Abstract
In arterioles of the hamster cheek pouch, vasodilatation and vasoconstriction can spread via the conduction of electrical signals through gap junctions between cells that comprise the vessel wall. However, conduction in resistance networks supplying other tissues has received relatively little attention. In anaesthetized hamsters, we have investigated the spread of dilatation and constriction along feed arteries and arterioles of the retractor muscle, which is contiguous with the cheek pouch.
When released from a micropipette, acetylcholine (ACh) triggered vasodilatation that spread rapidly along feed arteries external to the muscle and arterioles within the muscle. Responses were independent of changes in wall shear rate, perivascular nerve activity, or release of nitric oxide, indicating cell-to-cell conduction.
Vasodilatation conducted without decrement along unbranched feed arteries, yet decayed markedly in arteriolar networks. Thus, branching of the conduction pathway dissipated the vasodilatation.
Noradrenaline (NA) or a depolarizing KCl stimulus evoked constriction of arterioles and feed arteries of the retractor muscle that was constrained to the vicinity of the micropipette. This behaviour contrasts sharply with the conduction of vasodilatation in these microvessels and with the conduction of vasoconstriction elicited by NA and KCl in cheek pouch arterioles.
Focal electrical stimulation produced constriction that spread rapidly along feed arteries and arterioles. These responses were inhibited by tetrodotoxin or prazosin, confirming the release of NA along perivascular sympathetic nerves, which are absent from arterioles studied in the cheek pouch. Thus, sympathetic nerve activity co-ordinated the contraction of smooth muscle cells as effectively as the conduction of vasodilatation co-ordinated their relaxation.
In the light of previous findings in the cheek pouch, the properties of vasoconstriction and vasodilatation in feed arteries and arterioles of the retractor muscle indicate that substantive differences can exist in the nature of signal transmission along microvessels of tissues that differ in structure and function.
Vasomotor responses initiated at discrete locations in a resistance network can spread to encompass multiple branches and thereby co-ordinate the distribution and magnitude of tissue blood flow. The mechanisms underlying this behaviour have been most extensively studied in arterioles supplying the epithelial region of the hamster cheek pouch (Segal & Duling, 1986, 1989; Little et al. 1995; Xia & Duling, 1995; Welsh & Segal, 1998). In these vessels, vasodilatation evoked by acetylcholine (ACh) spreads via the conduction of hyperpolarization (Welsh & Segal, 1998), which results in the co-ordinated relaxation of smooth muscle cells along the vessel wall. In turn, vasoconstriction triggered by noradrenaline (NA) or a concentrated KCl stimulus spreads via the conduction of depolarization and smooth muscle contraction (Xia & Duling, 1995; Welsh & Segal, 1998). Although opposite in polarity, the conduction of both vasodilatation and vasoconstriction reflects functional coupling among vascular (endothelial, smooth muscle) cells, with gap junctions serving a key role in the coupling process (Segal & Duling, 1986, 1989; Little et al. 1995).
Mechanisms of vasomotor control can vary between vessels and the tissues they supply. The cheek pouch is a specialized epithelial tissue with low metabolic demand. Unlike the cheek pouch, skeletal muscle experiences tremendous increases in blood flow in response to muscle fibre contraction (Laughlin & Armstrong, 1982; Welsh & Segal, 1997). Moreover, muscle blood flow is controlled not only by arterioles embedded within the muscle fibres, but ‘ascends’ to encompass the feed arteries that lie external to the muscle (Hilton, 1959; Folkow et al. 1971; Williams & Segal, 1993; Welsh & Segal, 1997). The wall morphology of feed arteries supplying the retractor muscle consists of a circumferential layer of smooth muscle cells surrounding a monolayer of endothelial cells aligned with the vessel axis (Welsh & Segal, 1996; S. Segal, unpublished observations). This organization is similar to that described for arterioles in several tissues, including those of the cheek pouch and skeletal muscle (Haas & Duling, 1997). Nevertheless, the functional properties of signal transmission along the vessel wall may differ with location of and within a resistance network.
In the present study, our goal was to determine whether variability exists in the properties of conducted vasomotor responses between tissues that differ in function and between feed arteries and arterioles that supply the same tissue. Experiments were performed in vivo using the hamster retractor muscle. This thin, parallel-fibred muscle is contiguous with the cheek pouch (West, 1958) and enables ready access to extraparenchymal feed arteries as well as intramuscular arterioles (Nakao & Segal, 1995; Welsh & Segal, 1996, 1997). We tested the hypothesis that vasodilatation and vasoconstriction would spread along arterioles and feed arteries of the retractor muscle in the manner shown for arterioles of the hamster cheek pouch (Segal et al. 1989; Welsh & Segal, 1998).
METHODS
Animal care and use
All procedures were approved by the Institutional Animal Care and Use Committee of The John B. Pierce Laboratory and performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Research Council (USA).
Male golden hamsters (n = 23 hamsters; 80-110 g) were maintained at 24°C on a 14 h-10 h light-dark cycle and provided with rodent chow and water ad libitum. Each hamster was anaesthetized with pentobarbital sodium (60 mg kg−1, i.p.) and tracheotomized (PE 190 tubing, Clay Adams) to maintain airway patency. The right carotid artery was cannulated (PE 50) for monitoring arterial pressure; a second cannula (PE 50) was secured in the left femoral vein to replace fluids and to maintain anaesthesia during experiments (10 mg pentobarbital (ml isotonic saline)−1, infused at 410 μl h−1). Oesophageal temperature was measured continuously with a thermocouple and maintained at 37-38°C using conducted heat.
An incision was made through the skin overlying the right retractor muscle. The exposed tissue was superfused continuously (4-5 ml min−1) with a bicarbonate-buffered physiological saline solution (composition (mM): NaCl, 131.9; KCl, 4.7; CaCl2, 2.0; MgSO4, 1.2; and NaHCO3, 18) equilibrated with 95 % N2 and 5 % CO2 (pH 7.4, 34-35°C). All reagents were obtained from Sigma. Using a stereo microscope (DRC, Zeiss), connective tissue covering the dorsal surface of the muscle was carefully dissected away and each end of the muscle was secured in an atraumatic clamp (Welsh & Segal, 1996). The in situ muscle length was recorded, then the muscle was severed from its origin and insertion and reflected to expose the ventral surface. Nerve bundles and superficial connective tissue were removed to optimize visibility and the access of micropipettes to microvessels. The preparation was then repositioned onto a custom-built transparent acrylic platform (Technical Services, The John B. Pierce Laboratory, Inc.) for viewing the retractor muscle and its microvascular supply (Nakao & Segal, 1995; Welsh & Segal, 1996). Each end of the muscle was connected to a micrometer spindle and in situ length was restored; all experiments were performed at this position. At the end of the day's experiments, an overdose of pentobarbital was delivered through the venous cannula.
