Abstract
Segments of isolated guinea-pig intestine, 12 mm long, were distended slowly by intraluminal fluid infusion or by mechanical stretch as either a tube or flat sheet. In all cases, at a constant threshold length, a sudden, large amplitude contraction of the circular muscle occurred orally, corresponding to the initiation of peristalsis.
Circumferential stretch of flat sheet preparations evoked graded contractions of the longitudinal muscle (the ‘preparatory phase’), which were maintained during circular muscle contraction. This suggests that the lengthening reported during the emptying phase of peristalsis is due to mechanical interactions.
The threshold for peristalsis was lower with more rapid stretches and was also lower in long preparations (25 mm) compared with short preparations (5-10 mm), indicating that ascending excitatory pathways play a significant role in triggering peristalsis.
Stretching a preparation beyond the threshold for peristalsis evoked contractions of increasing amplitude; thus peristalsis is graded above its threshold. However, during suprathreshold stretch maintained at a constant length, contractions of the circular muscle quickly declined in amplitude and frequency.
Circular muscle cells had a resting membrane potential approximately 6 mV more negative than the threshold for action potentials. During slow circumferential stretch, subthreshold graded excitatory motor input to the circular muscle occurred, prior to the initiation of peristalsis. However, peristalsis was initiated by a discrete large excitatory junction potential (12 ± 2 mV) which evoked bursts of smooth muscle action potentials and which probably arose from synchronized firing of ascending excitatory neuronal pathways.
The movements of the small intestine are essential for the normal processes of digestion to take place, since they are responsible for mixing food with digestive juices, exposing chyme to the surface for absorption of nutrients and propelling material along the gastrointestinal tract. The co-ordinated patterns of contraction and relaxation of the smooth muscle of the intestinal wall are due to the interaction of myogenic and neurogenic mechanisms.
In 1899, Bayliss and Starling proposed the law of the intestine, stating that ‘local stimulation of the gut produces excitation above and inhibition below the excited spot’. These two polarized reflexes, sometimes called the ‘ascending excitatory reflex’ and the ‘descending inhibitory reflex’ could be evoked by distending the gut with a balloon or by introducing a semisolid bolus into the lumen. Typically, the reflexes were graded in amplitude according to the degree of distension (Bayliss & Starling, 1899; Cannon, 1912). The two polarized reflexes, in combination called the ‘myenteric reflex’ (Cannon, 1912), have been proposed to underlie the propagating contraction observed during peristalsis. It has been widely accepted that the combination of contraction oral to a bolus and relaxation aborally leads to propulsion of the bolus and thus triggers a new set of reflexes, leading to propulsion.
Since the pioneering studies of Gayda and Trendelenburg (Gayda, 1913; Trendelenburg, 1917), peristalsis has been widely studied in isolated specimens of small intestine using fluid distension as a stimulus. A number of modifications to the original method have been developed (Kosterlitz et al. 1956; Bülbring et al. 1958; Holzer & Lembeck, 1979; Tonini et al. 1981), but these methods all rely on filling isolated tubes of small intestine with fluid until peristalsis is evoked. During the filling phase, Trendelenburg (1917) and subsequent authors (Kosterlitz & Lees, 1964) reported a graded contraction of the longitudinal muscle (the ‘preparatory phase’ of peristalsis) and that this was followed, at a sharp threshold, by a powerful contraction of the circular muscle at the oral end of the segment which then propagated aborally, propelling the contents before it. Collectively, this sequence of events has been called the ‘peristaltic reflex’ by many authors (Magnus, 1904; Cannon, 1912; Kosterlitz et al. 1956; Bülbring et al. 1958; Costa & Furness, 1976; Holzer & Lembeck, 1979), although others referred to it as ‘peristalsis’. It has been shown that the ascending excitatory neuronal pathways and descending inhibitory neuronal pathways that underlie the ‘law of the intestine’ contribute to peristalsis in the isolated guinea-pig small intestine (Waterman et al. 1994b). However, it has also become clear that the initiation of peristalsis by fluid distension can be dissociated from the ascending excitatory reflex, by its threshold, refractoriness and susceptibility to fatigue and hence must involve additional neuronal mechanisms (Tonini et al. 1996).
This study aimed to identify the mechanisms underlying the abrupt initiation of peristalsis, without the complications of fluid displacement that occur in tubular preparations of gut during the propagating contractions that cause emptying of the segment. In order to achieve this, we have developed a flat sheet preparation of small intestine which can be circumferentially stretched in a controlled fashion using a microprocessor-controlled stepper motor with in-series force transducer. Using this device, fluid-mechanical coupling between adjacent regions of intestine does not occur after the initiation of peristaltic contractions and it is possible to make stable intracellular recordings from smooth muscle without the use of drugs to immobilize the preparation. We have shown that circular muscle contractions, corresponding to the initiation of peristalsis, are evoked by an abrupt compound excitatory junction potential (EJP) that evokes a burst of smooth muscle action potentials. We have also examined interactions between circular and longitudinal muscle layers and the effects of suprathreshold stretch and maintained stretch, under controlled conditions, to determine the characteristics of the neuronal pathways that contribute to this motor pattern.
METHODS
Adult guinea-pigs of either sex, weighing between 250 and 430 g, were killed humanely by stunning and bleeding, a method approved by the Animal Welfare Committee of Flinders University. Short segments of small intestine, flushed of their contents, were placed in a Petri dish containing Krebs solution (mM: NaCl, 118; KCl, 4·75; NaH2PO4, 1·0; NaHCO3, 25; MgSO4, 1·2; CaCl2, 2·5; glucose, 11; bubbled with 95 % O2-5 % CO2). Preparations were set up as described below and allowed to equilibrate for at least 60 min before experiments started and were then allowed 3-5 min rest periods between stretches. After the equilibration period of 60 min, preparations were stretched at various rates. Many preparations showed spontaneous repetitive contractions of the circular muscle prior to the first stretch but these usually disappeared after one to two stretches. In about 10 % of preparations, spontaneous circular muscle contractions persisted throughout the recording period, making identification of the initiation of peristalsis impossible; these preparations were discarded. All data were recorded onto Maclab recording systems (ADI, Castle Hill, NSW, Australia) with a sampling frequency of 100 Hz for mechanical recordings and 1-2 kHz for electrophysiological recordings.
Flat, open preparations
Segments of flushed ileum were opened along the mesenteric border and pinned out flat. An array of hooks, resembling a miniature garden rake, made from 200 μm diameter entomological pins attached to a stainless steel frame, was attached to one edge of the preparation and to a purposed-designed tissue stretcher (see below). The other edge of the preparation was pinned to the Sylgard (Dow Corning, Midland, MI, USA) base of an organ bath (either 5 or 30 ml volume) and the whole preparation was superfused with Krebs solution at 35-36°C at a rate of either 3 or 15 ml min−1 depending on organ bath volume.
In preparations where intracellular recordings were made from circular smooth muscle cells, a second row of 50 μm diameter pins was inserted 2-3 mm from the fixed edge of the preparation and the mucosa and submucosa were removed from this area. A small area of circular muscle was mechanically isolated within this immobilized region using 40-60 tungsten pins made of 20 μm diameter wire, placed in rows 140-150 μm apart, with care being taken not to disrupt circumferential continuity with the rest of the preparation (see Fig. 1) Conventional intracellular recordings were made from circular muscle cells between the rows of fine tungsten pins using an Axoclamp-2A amplifier (Axon Instruments). Microelectrodes were filled with 0·5 M KCl and had resistances of 60-100 MΩ. Focal electrical stimulation was applied to the circular muscle 1 mm circumferential to the recording site using a pair of 25 μm diameter platinum iridium wires, insulated to within 50 μm of their tips, with pulses of 5-15 V, 0·25 ms in duration, delivered by a Grass S88 stimulator via a Grass SIU isolation unit.
Figure 1. Flat sheet preparation used to study the effects of slow stretch on motor activity in the circular smooth muscle of the isolated guinea-pig ileum.
