Abstract
Single-unit recordings were made from 19 postganglionic muscle vasoconstrictor axons via tungsten microelectrodes in the peroneal nerve in seven healthy subjects with many multi-unit sympathetic discharges at rest (‘high group’, 75 ± 5 multi-unit bursts per 100 heart beats, mean ± s.e.m.). The results were compared with previous data from 14 units in subjects with 21 ± 2 multi-unit bursts per 100 heart beats (‘low group’).
In the ‘high group’ the units fired spontaneously in 35 ± 4 % of all cardiac intervals. One unit only ever fired once per cardiac interval, 14 units (74 %) generated maximally two to three spikes, and four units (21 %) up to four to five spikes. Of those cardiac intervals in which a unit fired, a single spike occurred in 78 %, two spikes in 18 %, three spikes in 4 % and four spikes in less than 1 % of cardiac intervals. Measured as the inverse of all interspike intervals, the mean rate was 0.33 ± 0.04 Hz and the mean intraburst frequency 22.2 ± 1.6 Hz. Most results were similar to those in the ‘low group’, but in the ‘low group’ heart rate was higher (64.5 vs. 50.4 beats min−1) and mean firing frequency was higher (0.49 ± 0.06 Hz).
During increases of multi-unit burst activity evoked by sustained inspiratory-capacity apnoea the firing probability of nine units in the ‘high group’ increased from 33 ± 6 to 56 ± 3 % of the cardiac intervals. Simultaneously, the incidence of single spikes decreased and the incidence of multiple spikes per cardiac interval increased, resulting in an increase of mean firing frequency from 0.23 ± 0.04 Hz at rest to 1.04 ± 0.14 Hz during the apnoea.
We conclude that single muscle vasoconstrictor neurones usually fire only a solitary spike during sympathetic bursts both in subjects with a high and in subjects with a low number of bursts at rest. Presumably, differences in the numbers of bursts are due mainly to differences in firing probability and recruitment of sympathetic fibres. During acute increases of multi-unit activity, both increases in discharge frequency and recruitment of additional neurones contribute to the increased intensity of an individual sympathetic burst.
Microelectrode recordings from peripheral nerves in awake human subjects have shown that the sympathetic outflow to the muscle vascular bed occurs as bursts of impulses with an obvious cardiac rhythmicity (Hagbarth & Vallbo, 1968; Delius et al. 1972; Sundlöf & Wallin, 1978). Selective recordings from single postganglionic axons have further shown that individual vasoconstrictor neurones generally fire only once in a given sympathetic burst, with few instances of multiple firing (Macefield et al. 1994). From this it was concluded that the intensity of a multi-unit burst is determined not so much by the number of spikes a given postganglionic neurone contributes to the burst, but more by the number of neurones active. However, these single-unit recordings were made in subjects with low levels of spontaneous muscle sympathetic activity, and it is known that the amount of resting activity varies from subject to subject (Sundlöf & Wallin, 1978; Fagius & Wallin, 1993). It could be argued, therefore, that our conclusions on the firing patterns of single units would not hold in subjects with high levels of resting muscle sympathetic activity. Hence, the purpose of the present study: to compare firing properties of individual muscle vasoconstrictor neurones between subjects with high and low levels of multi-unit nerve traffic. To this end we have made unitary recordings from a group of subjects with high resting levels of multi-unit muscle sympathetic activity and compared the results with data from the study of Macefield et al. (1994). The recordings were made both at rest and during a maximal inspiratory breath-hold, a manoeuvre known to augment muscle sympathetic outflow (Macefield & Wallin, 1995).
METHODS
Subjects
Recordings were made from seven healthy males ranging in age from 24 to 61 years (mean, 36 years). Each subject provided informed written consent to the procedures, which were approved by the human ethics committee of the University of Göteborg. Subjects were selected because they had been noted in previous recordings to possess a high level of muscle sympathetic activity at rest.
General procedures
The subject lay supine with Ag-AgCl ECG electrodes on the chest, a respiration transducer wrapped around the lower thorax or upper abdomen and a Finapres cuff (Finapres, Ohmeda, CO, USA) placed on the middle finger for continuous monitoring of arterial blood pressure. The thigh was supported by a vacuum cast and the common peroneal nerve located by palpation and electrical stimulation via a surface probe. A laboratory-produced tungsten microelectrode of relatively low impedance was inserted percutaneously into a motor fascicle of the nerve and a site was located in which spontaneous pulse-synchronous sympathetic activity could be recorded. A nearby subdermal electrode with a large uninsulated tip served as reference. The number of multi-unit bursts and heart beats at rest were recorded during 5 min of quiet breathing, so as to allow measurement of burst incidence (bursts per 100 heart beats). Following removal of this microelectrode a second, high-impedance microelectrode (type 25-10-1, Frederick Haer Co., Brunswick, ME, USA; type TM33B20, World Precision Instruments, Sarasota, FL, USA) was then inserted into a motor fascicle of the same peroneal nerve. When a site was located in which multi-unit muscle sympathetic activity could be recorded, small electrode adjustments were made until a position was found in which individual spikes stood out from the noise. After resting activity had been recorded from a putative unitary site the subject was asked to make a maximal voluntary inhalation and to hold the lungs inflated against a closed glottis (inspiratory-capacity apnoea) for 40-120 s (Macefield & Wallin, 1995).