Video microscopy
The acrylic platform was transferred to the stage of an intravital microscope (ACM, Zeiss) and the preparation equilibrated for 60 min. Microvessels were observed in bright field through × 10 widefield eyepieces and a × 25 objective (Leitz, numerical aperture (n.a.) = 0.35) during transillumination (condenser n.a. = 0.32) from a 100 W halogen lamp. The image was directed to a CCD video camera (C2400, Hamamatsu) coupled to a video monitor (PVM 1343MD, Sony). All of the vessels studied demonstrated brisk and reversible dilatation in response to sodium nitroprusside (10 μM) delivered in the superfusion fluid.
Microiontophoresis
Borosilicate glass capillaries were pulled (model P-87; Sutter Instruments) to produce micropipettes with a tip internal diameter of ∼1 μm, then filled with acetylcholine (ACh, 1 M), noradrenaline (NA, 0.5 M), or KCl (2 M). A stimulus micropipette was secured to a Leitz micromanipulator and connected to an iontophoresis programmer (model 160; World Precision Instruments) via a Ag-AgCl wire; a reference Ag-AgCl wire was secured at the edge of the preparation. The microscope stage was designed so that the micromanipulators moved as a unit with the hamster preparation, enabling responses to be investigated at remote locations without disturbing the position of the micropipettes.
Based upon previous (Segal et al. 1989; Kurjiaka & Segal, 1995a) and present (data not shown) stimulus-response relationships, microiontophoresis current (1 μA for 1 s) for ACh and NA stimuli was standardized to elicit peak responses at the site of release. For KCl, stimuli of up to 5 s duration were also evaluated. Control experiments verified negligible diffusion or convection of the stimulus from the site of release, a lack of tachyphylaxis, and the absence of non-specific responses to microiontophoresis (Segal, 1991; Kurjiaka & Segal, 1995a). A separate, identical stimulus was applied for observing each site along a vessel, with 2-3 min allowed for complete recovery between stimuli.
Perivascular nerve activation
Perivascular sympathetic nerves were stimulated electrically with 1 ms pulses (at 100 V, 32 Hz) for 5 s to elicit a peak response (S48 Grass stimulator) using a micropipette positioned as above and filled with 0.9 % saline (Kurjiaka & Segal, 1995b; Welsh & Segal, 1996). Functional experiments have confirmed that vasodilator nerves are absent from feed arteries and arterioles of the retractor muscle (Welsh & Segal, 1996, 1997).
Measurements and calculations
Data were acquired at 40 Hz using a MacLab system (CB Sciences, Milford, MA, USA) coupled to a Macintosh IIVX computer. The diameter (D) of the vessel lumen was measured as the width of the red blood cell column from the monitor using a video calliper (Microcirculation Research Institute, Texas A & M University, College Station, TX, USA) calibrated against a stage micrometer (100 × 0.01 = 1 mm; Graticules, Tonbridge, Kent, UK). Spatial resolution on the monitor screen was ∼1 μm with a total magnification of × 1140. Vasomotor responses were evaluated at five locations along a vessel, as defined below in ‘Experimental stimuli’.
At a given diameter, an increase in blood flow through a vessel will increase luminal shear stress (wall shear rate (WSR) × blood viscosity) and thereby stimulate vasodilator production (e.g. nitric oxide, NO) by the endothelium (Davies, 1995). To determine whether responses to ACh (see Results) reflected flow-induced vasodilatation (Pohl et al. 1986; Smiesko et al. 1989; Davies, 1995), WSR was followed continuously and quantified at rest (control), at 1 s prior to the onset of dilatation, and at the peak diameter response. Respective values were averaged over 1 s (i.e. 5-6 cardiac cycles). Centreline red blood cell velocity (Vrbc) was monitored continuously in feed arteries using an optical Doppler velocimeter (Borders & Granger, 1984); mean red blood cell velocity (Vm) was calculated as Vrbc/1.6 and WSR was calculated as 8Vm/D (Kurjiaka & Segal, 1995a; Welsh & Segal, 1996). These measurements were more readily obtained in feed arteries due to the lack of surrounding muscle fibres.
The temporal nature of feed artery responses to ACh (see below) was evaluated in two ways. First, ‘response duration’ was taken as the interval between the onset of vasodilatation and 50 % of recovery to resting diameter. Second, the ‘time to peak response’ was taken as the interval between the onset of vasodilatation and attainment of peak diameter.
Experimental stimuli
In the present experiments, we tested whether ACh, NA and KCl can trigger the conduction of vasomotor responses in feed arteries and arterioles of the retractor muscle in the manner shown for arterioles of the cheek pouch (Segal et al. 1989; Welsh & Segal, 1998). A stimulus micropipette was positioned with the tip adjacent to the distal end of a feed artery, just before the point of entry of the vessel into the retractor muscle (Fig. 1). Alternatively, the tip was positioned at the distal end of an arteriole (see below). Vasomotor responses were evaluated at the site of stimulation (referred to as ‘local’ or ‘stimulus origin’) and at four sites upstream along the vessel (390, 780, 1170 and 1560 μm), defined using a calibrated eyepiece reticle (full scale = 390 μm at × 250 optical magnification).
Figure 1. Conducted vasodilatation in feed arteries and arterioles of retractor muscle.
