Abstract
The objective of the study was to investigate the amplitude and modulation of the human soleus Hoffmann (H) reflex during walking and during running at different speeds.
EMGs were recorded with surface electrodes from the soleus, the medial and lateral head of the gastrocnemius, the vastus lateralis and the anterior tibial muscles. The EMGs and the soleus H reflex were recorded while walking on a treadmill at 4.5 km h−1 and during running at 8, 12 and 15 km h−1.
The amplitudes of the M wave and the H reflex were normalized to the amplitude of a maximal M wave elicited by a supramaximal stimulus just after the H reflex to compensate for movements of the recording and stimulus electrodes relative to the nerve and muscle fibres. The stimulus intensity was set to produce M waves that had an amplitude near to 25% of the maximal M wave measured during the movements. As an alternative, the method of averaging of sweeps in sixteen intervals of the gait cycle was applied to the data. In this case the amplitude of the H reflex was expressed relative to the maximal M wave measured whilst in the standing position.
The amplitude of the H reflex was modulated during the gait cycle at all speeds. During the stance phase the reflex was facilitated and during the swing and flight phases it was suppressed. The size of the maximal M wave varied during the gait cycle and this variation was consistent for each subject although different among subjects.
The peak amplitude of the H reflex increased significantly (P = 0.04) from walking at 4.5 km h−1 to running at 12 and 15 km h−1 when using the method of correcting for variations of the maximal M wave during the gait cycle. The sweep averaging method showed a small but non-significant decrease (P = 0.3) from walking to running at 8 km h−1 and a small decrease with running speed (P = 0.3). The amplitude of the EMG increased from walking to running and with running speed.
The relatively large H reflex recorded during the stance phase in running indicates that the stretch reflex may influence the muscle mechanics during the stance phase by contributing to the motor output and enhancing muscle stiffness.
The modulation and amplitude of the human soleus H reflex during walking and running have been investigated by Capaday & Stein (1987). They reported that the transition from walking at 4.5 km h−1 to running at 8 km h−1 caused a statistically significant decrease in the peak amplitude of the human soleus H reflex. In an ensuing study Edamura et al. (1991) measured the soleus H reflex during walking at speeds from 2.0 to 7.5 km h−1 and during running from 5.0 to 9.0 km h−1. They also reported that the peak amplitude of the soleus H reflex was always lower during running than during walking. In the latter study the subjects were tested during both walking and running at identical speeds. The H reflex measuring technique bypasses the muscle spindle and measures the central component of the monosynaptic part of the stretch reflex, i.e. the efficacy of the transmission from I a afferents to the α-motoneurones in the spinal cord. We have previously reported that the amplitude of the maximal M wave (Mmax) in soleus muscle varied considerably but systematically during walking (Simonsen et al. 1995). To expand on our findings we proceeded to measure the maximal M wave amplitude and the H reflex amplitude during various motor tasks, including running. These experiments showed that the soleus H reflex was not lowered during running and we therefore decided to investigate this observation systematically. Since the highest running speed previously investigated was 9.0 km h−1, we decided to compare walking at 4.5 km h−1 with running at 8.0, 12.0 and 15.0 km h−1. Preliminary results of the present study have been published earlier in abstract form (Simonsen & Dyhre-Poulsen, 1995).
METHODS
Subjects
Seven male subjects gave voluntary written consent to participate in the experiments after being informed about the experimental conditions. The study was approved by the local ethics committee. The subjects were well trained but not specialized in running events. Their mean age was 28 years (range, 24–35 years), mean height was 1.85 m (range, 1.72–1.95 m) and mean body weight was 76 kg (range, 65–89 kg).
Experimental procedure
When the subjects arrived EMG electrodes were mounted and the site of nerve stimulation located. The maximal M wave and the H reflex amplitude were measured at rest in the standing position. EMG activity was measured during walking at 4.5 km h−1 and during running at 8.0, 12.0 and 15.0 km h−1 without nerve stimulation. Finally, the soleus H reflex was measured during the same walking and running conditions. The subjects were walking and running on a motor-driven treadmill (HS-1200; Technogym) and were allowed to rest freely during the recording sessions to avoid fatigue. The sessions were in random order and a total experimental session lasted about 4 h. All measurements were performed on the same day on the right leg.
Electromyography
Surface electrodes (Medicotest Q-10-A prefilled ECG electrodes) were mounted 2 cm apart (bipolar) over five muscles. The electrodes were placed as follows: for the soleus, about 15 cm above the calcaneus over the gastrocnemius aponeurosis but below the muscle fibres of the gastrocnemius heads; for the anterior tibial muscle about 12 cm below the caput fibulae; for the lateral and medial head of the gastrocnemius muscle, about 5 cm below the caput fibulae; and for the vastus lateralis muscle about 16 cm above the patella. The electrodes were connected to small custom-built preamplifiers (input impedance, 80 MΩ) taped to the skin. The EMG signals were then led through long shielded wires to custom-built amplifiers with a frequency response between 20 Hz and 10 kHz. The set-up permitted the subjects to move freely on the treadmill and the preamplifiers reduced movement artefacts effectively.
Goniometry and microswitches
The ankle joint position was measured with an electrogoniometer (M-series twin axis; Penny & Giles) placed on the lateral side of the ankle joint. Foot-ground contact was detected by microswitches placed under the heel and forefoot.