Data collection
Neural activity was amplified (× 50000), filtered (0.3-5.0 kHz), digitized at 12.8 kHz (12 bits) and stored on disk via the SC/ZOOM data acquisition and analysis computer system (Department of Physiology, University of Umeå, Sweden). The amplified and filtered nerve signal was also led to an audiomonitor and through a resistance-capacitance integrating circuit (time constant, 100 ms) to produce a mean voltage neurogram. This was digitized at 800 Hz and stored as 8 bits. The ECG channel was also digitized at 800 Hz (8 bits), and respiration and arterial pressure at 200 Hz (8 bits). Data were sampled in standard epochs of 300 s duration.
Data analysis
During off-line analysis the morphology of every spike of a candidate unit was carefully assessed using a spike recognition algorithm (Edin et al. 1988) incorporated in the SC/ZOOM software. Spike superimposition was used to ascertain whether a recording was, in all likelihood, from a single nerve fibre. This was validated by comparing the distribution of spike amplitudes for a given unit with that of the underlying noise. Noise was sampled over a 1-2 s period by lowering the amplitude threshold of the window discriminator below that of the spikes of interest, while maintaining the same time window (± 0.35 ms) as used by the spike detection algorithm incorporated in ZOOM; in this way spike-like noise was sampled with amplitudes ranging from some 10 nV to 15 μV. Figure 1 shows examples of the distributions of noise and spike amplitudes for two muscle vasoconstrictor units. For both units the variation in the amplitude of the spike signal was similar to that of the noise, suggesting that the variation in spike amplitude can be explained by the variation in the underlying noise. All units satisfied this criterion. For each unit the following parameters were determined: (1) probability of firing = the percentage of heart beats during which one or more spikes occurred, (2) probability of multiple firing = the number of heart beats with more than one spike in relation to all heart beats with any spike (as a percentage), and (3) mean firing frequency = the mean of the inverse of all interspike intervals. During inspiratory-capacity apnoea, analyses of unit firing were limited to cardiac intervals occurring during the static phase of the lung inflation (duration, 79.5 ± 8.6; range, 39.5-115.5 s), i.e. when maximal inflation had been achieved. During the same period the multi-unit activity was quantified by averaging the integrated nerve record, synchronized to the R-wave of the ECG. The result (expressed both as burst amplitude and burst area) was compared with the corresponding R-wave-triggered average obtained during a 5 min rest period prior to the apnoea.
Figure 1. Variation of spike amplitude in single unit recordings.
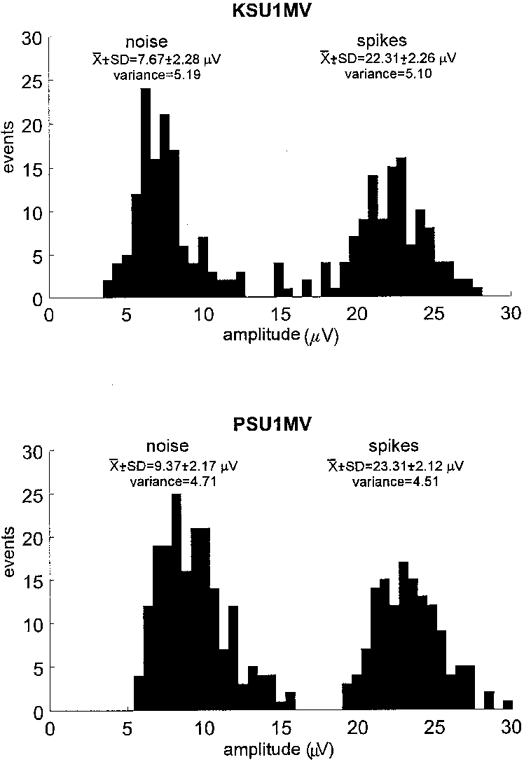
Histograms showing the distributions of spike amplitude and of the noise for two muscle vasoconstrictor units (KSU1MV and PSU1MV). For each unit the variances of the noise and spike amplitudes were not significantly different, indicating that variations in the spike amplitude can be accounted for by variations in the underlying noise.
Statistics
Variations in noise and signal amplitude for each unitary recording were compared using Levene's test for homogeneity of variance - a one-way ANOVA of the deviations from the means. To determine whether the data sets showed a normal distribution the Shapiro- Wilk test was used. Student's paired t test was used to compare firing characteristics of the same unit at rest and during maximal inspiratory apnoea. Student's unpaired t test was used to compare unit firing characteristics with similar data from Macefield et al. (1994). The non-parametric Mann-Whitney U test was used to compare data that were not normally distributed. All statistical evaluation of the data was performed using STATISTICA for Windows v.5.1 (StatSoft Inc., Tulsa, OK, USA). Values are expressed as means and, unless otherwise indicated, standard errors of the mean, and differences are considered statistically significant at P < 0.05.
RESULTS
The multi-unit burst incidence at rest was 75 ± 5 bursts per 100 heart beats (range, 63-96 bursts). The mean ±s.e.m. cardiac interval was 1.19 ± 0.05 s, corresponding to a mean heart rate of 50.4 beats min−1. In the group with few multi-unit bursts studied previously (Macefield et al. 1994) the mean cardiac interval was 0.93 ± 0.04 s, corresponding to a mean heart rate of 64.5 beats min−1 (significant difference, P < 0.001).