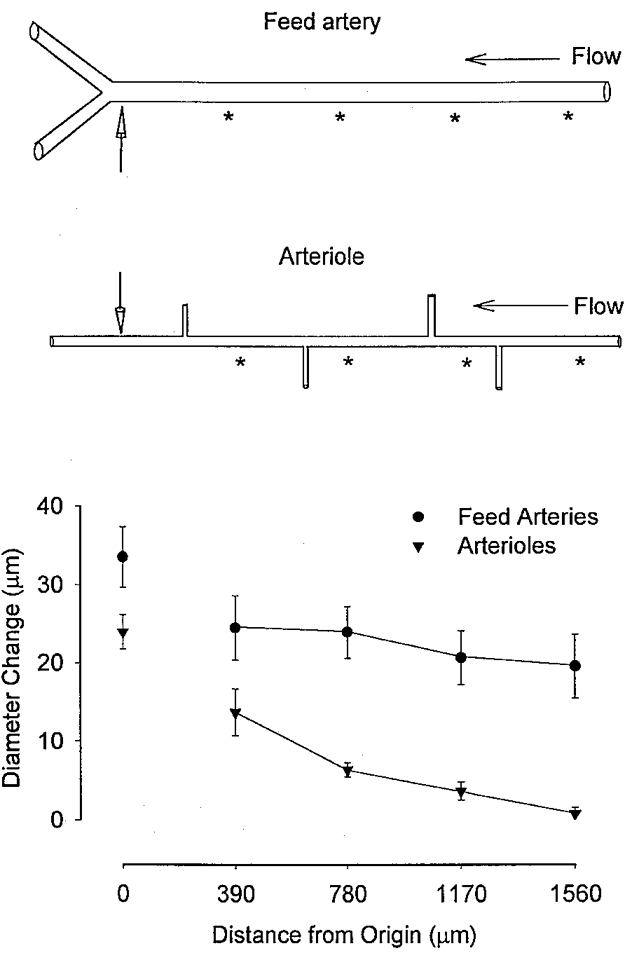
Top panel, schematic illustration of feed arteries and arterioles of hamster retractor muscle. Note the lack of branching in feed arteries (until they enter retractor muscle; not shown) and the extent of branching typically encountered along intramuscular arterioles. An acetylcholine (ACh) stimulus was delivered using microiontophoresis (1 μA for 1 s) at the ‘local’ site indicated by the location of the micropipette tip (vertical arrows); noradrenaline and KCl were applied in a similar fashion as detailed in Methods. Vasomotor responses (i.e. Diameter Change) were calculated (as peak response diameter - control diameter) at the stimulus origin and at defined distances (indicated by asterisks) along a vessel (i.e. Distance from Origin). Bottom panel illustrates summary data from 6 feed arteries (diameter: control, 68 ± 5 μm; peak, 102 ± 6 μm; n = 6) and 5 arterioles (control, 28 ± 4 μm; peak, 52 ± 6 μm). Note the significant (P < 0.05) decay of conducted responses along arterioles compared with feed arteries.
In vitro studies of isolated cheek pouch arterioles have suggested that a component of conducted vasodilatation evoked by ACh involves the production and release of NO (Doyle & Duling, 1997). Thus, once control measurements were obtained, a role for NO in the ACh response of retractor feed arteries was investigated by equilibrating the preparation (20 min) with the competitive nitric oxide synthase (NOS) inhibitor Nω-nitro-L-arginine (LNA, 50 μM). To test the specificity of the action of LNA, L-arginine (1 or 2 mM) was added to the superfusate during LNA exposure and responses to ACh were re-evaluated. As with the evaluation of WSR (above) the effect of LNA on conduction was more readily studied in feed arteries due to their greater accessibility.
The effect of NA release from perivascular sympathetic nerves in response to electrical stimulation was evaluated by adding prazosin (0.1 μM) or tetrodotoxin (TTX, 1 μM) to the superfusate (Vonderlage, 1981; Kurjiaka & Segal, 1995b; Welsh & Segal, 1996). To evaluate responses to a vasoconstrictor (i.e. depolarizing) stimulus that acts independently of receptors (Xia & Duling, 1995; Segal & Neild, 1996; Welsh & Segal, 1998), KCl was delivered in the manner used for ACh and NA.
Influence of vessel branching
In vitro studies have shown that the branching characteristics of a resistance network can influence the distance over which signals spread from cell to cell along microvessels (Segal & Neild, 1996). However, there is a paucity of information with respect to the effect of branching on the conduction of vasomotor responses in vivo. To evaluate the behaviour of unbranched feed arteries in the light of responses in branching networks, we investigated the properties of conduction along arterioles, which branch extensively (typically 4-6 times along segments studied here; Fig. 1) within the retractor muscle (see Fig. 1 of Nakao & Segal, 1995). For these experiments, a stimulus micropipette was positioned with its tip at the distal end of a second- or third-order arteriolar segment (Fig. 1) and diameter responses were evaluated locally and at distances along the segment as defined above.
Statistics
Summary data are reported as means ±s.e.m. Repeated measures analysis of variance (ANOVA) was used to determine whether the magnitude of vasomotor responses changed with distance and to evaluate the effects of LNA. When significant F-ratios were found with ANOVA, post hoc analyses were performed using Tukey's comparisons. Where appropriate, responses at a given site were compared using Student's paired t tests. Results were considered statistically significant with P < 0.05.
RESULTS
Mean arterial pressure in anaesthetized hamsters was typically ≥ 90 mmHg and stable throughout experimental procedures. Robust vasomotor tone was confirmed in all experiments by local responses to ACh (1 μA for 1 s); these responses were not different from the peak diameters recorded during exposure to 10 μM sodium nitroprusside. The distal portion of feed arteries was observed clearly for a length of nearly 2 mm; this typically represents half of the total length that these vessels span from the thoracodorsal artery.
Vasodilatation
In feed arteries and arterioles (control diameter, 68 ± 5 and 28 ± 4 μm, respectively), local dilatations in response to ACh were greater (P < 0.05) than the conducted responses observed at proximal locations (Fig. 1). The amplitude of the conducted vasomotor response did not change with distance along feed arteries (Fig. 1). In contrast, dilatation decayed progressively with distance along arterioles (Fig. 1). In all cases, once vasodilatation was initiated (typically ∼3 s following an ACh stimulus), the entire vessel dilated with no measurable time delay between locations along the vessel.
Wall shear rate
As illustrated in Fig. 2, wall shear rate (WSR) in feed arteries averaged ∼1000 s−1 at rest (1063 ± 165 s−1; n = 6). Measured > 1 mm upstream from the stimulus, WSR increased slightly (to ∼1237 ± 151 s−1) but not significantly (P = 0.11) at 1 s prior to the onset of vasodilatation, then fell significantly (P < 0.05) to 789 ± 85 s−1 as dilatation ensued.
