly non-uniform distribution in many cell types, including neurons and other polarized secretory cells. We have examined whether this can be mimicked in a polarized epithelial cell line (Madin-Darby canine kidney), which has been used extensively to study the targeting of proteins.
We expressed the VDCC α1A, α1B or α1C subunits either alone or in combination with accessory subunits α2-δ and the different β subunits, and examined their localization immunocytochemically. An α1 subunit was only targeted to the plasma membrane if co-expressed with the accessory subunits.
The combination α1C/α2-δ and all β subunits was always localized predominantly to the basolateral membrane. It has been suggested that this is equivalent to somatodendritic targeting in neurons.
In contrast, the α1B subunit was expressed at the apical membrane with all the accessory subunit combinations, by 24 h after microinjection. This membrane destination shows some parallels with axonal targeting in neurons.
The α1A subunit was consistently observed at the apical membrane in the combinations α1A/α2-δ/β1b or β4. In contrast, when co-expressed with α2-δ/β2a, α1A was clearly targeted to the basolateral membrane.
In conclusion, the VDCC α1 subunit appears to be the primary determinant for targeting the VDCC complex, but the β subunit can modify this destination, particularly for α1A.
Voltage-dependent calcium channels (VDCCs) are heteromeric complexes consisting of a channel-forming α1 subunit and accessory α2-δ and β subunits. There are at least eight cloned and expressed α1 subunits (Perez-Reyes & Schneider, 1994; Perez-Reyes et al. 1998), at least six of which (α1A-E and G) are found in the nervous system. The α1 subunit determines the characteristics of the channel and some of the cloned channels have been attributed to functionally identified channels. The N-type channel is believed to be encoded by the α1B clone (De Waard et al. 1994), P/Q-type channels by α1A (Gillard et al. 1997) and L-type channels by α1C and α1D (Birnbaumer et al. 1994). The assignment of the α1E clone to a native channel has been controversial. It has been suggested that it encodes either an R-type (residual) (Randall & Tsien, 1995) or a subset of low voltage-activated T-type channels (Bourinet et al. 1996). The α1G subunit encodes a T-type channel (Perez-Reyes et al. 1998).
The accessory subunits, particularly the intracellular β subunit, have been shown to have marked effects on the properties of α1 subunits (apart from α1G), including modification of kinetics, amplitude and targeting of the complex to the plasma membrane (Singer et al. 1991; Brice et al. 1997). There are four β subunits, all of which are expressed in the nervous system (Perez-Reyes & Schneider, 1994). We have shown previously that the β subunit has a chaperone-like effect, promoting functional expression of the VDCC α1A subunit at the plasma membrane of COS-7 cells (Brice et al. 1997), and similar results have been obtained for cardiac α1C with β2a (Chien et al. 1995).
In neurons, and other polarized cells, VDCCs must be sorted to the correct membrane domain for the proper functioning of the cell, as the different subtypes of VDCC have very different biophysical properties and potential for modulation (Dolphin, 1998). For example, in neurons certain subtypes of VDCC are essential to provide Ca2+ for neurotransmitter release. Studies have shown that the N-and P/Q-type channels are particularly involved, either individually or in combination with each other (Turner et al. 1993; Wheeler et al. 1994), indicating a presynaptic localization for these channels. Nevertheless, these VDCCs have also been observed on the soma and dendrites of a number of neuronal cell types (Westenbroek et al. 1992). In contrast, the L-type channels α1C and α1D are not involved in transmitter release, but have been implicated in the entry of Ca2+ into cell bodies and dendrites for control of other cellular processes such as gene expression (Bito et al. 1996). They have also been localized electrophysiologically and immunocytochemically in these regions (Hell et al. 1993; Pearson et al. 1995; Melliti et al. 1996). How the differential targeting of the VDCCs to different neuronal locations is achieved is not known. The aim of these experiments was, firstly, to study whether there is a polarized distribution of VDCCs when expressed in a polarized epithelial cell line, in order to provide a model system in which to study the calcium channel domains involved in the differential targeting that occurs in neurons and other polarized cells. Secondly, we wished to investigate whether the accessory β subunits contributed to the polarized distribution of α1 subunits.
To examine the role of subunit composition in the control of targeting of the VDCCs we used Madin-Darby canine kidney (MDCK) cells as a model system. MDCK cells have been shown in a number of studies to have a sorting mechanism analogous to that in neurons for several different protein species (Dotti & Simons, 1990). It has recently been demonstrated that neuronal expression of exogenous dendritically targeted proteins in neurons is paralleled by basolateral sorting in MDCK cells, and proteins that are apically sorted in MDCK cells show both axonal and dendritic targeting in neurons (Jareb & Banker, 1998).
By expressing subunits of the VDCCs either alone or in combination in MDCK cells, we have shown firstly that there is differential targeting of different VDCC combinations, and secondly that the α1 subunit is the primary determinant of targeting, but that different combinations of α1 and β subunits can alter the destination of the complex.
METHODS
cDNA constructs
The cDNAs used in this study were all cloned from rat brain except for α1B which was from rabbit brain. α1A (M64373), α1C (M67515) and β1b (X11394) were obtained in the vertebrate expression vector pMT2 (Genetics Institute, Cambridge, MA, USA) (Swick et al. 1992). Full-length cDNAs for α1B (Genbank accession number D14157), α2-δ (M86621), β2a (M80545), β3 (M88751) and β4 (M80545) were subcloned into pMT2 using standard molecular biology techniques. Multiple restriction digests were used to verify correct orientation of all inserts. The wild-type and truncated CD44 cDNAs were supplied in the pSRα vector (Neame & Isacke, 1993).