Using the high-impedance microelectrodes, unitary recordings were made from 19 neurones. These fired with an overt cardiac rhythmicity, supporting their identification as muscle vasoconstrictor neurones. An example of spontaneous activity in one such neurone is shown in Fig. 2A. This unit did not fire with every burst, but when it did fire it generally discharged only one spike. The consistent amplitude and shape of the superimposed action potentials in Fig. 2A support the conclusion that the spikes originated from a single axon. All units generated spikes that were triphasic and with a dominant negativity. Spike amplitudes ranged from 8.4 to 29.1 μV (mean ±s.e.m., 19.8 ± 1.2 μV).
Figure 2. Activity of a single muscle vasoconstrictor neurone at rest.
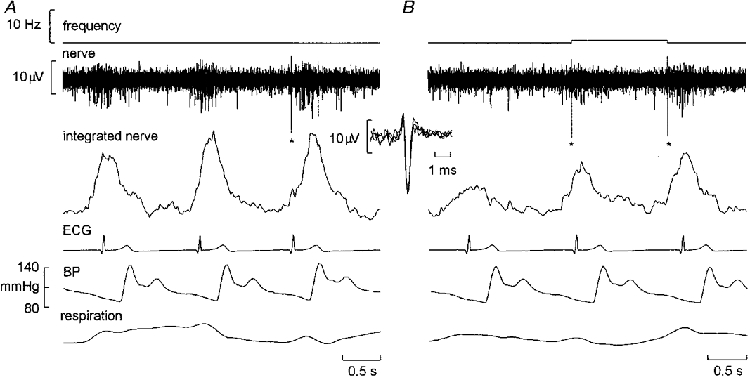
Recording from a subject with a high number of multi-unit sympathetic bursts (indicated by the integrated nerve record), in which a solitary large spike (asterisks) occurred in one of the sympathetic bursts in A and in two of the bursts in B. Despite the high burst probability, in each burst the unit fired only once. The inset shows the three superimposed spikes from A and B, the uniform morphology of the negative-going action potential suggesting that each spike was generated by the same unmyelinated sympathetic axon. Inspiration upwards in respiration trace.
Firing properties of single neurones at rest
Despite the high multi-unit burst incidence, the firing probabilities of the single units was low. When expressed as the percentage of heart beats during which spike(s) occurred, the firing probability of a single unit was 34.9 ± 3.6 % (range, 6-60 %). The mean discharge frequency, calculated as the inverse of all interspike intervals, was 0.33 ± 0.04 Hz (range, 0.09-0.69 Hz) across units. Counting only interspike intervals < 500 ms, i.e. only those intervals occurring within a sympathetic burst, the mean instantaneous frequency - corresponding to the mean intraburst frequency - was 22.2 ± 1.6 Hz. The distribution of instantaneous frequencies was very wide but strongly skewed towards low firing rates (Fig. 3). The maximum instantaneous frequency was 230 Hz and occurred as an isolated doublet, which approaches the limit imposed by the absolute refractory period of mammalian C fibres (see Discussion). An example of a short interspike interval occurring within a sympathetic burst is shown in Fig. 4.
Figure 3. Distribution of firing rates for all muscle vasoconstrictor neurones.
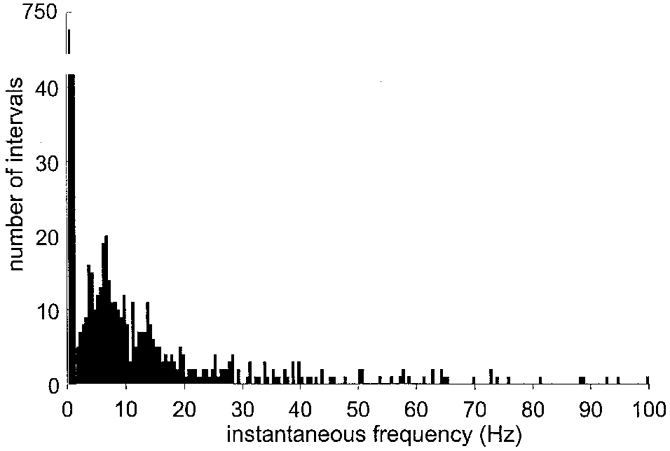
Pooled data from 19 muscle vasoconstrictor neurones. Note break of the y-axis, which indicates that most interspike intervals were long (i.e. that most instantaneous frequencies were low).
Figure 4. High-frequency discharges in a muscle vasoconstrictor neurone.
