Abstract
The firing of single sympathetic neurones was recorded via tungsten microelectrodes in cutaneous fascicles of the peroneal nerve in awake humans. Studies were made of 17 vasoconstrictor neurones during cold-induced cutaneous vasoconstriction and eight sudomotor neurones during heat-induced sweating. Oligounitary recordings were obtained from 8 cutaneous vasconstrictor and 10 sudomotor sites. Skin blood flow was measured by laser Doppler flowmetry, and sweating by changes in skin electrical resistance within the innervation territory on the dorsum of the foot.
Perispike time histograms revealed respiratory modulation in 11 (65 %) vasoconstrictor and 4 (50 %) sudomotor neurones. After correcting for estimated conduction delays, the firing probability was higher in inspiration for both classes of neurone. Measured from the oligounitary recordings, the respiratory modulation indices were 67.7 ± 3.9 % for vasoconstrictor and 73.5 ± 5.7 % for sudomotor neurones (means ± s.e.m.). As previously found for sudomotor neurones, cardiac rhythmicity was expressed by 7 (41 %) vasoconstrictor neurones, 5 of which showed no significant coupling to respiration. Measured from the oligounitary records, the cardiac modulation of cutaneous vasoconstrictor activity was 58.6 ± 4.9 %, compared with 74.4 ± 6.4 % for sudomotor activity.
Both vasoconstrictor and sudomotor neurones displayed low average firing rates (0.53 and 0.62 Hz, respectively). The percentage of cardiac intervals in which units fired was 38 % and 35 %, respectively. Moreover, when considering only those cardiac intervals when a unit fired, vasoconstrictor and sudomotor neurones generated a single spike 66 % and 67 % of the time. Rarely were more than four spikes generated by a single neurone.
We conclude that human cutaneous vasoconstrictor and sudomotor neurones share several properties: both classes contain subpopulations that are modulated by respiration and/or the cardiac cycle. The data suggest that the intensity of a multi-unit burst of vasoconstrictor or sudomotor impulses is probabably governed primarily by firing incidence and the recruitment of additional neurones, rather than by an increase in the number of spikes each unit contributes to a burst.
Respiratory modulation in the firing of postganglionic sympathetic neurones has been studied extensively in the anaesthetized cat and rat. In the cat, single muscle vasoconstrictor neurones fire during inspiration, whereas most cutaneous vasoconstrictor neurones show no modulation and sudomotor neurones discharge primarily in expiration (Boczek-Funcke et al. 1992a, b). In the rat, on the other hand, muscle and cutaneous vasoconstrictor neurones fire only during expiration (Häbler et al. 1993, 1994, 1996; Johnson & Gilbey, 1996). However, Johnson & Gilbey (1996) recently showed that while the firing of some cutaneous vasoconstrictor neurones displayed respiratory rhythmicity, the discharge of others was independent of the central or peripheral respiratory rhythm. No evidence of cardiac rhythmicity was reported. In humans, single postganglionic muscle vasoconstrictor neurones fire with a respiratory rhythm, in addition to the dominant cardiac rhythm (Macefield et al. 1994). Furthermore, the respiratory periodicity is independent of the associated changes in blood pressure, so it cannot be explained by baroreceptor influences (Macefield & Wallin, 1995). A subpopulation of single human sudomotor neurones also express a cardiac rhythmicity, albeit weak, but it is unknown whether they are modulated by respiration (Macefield & Wallin, 1996). In the present study we continued our assessment of the firing properties of single sympathetic neurones in awake humans, by recording from single vasoconstrictor fibres innervating blood vessels in the skin on the dorsum of the foot.
While multi-unit bursts of sympathetic activity in human cutaneous nerve fascicles often contain both vasoconstrictor and sudomotor (and in some instances vasodilator or pilomotor) impulses, it is known that exposing subjects to a cold or a warm environment can bias the sympathetic outflow towards essentially ‘pure’ vasoconstrictor or sudomotor neural traffic, respectively (Bini et al. 1980a, b). In the present study, vasoconstrictor activity was induced by whole-body cooling and external arousal stimuli that could lead to coactivation of sudomotor neurones were minimized. While multi-unit skin sympathetic activity may display a respiratory rhythm (Delius et al. 1972b; Hagbarth et al. 1972; Bini et al. 1980B), it is unknown whether each class of cutaneous sympathetic neurones expresses respiratory modulation. The primary purpose of the present investigation was to determine whether single cutaneous vasoconstrictor neurones exhibit respiratory and/or cardiac rhythmicity. Furthermore, we have reanalysed our recordings from single sudomotor neurones to assess whether these neurones fire with a respiratory periodicity.
METHODS
Subjects
Nine successful experiments were performed on three male and five female subjects (age range 23-49 years). Each subject provided informed written consent to the procedures, which were approved by the human ethics committee of the University of Göteborg.
General procedures
The subject lay supine on a bed wearing only a light top and underpants. ECG surface electrodes were applied to the chest, a strain gauge-based respiration transducer was wrapped around the thorax or abdomen for recording respiratory movements, and the sensor of a photo-plethysmography arterial pressure monitor (Finapres, Ohmeda, CO, USA) was placed on the middle phalanx of the long finger. The thigh was supported by a vacuum cast and the common peroneal nerve located by electrical stimulation via a surface probe. The nerve recording technique has been described previously (Bini et al. 1980B). In short, a tungsten microelectrode (type 25-10-1, Frederick Haer Co., Brunswick, ME, USA; type TM33B20, World Precision Instruments, Sarasota, FL, USA) was inserted into a cutaneous fascicle of the common peroneal nerve and a site was located in which spontaneous and evoked multi-unit sympathetic activity could be recorded. All fascicular innervation territories were in the non-hairy dorsal aspect of the foot or in the non-hairy region of skin between the first and second toe; we specifically avoided the cutaneous fascicle supplying the hairy skin on the lateral side of the leg.
To monitor changes in skin blood flow a laser Doppler flow probe (Periflux 4001, Perimed AB, Sweden) was applied to the skin within the innervation territory of the fascicle. The instrument was calibrated with respect to the minimum and maximum electrical signal at a constant gain setting and expressed as 0 % and 100 % flux (1-10 V). A surface Ag-AgCl electrode was applied within the receptive field for monitoring changes in skin resistance (modified van Gough GSR module IGSR/7A), and referenced to an electrode outside the field. The measuring current was 12 μA and the bandwidth 0.7-100 Hz. All experiments were performed during late winter; cooling was induced by opening a window of the laboratory. Skin temperature was monitored via a thermocouple on the distal pad of the second toe and allowed to fall to below 22°C. The rate of cooling was adjusted so shivering did not occur. All subjects displayed piloerection in the skin of the leg, but none was noted on the dorsum of the foot. The cooling led to increased spontaneous multi-unit sympathetic activity associated with marked skin vasoconstriction, as indicated by low laser Doppler flux. The nerve recording electrode was then manipulated until unitary discharges appeared out of the multi-unit bursts. Resting activity was recorded while subjects were relaxed with eyes closed, breathing quietly and with external sounds and other stimuli kept to a minimum to avoid arousals and the possible coactivation of sudomotor neurones (i.e. ‘cold sweat’). Presumably, the spontaneous neural activity so recorded reflects primarily thermoregulatory rather than emotionally driven vasoconstrictor traffic. In addition to cooling, we also reanalysed data from our previously published recordings of sudomotor activity obtained during heat-induced sweating (Macefield & Wallin, 1996).
