Abstract
Adenosine influences the vectorial transport of Na+ and HCO3− across kidney epithelial cells. However, its action on effector proteins, such as the Na+-H+ exchanger NHE3, an epithelial brush border isoform of the Na+-H+ exchanger (NHE) gene family, is not yet defined.
The present study was conducted in Xenopus laevis distal nephron A6 epithelia which express both an apical adenosine receptor of the A1 type (coupled to protein kinase C (PKC)) and a basolateral receptor of the A2 type (coupled to protein kinase A (PKA)). The untransfected A6 cell line expresses a single NHE type (XNHE) which is restricted to the basolateral membrane and which is activated by PKA.
A6 cell lines were generated which express exogenous rat NHE3. Measurements of side-specific pHi recovery from acid loads in the presence of HOE694 (an inhibitor with differential potency towards individual NHE isoforms) detected an apical resistant Na+-H+ exchange only in transfected cell lines. The sensitivity of the basolateral NHE to HOE694 was unchanged, suggesting that exogenous NHE3 was restricted to the apical membrane.
Stimulation of the apical A1 receptor with N6-cyclopentyladenosine (CPA) inhibited both apical NHE3 and basolateral XNHE. These effects were mimicked by the addition of the protein kinase C (PKC) activator phorbol 12-myristate 13-acetate (PMA) and partially prevented by the PKC inhibitor calphostin C which also blocked the effect of PMA.
Stimulation of the basolateral A2 receptor with CPA inhibited apical NHE3 and stimulated basolateral XNHE. These effects were mimicked by 8-bromo-cAMP and partially prevented by the PKA inhibitor H89 which entirely blocked the effect of 8-bromo-cAMP.
In conclusion, CPA inhibits rat NHE3 expressed apically in A6 epithelia via both the apical PKC-coupled A1 and the basolateral PKA-coupled A2 adenosine receptors.
Adenosine has been identified as a mediator essential for homeostatic regulation of kidney function. Endogenous adenosine generated by adenosine triphosphate (ATP) dephosphorylation (Mi & Jackson, 1995) participates in regulation of renin release and glomerular haemodynamics and influences renal electrolyte transport by regulating a variety of plasma membrane ion channels and transporters (Friedlander & Amiel, 1995; Osswald et al. 1997). Increased local production of adenosine which associates with hypernatraemia, ischaemia or administration of nephrotoxic substances reflects an imbalance between energy supply and energy demand during active transport and has been implicated in acute renal failure (Osswald & Gleiter, 1993a, b). Due to the kidney-specific relationship between energy consumption and tubular Na+ reabsorption, it is intriguing to consider adenosine regulation of Na+ re-absorption (in addition to modulation of renal blood flow and glomerular filtration rate) as an important factor in the pathophysiological control of renal solute and fluid re-absorption.
A few studies only have so far addressed adenosine action on renal Na+ transport. From these studies the picture emerges that modulation of renal Na+ reabsorption is a function of the expression of adenosine receptor subtypes on target cells. This is exemplified in primary cultures of rat inner medullary collecting duct cells (Yagil et al. 1994) and most likely also in rat medullary thick ascending limbs (Seney & Seikali, 1989) where activation of A1 receptors inhibit Na+ reabsorption, and in A6 cells (a cell line derived from Xenopus laevis kidney) where activation of A2 receptors have been shown to stimulate transepithelial Na+ transport (Lang et al. 1985; Casavola et al. 1996).
It is generally accepted that the proximal tubule of the kidney reabsorbs the majority of the filtered load of sodium. Current evidence mainly coming from cultured cells but also from native tissue strongly suggests that NHE3, which encodes an apical amiloride resistant isoform of the Na+-H+ exchanger (NHE) family, promotes basal- and hormone-stimulated active Na+ transport in the mammalian proximal tubule (Yun et al. 1995; Orlowski & Grinstein, 1997; Wakabayashi et al. 1997). In addition, NHE3 is important in HCO3− reabsorption; yet effects of adenosine on NHE3 activity have not been elucidated.
Therefore, in the present study we wished to determine whether adenosine acutely modulates the activity of NHE 3. To understand the underlying signalling mechanism(s), experiments were designed to evaluate changes in NHE3 activity in response to either A1 or A2 receptor activation. This was accomplished: (i) by stable transfection of cDNA encoding the Na+-H+ exchanger NHE3 (rat isoform) into A6/C1 cells that are devoid of the functional apical Na+-H+ exchanger (Guerra et al. 1993; Casavola et al. 1996) and are expressing A1 adenosine receptors on the apical side and A2 adenosine receptors on the basolateral aspect of the cell surface (Casavola et al. 1997), and (ii) by using a series of selective inhibitors of the adenosine effector systems.
The data show that A1 receptor activation decreases NHE 3 activity by a PKC-dependent mechanism and A2 receptor activation by a PKA-dependent mechanism. Based on the pattern of the pharmacological regulation of the transfected and endogenous Na+-H+ exchanger by PKC and PKA agonists, it is suggested that the endogenous Na+-H+ exchanger (XNHE) in A6/C1 cells is functionally distinct from the ubiquitous NHE 1 isoform (Wakabayashi et al. 1997), the piscine β-NHE isoform (Borgese et al. 1992) and the isoform of the exchanger studied in Xenopus laevis oocytes (Busch et al. 1995).
METHODS
Solutions
Media used in the fluorimetric pH measurements included Na+ medium composed of (mM): 110 NaCl, 3 KCl, 1 CaCl2, 0.5 MgSO4, 1 KH2PO4, 5 glucose and 10 Hepes buffered to pH 7.5 with Tris. TMA medium consisted of (mM): 110 tetramethylammonium chloride (TMACl), 3 KCl, 1 CaCl2, 0.5 MgSO4, 1 KH2PO4, 5 glucose and 10 Hepes buffered to pH 7.5 with Tris. KCl medium contained (mM): 105 KCl, 8 NaCl, 1 CaCl2, 0.5 MgSO4, 1 KH2PO4, 5 glucose and 10 Hepes buffered to various pH values for calibration of the intracellular BCECF (2′,7′-bis(carboxymethyl)-5(6)-carboxyfluorescein-acetoxymethyl ester; Molecular Probes, Eugene, OR, USA) signal.
Cell culture
Experiments were performed with A6/C1 cells, a subclone of A6-2F3 cells that were selected by ring cloning on the basis of high transepithelial resistance and responsiveness to aldosterone and vasotocin (Verrey, 1994). A6/C1 cell cultures were maintained in 0.8 × concentrated DMEM (Life Technologies, Gibco, Basel, Switzerland), containing 25 mM NaHCO3, 10 % heat-inactivated fetal bovine serum (Life Technologies, Gibco), 50 i.u. ml−1 penicillin and 50 μg ml−1 streptomycin (final osmolality: 220–250 mosmol kg−1). Cells were incubated in a humidified 95 % air-5 % CO2 atmosphere at 28°C and subcultured weekly by trypsinization using a Ca2+-Mg2+-free salt solution containing 0.25 % (w/v) trypsin and 1 mM EGTA. Cells generally reached confluency between 7 to 8 days after seeding when the culture medium was changed three times a week. Studies on A6/C1 cells were performed between passage 114 to 128.
Stable transfection and expression of NHE3 cDNA
Full-length rat NHE3 cDNA (nucleotides 50–4980) originally obtained by Dr John Orlowski (Montreal, Canada) and Dr Gary Shull (Cincinnati, OH, USA) was subcloned into the mammalian expression plasmid pCMV-5 (gift from Dr David Russel, Dallas, TX, USA) as described previously (Moe et al. 1995). A6/C1 cells grown to 20–25 % confluence in 35 mm tissue culture dishes were co-transfected with 10 μg NHE3 cDNA in the pCMV-5 expression plasmid and 0.5 μg of a selection marker termed p3′SS-ΔLacI in 1 ml serum-free culture medium (lacking antibiotics) using the lipophilic reagent polybrene (15 μg (10 μg)−1 cDNA) (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) (Brewer, 1994). The p3′SS-ΔLacI vector which confers resistance to hygromycin B was generated from the eucaryotic lac repressor expression vector p3 ‘SS (Strategene AG, Basel, Switzerland) by excising a Xba I−Eco RV fragment (nucleotides 1337–2157) in the coding region of the lacI gene. Starting from transfection, cells were incubated for 6 h in a humidified 95 % air-5 % CO2 atmosphere at 28°C. Solution was then replaced by serum-free culture medium containing 30 % dimethylsulphoxide (DMSO) and cells were allowed to rest for exactly 5 min at room temperature. Thereafter, cells were carefully washed with 2 ml culture medium (composition as given above) and incubated in culture medium in a humidified 95 % air- 5 % CO2 atmosphere at 28°C for 24 h. Culture medium was then exchanged and cells were maintained for an additional 12 h in the incubator. To start selection, cells were trypsinized and transferred to 100 mm culture plates, where they were exposed to 450 μg hygromycin B (ml culture medium)−1 (Calbiochem, Luzern Switzerland) over a period of 2–3 weeks. Hygromycin B-resistant colonies were harvested by ring cloning and were further selected on the basis of expression of NHE3 activity (defined as apical transport activity which is sensitive to inhibition by 8-bromo-cAMP). Cell clones expressing NHE3 activity (A6/NHE3 cells) were maintained in culture as described above and exposed to hygromycin B during growth in order to maintain selection pressure. As addition of dexamethasone has been shown to accelerate differentiation and to improve the electrical resistance of A6 cell layers (Preston et al. 1988), both untransfected and transfected A6 cell lines were grown for 7–8 days in the presence of 1 μM dexamethasone prior to their introduction into experiments.