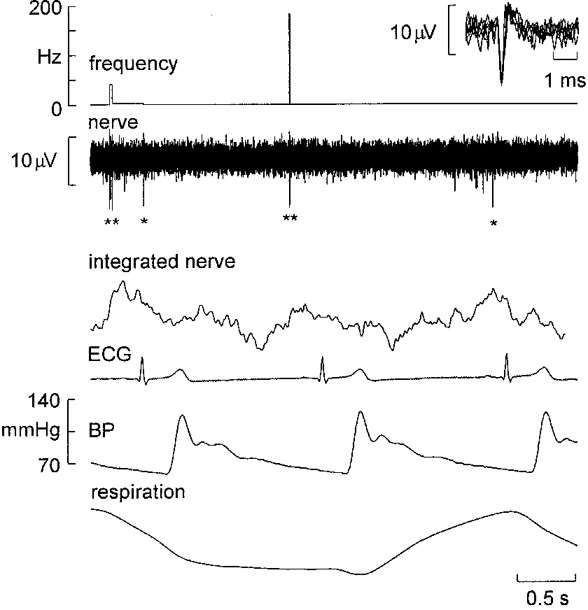
Recording from a single muscle vasoconstrictor neurone that occasionally generated short interspike intervals. In the first illustrated sympathetic burst (indicated by the integrated nerve record) the unit fired three times (indicated by asterisks), in the second burst twice and in the third burst once. Note the high instantaneous frequency associated with the second burst. The 6 superimposed action potentials shown in the inset support the unitary integrity of the recording. Inspiration upwards in respiration trace.
The probability of multiple firing was also low and no unit fired more than five spikes during a single cardiac interval. One unit only ever generated a single spike, fourteen units (74 %) generated a maximum of two to three spikes and four units (21 %) up to four to five spikes (Fig. 5A). Calculated from the average values from each unit, and considering only those cardiac intervals in which the units fired, a single spike was generated in 78 ± 4 % of intervals, two spikes in 18 ± 3 %, three spikes in 4 ± 1 % and four spikes in only 0.5 ± 0.3 % of the cardiac intervals in which the units discharged. Pooled data from all units are shown in Fig. 5B. Table 1 summarizes the data and includes a comparison with data reanalysed from our study in subjects with low multi-unit burst incidence (Macefield et al. 1994). Despite the marked difference in resting sympathetic activity, as assessed from multi-unit records (75 vs. 21 bursts per 100 heart beats), the firing characteristics of single neurones in the two studies were mostly similar. There were, however, some quantitative differences: the mean firing frequency was lower (0.33 vs. 0.49 Hz, P < 0.05) and the incidence of multiple firing within a single heart beat was lower (4 spikes generated in 0.5 vs. 6.0 % of heart beats, P < 0.05; Mann- Whitney U test) in subjects with a high multi-unit burst incidence. On the other hand, there was a tendency for units to have a higher firing probability in subjects with a high multi-unit burst incidence (34.9 vs. 25.3 %, P = 0.06; t test).
Figure 5. Firing pattern of single muscle vasoconstrictor neurones.
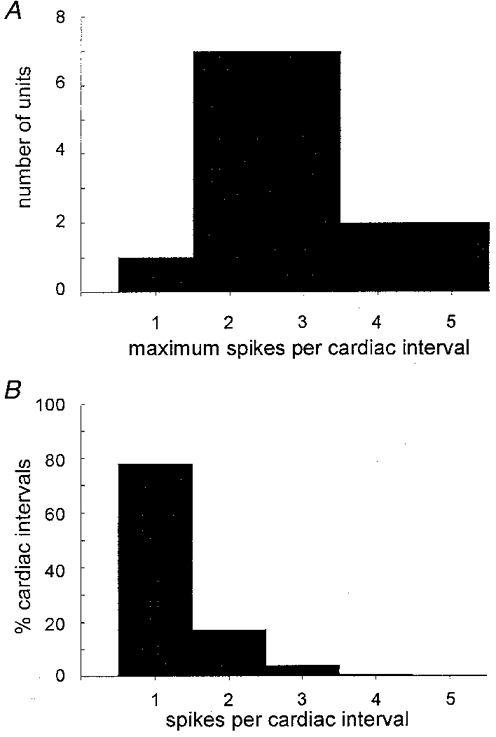
A, histogram showing the maximum number of spikes generated per cardiac interval for all units. B, histogram showing the percentage of cardiac intervals in which a unit generated 1, 2, 3, 4 or 5 spikes. Only those cardiac intervals in which the units were active are included; data pooled from all units.
Table 1.
Mean data on multi-unit burst incidence, single unit firing probability, mean firing rate and, measured only from those cardiac intervals in which the units fired, the percentage of heart beats in which units generated 1, 2, 3 or 4 spikes
| Percentage of heart beats generating: | |||||||
|---|---|---|---|---|---|---|---|
| Burst probability | Firing probability | Mean firing frequency (Hz) | 1 spike (%) | 2 spikes (%) | 3 spikes (%) | 4 spikes (%) | |
| High MSA | 0.749 ± 0.049 | 0.349 ± 0.036 | 0.33 ± 0.04 | 77.6 ± 3.6 | 18.1 ± 2.9 | 3.6 ± 1.1 | 0.5 ± 0.3 |
| Low MSA | 0.210 ± 0.022* | 0.253 ± 0.030 | 0.49 ± 0.06* | 65.9 ± 5.5 | 18.9 ± 2.6 | 7.1 ± 2.2 | 6.0 ± 2.4* |
Data are from normal subjects with high muscle sympathetic activity (high MSA) recorded in the present study; data from normal subjects with low activity (low MSA) reanalysed from Macefield et al. (1994). In both groups burst probability was calculated with n = 7 (i.e. 7 subjects), whereas the other parameters were calculated with n = 19 for high MSA and n = 14 for low MSA.
