Abstract
The effects of the NH2-terminal fragments of M130, a 130 kDa regulatory subunit of smooth muscle myosin phosphatase, on contraction and myosin light chain phosphorylation were investigated in Triton X-100-permeabilized porcine renal artery.
Incubation of the permeabilized fibres with M1301-633 (a fragment containing amino acid residues 1-633) or M13044-633 enhanced the Ca2+-induced contraction and shifted the [Ca2+]i-force relationship to the left (EC50 of Ca2+: 330 nM, control, without fragment; 145 nM, M1301-633; 163 nM, M13044-633). Pre-incubation for 1-3 h was needed for these long constructs.
M1301-374, M130304-511 and M130297-374, i.e. relatively short constructs compared with M1301-633 and M13044-633, also induced leftward shifts of the [Ca2+]i-force relationship (EC50 of Ca2+: 65 nM, 72 nM and 180 nM, respectively). However, these required no pre-incubation.
Deletion of residues 304-374 from the most potent construct, M1301-374, abolished the Ca2+-sensitizing effect.
Wortmannin inhibited the enhancement of contraction induced by M130 fragments when added before contraction was initiated and partially inhibited the effects when added after steady-state contraction.
M1301-374 slowed the rate of relaxation in Ca2+-free medium. The time for 50 % relaxation with this fragment was 510 ± 51 s, compared with 274 ± 14 s for control.
The levels of myosin light chain phosphorylation (22.4 %) and force (34.5 %) obtained with 300 nM Ca2+ were increased by 3 μM M1301-374 to 35.7 and 92.2 %, respectively. However, M1301-374 had no effect on the phosphorylation-force relationship.
In conclusion, the NH2-terminal M130 fragments containing residues 304-374 inhibited myosin phosphatase, increased myosin light chain phosphorylation and increased the Ca2+ sensitivity of the contractile apparatus in permeabilized porcine renal artery.
Reversible phosphorylation of the myosin light chains (MLCs) is one of the most important mechanisms for the regulation of smooth muscle contraction and relaxation (Hartshorne, 1987; Somlyo & Somlyo, 1994). The extent of MLC phosphorylation is regulated by the balance of two processes: phosphorylation and dephosphorylation catalysed by MLC kinase (MLCK) and MLC phosphatase (MLCP), respectively (Somlyo & Somlyo, 1994). Since the activity of MLCK is regulated by the Ca2+-calmodulin complex, the cytosolic Ca2+ concentration ([Ca2+]i) has long been regarded as the major determinant of smooth muscle contraction, while the activity of MLCP was assumed to remain constant (Hartshorne, 1987). However, simultaneous measurement of [Ca2+]i and force revealed that the relationship between [Ca2+]i and force in smooth muscle varies with the type of stimulation inducing contraction or relaxation (Morgan & Morgan, 1984; Kanaide, 1995). This was also clearly demonstrated using permeabilized fibres, in which agonist and GTP-γ-S increased force at a constant [Ca2+]i level (Nishimura et al. 1988). Thus, it is now widely accepted that alteration of the Ca2+ sensitivity of the contractile apparatus as well as changes in the Ca2+ level are important for the regulation of smooth muscle contraction (Somlyo & Somlyo, 1994). Concerning the mechanism for this change in Ca2+ sensitivity, it has been suggested that regulation of MLCP activity is one of the important mechanisms in the regulation of Ca2+ sensitivity (Somlyo et al. 1989). More recent experimental evidence suggests that inhibition of MLCP activity is linked to an increase in Ca2+ sensitivity (Kitazawa et al. 1991; Gong et al. 1995) and that the activation of MLCP is linked to a decrease in Ca2+ sensitivity (Wu et al. 1996; Lee et al. 1997).
Smooth muscle MLCP has been isolated and cloned from chicken gizzard and pig bladder (Alessi et al. 1992; Shimizu et al. 1994; Shirazi et al. 1994). MLCP is a type 1 protein phosphatase (Cohen, 1989; Okubo et al. 1993) and is composed of three subunits: a catalytic subunit of 38 kDa (PP1c) and two regulatory subunits of 110-130 kDa (referred to as M110 for the rat isoform and M130 for the chicken isoform) and 20 kDa (M20) (Chen et al. 1994; Shimizu et al. 1994; Haystead et al. 1995). The major activity towards MLC in smooth muscle is thought to be due to the heterotrimeric holoenzyme (Hartshorne et al. 1998). M130 has been shown to perform two major regulatory functions: one is to target the phosphatase to myosin (Shimizu et al. 1994; Ichikawa et al. 1996A; Johnson et al. 1996; Hirano et al. 1997) and the other is to modulate catalytic activity depending on its phosphorylation state (Trinkle-Mulcahy et al. 1995; Ichikawa et al. 1996b; Kimura et al. 1996). The former function resides on an NH2-terminal part of M130 (Okubo et al. 1993; Haystead et al. 1995; Ichikawa et al. 1996A; Johnson et al. 1996; Hirano et al. 1997). NH2-terminal fragments of M130 have been shown to bind to myosin, interact with PP1c and substrate (phosphorylated MLC) and activate PP1c activity towards MLC (Shimizu et al. 1994; Ichikawa et al. 1996A; Johnson et al. 1996; Hirano et al. 1997). A region of M130 comprising residues 1-38 is essential for PP1c binding (Johnson et al. 1996; Hirano et al. 1997), and an ankyrin repeat structure (Shimizu et al. 1994) has been suggested to contain a weaker second binding site for PP1c and a binding site for MLC (Hirano et al. 1997). Activation of PP1c activity towards MLC requires both sites (Johnson et al. 1996; Hirano et al. 1997), but it has been suggested that an additional sequence of M130, a region containing an acidic amino acid cluster (Shimizu et al. 1994), is also necessary for the activation (Hirano et al. 1997).
In spite of these numerous in vitro studies using cell-free systems, there have been only a few reports describing the effect of M130 on smooth muscle contraction (Haystead et al. 1995; Gailly et al. 1996). These experiments indicated that M110 fragments stimulated PP1c activity. However, it should be noted that in these experiments the endogenous phosphatases were inhibited irreversibly by the phosphatase inhibitor microcystin (Honkanen et al. 1990; MacKintosh et al. 1995), and subsequently the effects on the relaxation rate of permeabilized fibres of exogenously added NH2-terminal M110 fragments in combination with the purified PP1c were investigated. In the present study, we investigated the effects of exogenously added M130 fragments on the contraction of Triton X-100-permeabilized smooth muscle fibre preparations from porcine renal artery. We determined the MLC phosphorylation level and the contraction amplitude of the permeabilized smooth muscle fibres using a series of NH2-terminal fragments of chicken gizzard M130, since M130 is highly conserved among human, rat and chicken (Hartshorne et al. 1998), and a porcine homologue has not been obtained. We found that exogenously added M130 fragments increased the Ca2+ sensitivity of the contractile apparatus. We suggest that this increase in Ca2+ sensitivity is due to inhibition of the endogenous MLCP, since MLC phosphorylation was also increased by M130 fragments at a constant [Ca2+]i and the relaxation rate was reduced. The specific M130 domain responsible for this increase in Ca2+ sensitivity was the NH2-terminal M130 fragment (residues 304-374) containing the acidic amino acid cluster.