Experiments on A6/NHE3 cells were carried out on passages 3 to 36. The data that are presented from A6/NHE3 cells were obtained from two different cell clones (clones 6 s and 9 s).
Measurement of intracellular pH and Na+-H+ exchange activity
For pH experiments, cells were seeded onto collagen-coated coverslips with a 1.5 mm hole punched in the centre covered by a Teflon filter (Millicell-CM, 0.4 μm pore size; Millipore, Eschborn, Germany) as described previously (Helmle-Kolb et al. 1997). Cells were incubated for 60 min with 4.2 μM BCECF AM in Na+ medium containing 50 μM probenecid to minimize possible dye leakage. Dye-loaded cells were mounted into a chamber and perfused continuously at 25°C with Na+ medium (at a rate of 0.5 ml min−1) on the stage of an inverted microscope (Zeiss IM35) equipped with a Leitz × 50 water immersion objective. BCECF was excited sequentially by positioning 390–440 nm and 475–490 nm band pass filters in front of a xenon lamp. The excitation light was directed to the cells via a 510 dichroic mirror and emission was collected by a 515–565 nm band pass filter. To minimize dye bleaching a neutral density filter was placed into the excitation light pass. Fluorescence data were recorded every 5 or 10 s by irradiating the cells for 7 ms at each of the wavelengths. Data were processed and corrected for autofluorescence (measured in cells grown on the filter support but not loaded with BCECF) as previously detailed (Helmle-Kolb et al. 1997). Intracellular pH (pHi) was estimated from the ratio of BCECF fluorescence calibrated intracellularly by using the K+-nigericin approach (Helmle-Kolb et al. 1997).
Na+-H+ exchange activity was measured as initial rate of Na+-dependent recovery of intracellular pH from an acid load in the absence of HCO3− as described previously (Helmle-Kolb et al. 1997). In brief, cells were acid loaded by application of 40 mM NH4Cl (in Na+ medium). Subsequent removal of NH4Cl and perfusion with sodium-free TMA medium caused the pHi to decrease. Once a stable fluorescence signal was reached, asymmetric expression of Na+-H+ exchanger activity was assessed by measuring Na+-dependent alkalinization as a function of Na+ addition to either the apical or basolateral perfusate.
Effects of agonists on NHE activity were determined by evaluating pHi recovery rates of a single cell layer under control conditions and after repeated cell acidification under test conditions. Prior to adopting this protocol, we verified that rates of pHi recovery after consecutive NH4 pulses were virtually identical within a cell population when examined from the same starting acid pHi value. An example of a typical control experiment examining the reproducibility Na+-H+ exchange activity in either the apical or basolateral membrane of NHE3-transfected A6 cells upon repetitive acidification is given in Fig. 1. Following an NH4 pulse, cells underwent an acidification and adopted on average an intracellular acid pH of 6.37 ± 0.016 (n = 15, the number of independent experiments from cells of different cell passages) in Na+-free medium. Upon introduction of Na+ to either the apical (Fig. 1A) or basolateral cell surface (Fig. 1B) a rapid alkalinization ensued (in four independent experiments initial rates of pHi recovery were observed to be 0.158 ± 0.012 pH units min−1 upon apical Na+ addition and 0.236 ± 0.013 pH units min−1 upon basolateral Na+ addition). Importantly, in pairwise comparisons, pHi recovery rates following a second NH4 pulse were indistinguishable from those measured under control conditions (rates of pHi recovery at pHi 6.36 ± 0.016 were 0.167 ± 0.014 pH units min−1 upon readdition of apical Na+ and 0.0223 ± 0.016 pH units min−1 upon readdition of basolateral Na+), thus demonstrating the validity of the protocol for comparison of the effect of agonists on basal rate of transport activity. Unless otherwise stated, agonists were introduced into experiments after monitoring of basal transport rates. Cells were then incubated in the presence of agonists for at least 15 min prior to recording their effect on Na+-H+ exchange activity. Concentrations of agonist were chosen to be the same as those that have recently been shown to elicit maximal or near- maximal responses in A6/C1 cells (Casavola et al. 1997).
Figure 1. Comparison of rates of Na+-H+ exchange activity in A6/NHE3 cells following repeated acidification of intracellular pH.
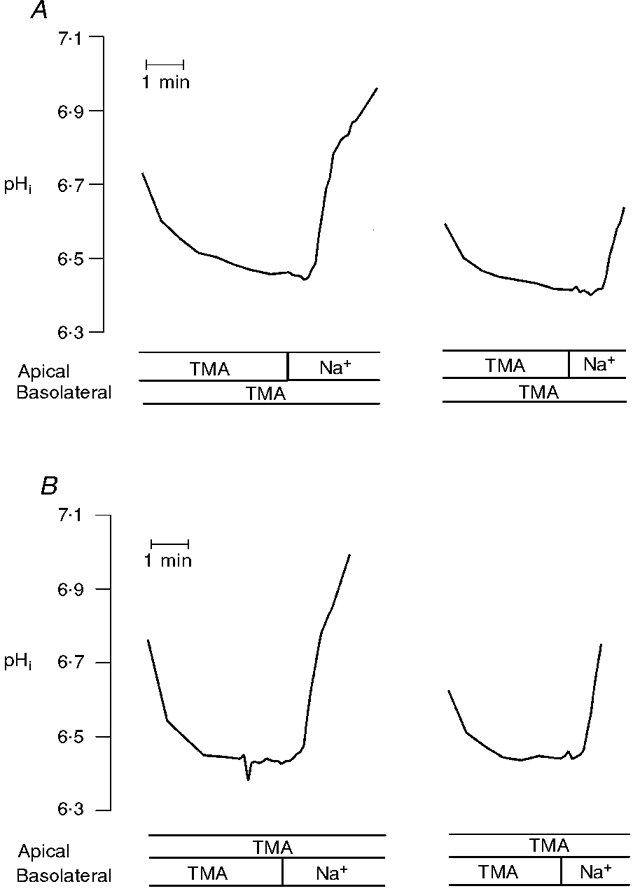
Na+-H+ exchange activity was studied as Na+-dependent recovery of intracellular pH using microspectrofluorometry. For study of the influence of repeated acid loading on the rate of transport activity, confluent cell cultures were pulsed with NH4Cl which was then replaced by TMA medium (onset of data presentation). Recovery of pHi from an acid load was introduced by addition of Na+ medium. When rates of alkalinization approached original levels (data not shown), cells were again subjected to a protocol of NH4Cl prepulse and withdrawal followed by Na+ addition. A, pHi recovery in response to apical Na+ addition to acid-loaded cells; B, pHi recovery in response to basolateral Na+ addition to acid-loaded cells. The tracings are representative of four similar experiments. TMA, perfusion with TMA medium; Na+, perfusion with Na+ medium.
Total RNA preparation, reverse transcription (RT) and polymerase chain reaction (PCR)
Total RNA was extracted from cells grown to confluence on 60 mm culture dishes with TRIzol (Life Technologies, Gibco), following the manufacturer's instructions. Deoxyribonuclease I (Promega GmbH, Mannheim, Germany) treatment was performed to remove any potential genomic DNA contamination.
RT and PCR was carried out essentially as described (Helmle-Kolb et al. 1997). In brief, 10 μg of total RNA denatured for 3 min at 65°C were used for RT with (dT15) primers and avian myeloblastoma virus reverse transcriptase (Promega GmbH). The reaction was carried out in a 20 μl reaction container with 2 μl of 10 × reaction buffer (100 mM Tris-HCl, pH 8.8, 500 mM KCl and 1 % Triton X-100), 5 mM MgCl2, 0.5 μg (dT15) primers, 1 mM of each deoxyribonucleotide (dNTP), 10 i.u. ribonuclease inhibitor (RNAsin) and 12.5 i.u. avian myeloblastoma virus reverse transcriptase and incubated at 42°C for 1 h. To control for cDNA origin, each reaction was performed in parallel with an otherwise identical reaction to which no reverse transcriptase was added. After RT, 2 μl of the first strand synthesis product were amplified by PCR in a 50 μl reaction using 0.2 μM of each NHE3 primer in the presence of 2 mM MgCl2, 0.2 mM dNTPs, 1.25 i.u. Taq polymerase (Promega GmbH), and 5 μl of 10 × reaction buffer (composition as given above). For control, expression of the amino acid permease-related protein ASUR4 (Spindler et al. 1997) was tested. The temperature profile for PCR reaction was: hot start (95°C for 2 min); followed by 40 cycles consisting of denaturing at 95°C (for 40 s), annealing at 48°C (40 s), and extension at 72°C (for 40 s); final extension was at 72°C (for 10 min). PCR products were separated on 1.5 % agarose gels stained with ethidium bromide. Bands of the expected sizes were visualized under UV light.