Data collection
Neural activity was amplified (× 50000), filtered (0.3-5.0 kHz), digitized at 12.8 kHz (12 bits) and stored on magnetic and optical media via the SC/ZOOM data acquisition and analysis computer system developed at the Department of Physiology, University of Umeå, Sweden. The amplified and filtered nerve signal was also led to an audiomonitor and through a resistance-capacitance integrating circuit (time constant 100 ms). The latter output, the mean voltage neurogram, was digitized at 800 Hz and stored as 8 bits. The ECG channel was also digitized at 800 Hz, and the blood flow, skin resistance, respiration and arterial pressure signals at 200 Hz; each of these was stored as 8 bits. Data were sampled in standard epochs of 300 s duration.
Data analysis
During off-line analysis the morphology of every spike of a candidate unit was carefully assessed using a spike recognition algorithm (Edin et al. 1988) incorporated in the SC/ZOOM software. To accept a unit as deriving from a single fibre, the distribution of spike amplitudes was compared with that of the underlying noise, the idea being that the variations in spike amplitude must not exceed the variations in noise upon which the signal rides. Noise was sampled over a 1-2 s period by lowering the amplitude threshold of the window discriminator below that of the spikes of interest, while maintaining the same time window (± 0.35 ms) as used by the spike detection algorithm incorporated in ZOOM; in this way spike-like noise was sampled with amplitudes ranging from some 10 nV to 15 μV. Figure 1 shows examples of the distributions of noise and spike amplitudes for two cutaneous vasoconstrictor units. For both units the variation of spike amplitude was similar to the variation in noise amplitude, suggesting that the variation in the former can be explained by the variation in the latter. All unitary recordings satisfied this criterion.
Figure 1. Variation of spike amplitude in single unit recordings.
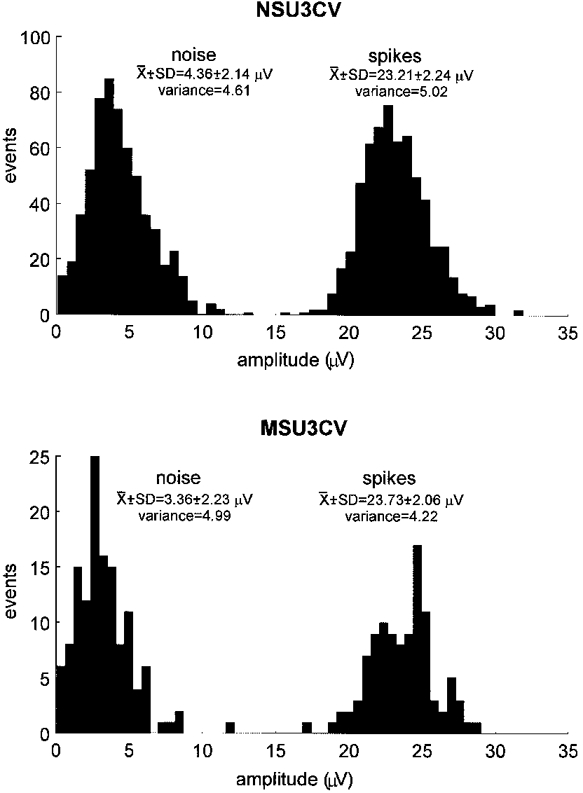
Histograms showing the distributions of spike amplitude and of the underlying noise for two cutaneous vasoconstrictor units. For each unit the variances of the noise and spike amplitudes were not significantly different, indicating that variations in the signal can be accounted for by variations in the noise.
The computer measured the times at which an ECG R-wave occurred, the systolic and diastolic pressures of each beat, the peak rate of fall in arterial pressure (calculated from the first-time derivative), and the absolute pressure at which the rate of fall was greatest. Cursors were placed at the onset, peak and end of a sympathetic burst (measured from the integrated nerve signal), the first and last spikes a unit fired in the burst, and the two R-waves before the burst. The latency of the first spike in a burst, and the onset and peak latencies of the integrated nerve signal were measured from the R-wave that preceded the spike by approximately 1 s. Cardiac interval was measured between R-wave ‘n’ and R-wave ‘n - 1′, and pulse pressure (systolic minus diastolic) and mean pressure calculated for the beat ‘n - 1′.
To illustrate possible cardiac rhythmicity, perispike time histograms were plotted with respect to the time of occurrence of each ECG R-wave over a time window of 4-5 s, so as to allow three to four cycles of the ECG to be identified. Respiratory rhythmicity was measured in a similar fashion, using the peak of inspiration (measured from the thoracic/abdominal expansion) to trigger the histograms over a time window of 10-14 s. Modulation of oligounitary sympathetic activity was assessed by measuring the largest peak-valley difference in the perispike time histograms, using bin widths of 50 ms (cardiac interval) or 500 ms (respiration), and expressing this as a percentage of the peak amplitude.
Statistics
Variations in noise and signal amplitude for each unitary recording were compared using Levene's test for homogeneity of variance, essentially a one-way ANOVA of the absolute deviations from the respective means. All statistical evaluation of the data was performed using STATISTICA for Windows v. 5.1 (StatSoft Inc., Tulsa, OK, USA). Values are expressed as means and standard errors of the mean, and differences considered statistically significant at P < 0.05.
RESULTS
Identification of vasoconstrictor units
Successful unitary recordings were made from 17 intrafascicular sites. A typical example of a unitary recording of spontaneous activity is shown in Fig. 2A, in which five large spikes appeared during the illustrated period. Arousal, evoked by an unexpected tap on the wrist (Fig. 2A) activated the neurone but no further fall in skin blood flow occurred, illustrating the essentially maximal cutaneous vasoconstriction. The relatively consistent amplitude and shape of the superimposed action potentials (Fig. 2A) suggest that the spikes originated from a single axon, the negative polarity and triphasic shape suggesting that the axon was unmyelinated. For all units recorded spike amplitudes ranged from 13.1 to 30.2 μV (mean ±s.e.m. 20.5 ± 1.3 μV).
Figure 2. Recording from a single cutaneous vasoconstrictor neurone in the cold state.