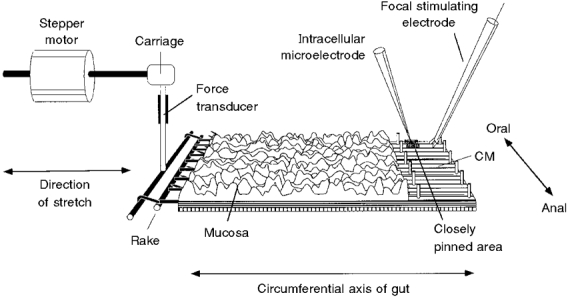
One edge was pinned to the base of the organ bath and the other was connected via a claw, resembling a miniature garden rake, to an inflexible force transducer. The force transducer was mounted on the carriage of the tissue stretcher, which was driven by a stepper motor and used to stretch the preparation in the circumferential direction. In some preparations, the mucosa and submucosa were removed from the right-hand edge of the preparation, exposing the circular muscle (CM). A line of 50 μm diameter pins restricted movement of 2-3 mm of tissue along the pinned edge of the preparation and within this region 40-60 fine pins (made from 20 μm diameter tungsten wire) were placed in serried rows 140 μm apart to immobilize a small area of circular muscle. Care was taken to ensure that none of the pins was aligned circumferentially with the immobilized area, thus ensuring that this region remained functionally connected with the bulk of smooth muscle in the preparation. Conventional intracellular recordings were made from the immobilized area and a focal stimulating electrode was placed 1 mm circumferential to the pinned area to stimulate motor axons within the circular muscle.
Comparison with tubular preparations
In one set of experiments, tubular preparations of intestine were ligated to fixed cannulae and distended by fluid infusion from a syringe pump (Harvard, model 975, South Natick, MA, USA). This was recorded by a JVC TK1280E video camera and stored on a Panasonic AG7355 SVHS video recorder. Images were then played back into the AV input of a Powermac 7500/100 computer using Image version 1.62 (NIH, Bethesda, MD, USA) and diameters of the preparation were measured at the oral and aboral ends and in the middle, using the same software. The ligatures holding the preparation onto the inlet and outlet catheters were then cut and the same tubular preparation was stretched by a rake attached to the tissue stretcher, pulling against a stainless steel tube passed through the lumen (see Fig. 2). The same preparation was then cut open into a flat sheet, pinned at one end and stretched circumferentially as shown in Fig. 1.
Figure 2. Set-up used to stretch the same tubular preparation of guinea-pig ileum via infusion of Krebs solution into the lumen or by the tissue stretcher.
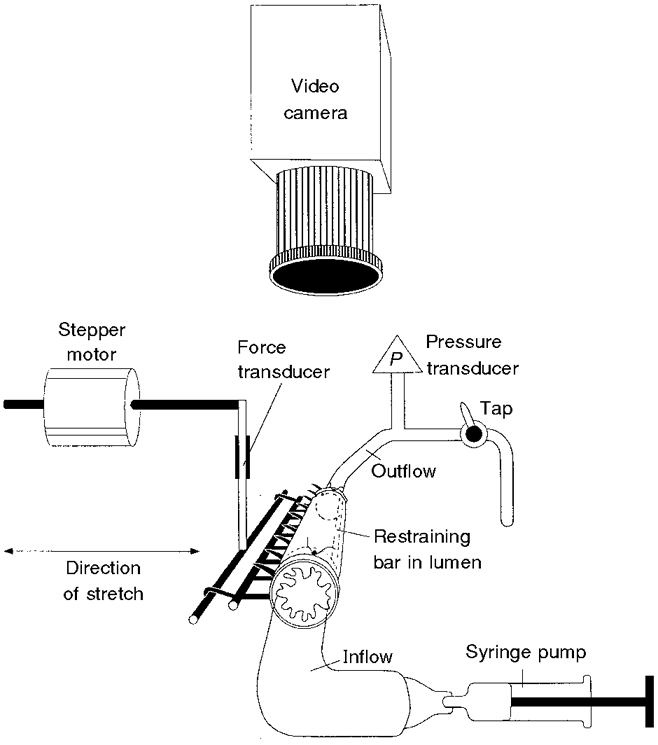
A 12 mm length of ileum was ligated to the inflow and outflow cannulae (3 mm outer diameter), with the restraining bar (of approximately 1 mm outer diameter) running through the lumen. A miniature ‘rake’ was inserted though the wall of the intestine along its length and initially allowed to hang beneath the preparation. Krebs solution was infused into the lumen at 2·7 μl s−1 from a syringe pump until peristalsis was evoked. Intraluminal pressure (P) was monitored by a pressure transducer attached to the outflow cannula and the experiment was recorded with a video camera positioned above the preparation. After stable recordings had been obtained, the ligatures were cut and the ‘rake’ was hooked onto the transducer on the tissue stretcher. The preparation remained anchored by the restraining bar through the lumen and was stretched at 100 μm s−1 until a sudden contraction marked the initiation of peristalsis.
Mechanical recordings at multiple points along the preparation
In some preparations, recordings were made to monitor circular muscle contractions at several points along the length of the preparation. To achieve this, a row of pins was placed 2-3 mm from the fixed edge of the preparation. Small cuts were made approximately 1·5 mm apart to isolate mechanically a peninsula of circular muscle from the neighbouring muscle orally and aborally within this area, while maintaining continuity circumferentially with the bulk of the preparation. A fine cotton ligature was tied to the end of the peninsula and attached, via a pulley, to an isotonic transducer (Harvard Bioscience 52-9511) with a counterweight of 200 mg mass (see Fig. 5). In this way, contractions of the circular muscle at the both ends and in the middle of the preparation could be monitored simultaneously and independently, while the bulk of the preparation was stretched by the tissue stretcher. A fourth isotonic transducer was connected to the oral end of the preparation to monitor changes in length of the preparation during slow stretch.
Figure 5. Preparation used to record circular and longitudinal muscle contractions at multiple points during slow circumferential stretch.
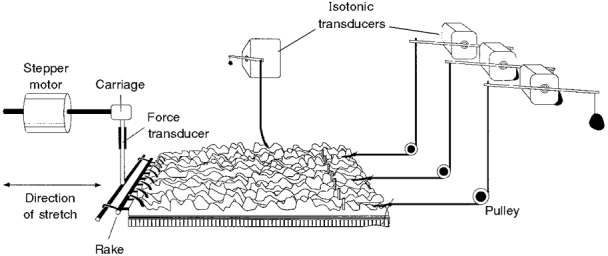
The preparation was set up in a manner similar to that shown in Fig. 1 but without removal of the mucosa or submucosa. Four short notches (2 mm long) were cut into the immobilized edge of the preparation and the resulting peninsulas at the oral, middle and anal ends of the preparation, each 1-1·5 mm wide, were attached to isotonic transducers via a pulley, with a counterweight of 200 mg. The end of the preparation was also attached to an isotonic transducer by a long (30 cm) cotton thread to monitor longitudinal muscle contractions, with minimal interference from circular muscle movements.
Tissue stretcher
The tissue stretcher consisted of a stepper motor-driven linear actuator (Radio Spares, Sydney, Australia) with a step amplitude of 25 μm. Pulses to the stepper motor were provided by a dedicated circuit board (Radio Spares) via a purpose-built, microprocessor-based controller, made in the Biomedical Engineering Workshop at Flinders Medical Centre. The user could specify rates of stretch from 5 μm to 5 mm s−1 for distances of 10 μm to 20 mm, held for 10 ms to 100 s, with return speeds of 5 μm to 5 mm s−1. A force transducer (DSC no.46-1001-01, Kistler-Morse, Redmond, WA, USA) was mounted on the carriage driven by the linear actuator. The output of the transducer was converted to a voltage signal by a dedicated bridge circuit (Radio Spares). The end of the transducer on the tissue stretcher was connected via a short Perspex rod and the array of hooks to the edge of the preparation and thus recorded the tension of the circular muscle along the entire length of the stretched region (Fig. 1).
Tetrodotoxin (TTX), where used, was obtained from Sigma, stored in aqueous solution at 10−2 mol l−1 and used at a final concentration of 0·6 μM. Statistical analysis was by repeated measures analysis of variance with Scheffé's post hoc tests. Results were considered significant when P < 0·05. Values are expressed as means ± standard error of the mean (s.e.m.); n refers to number of animals.