NHE3 primers used for PCR reaction encode a sequence of 441 bp in the cytoplasmic tail of NHE3 (starting from amino acid (aa) 684 until aa 837) Primer sequences were: 5′-ACAGAAGCGGAGGAATAG-3′ (sense strand) and 5′-CATGTGTGTGGACTCAGG-3′ (antisense strand).
Primers sets for ASUR4 detect a 501 bp sequence (aa 244 to aa 410). Sequences were: 5′-GTCCTGGCATTGTACAGT-3′ (sense strand) and 5′-CAGGGCTACGCAAAGCCA-3′ (antisense strand).
Southern blot analysis of PCR products
For Southern blot analysis of PCR products, gels were denatured (in 0.5 M NaOH, 1.5 M NaCl) at room temperature for 30 s, vacuum transferred (Bio-Rad, Glattbrugg, Switzerland) to a nylon filter (Pall, Biodyne B transfer membrane), and UV crosslinked at 312 nm for 5 min. The nylon filter was pre-hybridized for 30 min in hybridization solution (containing 5 × Denhardt's reagent (containing per 500 ml: 5 g Ficoll (type 400: Pharmacia), 5 g polyvinylpyrrolidone and 5 g bovine serum albumin (fraction V, Sigma-Aldrich Chemie), made up to 500 ml with H2O), 5 × saline-sodium citrate buffer (SSC), 0.5 % SDS, and 100 μg ml−1 denatured herring sperm DNA) at 62°C. Hybridization was carried out for 5 h using 10 ml of hybridization solution containing 25 μl of [32P]-labelled 3 kb rat NHE3 cDNA (10−6 c.p.m. ml−1) (the 3 kb NHE3 cDNA fragment was obtained from the pMVC5/NHE3rat construct by digestion with the restriction enzyme Bam HI (Biofinex) followed by electrophoresis on a 1.5 % agarose gel and extraction with the gene clean II kit (Bio 101, 1070 Joshua Weg, Vista, CA, USA)). The probes obtained were labelled using the commercially available oligolabelling kit from Pharmacia (Freiburg im Breisgau, Germany). The blots were washed twice for 5 min at room temperature in 2 × SSC and 0.1 % SDS and twice for 30 min at 65°C in 0.5 × SSC and 0.1 % SDS, and then analysed by autoradiography.
Other methods
Monolayer resistance was measured on A6 cells grown on collagen-coated polycarbonate filter rings (Transwell, 0.4 μm pore size, 4.7 cm2, CorningCostar, Cambridge, MA, USA) in a modified Ussing chamber with an automatic voltage-clamp apparatus connected to a dual-channel recorder as described (Verrey, 1994). For experiments culture medium was replaced by Na+ medium (composition as given above) and measurements were carried out at room temperature.
Data presentation
Results are presented as means ± standard error (s.e.m). Statistical significance was established by analysis of variance (ANOVA). A probability (P) < 0.05 was considered statistically significant.
RESULTS
Functional expression of NHE3 in transfected cells
Three complementary approaches were used to confirm NHE3 expression in transfected A6 cells (A6/NHE 3). First, RT-PCR of the NHE3 region corresponding to the C terminus of the protein served to verify that the NHE3 transcript was expressed in hygromycin- (selection marker) resistant cell clones. Second, inhibitor sensitivity (HOE694, (3-methylsulphonyl-4-piperidinobenzoyl)guanidine methanesulphonate), kindly provided by Dr H. Lang, (Hoechst, Germany) was used to document that endogenous and transfected NHE activities can be separated pharmacologically. Third, analysis of cAMP responses was performed to establish that endogenous and transfected NHE activities can be separated functionally.
Figure 2 shows the results of a RT-PCR analysis on expression of NHE3 transcripts. As shown in Fig. 2A, primers specific for rat NHE3 detected a product of 441 bp when PCR reactions were carried out on cDNA templates obtained by reverse transcription of RNA samples from NHE3 transfectants (clone 6 s and clone 9 s; Fig. 2A, lanes 4 and 6). A product of similar size was obtained with the linearized pCMV-5 expression plasmid containing the full length NHE3 cDNA (Fig. 2A, lane 9). No discernable products were detected when cDNA obtained by reverse transcription of A6/C1 cell RNA (parent cell line) served as a template (Fig. 2A, lane 2). Primers specific for AZUR4 (Spindler et al. 1997) resulted in a product of 501 bp with RNA from A6/C1 and A6/NHE3 cells (Fig. 2B), thereby confirming the selected RT-PCR reaction sequence. Samples analysed in the absence of reverse transcriptase prevented the appearance of the 441 bp and 501 bp RT-PCR products, ruling out contamination of samples with genomic DNA. That A6/NHE3 transfectants are indeed expressing the transcript encoding NHE3 was documented by Southern blot analysis. As shown in Fig. 2C, the NHE3 cDNA probe hybridized with cDNA obtained from A6/NHE3 cells, but not from A6/C1 cells.
Figure 2. Analysis of transcripts for NHE3 in A6/C1 and A6/NHE3 cells (clone 6 s and clone 9 s).
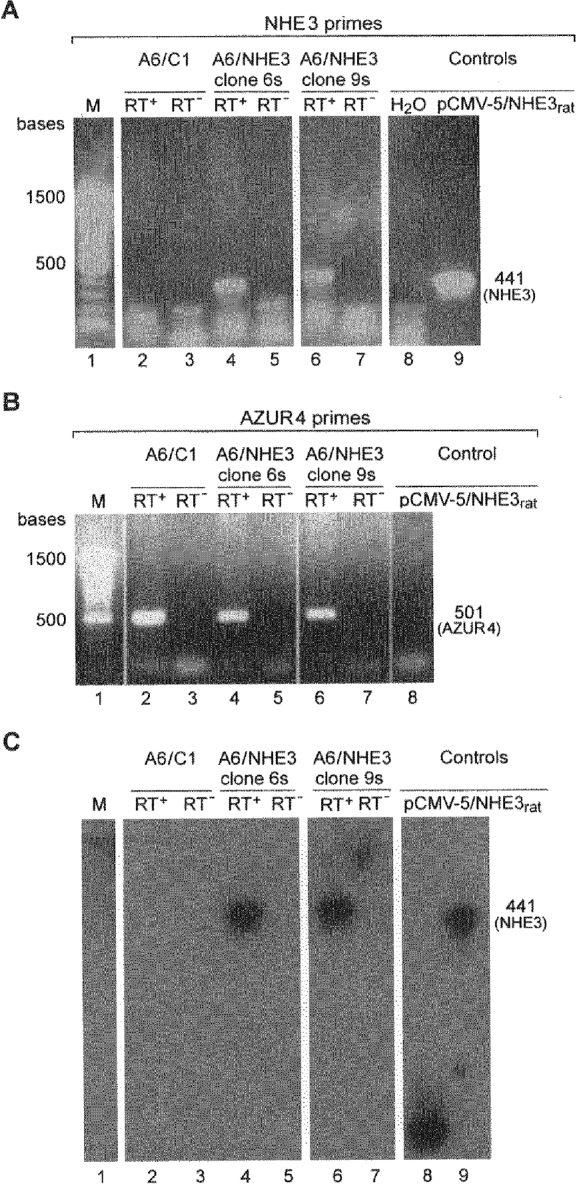
Total RNA was extracted from A6/C1 or A6/NHE3 cells and used to detect message for NHE3 (panel A) or AZUR4 (positive control; panel B) with RT-PCR. Southern blot hybridization (*, see below) to [32P]-labelled, 3 kb NHE3 cDNA was then used to determine the identity of PCR products obtained with the NHE3 primer set (panel C). RT+, RT reactions performed in the presence of reverse transcriptase; RT−, RT reactions performed in the absence of reverse transcriptase. pCMV-5/NHE3rat, PCR reaction performed with the linearized expression plasmid containing the full sequence of rat NHE3; M, molecular mass marker (100 bp ladder from Life Technologies, Gibco). *Note that the band in lane 8 of the Southern blot represents hybridization of pCMV-5/NHE3 to 3 kb NHE3 cDNA, because PCR probes were run on a single agarose gel with an upper and lower slit row.