Cell culture and microinjection
Monkey COS-7 cells were cultured and transfected as previously described (Brice et al. 1997). MDCK cells were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10 % fetal calf serum, 100 i.u. ml−1 penicillin and 100 μg ml−1 streptomycin, at 37°C. For the experiments, the MDCK cells were grown on Costar transwell filters, which allow the cells to become fully polarized. Cells were plated onto the filters at 106 cells per well, and the medium was changed every 2–3 days. After 10–17 days on the filters, the cells were used for microinjection, but only if they were fully confluent and the monolayer intact when examined at a magnification of × 400. The nuclei of individual cells were microinjected with cDNA (0.1 μg μl−1) using an Eppendorf microinjector, within an area marked on the filter, so that it could be located for subsequent visualization. Cells were then returned to the incubator. Each subunit combination was microinjected into at least three sets of polarized MDCK cells.
Immunocytochemistry
COS-7 cells were fixed 48 h after transfection. MDCK cells were fixed 6 or 24 h after microinjection. The cells were washed twice in Tris-buffered saline (TBS; 154 mM NaCl, 20 mM Tris, pH 7.4), then fixed in 4 % paraformaldehyde in TBS as described (Brice et al. 1997). The cells were permeabilized in 0.02 % Triton X-100 in TBS and incubated with blocking solution (20 % (v/v) goat serum, 4 % (w/v) bovine serum albumin (BSA), 0.1 % (w/v) dl-lysine in TBS). The cells were incubated for 14 h at 4°C with the appropriate primary antibody diluted 1 : 500 in 10 % goat serum, 2 % BSA and 0.05 % dl-lysine. The VDCC antibodies used in this study were raised in rabbits against specific peptides derived from the sequences of α1A, α1B, α1C and α2 subunits. The α1B antibody was affinity-purified on a Sepharose 4B-immunizing peptide column (stock concentration, 100 μg ml−1); the others were used as antisera. Their specificity has been described previously (Berrow et al. 1995; Brickley et al. 1995; Brice et al. 1997; Wyatt et al. 1997; Stephens et al. 1998). None of the α1 subunit antibodies utilized in this study cross-react with the other α1 subunits. These primary antibodies were detected using biotin-conjugated goat anti-rabbit IgG (1 : 200) (Sigma), then streptavidin-fluorescein isothiocyanate (FITC) (1 : 100) (Molecular Probes, Eugene, OR, USA). The chicken anti-canine Na+,K+-ATPase α subunit antiserum was used at a dilution of 1 : 500 (Chemicon, Temecula, CA, USA) and the secondary goat anti-chicken IgG-FITC was used at a dilution of 1 : 250 (Southern Biotechnology Associates, Inc., Birmingham, AL, USA). The monoclonal antibody CD44 (1 : 200) was detected using goat anti-mouse IgG-FITC conjugate (1 : 200; Molecular Probes). Filters were mounted with Vectorshield (Vector Laboratories, Burlingame, CA, USA) between a coverslip and a microscope slide. Cells were examined on either an MRC 600 or MRC 1024 laser scanning confocal microscope (Bio-Rad, Hemel Hempstead, UK). For MDCK cells, confocal images are shown in the X-Y plane either at the level of the apical plasma membrane or midway through the cells (as stated), and in the X-Z plane (see Fig. 2A). An average of eight typical cells was examined from each experiment. The n values given in the figure legends refer to the number of separate experiments performed, therefore for each condition a total of ∼8 ×n cells have been visually examined. Quantification was performed on X-Z sections using Imagequant software (Molecular Dynamics, Sunnyvale, CA, USA).
Figure 2. Polarized phenotype of MDCK cells.

A, schematic diagram of an MDCK cell, showing the planes in which the confocal images were taken. In the X-Y plane the images were either midway (mid) through the cell or at the apical surface (as stated). In B and C, the polarized distribution of the endogenous Na+,K+-ATPase α subunit was examined to verify the polarized phenotype of the MDCK cells used in this study. Cells that had grown on filters for 10–16 days were fixed and permeabilized, then immunolabelled with polyclonal anti-canine Na+,K+-ATPase and examined on a confocal microscope (n = 6). Images are shown in the X-Y plane (mid; B), and in the X-Z plane (C). For the X-Z plane, the apical and basal surfaces of the MDCK cells are shown by arrows, and the vertical columns representing lateral staining are indicated by arrowheads. Scale bar, 15 μm.
RESULTS
Plasma membrane expression of α1 subunits in COS-7 cells
VDCC subunits were expressed individually or in combination by transfection in COS-7 cells, and their immunocytochemical localization determined after 48 h. When α1A (Brice et al. 1997), α1B or α1C subunits (Fig. 1A) were expressed alone, immunostaining was observed throughout the cell cytoplasm, with little localization to the plasma membrane. In contrast, when the same α1 subunits were expressed together with α2-δ and the β1b subunit, immunostaining was observed largely at the plasma membrane (Fig. 1B, arrows) in both panels, confirming results previously obtained for α1A (Brice et al. 1997). For α1C in combination with α2-δ and β1b, immunostaining was also observed intracellularly in perinuclear regions (Fig. 1B, arrowhead in lower panel). Similar results were observed for these α1 subunits in combination with other β subunits (results not shown).
Figure 1. Distribution of α1B and α1C transfected in COS-7 cells.

COS-7 cells were transfected with α1B (upper panel) or α1C (lower panel), either alone (A), or together with β1b and α2-δ (B). Cells were fixed 48 h after transfection. The distribution is largely intracellular for the α1 subunits transfected alone, but shows membrane localization when the α1 subunits are transfected together with the accessory subunits (arrows). For α1C, punctate perinuclear staining was also observed (arrowhead). Scale bar, 15 μm.
