Abstract
Whole-cell clamp recordings of the compound synaptic current elicited by afferent stimulation of Schaffer collaterals showed that blockade of the NMDA, AMPA and GABAA receptor-mediated components by 6-nitro-7-sulphamoyl- benzo(f)quinoxaline-2,3-dione (NBQX), 3-((R)-2-carboxypiperazine-4-yl)propyl-1-phosphonate (R-CPP) and picrotoxin, respectively, left a small residual current in 39 out of 41 CA1 pyramidal neurones in organotypic cultures and 9 out of 16 CA1 cells in acutely prepared slices.
This current represented 2.9 ± 0.4% of the compound evoked synaptic response in organoypic cultures and 1.4 ± 0.5% in slices. It was characterized by a slightly rectifying I–V curve and a reversal potential of 3.4 ± 5.1 mV.
This residual current was insensitive to blockers of GABAB, purinergic, muscarinic and 5-HT3 receptors, but it was essentially blocked by the nicotinic receptor antagonist d-tubocurarine (91 ± 4% blockade; 20 μm), and partly blocked by α-bungarotoxin (200 nm) and methyllycaconitine (10 nm), two antagonists with a higher selectivity for α7 subunit-containing nicotinic receptors (48 ± 3% and 55 ± 11% blockade, respectively).
The residual current was of synaptic origin, since it occurred after a small delay; its amplitude depended upon the stimulation intensity and it was calcium dependent and blocked by the sodium channel antagonist tetrodotoxin.
We conclude that afferent stimulation applied in the stratum radiatum evokes in some hippocampal neurones a small synaptic current mediated by activation of neuronal nicotinic receptors.
Despite its predominant role in the peripheral nervous system and particularly at the motor endplate where it was first discovered, fast nicotinic cholinergic transmission has been found up to now only exceptionally in the central nervous system. One classic example is the synaptic activation of the Renshaw cell in the spinal cord, which receives a cholinergic nicotinic input from motoneurones (Eccles et al. 1954). Similarly, synaptic nicotinic potentials have been reported in brainstem vagal motoneurones (Zhang et al. 1993) and neuronal nicotinic acetylcholine receptor (nAChR) mediated responses could at least in part mediate dopamine release in the striatum (Clarke et al. 1987; Futami et al. 1995). So far, however, there has been no report of fast cholinergic transmission in mammalian cortical regions.
Contrasting with this, nicotine has been shown to play a major role in modulating brain functions (Wonnacott, 1997). Recent developments in gene technology have revealed the presence in many brain areas of mRNA coding for different nicotinic receptor subtypes (Goldman et al. 1987; Sargent, 1993). Furthermore, the presence of these mRNAs correlates well with reports of the existence of specific binding sites for both ACh analogues and the selective nicotinic receptor antagonist α-bungarotoxin (α-BgTX; Clarke et al. 1985). In addition, electrophysiological studies made on dissociated hippocampal neurones have demonstrated the existence of functional nicotinic receptors and identified several types of nicotinic currents (Alkondon & Albuquerque, 1993; Albuquerque et al. 1997). Finally, strong evidence implicates nicotinic acetylcholine receptors in behaviour, learning and memory as well as in the reinforcing actions of nicotine (Role & Berg, 1996; Picciotto et al. 1998).
A major issue, therefore, has been to understand whether neuronal nAChRs have purely a modulatory role, representing mainly targets for the low concentrations of nicotine that reach the brain during smoking, or whether, as found in the peripheral nervous system, they also mediate fast synaptic transmission. This is not unlikely considering the evidence showing the existence in the hippocampus of a major cholinergic input as well as a few cholinergic cells (Frotscher & Leranth, 1985; Frotscher et al. 1986, but see Oh et al. 1992). To examine this issue, we used here hippocampal slices and slice cultures (Stoppini et al. 1991) and have analysed whole-cell recordings of the synaptic currents evoked in CA1 pyramidal neurones in response to afferent stimulation of Schaffer collaterals. Through a pharmacological dissection of the components of these evoked currents, we provide evidence for the existence in the hippocampus of a fast current of synaptic origin that appears to be mediated by nicotinic receptors. A summary of this work was published in abstract form (Hefft et al. 1997).