Figure 2. Red blood cell velocity and wall shear rate during conducted vasodilatation.
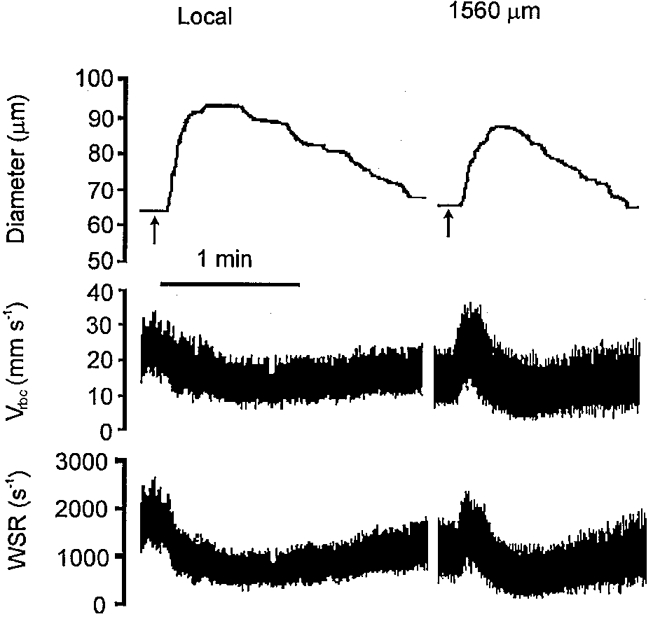
Representative record of feed artery diameter, red blood cell velocity (Vrbc), and wall shear rate (WSR) at the site of stimulation (Local) and at a conducted site (1560 μm upstream) in response to ACh microiontophoresis (1 μA for 1 s, given at arrows). At both locations, Vrbc and WSR were stable at rest for at least 1 min prior to the stimulus. At 1560 μm upstream, note the transient increase in WSR during the ≈3 s delay between delivery of the stimulus and onset of vasodilatation; WSR then fell below rest as dilatation ensued.
Influence of NO
The effects of LNA treatment are presented in Fig. 3 (and see legend). During equilibration with LNA, feed artery diameter decreased by 15-20 % (P < 0.05). Nevertheless, LNA had no effect on the change in diameter triggered by ACh, either locally or at distances along the vessel. Whereas the time-to-peak dilatation (typically 14-15 s) was also unaffected by LNA, the duration of responses to ACh was significantly attenuated (P < 0.05) at each site along the vessel. The addition of excess L-arginine reversed the effects of LNA on both resting diameter and the duration of vasodilator responses, confirming the competitive inhibition of NOS activity by LNA.
Figure 3. Effect of nitric oxide synthase inhibition on conducted vasodilatation.
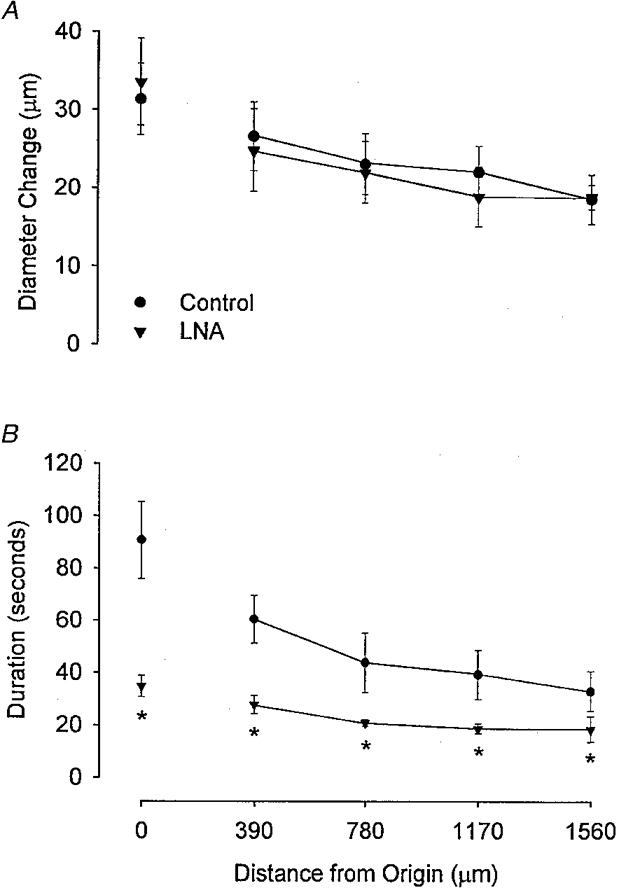
Summary data (n = 6) for the amplitude (A, Diameter Change) and duration (B) of vasodilator responses to ACh microiontophoresis (1 μA, 1 s) in hamster feed arteries under control conditions and in the presence of 50 μM LNA in the superfusate. Distance from Origin as in Fig. 1; Duration is defined in Methods. Note the LNA-induced attenuation of response duration with no effect on response amplitude. Control diameter at the local site (68 ± 6 μm) was significantly (P < 0.05) reduced (to 57 ± 5 μm) with LNA. L-Arginine (1 or 2 mM) added to the superfusate in the presence of LNA restored resting diameter (to 69 ± 3 μm) and reversed the attenuation in response duration (e.g. at the local site, from 36 ± 6 s with LNA to 76 ± 16 s with L-arginine). * Significantly different from control, P < 0.05.
Additional experiments (n = 4) were performed to test whether the lack of an effect of LNA on the peak vasodilator response to ACh was due to supra-threshold stimulation of feed arteries (control diameter, 63 ± 3 μm). With ejection current maintained at 1 μA, vasodilatation at the local site increased (P < 0.05), both in amplitude (from 17 ± 3 to 36 ± 6 μm) and duration (from 19 ± 5 to 68 ± 5 s) as pulse duration increased from 125 to 1000 ms (the standardized stimulus). Coincidentally, conducted vasodilatation (e.g. at a distance of 1560 μm) also increased (P < 0.05) in amplitude (from 11 ± 2 to 22 ± 2 μm) and duration (from 16 ± 4 to 30 ± 7 s). Treatment with LNA did not significantly affect these changes in diameter, but did attenuate (P < 0.05) the duration of local and conducted responses by an average of 52 and 26 %, respectively. These findings indicate that the release of NO consistently prolonged both local and conducted responses to ACh throughout this range of stimuli, but that a NO-independent (and stimulus-sensitive) mechanism underlies both local and conducted vasodilatation.