MDCK cells produce a polarized phenotype in culture
To establish that the MDCK cells produce a polarized phenotype under the culture conditions used (Fig. 2A), the α subunit of the endogenous Na+,K+-ATPase was labelled with specific canine antibodies. The resultant staining was basolaterally localized (Fig. 2B and C; n = 6/6), consistent with previous studies in polarized MDCK cells (Muth et al. 1998). For the second phenotype control, the cells were microinjected with wild-type murine CD44 cDNA or a mutated CD44 cDNA which had its terminal 3′ domain deleted (Neame & Isacke, 1993). We observed that wild-type CD44 was targeted to the basolateral membrane whereas the mutated CD44 was apically targeted (n = 3/3 for both constructs, data not shown). This is in agreement with previous studies (Neame & Isacke, 1993).
Distribution of α1 subunits in MDCK cells
VDCC subunits were expressed individually or in combination in differentiated MDCK cells by microinjection of the cDNAs into the nuclei of cells that had formed a confluent polarized monolayer (see Fig. 2A). After 24 h, the cells were fixed and the VDCC subunit distribution investigated with specific antibodies. Initially we examined, using immunocytochemical methods, whether endogenous calcium channel subunits were present in confluent monolayers of MDCK cells. Of the subunit antibodies examined - α1A, α1B, α1C, α1D, α2 and β - none produced positive immunostaining for endogenous subunits in the polarized cells (see Fig. 3A for α1A, α1B and α1C). When the α1 subunits were expressed alone, immunostaining for α1A, α1B or α1C was substantial (Fig. 3B), but was located intracellularly, as indicated from both X-Y and X-Z sections, with no distinct plasma membrane staining in any of the cells observed (Fig. 3B and Fig. 4a). This is consistent with the results obtained in COS-7 cells, where α1 subunits are not plasma membrane-associated unless expressed with accessory subunits (Fig. 1) (Brice et al. 1997). Also in agreement with the results in COS-7 cells, co-expression of accessory subunits, α2-δ and all β subunits (β1b, β2a, β3 or β4) had clear effects on the distribution of the expressed VDCC α1 subunits in MDCK cells, for all three α1 subunits examined here, causing plasma membrane association.
Figure 3. Heterologous expression of VDCC α1 subunits in MDCK cells.

Polarized MDCK cells (grown on filters for 10–16 days) were either not microinjected (A), or microinjected with α1A (left; n = 5), α1B (centre; n = 4) or α1C (right; n = 4) cDNAs (B). Cells were fixed 24 h after microinjection, permeabilized and immunolabelled with the specific anti-peptide α1 antibodies for α1A, α1B or α1C, respectively. Cells were examined using a confocal microscope in the X-Y plane through the centre of the cell (X-Y mid in A and upper panel in B) and in the X-Z plane (lower panel in B). Scale bar, 15 μm. For this and the following figures showing confocal images, the numbers in parentheses indicate the number of separate experiments performed. In each experiment at least 8 cells were examined and the results shown are representative of all these cells.
Figure 4. Quantification of heterologous expression of α1 subunits in combination with α2-δ and different β subunits in MDCK cells.

Quantification was performed on X-Z sections, by determining the percentage of total immunostaining for the specific α1 subunit in each of the 3 compartments making up the cell, apical (▪), basolateral (□) and intracellular (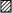 ), using Imagequant software. The results are given for α1C (A), α1B (B) and α1A (C). a,α1 subunit expressed alone, cells fixed 24 h after injection. b and c,α1 subunit expressed together with the α2-δ subunit and the specified β subunit, for either 6 h (b) or 24 h (c). Results are given as means ±s.e.m. for the number (n) of X-Z sections that were subjected to quantitative analysis for each condition. The statistical significances of the differences between apical and either intracellular (a) or basolateral (b and c) distributions were determined by Student's paired t test, and are shown above the column illustrating the greater immunostaining. *P < 0.05, **P < 0.01.
), using Imagequant software. The results are given for α1C (A), α1B (B) and α1A (C). a,α1 subunit expressed alone, cells fixed 24 h after injection. b and c,α1 subunit expressed together with the α2-δ subunit and the specified β subunit, for either 6 h (b) or 24 h (c). Results are given as means ±s.e.m. for the number (n) of X-Z sections that were subjected to quantitative analysis for each condition. The statistical significances of the differences between apical and either intracellular (a) or basolateral (b and c) distributions were determined by Student's paired t test, and are shown above the column illustrating the greater immunostaining. *P < 0.05, **P < 0.01.
Distribution of α1C co-expressed with accessory subunits
The immunostaining observed for the α1C subunit expressed with the accessory subunits was the most consistent of the three α1 subunits examined, with a similar localization pattern being observed for all four β subunits. By 6 h after microinjection, the α1C subunit in combination with α2-δ and all β subunits was observed largely at the basolateral membrane, with little immunolocalization at the apical membrane, but with some discrete punctate staining in intracellular, including perinuclear, regions (Figs 4Ab and 5).
Figure 5. Polarized expression of α1C in combination with α2-δ and different β subunits in MDCK cells.

Polarized MDCK cells were microinjected with α1C cDNA and the accessory subunit cDNAs as indicated. Cells were fixed and permeabilized, 6 h after microinjection with α1C/α2-δ/β1b (n = 4), α1C/α2-δ/β2a (n = 3), α1C/α2-δ/β3 (n = 3) or α1C/α2-δ/β4 (n = 3), followed by immunolabelling with anti-α1C antibodies. Cells were examined on a confocal microscope in the X-Y (mid) plane (upper panel) and X-Z plane (lower panel). Scale bar, 15 μm.