The H reflex
The soleus H reflex was elicited by stimulation of the tibial nerve using an AgCl cathode in the popliteal fossa (Medicotest Q-10-A) and a 40 mm diameter anode placed over the patella. The optimum site of nerve stimulation was first located by a hand-held electrode and it was a criterion that the soleus I a afferents could be stimulated selectively at low stimulus intensities. The stimulus was a 1 ms square pulse delivered by a custom-built constant current stimulator. The maximal M wave amplitude (Mmax) and the maximal H reflex amplitude were measured during rest with the subjects in a standing position. The stimulus intensity necessary to elicit Mmax was determined and then increased by a factor of 2 prior to measurement of Mmax. This doubled stimulus intensity was also used to elicit Mmax during locomotion. The stimuli were given every 2 s during locomotion, which was slightly out of phase with the gait cycle. In this way the stimuli were dispersed randomly over the gait cycle. An integrator was reset by the microswitch placed under the subject's heel and a ramp function was generated from 0 to 2 V over a 2 s period. The amplitude of the ramp function at the time of stimulation was used to compute the time interval that had elapsed since the preceding heel strike. A second, but supramaximal, stimulus elicited a maximal M wave 60 ms after the first stimulus. The Mmax therefore appeared just after the H reflex elicited by the first stimulus (Fig. 1). The experiments were controlled by a ‘self-taught’ computer program written in ASYST and running on a personal computer (Dyhre-Poulsen et al. 1993; Voigt et al. 1998). The gait cycle was divided into twenty time slices for which the computer ‘taught’ itself the appropriate stimulus intensity, which varied during the gait cycle. The peak-to-peak amplitude of the M wave was assumed to reflect the effective stimulus strength and the computer calculated the stimulus intensity necessary to evoke an M wave that was 25 % of the actual Mmax measured in the same sweep. Thus the computer program would go through the following procedure every 2 s: (1) determine the actual time slice (out of 20) in the gait cycle by measuring the actual value of the ramp signal reset by the preceding heel strike; (2) look up the last notified stimulus strength for the actual time slice; (3) send the stimulus information to the stimulator and trigger the stimulator; (4) sample the soleus EMG signal at 20 kHz for 120 ms; (5) measure the peak-to-peak amplitudes of the M wave, the H reflex and Mmax; (6) determine whether to accept or reject the sweep (the target amplitude for the M wave was 25 ± 10 % of Mmax measured in the same sweep); (7) store the sweep and the measured results on disk; and (8) adjust and update the stimulus strength for the actual time slice.
Figure 1. H reflex modulation of one subject during walking at 4.5 km h−1.
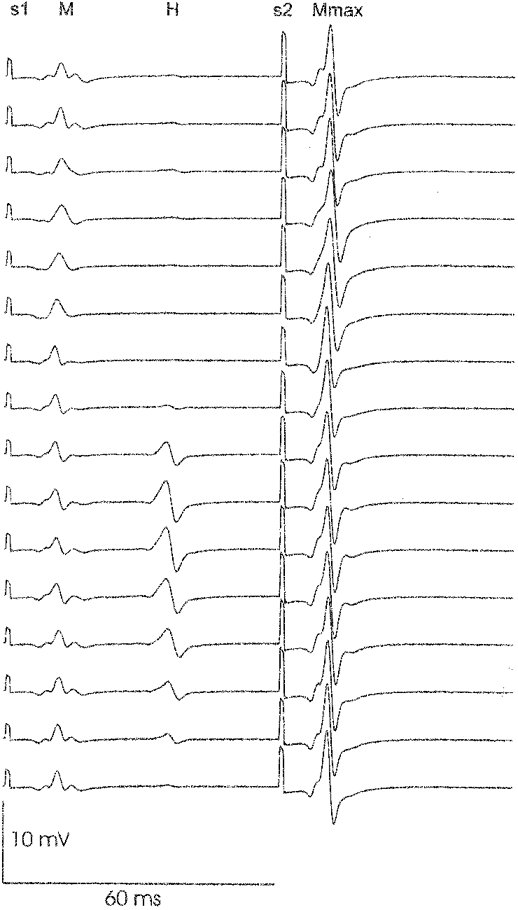
The gait cycle was divided into 16 time slices starting at the bottom of the figure corresponding to heel strike. In each slice 5 events are visible, from left to right: s1, the first stimulus artefact; M, the M wave; H, the H reflex; s2, the second stimulus artefact; and Mmax, the maximal M wave. The duration of each sweep was 120 ms. The H reflex appeared after approximately 35 ms and the second stimulus (s2) was elicited 60 ms after the first stimulus (s1). The signals in each time slice represent an average of multiple sweeps obtained by the method described earlier by Capaday & Stein (1987). It can be seen that the H reflex was facilitated during the stance phase, but suppressed during the swing phase.
During the first 5–10 min of an experimental session the program showed a low score of acceptable stimulations, but after having ‘learned’ how to stimulate a specific subject, almost every stimulation resulted in acceptable M waves.
The ‘learning algorithm’ of the program was quite simple. Initially the stimulus intensity in all twenty time slices was set to 25 % of Mmax measured at rest. At each stimulation the computer program measured the amplitude of Mmax and calculated the target 25 % M wave. If the measured M wave differed from the target then the program would calculate an estimated new stimulus intensity based on the reference excitability curve of the actual subject and store the new value to be used next time. In practice it was found convenient to add or subtract only 50 % of the estimated difference in stimulus intensity. In this way the program would gradually turn up or turn down the stimulus intensity in each time slice during the experiment.
The purpose of using the ‘self-teaching’ program was (i) to adjust the stimulus strength to fluctuations of the maximal M wave during the gait cycle and (ii) to avoid subject fatigue by reducing the total experimental time.
Signal treatment and calculations
During off-line analysis the gait cycle was divided into sixteen time slices (to match previous studies) and the peak-to-peak amplitudes of the H reflex, expressed as a percentage of the actual Mmax in every sweep, were averaged for each time slice.
The H reflex amplitude was also calculated as the percentage of Mmax during standing. During this procedure the selection process was repeated off-line to accept only M waves 25 ± 10 % of Mmax during standing. Then the accepted sweeps were averaged for each of the sixteen time slices by the method described by Capaday & Stein (1986) (Fig. 1). The background EMG was thereby removed by the averaging procedure. Finally the peak-to-peak amplitude of the H reflex was measured for each slice and expressed as a percentage of Mmax during standing at rest to compare the two different methods.