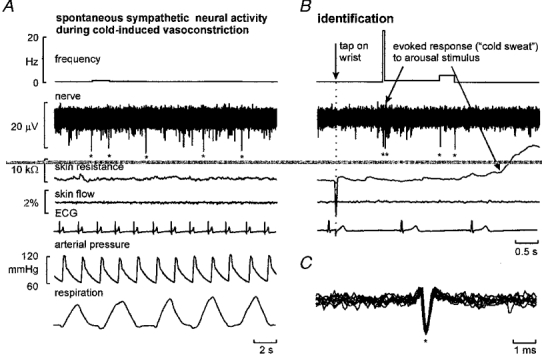
A, five spikes (asterisks) occurring spontaneously. Skin blood flow and spontaneous changes in skin electrical resistance (indicative of sweating) were negligible. B, the unit generated two spikes following an arousal stimulus, associated with a decrease in skin resistance (upward deflection) (due to coactivation of sudomotor neurones) but no further reduction in skin blood flow. C, superimposed spikes from A and B suggest that they were recorded from a single axon.
General firing properties of cutaneous vasoconstrictor neurones
Calculated as the inverse of all interspike intervals < 30 s the average firing rate of the 17 units was 0.53 ± 0.11 Hz (range 0.08-2.04 Hz). The firing pattern was highly irregular and, as indicated in Fig. 3A, the range of instantaneous frequencies was broad. However, the distribution was strongly skewed towards low firing rates: the median value was 1.17 Hz and the lower and upper quartiles 0.44 Hz and 4.83 Hz, respectively. Short interspike intervals, while occurring rarely, could approach 5 ms (maximum instantaneous frequency 146 Hz), but only as short bursts of impulses (doublets or triplets). The two units in Fig. 4A and C generated peak instantaneous frequencies of 108 and 118 Hz, respectively.
Figure 3. Distribution of firing rates for all cutaneous vasoconstrictor neurones.
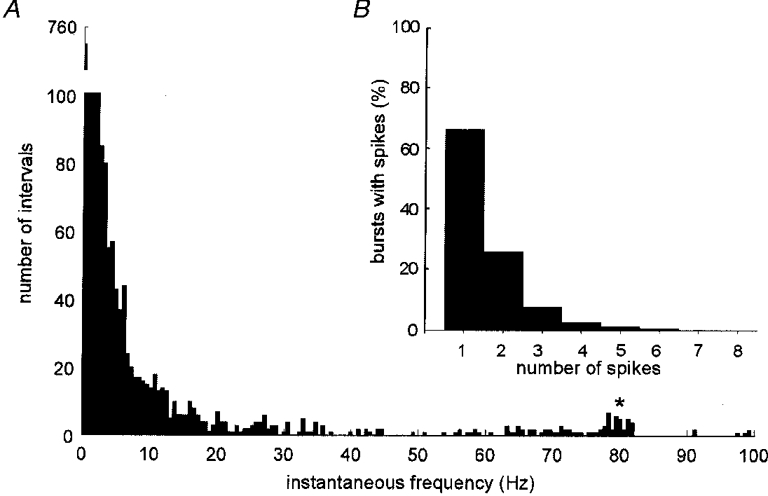
A, pooled data from 17 cutaneous vasoconstrictor neurones. * Short intervals from unit firing doublets. Note break of the y-axis. B, number of sympathetic bursts in which a unit fired one or more spikes.
Figure 4. Multiple firing in cutaneous vasoconstrictor neurones.
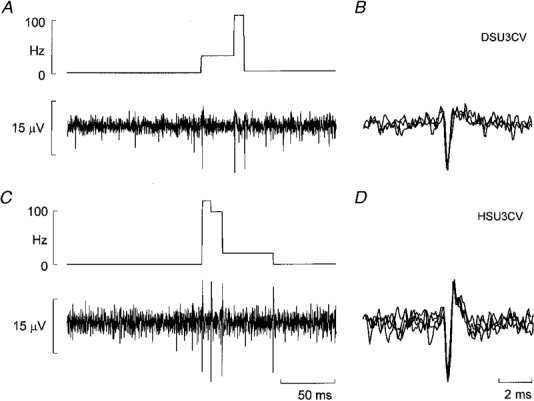
A and C, examples from two units in which multiple spikes were generated in a sympathetic burst. B and D, superimposed spikes from the left panels demonstrating the reproducibility of shapes, suggesting that the respective spikes in each panel derived from a single neurone.
One unit was interesting in that it alternated loosely between generating single spikes and doublets in which instantaneous frequencies were centred around 80 Hz (see asterisk in Fig. 3A). In addition to the low average firing rates, the number of spikes a unit contributed to a sympathetic burst was quite low (Fig. 3A). Measured as the number of spikes that occurred within a period defined by two successive R-waves, and pooling the data from all units, the firing of a single neurone occurred in only 38 ± 5 % (range 7-86 %) of all cardiac intervals (mean ±s.d. cardiac interval = 1.19 ± 0.26 s). Considering only those cardiac intervals in which a unit fired, only one spike was generated in 66 % of intervals, two spikes in 24 % and three spikes in only 7 % of intervals (Fig. 3A). The maximum number that could be attributed to the firing of a single neurone varied widely: while four units generated only 1-2 spikes per burst, the majority (n = 9) generated up to 3-4 spikes per burst and three units up to 5-7 spikes. The unit with the highest mean firing rate (2.0 Hz) and the highest firing incidence (86 % of all cardiac intervals) generated up to 14 spikes in a single cardiac interval.
Respiratory and cardiac modulation of cutaneous vasoconstrictor activity
Perispike time histograms revealed respiratory modulation in the discharge of 11 of the 17 vasoconstrictor neurones (65 %). Graphical data from four such units are shown in Fig. 5A-D. The perispike time histograms (upper panels) each show a respiratory rhythm, as evidenced by the period of the peaks being identical to that of respiration (lower panels). After allowing approximately 1 s for the conduction delay from brainstem to recording site, the respiratory modulation could be explained primarily by an increase in firing probability during inspiration, with the decrease commencing before the peak of inspiration and continuing in expiration. In addition to respiratory modulation, the firing of seven units (41 %) was temporally coupled to the cardiac cycle. The discharge of only one of these was linked to the respiratory cycle as well. Data from four units are illustrated in Fig. 6. One unit was modulated by neither the respiratory nor the cardiac cycle.
Figure 5. Respiratory rhythmicity in single cutaneous vasoconstrictor neurones.