Vasoconstriction
In feed arteries and arterioles (resting diameters, 60 ± 4 and 39 ± 4 μm, respectively), microiontophoresis of NA produced constrictions of 31 ± 2 and 16 ± 2 μm, respectively. However, the diameter of neither feed arteries nor arterioles was affected at distances of 390 to 1560 μm upstream from the stimulus (Fig. 4). In a similar manner, KCl constricted feed arteries locally (by 26 ± 1 μm) yet had no effect on vessel diameter at upstream locations (Fig. 4). Extending the KCl stimulus for up to 5 s did not appreciably alter the nature of the responses; in some cases the local response was increased, but conduction did not occur. In one experiment, we confirmed that KCl produced local constriction of an arteriole without a remote response along the vessel; however, skeletal muscle fibres typically contracted when KCl was released within the tissue, curtailing further measurements.
Figure 4. Lack of conducted vasoconstriction along feed arteries and arterioles.
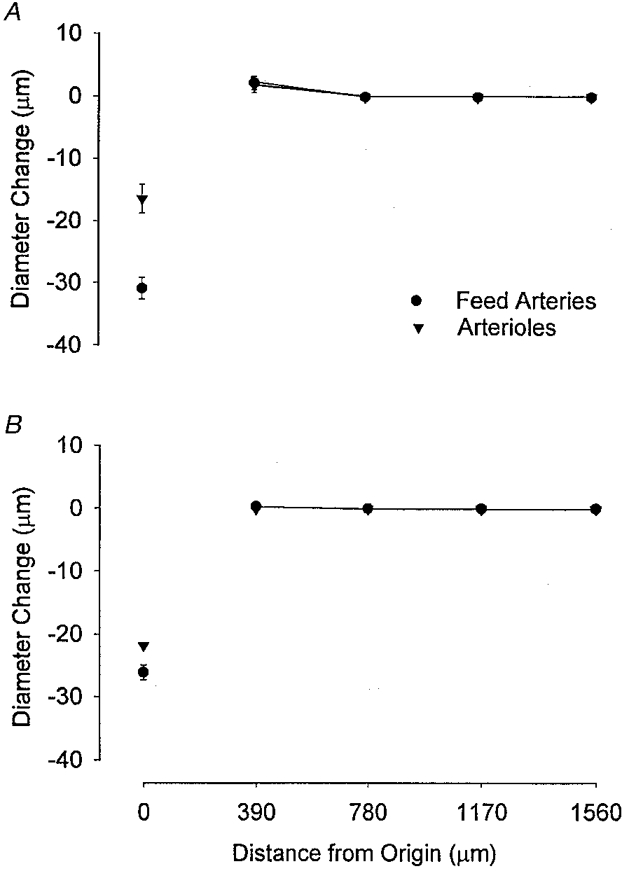
Summary data for vasoconstrictor responses triggered by NA (A) and KCl (B) microiontophoresis (1 μA, 1-5 s) onto feed arteries (•; n = 4-6) or arterioles (▾; n = 7 with NA, n = 1 with KCl) of hamster retractor muscle. Diameter Change and Distance from Origin as in Fig. 1. For both NA and KCl, note local vasoconstriction with lack of a response at upstream sites (• and ▾ are superimposed). Control diameters at stimulus origin: feed arteries, 60 ± 4 μm; arterioles, 39 ± 4 μm.
Electrical stimulation at the distal end of a feed artery evoked vasoconstriction that spread along the entire vessel (Fig. 5) and into arterioles (data not shown). The local response was greater (P < 0.05) than responses recorded at upstream sites, which were not significantly different from each other (Fig. 5). Conducted vasodilatation in these same vessels (evoked by ACh microiontophoresis) was similar in magnitude, although opposite in direction (Fig. 5). The spread of vasoconstriction evoked by electrical stimulation was inhibited by the addition of 1 μM TTX or prazosin to the superfusion solution, indicating the activation of perivascular sympathetic nerves (Kurjiaka & Segal, 1995B; Welsh & Segal, 1996).
Figure 5. Sympathetic vasoconstriction and conducted vasodilatation along feed arteries.
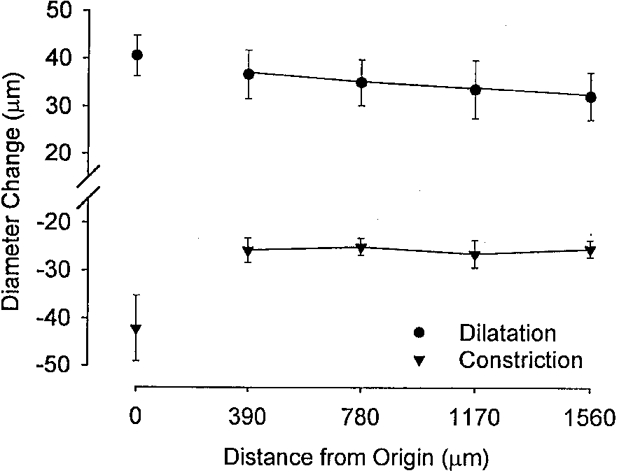
Summary data (n = 4) for constriction in response to perivascular nerve stimulation (1 ms pulses at 32 Hz for 5 s) and dilatation in response to ACh microiontophoresis (1 μA for 1 s) applied at the distal end of feed arteries using micropipettes (as illustrated in Fig. 1). Diameter Change and Distance from Origin as in Fig. 1. Note the persistence of both responses along the same vessels. Control diameter at stimulus origin, 71 ± 4 μm.
Control experiments (Welsh & Segal, 1996, 1997; S. Segal & G. Emerson, unpublished observations; n≥ 4 in all cases) performed on feed arteries and arterioles of the retractor muscle demonstrated the following relationships. (1) Neither prazosin nor TTX altered resting diameter. (2) Neither local nor conducted vasodilatation in response to ACh was altered during inhibition of perivascular nerves with TTX. (3) Vasoconstriction in response to NA microiontophoresis also did not spread during nerve inhibition with TTX.