In MDCK and other polarized cells, some proteins have been observed to be targeted initially to one membrane, only later to be transcytosed to the other membrane domain. Transcytosis, when it occurs, is most frequently observed from the basolateral to the apical membrane (Mostov et al. 1992), with basolaterally associated proteins more usually being directly targeted (Dotti & Simons, 1990; Jareb & Banker, 1998). To investigate if transcytosis of the VDCC complexes occurs in this polarized system, the same batches of injected cells were also left for a longer time (24 h) before fixation and immunocytochemistry, to examine whether the route of the α1 subunit to its final destination seen at 24 h was direct or indirect. At 24 h after microinjection of α1C with α2-δ and any of the β subunits (β1b, β2a, β3 or β4), immunostaining for the α1C subunit was still observed basolaterally, with much less associated with the apical membrane (Fig. 4Ac), indicating that targeting was directly to the basolateral membrane domain.
Distribution of α1B co-expressed with accessory subunits
The α1B subunit was clearly observed at the plasma membrane when co-expressed with all α2-δ/β combinations. However, this distribution was opposite to that of α1C. At 24 h after microinjection, the localization pattern of the α1B subunit was entirely apical, for all combinations of accessory subunits, with minimal staining associated with the basolateral membrane, and little intracellular staining except for the combination containing β3 (Fig 4Bc and Fig 6).
Figure 6. Polarized expression of α1B in combination with α2-δ and different β subunits in MDCK cells.

Polarized MDCK cells were microinjected with α1B cDNA and the accessory subunit cDNAs as indicated. Cells were fixed and permeabilized, 24 h after microinjection with the combinations α1B/α2-δ/β1b (n = 4), α1B/α2-δ/β2a (n = 3), α1B/α2-δ/β3 (n = 3) or α1B/α2-δ/β4 (n = 3), followed by immunolabelling with anti-α1B antibodies. Cells were examined on a confocal microscope in the apical X-Y plane (upper panel) and X-Z plane (lower panel). Scale bar, 15 μm.
To investigate whether transcytosis had occurred for α1B to reach the apical membrane, localization was also investigated at the earlier time of 6 h. At this time point, when α1B was co-expressed with α2-δ/β1b, β3 or β4, clear apical staining was already observed in all of the cells examined (Fig. 4Bb). In contrast, cells expressing α1B/α2-δ/β2a showed little differential targeting by 6 h, α1B immunoreactivity being observed at both basolateral and apical membranes, as well as intracellularly (Fig. 4Bb).
Distribution of α1A co-expressed with accessory subunits
The α1A subunit was the α1 species whose immunolocalization was most affected by the β subunit with which it was co-expressed. The staining for α1A co-injected with α2-δ and either β1b or β4 cDNA was predominantly at the apical membrane at both 6 and 24 h (Fig 4Cb,c and Fig 7). In contrast, the localization of the α1A/α2-δ/β2a combination was predominantly confined to the lateral membrane, with some intracellular staining, at both time points and the α1A/α2-δ/β3 combination showed no selective localization at either time point (Fig 4Cb,c and Fig 7).
Figure 7. Polarized expression of α1A in combination with α2-δ and different β subunits in MDCK cells.

Polarized MDCK cells were microinjected with α1A cDNA and the accessory subunit cDNAs as indicated. Cells were fixed and permeabilized, 6 or 24 h after microinjection with α1A/α2-δ/β1b (n = 3), α1A/α2-δ/β2a (n = 3), α1A/α2-δ/β3 (n = 3) or α1A/α2-δ/β4 (n = 3), then immunolabelled with anti-α1A antibodies. Cells were examined on a confocal microscope, and images are shown in the apical X-Y plane (upper panel) and X-Z plane (lower panel). The image for the α1A/α2-δ/β1b combination was taken at 6 h, but identical results were obtained at 24 h. The other images were taken at 24 h. Scale bar, 15 μm.
DISCUSSION
In many secretory cells there is polarization of channels and the secretory apparatus (Schroeter et al. 1997). In neurons, the VDCC subtypes are differentially distributed between cell bodies, dendrites and presynaptic terminals (Westenbroek et al. 1992; Day et al. 1996) but how this distinct targeting is achieved is unknown. By expressing the VDCC subunits in polarized MDCK cells, we have dissected the involvement and contribution of the subunits to the targeting of the VDCC complex. MDCK cells and neurons are functionally very different, but both have a polarized phenotype. Moreover, MDCK cells have been shown to have a membrane protein sorting mechanism which is similar in some respects to that in neurons. Basolateral targeting in MDCK cells was initially proposed to be equivalent to somatodendritic targeting in neurons, whereas apical targeting in MDCK cells was suggested to bear similarities to axonal targeting in neurons (Dotti & Simons, 1990). More recently this view has been partially revised. It has been found that while basolateral sorting in MDCK cells does correspond to somatodendritic targeting in hippocampal neurons, proteins that are apically targeted in MDCK cells correspond to those that are targeted both axonally and dendritically in hippocampal neurons (Jareb & Banker, 1998). This indicates that for membrane proteins that are selectively targeted to axons or presynaptic terminals, additional sorting signals must exist.
In the present study we have used the technique of microinjecting MDCK cells that had already formed their polarized phenotype, because transient transfection of non-differentiated cells, followed by expression of the heterologous protein during the development of the differentiated phenotype, may lead to aberrant targeting of proteins (Haller & Alper, 1995). We expressed α1A, α1B or α1C subunits either alone or in combination with the accessory subunits α2-δ and β1b, β2a, β3 or β4 in polarized MDCK cells, and detected the localization of the subunits using immunocytochemical techniques. The focus of the study was the immunolocalization of α1 subunits and the influence exerted by the different β subunits. The β subunit is an entirely intracellular hydrophilic protein that interacts with the α1 subunit in at least one position (Pragnell et al. 1994). We found that all α1 subunits expressed alone in MDCK cells were intracellularly localized, consistent with results obtained in COS-7 and other cells (Fig. 1) (Chien et al. 1995; Brice et al. 1997). However, if co-expressed with α2-δ and a β subunit, immunostaining for all α1 subunits examined was observed at the plasma membrane, an observation also made in the other cell lines and leading to the suggestion of a chaperone role for β subunits (Fig. 1) (Brice et al. 1997).