Significant difference between the two groups.
Firing properties of single neurones during inspiratory-capacity apnoea
In five subjects, the unitary integrity of nine recording sites was preserved during inspiratory-capacity apnoea. Figure 6 shows the increase in multi-unit burst incidence and amplitude during the static phase of the lung inflation. Measured from the integrated nerve records in the same subjects, mean burst amplitude during the static phase was double that at rest (mean increase, 214 ± 30 %). During the same time mean firing rates increased from 0.23 ± 0.04 Hz at rest to 1.04 ± 0.14 Hz during the apnoea. An example of a unitary recording during the apnoea is shown in Fig. 7.
Figure 6. Augmentation of muscle sympathetic activity during inspiratory apnoea.
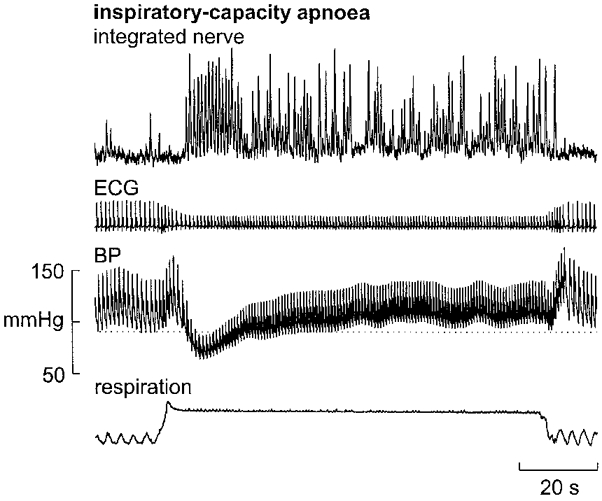
Example of the sustained increase in muscle sympathetic activity (multi-unit recording) generated during a maximal inspiratory breath-hold (inspiratory-capacity apnoea) against a closed glottis. Note the overall increase in burst probability and amplitude during the static phase of the inflation. Note also that diastolic pressure is above the resting level (dotted horizontal line) during most of the apnoea. Inspiration upwards in respiration trace.
Figure 7. Firing of a single muscle vasoconstrictor neurone during inspiratory apnoea.
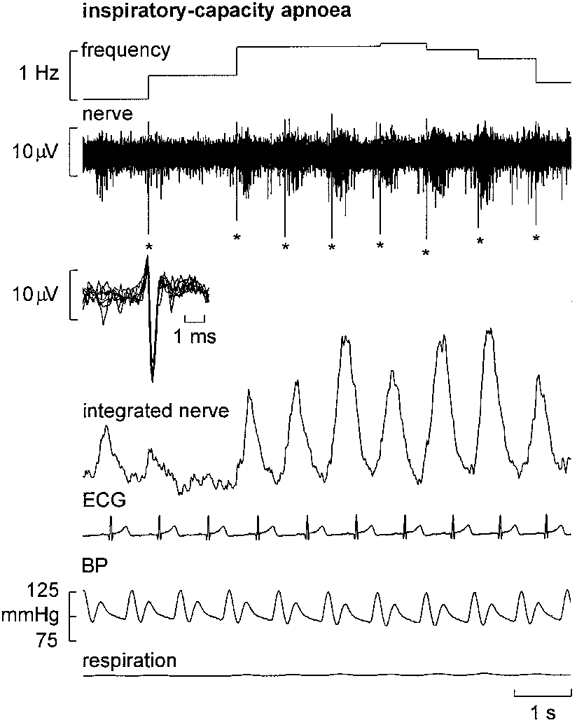
Recording from a single unit during the static phase of a maximal inspiratory breath-hold (note the flat respiration trace). Despite the high intensity of the muscle sympathetic bursts, the unit only fired once (indicated by asterisks) in each of the illustrated bursts. Superimposed spikes in the inset confirm the unitary integrity of the recording.
Using paired data the mean firing probabilities of the nine units increased significantly from 32.5 ± 6.2 % (range, 13.3-59.4 % across units) at rest to 56.3 ± 3.1 % (range, 45.3-76.6 %) during the static phase of an inspiratory-capacity apnoea. There was an inverse linear correlation (r = -0.89,P < 0.01) between the firing probability at rest and the change of firing probability during the apnoea, i.e. units with low firing probability at rest displayed greater increases during the apnoea than units with high firing probability at rest. The percentage of cardiac intervals in which a solitary spike was generated (computed from those intervals in which units fired) decreased from 85.1 ± 4.5 % at rest to 61.3 ± 5.6 % during the apnoea (P = 0.009). Correspondingly, the percentage of cardiac intervals in which a unit fired twice increased from 11.5 ± 2.7 % at rest to 26.7 ± 2.2 % during the apnoea (P = 0.005). The percentage of intervals in which a unit generated three or four spikes also increased respectively from 2.9 ± 1.8 % and 0.4 ± 0.4 % at rest to 9.5 ± 3.4 % and 2.0 ± 1.0 % during the apnoea, although these changes failed to reach statistical significance. Data from all units on firing probability, probability of multiple firing and mean frequency are summarized in Fig. 8, and Fig. 9 shows histograms summarizing the changes in probability of firing single or multiple spikes during the apnoea.