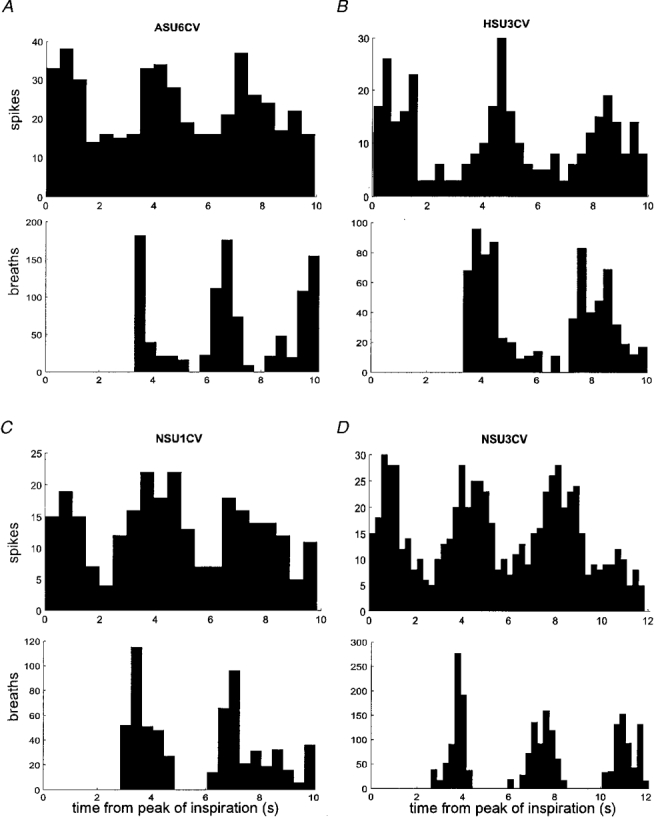
Perispike time histograms showing the distribution of spikes with respect to the peak of inspiration and corresponding diagram showing the time of occurrence of inspiratory peaks. Each unit exhibited temporal coupling to the respiratory cycle.
Figure 6. Cardiac rhythmicity in cutaneous vasoconstrictor neurones.
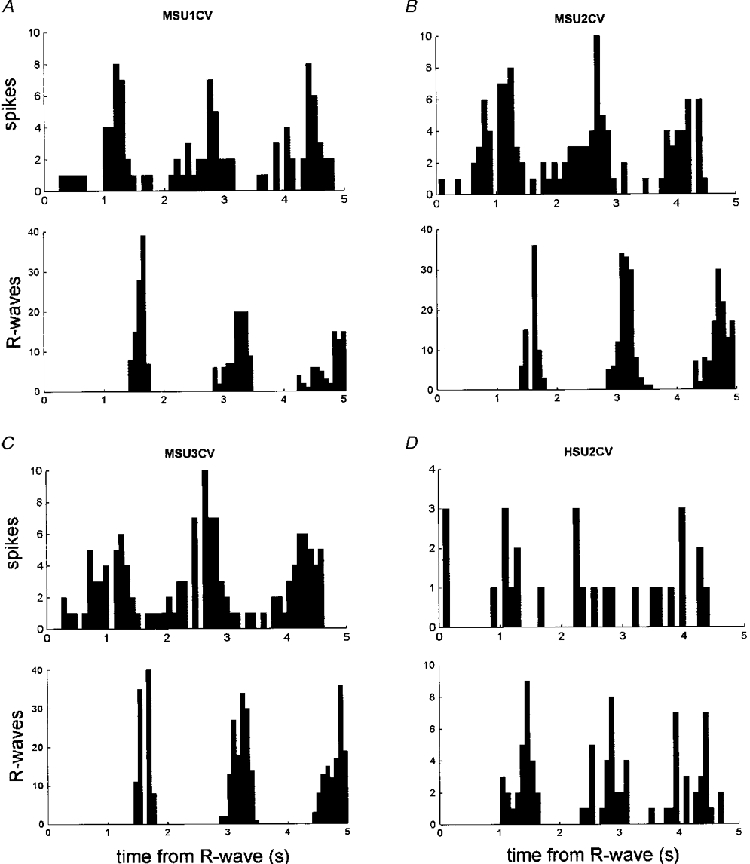
Perispike time histograms showing the distribution of spikes with respect to the R-wave of the ECG and corresponding diagram showing the time of occurrence of R-waves.
Linear regression analyses were performed to determine whether the firing probability (spikes per cardiac interval) or spike onset latency (measured from the relevant R-wave) of the units were significantly coupled to cardiac interval, diastolic pressure, rate of fall in blood pressure, etc., in a manner that would support their firing being influenced by baroreceptor stimuli. Despite the presence of cardiac rhythmicity none of vasoconstrictor neurones exhibited a significant correlation with any cardiovascular parameter.
Oligounitary recordings of cutaneous vasoconstrictor activity, each comprising a few active units, were made from eight intrafascicular sites in six experiments (6 subjects). As illustrated by the perispike time histogram for two oligounitary sites in Fig. 7, cardiac as well as respiratory modulation could be observed, indicating that our sample of single units displaying cardiac rhythmicity was representative of the population of vasoconstrictor neurones. Modulation indices were 67.7 ± 3.9 % (range 59.5-79.2 %) for the respiratory cycle and 58.6 ± 4.9 % (range 43.8-86.7 %) for the cardiac cycle (P = 0.058, Mann-Whitney U test).
Figure 7. Respiratory and cardiac rhythmicity in oligounitary vasoconstrictor recordings.
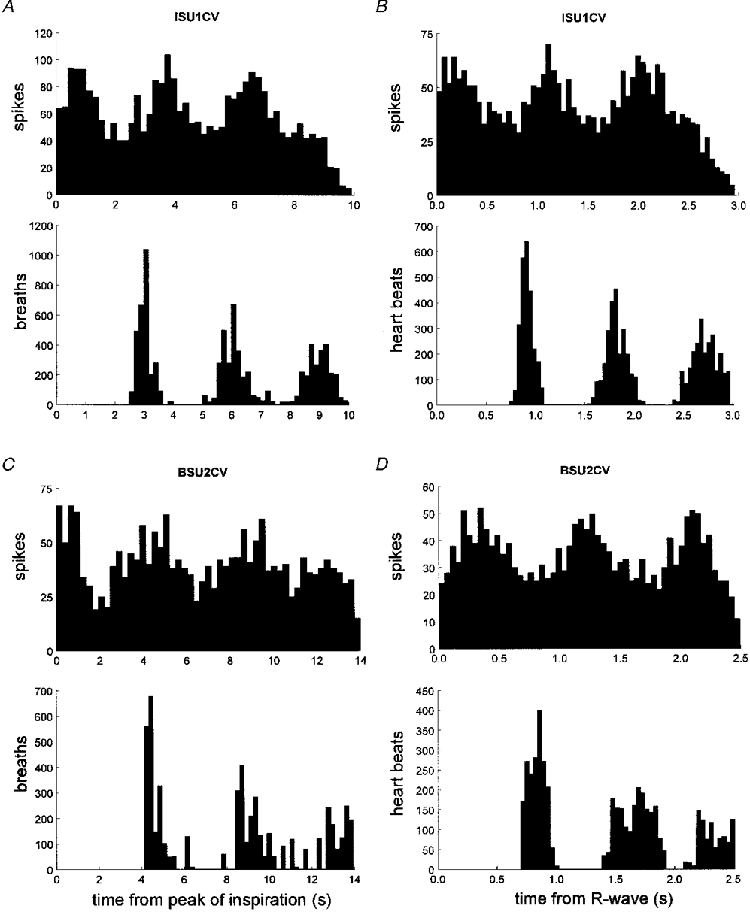
Respiratory (A and B) and cardiac (C and D) rhythmicity in two oligounitary cutaneous vasoconstrictor recordings. Same format as in Figs 5 and 6
Respiratory modulation of sudomotor activity
The discharge patterns of eight single sudomotor neurones recorded during heat-induced sweating, previously described with respect to their general firing properties and cardiac modulation (Macefield & Wallin, 1996), were reanalysed to assess whether firing probability was affected by respiration. In addition, we analysed both the respiratory and cardiac modulation of 10 oligounitary sudomotor recordings from seven experiments (6 subjects) of the same series. Respiratory modulation, as detected by perispike time histograms, could be observed in four of the eight units (50 %). Figure 8A-C shows data from three of these units and Fig. 8A data from one unit without apparent respiratory modulation. As for cutaneous vasoconstrictor neurones, firing incidence was higher in inspiration than in expiration (after allowing 1 s for peripheral conduction delay).