The α1C subunit was always localized preferentially to the basolateral membrane of MDCK cells, when co-expressed with α2-δ and any β subunit, at both time points examined, although a punctate intracellular component was also labelled. This punctate perinuclear α1C staining was also seen in COS-7 cells (Fig. 1), and has been observed in other expression systems (Chien et al. 1995; Grabner et al. 1998). It may represent a pool of L-type channels associated with intracellular membranes. The basolateral sorting of α1C is equivalent to somatodendritic targeting of all α1C-containing complexes in neurons. This observation is consistent with those of others, who have detected the involvement of L-type channels in gene transcription (Misra et al. 1994), a postsynaptic role in LTP (Mulkeen et al. 1987), immunohistochemical localization of α1C and α1D subunits to the somata and dendrites of neurons (Hell et al. 1993; Wyatt et al. 1997), and a postsynaptic role in the release of dynorphin from hippocampal granule cell bodies (Simmons et al. 1995). While functional studies cannot distinguish between α1C and α1D, electrophysiological examination reveals a Bay K 8644-sensitive L-type current component in many neuronal cell bodies (Pearson et al. 1995; Melliti et al. 1996). In contrast, recent studies have failed to show a role for L-type channels in presynaptic release of neurotransmitters (Regehr & Mintz, 1994), again consistent with the targeting demonstrated here.
Like the α1C channels, α1B appeared to reach steady-state localization at 24 h to a single membrane domain for co-expression with all β subunits, although in this case it was the apical membrane. When α1B was co-expressed with β1b or β4, and to a lesser extent β3, this apical destination was already observed at 6 h. In contrast, the immunolocalization of α1B co-expressed with α2-δ and β2a was substantial at the basolateral as well as the apical membrane at 6 h, and this became largely apical by 24 h. Further experiments at additional early time points, and with selective in vivo labelling of the α1 subunits expressed at the different membrane compartments, will be required to determine whether this apparent change in targeting represents transcytosis of the α1B/α2-δ/β2a combination from the basolateral to the apical membrane. Transcytosis by this route (basolateral to apical) has been observed for several proteins, including the polymeric immunoglobulin receptor (Mostov et al. 1992). It is believed that transcytosis from one membrane compartment to the other is signal mediated (Odorizzi et al. 1996).
The α1A subunit showed most variation in plasma membrane targeting with the different β subunits. It was always targeted to the apical domain when expressed with α2-δ and either β1b or β4. However, when co-expressed with α2-δ/β2a, the destination of the α1A-containing complex was the basolateral membrane. For the α1A/α2-δ/β3 combination there was no selective targeting to either membrane domain, and there was also substantial intracellular staining. This may be related to the low affinity of β3, compared with the other β subunits, for its binding site on the intracellular I-II loop of α1A (De Waard et al. 1995), and thus a failure of α1 trafficking. Whether the intracellular immunolocalization constitutes α1 subunits that have been endocytosed or an intracellular pool of channels that have not reached the plasma membrane remains to be determined.
It is as yet unknown how VDCCs obtain the differential distribution that is found in neurons. There have been many studies on the localization of VDCCs in the nervous system, either directly by immunohistochemistry or indirectly by electrophysiological or neurotransmitter release studies (Westenbroek et al. 1992; Luebke et al. 1993). Neurotransmitter release from hippocampal, cerebellar and other neurons shows dependence on Ca2+ entry through P/Q- and N-type channels (Luebke et al. 1993; Regehr & Mintz, 1994; Huston et al. 1995; Forsythe et al. 1998), implying a presynaptic localization for the α1A and α1B VDCC subunits. In contrast, in rat cerebellar Purkinje cells, the α1A subunit is localized to the soma and distal dendrites; additionally, high levels of β2a and β4 mRNA are detected in these cells (Westenbroek et al. 1995). Nevertheless, it is not known which of the accessory subunits are associated with the functional α1A-containing VDCC complexes in Purkinje cells (Mintz et al. 1992). The results obtained here in the MDCK cells would predict that if β2a and β4 are the predominant β subunits in Purkinje neurons, the somatodendritic α1A subunits would be associated with the β2a subunit, whereas the presynaptic α1A channels would be bound to β4. Supporting evidence for this hypothesis comes from electrophysiological data on the Purkinje cells, which are unusual in their somatic current being predominantly P-type, with its characteristically slow inactivation kinetics (Mintz et al. 1992). A unique property of rat β2a, in contrast to other β subunits, is its ability to attenuate current inactivation, suggesting that the somatic P-type currents in Purkinje cells represent α1A associated with a β2a subunit (De Waard & Campbell, 1995). Furthermore, in the calyx of Held, where the presynaptic current has been recorded directly, it was classified pharmacologically as P-type, but exhibited marked inactivation (Forsythe et al. 1998). This suggests that it is likely to be associated with a β subunit other than β2a.
Our results suggest that VDCC β subunits can exert an effect on the targeting of the VDCC complex, particularly for the α1A subunit. This provides a possible mechanism for the major cerebellar deficit found in the lethargic (Lh/Lh) mutant mouse, which has a point mutation that results in a truncated and non-functional β4 subunit (Burgess et al. 1997). Since in expression studies all β subunits are able to interact with the α1A subunit (De Waard & Campbell, 1995; Brice et al. 1997), until now it has been unclear how only the absence of β4 could produce such effects. However, if β4 is one of only two β subunits able to target α1A to presynaptic sites, and the other, β1b, has only low expression in the adult brain (Ludwig et al. 1997; McEnery et al. 1998), it is quite conceivable that the loss of β4 could produce an alteration in the targeting of α1A, and produce major defects in cerebellar development and function. It has also been shown that β1b levels are increased in lethargic mice, which may be an adaptive developmental response to the lack of β4 (McEnery et al. 1998).