RESULTS
Circumferential stretch in tubular and flat sheet preparations
Fluid was infused at a rate of 2·7 μl s−1 into a 12 mm length of small intestine tied to inlet and outlet catheters (see Fig. 2). The intraluminal pressure increased gradually during this period, until the threshold for the initiation of peristalsis was reached. At this point there was a sudden increase in intraluminal pressure (Fig. 3A) and a marked contraction of the circular muscle occurred at the oral end of the preparation, as seen in video images (Fig. 3B). The increase in intraluminal pressure had a duration at half-peak amplitude of 5·2 ± 0·4 s (n= 6). The mean diameter of the preparation at the threshold for the initiation of peristalsis was 5·3 ± 0·3 mm (n= 6), calculated from video images, which corresponded to a circumference of 18·0 ± 0·9 mm (calculated from π× diameter). However, while the intestine appeared to be distended concentrically (i.e. it had a circular cross-section along its length), there was a significant tendency for the oral end of the preparation to be less distended than the anal end prior to the initiation of peristalsis (see Fig. 3). The mean calculated circumference just prior to the initiation of peristalsis was 16·8 ± 0·8 mm for the oral end, 18·5 ± 0·9 mm at the middle of the preparation and 18·6 ± 1·0 mm near the aboral end (F(2,12) = 17·795, P < 0·05). The simplest explanation for this is that the aboral end of the preparation was more compliant than the oral end, presumably due to the asymmetric activation of descending inhibitory or ascending excitatory reflex pathways. Because the expulsion of contents was prevented, the total intraluminal volume remained constant during the contraction; however, a marked redistribution of contents occurred. As the oral end of the segment contracted, the aboral end was further distended by the displaced contents, to a diameter of over 7 mm (Fig. 3B). Following drainage of the contents through the outlet catheter, intraluminal pressure fell to control levels and the contractions of the circular muscle stopped.
Figure 3. Pressure and video recordings of a 12 mm preparation distended by infusion of fluid at 2·7 μl s−1.
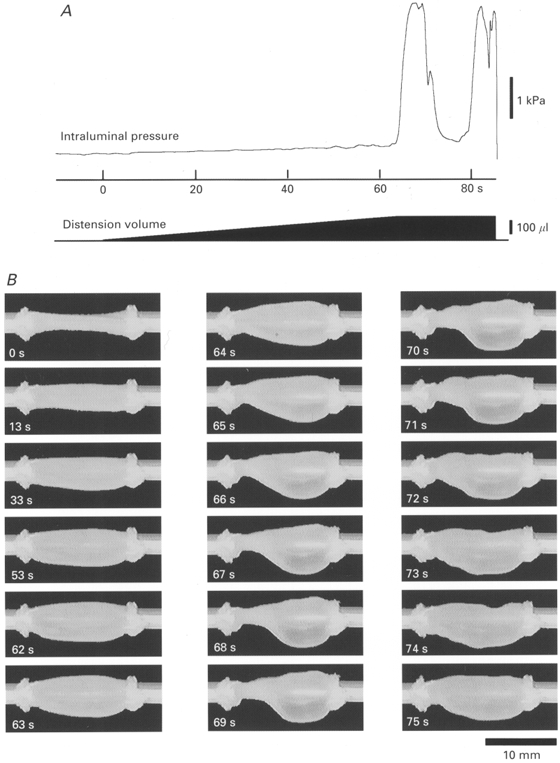
A shows the intraluminal pressure recording and a schematic representation of the infusion volume. In B, the appearance of the preparation at various times after the start of infusion (at 0 s) is shown on each image (oral is to the left). After the start of the infusion, intraluminal pressure increased gradually, corresponding to the slow distension of the preparation (from 0 to 60 s). Note that during this period there was a tendency for the aboral end of the preparation to be more distended than the oral end. At 64 s a substantial contraction of the circular muscle occurred at the oral end of the preparation, corresponding to the start of the pressure wave in A, and the syringe pump was switched off. The contraction increased in amplitude and spread along the preparation, displacing contents into the aboral region which became dramatically distended (expulsion of contents was prevented by the outflow tap being closed). By 70-72 s, the contraction started to wane and intraluminal pressure fell. A second wave of peristalsis started at 77-80 s. The outflow tap was opened at 86 s, draining the contents.
The same preparations were then stretched in two other ways for comparison. The ligatures at the end of the preparation were cut, leaving the preparation anchored by the steel tubing running through the lumen (see Fig. 2). The preparation was then attached to the tissue stretcher via the rake inserted through the mesenteric border of the intestinal wall (Fig. 2). Slow circumferential stretch of the tubular preparation at 100 μm s−1 produced a tension trace that resembled the intraluminal pressure trace during distension by fluid infusion (compare Fig. 4A and B). Essentially, during the slow circumferential stretch, tension increased gradually until the threshold for peristalsis was reached, when a large contraction of the circular muscle occurred abruptly (Fig. 4B). The mean peak tension of the circular muscle contraction was 123 ± 18 mN and the mean duration of the contraction at half-peak amplitude was 4·6 ± 0·9 s (n= 6). The mean threshold length of the preparation, at which the contraction occurred, corresponded to a circumference of 19·2 ± 0·7 mm. It should be noted that the tension recorded by the transducer on the tissue stretcher reflected the sum of tension evoked by circular muscle contractions at all points along the 12 mm length of the preparation.
Figure 4. Peristalsis evoked in the same piece of tissue, as a tube and as a flat sheet.
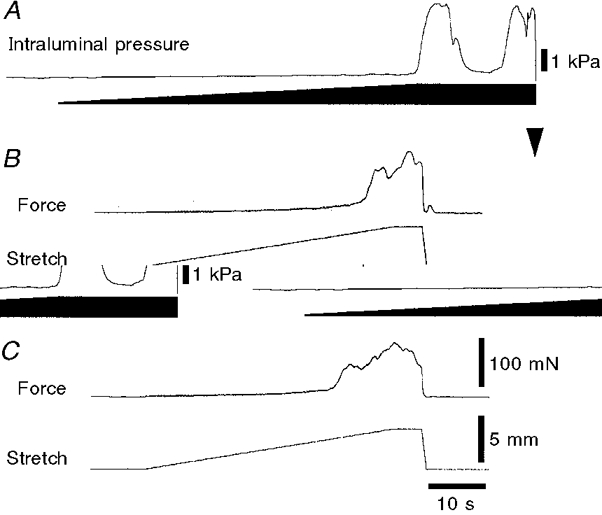
The effects of distension by slow fluid infusion (A), circumferential stretch of the intact tube with the tissue stretcher (B) and circumferential stretch of the flat sheet (C) are shown for the same segment of ileum. The trace in A is the same as that shown in Fig. 3A and the downward arrowhead marks the point at which the contents were drained from the preparation. Note that in each case, stretch evoked a small increase in wall tension (measured as pressure in A, and as force in B and C) until a threshold length, at which point there was a powerful contraction of the circular muscle, corresponding to the initiation of peristalsis. With fluid infusion maintained for 10 s (A), a second peristaltic pressure wave was recorded; similar findings were obtained with preparations stretched with the tissue stretcher, when suprathreshold lengths were maintained (see Fig. 7). Calibration bars in C also apply to B.
The same preparations were then opened up along the mesenteric border to make a flat sheet preparation and the free edge was pinned to a Sylgard block in the recording chamber (as shown in Fig. 1). After a further equilibration period of 30 min, the preparation was stretched at 100 μm s−1 every 3 min. During stretch, flat sheet preparations developed a small amount of tension until a threshold length was reached, when an abrupt contraction of the circular muscle led to a large increase in tension (see Fig. 4C). The threshold length of the preparations was 16·0 ± 0·9 mm (n= 6), the peak force was 91 ± 9 mN (n= 6) and the duration at half-peak amplitude was 5·8 ± 1·3 s (n= 6). This demonstrated that the abrupt initiation of peristalsis that is evoked by fluid infusion in tubular preparations can be mimicked by circumferential stretch of the tube using the tissue stretcher. In addition, stretch of the same tissue, opened up as a flat sheet, also evoked a large amplitude contraction of the circular muscle at a sharp threshold, mimicking the initiation of peristalsis.
Flat sheet preparations
There was a high degree of variability in the threshold for peristalsis in flat sheets partly depending on the size of the animal. When preparations were initially set up, the tissue was stretched to take up the slack, giving a resting length that was maintained for the duration of the experiment. The mean resting length for 37 animals was 13·7 ± 0·2 mm and the mean length at which peristalsis was inititated was 17·0 ± 0·2 mm (n= 37). There was a significant correlation (r= 0·669, n= 37, P < 0·05) between animal weight and resting length and a weaker, but still significant correlation, between animal weight and threshold length for peristalsis (r= 0·394, n= 37, P < 0·05). Thus, not unexpectedly, larger animals have intestines of larger resting circumference and also have a correspondingly higher threshold circumference at which peristalsis is initiated.