The EMG signals recorded without stimulation were sampled at 1000 Hz, high-pass filtered at 20 Hz and low-pass filtered at 500 Hz (fourth order Butterworth digital filters with no phase lag). After full wave rectification linear envelopes were obtained with a 15 Hz low-pass filter. Then twelve sweeps, corresponding to twelve step cycles, were averaged. The goniometer signal was low-pass filtered at 50 Hz and averaged over twelve step cycles. The onset and offset of the EMG signals were determined visually on a computer monitor with an accuracy of 1 ms. Peak amplitude, mean amplitude and integrated EMG (IEMG) were calculated for the activity period of each muscle. IEMG was defined as the area under the linear envelope expressed in microvolt seconds.
Statistical analysis
Differences in the selected parameters were tested between walking and the three running speeds by the Friedman test for several related samples (two-tailed) (Conover, 1980). The level of significance was set to 5 % in all cases. Only one P value for each parameter is returned by this test; however, when P < 0.05 was obtained, differences between the four gait conditions were examined by multiple comparisons (Conover, 1980).
RESULTS
The mean amplitude of the maximal M wave during standing was 9.1 mV (range, 7.2–10.7 mV) for the seven subjects. The maximal H reflex, also measured during standing, was on average 5.2 mV (range, 3.1–8.2 mV) corresponding to 56 % of Mmax. The target M wave amplitude was set to 25 % of Mmax measured during locomotion. However, a range of ±10 % was considered acceptable. Off-line analysis of the data from one subject showed no change in the mean H reflex amplitude when the acceptance criteria were narrowed to 25 ± 5 %. Table 1 displays the amplitudes of the average M waves elicited for each subject and the standard deviations. On average, the M wave amplitude seemed to decrease from 2.1 mV during walking to 1.91 mV during running at 15 km h−1. However, no statistically significant differences regarding the M wave amplitudes were observed between the four gait conditions.
Table 1.
M wave and H reflex amplitudes
| Subject | Mean Mmax(mV) | s.d.Mmax(mV) | Mean M(mV) | s.d.M(mV) | Peak H reflex(%Mmax) | Peak H reflex(C&S)(%Mmax) | |
|---|---|---|---|---|---|---|---|
| A. 4.5 km h−1 | |||||||
| BL | 9.07 | 0.46 | 2.14 | 0.53 | 28.6 | 33.2 | |
| LK | 7.30 | 0.40 | 1.71 | 0.40 | 47.1 | 68.1 | |
| JM | 5.75 | 1.80 | 1.42 | 0.55 | 42.6 | 38.2 | |
| NI | 8.98 | 0.63 | 2.32 | 0.50 | 43.2 | 53.1 | |
| SO | 11.45 | 0.75 | 2.68 | 0.57 | 47.7 | 52.3 | |
| MO | 10.25 | 1.06 | 2.40 | 0.50 | 41.0 | 48.6 | |
| HE | 8.21 | 0.77 | 2.00 | 0.52 | 38.0 | 42.7 | |
| Mean | 8.72 | 0.84 | 2.10 | 0.51 | 41.2 | 48.0 | |
| B. 8.0 km h−1 | |||||||
| BL | 8.46 | 0.49 | 1.96 | 0.48 | 33.2 | 31.7 | |
| LK | 6.90 | 0.67 | 1.61 | 0.41 | 50.5 | 68.3 | |
| JM | 6.64 | 2.89 | 0.96 | 0.84 | 54.9 | 38.2 | |
| NI | 8.41 | 0.97 | 2.11 | 0.53 | 52.5 | 50.8 | |
| SO | 10.98 | 1.02 | 2.65 | 0.58 | 47.7 | 47.3 | |
| MO | 11.02 | 1.62 | 2.67 | 0.65 | 50.0 | 53.2 | |
| HE | 8.85 | 1.62 | 2.09 | 0.60 | 36.4 | 38.9 | |
| Mean | 8.75 | 1.33 | 2.01 | 0.58 | 46.5 | 44.2 | |
| C. 12.0 km h−1 | |||||||
| BL | 7.99 | 0.52 | 2.00 | 0.49 | 37.8 | 31.9 | |
| LK | 7.07 | 0.61 | 1.69 | 0.46 | 52.9 | 58.4 | |
| JM | 6.62 | 3.10 | 1.46 | 0.71 | 44.4 | 20.7 | |
| NI | 7.39 | 1.17 | 1.98 | 0.46 | 60.9 | 46.5 | |
| SO | 9.05 | 0.94 | 2.22 | 0.54 | 41.2 | 36.0 | |
| MO | 11.35 | 1.72 | 2.67 | 0.76 | 55.7 | 62.6 | |
| HE | 8.28 | 1.96 | 2.00 | 0.63 | 43.7 | 41.8 | |
| Mean | 8.25 | 1.43 | 2.00 | 0.58 | 48.1 | 43.7 | |
| D. 15.0 km h−1 | |||||||
| BL | 7.95 | 0.62 | 1.84 | 0.42 | 36.8 | 37.0 | |
| LK | 6.73 | 0.64 | 1.51 | 0.64 | 52.8 | 57.9 | |
| JM | 6.80 | 3.68 | 1.69 | 1.12 | 61.0 | 11.3 | |
| NI | 7.39 | 1.20 | 1.89 | 0.48 | 54.1 | 46.5 | |
| SO | 7.63 | 1.37 | 1.81 | 0.56 | 41.6 | 23.5 | |
| MO | 11.04 | 1.69 | 2.79 | 0.79 | 47.9 | 44.7 | |
| HE | 7.98 | 2.15 | 1.85 | 0.66 | 49.5 | 42.8 | |
| Mean | 7.93 | 1.62 | 1.91 | 0.67 | 49.1 | 37.7 | |
Columns are, from left to right: the mean maximal M wave amplitude(Mmax)with standard deviation(S.D.) measured during locomotion, the mean M wave amplitude(M) with S.D. used as the conditioning stimulus during locomotion, the peak H reflex measured during locomotion expressed as the percentage of Mmax also measured during locomotion, and the peak H reflex measured during locomotion but expressed as the percentage of Mmax during standing(peak H reflex(C&S)). A, walking at 4.5 km h−1. B, running at 8.0 km h−1. C, running at 12.0 km h−1. D, running at 15.0 km h−1.