Figure 8. Respiratory rhythmicity in single sudomotor neurones.
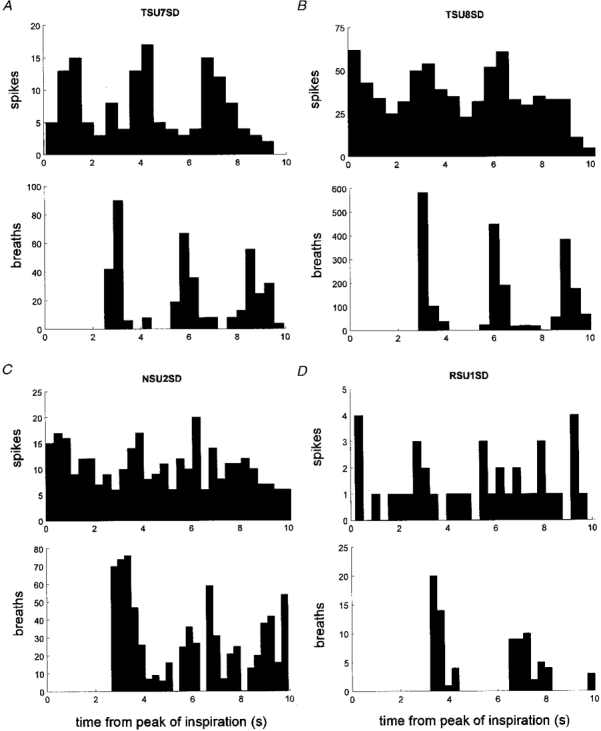
Perispike time histogram between unit firing and the peak of inspiration and corresponding diagrams showing the time of occurrence of subsequent inspiratory peaks. Data from four sudomotor neurones reanalysed from Macefield & Wallin (1996).
Perispike time histograms indicated that all oligounitary sudomotor sites displayed respiratory as well as cardiac modulation (Fig. 9). Modulation indices ranged from 41.5 to 92.4 % for the respiratory rhythm and 46.3 to 100 % for the cardiac rhythm. There was no significant difference between the mean values: 73.5 ± 5.7 and 74.4 ± 6.4 %, respectively. The cardiac modulation index for the oligounitary sudomotor sites was higher than that for the oligounitary vasoconstrictor sites (P = 0.042, Mann-Whitney U test), whereas there was no significant difference in the magnitude of the respiratory modulation.
Figure 9. Respiratory and cardiac rhythmicity in oligounitary sudomotor recordings.
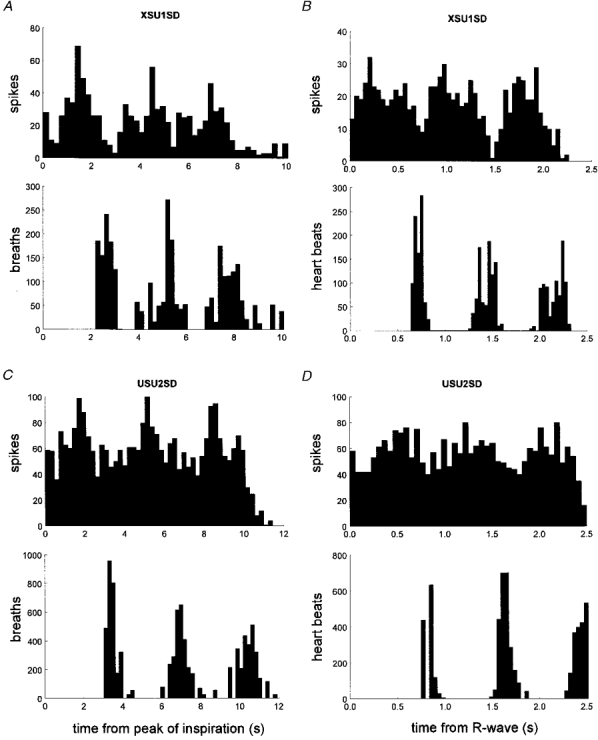
Respiratory rhythmicity (A and B) and cardiac rhythmicity (C and D) in two oligounitary recording sites. Same format as in Fig. 7.
DISCUSSION
This is the third study in a series that has examined the firing properties of single, type-identified, sympathetic postganglionic axons in the peroneal nerves of relaxed awake human subjects. In the first, we recorded from 14 vasoconstrictor units supplying the pretibial skeletal muscle vascular bed (Macefield et al. 1994), in the second from eight sudomotor units supplying sweat glands on the dorsum of the foot (Macefield & Wallin, 1996), and in the present study from 17 putative vasoconstrictor units innervating skin blood vessels on the dorsum of the foot. The results extend our knowledge of the sympathetic nervous system by describing the discharge behaviour of specific classes of neurones without the confounding effects of general anaesthesia. The three populations share certain similarities, yet are also characterized by specific differences.