Figure 8. Changes of firing characteristics of single vasoconstrictor neurones during inspiratory apnoea.
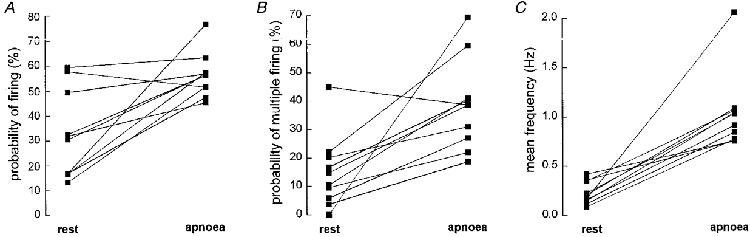
Probability of firing (A), probability of multiple firing (B) and mean unit frequency (C) at rest and during inspiratory apnoea. Data from 9 units in 5 subjects.
Figure 9. Incidence of multiple firing at rest and during inspiratory apnoea.
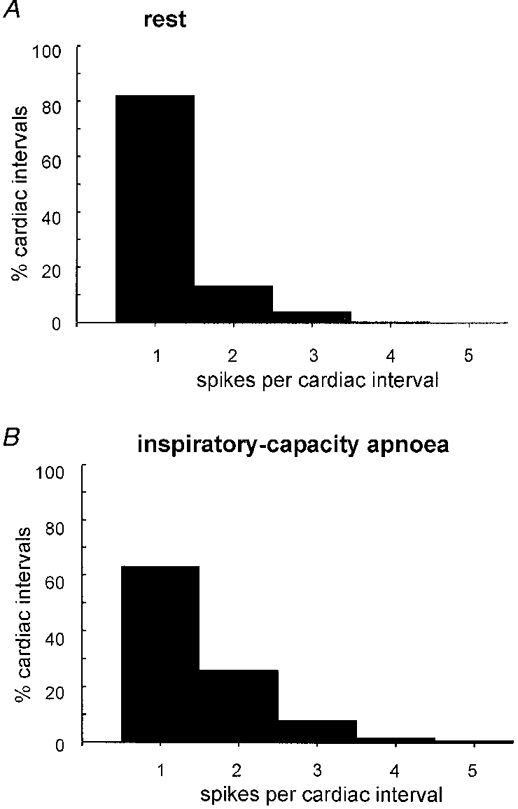
Histograms showing the percentage of cardiac intervals in which a unit generated 1, 2, 3, 4 or 5 spikes at rest (A) or during the static phase of an inspiratory-capacity apnoea (B). Only those cardiac intervals in which the units were active are included; data pooled from 9 units studied in both conditions.
DISCUSSION
There were three main findings in the present study. (1) Qualitatively, the firing characteristics of single human muscle vasoconstrictor units are similar in subjects with high and low levels of spontaneous multi-unit sympathetic activity: in both groups an individual axon contributes preferentially only one spike to a sympathetic burst, and the probability of multiple firing is low. (2) Quantitatively, firing probabilities tended to be higher, whereas mean firing frequencies were significantly lower in subjects with high than in subjects with low multi-unit burst incidence. (3) During acute increases in multi-unit sympathetic drive the mean unit firing frequency increased, due to increases of both the firing probability and the incidence of multiple firing.
Methodological considerations
As in previous studies of single sympathetic neurones in humans (Macefield et al. 1994; Macefield & Wallin, 1996, 1999), a main technical difficulty was to decide if a recording was truly unitary. To accept a unit our requirements were that it should fire in association with spontaneous multi-unit discharges, that the spike morphology agreed with that of an extracellular recording from a C fibre, and that the shape was reproducible between successive spikes. In addition, the variations in spike amplitude should not exceed the variations in noise amplitude. In spite of these precautions it is difficult to exclude with certainty the possibility that two units of identical shape and size may be active in the same recording. The risk is probably highest when multiple spikes are generated with short interspike intervals and, therefore, the maximum instantaneous frequency observed in the present study (230 Hz) may be an overestimate. However, we could not exclude any of the relevant spikes on the basis of shape, and while the frequency is high, it is not impossible. For instance, it is known that sensory C fibres in human subjects can sustain high firing rates of approximately 100 Hz for brief periods (Nordin, 1990; Vallbo et al. 1993). The refractory period is 2 ms for sympathetic postganglionic axons in the cat (Grundfest & Gasser, 1938) and sensory C fibres in the hippocampus of the guinea-pig (Andersen et al. 1978), i.e. the theoretical maximum instantaneous frequency would be approximately 500 Hz. If a single postganglionic neurone is driven by two (or more) preganglionic neurones possessing strong synapses (McLachlan et al. 1997) there will be chance occurrences in which impulses from the preganglionic neurones impinge on the postganglionic neurone at essentially the same time: this will evince itself as the occasional generation of very high instantaneous frequencies.
Firing properties of muscle vasoconstrictor neurones
Despite the high multi-unit burst incidence of the present subjects (75 bursts per 100 heart beats), an average individual vasoconstrictor neurone fired in only 35 % of the heart beats. This firing probability was similar to the firing probability of single sudomotor (35 %; Macefield & Wallin, 1996) and cutaneous vasoconstrictor neurones (38 %; Macefield & Wallin, 1999). In addition, when measured from those cardiac intervals in which the units fired, the percentage of intervals in which an individual neurone generated only one spike (78 %) was also comparable to that found for sudomotor neurones (67 %) and cutaneous vasoconstrictor neurones (66 %).