METHODS
Interface-type organotypic hippocampal slice cultures (300 μm) were prepared from 14-day-old rats (Sprague-Dawley) according to the method described by Stoppini et al. (1991). Briefly, 14-day-old neonate Sprague-Dawley rats were decapitated following a brief anaesthesia with ethrane (4 %), the brain removed and the hippocampus dissected out. Experiments were carried out in accordance with the guidelines of the animal welfare committee (authorization 31.1.1024.1190). Slices were prepared using a chopper and maintained at the interface on a porous and transparent membrane (Millicell-CM, Millipore). They were maintained in culture in a medium containing 50 % MEM (+ 25 mM Hepes, 4 mM NaHCO3+ 5 mM Tris), 25 % horse serum and 25 % Hanks’ solution, pH 7.2.
Bicuculline, D-AP5, 3-((R)-2-carboxypiperazine-4-yl)propyl-1-phosphonate (R-CPP), 6-Nitro-7-sulphamoyl-benzo(f)quinoxaline-2,3-dione (NBQX), and were from Tocris Cookson Ltd, Bristol, UK; tetrodotoxin (TTX) was from Latoxan, France; QX-314 was from Alomone Laboratories, Jerusalem, Israel; d-tubocurarine and α-bungrotoxin were from Fluka Chemie AG, Buchs, Switzerland; and methyllycaconitine (MLA) was from Research Biochemicals International, Switzerland.
Recordings were performed between 4 and 10 days after tissue explantation. Most experiments were carried out using hippocampal organotypic cultures, but some of them were done, when mentioned, with hippocampal slices prepared from 18- to 25-day-old rats. For whole-cell recordings of synaptic currents, the slice cultures were transferred on a nylon grid in an interface chamber (1.5 ml; flow of 1 ml min−1). The bath solution contained (mM): 124 NaCl, 3 KCl, 1.25 NaH2PO4, 24 NaHCO3, 10 glucose, 1.5 MgCl2, 2 CaCl2; it was continuously bubbled with 95 % O2 and 5 % CO2 (pH 7.4, 290–295 mosmol l−1). The fire-polished low resistance patch pipettes (1–3 MΩ) were filled with intracellular saline containing (mM): 110 CsCH3SO3, 5 NaCl, 10 EGTA in 30 CsOH, 1 MgCl2, 1 CaCl2, 10 Hepes, 2 Na2ATP, 20 TEACl and 5 QX-314 (pH 7.2–7.4, 290 mosmol l−1). Gigaseals (10–20 GΩ) were obtained in stratum pyramidale of area CA1 by blind searching. In most experiments carried out on acutely prepared hippocampal slices, whole-cell patch recording were obtained under visual control using an infrapatch set-up (Luigs & Neumann, Germany). CA1 pyramidal cells were identified through their electrophysiological characteristics and in some instances by intracellular injection of Lucifer Yellow. Neurones were held at Vrest (−60 to −65 mV), and synaptic currents evoked at 0.016–0.033 Hz by a bipolar stimulation electrode placed in the stratum radiatum. A cut was made between CA3 and CA1 to prevent recurrent excitation. The ratio Rinput/Rseries was continuously monitored by a 10 mV-50 ms de- or hyperpolarizing voltage step preceding nerve fibre stimulation by 130 ms. All experiments were performed at 34°C. Currents were recorded with an Axopatch 200A amplifier filtered at 5 kHz, digitized at 10 kHz and stored on hard disk for off-line data analysis. All illustrated responses are averages of four to five consecutive current traces.
RESULTS
As expected from previous work, afferent stimulation of Schaffer collaterals in the stratum radiatum of organotypic slice cultures (Stoppini et al. 1991) evoked large currents reflecting the activation of excitatory and inhibitory synapses (Fig. 1A). These responses were readily inhibited by application of the AMPA/kainate and NMDA receptor antagonists NBQX (5 μM) and R-CPP (10 μM) and by the GABAA receptor antagonist bicuculline (10 μM). Note that GABAB-mediated responses were suppressed in these experiments by addition of QX-314 (5 mM) to the intracellular medium (Nathan et al. 1990). Surprisingly, however, we found in a majority of pyramidal neurones in organotypic slice cultures (39 out of 41 cells tested) and in a smaller proportion of cells in acutely prepared hippocampal slices (9 out of 16 cells tested) that blockade of excitatory and inhibitory transmission left a small inward current. In 10 experiments carried out in organotypic cultures in which the size of this residual current was precisely analysed during blockade of excitatory and inhibitory transmission, it corresponded to 2.9 ± 0.4 % (n = 10) of the initial compound postsynaptic response (10 out of the 10 cells tested exhibited the residual current; Fig. 1A). In acutely prepared slices, only 9 out of 16 cells tested exhibited this residual current. In these nine cells, it represented 2.5 ± 0.3 % of the compound postsynaptic response (n = 9), but 1.4 ± 0.4 % when considering the 16 cells tested (n = 16). To evoke this residual current, the stimulation intensity was usually adjusted so as to evoke a compound postsynaptic current of approximately 1 nA in pyramidal neurones prior to the application of the blockers. This corresponded to a suprathreshold, but not maximal, stimulation since synaptic responses of 5–10 nA could easily be obtained by increasing further the stimulation strength.