From the video analysis (Fig. 3B) it was clear that, during fluid distension of a short length of gut, the contraction of the circular muscle started at the oral end of the preparation. It is also well established that prior to peristalsis there is typically a graded shortening of the longitudinal muscle during the ‘preparatory phase’ (Trendelenburg, 1917; Kosterlitz & Lees, 1964) although this was not evident in Fig. 3 because the ends of the preparation were fixed by the ligatures. With the tissue stretcher, recordings of circular muscle tension reflected the integrated activity along the entire length of the preparation and the stiffness of the force transducer prevented circular muscle shortening, thus the site of initiation was not visible. We determined whether or not contraction started at the oral end of flat sheet preparations and whether there was activation of the longitudinal muscle layer during stretch, using the set-up shown in Fig. 5. Essentially a flat sheet preparation was stretched circumferentially, whilst recordings were made with three isotonic transducers from 1-1·5 mm wide ‘peninsulas’ of circular muscle at the fixed edge of the preparation, located at the oral, middle and anal ends of the preparation. These peninsulas were functionally connected to the main body of the preparation but were able to shorten during their contractions. A fourth isotonic transducer recorded shortening of the longitudinal muscle.
As shown in Fig. 6A, in most preparations the longitudinal muscle was spontaneously active, even in the absence of stretch. During slow stretch there was a net shortening of the longitudinal muscle, with an increase in amplitude of the phasic contractions, which occurred at a frequency of 10-16 contractions per minute. During the circular muscle contractions marking the initiation of peristalsis, recorded with the force transducer of the tissue stretcher, the longitudinal muscle remained contracted or even increased its contraction, lengthening again only when the stretch was removed. The large amplitude circular muscle contractions usually appeared first at the oral end of the preparation (Fig. 6A), appearing with a slight delay at the middle and aboral recording sites (see Fig. 6C). Interestingly, the amplitude of the circular muscle contractions was usually lower at the aboral recording site. In the presence of TTX (0·6 μM) to block neuronal activity, active contractions of both the circular and longitudinal muscle layer were substantially reduced (Fig. 6B), although some passive mechanical changes in length and tension still occurred.
Figure 6. Contractions of circular (CM) and longitudinal muscle (LM) evoked by slow circumferential stretch of a flat sheet preparation.
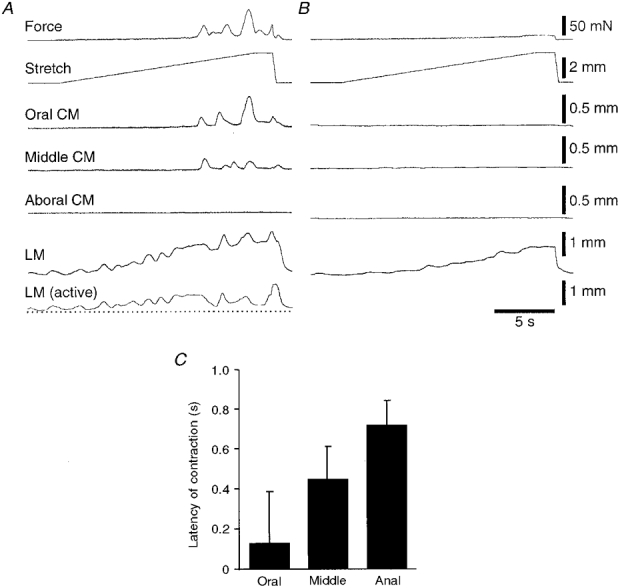
A, the traces at the top show the slow ramp stretch (at 100 μm s−1 for approximately 3 mm) and the circular muscle force, showing the abrupt onset of contraction in the circular muscle. There was spontaneous contractile activity in the longitudinal muscle prior to stretch, which was enhanced during the stretch, and maintained during the initiation of peristalsis. The first area of circular muscle to contract was at the oral end of the preparation, with later and smaller contractions occurring further anally; in this particular run no contraction was recorded at the aboral end of the tissue. B, all contractile activity, in both circular and longitudinal muscle layers, was abolished by addition of 0·6 μM TTX to the bath for 6 min, although passive changes in length and tension of the muscle were still present, especially in the longitudinal muscle. The bottom trace in A shows the longitudinal muscle trace in B (in the presence of TTX) subtracted from the control response, reflecting the active, neuronally mediated response of the longitudinal muscle to stretch. C shows the mean latency of circular muscle contractions, relative to the initiation of peristalsis, recorded with the tissue stretcher for the oral, middle and anal ends of 4 preparations, showing that the oral site contracted, on average, before the other sites. In most cases the circular muscle contraction at the oral end of the preparation was largest and it diminished as it propagated along the preparation. In some cases, the aboral end of the tissue failed to contract at all - in these cases latency measurements could not be made and these traces were not included in the analysis.
Extended and maintained stretches
In tubular preparations distended by fluid, the initiation of peristalsis generally leads to aboral propulsion of the contents, thus modifying the distribution of the stretch stimulus to the preparation. With the tissue stretcher it was possible to stretch the preparation in a controlled fashion along its whole length, to lengths beyond the threshold for peristalsis. When the stretch was maintained just above the threshold, several contractions could be evoked, although these quickly declined in both amplitude and frequency (Fig. 7A). Combined results for six preparations are shown in Fig. 7B, which characterizes the decrease in contractile activity during a maintained suprathreshold stretch. If a stretch was continued at a constant rate beyond the threshold for peristalsis, the circular muscle contracted repeatedly, with increasing force (Fig. 8A). This demonstrates that although peristalsis normally starts abruptly, beyond its threshold length the contractions are graded in amplitude. Often the separate contractions evoked by continuing stretch fused together to make tetanic contractions lasting 10 s or longer. Again, when the tissue stretcher was stopped and a constant length maintained, contractile activity quickly declined in both frequency and amplitude (Fig. 8A). Combined data from six preparations (Fig. 8B) showed the correlation between peak contractile amplitude and the degree of stretch beyond the resting length.
Figure 7. Effects of maintained, suprathreshold stretch.
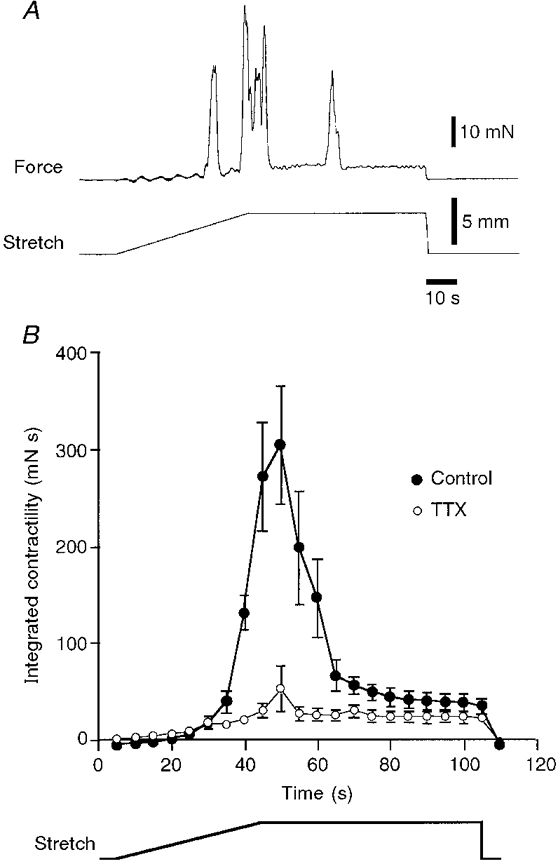
A, typical trace showing the effects of maintained suprathreshold stretch revealing a rapid run-down in peristaltic contractions. The threshold for peristalsis corresponded to a stretch of 3·0 mm. When the stretch was continued to 4·5 mm, another contraction of greater peak amplitude was evoked during the stretch. However, when the constant stretch of 4·5 mm was maintained, contraction amplitude declined, as did the frequency of contractions. B, mean contractile activity (measured as the integral of force over time, in 5 s time bins) plotted for 6 preparations (•), each stretched by 4·5 mm. It is clear that there was an increase in contractile activity after the threshold was reached, but that when a constant level of stretch was maintained, contractile activity declined rapidly. In the presence of 0·6 μM TTX (^) a lower level of muscle activity occurred throughout the maintained stretch, indicating that although peristaltic contractions disappeared during maintained stretch, there was still on-going excitatory neuronal input to the muscle.