When comparing vasoconstrictor unit firing characteristics in subjects with high and low levels of multi-unit muscle sympathetic activity, the quantitative differences were few and small. Interestingly, there was no tendency for subjects with a high multi-unit burst incidence to have higher firing frequencies and/or a higher incidence of multiple firing in individual units. On the contrary, in such subjects the units had slightly, but significantly, lower mean firing frequencies than the units in subjects with a low multi-unit burst incidence. The lower mean firing frequency is in all probability coupled to the differences in heart rate between the groups. Due to the strong arterial baroreflex influence on muscle sympathetic activity, each systolic pressure wave causes a complete inhibition of activity such that sympathetic impulses occur only in association with diastoles (Delius et al. 1972; Sundlöf & Wallin, 1978). In the present context the interesting consequence is that subjects with similar unit firing probabilities but different heart rates will have different mean unit firing frequencies. In agreement with a previous report (Wallin, 1981), the subjects in the present study with a high multi-unit burst incidence had lower heart rates than those subjects with a low burst incidence in the study of Macefield et al. (1994). The difference in heart rate was large enough to lead to a lower mean firing frequency in the subjects with a high multi-unit burst incidence, in spite of their units showing a tendency for higher firing probability and having fewer heart beats with four or more spikes. Thus, the present findings indicate that changes of heart rate per se may influence not only cardiac output, but also the strength of peripheral sympathetic nerve traffic. Since subjects with a high multi-unit burst incidence have slightly lower mean firing frequencies than subjects with a low burst incidence, the present results also imply that a high multi-unit burst incidence in resting subjects is due primarily to such subjects having a higher number of active sympathetic neurones than subjects with a low burst incidence. Thus, the main reason for differences in burst incidence is probably differences in recruitment of sympathetic fibres.
Both in subjects with a high and low incidence of muscle vasoconstrictor bursts the mean firing rates of the units were low and similar to those of sudomotor (0.62 Hz; Macefield & Wallin, 1996) and cutaneous vasoconstrictor neurones (0.53 Hz; Macefield & Wallin, 1999). In the latter studies there was an increased sympathetic drive to the target tissue - heat-induced sweating or cold-induced cutaneous vasoconstriction - whereas the activity of the muscle vasoconstrictor neurones was recorded at rest. The similarities of firing rates despite the differences in experimental conditions, and the similarities to the firing rates found in anaesthetized experimental animals (Jänig, 1985; Meckler & Weaver, 1988; Stein & Weaver, 1988) raise the possibility that such low frequencies prevail not only at rest but under a variety of conditions. The mechanism(s) restricting unit firing frequency may be located in sympathetic ganglia or in the central nervous system. One possible contributing alternative is that the discharge of an individual postganglionic neurone is governed primarily by the firing of a single preganglionic neurone possessing a strong synaptic connection. Multiple firing and the generation of short interspike intervals would then occur only rarely, when one (or several) additional preganglionic neurone(s) with sufficiently strong synapses fire(s) almost at the same time as the first neurone, thereby increasing the likelihood that the postganglionic neurone generates multiple action potentials. This would agree with the firing characteristics of superior cervical sympathetic ganglion cells in the rat (McLachlan et al. 1997).
Gradation of burst intensity by single sympathetic neurones
While single sympathetic neurones have a low firing probability and tend to contribute only one spike to a sympathetic burst, our data show that increases may occur when the sympathetic drive is elevated. During the maximal inspiratory breath-hold there was a sustained increase in multi-unit muscle sympathetic activity (Macefield & Wallin, 1995). At the same time the firing probability of the units increased, the percentage of sympathetic bursts in which a solitary spike was generated decreased, and the probability of multiple firing increased, the result being an increase in mean firing frequency. The findings indicate that an increase in multiple firing of already active neurones may contribute to the intensity of a sympathetic burst. However, also during the breath-hold only one spike occurred during as many as 61 % of the multi-unit bursts, suggesting that multiple firing is a fairly weak mechanism. Consequently, increases in firing probability and recruitment of additional neurones may still be the primary means by which burst intensity is augmented.
The present data give some clues to mechanisms contributing to the strength of multi-unit muscle sympathetic activity. Interindividual differences in resting sympathetic burst incidence are highly reproducible over a long time (Sundlöf & Wallin, 1977; Fagius & Wallin, 1993). Our results suggest that such differences primarily reflect interindividual differences in the number of active vasoconstrictor neurones. Manoeuvres or conditions that result in acute changes of sympathetic traffic lead to changes of burst incidence and/or burst amplitude (or area). Such changes probably reflect the combination of (1) a change in the number of neurones active (recruitment or decruitment), (2) a change in the firing probabilities of individual neurones, and (3) a change in the number of spikes an individual neurone contributes to a burst.