Figure 1. A fraction of the evoked postsynaptic current recorded in hippocampal CA1 pyramidal neurons is resistant to glutamate and GABA receptor antagonists.

A, graph showing the changes in size of the compound postsynaptic current (cPSC) recorded before and after bath application of NBQX (5 μM), R-CPP (10 μM) and bicuculline (10 μM) in an organotypic culture. The traces on the right are representative evoked response recorded before (a) and after (b) drug application. As shown, a small current measuring 2.9 ± 0.4 % of the initial cPSC could still be evoked following drug application (n = 10; the 10 cells tested exhibited the residual current). This residual current was obtained in 39 out of 41 cells in organotypic slice cultures and in 9 out of 16 cells in acutely prepared slices (mean size: 1.4 ± 0.4 %). B, application of additional antagonists of excitatory and inhibitory transmission did not affect the residual current. The graph shows the results of 3 experiments made on organotypic cultures in which CNQX (20 μM), D-AP5 (100 μM) and picrotoxin (100 μM) were added to previous concentrations of NBQX (5 μM), R-CPP (20 μM) and bicuculline (20 μM). Data are means ±s.e.m. of the amplitude of the evoked current. The trace on the right is a representative response recorded in the presence of these antagonists.
Controls made in organotypic slice cultures showed that this residual current was unaffected by increasing the concentrations of the glutamate and GABAA receptor inhibitors (NBQX, 5 μM; R-CPP, 20 μM, bicuculline, 20 μM; n = 5) or by further addition of CNQX (20 μM), D-AP5 (100 μM) and picrotoxin (100 μM) to the previous concentrations of the other inhibitors (Fig. 1B; n = 4). The residual current was therefore insensitive to antagonists of known glutamate and GABA receptors.
This inward current showed a reversal potential of 3.4 ± 5.1 mV and a current-voltage relationship characterized by a slight rectification (Fig. 2A). A pharmacological analysis showed that the residual current remained insensitive to application of suramin (50 μM), atropine (1 μM) and Y-25130 (20 μM), compounds which are known to block purinergic, muscarinic and 5-HT3receptors, respectively (data not shown; Cole & Nicoll, 1983; Edwards et al. 1992; Kagami et al. 1992). Also, the current could not be due to the triggering of fast activating dendritic Na+ channels, since QX-314 was routinely present in the intracellular medium. Interestingly, however, addition of d-tubocurarine (20 μM), a nAChR antagonist, to the perfusion medium strongly reduced this current both in organotypic cultures and slices, thereby suggesting that it could be attributed to the activation of nAChRs (Fig. 2).
Figure 2. Current to voltage relationship of the residual current evoked in CA1 neurones of organotypic cultures.
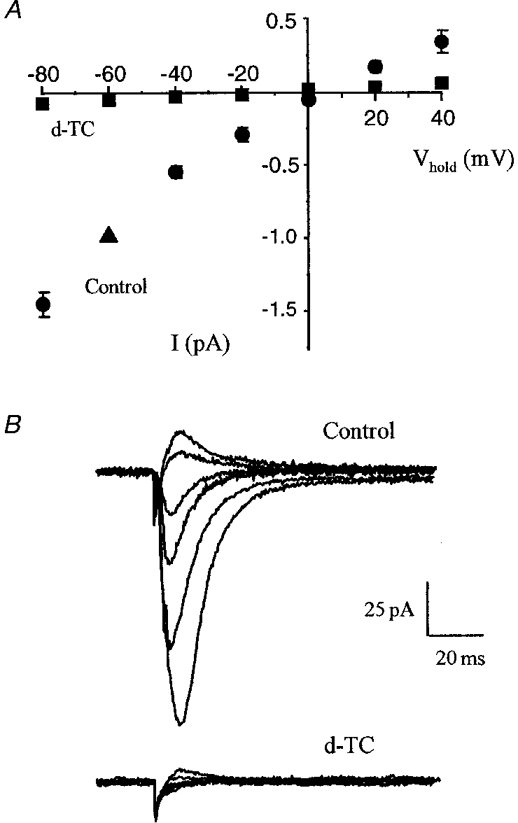
A, responses were recorded using whole-cell voltage clamp at potentials varying between −80 and +40 mV. The graph illustrates the I-V relationship of the residual current recorded in the presence of GABAA and glutamate antagonists (control). Values were obtained in 3 different cells and were normalized for the size of the current recorded at −60 mV (100 %, ▴). Application of d-tubocurarine (20 μM; d-TC) essentially eliminated the evoked response. The I-V curves were recorded 15–20 min after drug application. B, representative responses recorded at holding potentials between −80 and +40 mV (steps of 20 mV) before and after application of d-TC (20 μM).