Figure 3 illustrates experiments on the time course of Na+-dependent pHi recovery from an acid load in A6/C1 and A6/NHE3 cells as a function of apical or basolateral Na+ addition. In agreement with earlier observations (Guerra et al. 1993) only addition of basolateral Na+ initiated pHi recovery in A6/C1 cells (0.192 ± 0.039 pH units min−1; n = 20) (Fig. 3A). In A6/NHE3 cells substantial pHi recovery rates were observed upon addition of both apical Na+ (0.167 ± 0.014 pH units min−1; n = 48) (Fig. 3B, left) and basolateral Na+ (0.223 ± 0.0164 pH units min−1; n = 46) (Fig. 3B, right). As monolayer resistance was observed to be 7.16 ± 0.72 and 6.82 ± 0.22 kΩ cm2 in A6/C1 and A6/NHE3 cells, respectively, the occurrence of pHi transients in response to apical Na+ addition to acid-loaded A6/NHE3 cells is unlikely to arise from paracellular ion leak.
Figure 3. Na+ dependence and polarity of pHi recovery in acid-loaded A6/C1 and A6/NHE3 cells.
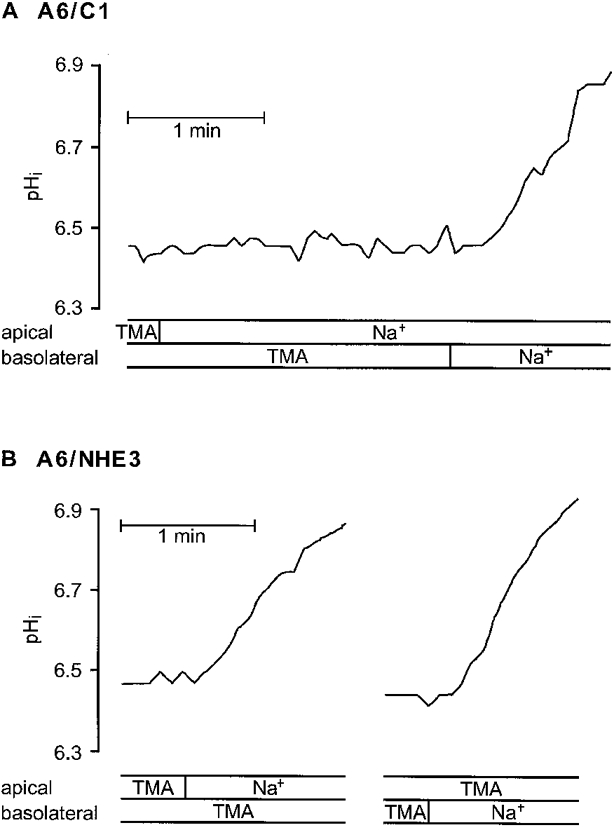
Confluent cell cultures of A6/C1 or A6/NHE3 cells on filter support were examined for pHi change by microspectrofluorometry using the dye BCECF as detailed in Methods. Cells in Na+ medium were pulsed with 40 mM NH4Cl and then perfused with Na+-free medium (TMA). Recovery of pHi from an acid load was next induced by re-introduction of Na+ medium (Na+) to either the apical or the basolateral cell surface. Fluorescence ratio traces from A6/C1 cells (A) and from A6/NHE3 cells (B), representative of at least four independent experiments.
Although preceding experiments suggest that NHE3 is present in A6/NHE3 cells, they do not define the contribution of NHE3 to apical and/or basolateral Na+-H+ exchange activity. To assess the fraction of Na+-H+ exchange mediated by NHE3 we have used the NHE specific inhibitor, HOE694, which inhibits Na+-H+ exchanger isoforms at widely differing concentrations (Noël & Pouysségur, 1995), thus being capable of distinguishing between ‘resistant’ (NHE3) and sensitive isoforms (NHE1 and NHE2) of the Na+-H+ exchanger. As shown in Table 1, basolateral pHi recovery of both A6/C1 and A6/NHE3 cells was progressively inhibited with increasing concentrations of HOE694, with 85 % inhibition occurring at 10−4 M HOE694 in NHE3 transfectants. Apical pHi recovery in the A6/NHE3 cells was found to be less sensitive to HOE694. Of note, there was no significant difference between inhibition profiles of both apical and basolateral NHE activities in clone 6 s and clone 9 s and basolateral NHE activities in untransfected and transfected cell lines (Table 1). Together, inhibition data are indicative of a preferential distribution of NHE3 on the apical side of A6/NHE3 cells. Whether Na+-dependent pHi recovery in the apical membrane is consequent to expression of NHE3 alone or a combination of NHE3 and endogenous Na+-H+ exchanger (XNHE) cannot be determined by the present findings, since the difference in inhibitor sensitivity of apical and basolateral Na+-H+ exchange activity was so small.
Table 1.
Concentration response profiles for HOE694 on Na+–H+ exchange activities in A6/C1 and A6/NHE3 cells
| Cell type | NHE activity | [HOE694] | |||
|---|---|---|---|---|---|
| 5 ± 10−5 M | 7.5 ± 10−5 M | 10−4 M | 10−3 M | ||
| A6/C1 | Basolateral | 57.6 ± 4.3*‡§(4) | 69.0 ± 6.2*‡§(3) | 96.8 ± 3.2*‡§(3) | — |
| A6/NHE3, clone 6s | Apical | 28.0 ± 3.7*†(5) | 41.3 ± 4.5*†(3) | 48.7 ± 4.3*†(4) | 94.5 ± 3.3*(4) |
| Basolateral | 59.7 ± 5.3*(4) | 64.9 ± 1.8*(4) | 85.5 ± 1.8*(3) | — | |
| A6/NHE3, clone 9s | Apical | 33.4 ± 2.5*†(5) | 39.7 ± 6.2*†(4) | 46.1 ± 5.7*†(7) | — |
| Basolateral | 57.8 ± 4.8*(4) | 74.1 ± 5.7*(5) | 89.8 ± 4.6*(3) | — | |
Values are the mean ± s.e.m. of the NHE activity inhibition (%) with the number of experiments performed under identical conditions (n) given in parentheses. Confluent cell cultures of A6/C1 and A6/NHE3 cells were examined for Na+-dependent recovery of pHi from an acid load as described in Fig. 3. Effects of HOE694 were studied after repetitive acidification of cell cultures to control acid pHi values. Data represent the initial decrease of pHi recovery rates resulting from a 2 min exposure of either the apical or basolateral cell surface to indicated concentrations of HOE694.
Significant vs. control
significant vs. basolateral effect of HOE694
significant compared with apical NHE inhibition in clone 6s
significant compared with apical NHE inhibition in clone 9s.
Several lines of evidence suggest that the behaviour of the A6 cell Na+-H+ exchanger and NHE3 to PKA activation is likely to be diametrically opposed, the former is stimulated (Guerra et al. 1993) while the latter is inhibited (Helmle-Kolb et al. 1990; Moe et al. 1995; Cabado et al. 1996). This prompted us to validate the fraction of contribution of NHE 3 to A6 cell Na+-H+ exchange activity by measuring the rates of Na+-dependent pHi recovery in acid-loaded cells pretreated with or without 8-bromo-cAMP. As shown in Fig. 4, 8-bromo-cAMP (10−4 M for 15 min) significantly stimulated basolateral NHE activity in A6/C1 cells (by 29 %) as well as in A6/NHE3 cells (by 29 % in clone 6 s and 33 % in clone 9 s), but decreased NHE activity located on the apical side of A6/NHE3 cells (by 29 % in clone 6 s and by 21 % in clone 9 s). Stimulation of basolateral NHE activity by 8-bromo-cAMP was not significantly different between untransfected and transfected cell lines. Likewise, the percentage of inhibition of apical NHE activity was not significantly different between transfected cell cultures (clones 6 s and 9 s), although 8-bromo-cAMP inhibited apical activity in clone 6 s slightly more. As expected, 8-bromo-cAMP-induced changes of NHE activities in A6/NHE3 cells (clone 6 s) were prevented by the PKA antagonist, H89, which when tested alone had no influence on rates of Na+-H+ exchange. This indicated that the stimulatory and inhibitory actions of 8-bromo-cAMP were mediated by PKA. Importantly, in experiments evaluating the effect of 8-bromo-cAMP in the presence of HOE694 concentrations that completely blocked NHE activity located in the basolateral membrane of A6 cells (see Table 1), 8-bromo-cAMP-evoked responses of apical Na+-H+ exchanger (clones 6 s and 9 s) were barely distinguishable from those obtained in the absence of the inhibitor. These results imply that endogenous XNHE activity is either absent from the apical side or completely unresponsive to PKA agonists when expressed in the apical membrane context of A6/NHE3 cells.