In neurons, the N-type or α1B VDCCs can clearly be detected in the distal dendrites, as well as playing an important role in transmitter release at many synapses (Westenbroek et al. 1992; Luebke et al. 1993). In contrast, in MDCK cells we found predominantly apical targeting of α1B, with basolateral localization, which is reported as being equivalent to somatodendritic targeting, being only transient. However, it has recently been found that apical targeting in MDCK cells occurs for proteins that are sorted to both axons and dendrites in neurons, suggesting that apical targeting corresponds to a permissive, but not exclusive, pathway for axonal sorting in neurons (Jareb & Banker, 1998). This would be in agreement with our results for α1B.
We have used MDCK cells to examine the polarized distribution of VDCC complexes. Whilst this is not an ideal model for the study of polarized trafficking of neuronal proteins (Jareb & Banker, 1998), these cells do show certain important parallels (Dotti & Simons, 1990; Jareb & Banker, 1998), and have been widely studied for this purpose. Using this system, we have been able to show that for VDCCs it is a combination of the specific α1 and β subunit within a complex that determines to which membrane domain the channel is delivered, but the α1 subunit provides the basis for the ultimate destination. The results imply that functional P/Q-type VDCCs targeted to either somatodendritic or axonal regions may be associated predominantly with different β subunits in each domain, whereas α1C and α1B channels in combination with all β subunits possess sorting signals that determine their differential localization. The nature of these signals remains to be elucidated and is currently under investigation.
Acknowledgments
We thank the following for the generous gifts of cDNAs: T. Snutch (UBC, Vancouver, Canada) for α1A, α1C and β1b; H. Chin (NIH, USA) for α2-δ; Y. Mori (Seriken, Okazaki, Japan) for α1B; E. Perez-Reyes (Loyola, USA) for β2a, β3 and β4; Genetics Institute (CA, USA) for pMT-2; and C. Isacke (Imperial College London, UK) for wild-type and truncated CD44 and monoclonal antibody. MDCK cells were a kind gift from S. Moss (Department of Pharmacology, UCL, London). We would also like to thank Professor C. Hopkins (UCL, London) for the use of the MRC Laboratory of Cell and Molecular Biology confocal microscope, Dr S. Moss and Dr C. Connolly for valuable advice during the course of these studies, Drs N. S. Berrow and K. M. Page for subcloning the cDNA constructs and Ms M. Li for technical assistance. We gratefully acknowledge the financial support of The Wellcome Trust and the MRC. N. L. B. was an MRC PhD student. This work has benefited from the use of the Seqnet facility (Daresbury, UK).
References
- Berrow NS, Campbell V, Fitzgerald EG, Brickley K, Dolphin AC. Antisense depletion of β-subunits modulates the biophysical and pharmacological properties of neuronal calcium channels. The Journal of Physiology. 1995;482:481–491. doi: 10.1113/jphysiol.1995.sp020534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaumer L, Campbell KP, Catterall WA, Harpold MM, Hofmann F, Horne WA, Mori Y, Schwartz A, Snutch TP, Tanabe T, Tsien RW. The naming of voltage-gated calcium channels. Neuron. 1994;13:505–506. doi: 10.1016/0896-6273(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca2+- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- Bourinet E, Zamponi GW, Stea A, Soong TW, Lewis BA, Jones LP, Yue DT, Snutch TP. The α1E calcium channel exhibits permeation properties similar to low-voltage-activated calcium channels. Journal of Neuroscience. 1996;16:4983–4993. doi: 10.1523/JNEUROSCI.16-16-04983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brice NL, Berrow NS, Campbell V, Page KM, Brickley K, Tedder I, Dolphin AC. Importance of the different β subunits in the membrane expression of the α1A and α2 calcium channel subunits: studies using a depolarisation-sensitive α1A antibody. European Journal of Neuroscience. 1997;9:749–759. doi: 10.1111/j.1460-9568.1997.tb01423.x. [DOI] [PubMed] [Google Scholar]
- Brickley K, Campbell V, Berrow N, Leach R, Norman RI, Wray D, Dolphin AC, Baldwin S. Use of site-directed antibodies to probe the topography of the α2 subunit of voltage-gated Ca2+ channels. FEBS Letters. 1995;364:129–133. doi: 10.1016/0014-5793(95)00371-f. [DOI] [PubMed] [Google Scholar]
- Burgess DL, Jones JM, Meisler MH, Noebels JL. Mutation of the Ca2+ channel beta subunit gene Cchb4 is associated with ataxia and seizures in the lethargic (lh) mouse. Cell. 1997;88:385–392. doi: 10.1016/s0092-8674(00)81877-2. [DOI] [PubMed] [Google Scholar]
- Chien AJ, Zhao XL, Shirokov RE, Puri TS, Chang CF, Sun D, Rios E, Hosey MM. Roles of a membrane-localized β subunit in the formation and targeting of functional L-type Ca2+ channels. Journal of Biological Chemistry. 1995;270:30036–30044. doi: 10.1074/jbc.270.50.30036. 10.1074/jbc.270.50.30036. [DOI] [PubMed] [Google Scholar]
- Day NC, Shaw PJ, McCormack AL, Craig PJ, Smith W, Beattie R, Williams TL, Ellis SB, Ince PG, Harpold MM, Lodge D, Volsen SG. Distribution of α1A, α1B and α1E voltage-dependent calcium channel subunits in the human hippocampus and parahippocampal gyrus. Neuroscience. 1996;71:1013–1024. doi: 10.1016/0306-4522(95)00514-5. 10.1016/0306-4522(95)00514-5. [DOI] [PubMed] [Google Scholar]