To investigate further the nature of the receptor responsible for the residual current, we then tested the action of various compounds known to antagonize this class of receptor. Work carried out using both cultured and native hippocampal neurones showed that these cells express different subtypes of neuronal nAChRs displaying important physiological and pharmacological differences (Albuquerque et al. 1997). As shown in Fig. 2, bath application of 20 μM d-tubocurarine essentially abolished the inward current. In four experiments on organotypic cultures, d-tubocurarine blocked the residual current by 91 ± 4 %. Similar results were obtained in six experiments in hippocampal slices. In an attempt to discriminate between α-bungarotoxin (α-BgTX) sensitive and insensitive subtypes, we then tested the effect of this toxin. Addition of 200 nM α-BgTX to the bath reduced by 48 % the amplitude of the residual current recorded in four organotypic cultures (Fig. 3A). In agreement with previous observations made on α-BgTX sensitive nAChRs, this inhibition was irreversible. The possible contribution of an α-BgTX sensitive receptor was further reinforced when examining the effect of methyllycaconitine (MLA), a highly potent nicotinic antagonist. Given its 104-fold higher affinity for subunits of the α7-subtype over other known nAChRs (Alkondon et al. 1992), MLA can be used as a pharmacological tool to discriminate between different ACh-evoked currents. We found that addition of 10 nM MLA to five organotypic cultures inhibited by 55 % the residual current and that 80 % blockade was reversibly achieved upon addition of 1 μM MLA to the perfusion medium (n = 3; Fig. 3B and C). Similar results were obtained in three slices. In the light of the high specificity of both α-BgTX and MLA for one of the neuronal nAChR subtypes, these data suggested that α7-containing receptors were activated and contributed to the fast inward current response.
Figure 3. Blockade of the residual synaptic current by nicotinic acetylcholine receptor antagonists.
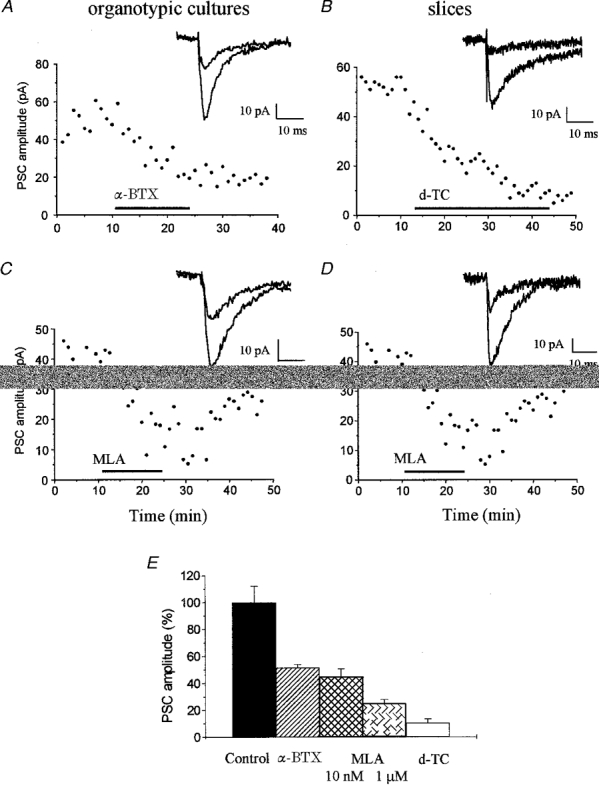
All experiments illustrated in this figure were carried out in the continuous presence of NBQX (5 μM), R-CPP (20 μM) and bicuculline (20 μM). Experiments on the left were carried out on organotypic cultures and on the right on acutely dissected hippocampal slices. A, illustration of an experiment made with an organotypic slice culture showing the irreversible blockade of the residual current produced by application of α-BgTX (200 nM, in serum bovine albumin at 20 mg ml−1). The responses illustrated on the right were recorded before and during application of α-BgTX. B, experiment made on a hippocampal slice showing the blockade of the residual current produced by 20 μM d-tubocurarine. C and D, illustration of the effect of 10 nM MLA, a nicotinic receptor antagonist, on the residual current recorded in an organotypic culture (C) and hippocampal slice (D). The traces on the right were recorded before and during application of MLA. E, bar graph summarizing the effects of MLA (10 nM and 1 μM), α-bungarotoxin (200 nM) and d-tubocurarine (20 μM) on the peak amplitude of the residual current recorded in organotypic cultures. Data are mean ±s.e.m. of 3–5 experiments. Drug effects were measured 15 min after application. Differences from control are statistically significant (P < 0.01, Student's t test).