Methodological considerations
In microneurographic recordings from single sympathetic neurones signal-to-noise ratios are smaller than those achieved by fibre splitting in experimental animals. We considered the recordings to be unitary if spike superimposition showed little variation in shape, and if variations in spike amplitude were explicable by the variation of the underlying noise. Nevertheless, uniformity of spike morphology can never exclude the possibility that two units of practically identical amplitude and shape have been recorded simultaneously. However, this is not limited to human recordings, and as an example, extracellular recordings from single postganglionic sympathetic axons innervating the rat tail artery show similar variations in spike amplitude and shape (Johnson & Gilbey, 1996). All unitary action potentials recorded in the present study were triphasic with a dominant negative-going phase, consistent with an extracellular recording from a C fibre. This is similar to that of muscle vasoconstrictor units (Macefield et al. 1994) yet different from that of sudomotor neurones, which presented a more bipolar shape (Macefield & Wallin, 1996). The latter presumably reflects the underlying biophysical properties of different C fibre classes; conduction velocities of the postganglionic axons of human sudomotor neurones are faster than those of the muscle or cutaneous vasoconstrictor neurones (Fagius & Wallin, 1980).
Identification of cutaneous vasoconstrictor neurones
The present recordings were made during whole-body cooling in an environment designed to minimize external disturbances likely to induce arousal/emotional reactions. The cooling caused a reduction in skin temperature, piloerection and cutaneous vasoconstriction, and there was no evidence of sweating (no skin resistance variations). As shown previously, the increased multi-unit sympathetic activity in cutaneous fascicles seen under such conditions is dominated by vasoconstrictor impulses (Bini et al. 1980a, b), and even though it cannot be excluded, a contribution of sudomotor neurones to our unit sample is unlikely.
Because the laser Doppler flux was so low, spike-triggered averaging of the flux signal was not a useful approach to unit identification. While contamination by sudomotor neurones is unlikely, pilomotor neurones may have contributed to the sample, particularly as all subjects had raised hairs and ‘goose-bumps’ on the legs. We specifically avoided those cutaneous fascicles of the peroneal nerve supplying the lateral side of the leg, which is richly endowed with hairs, and recorded only from those fascicles supplying the dorsum of the foot; much of the skin on the dorsal surface of the foot is devoid of hairs and, it is reasonable to suggest, pilomotor fibres destined to this area. In the anaesthetized cat noxious skin stimulation inhibits the ongoing discharge of cutaneous vasoconstrictor neurones, yet has no effect on pilomotor neurones (Jänig, 1990). However, this method of differentiating between the two types of neurones could not be applied since painful stimuli also induces arousal-related coactivation of sudomotor neurones.
General firing properties of cutaneous vasoconstrictor neurones
In spite of the subjects being cooled and displaying marked skin vasoconstriction, mean firing rates were low (0.53 Hz). This is similar to the mean firing frequency of human sudomotor neurones (0.62 Hz) during heat-induced sweating (Macefield & Wallin, 1996), and of muscle vasoconstrictor neurones (0.47 Hz) at rest (Macefield et al. 1994). The frequencies agree well with values found in anaesthetized experimental animals (Jänig, 1985; Meckler & Weaver, 1988; Stein & Weaver, 1988). The similarities between different classes of human sympathetic neurones extended also to other firing characteristics. Cutaneous vasoconstrictor neurones fired in only 38 % of all heart beats, sudomotor neurones in 35 % and muscle vasoconstrictor neurones in 21 % of heart beats, and the percentage of cardiac intervals in which a unit generated only one spike was remarkably similar for all three populations: 66 % (cutaneous vasoconstrictor), 67 % (sudomotor) and 68 % (muscle vasoconstrictor). Since multiple firing was rare in all studies it seems likely that the intensity of a sympathetic burst is governed largely by the recruitment of additional neurones, and that increases in the number of spikes an individual unit generates is of lesser importance.
The distribution of instantaneous firing rates was broad, with very short interspike intervals occasionally being generated. The risk of not recognizing the recruitment of a second unit is probably greatest when several spikes occur with short intervals. Therefore it is difficult to exclude the possibility that the maximum number of spikes and the highest instantaneous firing frequencies exhibited by a unit may be overestimated. On the other hand we could not exclude very short intervals unless the spikes differed in amplitude and shape, and it is known that non-myelinated afferents in human subjects can sustain high frequency discharges of approximately 100 Hz (Nordin, 1990; Vallbo et al. 1993). The reason for the irregular firing pattern and widely varying instantaneous firing frequencies may be that a single postganglionic neurone is driven by two (or more) preganglionic neurones possessing strong synapses (McLachlan et al. 1997). Regardless of the cause, it is likely that such short interspike intervals may increase the efficacy of the neuroeffector transfer function, as we and others have pointed out previously. For instance, the responses of some target tissues are greater when sympathetic axons are stimulated with bursts of irregular stimuli that include high frequencies than when stimulated evenly (Andersson et al. 1982; Andersson, 1983; Nilsson et al. 1985; Kirnöet al. 1991; Polenov et al. 1991; Kunimoto et al. 1991, 1992).
Respiratory rhythmicity of vasoconstrictor and sudomotor neurones
The discharge of many cutaneous vasoconstrictor and sudomotor neurones was modulated by respiration, and all oligounitary sites displayed respiratory modulation. After correcting for conduction delays (approximately 1 s from the brainstem to the recording site in the leg, see Fagius & Wallin, 1980), this modulation could be explained primarily by an increased probability of firing during inspiration for both classes of neurones. This is the same pattern seen in muscle vasoconstrictor neurones in the anaesthetized cat, but as described in the Introduction the pattern may vary across species and type of sympathetic neurone.
Cardiac rhythmicity of vasoconstrictor and sudomotor neurones
Both the single and oligounitary recordings provided evidence of a subpopulation of cutaneous vasoconstrictor neurones exhibiting cardiac rhythmicity. This agrees with observations in the cat (Jänig, 1985). On the other hand, cardiac rhythmicity has not been detected in previous multi-unit recordings of human skin sympathetic activity dominated by vasoconstrictor traffic (Hagbarth et al. 1972; Wallin et al. 1975; Bini et al. 1980b, 1981; Vissing et al. 1994). However, only one of these human studies (Bini et al. 1981) used a quantitative analytical method (ECG-triggered averaging) to search for cardiac rythmicity in the cold state, and in that study the nerve recordings were made from the median nerve innervating the palm of the hand. Although all studies do not agree, there is haemodynamic evidence suggesting that glabrous skin may not be influenced by arterial baroreflexes (for references see Roddie, 1983; Eckberg & Sleight, 1992), which may be the reason for the lack of cardiac rhythmicity of median nerve vasoconstrictor activity. If only a subpopulation of peroneal vasoconstrictor neurones exhibits cardiac rhythmicity, this may be difficult to distinguish by mere inspection of a mean voltage neurogram. Thus, the cardiac rhythmicity seen so clearly in our ECG-triggered averaging procedure may have been overlooked in previous peroneal recordings not subjected to this type of quantitative evaluation.