- De Waard M, Campbell KP. Subunit regulation of the neuronal α1A Ca2+ channel expressed in Xenopus oocytes. The Journal of Physiology. 1995;485:619–634. doi: 10.1113/jphysiol.1995.sp020757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Waard M, Witcher DR, Campbell KP. Functional properties of the purified N-type Ca2+ channel from rabbit brain. Journal of Biological Chemistry. 1994;269:6716–6724. [PubMed] [Google Scholar]
- De Waard M, Witcher DR, Pragnell M, Liu H, Campbell KP. Properties of the α1-β anchoring site in voltage-dependent Ca2+ channels. Journal of Biological Chemistry. 1995;270:12056–12064. doi: 10.1074/jbc.270.20.12056. 10.1074/jbc.270.20.12056. [DOI] [PubMed] [Google Scholar]
- Dolphin AC. Mechanisms of modulation of voltage-dependent calcium channels by G proteins. The Journal of Physiology. 1998;506:3–11. doi: 10.1111/j.1469-7793.1998.003bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti CG, Simons K. Polarized sorting of viral glycoproteins to the axon and dendrites of hippocampal neurons in culture. Cell. 1990;62:63–72. doi: 10.1016/0092-8674(90)90240-f. [DOI] [PubMed] [Google Scholar]
- Forsythe ID, Tsujimoto T, Barnes-Davies M, Cuttle MF, Takahashi T. Inactivation of presynaptic calcium current contributes to synaptic depression at a fast central synapse. Neuron. 1998;20:797–807. doi: 10.1016/s0896-6273(00)81017-x. 10.1016/S0896-6273(00)81017-X. [DOI] [PubMed] [Google Scholar]
- Gillard SE, Volsen SG, Smith W, Beattie RE, Bleakman D, Lodge D. Identification of pore-forming subunit of P-type calcium channels: An antisense study on rat cerebellar Purkinje cells in culture. Neuropharmacology. 1997;36:405–409. doi: 10.1016/s0028-3908(97)00046-4. 10.1016/S0028-3908(97)00046-4. [DOI] [PubMed] [Google Scholar]
- Grabner M, Dirksen RT, Beam KG. Tagging with green fluorescent protein reveals a distinct subcellular distribution of L type and non L type Ca2+ channels expressed in dysgenic myotubes. Proceedings of the National Academy of Sciences of the USA. 1998;95:1903–1908. doi: 10.1073/pnas.95.4.1903. 10.1073/pnas.95.4.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller C, Alper SL. Assessment of heterologous membrane protein polarity in transiently transfected MDCK cells. Cytotechnology. 1995;17:71–82. doi: 10.1007/BF00749394. [DOI] [PubMed] [Google Scholar]
- Hell JW, Westenbroek RE, Warner C, Ahlijanian MK, Prystay W, Gilbert MM, Snutch TP, Catterall WA. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel α1 subunits. Journal of Cell Biology. 1993;123:949–962. doi: 10.1083/jcb.123.4.949. 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston E, Cullen GP, Burley JR, Dolphin AC. The involvement of multiple calcium channel sub-types in glutamate release from cerebellar granule cells and its modulation by GABAB receptor activation. Neuroscience. 1995;68:465–478. doi: 10.1016/0306-4522(95)00172-f. 10.1016/0306-4522(95)00172-F. [DOI] [PubMed] [Google Scholar]
- Jareb M, Banker G. The polarised sorting of membrane proteins expressed in hippocampal neurons using viral vectors. Neuron. 1998;20:855–867. doi: 10.1016/s0896-6273(00)80468-7. 10.1016/S0896-6273(00)80468-7. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Flockerzi V, Hofmann F. Regional expression and cellular localization of the α1 and β subunit of high voltage-activated calcium channels in rat brain. Journal of Neuroscience. 1997;17:1339–1349. doi: 10.1523/JNEUROSCI.17-04-01339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebke JI, Dunlap K, Turner TJ. Multiple calcium channel types control glutamatergic synaptic transmission in the hippocampus. Neuron. 1993;11:895–902. doi: 10.1016/0896-6273(93)90119-c. 10.1016/0896-6273(93)90119-C. [DOI] [PubMed] [Google Scholar]
- McEnery MW, Copeland TD, Vance CL. Altered expression and assembly of N-type calcium channel α1B and β subunits in epileptic lethargic (lh/lh) mouse. Journal of Biological Chemistry. 1998;273:21435–21438. doi: 10.1074/jbc.273.34.21435. 10.1074/jbc.273.34.21435. [DOI] [PubMed] [Google Scholar]
- Melliti K, Bournaud R, Bastide B, Shimahara T. Nifedipine-sensitive intramembrane charge movement in Purkinje cells from mouse cerebellum. The Journal of Physiology. 1996;490:363–372. doi: 10.1113/jphysiol.1996.sp021150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz IM, Adams ME, Bean BP. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992;9:85–95. doi: 10.1016/0896-6273(92)90223-z. 10.1016/0896-6273(92)90223-Z. [DOI] [PubMed] [Google Scholar]
- Misra RP, Bonni A, Miranti CK, Rivera VM, Sheng M, Greenberg ME. L-type voltage-sensitive calcium channel activation stimulates gene expression by a serum response factor-dependent pathway. Journal of Biological Chemistry. 1994;269:25483–25493. [PubMed] [Google Scholar]
- Mostov K, Apodaca G, Aroeti B, Okamoto C. Plasma membrane protein sorting in polarized epithelial cells. Journal of Cell Biology. 1992;116:577–583. doi: 10.1083/jcb.116.3.577. 10.1083/jcb.116.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkeen D, Anwyl R, Rowan MJ. Enhancement of long-term potentiation by the calcium channel agonist Bayer K8644 in CA1 of the rat hippocampus in vitro. Neuroscience Letters. 1987;80:351–355. doi: 10.1016/0304-3940(87)90481-2. 10.1016/0304-3940(87)90481-2. [DOI] [PubMed] [Google Scholar]