The modulation of the soleus H reflex during walking was similar to that in earlier reports (Capaday & Stein, 1986, 1987; Crenna & Frigo, 1987; Simonsen et al. 1995). The amplitude of the reflex increased during the stance phase and the reflex was suppressed during the swing phase (Fig 2 and Fig 3). The modulation of the reflex during running was also in accordance with previous reports (Capaday & Stein, 1987; Edamura et al. 1991) as the reflex was facilitated during the stance phase and suppressed during the swing and flight phases (Fig 2 and Fig 3).
Figure 2. Recordings from the same subject illustrated for 4 gait situations.
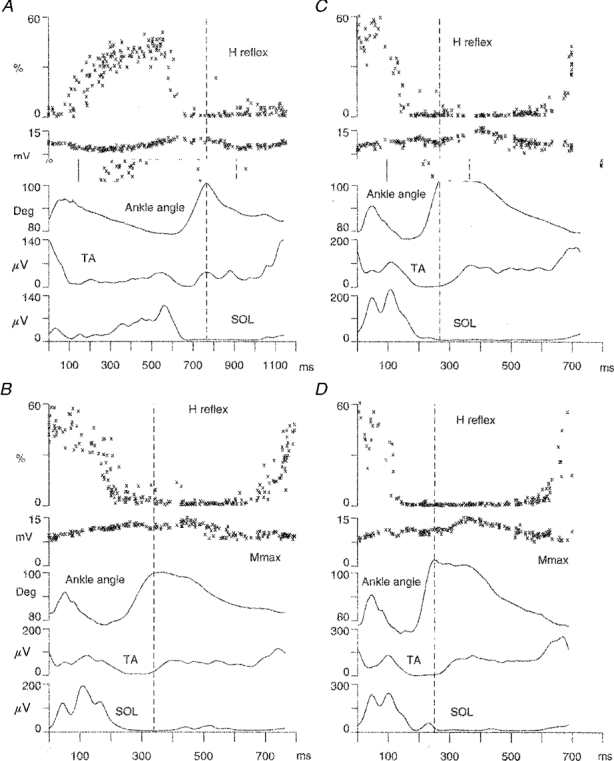
The gait situations were: walking at 4.5 km h−1 (A); running at 8 km h−1 (B); running at 12 km h−1 (C); and running at 15 km h−1 (D). For each of the 4 figures the following recordings are shown from bottom to top: the soleus EMG (SOL) and the tibialis anterior EMG (TA) (linear envelopes averaged from 12 sweeps); the ankle angle; the amplitude of the maximal M wave (Mmax) of the soleus muscle; and finally the amplitude of the soleus H reflex expressed as percentage Mmax and corrected for variations of Mmax during the gait cycle. For the H reflex and Mmax, each cross represents one stimulation given every 2 s. The stance phase starts on the left at 0 ms and ends at the vertical dashed line. Note that the scales for the soleus and anterior tibial EMG differ from A to D.
Figure 3. H reflex modulations.
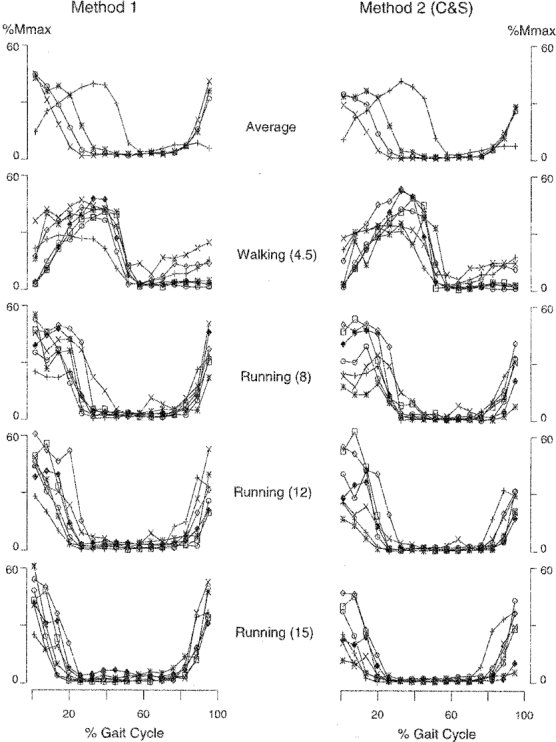
In the top tracings, the modulation of the soleus H reflex is shown as the average from 7 subjects during walking at 4.5 km h−1 (+), running at 8 km h−1 ( ), running at 12 km h−1 (^) and running at 15 km h−1 (×). In the left column the results were obtained by the method of the present study, in which the maximal M wave (Mmax) was measured in every sweep (Method 1). In the right column the same data were obtained by the method described by Capaday & Stein (1987) (Method 2 (C & S)). For each of the two methods the 4 lower tracings show individual data for each subject and for each velocity investigated. Each of the 7 subjects is represented by a specific symbol. All data are expressed as a percentage of Mmax. For Method 1, Mmax was measured in every sweep while for Method 2, the Mmax measured in the standing position was used for normalization. For both methods, only M waves between 15 and 35 % of Mmax were accepted. The gait cycle was divided into 16 time slices and normalized with heel strike at 0 and 100 %.