DISCUSSION
We have investigated the properties by which vasomotor responses triggered by the focal application of ACh, NA and KCl spread along the arterioles and feed arteries that supply the retractor muscle of the hamster. Both dilatation and constriction were hypothesized to conduct in the manner previously shown (using the same stimuli) for arterioles supplying the hamster cheek pouch (Segal et al. 1989; Welsh & Segal, 1998). Analogous to observations in the cheek pouch, ACh triggered dilatation that conducted along arterioles and decayed markedly with distance (Fig. 1). However, vasodilatation conducted along feed arteries with negligible decrement. This difference between arterioles, which branch extensively within the tissue, and feed arteries, which typically do not branch, supports the contention that branching of the resistance network dissipates the conduction of vasomotor responses (Segal & Neild, 1996).
The constrictions evoked by the release of either NA or KCl from a micropipette were constrained to the stimulus site (Fig. 4), as was the response to electrical stimulation during nerve blockade. This behaviour is in striking contrast to the conduction of vasoconstriction in the cheek pouch (Segal et al. 1989; Xia & Duling, 1995). In turn, this difference between tissues suggests that smooth muscle cells of retractor arterioles and feed arteries are not functionally coupled to the extent shown for those of cheek pouch arterioles of similar size and wall morphology. Despite the inability of feed arteries and arterioles of the retractor muscle to conduct vasoconstriction beyond the site of stimulation, the activation of perivascular sympathetic nerves did evoke vasoconstriction that spread along these vessels via the release of NA onto smooth muscle cells (Fig. 5; Vonderlage, 1981; Welsh & Segal, 1996). It should be recognized that this neural mechanism for vasoconstriction is absent from arterioles in epithelial cheek pouch (Joyner et al. 1983). Thus, we hypothesize that regional differences in vascular innervation may modulate the biophysical determinants of smooth muscle cell coupling in arterioles and feed arteries.
Vasodilatation
In contrast to the decay of responses to ACh along arterioles in the cheek pouch (Segal & Duling, 1986), cremaster muscle (Segal, 1991) and retractor muscle (Fig. 1), vasodilatation in the retractor muscle spread without decrement along feed arteries. To determine whether flow-induced vasodilatation contributed to this behaviour, WSR was evaluated in feed arteries prior to and during vasomotor responses to ACh. As illustrated in Fig. 2, the observed rapid (2-3 s) changes in WSR were in contrast to the 8-40 s delay that typifies the onset of flow-induced vasodilatation following the elevation of luminal shear stress (Pohl et al. 1986; Smiesko et al. 1989). Further, the prompt and significant fall in WSR observed here (see Results) is unlike the more gradual restoration of WSR that accompanies vasodilatation induced by elevated flow (Pohl et al. 1986; Smiesko et al. 1989). Thus, flow-induced vasodilatation was not apparent in the present experiments.
The endothelial cell layer appears critical to both the initiation and conduction of the electrical signals that give rise to vasodilatation. This conclusion is based upon in vivo electrophysiological measurements and cell labelling using dye microinjection (Welsh & Segal, 1998), immunostaining for gap junctions (Little et al. 1995), and morphological analyses of the arteriolar wall (Haas & Duling, 1997). As shown in feed arteries (Fig. 3), the inhibition of endothelial NOS with LNA produced vasoconstriction that was reversed by L-arginine, demonstrating basal release of NO during these experiments. However, LNA had no effect on the amplitude (i.e. change in diameter) of either local or conducted responses to ACh stimuli. Previous findings have shown that an increase in tone above the resting level (e.g. with elevated oxygen (Segal, 1991; Kurjiaka & Segal, 1995b)) does not affect conducted vasodilatation. Nevertheless, and consistent with the behaviour of cheek pouch arterioles (Doyle & Duling, 1997), the inhibition of NOS activity diminished the duration of both local and conducted responses by nearly a half (Fig. 3) and did so throughout an 8-fold range in the ACh stimulus (see Results).
In cheek pouch arterioles, intracellular recordings have indicated that ACh evokes hyperpolarization that conducts along the endothelium and into smooth muscle, without direct electrical coupling between respective cell layers (Welsh & Segal, 1998). We suggest that the ACh-induced dilatation along feed arteries and arterioles of the retractor muscle reflects the corresponding hyperpolarization and relaxation of smooth muscle cells. Given that LNA had no effect on the amplitude of the vasodilator responses (Fig. 3), our present data suggest that the initiation of hyperpolarization by ACh is largely independent of NOS activity and may thus reflect the rapid release of an endothelium-derived hyperpolarizing factor (Campbell et al. 1996). In contrast, reductions in the duration of vasomotor responses with LNA (Fig. 3) indicate a role for NO in maintaining dilatation, perhaps through sustaining the hyperpolarization (Murphy & Brayden, 1995). This interpretation is consistent with the view that separate, yet complimentary, signalling pathways co-ordinate the magnitude and the time course of smooth muscle cell relaxation during conducted responses to ACh (Doyle & Duling, 1997).
Applying cable theory to conduction in arteriolar networks (Hirst & Neild, 1978; Segal & Neild, 1996), passive (i.e. electrotonic) decay should have decreased the conducted response by ∼70 % at 1560 μm from the stimulus origin. This clearly did not occur in feed arteries (Fig. 1). Such a lack of decay in the conduction of vasodilatation along feed arteries implies highly effective coupling between (and transmission along) the cells that comprise the conduction pathway. This may in turn reflect an extraordinarily long length constant along endothelial cells, and/or the contribution of an active (i.e. regenerative) signalling process that is intrinsic to the vessel wall (Segal & Neild, 1996). Further experiments based upon electrophysiological measurements will be required to distinguish these possibilities, and to determine whether the electrotonic (i.e. passive membrane) properties described for arterioles (Hirst & Neild, 1978) apply to feed arteries.