Figure 8. Effects of stretching beyond the threshold for the initiation of peristalsis.
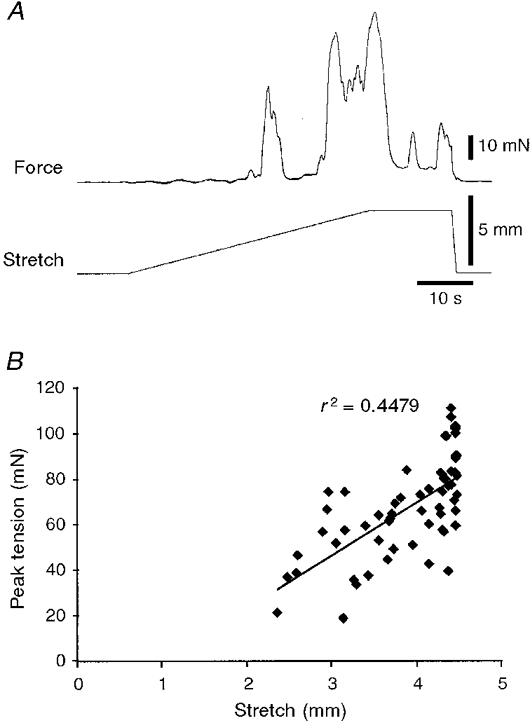
A, slow circumferential stretch at 100 μm s−1 initiated peristalsis at a threshold length change of 2·6 mm. Continuing the stretch to 4·5 mm evoked two further major contractions of increasing amplitude. Typically, during the plateau phase of the stretch, subsequent contractions were reduced in amplitude and eventually disappeared altogether (see Fig. 7). B shows that there was a significant correlation between the peak tension of the phasic contractions evoked by stretch and the degree of stretch (n= 6). This indicates that although the first contraction occurs at a particular threshold length, peristalsis is not an all-or-nothing phenomenon, but rather is graded with increasing degrees of stretch.
Effect of rate of stretch and length of preparation
The rate of stretch had a strong influence on the threshold length at which peristalsis was initiated in 12 mm long preparations. Faster rates of stretch evoked circular muscle contractions at shorter threshold lengths than slower rates of stretch. Data from six preparations are summarized in Fig. 9A. The length of the preparation also affected the threshold length, when preparations were stretched at a rate of 100 μm s−1. A 25 mm long flat sheet preparation was set up on an extended ‘rake’ and stretched at 100 μm s−1 until a constant threshold length was determined. Approximately 5 mm of tissue was then cut off the aboral end of the tissue, in situ, and removed from the ‘rake’ and the new threshold for peristalsis was determined, after allowing at least 20 min for re-equilibration. Data from six preparations are summarized in Fig. 9B, demonstrating the significantly shorter threshold length in longer preparations. To test whether the threshold for peristalsis changed spontaneously over the course of long experiments, six preparations (12 mm in length) were stretched repeatedly at 4 min intervals for 3 h after a 1 h warm-up period. The threshold in the first 30 min was 2·9 ± 0·2 mm and 150 min later it was 2·7 ± 0·2 mm (n= 6), indicating that after initial equilibration the threshold did not change significantly over the time course of these experiments.
Figure 9. Effects of rate of stretch and the length of the preparation on the threshold for peristalsis.
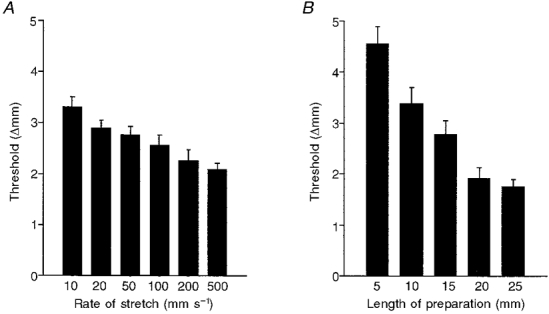
Each graph shows combined data from 6 preparations; error bars represent s.e.m.A, less stretch was required to evoke peristalsis with faster rates of stretch indicating that there must be some run-down in the excitatory pathways prior to the initiation of peristalsis. B, shorter preparations (down to 5 mm in length) required more stretch to evoke circular muscle contractions than did longer preparations (up to 25 mm in length).
Electrical activity of circular smooth muscle cells
Intracellular recordings were made from smooth muscle cells in the circular muscle layer during slow circumferential stretch of flat sheet preparations that were 12 mm in length as described in the Methods and illustrated in Fig. 1. All recordings were made within 2·5 mm of the oral end of the preparation.
Smooth muscle cells in the immobilized area had mean resting membrane potentials of -55 ± 2 mV (n= 9) and rarely showed spontaneous activity. A single shot stimulus (0·25 ms duration, 15 V) delivered to the focal stimulating electrode was used to evoke an inhibitory junction potential (IJP) to ascertain that the electrode tip was within the circular muscle layer and to establish that the preparation had recovered from the pinning. Single shot IJPs had a mean amplitude of 15 ± 1 mV (n= 9). During slow stretch at 100 μm s−1, recordings from the circular muscle within 1-3 mm of the oral end of the preparation revealed a consistent pattern of activation. After 1-2 mm of stretch from the resting length, a slow, graded depolarization of the smooth muscle, presumably due to asynchronous firing of excitatory motor neurones, was seen in all preparations (Fig. 10A). This reached a peak value of 4 ± 1 mV (n= 9; range, 0-7 mV; median value, 4 mV) and was usually associated with slow fluctuations in membrane potential. At a sharp threshold value, a large, discrete EJP was evoked with superimposed smooth muscle action potentials. The action potentials appeared to correlate well with the individual phasic contractions that combined to make up the contraction at the initiation of peristalsis (Fig. 10B). The peak value of the EJP associated with the initiation of peristalsis (measured relative to the resting membrane potential) was 12 ± 2 mV (n= 9). The number of action potentials evoked was very variable between preparations, varying from one to 17 (mean = 6 ± 2 action potentials; n= 9). In addition, the amplitude of the action potentials was also highly variable (mean maximum amplitude, 21 ± 3 mV; n= 9). In most preparations, action potentials were often attenuated at the start of the recording period, but increased in amplitude over the next 2-5 h. Often, within a train of large amplitude action potentials, a few attenuated potentials were seen. We assume that these represent action potentials generated at a site at some distance from the recording microelectrode, conducted passively to the recording site. It is possible that damage caused by the removal of the submucosa or by close pinning may have prevented the reliable initiation of full action potentials in the dissected region. The mean threshold for action potentials was -48 ± 2 mV, or about 6-7 mV above resting membrane potential. Application of TTX (0·6 μM) to the bath, to block nerve-mediated activity, abolished the graded depolarization, the large synchronous EJP and the contraction at the initiation of peristalsis (Fig. 10C) in all preparations tested (n= 5).
Figure 10. Intracellular recordings from circular muscle (CM) cells during slow, circumferential stretch.
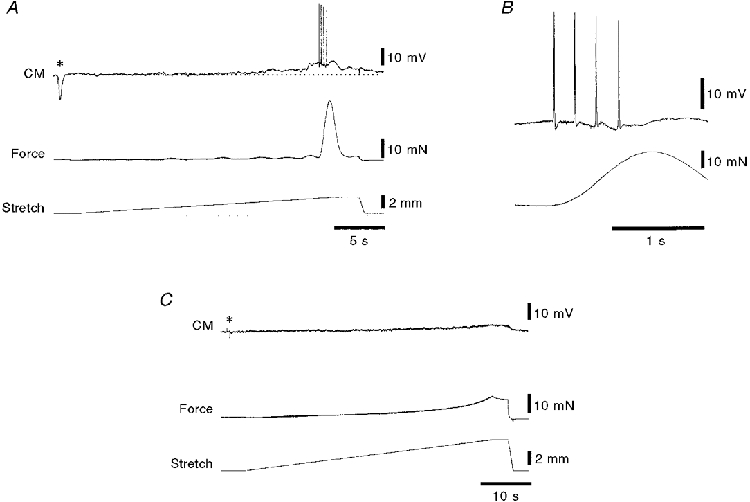
A, typical recording from a circular muscle cell located 2 mm from the oral end of a 12 mm long preparation showing the sudden contraction of the circular muscle after the preparation had been stretched 2·4 mm beyond its resting length of 12 mm. A single electrical stimulus (0·25 ms, 15 V) was applied via focal stimulating electrodes 1 mm circumferential to the recording site (asterisk) evoking a large inhibitory junction potential, confirming that the recording electrode was in a circular muscle cell. A small graded depolarization (about 3 mV in amplitude) occurred prior to the initiation of peristalsis (above the resting membrane potential shown by the dotted line). Just before the initiation of peristalsis there was a large compound excitatory junction potential (7 mV above resting membrane potential) with superimposed smooth muscle action potentials. In B, the same recording is shown on a faster time base, revealing that the burst of action potentials preceded the start of the contraction. This is consistent with the contractile activity appearing first at the oral end of the preparation close to where the recording was made. C, TTX (0·6 μM for 15 min) abolished the inhibitory junction potential (asterisk), and most electrical and contractile activity, leaving just a small myogenic depolarization of approximately 2 mV at the highest level of stretch. Note the different time scales in A, B and C and that the preparation was stretched 5 mm in C.