METHODS
Materials
Oligonucleotides were synthesized by Sawady Technology (Tokyo, Japan) or Hokkaido System Science (Sapporo, Japan). Deoxyribonucleoside triphosphates (dATP, dCTP, dTTP, dGTP) were purchased from Takara (Tokyo, Japan). The expression vectors (pQE vectors) for hexahistidine-tagged proteins were purchased from Qiagen (Hilden, Germany). Mouse monoclonal anti-MLC (20 kDa) antibody, horseradish peroxidase-conjugated goat anti-mouse IgM antibody, creatine phosphate kinase, wortmannin, poly-L-glutamate (molecular weight, 2000-15 000), poly-L-aspartate (molecular weight, 5000-15 000) and low molecular weight heparin (approximate molecular weight, 3000) were purchased from Sigma. Calmodulin was purchased from Seikagaku Kogyo (Tokyo, Japan). Triton X-100 was purchased from Katayama Chemicals (Osaka, Japan).
Preparation of mutant proteins of the chicken gizzard M130
The NH2-terminal fragments of the 130 kDa regulatory subunit (M130) of chicken gizzard myosin phosphatase were expressed as hexahistidine-tagged recombinant proteins using pQE expression vectors. Escherichia coli strain JM109 or M15[pREP4] (Qiagen) was used as a bacterial host for expression. Plasmids for the expression of the fragments corresponding to amino acid residues 1-296 (M1301-296), 1-374 (M1301-374), 1-633 (M1301-633) and 304-511 (M130304-511) have been described previously (Fig. 1) (Hirano et al. 1997). cDNAs for the other fragments (M13044-633 and M130297-374) were obtained by PCR amplification with either pQE-M1301-374 or pQE-M1301-633 as template DNA (Fig. 1). LA Taq DNA polymerase (Takara) was used for the PCR reaction. The sense and antisense primers were designed to contain BamHI and Sal I sites, respectively, to enable ligation of the PCR products to the vector plasmids. The BamHI- and Sal I-digested PCR products were ligated to the BamHI- and Sal I-digested pQE31 vector using T4 ligase (DNA Ligation kit ver.2, Takara).
Figure 1. Schematic representation of the M130 fragments.
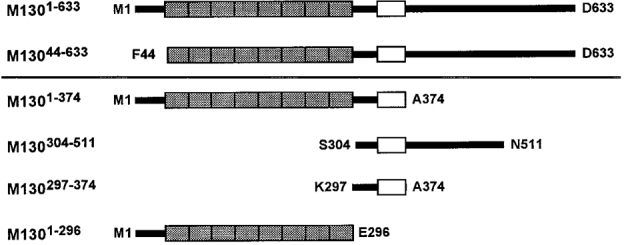
The NH2- and COOH-terminal residues are indicated by the single letter amino acid codes and the abbreviation used for each fragment is given. The shaded box area indicates the 8 ankyrin repeats, and the open box area is the acidic amino acid cluster (Shimizu et al. 1994).
The expression of M130 fragments in E. coli was as described previously (Hirano et al. 1997). Purification of expressed protein was performed according to the manufacturer's instructions (Qiagen) with minor modifications. In brief, the bacterial pellet was resuspended in sonication buffer (50 mM sodium phosphate, 300 mM NaCl, pH 8.0) and homogenized with an ultrasonic disrupter (UD-201, Tomy, Tokyo, Japan). The homogenate was centrifuged at 25 000 g for 10 min at 4°C, and the supernatant obtained was mixed for 1 h with nickel-nitrilotriacetic acid- agarose resin (Qiagen) equilibrated with sonication buffer. The resin was loaded onto a column and washed extensively in sonication buffer and then in wash buffer (50 mM sodium phosphate, 300 mM NaCl, 10 % glycerol, pH 6.0) until the absorbance of the eluate at 280 nm from each of the washes was less than 0.01. Then the resin was washed with three volumes of wash buffer containing 50 mM imidazole. The histidine-tagged recombinant proteins were eluted in wash buffer containing 100 mM imidazole. Every fraction of the eluate was evaluated by SDS-PAGE. Fractions of greater than 70 % purity were pooled and dialysed against 100 mM potassium methanesulphonate and 20 mM Tris-maleate (pH 7.0). The dialysate was cleared by centrifugation at 11 000 g at 4°C for 10 min and concentrated using Centricon 10 (Amicon, Tokyo, Japan).
Preparation of porcine renal artery
Fresh kidneys from pigs of either sex were obtained from a local slaughterhouse. They were brought back to the laboratory in pre-aerated physiological salt solution (mM: NaCl, 123; KCl, 4.7; NaHCO3, 15.5; KH2PO4, 1.2; MgCl2, 1.2; CaCl2, 1.25; and d-glucose, 11.5). The kidney was then cut open and the distal portions of the interlobular arteries were isolated. Arterial segments with an interior diameter of 200-250 μm were chosen. The fat and adventitia were mechanically removed under a binocular microscope. The segments were then cut into 500 μm wide vascular rings. The tissue and arterial segments were kept in physiological salt solution aerated with 95 % O2 and 5 % CO2 during all preparative procedures.
Permeabilization and force measurements
Arterial rings thus obtained were permeabilized with 1 % Triton X-100 in Ca2+-free cytoplasmic substitution solution (CSS (mM): EGTA, 10; potassium methanesulphonate, 100; MgCl2, 3.38; Na2ATP, 2.2; creatine phosphate, 10; and Tris-maleate, 20; pH 6.8) at 24-25°C for 30 min. Measurement of isometric force was performed at 24-25°C as described previously (Nishimura et al. 1988). Briefly, the tissue was mounted onto two tungsten wires bathed in wells filled with Ca2+-free CSS on a plate, by passing the tungsten wires through the lumen of the arterial ring. One of the wires was fixed and the other was connected to a force transducer (U gauge, Minebea, Japan). The tissue was then stretched to two times its resting diameter, and was allowed to relax completely in Ca2+-free CSS for 30 min. The extent of force development was expressed as a percentage, assigning values of 0 and 100 % to the force in Ca2+-free CSS (resting state) and 10 μM Ca2+ CSS (maximum contraction), respectively. The Ca2+ CSS containing the indicated concentration of free Ca2+ was prepared by adding an appropriate amount of CaCl2, using the EGTA-Ca2+ binding constant of 106 M−1 (Saida & Nonomura, 1978). All solutions used for force measurement of permeabilized fibres were supplemented with 2 μM calmodulin and 50 U ml−1 creatine phosphate kinase.
The fragments of M130 were applied directly in CSS. Tissues treated with M1301-633 or M13044-633 were incubated in CSS containing 3 μM recombinant proteins at 4°C for 3 h before force measurements (see Fig. 2 for determination of incubation time). In control experiments for this protocol, tissues were treated in the same way except that the protein solution was replaced with an equal volume of the dialysis buffer. The [Ca2+]i-force relationship of the Ca2+-induced contraction obtained after a 3 h incubation in the vehicle-containing CSS did not differ from that obtained without the 3 h incubation (data not shown).
Figure 2. Effects of M1301-633 and M13044-633 on the [Ca2+]i-force relationship of the Ca2+-induced contraction in 1 % Triton X-100-permeabilized porcine renal artery.