The cardiac modulation of single- and multi-unit muscle vasoconstrictor activity is very pronounced (Delius et al. 1972A; Macefield et al. 1994), and known to be evoked primarily from arterial baroreceptors (Wallin et al. 1975; Wallin & Eckberg, 1982). In contrast, only subpopulations of cutaneous vasoconstrictor and sudomotor neurones display a cardiac rhythmicity (the present data and Macefield & Wallin, 1996) which is weaker than that of muscle vasoconstrictor neurones. Furthermore the present data suggest that cardiac rhythmicity of cutaneous vasoconstrictor activity was weaker than that of the sudomotor activity, even if measuring modulation in terms of the peak-valley difference in a histogram gives little indication of how tight the temporal coupling is. The source of the cardiac rhythmicity of the cutaneous vasoconstrictor neurones is unclear. Unlike muscle vasoconstrictor units (Macefield et al. 1994), the firing probability or spike onset latency of the cutaneous vasoconstrictor units was not correlated to cardiac interval, diastolic pressure or other cardiovascular parameters. Given that skin sympathetic activity in human subjects is not greatly influenced by the arterial baroreceptors (Hagbarth et al. 1972; Delius et al. 1972b; Wallin et al. 1975; Bini et al. 1980b, 1981), the low-pressure (cardiopulmonary) baroreceptors may be seen as a potential source of cardiac rhythmicity. Such receptors are believed to reduce sudomotor activity, as lower-body negative pressure (which unloads the cardiopulmonary baroreceptors) and head-up tilt decrease sudomotor traffic in sweating subjects (Dodt et al. 1995) - an effect opposite to the negative feedback these receptors exert on muscle vasoconstrictor activity (Sundlöf & Wallin, 1978). However, lower body negative pressure has no effect on skin sympathetic nerve activity or skin blood flow in thermoneutral conditions (Vissing et al. 1994, 1997), arguing against a role of the low-pressure baroreceptors in the cardiac modulation of cutaneous vasoconstrictor activity. Whether the effect would be different in the cold state is unclear.
Conclusions
With the present study we now have single unit data from three sets of human sympathetic fibres: muscle vasoconstrictor, skin vasoconstrictor and sudomotor neurones. Despite the varying degrees of sympathoexcitation prevailing in each set of experimental conditions, all units present low levels of spontaneous activity, with most units firing only once in a sympathetic burst. In all three groups the firing of some units is modulated by respiration, inspiration decreasing the probability of firing of the muscle vasoconstrictor neurones, yet increasing the firing incidence of the cutaneous vasoconstrictor and sudomotor neurones. And while the cardiac modulation of firing is not as tightly coupled to the ECG as for muscle vasoconstrictor neurones, both types of cutaneous sympathetic neurones contain subclasses that do exhibit cardiac rhythmicity.
Acknowledgments
This work was supported by the Swedish Medical Research Council, grant no. 3546. V. G. M. is supported by the National Health and Medical Research Council of Australia.
References
- Andersson PO. Comparative vascular effects of stimulation continuously and in bursts of the sympathetic nerves to cat skeletal muscle. Acta Physiologica Scandinavica. 1983;118:343–348. doi: 10.1111/j.1748-1716.1983.tb07281.x. [DOI] [PubMed] [Google Scholar]
- Andersson PO, Bloom SR, Edwards A, Järhult J. Effects of stimulation of the chorda tympani in bursts on submaxillary responses in the cat. The Journal of Physiology. 1982;322:469–483. doi: 10.1113/jphysiol.1982.sp014050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bini G, Hagbarth K-E, Hynninen P, Wallin BG. Regional similarities and differences in thermoregulatory vaso- and sudomotor tone. The Journal of Physiology. 1980a;306:553–565. doi: 10.1113/jphysiol.1980.sp013414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bini G, Hagbarth K-E, Hynninen P, Wallin BG. Thermoregulatory and rhythm-generating mechanisms governing the sudomotor and vasoconstrictor outflow in human cutaneous nerves. The Journal of Physiology. 1980b;306:537–552. doi: 10.1113/jphysiol.1980.sp013413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bini G, Hagbarth K-E, Wallin BG. Cardiac rhythmicity of skin sympathetic activity recorded from peripheral nerves in man. Journal of the Autonomic Nervous System. 1981;4:17–24. doi: 10.1016/0165-1838(81)90003-5. [DOI] [PubMed] [Google Scholar]
- Boczek-Funcke A, Dembowsky K, Häbler HJ, Jänig W, Michaelis M. Respiratory-related activity patterns in preganglionic neurones projecting into the cat cervical sympathetic trunk. The Journal of Physiology. 1992b;457:277–296. doi: 10.1113/jphysiol.1992.sp019378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boczek-Funcke A, Häbler HJ, Jänig W, Michaelis M. Respiratory modulation of the activity in sympathetic neurones supplying muscle, skin and pelvic organs in the cat. The Journal of Physiology. 1992a;449:333–361. doi: 10.1113/jphysiol.1992.sp019089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius W, Hagbarth K-E, Hongell A, Wallin BG. General characteristics of sympathetic activity in human muscle nerves. Acta Physiologica Scandinavica. 1972a;84:65–81. doi: 10.1111/j.1748-1716.1972.tb05158.x. [DOI] [PubMed] [Google Scholar]
- Delius W, Hagbarth K-E, Hongell A, Wallin BG. Manouevres affecting sympathetic ouflow in human skin nerves. Acta Physiologica Scandinavica. 1972b;84:177–186. doi: 10.1111/j.1748-1716.1972.tb05168.x. [DOI] [PubMed] [Google Scholar]
- Dodt C, Gunnarson T, Elam M, Karlsson T, Wallin BG. Central blood volume influences sympathetic sudomotor nerve traffic in warm humans. Acta Physiologica Scandinavica. 1995;155:41–51. doi: 10.1111/j.1748-1716.1995.tb09946.x. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Sleight P. Human Baroreflexes in Health and Disease. Oxford: Clarendon Press; 1992. pp. 278–280. [Google Scholar]
- Edin BB, Bäckström PA, Bäckström LO. Single unit retrieval in microneurography: a microprocessor-based device controlled by an operator. Journal of Neuroscience Methods. 1988;24:137–144. doi: 10.1016/0165-0270(88)90057-x. 10.1016/0165-0270(88)90057-X. [DOI] [PubMed] [Google Scholar]
- Fagius J, Wallin BG. Sympathetic reflex latencies and conduction velocities in normal man. Journal of the Neurological Sciences. 1980;47:433–448. doi: 10.1016/0022-510x(80)90098-2. 10.1016/0022-510X(80)90098-2. [DOI] [PubMed] [Google Scholar]