DISCUSSION
The effects of slow circumferential stretch of flat sheet preparations of guinea-pig ileum on the circular muscle were studied. In isolated tubular preparations of small intestine, radial distension by slow fluid infusion evokes the motor pattern of peristalsis; this is mimicked in flat sheet preparations by controlled circumferential stretch. Like peristalsis evoked by fluid infusion, the contraction of the circular muscle starts primarily at the oral end of the preparation. There is also a graded shortening of the longitudinal muscle layer prior to the contraction, similar to that which occurs during the preparatory phase of peristalsis. These results indicate that the circuitry required for the preparatory phase and for the abrupt initiation of peristalsis do not require full circumferential continuity of the gut and that simple circumferential stretch is sufficient to evoke these motor patterns.
Requirements for peristalsis
The present study confirmed that slow distension evokes peristalsis in a 12 mm long tube of guinea-pig small intestine, as seen by a sudden contraction of the circular muscle at the oral end of the preparation and an increase in intraluminal pressure (Tonini et al. 1996). Circumferential stretch of the same preparation, using the tissue stretcher, also evoked a large contraction of the circular muscle at a sharp threshold value. This indicates that stretch alone is sufficient to evoke peristalsis and that transmural pressure is not required. It would appear that the reflex pathways activated by specific mechanical deformation of the mucosa (Raiford & Mulinos, 1934; Smith & Furness, 1988) are also not essential for the initiation of peristalsis, although the possibility that mucosal stimulation contributes to peristalsis under physiological conditions in vivo cannot be ruled out. Indeed, the mucosa itself was also stretched linearly during these experiments, although the villi were not compressed or distorted. These data are consistent with previous reports that peristalsis can occur after damage or removal of the mucosa (Ginzel, 1959; Tsuji & Yokoyama, 1982). Opening up the preparation into a flat sheet and stretching it circumferentially evoked an abrupt increase in circular muscle tension which was initiated at the oral end of the preparation. This demonstrates that full circumferential continuity is not required for the initiation of peristalsis. Anatomical studies have demonstrated that enteric neurones project for up to 130 mm aborally, 10 mm circumferentially and up to 14 mm orally (Brookes et al. 1995b, 1997; Song et al. 1996). Clearly the motor pattern of peristalsis does not require all of these projections to be intact since it can be evoked in preparations as short as 12 mm. However, we have also shown in flat sheet preparations that longer preparations require less stretch to initiate peristalsis. Since the peristalsis starts at the oral end, this suggests that ascending excitatory pathways play an important role in setting the threshold for initiation (Waterman et al. 1994b).
Longitudinal muscle
Peristalsis represents a co-ordinated pattern of motor activity in the intestine that has been widely studied in vitro, but is also known to occur in vivo. During the filling phase, a graded shortening of the longitudinal muscle has been widely described (Trendelenburg, 1917; Kosterlitz et al. 1956; Kosterlitz & Lees, 1964; Kottegoda, 1970; Tonini et al. 1981; Yokoyama & Ozaki, 1990), but after the initiation of the circular muscle contraction, the longitudinal muscle lengthens. It has been shown that longitudinal muscle motor neurones can be activated by both ascending and descending pathways activated by stretch in the guinea-pig ileum (Smith et al. 1992). It was suggested that there may be a reciprocal innervation of the circular and longitudinal muscle layers so that when the circular muscle layer contracts, the longitudinal muscle layer is actively inhibited (Kottegoda, 1970). However, other authors have suggested that the longitudinal and circular muscle layers contract synchronously (Bayliss & Starling, 1899; Yokoyama & Ozaki, 1990; Smith & Robertson, 1998). The results of the present study suggest that the longitudinal muscle layer remains contracted throughout the duration of the circular muscle contraction; there is no evidence to support the existence of reciprocal inhibition. A recent study by Smith & Robertson (1998) of guinea-pig colon reached a similar conclusion about the longitudinal muscle just oral or anal to the region actually involved in peristalsis.
It is perhaps not surprising that the longitudinal muscle layer is not actively inhibited during peristalsis in the guinea-pig ileum, since morphological studies have suggested that there is little inhibitory motor innervation of this layer in the guinea-pig ileum (Brookes et al. 1992) and intracellular recordings have shown relatively small IJPs (Bywater & Taylor, 1986). It is likely that, in tubular preparations, the lengthening of longitudinal muscle that is observed during the emptying phase of peristalsis is due to simple mechanical interactions between the layers due to the stronger contractility of the circular muscle and the conservation of total volume of the wall tissue during lumen-occlusive contractions (Gregory & Bentley, 1968; Wood & Perkins, 1970). It is also likely that the mesh of connective tissue within the intestinal wall may contribute to such interactions, analogous to the way that stretching a knitted sleeve longitudinally decreases its diameter. In the flat sheet preparation of the present study, such mechanical interactions may be expected to be less powerful than in intact tubes with contents. The longitudinal muscle shortening that occurs during filling of the intestine will have the effect of increasing the average diameter of the intestine for any given volume of contents, and may thus interact mechanically with circular muscle activity, but may not be part of the same neuronal pattern generator that controls the emptying phase of peristalsis.
Why does peristalsis start abruptly?
The contraction of the longitudinal muscle layer is graded during filling; in contrast, circular muscle contraction usually starts very abruptly. This is not because the circular muscle is incapable of generating graded responses; it is well established that the ascending excitatory reflex, evoked by rapid distension (usually with an intraluminal balloon), is graded with stimulus intensity (Bayliss & Starling, 1899; Costa & Furness, 1976; Smith et al. 1990; Holzer et al. 1993; Tonini et al. 1996). In part, differences in the patterns of contraction are probably due to the different basal level of excitability of the two muscle layers. In most preparations, the longitudinal muscle layer was spontaneously contractile, even in the absence of stretch. Thus graded excitatory motor input would be expected to increase contractility in a graded fashion. In contrast, the circular muscle in most preparations was normally quiescent, having a membrane potential about 6 mV below the threshold for action potential generation. In fact, intracellular recordings in this study have shown that there is a graded excitatory motor input to the circular muscle, prior to the initiation of peristalsis, but that this does not normally reach threshold for triggering action potentials. Instead, we have shown that the initiation of contraction is due to a synchronized burst of firing in excitatory motor neurones that generates a large, discrete EJP at the oral end of the preparation. It is this sudden large depolarization that brings the muscle to threshold for the generation of action potentials and hence causes the abrupt initiation of peristalsis. Tsuchiya (1972) reported graded depolarization without action potentials during fluid infusion into tubular preparations of guinea-pig ileum. Rapid EJPs with superimposed muscle action potentials occurred only during the emptying phase of peristalsis in those preparations. However, it was suggested (Tsuchiya, 1972) that the recordings were from longitudinal muscle cells. It seems likely that some of those recordings may have inadvertently been made from the thicker circular smooth muscle layer, given the results presented here. In the present study impaled cells were identified as circular muscle cells, for each impalement, by the presence of a large amplitude IJP evoked by single electrical stimuli (Bywater & Taylor, 1986). Longitudinal muscle cells (without large IJPs) were recorded accidentally on several occasions: these had spontaneous action potentials at rest (S. J. H. Brookes, unpublished observations).