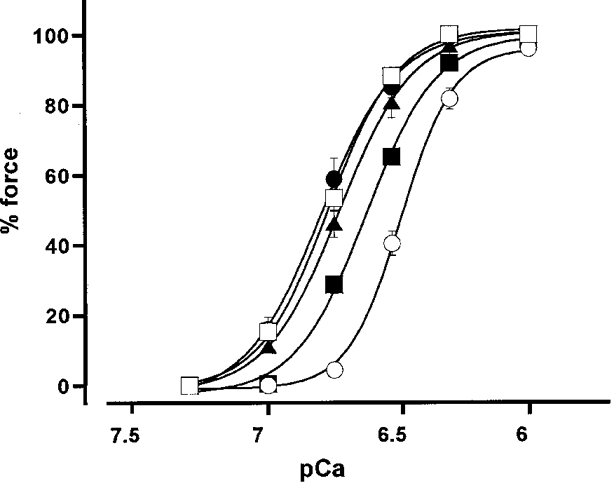
The permeabilized fibres were pretreated with 3 μM M1301-633 for 1 (▪), 2 (▴) and 3 h (•) or 3 μM M13044-633 for 3 h (□) at 4 °C in Ca2+-free cytoplasmic substitution solution (CSS) (see Methods for composition). Then, the fibres were mounted and connected to a force transducer in the buffer without M130 fragments, and contraction was initiated by incrementing the Ca2+ concentration. The [Ca2+]i-force relationship curve of the control contraction (○) was obtained in permeabilized fibres treated with vehicle (dialysis buffer) for 3 h at 4 °C. Force development is expressed as a percentage of that obtained with 10 μM Ca2+. Data are means ±s.e.m. (n = 3-4).
Measurement of MLC phosphorylation
The extent of MLC phosphorylation in permeabilized fibres was determined using the urea-glycerol gel electrophoresis technique (Persechini et al. 1986), followed by immunoblot detection with a specific mouse monoclonal anti-MLC antibody. Arterial ring preparations were obtained, permeabilized and treated in a similar way as described for the force study, except that the tissues were not attached to tungsten wires. At the indicated times, arterial rings were transferred into 90 % acetone, 10 % trichloroacetic acid and 10 mM dithiothreitol (DTT) pre-chilled at -80°C to stop the reaction. Tissues were then washed extensively and stored in acetone containing 10 mM DTT at -80°C. After the tissue had been dried to remove acetone, it was extracted in sample buffer (8 M urea, 20 mM Tris-base, 23 mM glycine, 0.004 % Bromophenol Blue and 10 mM DTT) at room temperature for 1 h. The supernatant was subjected to electrophoresis on a 10 % polyacrylamide, 40 % glycerol gel, followed by transfer onto polyvinylidene difluoride membrane (BioRad, Hercules, CA, USA) in 10 mM Na2HPO4 (pH 7.6). The 20 kDa MLC, in both unphosphorylated and phosphorylated forms, was detected by a specific anti-MLC antibody (× 200 dilution), and a horseradish peroxidase-conjugated secondary antibody (× 1000 dilution). The immune complex was detected using the enhanced chemiluminescence technique (ECL plus kit; Amersham, Buckinghamshire, UK). X-OMAT AR film (Kodak, Rochester, NY, USA) was used to detect light emission. After scanning the X-ray film on an Epson colour scanner GT-9500, the density of unphosphorylated and phosphorylated MLCs was determined by Gel Plotting Macros of the NIH image version 1.61 (National Institutes of Health, USA). The percentage of the phosphorylated form in total MLC (sum of unphosphorylated and phosphorylated forms) was calculated to indicate the extent of MLC phosphorylation.
Other methods
Nucleotide sequences of the expression plasmids were determined with the dye-termination method on an ABI Prism 310 automated sequencer (Applied Biosystem, Foster City, CA, USA). The sequence data were analysed with the Wisconsin Sequence Analysis Package (Genetic Computer Group, Madison, WI, USA). SDS-PAGE and the immunoblot technique were as described (Lemmli, 1970; Towbin et al. 1979). The concentration of protein was determined by the Bradford method (Bradford, 1976) with bovine serum albumin as the standard (Pierce, Rockford, IL, USA).
Data analysis
The EC50 value, the concentration required to induce a force of 50 % of the maximum response, was determined by fitting the concentration-response curves to a four-parameter logistic model (De Lean et al. 1978). The data are expressed as means ±s.e.m. Student's t test was used to determine statistically significant differences. P < 0.05 was considered to be of statistical significance.
RESULTS
Effect of M130 fragments on Ca2+ sensitivity in permeabilized porcine renal artery
In the Triton X-100-permeabilized porcine renal artery, a stepwise increment of Ca2+ concentration caused a stepwise increase in force. In the presence of 2 μM calmodulin, force development was observed at Ca2+ concentrations higher than 180 nM. The maximum force development was obtained at 1 μM Ca2+; there was no further force development above 1 μM Ca2+. Therefore, the level of force obtained with 10 μM Ca2+ was assigned as 100 %. Incubating permeabilized fibres in CSS containing dialysis buffer for 1, 2 and 3 h (for the control experiment) did not shift the [Ca2+]i-force curves (data not shown). Thus, Fig. 2 shows the control [Ca2+]i-force relationship obtained in fibres treated with control solution for 3 h. The concentration of Ca2+ required to obtain half the maximal force development (EC50) was 329.6 ± 12.1 nM (n = 4) (Fig. 2).
When M1301-633 was applied in 180 nM Ca2+ CSS, there was no further development of force (data not shown). However, in permeabilized fibres treated with 3 μM M1301-633 in CSS at 4°C for a longer period, the Ca2+-induced contraction was augmented and the [Ca2+]i-force relationship curve shifted to the left (compared with control), depending on the length of the treatment (Fig. 2). The EC50 values for Ca2+ were significantly decreased to 227.8 ± 7.8, 191.7 ± 10.7 and 144.5 ± 31.2 nM (n = 3-4,P < 0.01) by 1, 2 and 3 h treatment, respectively. Following treatment of the permeabilized fibres with M1301-633, this enhancement of Ca2+-induced contraction was observed even in the absence of M130 fragments in the bathing buffer. Similarly, treating the permeabilized fibres with 3 μM M13044-633 in CSS at 4°C for 3 h caused a leftward shift of the Ca2+-force curves. The EC50 value obtained in the M13044-633-treated fibres was 163.1 ± 5.6 nM (n = 4, P < 0.01) and did not differ significantly from that obtained with M1301-633. Thus, M13044-633 had a similar effect to that seen with M1301-633.
M1301-374, M130304-511 and M130297-374 were effective in augmenting the Ca2+-induced contraction, but without pretreatment (Fig. 3A, B and C). When these fragments were applied in 180 nM Ca2+ CSS, there was an instant development of force. Application of 3 μM M1301-374, M130304-511 or M130297-374 caused 81.7, 70.7 and 34.7 % force development, respectively. The effects of these M130 fragments were concentration dependent. Figure 3F shows a concentration-response curve for the M1301-374-induced contraction in 180 nM Ca2+ CSS. The fragment had enhancing effects at concentrations above 0.5 μM, and maximal enhancement was obtained at 3 μM. The concentration of M1301-374 required to induce half the maximal enhancement was 1.4 ± 0.2 μM (n = 3). The [Ca2+]i-force relationships of the Ca2+-induced contractions obtained in the presence of these fragments were shifted leftwards (Fig. 4). The EC50 values for Ca2+ obtained in the presence of 3 μM M1301-374, M130304-511 and M130297-374 were 65.2 ± 10.8, 72.3 ± 13.4 and 180.1 ± 13.5 nM (n = 3,P < 0.01), respectively.
Figure 3. Effect of M1301-374, M130304-511, M130297-374, M1301-296 and heparin on contraction in 1 % Triton X-100-permeabilized porcine renal artery.