Figure 4. Modulation of NHE activity by a PKA agonist/antagonist.
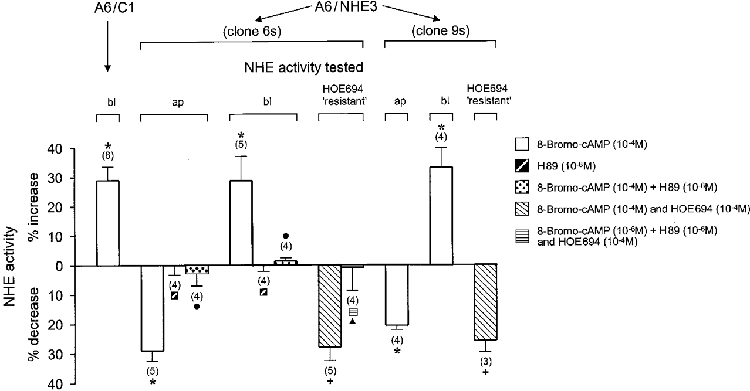
Na+-H+ exchange activity was assessed as initial rates of HOE694 inhibitable pHi recovery of A6 cell cultures from an acid load as described in Fig. 3. To test the effect of 8-bromo-cAMP (PKA agonist) and/or H89 (PKA antagonist), cell cultures were repetitively acidified to the same starting acid pHi value, and pHi recovery rates were observed either in the absence or presence of pharmacological agents added to the apical and basolateral cell surface. In all experiments, cells were exposed to different agents for 15 min prior to evaluating their effect on Na+-H+ exchange activity in the absence or presence of HOE694 that was included for a 2 min period in the apical perfusate. The number of experiments performed under identical experimental conditions is given in parentheses. ap, the change (expressed in %) of the activity of apical Na+-H+ exchanger in response to pharmacological agents; bl, the corresponding change (expressed in %) of basolateral Na+-H+ exchanger; HOE694 ‘resistant’, agonist-induced net change (expressed in %) of apical transport activity which was calculated as difference between the sum of Na+-dependent rate of pHi recovery measured in the presence of agonists and 10−4 M apical HOE694 and 10−4 M apical HOE694 alone. * Significant vs. control;+ significant vs. HOE694;. significant vs. 8-bromo-cAMP; ▴ significant vs. 8-bromo-cAMP plus HOE694.
As adenosine induces stimulation of PKA and PKC in A6 cells, we proceeded to test the sensitivity of the transfected Na+-H+ exchanger NHE3 to PKC activation. To this end, Na+-dependent pHi recovery rates in A6/NHE3 cells were analysed prior to and following a 15 min incubation with the PKC agonist, phorbol 12-myristate 13-acetate (PMA). In these and further experiments we abstained from comparison of A6/NHE3 data with A6/C1 cell data, because transfection, as judged from the preceding results, did not noticeably alter basolateral transport characteristics. As illustrated in Fig. 5, PMA (10−7 M) inhibited Na+-H+ exchange activity both in the apical and basolateral membrane of A6/NHE3 cells. The inert analogue 4α-phorbol had no effect on either of the transport activities (clone 6 s). Calphostin C, a specific inhibitor of PKC, effectively prevented the inhibitory action of PMA (clone 6 s), suggesting that downregulation of NHE activities in A6/NHE3 cells was indeed due to activation of PKC. Again, the presence of HOE694, even though it partially inhibited apical NHE activity, did not alter the fraction of Na+-H+ exchange activity inhibited by PMA (clones 6 s and 9 s). Likewise, HOE694 did not influence typical responses of apical Na+-H+ exchanger (clone 6 s) to PMA and calphostin C. Since regulation of NHE3 in A6 cells largely resembles protein kinase regulation of the renal brush border membrane Na+-H+ exchanger in other cell systems (Helmle-Kolb et al. 1997, 1990; Levine et al. 1993; Azarani et al. 1995; Cabado et al. 1996), the present data are interpreted to indicate that the A6 cell model is suited for study of the mechanism of NHE3 modulation by biological stimuli acting through PKA and PKC.
Figure 5. Modulation of NHE activities by a PKC agonist/antagonist.
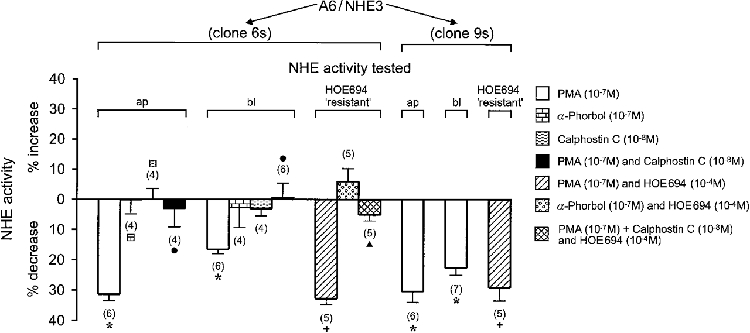
The effect of PMA (PKC agonist) and/or calphostin C (PKC antagonist) on Na+-H+ exchange activities of confluent A6/NHE3 cells was tested as indicated in the legend to Fig. 4. Exposure to different agents (which were included in the apical and basolateral perfusate) was for 15 min, except for HOE694 which was added to the apical perfusate 2 min prior to evaluating the influence of different agents on Na+-dependent rate of alkalinization. The number of experiments performed under identical experimental conditions is given in parentheses. ap, change (expressed in %) of the activity of apical Na+-H+ exchanger in response to pharmacological agents; bl, corresponding change (expressed in %) of basolateral Na+-H+ exchanger; HOE694 ‘resistant’, change (expressed in %) of apical transport rates in response to pharmacological agents and HOE694 when normalized to inhibition of 10−4 M HOE694 alone. * Significant vs. control; + significant vs. HOE694;. significant vs. PMA; ▴ significant vs. PMA plus HOE694.
Influence of adenosine on the Na+-H+ exchanger activity in A6/NHE3 cells
Distinct adenosine receptor subtypes have previously been found to be expressed in A6/C1 cells: A1 receptors functionally coupled to the phospholipase C-PKC effector system were shown to reside on the apical cell surface, whereas A2 receptors coupled to the adenylate cyclase-PKA effector system were found to be expressed on the basolateral side (Casavola et al. 1997). Based on these earlier observations, addition of either apical or basolateral adenosine (in the absence or presence of PKC or PKA antagonists) would therefore provide a useful means of determining whether activation of A1 or A2 receptors is associated with NHE 3 regulation. As preceding results indicate that all NHE regulation in clones 6 s and 9 s is virtually identical, experiments testing adenosine effects were conducted on clone 6 s only. As can be seen from Fig. 6A, apical addition of the metabolically stable adenosine analogue N6-cyclopentyladenosine (CPA, 10−6 M for 15 min), significantly reduced NHE activity located in the apical membrane (by 31 %) and in the basolateral membrane (by 22 %). Calphostin C abrogated CPA inhibition of apical NHE activity only partially, but evoked a significant reduction of the CPA response of basolateral Na+-H+ exchanger. H89 had no significant effect on transport responses elicited by apical CPA. To verify that CPA indeed inactivated NHE3, experiments were also conducted in the presence of HOE694. As shown in Fig. 6A, HOE694 concentrations that specifically inactivated XNHE activity had no effect on CPA-induced downregulation of apical Na+-H+ exchanger. As is also shown, calphostin C prevented CPA inhibition of apical Na+-H+ exchange activity with a similar efficacy as in the corresponding experiments performed in the absence of the inhibitor. Furthermore, as observed in the experiments above, H89 was unable to prevent inhibition of apical Na+-H+ exchanger by CPA. Of note, the A1 receptor blocker CPX (1,3-dipropyl-8-cyclopentylxanthine) inhibited CPA-induced downregulation of apical Na+-H+ exchanger effectively without appreciably altering basal NHE3 activity (compared with controls, transport rates were altered by 0.3 ± 3.7 %, n = 5, in the presence of CPA and CPX, and by 5.1 ± 3.4 %, n = 4, in the presence of CPX alone).
Figure 6. CPA effects on NHE activities of A6/NHE3 cells in clone 6 s, in the absence and presence of PKC or PKA antagonists.