Conclusions
Single postganglionic muscle vasoconstrictor neurones in healthy subjects with high and low levels of resting muscle sympathetic activity show the same tendency to contribute only one spike to a sympathetic burst. However, when the sympathetic drive is elevated acutely the incidence of multiple firing increases, indicating that an increase in discharge frequency, as well as the recruitment of additional neurones, is a potential mechanism by which the intensity of a sympathetic burst can be graded.
Acknowledgments
This work was supported by the Swedish Medical Research Council, grant no. 3546. V. G. M. is supported by the National Health and Medical Research Council of Australia.
References
- Andersen P, Silfvenius H, Sundberg SH, Sveen O, Wigström H. Functional characteristics of unmyelinated fibres in the hippocampal cortex. Experimental Brain Research. 1978;144:11–18. doi: 10.1016/0006-8993(78)90431-6. [DOI] [PubMed] [Google Scholar]
- Delius W, Hagbarth K-E, Hongell A, Wallin BG. General characteristics of sympathetic activity in muscle nerves. Acta Physiologica Scandinavica. 1972;84:65–81. doi: 10.1111/j.1748-1716.1972.tb05158.x. [DOI] [PubMed] [Google Scholar]
- Edin BB, Bäckström PA, Bäckström LO. Single unit retrieval in microneurography: a microprocessor-based device controlled by an operator. Journal of Neuroscience Methods. 1988;24:137–144. doi: 10.1016/0165-0270(88)90057-x. 10.1016/0165-0270(88)90057-X. [DOI] [PubMed] [Google Scholar]
- Fagius J, Wallin BG. Long-term variability and reproducibility of resting human muscle nerve sympathetic activity at rest, as reassessed after a decade. Clinical Autonomic Research. 1993;3:201–205. doi: 10.1007/BF01826234. [DOI] [PubMed] [Google Scholar]
- Grundfest H, Gasser HS. Electrical properties of mammalian nerve fibres of slowest conduction. American Journal of Physiology. 1938;123:307–318. [Google Scholar]
- Hagbarth K-E, Vallbo Å B. Pulse and respiratory grouping of sympathetic impulses in human peripheral nerves. Acta Physiologica Scandinavica. 1968;74:96–108. doi: 10.1111/j.1748-1716.1968.tb04218.x. [DOI] [PubMed] [Google Scholar]
- Jänig W. Organization of the lumbar sympathetic outflow to skeletal muscle and skin of the cat hindlimb and tail. Review of Physiology, Biochemistry and Pharmacology. 1985;102:119–213. doi: 10.1007/BFb0034086. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Wallin BG. Effects of static lung inflation on sympathetic activity in human muscle nerves at rest and during asphyxia. Journal of the Autonomic Nervous System. 1995;53:148–158. doi: 10.1016/0165-1838(94)00174-i. 10.1016/0165-1838(94)00174-I. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Wallin BG. Discharge behaviour of single sympathetic neurones innervating human sweat glands. Journal of the Autonomic Nervous System. 1996;61:277–286. doi: 10.1016/s0165-1838(96)00095-1. 10.1016/S0165-1838(96)00095-1. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Wallin BG. Respiratory and cardiac modulation of single sympathetic vasoconstrictor and sudomotor neurones to human skin. The Journal of Physiology. 1999;516:303–314. doi: 10.1111/j.1469-7793.1999.303aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield VG, Wallin BG, Vallbo Å B. The discharge behaviour of single vasoconstrictor motoneurones in human muscle nerves. The Journal of Physiology. 1994;481:799–809. doi: 10.1113/jphysiol.1994.sp020482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan EM, Davies PJ, Häbler HJ, Jamieson J. On-going and reflex synaptic events in rat superior cervical ganglion cells. The Journal of Physiology. 1997;501:165–182. doi: 10.1111/j.1469-7793.1997.165bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckler RL, Weaver LC. Characteristics of ongoing and reflex discharge of single splenic and renal sympathetic postganglionic fibres in cats. The Journal of Physiology. 1988;396:163–175. doi: 10.1113/jphysiol.1988.sp016955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordin M. Low-threshold mechanoreceptive and nociceptive units with unmyelinated (C) fibres in the human supraorbital nerve. The Journal of Physiology. 1990;426:229–240. doi: 10.1113/jphysiol.1990.sp018135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein RD, Weaver LC. Multi- and single-fibre mesenteric and renal sympathetic responses to chemical stimulation of intestinal receptors in cats. The Journal of Physiology. 1988;396:155–172. doi: 10.1113/jphysiol.1988.sp016956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundlöf G, Wallin BG. The variability of muscle nerve sympathetic activity in resting recumbent man. The Journal of Physiology. 1977;272:383–397. doi: 10.1113/jphysiol.1977.sp012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundlöf G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. The Journal of Physiology. 1978;274:621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo Å B, Olausson H, Wessberg J, Norrsell U. A system of unmyelinated afferents for innocuous mechanoreception in the human skin. Brain Research. 1993;628:301–304. doi: 10.1016/0006-8993(93)90968-s. 10.1016/0006-8993(93)90968-S. [DOI] [PubMed] [Google Scholar]
- Wallin BG. Relationship between sympathetic outflow to muscles, heart rate and plasma noradrenaline in man. In: Delius W, Gerlach E, Grobecker H, Kübler W, editors. Catecholamines and the Heart. Berlin: Springer Verlag; 1981. pp. 11–17. [Google Scholar]