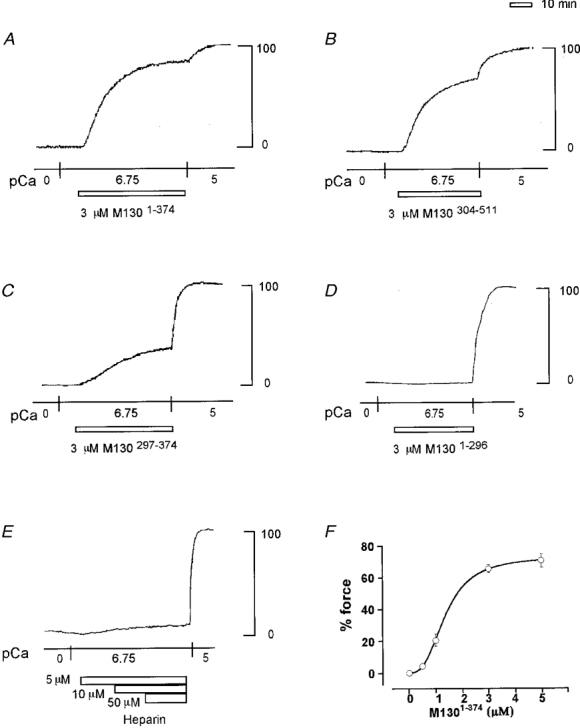
A-E, representative recordings showing the effect of 3 μM M1301-374 (A), 3 μM M130304-511 (B), 3 μM M130297-374 (C), 3 μM M1301-296 (D) and 5, 10 and 50 μM heparin (E) on the force development in 180 nM Ca2+ CSS. F, concentration-response curve for the contraction induced by M1301-374 in 180 nM Ca2+ CSS in 1 % Triton X-100-permeabilized porcine renal artery. Force development is expressed as a percentage of that obtained with 10 μM Ca2+. Data are means ±s.e.m. (n = 3).
Figure 4. [Ca2+]i-force relationship curves for contractions induced by incrementing Ca2+ levels in the absence and presence of M130 fragments.
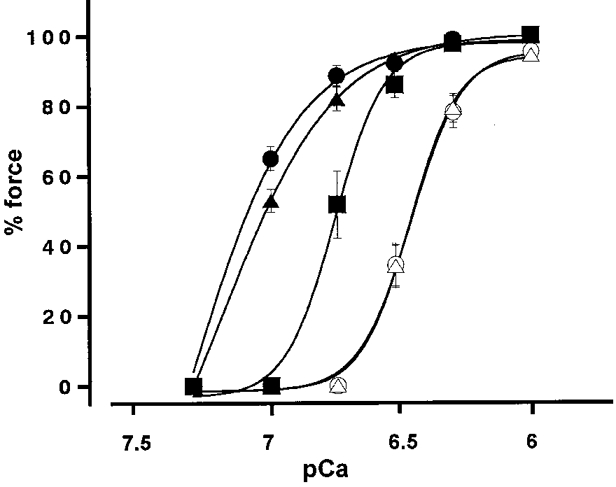
○, control; ▵, 3 μM M1301-296; ▪, 3 μM M130297-374; ▴, 3 μM M130304-511; and •, 3 μM M1301-374. Force development is expressed as a percentage of that obtained with 10 μM Ca2+. Data are means ±s.e.m. (n = 3-4).
In contrast, 3 μM M1301-296 did not induce a contraction in 180 nM Ca2+ CSS (Fig. 3A). Higher concentrations of M1301-296, up to 5 μM, had no effect on the Ca2+-induced contraction (data not shown). The [Ca2+]i-force relationship obtained in the presence of 3 μM M1301-296 overlapped the control relationship obtained without M130 fragments (Fig. 4). The EC50 value obtained in the presence of M1301-296 was 340.3 ± 22.2 nM (n = 3). This value did not significantly differ from the control value (351.4 ± 26.4 nM). Pretreatment of permeabilized fibres with 3 μM M1301-296 for 3 h had no effect on the Ca2+-induced contraction or on its [Ca2+]i-force relationship curve (data not shown).
Among the NH2-terminal fragments of M130 evaluated in the present study, M130297-374 was the shortest fragment to induce augmentation of the Ca2+-induced contraction in Triton X-100-permeabilized porcine renal artery. Since M130297-374 contains a cluster of acidic amino acids (Shimizu et al. 1994), there was the possibility that negative charge and not the specific structure of the M130 fragments was linked to the augmentation of the Ca2+-induced contraction. Thus, we examined the effects of poly-L-glutamate (molecular weight, 2000-15 000), poly-L-aspartate (molecular weight, 5000-15 000) and low molecular weight heparin (approximate molecular weight, 3000) on the Ca2+-induced contraction. Neither poly-L-glutamate nor poly-L-aspartate, up to 5 μM, induced force in either 180 or 300 nM Ca2+ CSS (data not shown). The multiple negative charge carrier heparin had no effect up to 1 μM, although 5 μM heparin induced a small but significant development of force in 180 nM Ca2+ CSS. However, the extent of force development induced by 5 μM heparin in 180 nM Ca2+ CSS was 5 %, which was much lower than that obtained with M1301-374 (Fig. 3A). Further increments in the concentration of heparin up to 50 μM did not cause further force development.
Effect of wortmannin on the Ca2+ sensitization induced by M130 mutants
Figure 5A and B shows the effect of wortmannin on the 180 nM Ca2+-induced contractions of permeabilized fibres treated with 3 μM M1301-633 at 4°C for 3 h. Wortmannin was used as an inhibitor of MLCK because only two kinases, MLCK and phosphatidyl inositol 3-kinase, are reported to be the major target kinases of wortmannin (Yano et al. 1993). When applied during the steady state of the 180 nM Ca2+-induced contraction, 10 μM wortmannin relaxed contraction by 39 % (Fig. 5A). However, the inhibition was partial even with 30 and 100 μM wortmannin. In contrast, when 10 μM wortmannin was applied 5 min before and during the application of 180 nM Ca2+, force development was completely inhibited (Fig. 5A). Similarly, application of 10 μM wortmannin before the induction of contraction completely inhibited the subsequent force development induced by 3 μM M1301-374, M130304-511 and M130297-374 in 180 nM Ca2+ CSS (Fig. 5A).
Figure 5. Effects of wortmannin on the enhancement of Ca2+-induced contraction by M130 fragments in 1 % Triton X-100-permeabilized porcine renal artery.