We suggest that the mechanism underlying the generation of the discrete, large EJPs is likely to be due to bursting activity in ascending interneurones. In the guinea-pig ileum, the great majority of excitatory motor neurones project only 1-2 mm orally to the circular muscle (Brookes et al. 1991). The fact that the contraction starts at the oral end of the preparation suggests that the excitatory motor neurones at the oral end are activated by ascending pathways. This is supported by the observation that longer preparations (which have longer intact ascending pathways) require less stretch to initiate peristalsis than do shorter preparations. Intracellular recordings from the one class of ascending interneurones in the guinea-pig small intestine have revealed that these cells synapse with other cells of the same class and thus are organized into functional chains running up the intestine, with outputs onto excitatory motor neurones (Brookes et al. 1995a). As a population, they are also capable of firing in a bursting pattern (Brookes et al. 1997). We suggest that ascending interneurones all along the preparation are activated by circumferential stretch, probably by stretch-activated primary afferent neurones (Kunze et al. 1998) and that, for reasons that are not yet clear, tend as a population to fire in synchronized bursts. By summation, their output onto excitatory motor neurones would be most powerful at the oral end of the preparation, ensuring the oral initiation of peristalsis. This hypothesis will require direct testing by recording from these neurones during the initiation of peristalsis.
The role of inhibitory motor neurones
In the present study, no evidence was found for large compound IJPs in circular muscle cells, although single electrical stimuli evoked IJPs up to 20 mV in amplitude. The absence of IJPs suggests that synchronized firing of inhibitory motor neurones does not occur during slow stretch and the initiation of peristalsis. However, inhibitory motor neurones may be active in these preparations, but firing tonically rather than phasically. During radial distension of intestine, it has been established that there is an accommodatory reflex in the circular muscle, which serves to reduce intraluminal pressure (Furness & Costa, 1977; Tonini & Costa, 1990; Waterman et al. 1994a) and which may be the functional role of the on-going inhibitory drive to circular muscle described in both small and large intestines (Wood & Marsh, 1973; Tonini et al. 1974). In this study, during slow fluid distension, the diameter of tubes of intestine was significantly greater at the aboral end compared to orally, prior to the initiation of peristalsis. This suggests that there was greater compliance aborally, presumably due to polarized inhibitory motor pathways. It has previously been shown that inhibitory motor neurones in the guinea-pig small intestine project aborally before entering the circular muscle, for distances of 0·5-25 mm (Brookes et al. 1991). The polarization of this pathway would also tend to favour an oral start to the emptying phase of peristalsis (since inhibition would be least at the oral end). A role for inhibitory motor neurones in the initiation of peristalsis has been demonstrated by several studies that have shown a decrease in the threshold, or an increase in frequency, of peristalsis induced by blockers of nitric oxide synthase (Suzuki et al. 1994; Waterman & Costa, 1994; Holzer et al. 1997). The apparent absence of IJPs is perhaps not surprising since the slow inhibition caused by nitric oxide is associated with little hyperpolarization in this preparation (Lyster et al. 1992). It is also clear that rapid apamin-sensitive IJPs evoked by single motor neurones are of very small amplitude (Bornstein et al. 1986) and thus are not likely to be visible during unsynchronized tonic firing by a large population of inhibitory motor neurones. It is interesting that large amplitude, apamin-sensitive IJPs can be evoked by balloon distension (Smith et al. 1990) or by gross mechanical stimulation of the mucosa (Smith & Furness, 1988; Yuan et al. 1991) presumably because the firing of many inhibitory motor neurones was synchronized by the rapid onset of strong stimuli.
Myogenic mechanisms in peristalsis
In a recent study (Huizinga et al. 1998), it was reported that propulsive peristalsis in the proximal small intestine of the mouse only occurs in the presence of aborally propagating slow waves with superimposed smooth muscle action potentials. In that study it was reported that, in W/Wv mice, which lack interstitial cells of Cajal and slow wave activity (Ward et al. 1994; Huizinga et al. 1995), neuronal activity alone was insufficient to evoke propagating peristalsis. The authors concluded that slow wave activity may be essential for normal peristalsis. This does not appear to be the case in the guinea-pig small intestine where slow wave activity is rarely recorded in circular muscle cells in flat sheet preparations (Smith, 1989), and was never recorded in this study. However, it is clear that the circular muscle of the guinea-pig ileum is capable of generating slow waves in vivo (Galligan et al. 1985) but that this mechanism is normally suppressed in vitro perhaps by the presence of endogenous prostanoids (Maggi et al. 1994). In the present study, muscle action potentials always preceded contractions although the circular muscle did not have detectable slow waves. It would seem likely that the neurogenic mechanisms and myogenic depolarizations would normally work in concert, reinforcing one another to give rise to propagating peristaltic contractions.
The flat sheet preparation presented in this study will allow the systematic analysis of the different pathways and mechanisms that contribute to the motor pattern of peristalsis in the guinea-pig small intestine. By opening up the intestine it will be possible to separate the mechanical and fluid/mechanical interactions from the myogenic and neurogenic mechanisms that underlie peristalsis and study the various components of this motor pattern separately.
Acknowledgments
This work was supported by a project grant from the NH and MRC of Australia. S. J. H. B. was supported, during part of this study, by a Senior Research Fellowship in Digestive Sciences of the Gastroenterological Society of Australia. We would like to thank the staff of the Biomedical Engineering Workshop for their considerable help with the developing of the tissue stretcher used in this study.
References
- Bayliss WM, Starling EH. The movements and innervation of the small intestine. The Journal of Physiology. 1899;24:99–143. doi: 10.1113/jphysiol.1899.sp000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein JC, Costa M, Furness JB, Lang RJ. Electrophysiological analysis of projections of enteric inhibitory motoneurones in the guinea-pig small intestine. The Journal of Physiology. 1986;370:61–74. doi: 10.1113/jphysiol.1986.sp015922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes SJH, Bywater RAR, Costa M. Characterisation of excitatory motor neurones to the circular muscle of the guinea-pig small intestine. Proceedings of the Australian Physiological and Pharmacological Society. 1995a;6:43. [Google Scholar]
- Brookes SJH, Meedeniya ACB, Jobling P, Costa M. Orally projecting interneurones in the guinea-pig small intestine. The Journal of Physiology. 1997;505:473–491. doi: 10.1111/j.1469-7793.1997.473bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes SJH, Song ZM, Ramsay GA, Costa M. Long aboral projections of Dogiel type II, AH neurons within the myenteric plexus of the guinea pig small intestine. Journal of Neuroscience. 1995b;15:4013–4022. doi: 10.1523/JNEUROSCI.15-05-04013.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes SJH, Song ZM, Steele PA, Costa M. Identification of motor neurons to the longitudinal muscle of the guinea pig ileum. Gastroenterology. 1992;103:961–973. doi: 10.1016/0016-5085(92)90030-3. [DOI] [PubMed] [Google Scholar]
- Brookes SJH, Steele PA, Costa M. Identification and immunohistochemistry of cholinergic and non-cholinergic circular muscle motor neurons in the guinea-pig small intestine. Neuroscience. 1991;42:863–878. doi: 10.1016/0306-4522(91)90050-x. [DOI] [PubMed] [Google Scholar]
- Bülbring E, Lin RCY, Schofield G. An investigation of the peristaltic reflex in relation to anatomical observations. Quarterly Journal of Experimental Physiology. 1958;43:26–37. doi: 10.1113/expphysiol.1958.sp001305. [DOI] [PubMed] [Google Scholar]