Several properties of this inward current were indicative of its synaptic origin (Fig. 4). First there was a short delay (1.8–2.5 ms) between the stimulus artefact and the foot of its rising phase (Fig. 4A). This delay was similar to that of the compound excitatory synaptic current recorded under control conditions. Second, changing the stimulus intensity resulted, as expected for synaptic transmission, in a response that gradually increased up to saturation (Fig. 4B). Third, the current was reversibly abolished upon reduction of the external Ca2+ (0.1 mM Ca2+-4 mM Mg2+), a condition that is known to block synaptic transmission (Fig. 4C). Finally, addition of 100 nM tetrodotoxin reversibly abolished the evoked current, thereby confirming its dependence upon a regenerative action potential (Fig. 4D).
Figure 4. Synaptic origin of the residual current.
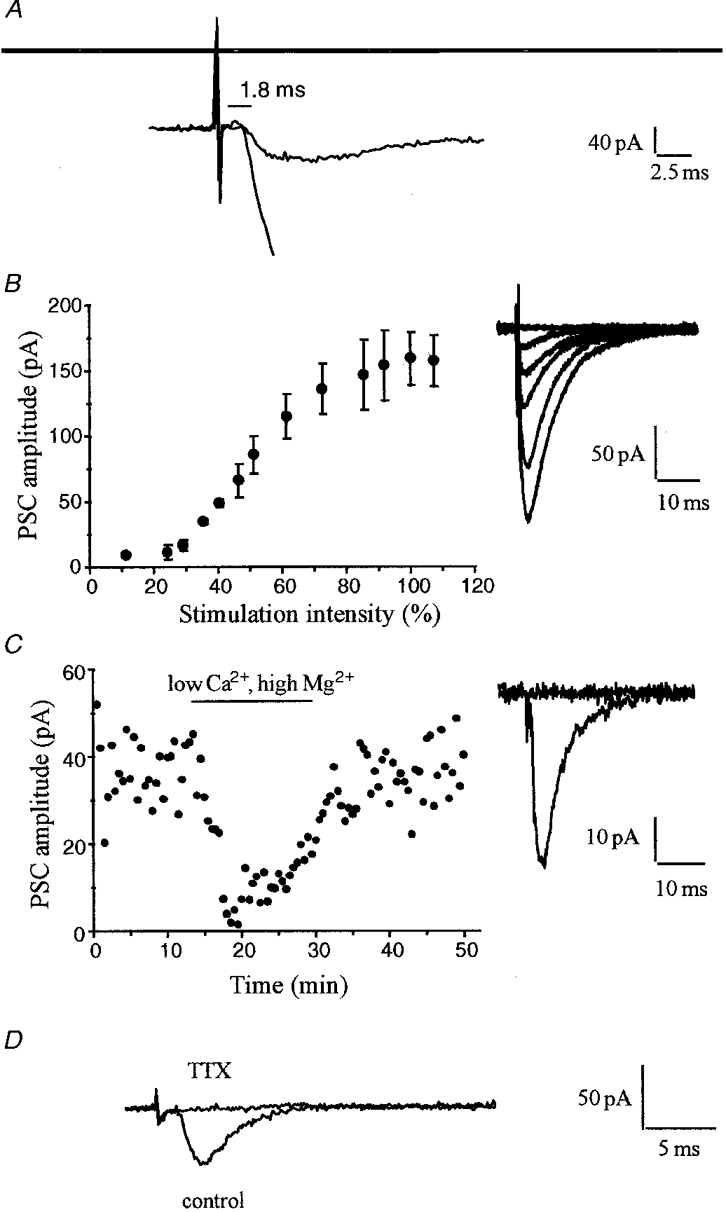
A, superimposed traces illustrating at magnified time and current scales the delay of about 1.8 ms existing between the end of the stimulus artifact and the onset of the compound EPSC recorded before blockade of excitatory and inhibitory transmission and the residual current. B, input-output curve illustrating the gradual increase in size of the residual current produced by increasing the stimulation intensity (expressed as a percentage of the stimulus that generated the maximal response). The traces on the right are averaged EPSCs recorded at various stimulation intensities. C, blockade of the residual evoked current by perfusion of a medium containing a low Ca2+ and high Mg2+ concentration (0.1 mM and 4 mM, respectively) in the continuous presence of GABA and glutamate receptor antagonists (similar results were obtained in 3 additional experiments). The traces on the right are EPSCs recorded before and during low Ca2+-high Mg2+ perfusion. D, dependence of the residual current upon regenerative action potentials. The traces are EPSCs recorded before and during application of TTX (0.1 mM). GABA and glutamate receptor antagonists were present throughout the experiment.