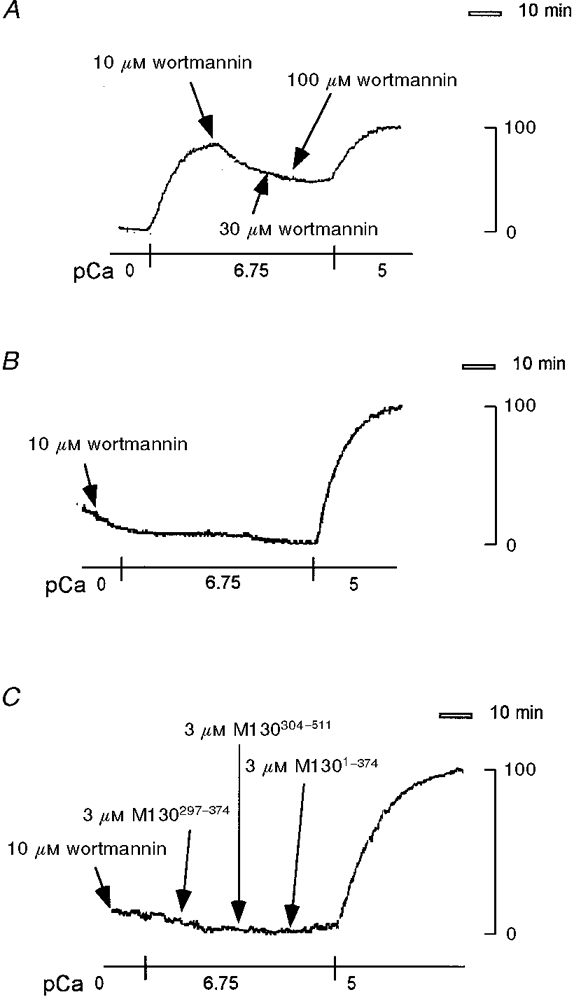
A and B, permeabilized fibres were treated with 3 μM M1301-633 in CSS at 4 °C for 3 h, and 10, 30 and 100 μM wortmannin was applied at the times indicated by arrows after the 180 nM Ca2+-induced contraction reached a steady state (A), or 10 μM wortmannin was applied 5 min before the induction of contraction by 180 nM Ca2+ CSS (B). C, after treatment with 10 μM wortmannin for 5 min in CSS and exposure to 180 nM Ca2+ CSS, permeabilized fibres were consecutively stimulated with 3 μM M130297-374, 3 μM M130304-511 and 3 μM M1301-374, at the times indicated by arrows. These are representative traces of three independent experiments, which yielded similar results.
Effect of M1301-374 on relaxation in permeabilized porcine renal artery
Figure 6 shows the effect of M1301-374 on the relaxation induced by exposure to Ca2+-free CSS in the permeabilized porcine renal artery. The fibres were contracted by 10 μM Ca2+ CSS, and then relaxation was induced by exposure to Ca2+-free CSS, in either the presence or the absence of 3 μM M1301-374. M1301-374 was applied 5 min before exposure to the Ca2+-free CSS, when the precontraction induced by 10 μM Ca2+ was in the sustained phase. There was no further force development by the application of M1301-374. The relaxing solution contained 10 μM wortmannin to inhibit any MLCK activity in the Ca2+-free CSS. Thus, the relaxation rate was considered to represent myosin phosphatase activity. As shown in Fig. 6, M1301-374 significantly slowed relaxation rate. The time to cause a 50 % reduction of force obtained in the presence of M1301-374 (510.0 ± 51.4 s, n = 4) was significantly (P < 0.001) longer than that obtained in its absence (control relaxation, 273.6 ± 13.5 s, n = 4).
Figure 6. Effect of M1301-374 on relaxation in 1 % Triton X-100-permeabilized porcine renal artery.
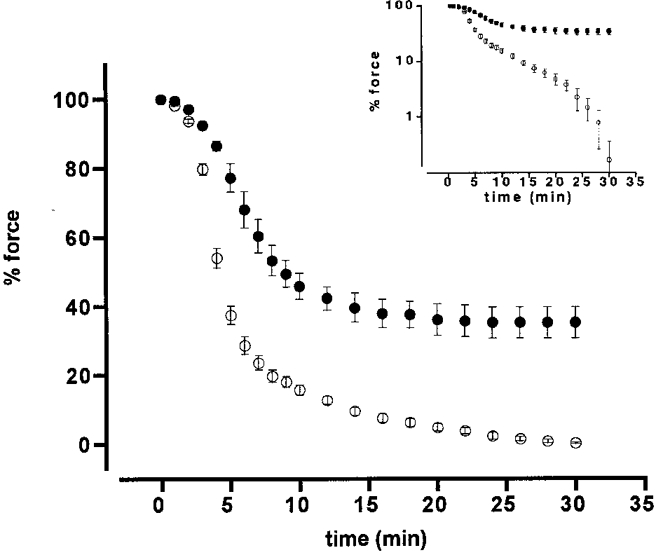
The permeabilized fibres were contracted with 10 μM Ca2+ CSS, and then relaxed by exposure to Ca2+-free CSS containing 10 μM wortmannin in either the presence (•) or the absence (○) of 3 μM M1301-374. M1301-374 was applied 5 min prior to exposure to Ca2+-free CSS. Force development is expressed as a percentage of that obtained with 10 μM Ca2+. Data are means ±s.e.m. (n = 4). Inset, a semilog plot of the same data, showing a decrease of force regression during relaxation for fibres in the presence of 3 μM M1301-374 (▪) compared with that in control fibres (○).
MLC phosphorylation during the M1301-374-induced enhancement of contractions
The effects of the M1301-374 fragment on the level of MLC phosphorylation and the relationship between the extent of MLC phosphorylation and developed force were examined. The level of MLC phosphorylation was determined in arterial tissues under the unloaded condition, while force measurement was performed under the isometrically loaded condition. The extent of MLC phosphorylation with no developed tension (0 % tension) obtained in the Ca2+-free CSS was 10.9 ± 2.7 % (n = 4) (Fig. 7A, column 1). During the steady-state contractions induced by 300 nM, 1 μM and 10 μM Ca2+, the extent of MLC phosphorylation significantly increased to 22.4 ± 4.0 % (n = 4,P < 0.05), 41.1 ± 6.4 % (n = 3,P < 0.005), and 64.3 ± 1.9 % (n = 3,P < 0.001), and force developed to 34.5 ± 6.2, 95.5 ± 2.9 and 100 % (n = 3), respectively (Fig. 7A, columns 2, 4 and 5, respectively). Addition of 3 μM M1301-374 in 300 nM Ca2+ CSS induced a further force development (92.2 ± 2.0 %, n = 3) associated with a significant increase in MLC phosphorylation (35.7 ± 4.2 % at 30 min after the induction of contraction; n = 4,P < 0.01) (Fig. 7A, column 3). The relationship between the level of MLC phosphorylation and the extent of force obtained with 300 nM Ca2+ in the presence of 3 μM M1301-374 was similar to that obtained with 1 μM Ca2+ in the absence of M1301-374 (Fig. 7A). This is clearly demonstrated in Fig. 7B. The control phosphorylation-force curve was reconstructed from the values of MLC phosphorylation and force shown in Fig. 7A. The phosphorylation-force relationship obtained with 300 nM Ca2+ in the presence of M1301-374 fitted the control curve.
Figure 7. Effects of M1301-374 on MLC phosphorylation and on the relationship between MLC phosphorylation and force in 1 % Triton X-100-permeabilized porcine renal artery.
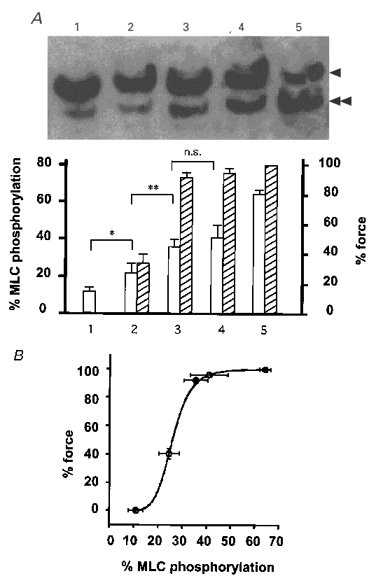
A, representative photograph of the MLC immunoblot (upper panel) and summary (lower panel) of MLC phosphorylation and force obtained by 30 min incubation in CSS (1), 300 nM Ca2+ (2), 300 nM Ca2+ plus 3 μM M1301-374 (3), 1 μM Ca2+ (4) and 10 μM Ca2+ (5). Single arrowhead, unphosphorylated MLC; double arrowhead, monophosphorylated MLC. □, MLC phosphorylation;  , force. The level of phosphorylation is expressed as a percentage of phosphorylated MLC in the total MLC (as described in Methods). Force development is expressed as a percentage of that obtained with 10 μM Ca2+. Values of force obtained in the absence of M1301-374 (columns 1, 2, 4 and 5) are from Fig. 4. Data are means ±s.e.m. (n = 3-4). * P < 0.05; ** P < 0.01; n.s., not significantly different. B, MLC phosphorylation-force relationship curve reconstructed from data shown in A. ○, phosphorylation-force relationship obtained with 0, 300 nM, 1 μM and 10 μM Ca2+ in the absence of M1301-374. •, phosphorylation-force relationship obtained with 300 nM Ca2+ in the presence of 3 μM M1301-374.