- Muth TR, Gottardi CJ, Roush DL, Caplan MJ. A basolateral sorting signal is encoded in the α subunit of the Na-K ATPase. Neuroscience Letters. 1998;80:351–355. doi: 10.1152/ajpcell.1998.274.3.C688. [DOI] [PubMed] [Google Scholar]
- Neame SJ, Isacke CM. The cytoplasmic tail of CD44 is required for basolateral localization in epithelial MDCK cells but does not mediate association with the detergent-insoluble cytoskeleton of fibroblasts. Journal of Cell Biology. 1993;121:1299–1310. doi: 10.1083/jcb.121.6.1299. 10.1083/jcb.121.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi G, Pearse A, Domingo D, Trowbridge IS, Hopkins CR. Apical and basolateral endosomes of MDCK cells are interconnected and contain a polarized sorting mechanism. Journal of Cell Biology. 1996;135:139–152. doi: 10.1083/jcb.135.1.139. 10.1083/jcb.135.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson HA, Sutton KG, Scott RH, Dolphin AC. Characterization of Ca2+ channel currents in cultured rat cerebellar granule neurones. The Journal of Physiology. 1995;482:493–509. doi: 10.1113/jphysiol.1995.sp020535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes E, Cribbs LL, Daud A, Lacerda AE, Barclay J, Williamson MP, Fox M, Rees M, Lee J. Molecular characterisation of a neuronal low-voltage-activated T type calcium channel. Nature. 1998;391:896–900. doi: 10.1038/36110. 10.1038/36110. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E, Schneider T. Calcium channels: structure, function, and classification. Drug Development Research. 1994;33:295–318. [Google Scholar]
- Pragnell M, De Waard M, Mori Y, Tanabe T, Snutch TP, Campbell KP. Calcium channel β-subunit binds to a conserved motif in the I-II cytoplasmic linker of the α1-subunit. Nature. 1994;368:67–70. doi: 10.1038/368067a0. 10.1038/368067a0. [DOI] [PubMed] [Google Scholar]
- Randall A, Tsien RW. Pharmacological dissection of multiple types of Ca2+ channel currents in rat cerebellar granule neurons. Journal of Neuroscience. 1995;15:2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr WG, Mintz IM. Participation of multiple calcium channel types in transmission at single climbing fiber to Purkinje cell synapses. Neuron. 1994;12:605–613. doi: 10.1016/0896-6273(94)90216-x. 10.1016/0896-6273(94)90216-X. [DOI] [PubMed] [Google Scholar]
- Schroeter S, Levey AI, Blakely RD. Polarised expression of the antidepressant-sensitive serotonin transporter in epinephrine-synthesising chromaffin cells of the rat adrenal gland. Molecular and Cellular Neuroscience. 1997;9:170–184. doi: 10.1006/mcne.1997.0619. 10.1006/mcne.1997.0619. [DOI] [PubMed] [Google Scholar]
- Simmons ML, Terman GW, Gibbs SM, Chavkin C. L-type calcium channels mediate dynorphin neuropeptide release from dendrites but not axons of hippocampal granule cells. Neuron. 1995;14:1265–1272. doi: 10.1016/0896-6273(95)90273-2. 10.1016/0896-6273(95)90273-2. [DOI] [PubMed] [Google Scholar]
- Singer D, Biel M, Lotan I, Flockerzi V, Hofmann F, Dascal N. The roles of the subunits in the function of the calcium channel. Science. 1991;253:1553–1557. doi: 10.1126/science.1716787. [DOI] [PubMed] [Google Scholar]
- Stephens GJ, Brice NL, Berrow NS, Dolphin AC. Facilitation of rabbit α1B calcium channels: involvement of endogenous Gβγ subunits. The Journal of Physiology. 1998;509:15–27. doi: 10.1111/j.1469-7793.1998.015bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick AG, Janicot M, Cheneval-Kastelic T, McLenithan JC, Lane DM. Promoter-cDNA-directed heterologous protein expression in Xenopus laevis oocytes. Proceedings of the National Academy of Sciences of the USA. 1992;89:1812–1816. doi: 10.1073/pnas.89.5.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner TJ, Adams ME, Dunlap K. Multiple Ca2+ channel types coexist to regulate synaptosomal neurotransmitter release. Proceedings of the National Academy of Sciences of the USA. 1993;90:9518–9522. doi: 10.1073/pnas.90.20.9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbroek RE, Hell JW, Warner C, Dubel SJ, Snutch TP, Catterall WA. Biochemical properties and subcellular distribution of an N-type calcium channel α1 subunit. Neuron. 1992;9:1099–1115. doi: 10.1016/0896-6273(92)90069-p. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Sakurai T, Elliott EM, Hell JW, Starr TVB, Snutch TP, Catterall WA. Immunochemical identification and subcellular distribution of the α1A subunits of brain calcium channels. Journal of Neuroscience. 1995;15:6403–6418. doi: 10.1523/JNEUROSCI.15-10-06403.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DB, Randall A, Tsien RW. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994;264:107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- Wyatt CN, Campbell V, Brodbeck P, Brice NL, Page KM, Berrow NS, Brickley K, Terracciano R, Naqvi RV, MacLeod KT, Dolphin AC. Voltage-dependent binding and calcium current inhibition by an anti-α1D subunit antibody in rat dorsal root ganglion neurones and guinea pig myocytes. The Journal of Physiology. 1997;502:307–319. doi: 10.1111/j.1469-7793.1997.307bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