DISCUSSION
The results of the present study provide evidence for the existence in the hippocampus of a fast synaptic current evoked by stimulation applied in the stratum radiatum and mediated by the activation of neuronal nAChRs. This finding is consistent with numerous previous reports showing the presence in the hippocampus of an important cholinergic input and the existence, in situ, of choline acetyltransferase positive cells (Frotscher & Leranth, 1985; Frotscher et al. 1986; but see Oh et al. 1992). It is also supported by the results of studies indicating that pyramidal neurones in the rat hippocampus transcribe mRNAs encoding a variety of nAChR subunits (Conroy et al. 1992; Vernallis et al. 1993) and actually express the corresponding proteins (Alkondon & Albuquerque, 1993; Albuquerque et al. 1997; but see Frazier et al. 1998). The present study now provides evidence that, in a significant proportion of pyramidal cells, afferent stimulation in the strratum radiatum triggers currents that are generated by activation of nicotinic receptors. The synaptic origin of this current was established by the existence of a synaptic delay, its modulation by graded electrical stimulation, its calcium dependence and its TTX sensitivity. These data thus provide a new mechanism for mediating acetylcholine and nicotine actions in this preparation, in addition to the reported modulation of glutamate and GABA release (McGehee et al. 1995; Gray et al. 1996).
An intriguing question arising from this conclusion concerns the origin of the presynaptic neurones or fibres responsible for this nicotinic transmission. Since Schaffer collaterals are glutamatergic, it is unlikely that the residual current reported here was directly due to their activation. Also, since the residual current was found in organotypic cultures, a preparation devoid of its extrinsic afferents, this nicotinic transmission is unlikely to arise from the stimulation of cholinergic fibres. Rather, a more plausible explanation is that the stimulation pulses directly activated cholinergic neurones present in the preparation. As suggested by immunohistochemical analyses (Frotscher & Leranth, 1985; Frotscher et al. 1986), these neurones are probably present only in small numbers in hippocampal slices. This may be the reason why the stimulation pulses used evoked a nicotinic synaptic current in only about half of the pyramidal cells tested in hippocampal slices. Note that, while these stimulation pulses were supratheshold for the generation of action potential in the postsynaptic cell, they were not maximal, activating probably around 50–200 synapses per neurone, a situation that may very well occur under physiological conditions. Also the higher proportion of cells exhibiting the nicotinic current in organotypic cultures could very well reflect the fact that all dendritic and axonal processes of neurones remain confined in the culture and that the level of interconnection between cells is usually higher in slice cultures (Gähwiler et al. 1997).
The pharmacological profile of the synaptic receptor involved here suggests that α7 or a closely related subunit participates in the receptor composition responsible for the residual evoked current. Indeed, about 50 % of the synaptic nicotinic current could be blocked by 10 nM MLA, a concentration at which this compound shows a high selectivity for α7-containing receptors (Alkondon et al. 1992). Also, the kinetic characteristics of this current were fast and therefore consistent with those of type I currents evoked by acetylcholine application on dissociated pyramidal neurones (Alkondon & Albuquerque, 1993; Albuquerque et al. 1997). Given the high calcium permeability at resting membrane potential of the α7 nAChR (Bertrand et al. 1993; Seguela et al. 1993; Albuquerque et al. 1997), one may consider that, despite the small size of this nicotinic response, it might generate calcium influx in dendrites and spines and thus influence Ca2+-dependent processes (Mulle et al. 1993; Rathouz & Berg, 1994). This is in agreement with the observation that the density of MLA-sensitive currents evoked by acetylcholine application is substantially higher on apical and basal dendrites of pyramidal neurones than on the soma (Albuquerque et al. 1997). This current could contribute there to modulate synaptic plasticity, induction of long-term potentiation or inhibitory mechanisms, and hence the computational properties of the dendritic tree. Indeed perfusion of nAChR agonists was found to facilitate the induction of long-term potentiation in the hippocampus (Hunter et al. 1994), illustrating that nicotinic synaptic transmission may play a role in this form of plasticity. Furthermore, the cholinergic neurones responsible for these nicotinic synaptic responses could be selectively activated during specific patterns of activity such as during theta rhythm and contribute in this way to information processing. Finally the nicotinic transmission found here could also be modulated by the constant presence of low concentrations of agonist during nicotine absorption. Hence the data presented here might shed a new light on the possible impact of neuronal nAChRs for CNS functions during both physiological and pathological conditions.