, force. The level of phosphorylation is expressed as a percentage of phosphorylated MLC in the total MLC (as described in Methods). Force development is expressed as a percentage of that obtained with 10 μM Ca2+. Values of force obtained in the absence of M1301-374 (columns 1, 2, 4 and 5) are from Fig. 4. Data are means ±s.e.m. (n = 3-4). * P < 0.05; ** P < 0.01; n.s., not significantly different. B, MLC phosphorylation-force relationship curve reconstructed from data shown in A. ○, phosphorylation-force relationship obtained with 0, 300 nM, 1 μM and 10 μM Ca2+ in the absence of M1301-374. •, phosphorylation-force relationship obtained with 300 nM Ca2+ in the presence of 3 μM M1301-374.
DISCUSSION
In the present study, we investigated the effects of a series of NH2-terminal fragments of chicken M130 on the Ca2+-induced contractions of permeabilized porcine renal artery. The major findings are as follows. (1) The NH2-terminal fragments of M130 induced contractions at a constant [Ca2+]i and shifted the [Ca2+]i-force relationship to the left. (2) This effect was completely abolished by deleting the amino acid sequence 304-374. (3) The contraction induced by M1301-374, the most potent construct, was accompanied by an increase in MLC phosphorylation. (4) The relationship between the level of MLC phosphorylation and force remained unchanged, irrespective of the presence or absence of M1301-374. (5) Wortmannin was more effective in inhibiting Ca2+ sensitization by the NH2-terminal fragments when applied before contraction was initiated, compared with the addition during steady-state contraction. (6) The rate of relaxation was decreased by treatment with M1301-374. Based on these findings, we propose that the NH2-terminal fragments of M130 could inhibit endogenous MLCP activity. Although the mechanism is still speculative, this is the first report showing that the NH2-terminal fragments of M130 increase MLC phosphorylation and Ca2+ sensitivity of permeabilized smooth muscle preparations.
The increases in Ca2+ sensitivity induced by the M130 fragments are clearly demonstrated in Figs 2, 3 and 4. Because [Ca2+]i was clamped in the permeabilized preparation, force developed at a constant level of Ca2+, thus indicating an increase in the Ca2+ sensitivity of the contractile apparatus. This increase in Ca2+ sensitivity was a marked effect (as opposed to a subtle change), because the M130 fragments induced almost 90 % of the maximal contraction at a Ca2+ concentration of 180 nM, which alone caused no contraction (Fig. 3A and B). The extent of the change was also noted by the quantitative evaluation of Ca2+ sensitivity, in which a marked leftward shift of the [Ca2+]i-force curve induced by the M130 fragments was observed (Figs 2 and 4). These results indicate that the M130 fragments caused an increase in Ca2+ sensitivity. However, there is the possibility that the histidine tag or other possible contaminating bacterial proteins might be responsible for the apparent Ca2+-sensitizing effects (Buning et al. 1996). This possibility may be ruled out by the observation that M1301-296 was inert even at concentrations up to 5 μM, with or without 3 h treatment (Fig. 3A). Thus, we conclude that the M130 fragments themselves induce an increase in Ca2+ sensitivity.
Since the Ca2+-sensitizing effect is due to the NH2-terminal fragments of M130, we attempted to identify the region responsible for this effect by using a series of truncated fragments. The Ca2+-sensitizing effect could not be abolished by deletion of amino acids 1-43 (M13044-633) or by deletion of the ankyrin repeats (M130304-511, M130297-374; Figs 1-4), both of which are important for the activation of PP1c (Johnson et al. 1996; Hirano et al. 1997; Hartshorne et al. 1998). Also, deletion of the amino acid sequence 375-633 (M1301-374) did not affect the Ca2+-sensitizing effect. However, the Ca2+-sensitizing effect was completely abolished by deletion of amino acids 304-374 (M1301-296; Fig. 3A). We thus conclude that an essential sequence required for the Ca2+-sensitizing effect may be located within residues 304 to 374. One characteristic of this area is a cluster of acidic amino acids (Shimizu et al. 1994).
In contrast to the present conclusion, previous studies indicated that the NH2-terminal fragments of rat M110, a rat homologue of M130, induced activation of PP1c in permeabilized rabbit portal vein (Haystead et al. 1995; Gailly et al. 1996). This discrepancy may have arisen for the following reasons. First, these authors investigated the effects of exogenously added NH2-terminal M110 fragments in combination with purified PP1c on the relaxation rate of permeabilized smooth muscle fibres after the endogenous phosphatases had been inhibited by an irreversible phosphatase inhibitor, microcystin (Honkanen et al. 1990; MacKintosh et al. 1995). In contrast, we investigated the effects of exogenously added M130 fragments on the endogenous MLCP activity. Second, the NH2-terminal M110 fragments (M1101-309 and M11039-309) used by others lack the acidic amino acid cluster that we suggest is responsible for the increase in Ca2+ sensitivity. Thus, the various reports are not necessarily in conflict, because M1301-296 (present study) had no effect on Ca2+ sensitivity, and this corresponds to the M1101-309 fragment used in the quoted studies. Third, the long constructs, e.g. M13044-633 or M1301-633, required 1-3 h incubation to influence Ca2+ sensitivity (Fig. 2). Although a long construct, M11011-612, was used by Gailly et al. (1996), an effect on Ca2+ sensitivity was not described. This could be due to the requirement for a preincubation period for these large constructs to show an effect. The requirement for a longer incubation time with M1301-633 and M13044-633 can be simply explained by the suggestion that diffusion of these constructs to the target sites was hindered due to their larger molecular size. However, there is a possibility that longer constructs were slowly proteolysed during the incubation at 4°C and only degradation products containing the acidic cluster were effective in enhancing Ca2+-induced contraction.
In order to clarify the mechanism for the Ca2+-sensitizing effect by the NH2-terminal fragments of M130, we examined the level of MLC phosphorylation. As shown in Fig. 7, the contraction induced by M1301-374 was accompanied by an increase in MLC phosphorylation. However, the relationship between the level of MLC phosphorylation and force remained unchanged, irrespective of the presence or absence of M1301-374. It was thus indicated that the Ca2+-sensitizing effect is due to an increase in MLC phosphorylation and not to other effects on the contractile apparatus (e.g. effect on cross-bridges or other regulatory components). Since [Ca2+]i was kept constant in the permeabilized preparation, the activity of MLCK should remain constant. Thus, the increase in MLC phosphorylation was considered to be due to inhibition of myosin phosphatase by the M130 fragments. This is supported by the observation that the relaxation rate was decreased by M1301-374 (Fig. 6). Since relaxation was induced in the presence of a MLCK inhibitor and in Ca2+-free CSS, the rate of relaxation should reflect myosin phosphatase activity. Evidence consistent with this idea is that wortmannin was more effective in inhibiting the Ca2+ sensitization by the NH2-terminal fragments when applied before the contraction was initiated, rather than after the contraction reached steady state.