), running at 12 km h−1 (^) and running at 15 km h−1 (×). In the left column the results were obtained by the method of the present study, in which the maximal M wave (Mmax) was measured in every sweep (Method 1). In the right column the same data were obtained by the method described by Capaday & Stein (1987) (Method 2 (C & S)). For each of the two methods the 4 lower tracings show individual data for each subject and for each velocity investigated. Each of the 7 subjects is represented by a specific symbol. All data are expressed as a percentage of Mmax. For Method 1, Mmax was measured in every sweep while for Method 2, the Mmax measured in the standing position was used for normalization. For both methods, only M waves between 15 and 35 % of Mmax were accepted. The gait cycle was divided into 16 time slices and normalized with heel strike at 0 and 100 %.
Using the sweep averaging method of Capaday & Stein (1987) and relating the peak H reflex amplitude to Mmax during standing showed that the reflex amplitude decreased from 48.0 % of Mmax during walking to 44.2 % during running at 8 km h−1. In contrast to the results of Capaday & Stein (1987) this decrease was small and statistically non-significant (Table 1 and Fig. 4). Using the method of correcting for fluctuations in Mmax showed that the peak H reflex amplitude increased from 41.2 % of Mmax during walking to 46.5 % during running at 8 km h−1 (Table 1 and Fig. 4). However, this difference was also not statistically significant. According to the statistical evaluation, the peak H reflex amplitude was therefore unchanged between walking at 4.5 km h−1 and running at 8 km h−1. The results from the faster running speeds followed the same pattern. Using the method of the present study showed a further increase in reflex amplitude and the statistical analysis showed significantly higher amplitudes during running at 12 and 15 km h−1 compared with walking (Table 1 and Fig. 4). The H reflex amplitude was 6.9 % higher during running at 12 km h−1 than during walking (P = 0.04), and at 15 km h−1 it was 7.9 % higher than during walking (P = 0.04) (the percentages refer to %Mmax and not percentage increase). No significant differences were observed between running conditions. When the method of Capaday & Stein (1987) was used, it showed that the reflex amplitude decreased with running speed, but no statistically significant differences were observed between the four conditions (P = 0.3) (Table 1 and Fig. 4).
Figure 4. Peak amplitude of the soleus H reflex during walking (4.5 km h−1) and during 3 running speeds.
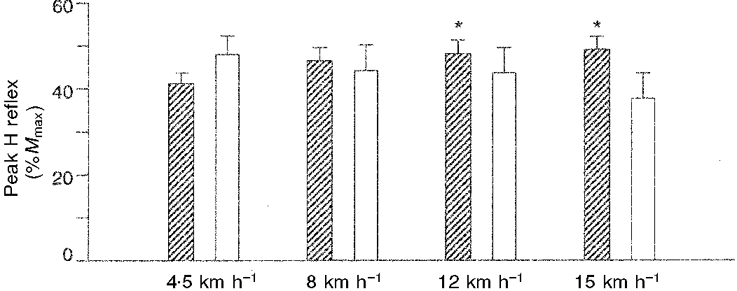
Hatched columns represent values obtained by the method of the present study where the maximal M wave (Mmax) was measured in every sweep. Open columns represent values obtained by the method of Capaday & Stein (1987) where variations of Mmax during the gait cycle are not accounted for. The bars are mean values of 7 subjects and the error bars are +s.e.m.* Statistically significantly different from walking at 4.5 km h−1 (P < 0.05).
In general, the mean and peak amplitudes of the EMG activity and the integrated EMG increased two- to three-fold from walking at 4.5 km h−1 to running at 8 km h−1 and further to running at 12 and 15 km h−1. Only eleven out of seventy-five comparisons resulted in non-significant differences (Table 2).
Table 2.
The mean, peak and IEMG for the soleus, medial gastrocnemius, vastus lateralis and anterior tibial muscles at 4.5 km h−1(walking) and for 3 running speeds
| 4.5 km h−1 | 8.0 km h−1 | 12.0 km h−1 | 15.0 km h−1 | |
|---|---|---|---|---|
| SOL mean | 72(10) | 134(21) | 151(21) | 165(20) |
| SOL peak | 160(16) | 346(54)n.s.12 | 385(67) | 401(50) |
| SOL IEMG | 107(18) | 383(85) | 487(93) | 556(93) |
| GM mean | 73(7) | 183(22) | 224(25)n.s.15 | 233(20) |
| GM peak | 192(13) | 452(65)n.s.12 | 516(56) | 588(48) |
| GM IEMG | 124(17) | 518(89) | 745(89)n.s.15 | 767(72) |
| GL mean | 38(7) | 131(16) | 164(19) | 203(25) |
| GL peak | 130(24) | 353(52) | 447(55) | 498(52) |
| GL IEMG | 62(15) | 364(66) | 514(76) | 719(100) |
| VL mean | 25(6) | 101(12) | 128(15) | 149(18) |
| VL peak | 64(17) | 301(44) | 382(57)n.s.15 | 423(42) |
| VL IEMG | 55(13) | 279(41) | 394(52) | 510(79) |
| TA mean | 50(6) | 81(7)n.s.12 | 100(10) | 127(14) |
| TA peak | 164(18) | 237(21)n.s.12 | 332(39) | 424(53) |
| TA IEMG | 105(44)n.s.8,12 | 126(19)n.s.12 | 163(21)n.s.15 | 217(32) |
SOL, soleus muscle; GM, medial gastrocnemius muscle; GL, lateral gastrocnemius muscle; VL, vastus lateralis muscle; TA, anterior tibial muscle; IEMG, integrated EMG. All values are the means of 7 subjects with the S.E.M. in parentheses. All comparisons across speeds are significant unless indicated by n.s. The number after n.s. denotes the velocities that are insignificant (P > 0.05).
In the present study a supramaximal stimulus was applied in every sweep to measure variations in Mmax. To test any possible influence of the supramaximal stimuli on the size of the H reflex, reference excitability curves with and without the supramaximal stimuli were recorded in two subjects during resting conditions (Fig. 5). It was clear from these measurements that the size of the H reflex was unaffected by the supramaximal stimuli (double stimulus condition). Theoretically, it is possible that the supramaximal stimulus given in every sweep could depress the size of the H reflex. However, we measured an increased H reflex during running, and the peak H reflex during walking was seemingly not lower than reported in previous studies. A direct comparison of amplitudes would require identical electrodes and inter-electrode distance. In the present study the inter-electrode distance was 2 cm, but this information is lacking in the previous reports (Capaday & Stein, 1987; Edamura et al. 1991).