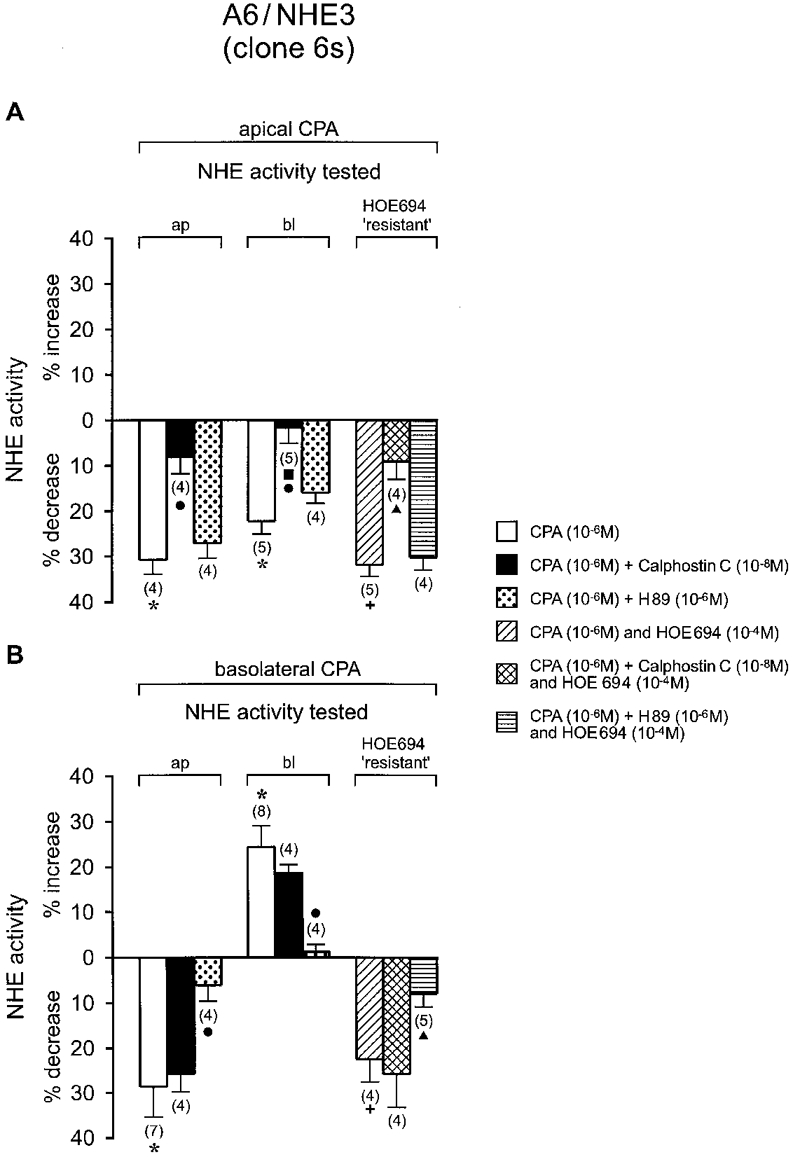
A, effect of apical CPA (adenosine analogue) on rate of Na+-H+ exchanger. B, effect of basolateral CPA on rate of Na+-H+ exchanger. NHE activities were observed under control and test conditions as indicated in Fig. 4. Except for CPA, agents were applied to the apical and basolateral aspect of the cells. Changes in rate of NHE activities were observed 15 min after application of pharmacological agents in the presence or absence of HOE694 (which was added to the apical perfusate 2 min prior to initiation of Na+-dependent cell alkalinization). The number of experiments performed under identical experimental conditions is given in parentheses. ap, change (expressed in %) of the activity of apical Na+-H+ exchanger in response to pharmacological agents; bl, corresponding change (expressed in %) of basolateral Na+-H+ exchanger; HOE694 ‘resistant’, change (expressed in %) of apical transport rates in response to pharmacological agents and HOE694 when normalized to inhibition of 10−4 M HOE694 alone. * Significant vs. control;+ significant vs. HOE694;. significant vs. CPA; ▴ significant vs. CPA plus HOE694.
Figure 6B illustrates the effect of basolateral CPA on the Na+-H+ exchanger. Treatment of cells with basolateral CPA (10−6 M for 15 min) significantly decreased apical NHE activity (by 29 %) but caused an increase of basolateral XNHE activity (by 24 %). As expected, H89 almost completely eliminated CPA inhibition of apical Na+-H+ exchanger and effectively reduced CPA activation of basolateral XNHE. In contrast, calphostin C was unable to negate transport responses following basolateral CPA addition. Of note, treatment with HOE694 had no influence on downregulation of apical NHEactivity by CPA (in the absence or presence of calphostin C). Likewise, there was no significant change in the degree of the reversal of CPA actions by H89 when experiments were done in the presence of HOE694. Together, these results indicate that the A1 receptor action on NHE3 activity in A6/NHE3 cells involves PKC, while A2 receptor action involves PKA.
DISCUSSION
The aim of the present study was to study regulation of the Na+-H+ exchanger NHE 3 by the adenosine analogue N6-cyclopentyladenosine (CPA). Since future studies aim to identify structural elements involved in the molecular mechanism of NHE3 regulation by CPA, the present studies were carried out in A6/C1 cells that lack endogenous expression of NHE 3 (see Fig. 2A and C, and Guerra et al. 1993; Casavola et al. 1997) and are able to activate the PKC and the PKA pathway in response to A1 or A2 receptor stimulation, respectively (see Figs 4 and 5, and Casavola et al. 1997).
The present functional data provide evidence that NHE3 is preferentially routed to the apical membrane of A6/C1 cells. This site of expression is in agreement with the reported location of NHE3 in the renal epithelia, including the proximal tubule, and the thin and thick ascending limbs of the loop of Henle of the rat kidney (Amemiya et al. 1995; Biemesderfer et al. 1997). It is also in agreement with the location of the transporter in cultured renal epithelial cells expressing either endogenous or transfected NHE3 activity, such as opossum kidney (OK) cells (Amemiya et al. 1995) and Madin-Darby canine kidney (MDCK) cells (Helmle-Kolb et al. 1997). That NHE3 is the major contributor to apical NHE activity in transfected A6/C1 cells was judged by the effect of HOE694 and 8-bromo-cAMP. The concentration of HOE694 required to inhibit ∼50 % of apical NHE activity was between 100 and 500 μM, and was similar to the concentration required for half-maximal inhibition of NHE3 in other cell systems (K0.5 = 650 μM) (Noël & Pouysségur, 1995). Interestingly, although the sensitivity of apical and basolateral Na+-H+ exchanger to HOE694 was significantly different at all concentrations tested, the potency of HOE694 to inhibit basolateral XNHE in A6/NHE3 cells and A6/C1 cells was rather weak. This finding could be interpreted to indicate that introduction of NHE3 into A6 cells may lead to random targeting of the transfected Na+-H+ exchanger to the apical and basolateral cell surface. This possibility, however, is very unlikely, because inhibition of basolateral Na+-H+ exchanger in A6/NHE3 was indistinguishable from that in A6/C1 cells (parent cell line). Furthermore, in the experiments examining PKA modulation of apical and basolateral Na+-H+ exchanger in A6/NHE3 cells we have shown that the PKA agonist 8-bromo-cAMP selectively inhibited apical NHE activity, whereas it stimulated basolateral NHE activity. Although consistent with earlier findings in OK cells, where NHE3 mediates apical transport (Helmle-Kolb et al. 1990), the cellular context has to be considered when accounting for the distinctive regulation of NHE3 by PKA (Wakabayashi et al. 1997), since responsiveness of NHE3 to agonists that elevate cAMP has been shown to depend largely on expression of cell-specific co-factors (Weinman et al. 1993; Yun et al. 1997) and most probably requires direct phosphorylation of the transport protein (Moe et al. 1995; Kurashima et al. 1997; Orlowski & Grinstein, 1997). As regards activation of basolateral XNHE activity by PKA, findings in A6/C1 and A6/NHE3 cells are comparable to PKA modulation of the trout β-NHE isoform (Borgese et al. 1992) and the exchanger from Xenopus laevis oocytes (XL-NHE) (Busch et al. 1995), but distinct from that reported for NHE1 (Noël & Pouysségur, 1995). PCR amplification and sequencing of the A6 cell exchanger revealed that XNHE is nearly identical to the XL-NHE in Xenopus laevis oocytes (Busch, 1997; F. Di Sole & F. Verrey, unpublished observations), thereby highlighting the possibility that amphibian NHEs are probably a distinct class of Na+-H+ exchanger.
In the present study we have shown that activation of PKC by PMA resulted in an inactivation of both apical NHE3 (Fig. 5) and basolateral XNHE activity in transfected (Fig. 5) and untransfected A6 cell cultures (data not shown). Despite earlier controversy, it is now generally accepted that PMA and/or PKC inhibit endogenous as well as transfected NHE3 activity in a variety of cells, including OK cells (Helmle-Kolb et al. 1990), NHE3 transfected Chinese hamster ovary cells (AP-1) (Kandasamy et al. 1995) and NHE3 transfected Chinese hamster lung fibroblasts (PS120) (Yip et al. 1997). Somewhat unexpected is the type of control of basolateral XNHE activity, since PKC has been reported to upregulate the activity of XL-NHE, which as stated above is highly homologous to XNHE. Although direct proof is as yet not available, a decrease in basolateral membrane area, as recently observed in A6 cells in response to PKC stimulation (Beron et al. 1997), could explain the overall inhibitory effect of PMA on basolateral XNHE activity. Alternatively, kidney-specific splicing of the amphibian Na+-H+ exchanger may be responsible for the ability of the exchanger to positively or negatively respond to PKC stimulation.