A possible explanation for the effect of M130 fragments could be that the negatively charged acidic amino acid cluster inhibits myosin phosphatase in a non-specific manner. Charged compounds, either positive or negative, are known to modulate PP1c activity (Bollen & Stalmans, 1992). For example, positively charged spermine was shown to increase MLC phosphorylation and cause contraction in both β-escin- and Triton X-100-permeabilized guinea-pig ileum (Swärd et al. 1995). Similarly, heparin, a multiple negative charge carrier, was shown to bind to PP1c and inhibit or stimulate activity depending on the substrates used (Bollen & Stalmans, 1992). To exclude this possibility, we examined the effects of negatively charged compounds such as poly-L-glutamate, poly-L-aspartate and heparin on the Ca2+-induced contractions of the permeabilized fibres. Among these compounds, only heparin produced a significant force, but this was much smaller than that seen with the M130 fragments. We thus consider that the inhibition of PP1c activity due to a non-specific charge effect is not responsible for the effect seen with the M130 fragments.
It is possible that amino acids 304-374 might act as an ‘inhibitory domain’ of M130 against PP1c. However, this region shows no similarity to the inhibitory regions of known PP1c inhibitors. It was noticed that the COOH-terminal part of M130, around the possible phosphorylation site (Ichikawa et al. 1996B), contains a region similar to the inhibitory domain of inhibitor-1 or the 32 kDa dopamine- and cAMP-regulated phosphoprotein (DARPP-32) (Hartshorne et al. 1998). Another possible explanation is that the inhibition of myosin phosphatase by M130 fragments may be due to an alteration of subunit interactions of the holoenzyme. Arachidonic acid was shown to interact with MLCP, disrupt the subunit interactions and thereby inhibit MLCP (Gong et al. 1992). It is thus possible that MLCP activity could be inhibited when one or more of the subunit interactions important for the activation of PP1c activity towards myosin are hindered by exogenously added M130 fragments. In other words, exogenously added M130 fragments might behave as a dominant negative. This is consistent with a report that amino acids 297-374 in addition to the PP1c-binding site (amino acids 1-38) and ankyrin repeats (amino acids 39-286) are required for activation of MLCP activity towards myosin (Hirano et al. 1997). If this is the case, the effects of M130 fragments would be expected to be stoichiometric with the endogenous M130. In the rabbit portal vein, the concentration of M130 was estimated to be 1.2 ± 0.3 μM (personal communication cited in Hartshorne et al. 1998). This value agrees with the concentration of M1301-374 required to induce the half-maximum Ca2+-sensitizing effect. M130 was shown to bind to both the catalytic subunit and myosin, and considered to target the catalytic subunit close to the substrate, i.e. myosin (Alessi et al. 1992; Shimizu et al. 1994). The activity of the catalytic subunit in the presence of M130 was greater with myosin as a substrate than with MLC (Ichikawa et al. 1996a). These results suggest that the interaction of M130 with myosin contributes to the regulation of MLCP activity. Thus, it is possible that exogenously added M130 fragments could inhibit the interaction of endogenous enzyme with myosin, and thereby inhibit phosphatase activity. These hypotheses are clearly speculative and further work is required to establish the molecular basis for the effect of the M130 fragments observed above.
In summary, the NH2-terminal fragments of M130, a regulatory subunit of MLCP, caused an increase in the Ca2+ sensitivity of the contractile apparatus in porcine renal artery permeabilized with Triton X-100. This Ca2+ sensitization was accompanied by an increase in MLC phosphorylation. Thus, we consider that the increase in Ca2+ sensitivity induced by the NH2-terminal fragments of M130 reflects an inhibition of the endogenous MLCP. A region essential for this Ca2+-sensitizing effect is the amino acid sequence 304 to 374.
Acknowledgments
We thank Dr David J. Hartshorne (University of Arizona, Tucson, AZ, USA) for comments and help with the manuscript. This study was supported in part by Grants-in-Aid for Scientific Research (No. 07407022, 10557072), for Scientific Research on Priority Areas (No. 10177222, 10177228), for Encouragement of Young Scientists (No. 10770308), and for Creative Basic Research Studies of Intracellular Signaling Network from the Ministry of Education, Science, Sports and Culture, Japan; and by The Vehicle Racing Commemorative Foundation; Kaibara Morikazu Medical Science Promotion Foundation; Kanae Foundation for Life & Socio-Medical Science; Japan Heart Foundation Research Grant; and Yokoyama Rinshoyakuri and Mochida Memorial Foundation for Medical and Pharmaceutical Research.
References
- Alessi D, MacDougall LK, Sola MM, Ikebe M, Cohen P. The control of protein phosphatase-1 by targetting subunits. The major myosin phosphatase in avian smooth muscle is a novel form of protein phosphatase-1. European Journal of Biochemistry. 1992;210:1023–1035. doi: 10.1111/j.1432-1033.1992.tb17508.x. [DOI] [PubMed] [Google Scholar]
- Bollen M, Stalmans W. The structure, role, and regulation of type 1 protein phosphatases. Critical Reviews in Biochemistry and Molecular Biology. 1992;27:227–281. doi: 10.3109/10409239209082564. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye-binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Buning H, Gartner U, von Schack D, Baeuerle PA, Zorbas H. The histidine tail of recombinant DNA binding proteins may influence the quality of interaction with DNA. Analytical Biochemistry. 1996;234:227–230. doi: 10.1006/abio.1996.0078. 10.1006/abio.1996.0078. [DOI] [PubMed] [Google Scholar]
- Chen YH, Chen MX, Alessi DR, Campbell DG, Shanahan C, Cohen P, Cohen PT. Molecular cloning of cDNA encoding the 110 kDa and 21 kDa regulatory subunits of smooth muscle protein phosphatase 1M. FEBS Letters. 1994;356:51–55. doi: 10.1016/0014-5793(94)01231-8. [DOI] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annual Review of Biochemistry. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- De Lean A, Munson PJ, Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. American Journal of Physiology. 1978;235:E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Gailly P, Wu X, Haystead TA, Somlyo AP, Cohen PT, Cohen P, Somlyo AV. Regions of the 110-kDa regulatory subunit M110 required for regulation of myosin-light-chain-phosphatase activity in smooth muscle. European Journal of Biochemistry. 1996;239:326–332. doi: 10.1111/j.1432-1033.1996.0326u.x. 10.1111/j.1432-1033.1996.0326u.x. [DOI] [PubMed] [Google Scholar]
- Gong MC, Fuglsang A, Alessi D, Kobayashi S, Cohen P, Somlyo AV, Somlyo AP. Arachidonic acid inhibits myosin light chain phosphatase and sensitizes smooth muscle to calcium. Journal of Biological Chemistry. 1992;267:21492–21498. [PubMed] [Google Scholar]