Figure 5. Recordings of the maximal M wave.
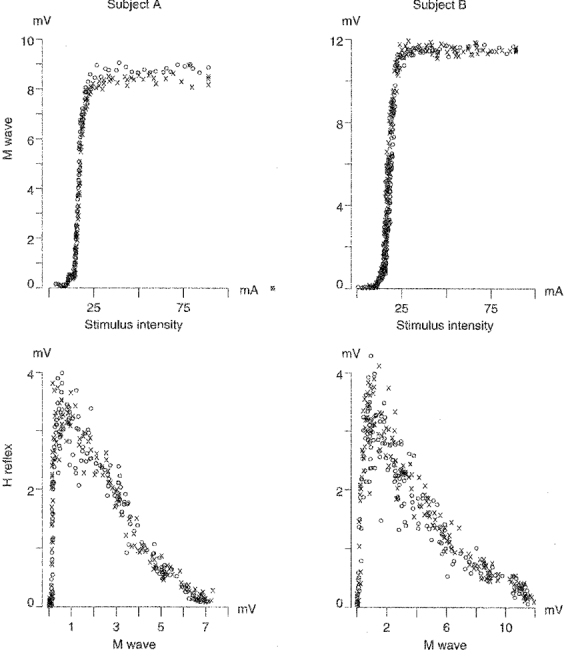
The upper panels show the relation between stimulus intensity and the recorded M wave for two subjects. It can be seen that the maximal M wave was achieved at a stimulation intensity of about 20 mA and stayed relatively constant up to the maximum capacity of the stimulator. ×, sweeps with a single stimulation; ^, sweeps with a double stimulation. In the latter condition the intensity of the second stimulus was set to 42 mA for both subjects. The lower panels show reference excitability curves of the same two subjects with (^) and without (×) the second but supramaximal stimulus applied to every sweep. It can be seen that the ‘double stimulus’ condition did not affect the amplitude of the H reflex.
The stimulus intensity necessary to elicit Mmax in the standing subjects was 19 mA on average (range, 12–21 mA). During locomotion these values were doubled to elicit Mmax. However, as the maximal output of the stimulator was 90 mA, the intensities used were far from the maximum capacity (Fig. 5).
DISCUSSION
Surprisingly, the results of the present study showed that the peak amplitude of the soleus H reflex was statistically similar during walking at 4.5 km h−1 and during running at 8 km h−1. Group mean values showed an increasing trend from walking to running using the method of the present study and a decreasing trend using the method introduced by Capaday & Stein (1987). However, both differences were statistically non-significant. For the two faster running speeds (12 and 15 km h−1) almost the same pattern was observed. The reflex amplitude decreased a little further but insignificantly using the method of Capaday & Stein (1987) while a relatively small but statistically significant increase was observed using the method of the present study. Despite the statistical probabilities it should be emphasized that large inter-individual differences were observed (Table 1). It was, however, clear that these differences were much smaller when using the method of the present study (Table 1 and Fig. 4). Unfortunately, group means and individual values are missing in the two previous studies. It therefore remains an open question if the observed variance is due to methodological problems or inter-individual differences.
Methodological considerations
It is possible that the supramaximal stimuli may disturb the movement pattern of the subjects. However, the stimuli given every 2 s were out of phase with the gait cycle, so that the subjects were unable to predict the stimulus in the step cycle. Furthermore, in several cases supramaximal stimuli were applied randomly rather than in every sweep, and this was never observed to influence the modulation or the amplitude of the H reflex. The duration of each step cycle was recorded and the use of supramaximal stimuli never appeared to influence this duration. The subjects verbally reported that the steps after the stimulus were perceived as normal steps.
In the present study M waves with an amplitude of 25 ± 10 % of Mmax were accepted as stimuli for the H reflex. Due to variations in Mmax during walking and running the size of these M waves was expressed relative to the actual Mmax measured in the same sweep. In the two previous studies the stimulus intensity was instead regulated to obtain a preset absolute M wave (in mV) during all phases of the movement (Capaday & Stein, 1987; Edamura et al. 1991). In these studies no values for Mmax during resting conditions were mentioned, which precludes comparison with the stimulus intensities in the present study. Theoretically, the use of exceedingly high stimulus intensities will decrease the size of the H reflex and confine the effects measured to the smaller motoneurones, i.e. those not involved in the formation of the M wave. Selective stimulation of I a afferents in the tibial nerve will often produce the largest H reflex in the soleus muscle, but the use of no M waves or very small ones is not practical during movement because the M wave is used as a measure and control of the effective stimulus strength. Using M waves below 15 % of Mmax presents a problem due to the underlying EMG during voluntary muscle activity in the stance phase. Although averaging removes the underlying EMG, this may still pose a problem since the size of the M wave must be measured in every sweep prior to averaging. Otherwise, wrong (too high or too low) stimulus strengths could have been applied. In the study of Capaday & Stein (1987), averaged M waves of about 2 mV are depicted in their Fig. 1 and about 1.5 mV in their Fig. 2. Moreover, they state that for some of their subjects it was necessary to use rather high M waves corresponding to the falling part of the M wave-H reflex recruitment curve. A comparison between the present and previous studies regarding the absolute size of the H reflex in millivolts is difficult due to the lack of mean values and inter-electrode distances in the previous reports. In the present study the peak H reflex during walking averaged 3.3 mV (range, 2.7–4.6 mV) while the previous reports graphically presented values of approximately 4.8, 2.9 and 2.7 mV (Edamura et al. 1991) and 8.0 and 1.8 mV (Capaday & Stein, 1987). Therefore, it seems unlikely that the stimulus intensities used in the present study were notably higher than those of the earlier studies.