The lack of branching along feed arteries would contribute further to sustaining the conducted response (Segal & Neild, 1996). In contrast, arteriolar segments in the retractor muscle are more highly branched (Nakao & Segal, 1995) and their responses decay over shorter distances (Fig. 1) than shown for arterioles in the cheek pouch (Segal et al. 1989) or cremaster muscle (Segal, 1991). Nevertheless, and of direct physiological importance, vasodilatation was consistently conducted along arterioles and feed arteries over distances that would enable the co-ordination of vasomotor activity (and blood flow control) between parent and daughter branches.
Vasoconstriction
In the light of the robust local constrictions in response to NA and KCl (Fig. 4), our findings indicate that smooth muscle cells of retractor microvessels are highly reactive and yet relatively (compared with cheek pouch arterioles) ineffective in conducting the vasoconstrictor response. This interpretation is supported further by the finding that electrical stimulation in the presence of neural blockade, when smooth muscle cells were directly activated (and presumably depolarized) by the microelectrode, evoked vasoconstriction that was also constrained to the stimulus vicinity (see Results).
Early studies of electrical conduction in arterioles focused on determining the passive membrane properties of the smooth muscle layer using minute levels of current injection (Hirst & Neild, 1978). In cheek pouch arterioles, smooth muscle readily conducts depolarization and vasoconstriction in response to the release of NA from micropipettes (Xia & Duling, 1995; Welsh & Segal, 1998). Due to its lack of sympathetic innervation (Joyner et al. 1983), and the barrier presented by the endothelium to circulating catecholamines (Lew et al. 1989), the physiological role of NA in the cheek pouch is questionable. In contrast, arterioles and feed arteries of the retractor muscle are richly invested with sympathetic nerves (Welsh & Segal, 1996). Based upon the present (Fig. 5) and previous (Welsh & Segal, 1996) data, the propagation of action potentials with release of NA along these nerve fibres appears to be the principal mechanism for co-ordinating the contraction of smooth muscle cells so as to increase vascular resistance.
The conduction of electrical signals from cell to cell is enhanced by low coupling resistance (e.g. through gap junctions) and high membrane resistance. Recent measurements in cheek pouch arterioles in vivo indicate that NA evokes depolarization of (and conduction along) the smooth muscle cell layer without changing the membrane potential of endothelial cells (Welsh & Segal, 1998). Thus, the lack of conducted vasoconstriction observed in arterioles and feed arteries of the retractor muscle may well reflect differences in coupling properties of their smooth muscle cells when compared with those of cheek pouch arterioles. Whereas recent evidence suggests that the expression of gap junctions between smooth muscle cells is prevalent throughout the vasculature (Christ et al. 1996), the effectiveness of gap-junctional coupling may be modulated by perivascular sympathetic nerves (Kurjiaka & Segal, 1995B). Indeed, little is known about whether the cable properties of smooth muscle cells vary between resistance microvessels that supply tissues that differ in structure, function and sympathetic innervation (Hirst & Edwards, 1989). Given the robust sympathetic innervation of arterioles and feed arteries of the retractor muscle (Fig. 5; Welsh & Segal, 1996), and the lack of such innervation of arterioles in the epithelial cheek pouch (Joyner et al. 1983), we hypothesize that regional differences in the conduction of vasoconstriction may reflect the presence (or absence) of sympathetic nerves and their potential influence on cell-to-cell coupling.
Conclusion
The present observations uniquely document tissue-specific differences in the mechanisms by which vasodilatation and vasoconstriction are transmitted along feed arteries and arterioles of the retractor skeletal muscle when compared with responses in arterioles of the epithelial cheek pouch. In the cheek pouch, conduction of vasoconstriction as well as vasodilatation reflects complimentary mechanisms for co-ordinating both increases and decreases in arteriolar resistance. In contrast, resistance vessels of the retractor (and other skeletal) muscle are richly innervated by sympathetic nerves. When activated, these nerves rapidly co-ordinate smooth muscle cell contraction throughout the resistance network, and can thereby redirect blood flow away from affected vascular beds. Substantive vasomotor tone persists in arterioles and feed arteries of the retractor muscle when sympathetic nerves are inhibited. Thus, independent of vasodilator nerves or flow-induced vasodilatation, the conduction of vasodilatation along arterioles and into feed arteries reflects a highly effective mechanism for increasing muscle blood flow.
Acknowledgments
We thank G. G. Emerson and T. O. Neild for valuable review and discussion of this study. This work was supported by grants RO1-HL56786 and RO1-HL41026 from the National Heart, Lung, and Blood Institute of the United States Public Health Service. S. S. Segal is an Established Investigator of the American Heart Association. D. G. Welsh and D. T. Kurjiaka were recipients of postdoctoral fellowships from the Heritage Affiliate of the American Heart Association.