- Gong MC, Kinter MT, Somlyo AV, Somlyo AP. Arachidonic acid and diacylglycerol release associated with inhibition of myosin light chain dephosphorylation in rabbit smooth muscle. The Journal of Physiology. 1995;486:113–122. doi: 10.1113/jphysiol.1995.sp020795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne DJ. Biochemistry of the contractile process in smooth muscle. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1987. pp. 423–482. [Google Scholar]
- Hartshorne DJ, Ito M, Erdödi F. Myosin light chain phosphatase: subunit composition, interactions and regulation. Journal of Muscle Research and Cell Motility. 1998;19:325–341. doi: 10.1023/a:1005385302064. 10.1023/A:1005385302064. [DOI] [PubMed] [Google Scholar]
- Haystead CM, Gailly P, Somlyo AP, Somlyo AV, Haystead TA. Molecular cloning and functional expression of a recombinant 72.5 kDa fragment of the 110 kDa regulatory subunit of smooth muscle protein phosphatase 1M. FEBS Letters. 1995;377:123–127. doi: 10.1016/0014-5793(95)01318-0. 10.1016/0014-5793(95)01318-0. [DOI] [PubMed] [Google Scholar]
- Hirano K, Phan BC, Hartshorne DJ. Interactions of the subunits of smooth muscle myosin phosphatase. Journal of Biological Chemistry. 1997;272:3683–3688. doi: 10.1074/jbc.272.6.3683. 10.1074/jbc.272.6.3683. [DOI] [PubMed] [Google Scholar]
- Honkanen RE, Zwiller J, Moore RE, Daily SL, Khatra BS, Dukelow M, Boynton AL. Characterization of microcystin-LR, a potent inhibitor of type 1 and type 2A protein phosphatases. Journal of Biological Chemistry. 1990;265:19401–19404. [PubMed] [Google Scholar]
- Ichikawa K, Hirano K, Ito M, Tanaka J, Nakano T, Hartshorne DJ. Interactions and properties of smooth muscle myosin phosphatase. Biochemistry. 1996a;35:6313–6320. doi: 10.1021/bi960208q. [DOI] [PubMed] [Google Scholar]
- Ichikawa K, Ito M, Hartshorne DJ. Phosphorylation of the large subunit of myosin phosphatase and inhibition of phosphatase activity. Journal of Biological Chemistry. 1996b;271:4733–4740. doi: 10.1074/jbc.271.9.4733. 10.1074/jbc.271.9.4733. [DOI] [PubMed] [Google Scholar]
- Johnson DF, Moorhead G, Caudwell FB, Cohen P, Chen YH, Chen MX, Cohen PT. Identification of protein-phosphatase-1-binding domains on the glycogen and myofibrillar targetting subunits. European Journal of Biochemistry. 1996;239:317–325. doi: 10.1111/j.1432-1033.1996.0317u.x. 10.1111/j.1432-1033.1996.0317u.x. [DOI] [PubMed] [Google Scholar]
- Kanaide H. Cytosolic calcium concentration-force relation in vascular smooth muscle. In: Nakano T, Hartshorne DJ, editors. Regulation of the Contractile Cycle in Smooth Muscle. Tokyo: Springer-Verlag; 1995. pp. 61–72. [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kitazawa T, Masuo M, Somlyo AP. G protein-mediated inhibition of myosin light-chain phosphatase in vascular smooth muscle. Proceedings of the National Academy of Sciences of the USA. 1991;88:9307–9310. doi: 10.1073/pnas.88.20.9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Li L, Kitazawa T. Cyclic GMP causes Ca2+ desensitization in vascular smooth muscle by activating the myosin light chain phosphatase. Journal of Biological Chemistry. 1997;272:5063–5068. doi: 10.1074/jbc.272.8.5063. 10.1074/jbc.272.8.5063. [DOI] [PubMed] [Google Scholar]
- Lemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacKintosh RW, Dalby KN, Campbell DG, Cohen PTW, Cohen P, MacKintosh C. The cyanobacterial toxin microcystin binds covalently to cysteine-273 on protein phosphatase 1. FEBS Letters. 1995;371:236–240. doi: 10.1016/0014-5793(95)00888-g. 10.1016/0014-5793(95)00888-G. [DOI] [PubMed] [Google Scholar]
- Morgan JP, Morgan KG. Stimulus-specific patterns of intracellular calcium levels in smooth muscle of ferret portal vein. The Journal of Physiology. 1984;73:673–699. doi: 10.1113/jphysiol.1984.sp015239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura J, Kolber M, Van Breemen C. Norepinephrine and GTP-γ-S increase myofilament Ca2+ sensitivity in α-toxin permeabilized arterial smooth muscle. Biochemical and Biophysical Research Communications. 1988;157:677–683. doi: 10.1016/s0006-291x(88)80303-6. [DOI] [PubMed] [Google Scholar]
- Okubo S, Erdödi F, Ito M, Ichikawa K, Konishi T, Nakano T, Kawamura T, Brautigan DL, Hartshorne DJ. Characterization of a myosin-bound phosphatase from smooth muscle. Advances in Protein Phosphatases. 1993;7:295–314. [Google Scholar]
- Persechini A, Kamm KE, Stull JT. Different phosphorylated forms of myosin in contracting tracheal smooth muscle. Journal of Biological Chemistry. 1986;261:6293–6299. [PubMed] [Google Scholar]
- Saida K, Nonomura Y. Characteristics of Ca2+- and Mg2+-induced tension development in chemically skinned smooth muscle fibers. Journal of General Physiology. 1978;72:1–14. doi: 10.1085/jgp.72.1.1. 10.1085/jgp.72.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Ito M, Miyahara M, Ichikawa K, Okubo S, Konishi T, Naka M, Tanaka T, Hirano K, Hartshorne DJ, Nakano T. Characterization of the myosin-binding subunit of smooth muscle myosin phosphatase. Journal of Biological Chemistry. 1994;269:30407–30411. [PubMed] [Google Scholar]
- Shirazi A, Iizuka K, Fadden P, Mosse C, Somlyo AP, Somlyo AV, Haystead TA. Purification and characterization of the mammalian myosin light chain phosphatase holoenzyme. The differential effects of the holoenzyme and its subunits on smooth muscle. Journal of Biological Chemistry. 1994;269:31598–31606. [PubMed] [Google Scholar]
- Somlyo AP, Kitazawa T, Himpens B, Matthijs G, Horiuti K, Kobayashi S, Goldman YE, Somlyo AV. Modulation of Ca2+-sensitivity and of the time course of contraction in smooth muscle: A major role of protein phosphatases? Advances in Protein Phosphatases. 1989;5:181–195. [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- Swärd K, Pato MD, Nilsson BO, Nordström I, Hellstrand P. Polyamines inhibit myosin phosphatase and increase LC20 phosphorylation and force in smooth muscle. American Journal of Physiology. 1995;269:C563–571. doi: 10.1152/ajpcell.1995.269.3.C563. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proceedings of the National Academy of Sciences of the USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinkle-Mulcahy L, Ichikawa K, Hartshorne DJ, Siegman MJ, Butler TM. Thiophosphorylation of the 130-kDa subunit is associated with a decreased activity of myosin light chain phosphatase in alpha-toxin-permeabilized smooth muscle. Journal of Biological Chemistry. 1995;270:18191–18194. doi: 10.1074/jbc.270.31.18191. 10.1074/jbc.270.31.18191. [DOI] [PubMed] [Google Scholar]
- Wu X, Somlyo AV, Somlyo AP. Cyclic GMP-dependent stimulation reverses G-protein-coupled inhibition of smooth muscle myosin light chain phosphate. Biochemical and Biophysical Research Communications. 1996;220:658–663. doi: 10.1006/bbrc.1996.0460. 10.1006/bbrc.1996.0460. [DOI] [PubMed] [Google Scholar]
- Yano H, Nakanishi S, Kimura K, Hanai N, Saitoh Y, Fukui Y, Nonomura Y, Matsuda Y. Inhibition of histamine secretion by wortmannin through the blockade of phosphatidylinositol 3-kinase in RBL-2H3 cells. Journal of Biological Chemistry. 1993;268:25846–25856. [PubMed] [Google Scholar]