It may be considered a problem that Mmax was elicited 60 ms after the stimulus for the conditioning M wave. However, the twenty time slices represented each 60 ms of the total walking cycle and Mmax never appeared to change considerably within one time slice. Off-line analysis where the Mmax modulation was shifted one time slice (60 ms) backwards did not show any visible differences in the H reflex modulation.
Variations in the maximal M wave
In the present study the variations of Mmax during the step cycle were systematic and consistent for each subject. However, the variation in the size of Mmax during the step cycle differed among the subjects. Figure 6 depicts the variation of Mmax and the modulation of the H reflex during running at 8 km h−1 for two subjects. The subject JM showed a large variation in Mmax with the lowest values during the stance phase. Subject NI, on the other hand, showed less variation in Mmax and the lowest values during the flight phase. Further, it is notable that using both the methodology of the present study and the one used in the previous studies would yield identical H reflex amplitude and modulation values for a subject with rather small variations in Mmax, while large discrepancies would be seen in a subject with a large variation in Mmax. In subject JM, Mmax was 10.6 mV during rest while it was only ∼5 mV during the stance phase of running (Fig. 6). Using M waves of about 25 % of resting Mmax in this phase of the movement would therefore result in lower H reflexes due to methodology (too high stimulation) rather than presynaptic or postsynaptic inhibition in the spinal cord. Post-experimentally we selected M waves close to 5 mV in subject JM during the stance phase of running and found H reflexes close to zero. This indicates that the true Mmax was about 5 mV in this phase of the movement for this subject. The stimulus intensity necessary to elicit Mmax in this subject during standing was 21 mA. Accordingly, during locomotion the stimulus intensity was set to 42 mA. However, as the measured amplitudes of the potentials were displayed on-line during the experiments the stimulus intensity was increased to 90 mA for this subject. This did not increase the rather low Mmax measured during the stance phase of running.
Figure 6. Two subjects with a large difference in variation of Mmax shown during running at 8 km h−1.
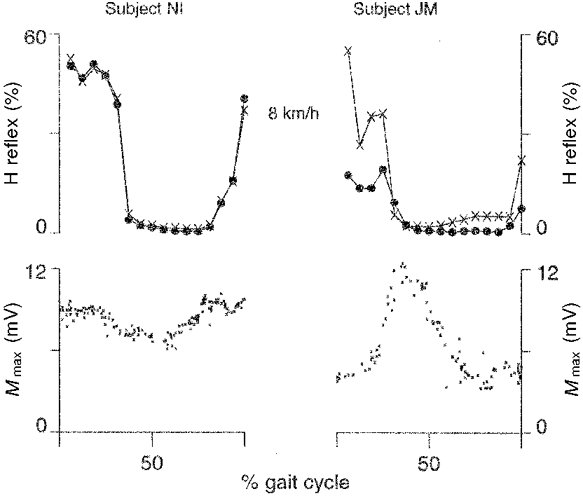
The subject on the left showed a rather small variation in Mmax during the gait cycle, while the subject on the right showed large variations. The H reflex is expressed as the percentage of the maximal M wave (Mmax) measured in every sweep (×) and during standing (•) (Capaday & Stein, 1987). For the subject shown on the right this variation resulted in a clearly lower H reflex amplitude during the stance phase of running when using the method of Capaday & Stein (1987). The discrepancy could not be demonstrated for the subject shown on the left. The reason for the disparity is likely to be the use of an excessively high stimulus intensity during the stance phase for the subject shown on the right (see text for further explanation).
The reason why Mmax varies in amplitude during the step cycle is most likely to be that the muscle fibres move under the skin with respect to the surface electrodes. This will change the muscle geometry and the electrical potentials will be conducted to the electrodes under circumstances constantly changing during the movement. This phenomenon has been shown to influence the recorded potentials significantly (Gerilowsky et al. 1989). The fibre movement is believed to be caused by shortening and lengthening of fibres and changes in the angle of fibre pennation (Gerilowsky et al. 1989). The results of the present study show that it is not only necessary to control the stimulus intensity due to movements of the tibial nerve relative to the stimulus electrodes as suggested by Capaday (1997), but that it may be even more important to compensate for variations in Mmax due to changes in muscle geometry.
Expression of the H reflex
The absolute amplitude in millivolts of the H reflex depends strongly on anatomical factors, i.e. differences in the thickness of axons from I a afferents and α-motoneurones. Expressing the H reflex as a fraction of the maximal M wave does not eliminate this problem on an individual basis. However, when a whole group of subjects is analysed the H reflex amplitude : Mmax ratio may provide a means for comparing the reflex excitability during different motor tasks and between different experiments. The H reflex may also be related to the level of voluntary EMG activity as used by Capaday & Stein (1986, 1987) and Edamura et al. (1991). In our opinion this is inappropriate in the case of walking and running, where the H reflex (after our normalization procedure) may increase by about 20 % while the soleus peak EMG increases by about 250 %.