Acknowledgments
This work was supported by a grant from the Swiss Science Foundation (Fonds National de la Recherche scientifique 31–40815.94 to D. M.) and by an MD-PhD fellowship to S. H.
References
- Albuquerque EX, Alkondon M, Pereira EF, Castro NG, Schrattenholz A, Barbosa CT, Bonfante-Cabarcas R, Aracava Y, Eisenberg HM, Maelicke A. Properties of neuronal nicotinic acetylcholine receptors: pharmacological characterization and modulation of synaptic function. Journal of Pharmacology and Experimental Therapeutics. 1997;280:1117–1136. [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. Journal of Pharmacology and Experimental Therapeutics. 1993;265:1455–1473. [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Wonnacott S, Albuquerque EX. Blockade of nicotinic currents in hippocampal neurons defines methyllycaconitine as a potent and specific receptor antagonist. Molecular Pharmacology. 1992;41:802–808. [PubMed] [Google Scholar]
- Bertrand D, Galzi JL, Devilliers-Thiéry A, Bertrand S, Changeux JP. Mutation at two distinct sites within the channel domain M2 alter calcium permeability of the neuronal alpha7 nicotinic receptor. Proceedings of the National Academy of Sciences of the USA. 1993;90:6971–6975. doi: 10.1073/pnas.90.15.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PB, Schwartz RD, Paul SM, Pert CB, Pert A. Nicotinic binding in rat brain: autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. Journal of Neuroscience. 1985;5:1307–1315. doi: 10.1523/JNEUROSCI.05-05-01307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PBS, Hommer DW, Pert A, Skirboll LR. Innervation of substantia nigra neurons by cholinergic afferents from the pedunculopontine nucleus in rats: neuroanatomical and electrophysiological evidence. Neuroscience. 1987;23:1011–1020. doi: 10.1016/0306-4522(87)90176-x. [DOI] [PubMed] [Google Scholar]
- Cole AE, Nicoll RA. Acetylcholine mediates a slow synaptic potential in hippocampal pyramidal cells. Science. 1983;221:1299–1301. doi: 10.1126/science.6612345. [DOI] [PubMed] [Google Scholar]
- Conroy WG, Vernallis AB, Berg DK. The alpha 5 gene product assembles with multiple acetylcholine receptor subunits to form distinctive receptor subtypes in brain. Neuron. 1992;9:679–691. doi: 10.1016/0896-6273(92)90031-8. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Fatt P, Koketsu K. Cholinergic and inhibitory synapses in a pathway from motoraxon collaterals to motoneurones. The Journal of Physiology. 1954;126:524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FA, Gibb AJ, Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992;359:144–147. doi: 10.1038/359144a0. 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an α-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurones, but not pyramidal cells. Journal of Neuroscience. 1998;18:1187–1195. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frotscher M, Leranth C. Cholinergic innervation of the rat hippocampus as revealed by choline acetyltransferase immunocytochemistry: a combined light and electron microscopic study. Journal of Comparative Neurology. 1985;239:237–246. doi: 10.1002/cne.902390210. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Schlander M, Leranth C. Cholinergic neurons in the hippocampus. A combined light- and electron- microscopic immunocytochemical study in the rat. Cell Tissue Research. 1986;246:293–301. doi: 10.1007/BF00215891. [DOI] [PubMed] [Google Scholar]
- Futami T, Takakusaki K, Kitai ST. Glutamatergic and cholinergic inputs from the pedunculo-pontine tegmental nucleus to dopamine neurons in the substantia nigra pars compacta. Neuroscience Research. 1995;21:331–342. doi: 10.1016/0168-0102(94)00869-h. 10.1016/0168-0102(94)00869-H. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH, Capogna M, Debanne D, McKinney RA, Thompson SM. Organotypic slice cultures: a technique has come of age. Trends in Neurosciences. 1997;20:471–477. doi: 10.1016/s0166-2236(97)01122-3. 10.1016/S0166-2236(97)01122-3. [DOI] [PubMed] [Google Scholar]