References
- Borders JL, Granger HJ. An optical Doppler intravital velocimeter. Microvascular Research. 1984;27:117–127. doi: 10.1016/0026-2862(84)90047-5. [DOI] [PubMed] [Google Scholar]
- Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circulation Research. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- Christ GJ, Spray DC, El-Sabban M, Moore LK, Brink PR. Gap junctions in vascular tissues. Evaluating the role of intercellular communication in the modulation of vasomotor tone. Circulation Research. 1996;79:631–646. doi: 10.1161/01.res.79.4.631. [DOI] [PubMed] [Google Scholar]
- Davies PF. Flow-mediated endothelial mechanotransduction. Physiological Reviews. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle MP, Duling BR. Acetylcholine induces conducted vasodilation by nitric oxide-dependent and -independent mechanisms. American Journal of Physiology. 1997;272:H1364–1371. doi: 10.1152/ajpheart.1997.272.3.H1364. [DOI] [PubMed] [Google Scholar]
- Folkow B, Sonnenschein RR, Wright DL. Loci of neurogenic and metabolic effects on precapillary vessels of skeletal muscle. Acta Physiologica Scandinavica. 1971;81:459–471. doi: 10.1111/j.1748-1716.1971.tb04924.x. [DOI] [PubMed] [Google Scholar]
- Haas TL, Duling BR. Morphology favors an endothelial cell pathway for longitudinal conduction within arterioles. Microvascular Research. 1997;53:113–120. doi: 10.1006/mvre.1996.1999. 10.1006/mvre.1996.1999. [DOI] [PubMed] [Google Scholar]
- Hilton SM. A peripheral arterial conducting mechanism underlying dilatation of the femoral artery and concerned in functional vasodilatation in skeletal muscle. The Journal of Physiology. 1959;149:93–111. doi: 10.1113/jphysiol.1959.sp006327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GD, Edwards FR. Sympathetic neuroeffector transmission in arteries and arterioles. Physiological Reviews. 1989;69:546–604. doi: 10.1152/physrev.1989.69.2.546. [DOI] [PubMed] [Google Scholar]
- Hirst GD, Neild TO. An analysis of excitatory junctional potentials recorded from arterioles. The Journal of Physiology. 1978;80:87–104. doi: 10.1113/jphysiol.1978.sp012374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner W, Campbell GT, Peterson C, Wagoner J. Adrenergic neurons: are they present on microvessels in cheek pouches of hamsters? Microvascular Research. 1983;26:27–35. doi: 10.1016/0026-2862(83)90052-3. 10.1016/0026-2862(83)90052-3. [DOI] [PubMed] [Google Scholar]
- Kurjiaka DT, Segal SS. Conducted vasodilation elevates flow in arteriolar networks of hamster striated muscle. American Journal of Physiology. 1995a;269:H1723–1728. doi: 10.1152/ajpheart.1995.269.5.H1723. [DOI] [PubMed] [Google Scholar]
- Kurjiaka DT, Segal SS. Interaction between conducted vasodilation and sympathetic nerve activation in arterioles of hamster striated muscle. Circulation Research. 1995b;76:885–891. doi: 10.1161/01.res.76.5.885. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Armstrong RB. Muscular blood flow distribution patterns as a function of running speed in rats. American Journal of Physiology. 1982;243:H296–306. doi: 10.1152/ajpheart.1982.243.2.H296. [DOI] [PubMed] [Google Scholar]
- Lew MJ, Rivers RJ, Duling BR. Arteriolar smooth muscle responses are modulated by an intramural diffusion barrier. American Journal of Physiology. 1989;257:H10–16. doi: 10.1152/ajpheart.1989.257.1.H10. [DOI] [PubMed] [Google Scholar]
- Little TL, Beyer EC, Duling BR. Connexin 43 and connexin 40 gap junctional proteins are present in arteriolar smooth muscle and endothelium in vivo. American Journal of Physiology. 1995;268:H729–739. doi: 10.1152/ajpheart.1995.268.2.H729. [DOI] [PubMed] [Google Scholar]
- Murphy ME, Brayden JE. Nitric oxide hyperpolarizes rabbit mesenteric arteries via ATP-sensitive potassium channels. The Journal of Physiology. 1995;486:47–58. doi: 10.1113/jphysiol.1995.sp020789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao M, Segal SS. Muscle length alters geometry of arterioles and venules in hamster retractor. American Journal of Physiology. 1995;268:H336–344. doi: 10.1152/ajpheart.1995.268.1.H336. [DOI] [PubMed] [Google Scholar]
- Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension. 1986;8:37–44. doi: 10.1161/01.hyp.8.1.37. [DOI] [PubMed] [Google Scholar]
- Segal SS. Microvascular recruitment in hamster striated muscle: role for conducted vasodilation. American Journal of Physiology. 1991;261:H181–189. doi: 10.1152/ajpheart.1991.261.1.H181. [DOI] [PubMed] [Google Scholar]
- Segal SS, Damon DN, Duling BR. Propagation of vasomotor responses coordinates arteriolar resistances. American Journal of Physiology. 1989;256:H832–837. doi: 10.1152/ajpheart.1989.256.3.H832. [DOI] [PubMed] [Google Scholar]
- Segal SS, Duling BR. Flow control among microvessels coordinated by intercellular conduction. Science. 1986;234:868–870. doi: 10.1126/science.3775368. [DOI] [PubMed] [Google Scholar]
- Segal SS, Duling BR. Conduction of vasomotor responses in arterioles: a role for cell-to-cell coupling? American Journal of Physiology. 1989;256:H838–845. doi: 10.1152/ajpheart.1989.256.3.H838. [DOI] [PubMed] [Google Scholar]
- Segal SS, Neild TO. Conducted depolarization in arteriole networks of the guinea-pig small intestine: effect of branching on signal dissipation. The Journal of Physiology. 1996;496:229–244. doi: 10.1113/jphysiol.1996.sp021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiesko V, Lang DJ, Johnson PC. Dilator response of rat mesenteric arcading arterioles to increased blood flow velocity. American Journal of Physiology. 1989;257:H1958–1965. doi: 10.1152/ajpheart.1989.257.6.H1958. [DOI] [PubMed] [Google Scholar]
- Vonderlage M. Spread of contraction in rabbit ear artery preparations in response to stimulation by norepinephrine. Circulation Research. 1981;49:600–608. doi: 10.1161/01.res.49.3.600. [DOI] [PubMed] [Google Scholar]
- Welsh DG, Segal SS. Muscle length directs sympathetic nerve activity and vasomotor tone in resistance vessels of hamster retractor. Circulation Research. 1996;79:551–559. doi: 10.1161/01.res.79.3.551. [DOI] [PubMed] [Google Scholar]
- Welsh DG, Segal SS. Coactivation of resistance vessels and muscle fibers with acetylcholine release from motor nerves. American Journal of Physiology. 1997;273:H156–163. doi: 10.1152/ajpheart.1997.273.1.H156. [DOI] [PubMed] [Google Scholar]
- Welsh DG, Segal SS. Endothelial and smooth muscle cell conduction in arterioles controlling blood flow. American Journal of Physiology. 1998;274:H178–186. doi: 10.1152/ajpheart.1998.274.1.H178. [DOI] [PubMed] [Google Scholar]
- West WT. Histologic study of living striated muscle fibers in situ in the cheek pouch of the golden hamster. American Journal of Anatomy. 1958;103:349–373. doi: 10.1002/aja.1001030303. [DOI] [PubMed] [Google Scholar]
- Williams DA, Segal SS. Feed artery role in blood flow control to rat hindlimb skeletal muscles. The Journal of Physiology. 1993;463:631–646. doi: 10.1113/jphysiol.1993.sp019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Duling BR. Electromechanical coupling and the conducted vasomotor response. American Journal of Physiology. 1995;269:H2022–2030. doi: 10.1152/ajpheart.1995.269.6.H2022. [DOI] [PubMed] [Google Scholar]