- Häbler H-J, Bartsch T, Jänig W. Two distinct mechanisms generate the respiratory modulation in fibre activity of the rat cervical sympathetic trunk. Journal of the Autonomic Nervous System. 1996;61:116–122. doi: 10.1016/s0165-1838(96)00066-5. 10.1016/S0165-1838(96)00066-5. [DOI] [PubMed] [Google Scholar]
- Häbler H-J, Jänig W, Krummel M, Peters OA. Respiratory modulation of the activity in postganglionic neurons supplying skeletal muscle and skin of the rat hindlimb. Journal of Neurophysiology. 1993;70:920–930. doi: 10.1152/jn.1993.70.3.920. [DOI] [PubMed] [Google Scholar]
- Häbler H-J, Jänig W, Michaelis M. Respiratory modulation in the activity of sympathetic neurones. Progress in Neurobiology. 1994;43:567–606. doi: 10.1016/0301-0082(94)90053-1. 10.1016/0301-0082(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Hagbarth KE, Hallin RG, Hongell A, Torebjörk HE, Wallin BG. General characteristics of sympathetic activity in human skin nerves. Acta Physiologica Scandinavica. 1972;84:164–176. doi: 10.1111/j.1748-1716.1972.tb05167.x. [DOI] [PubMed] [Google Scholar]
- Jänig W. Organization of the lumbar sympathetic outflow to skeletal muscle and skin of the cat hindlimb and tail. Review of Physiology, Biochemistry and Pharmacology. 1985;102:119–213. doi: 10.1007/BFb0034086. [DOI] [PubMed] [Google Scholar]
- Johnson CD, Gilbey MP. On the dominant rhythm in the discharges of single postganglionic sympathetic neurones innervating the rat tail artery. The Journal of Physiology. 1996;497:241–259. doi: 10.1113/jphysiol.1996.sp021764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirnö K, Kunimoto M, Lundin S, Elam M, Wallin BG. Can galvanic skin response be used as a quantitative estimate of sympathetic nerve activity in regional anesthesia? Anesthesia and Analgesia. 1991;73:138–142. [PubMed] [Google Scholar]
- Kunimoto M, Kirnö K, Elam M, Karlsson T, Wallin BG. Neuro-effector characteristics of sweat glands in the human hand activated by irregular stimuli. Acta Physiologica Scandinavica. 1992;146:261–269. doi: 10.1111/j.1748-1716.1992.tb09415.x. [DOI] [PubMed] [Google Scholar]
- Kunimoto M, Kirnö K, Elam M, Wallin BG. Neuroeffector characteristics of sweat glands in the human hand activated by regular neural stimuli. The Journal of Physiology. 1991;442:391–411. doi: 10.1113/jphysiol.1991.sp018799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield VG, Wallin BG. Modulation of muscle sympathetic activity during spontaneous and artificial ventilation and apnoea in humans. Journal of the Autonomic Nervous System. 1995;53:137–147. doi: 10.1016/0165-1838(94)00173-h. 10.1016/0165-1838(94)00173-H. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Wallin BG. Discharge behaviour of single sympathetic neurones innervating human sweat glands. Journal of the Autonomic Nervous System. 1996;61:277–286. doi: 10.1016/s0165-1838(96)00095-1. 10.1016/S0165-1838(96)00095-1. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Wallin BG, Vallbo Å B. The discharge behaviour of single vasoconstrictor motoneurones in human muscle nerves. The Journal of Physiology. 1994;481:799–809. doi: 10.1113/jphysiol.1994.sp020482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan EM, Davies PJ, Häbler HJ, Jamieson J. On-going and reflex synaptic events in rat superior cervical ganglion cells. The Journal of Physiology. 1997;501:165–182. doi: 10.1111/j.1469-7793.1997.165bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckler RL, Weaver LC. Characteristics of ongoing and reflex discharge of single splenic and renal sympathetic postganglionic fibres in cats. The Journal of Physiology. 1988;396:163–175. doi: 10.1113/jphysiol.1988.sp016955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson H, Ljung B, Sjöblom N, Wallin BG. The influence of the sympathetic impulse pattern on contractile responses of rat mesenteric arteries and veins. Acta Physiologica Scandinavica. 1985;123:303–309. doi: 10.1111/j.1748-1716.1985.tb07592.x. [DOI] [PubMed] [Google Scholar]
- Nordin M. Low-threshold mechanoreceptive and nociceptive units with unmyelinated (C) fibres in the human supraorbital nerve. The Journal of Physiology. 1990;426:229–240. doi: 10.1113/jphysiol.1990.sp018135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polenov SA, Lensman MV, Zasorin KV, Kolobanov SV. The effect of continuous and burst stimulation of rat sympathetic nerves on gastric submucosal microvessels in rats. In: Mulvany MJ, Aalkjaer C, Heagerty AM, Nyborg NCB, Strandgaard S, editors. Resistance Arteries, Structure and Function. Amsterdam: Elsevier Science Publishers; 1991. pp. 169–172. [Google Scholar]
- Roddie IC. Circulation to skin and adipose tissue. In: Shepherd JT, Abboud FM, editors. Handbook of Physiology, section 2, The Cardiovascular System, Peripheral Circulation and Organ Blood Flow. III. Bethesda, MD, USA: American Physiological Society; 1983. pp. 285–317. [Google Scholar]
- Stein RD, Weaver LC. Multi- and single-fibre mesenteric and renal sympathetic responses to chemical stimulation of intestinal receptors in cats. The Journal of Physiology. 1988;396:155–172. doi: 10.1113/jphysiol.1988.sp016956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundlöf G, Wallin BG. Effect of lower body negative pressure on human muscle nerve sympathetic activity. The Journal of Physiology. 1978;278:525–532. doi: 10.1113/jphysiol.1978.sp012322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo Å B, Olausson H, Wessberg J, Norrsell U. A system of unmyelinated afferents for innocuous mechanoreception in the human skin. Brain Research. 1993;628:301–304. doi: 10.1016/0006-8993(93)90968-s. 10.1016/0006-8993(93)90968-S. [DOI] [PubMed] [Google Scholar]
- Vissing SF, Scherrer U, Victor RG. Increase of sympathetic discharge to skeletal muscle but not to skin during mild lower body negative pressure. The Journal of Physiology. 1994;481:233–241. doi: 10.1113/jphysiol.1994.sp020434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissing SF, Secher U, Victor RG. Mechanisms of cutaneous vasoconstriction during upright posture. Acta Physiologica Scandinavica. 1997;159:131–138. doi: 10.1046/j.1365-201X.1997.573344000.x. 10.1046/j.1365-201X.1997.573344000.x. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Eckberg DL. Sympathetic transients caused by abrupt alterations of carotid baroreceptor activity in man. American Journal of Physiology. 1982;242:H185–190. doi: 10.1152/ajpheart.1982.242.2.H185. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Sundlöf G, Delius W. The effect of carotid sinus nerve stimulation on muscle and skin nerve sympathetic activity in man. Pflügers Archiv. 1975;358:101–110. doi: 10.1007/BF00583921. [DOI] [PubMed] [Google Scholar]