- Goldman D, Deneris E, Luyten W, Kochhar A, Patrick J, Heinemann S. Members of a nicotinic acetylcholine receptor gene family are expressed in different regions of the mammalian central nervous system. Cell. 1987;48:965–973. doi: 10.1016/0092-8674(87)90705-7. [DOI] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- Hefft S, Bertrand D, Muller D. Synaptic transmission mediated by nicotinic receptors in hippocampal slice cultures. Society for Neuroscience Abstracts. 1997;23:666. [Google Scholar]
- Hunter BE, de Fiebre CM, Papke RL, Kem WR, Meyer EM. A novel nicotinic agonist facilitates induction of long-term potentiation in the rat hippocampus. Neuroscience Letters. 1994;168:130–134. doi: 10.1016/0304-3940(94)90433-2. 10.1016/0304-3940(94)90433-2. [DOI] [PubMed] [Google Scholar]
- Kagami Y, Shigenobu S, Watanabe S. Neuroprotective effect of 5-HT3 receptor antagonist on ischemia-induced decrease in CA1 field potential in rat hippocampal slices. European Journal of Pharmacology. 1992;224:51–56. doi: 10.1016/0014-2999(92)94817-f. 10.1016/0014-2999(92)94817-F. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- Mulle C, Choquet D, Korn H, Changeux JP. Calcium influx through nicotinic receptor in rat central neurons: its relevance to cellular regulation. Neuron. 1992;8:135–143. doi: 10.1016/0896-6273(92)90115-t. [DOI] [PubMed] [Google Scholar]
- Nathan T, Jensen MS, Lambert JD. The slow inhibitory postsynaptic potential in rat hippocampal CA1 neurones is blocked by intracellular injection of QX-314. Neuroscience Letters. 1990;14:309–313. doi: 10.1016/0304-3940(90)90865-7. 10.1016/0304-3940(90)90865-7. [DOI] [PubMed] [Google Scholar]
- Oh JD, Woolf NJ, Roghani A, Edwards RH, Butcher LL. Cholinergic neurons in the rat central nervous system demonstrated by in situ hybridization of choline acetyltransferase mRNA. Neuroscience. 1992;47:807–822. doi: 10.1016/0306-4522(92)90031-v. 10.1016/0306-4522(92)90031-V. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Rathouz MM, Berg DK. Synaptic-type acetylcholine receptors raise intracellular calcium levels in neurons by two mechanisms. Journal of Neuroscience. 1994;14:6935–6945. doi: 10.1523/JNEUROSCI.14-11-06935.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Role LW, Berg DK. Nicotinic receptors in the development and modulation of CNS synapses. Neuron. 1996;16:1077–1085. doi: 10.1016/s0896-6273(00)80134-8. 10.1016/S0896-6273(00)80134-8. [DOI] [PubMed] [Google Scholar]
- Sargent PB. The diversity of neuronal nicotinic acetylcholine receptors. Annual Review of Neuroscience. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- Seguela P, Wadliche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. Journal of Neuroscience. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini L, Buchs P-A, Muller D. A simple method for organotypic cultures of nervous tissue. Journal of Neuroscience Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. 10.1016/0165-0270(91)90128-M. [DOI] [PubMed] [Google Scholar]
- Vernallis AB, Conroy WG, Berg DK. Neurons assemble acetylcholine receptors with as many as three kinds of subunits while maintaining subunit segregation among receptor subtypes. Neuron. 1993;10:451–464. doi: 10.1016/0896-6273(93)90333-m. 10.1016/0896-6273(93)90333-M. [DOI] [PubMed] [Google Scholar]
- Wonnacott S. Presynaptic nicotinic ACh receptors. Trends in Neurosciences. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. 10.1016/S0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- Zhang M, Wang YT, Vyas DM, Neuman RS, Bieger D. Nicotinic cholinoceptor-mediated excitatory postsynaptic potentials in rat nucleus ambiguus. Experimental Brain Research. 1993;96:83–88. doi: 10.1007/BF00230441. [DOI] [PubMed] [Google Scholar]