Functional implications
When Capaday & Stein reported a lower H reflex amplitude during running than walking, it was suggested that a reduction of the reflex gain during running could be necessary because of the potential danger of instability of the motoneurone pool (tremor) caused by saturation of the pool (Capaday & Stein, 1987). However, assuming the excitability of the H reflex in the present study was very close to maximum during running, it can be concluded that saturation of the motoneurone pool is not a potential danger during running. Instead we suggest that the stretch reflex (as measured by the H reflex) plays a significant role during running. Facilitation of the central component of the stretch reflex indicates strongly that part of the motor output comes from the stretch reflex unless the spindle afferents are deactivated by reduced γ-activation. The latter would seem unlikely since the stretch reflex and the H reflex, though not completely identical, never undergo changes in the opposite direction (Akazawa et al. 1982; Aldridge & Stein, 1982). At all three running speeds the duration of the stance phase was about 300 ms and the duration of the soleus lengthening contraction about 150–200 ms, as recorded by the goniometer (Fig. 2). Assuming an electrical latency for the stretch reflex of about 40 ms and an electromechanical delay of about 50 ms with respect to the muscle (Bigland-Ritchie et al. 1983) there is time for the stretch reflex to be elicited during a lengthening contraction in the stance phase which could then assist the subsequent shortening contraction. This would also correspond with Dietz et al. (1979), who found a significant contribution of the stretch reflex during sprint running evaluated by EMG. Finally, if the stretch reflex can enhance muscle stiffness, as reported by Hoffer & Andreassen (1981) and by Sinkjær et al. (1988), a considerable amount of elastic energy could be stored in the tendons and be re-used during push-off (Voigt et al. 1995b), possibly leading to increased mechanical efficiency during running (Voigt et al. 1995a).
Acknowledgments
This study was supported by The Danish Research Council for Sport and The Danish Elite Sports Association.
References
- Akazawa K, Aldridge JW, Steeves JD, Stein RB. Modulation of stretch reflexes during locomotion in the mesencephalic cat. The Journal of Physiology. 1982;329:553–567. doi: 10.1113/jphysiol.1982.sp014319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JW, Stein RB. Nonlinear properties of stretch reflex studied in the decerebrate cat. Journal of Neurophysiology. 1982;47:179–192. doi: 10.1152/jn.1982.47.2.179. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Johansson R, Lippold OC, Woods JJ. Contractile speed and EMG changes during fatigue of sustained maximal voluntary contractions. Journal of Neurophysiology. 1983;50:313–324. doi: 10.1152/jn.1983.50.1.313. [DOI] [PubMed] [Google Scholar]
- Capaday C. Neurophysiological methods for studies of the motor system in freely moving human subjects. Journal of Neuroscience Methods. 1997;74:201–218. doi: 10.1016/s0165-0270(97)02250-4. [DOI] [PubMed] [Google Scholar]
- Capaday C, Stein RB. Amplitude modulation of the soleus H-reflex in the human during walking and standing. Journal of Neuroscience. 1986;6:1308–1313. doi: 10.1523/JNEUROSCI.06-05-01308.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaday C, Stein RB. Difference in the amplitude of the human soleus H reflex during walking and running. The Journal of Physiology. 1987;392:513–522. doi: 10.1113/jphysiol.1987.sp016794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover WJ. Practical Nonparametric Statistics. 2. New York: John Wiley & Sons Inc.; 1980. [Google Scholar]
- Crenna P, Frigo C. Excitability of the soleus H-reflex arc during walking and stepping in man. Experimental Brain Research. 1987;66:49–60. doi: 10.1007/BF00236201. [DOI] [PubMed] [Google Scholar]
- Dietz V, Schmidtbleicher D, Noth J. Neuronal mechanisms of human locomotion. Journal of Neurophysiology. 1979;42:1212–1222. doi: 10.1152/jn.1979.42.5.1212. [DOI] [PubMed] [Google Scholar]
- Dyhre-Poulsen P, Simonsen EB, Voigt M, Bojsen-Møller F. Proceedings of the International Congress on Biomechanics XIV. Paris, France: 1993. How to record H-reflexes during natural movements; p. 374. [Google Scholar]
- Edamura M, Yang JF, Stein RB. Factors that determine the magnitude and time course of human H-reflexes in locomotion. Journal of Neuroscience. 1991;11:420–427. doi: 10.1523/JNEUROSCI.11-02-00420.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerilowsky L, Tsvetinov P, Trenkova G. Peripheral effects on the amplitude of monopolar and bipolar H-reflex potentials from the soleus muscle. Experimental Brain Research. 1989;76:173–181. doi: 10.1007/BF00253634. [DOI] [PubMed] [Google Scholar]
- Hoffer JA, Andreassen S. Regulation of soleus muscle stiffness in premammillary cats; intrinsic and reflex components. Journal of Neurophysiology. 1981;45:267–285. doi: 10.1152/jn.1981.45.2.267. [DOI] [PubMed] [Google Scholar]
- Simonsen EB, Dyhre-Poulsen P. Excitability of the soleus H reflex during running. Medicine and Science in Sports and Exercise. 1995;(suppl. 27):968. [Google Scholar]
- Simonsen EB, Dyhre-Poulsen P, Voigt M. Excitability of the soleus H reflex during graded walking in humans. Acta Physiologica Scandinavica. 1995;153:21–32. doi: 10.1111/j.1748-1716.1995.tb09830.x. [DOI] [PubMed] [Google Scholar]
- Sinkjær T, Toft E, Andreassen S, Hornemann BC. Muscle stiffness in human ankle dorsiflexors: intrinsic and reflex components. Journal of Neurophysiology. 1988;60:1110–1121. doi: 10.1152/jn.1988.60.3.1110. [DOI] [PubMed] [Google Scholar]
- Voigt M, Bojsen-Møller F, Simonsen EB, Dyhre-Poulsen P. The influence of tendon Youngs modulus, dimensions and instantaneous moment arms on the efficiency of human movement. Journal of Biomechanics. 1995a;28:281–291. doi: 10.1016/0021-9290(94)00071-b. [DOI] [PubMed] [Google Scholar]
- Voigt M, Dyhre-Poulsen P, Simonsen EB. Modulation of short latency stretch reflexes during human hopping. Acta Physiologica Scandinavica. 1998;163:181–194. doi: 10.1046/j.1365-201X.1998.00351.x. [DOI] [PubMed] [Google Scholar]
- Voigt M, Simonsen EB, Dyhre-Poulsen P, Klausen K. Mechanical and muscular factors influencing the performance in maximal vertical jumping after different pre-stretch loads. Journal of Biomechanics. 1995b;28:293–307. doi: 10.1016/0021-9290(94)00062-9. [DOI] [PubMed] [Google Scholar]