The results of this study provide direct evidence that the activity of NHE3 can be regulated by adenosine. Most notable, our studies suggest that stimulation of A1 receptors (linked to activation of PKC) and A2 receptors (linked to activation of PKA) produce qualitatively similar effects on NHE3 activity (inhibition of transport rate). That activation of A1 and A2 receptors contributes to NHE3 regulation is concluded from the following observations: (i) the ability of the A1 receptor blocker CPX to antagonize NHE3 modulation following apical adenosine addition, (ii) the ability of the PKA antagonist H89 to abrogate basolaterally evoked NHE regulation by adenosine in A6/NHE3 and A6/C1 cells, and finally, our recent findings that the A2 receptor blocker CSC (8-(3-chlorostyryl)caffeine) completely prevented adenosine effects in A6/C1 cells following basolateral nucleoside addition (Casavola et al. 1997).
However, as opposed to modulation of XNHE, A1 and A2 receptor activation of the PKC and PKA pathway appears not to account fully for the overall effect of adenosine on NHE3 activity. Inhibition of PKC by calphostin C prevented inactivation of NHE3 following apical adenosine only partially, and PKA antagonism by H89 did not fully avert transport responses stimulated by basolateral adenosine addition. This suggests that an, as yet unknown, mechanism participates in adenosine regulation of apical NHE3 activity in A6/NHE3 cells. Preliminary experiments in our laboratory indicate that stimulation of A3 receptors induces an elevation of intracellular [Ca2+] in A6/C1 cells. Interestingly, epithelial isoforms of the Na+-H+ exchanger (NHE2 and NHE3) have been suggested to become inhibited when [Ca2+]i increases (Cohen et al. 1990; Burns et al. 1991). Whether an increase in [Ca2+]i participates in adenosine modulation of apical NHE3 activity remains to be established.
Finally, our data confirm our previous observations (Casavola et al. 1997) that XNHE is under the control of adenosine. This points to a role of adenosine in acute regulation of intracellular pH, serving as a starting signal to change renal electrolyte excretion. In view of this argument, it is worth mentioning that K+ excretion has been observed to increase as intracellular pH rises (Gekle et al. 1996).
In conclusion, transfected A6/C1 cultures allowed us to confirm that adenosine decreases the activity of the renal brush border membrane Na+-H+ exchanger NHE3 with PKC playing a role in transport responses to A1 receptor activation and PKA being involved in transport responses effected by A2 receptor stimulation. With respect to kidney function, we can only speculate about the possible significance of inhibition of NHE3 by adenosine. NHE3 plays a role in net Na+ and HCO3− reabsorption in renal tubules. Short term regulation of NHE3 activity may be important in changing intraluminal sodium concentrations and altering Na+ reabsorption by the kidney, thus favouring reduction of oxygen consumption during ischaemic episodes. Under ischaemic conditions adenosine-dependent inhibition of NHE3 activity may also regulate the rate of bicarbonate reabsorption in the vulnerable outer renal medulla, which is routinely hypertonic. Furthermore, following intrarenal administration of adenosine, inhibition of NHE3 could explain the loss of Na+ that is not attributable to changes in renal haemodynamics (Yagil et al. 1994). In addition, responsiveness of NHE3 may also explain rapid reduction of salt and volume re-absorption in the proximal tubule that accompanies blood pressure challenge. It is noteworthy in this regard that Zhang and co-workers (Zhang et al. 1996) observed that acute pressure natriuresis promoted a removal of apical Na+-H+ exchangers in the proximal tubule. The validity of the above assumptions, however, remain to be established.
Acknowledgments
We thank Dr Steven J. Reshkin for helpful discussions, Dr Helene Hilfiker-Pfister for expert advice and help with RT-PCR and Southern blotting and Dr Hans-Jochen Lang from Hoechst for providing the HOE694 compound. We also thank Ellen Thelen for excellent graphical assistance and Jürgen Langer for secretarial assistance. Research was funded by the Swiss National Sciences Foundation (grant no. 32–30785.91) and the Sandoz Stiftung (Basel, Switzerland).
References
- Amemiya M, Loffing J, Lötscher M, Kaissling B, Alpern RJ, Moe OW. Expression of NHE-3 in the apical membrane of rat renal proximal tubule and thick ascending limb. Kidney International. 1995;48:1206–1215. doi: 10.1038/ki.1995.404. [DOI] [PubMed] [Google Scholar]
- Amemiya M, Yamaji Y, Cano A, Moe OW, Alpern RJ. Acid incubation increases NHE-3 mRNA abundance in OKP cells. American Journal of Physiology. 1995;269:C126–133. doi: 10.1152/ajpcell.1995.269.1.C126. [DOI] [PubMed] [Google Scholar]
- Azarani A, Goltzman D, Orlowski J. Parathyroid hormone and parathyroid hormone-related peptide inhibit the apical Na+/H+ exchanger NHE-3 isoform in renal cells (OK) via a dual signaling cascade involving protein kinase A and C. Journal of Biological Chemistry. 1995;270:20004–20010. doi: 10.1074/jbc.270.34.20004. 10.1074/jbc.270.34.20004. [DOI] [PubMed] [Google Scholar]
- Beron J, Forster I, Beguin P, Geering K, Verrey F. Phorbol 12-myristate 13-acetate down-regulates Na, K-ATPase independent of its protein kinase C site: decrease in basolateral cell surface area. Molecular Biology of the Cell. 1997;8:387–398. doi: 10.1091/mbc.8.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biemesderfer D, Rutherford PA, Nagy T, Pizzonia JH, Abu-Alfi AK, Aronson P. Monoclonal antibodies for high-resolution localization of NHE3 in adult and neonatal rat kidney. American Journal of Physiology. 1997;273:F289–299. doi: 10.1152/ajprenal.1997.273.2.F289. [DOI] [PubMed] [Google Scholar]
- Borgese F, Malapert M, Fievet B, Pouysségur J, Motais R. The cytoplasmic domain of the Na+-H+ exchangers (NHEs) dictates the nature of the hormonal response: behaviour of a chimeric human NHE1/trout NHE antiporter. Proceedings of the National Academy of Sciences of the USA. 1992;91:5431–5435. doi: 10.1073/pnas.91.12.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer CB. Cytomegalovirus plasmid vectors for permanent lines of polarized epithelial cells. Methods in Cell Biology. 1994;43:233–245. doi: 10.1016/s0091-679x(08)60606-8. [DOI] [PubMed] [Google Scholar]
- Burns KD, Homma T, Harris RC. Regulation of Na+-H+ exchange by ATP depletion and calmodulin antagonism in renal epithelial cells. American Journal of Physiology. 1991;261:F607–616. doi: 10.1152/ajprenal.1991.261.4.F607. [DOI] [PubMed] [Google Scholar]
- Busch S. Cloning and sequencing of the cDNA encoding for a Na+/H+ exchanger from Xenopus laevis oocytes (XL-NHE) Biochimica et Biophysica Acta. 1997;1325:13–16. doi: 10.1016/s0005-2736(97)00011-4. [DOI] [PubMed] [Google Scholar]
- Busch S, Wieland T, Esche H, Jacobs KH, Siffert W. G Protein regulation of the Na+/H+ antiporter in Xenopus laevis oocytes. Journal of Biological Chemistry. 1995;270:17898–17901. doi: 10.1074/jbc.270.30.17898. 10.1074/jbc.270.30.17898. [DOI] [PubMed] [Google Scholar]
- Cabado AG, Yu FH, Kapus A, Likacs G, Grinstein S, Orlowski J. Distinct structural domains confer cAMP sensitivity and ATP dependence to the Na+/H+ exchanger NHE3 isoform. Journal of Biological Chemistry. 1996;271:3590–3599. doi: 10.1074/jbc.271.7.3590. 10.1074/jbc.271.7.3590. [DOI] [PubMed] [Google Scholar]
- Casavola V, Guerra L, Reshkin SJ, Jacobson KA, Murer H. Polarization of adenosine effects on intracellular pH in A6 renal epithelial cells. Molecular Pharmacology. 1997;51:516–523. [PMC free article] [PubMed] [Google Scholar]
- Casavola V, Guerra L, Reshkin SJ, Jacobson KA, Verrey F, Murer H. Effect of adenosine on Na+ and Cl− currents in A6 monolayers. Receptor localization and messenger involvement. Journal of Membrane Biology. 1996;151:237–245. doi: 10.1007/s002329900074. [DOI] [PubMed] [Google Scholar]
- Cohen ME, Reinlib L, Watson AJ, Gorelick F, Rys-Sikora K, Tse M, Rood RP, Czernik AJ, Sharp GW, Donowitz M. Rabbit ileal villus cell brush border Na+/H+ exchange is regulated by Ca2+/calmodulin-dependent protein kinase II, a brush border membrane protein. Proceedings of the National Academy of Sciences of the USA. 1990;87:8990–8994. doi: 10.1073/pnas.87.22.8990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander G, Amiel C. Extracellular nucleotides as modulators of renal tubular transport. Kidney International. 1995;47:1500–1506. doi: 10.1038/ki.1995.212. [DOI] [PubMed] [Google Scholar]
- Gekle M, Golenhofen N, Oberleitner H, Silbernagl S. Rapid activation of Na+/H+ exchange by aldosterone in renal epithelial cells requires Ca2+ and stimulation of a plasma membrane proton conductance. Proceedings of the National Academy of Sciences of the USA. 1996;93:10500–10504. doi: 10.1073/pnas.93.19.10500. 10.1073/pnas.93.19.10500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra L, Casavola V, Vilella S, Verrey F, Helmle-Kolb C, Murer H. Vasopressin-dependent control of basolateral Na/H-exchange in highly differentiated A6-cell monolayers. Journal of Membrane Biology. 1993;135:209–216. doi: 10.1007/BF00211092. [DOI] [PubMed] [Google Scholar]
- Helmle-Kolb C, Di Sole F, Forgo J, Hilfiker H, Tse CM, Casavola V, Donowitz M, Murer H. Regulation of the transfected Na+/H+ exchanger NHE3 in MDCK cells by vasotocin. Pflügers Archiv. 1997;434:123–131. doi: 10.1007/s004240050372. [DOI] [PubMed] [Google Scholar]
- Helmle-Kolb C, Montrose MH, Murer H. Parathyroid hormone regulation of Na+/H+ exchange in opossum kidney cells: polarity and mechanisms. Pflügers Archiv. 1990;416:615–623. doi: 10.1007/BF00370605. [DOI] [PubMed] [Google Scholar]
- Kandasamy RA, Yu FH, Harris R, Boucher A, Hanrahan JW, Orlowski J. Plasma membrane Na+/H+ exchanger isoforms (NHE-1, −2, and −3) are differently responsive to second messenger agonists of the protein kinase A and C pathways. Journal of Biological Chemistry. 1995;270:29209–29216. doi: 10.1074/jbc.270.49.29209. [DOI] [PubMed] [Google Scholar]
- Kurashima K, Yu FH, Cabado AG, Szabo EZ, Grinstein S, Orlowski J. Identification of sites required for down-regulation of Na+/H+ exchanger NHE3 activity by cAMP-dependent protein kinase. Journal of Biological Chemistry. 1997;272:28672–28679. doi: 10.1074/jbc.272.45.28672. [DOI] [PubMed] [Google Scholar]
- Lang MA, Preston AS, Handler JS, Forrest JN., Jr Adenosine stimulates Na+ transport in kidney A6 epithelia in culture. American Journal of Physiology. 1985;249:C330–336. doi: 10.1152/ajpcell.1985.249.3.C330. [DOI] [PubMed] [Google Scholar]
- Levine SA, Montrose MH, Tse CM, Donowitz M. Kinetics and regulation of three cloned mammalian Na+/H+ exchangers stably expressed in a fibroblast cell line. Journal of Biological Chemistry. 1993;268:25527–25535. [PubMed] [Google Scholar]
- Mi Z, Jackson EK. Metabolism of exogenous cyclic AMP to adenosine in the rat kidney. Journal of Pharmacology and Experimental Therapeutics. 1995;273:728–733. [PubMed] [Google Scholar]
- Moe OW, Amemiya M, Yamaji Y. Activation of protein kinase A acutely inhibits and phosphorylates Na/H exchanger NHE-3. Journal of Clinical Investigation. 1995;96:2187–2194. doi: 10.1172/JCI118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montrose MH, Murer H. Regulation of intracellular pH by cultured opossum kidney cells. American Journal of Physiology. 1990;259:C110–120. doi: 10.1152/ajpcell.1990.259.1.C110. [DOI] [PubMed] [Google Scholar]
- Noël J, Pouysségur J. Hormonal regulation, pharmacology, and membrane sorting of vertebrate Na+/H+ exchanger isoforms. American Journal of Physiology. 1995;268:C283–296. doi: 10.1152/ajpcell.1995.268.2.C283. [DOI] [PubMed] [Google Scholar]
- Orlowski J, Grinstein S. Na+/H+ exchangers of mammalian cells. Journal of Biological Chemistry. 1997;272:22373–22376. doi: 10.1074/jbc.272.36.22373. [DOI] [PubMed] [Google Scholar]
- Osswald H, Gleiter C. Renale Wirkungen des Adenosins: mögliche Konsequenzen für die Nierentransplantation. (Renal effects of adenosine: possible consequences for kidney transplantation.) Zentralblatt für Chirurgie. 1993a;118:90–102. [PubMed] [Google Scholar]
- Osswald H, Gleiter C. Hypernatriämie und Nierenfunktion. (Hypernatremia and kidney function.) Zentralblatt für Chirurgie. 1993b;118:267–272. [PubMed] [Google Scholar]
- Osswald H, Muhlbauer B, Vallon V. Adenosine and tubuloglomerular feedback. Blood Purification. 1997;15:243–252. doi: 10.1159/000170342. [DOI] [PubMed] [Google Scholar]
- Preston AS, Muller J, Handler JS. Dexamethasone accelerates differentiation of A6 epithelia and increases response to vasopressin. American Journal of Physiology. 1988;255:C661–666. doi: 10.1152/ajpcell.1988.255.5.C661. [DOI] [PubMed] [Google Scholar]
- Seney FD, Jr, Seikaly MG. Adenosine inhibits Na+ uptake in the loop of Henle (abstract) Clinical Research. 1989;37:501. [Google Scholar]
- Spindler B, Mastroberardino L, Custer M, Verrey F. Characterization of early aldosterone-induced RNAs identified in A6 kidney epithelia. Pflügers Archiv. 1997;434:323–331. doi: 10.1007/s004240050403. [DOI] [PubMed] [Google Scholar]
- Verrey F. Antidiuretic hormone action in A6 cells: effect on apical Cl and Na conductances and synergism with aldosterone for NaCl reabsorption. Journal of Membrane Biology. 1994;138:65–76. doi: 10.1007/BF00211070. [DOI] [PubMed] [Google Scholar]
- Weinman EJ, Steplock D, Shenolikar S. cAMP-mediated inhibition of the renal brush border membrane Na+-H+ exchanger requires a dissociable phosphoprotein cofactor. Journal of Clinical Investigations. 1993;92:1781–1786. doi: 10.1172/JCI116767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi A, Shingekawa M, Pouysségur J. Molecular physiology of vertebrate Na+/H+ exchangers. Physiological Reviews. 1997;77:51–74. doi: 10.1152/physrev.1997.77.1.51. [DOI] [PubMed] [Google Scholar]
- Yagil C, Gurion K, Yagil Y. The effects of adenosine on transepithelial resistance and sodium uptake in the inner medullary collecting duct. Pflügers Archiv. 1994;427:225–232. doi: 10.1007/BF00374528. [DOI] [PubMed] [Google Scholar]
- Yip JW, Ko WH, Viberti G, Hunganir RL, Donowitz M, Tse CM. Regulation of the epithelial brush border Na+/H+ exchanger isoform 3 stably expressed in fibroblasts by fibroblast growth factor and phorbol esters is not through changes in phosphorylation of the exchanger. Journal of Biological Chemistry. 1997;272:18473–18480. doi: 10.1074/jbc.272.29.18473. [DOI] [PubMed] [Google Scholar]
- Yun CHC, Oh S, Zizak M, Steplock D, Tsao S, Tse C-M, Weinman EJ, Donowitz M. cAMP-mediated inhibition of the epithelial brush border Na+/H+ exchanger, NHE3, requires an associated regulatory protein. Proceedings of the National Academy of Sciences of the USA. 1997;94:3010–3015. doi: 10.1073/pnas.94.7.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun CHC, Tse C-M, Nath SK, Levine SA, Brant SR, Donowitz M. Mammalian Na+/H+ exchanger gene family: structure and function studies. American Journal of Physiology. 1995;269:G1–11. doi: 10.1152/ajpgi.1995.269.1.G1. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Mircheff AK, Hensley CB, Magyar CE, Warnock DG, Chambrey R, Yip K-P, Marsh DJ, Holstein-Rathlou N-H, McDonough AA. Rapid redistribution and inhibition of renal sodium transporters during acute pressure natriuresis. American Journal of Physiology. 1996;270:F1004–1014. doi: 10.1152/ajprenal.1996.270.6.F1004. [DOI] [PubMed] [Google Scholar]