- Bywater RAR, Taylor GS. Non-cholinergic excitatory and inhibitory junction potentials in the circular smooth muscle of the guinea-pig ileum. The Journal of Physiology. 1986;374:153–164. doi: 10.1113/jphysiol.1986.sp016072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon WB. Peristalsis, segmentation and the myenteric reflex. American Journal of Physiology. 1912;30:114–128. [Google Scholar]
- Costa M, Furness JB. The peristaltic reflex: an analysis of the nerve pathways and their pharmacology. Naunyn- Schmiedeberg's Archives of Pharmacology. 1976;294:47–60. doi: 10.1007/BF00692784. [DOI] [PubMed] [Google Scholar]
- Furness JB, Costa M. The participation of enteric inhibitory nerves in accommodation of the intestine to distension. Clinical and Experimental Pharmacology and Physiology. 1977;4:37–41. doi: 10.1111/j.1440-1681.1977.tb02375.x. [DOI] [PubMed] [Google Scholar]
- Galligan JJ, Costa M, Furness JB. Gastrointestinal myoelectric activity in conscious guinea pigs. American Journal of Physiology. 1985;249:G92–99. doi: 10.1152/ajpgi.1985.249.1.G92. [DOI] [PubMed] [Google Scholar]
- Gayda T. Beitrage zur Physiologie des überlebenden Dunndarms von Säugetieren. Pflügers Archiv. 1913;151:407–455. [Google Scholar]
- Ginzel KH. Are mucosal nerve fibres essential for the peristaltic reflex? Nature. 1959;184:1235–1236. doi: 10.1038/1841235b0. [DOI] [PubMed] [Google Scholar]
- Gregory JE, Bentley GA. The peristaltic reflex in the isolated guinea-pig ileum during drug-induced spasm of the longitudinal muscle. Australian Journal of Experimental Biology and Medical Science. 1968;46:1–16. doi: 10.1038/icb.1968.1. [DOI] [PubMed] [Google Scholar]
- Holzer P, Lembeck F. Effect of neuropeptides on the efficiency of the peristaltic reflex. Naunyn-Schmiedeberg's Archives of Pharmacology. 1979;307:257–264. doi: 10.1007/BF00505942. [DOI] [PubMed] [Google Scholar]
- Holzer P, Lippe IT, Tabrizi AL, Lenard L, Bartho L. Dual excitatory and inhibitory effect of nitric oxide on peristalsis in the guinea pig intestine. Journal of Pharmacology and Experimental Therapeutics. 1997;280:154–161. [PubMed] [Google Scholar]
- Holzer P, Schluet W, Maggi CA. Ascending enteric reflex contraction: roles of acetylcholine and tachykinins in relation to distension and propagation of excitation. Journal of Pharmacology and Experimental Therapeutics. 1993;264:391–396. [PubMed] [Google Scholar]
- Huizinga JD, Ambrous K, Der-Silaphet T. Co-operation between neural and myogenic mechanisms in the control of distension-induced peristalsis in the mouse small intestine. The Journal of Physiology. 1998;506:843–856. doi: 10.1111/j.1469-7793.1998.843bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Kosterlitz HW, Lees GM. Pharmacological analysis of intrinsic intestinal reflexes. Pharmacological Reviews. 1964;16:301–339. [PubMed] [Google Scholar]
- Kosterlitz HW, Pirie VW, Robinson JA. The mechanism of the peristaltic reflex in the isolated guinea-pig ileum. The Journal of Physiology. 1956;133:681–694. doi: 10.1113/jphysiol.1956.sp005618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottegoda SR. Peristalsis of the small intestine. In: Bülbring E, Jones A, Tomita T, editors. Smooth Muscle. London: Edward Arnold; 1970. pp. 525–541. [Google Scholar]
- Kunze WAA, Furness JB, Bertrand PP, Bornstein JC. Intracellular recordings from myenteric neurons of the guinea-pig ileum that respond to stretch. The Journal of Physiology. 1998;506:827–842. doi: 10.1111/j.1469-7793.1998.827bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyster D, Bywater R, Taylor GS, Watson MJ. Effects of a nitric oxide synthase inhibitor on non-cholinergic junction potentials in the circular muscle of the guinea pig ileum. Journal of the Autonomic Nervous System. 1992;41:187–196. doi: 10.1016/0165-1838(92)90058-o. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Patacchini R, Meini S, Giuliani S. Effect of longitudinal muscle-myenteric plexus removal and indomethacin on the response to tachykinin NK-2 and NK-3 receptor agonists in the circular muscle of the guinea-pig ileum. Journal of Autonomic Pharmacology. 1994;14:49–60. doi: 10.1111/j.1474-8673.1994.tb00589.x. [DOI] [PubMed] [Google Scholar]
- Magnus R. Versuche am überlebenden Dunndarm von Säugetieren. I. Mittheilung. Pflügers Archiv. 1904;102:123–151. [Google Scholar]
- Raiford T, Mulinos MG. The myenteric reflex as exhibited by the exteriorized colon of the dog. American Journal of Physiology. 1934;110:129–136. [Google Scholar]
- Smith TK. Spontaneous junction potentials and slow waves in the circular muscle of isolated segments of guinea-pig ileum. Journal of the Autonomic Nervous System. 1989;27:147–154. doi: 10.1016/0165-1838(89)90096-9. [DOI] [PubMed] [Google Scholar]
- Smith TK, Bornstein JC, Furness JB. Distension-evoked ascending and descending reflexes in the circular muscle of guinea-pig ileum: an intracellular study. Journal of the Autonomic Nervous System. 1990;29:203–217. doi: 10.1016/0165-1838(90)90146-a. [DOI] [PubMed] [Google Scholar]
- Smith TK, Bornstein JC, Furness JB. Convergence of reflex pathways excited by distension and mechanical stimulation of the mucosa onto the same myenteric neurons of the guinea pig small intestine. Journal of Neuroscience. 1992;12:1502–1510. doi: 10.1523/JNEUROSCI.12-04-01502.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Furness JB. Reflex changes in circular muscle activity elicited by stroking the mucosa: an electrophysiological analysis in the isolated guinea-pig ileum. Journal of the Autonomic Nervous System. 1988;25:205–218. doi: 10.1016/0165-1838(88)90025-2. [DOI] [PubMed] [Google Scholar]
- Smith TK, Robertson WJ. Synchronous movements of the longitudinal and circular muscle during peristalsis in the isolated guinea-pig distal colon. The Journal of Physiology. 1998;506:563–577. doi: 10.1111/j.1469-7793.1998.563bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Brookes SJH, Costa M. Projections of specific morphological types of neurons within the myenteric plexus of the small intestine of the guinea-pig. Cell and Tissue Research. 1996;285:149–156. doi: 10.1007/s004410050630. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Mizuno K, Gomi Y. Role of nitric oxide in the peristalsis in the isolated guinea-pig ileum. European Journal of Pharmacology. 1994;251:221–227. doi: 10.1016/0014-2999(94)90403-0. [DOI] [PubMed] [Google Scholar]
- Tonini M, Costa M. A pharmacological analysis of the neuronal circuitry involved in distension-evoked enteric excitatory reflex. Neuroscience. 1990;38:787–795. doi: 10.1016/0306-4522(90)90071-b. [DOI] [PubMed] [Google Scholar]
- Tonini M, Costa M, Brookes SJH, Humphreys CM. Dissociation of the ascending excitatory reflex from peristalsis in the guinea-pig small intestine. Neuroscience. 1996;73:287–297. doi: 10.1016/0306-4522(96)00040-1. [DOI] [PubMed] [Google Scholar]
- Tonini M, Frigo G, Lecchini S, D'Angelo L, Crema A. Hyoscine-resistant peristalsis in guinea-pig ileum. European Journal of Pharmacology. 1981;71:375–381. doi: 10.1016/0014-2999(81)90181-3. [DOI] [PubMed] [Google Scholar]
- Tonini M, Lecchini S, Frigo G, Crema A. Action of tetrodotoxin on spontaneous electrical activity of some smooth muscle preparations. European Journal of Pharmacology. 1974;29:236–240. doi: 10.1016/0014-2999(74)90021-1. [DOI] [PubMed] [Google Scholar]
- Trendelenburg P. Physiologische und pharmakologische Versuche uber die Dunndarmperistaltik. Naunyn-Schmiedeberg's Archives of Pharmacology. 1917;81:55–129. doi: 10.1007/s00210-006-0052-7. [DOI] [PubMed] [Google Scholar]
- Tsuchiya K. Electrical and mechanical activities of the longitudinal muscle in the peristaltic wave elicited by the intraluminal pressure raising. Rendic Gastroenterology. 1972;4:115–125. [Google Scholar]
- Tsuji S, Yokoyama S. Peristaltic reflex in the small intestine deprived of mucosa. Japanese Journal of Smooth Muscle Research. 1982;18:218–220. [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. The Journal of Physiology. 1994;480:91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman SA, Costa M. The role of enteric inhibitory motoneurones in peristalsis in the isolated guinea-pig small intestine. The Journal of Physiology. 1994;477:459–468. doi: 10.1113/jphysiol.1994.sp020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman SA, Costa M, Tonini M. Accommodation mediated by enteric inhibitory reflexes in the isolated guinea-pig small intestine. The Journal of Physiology. 1994a;474:539–546. doi: 10.1113/jphysiol.1994.sp020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman SA, Tonini M, Costa M. The role of ascending excitatory and descending inhibitory pathways in peristalsis in the isolated guinea-pig small intestine. The Journal of Physiology. 1994b;481:223–232. doi: 10.1113/jphysiol.1994.sp020433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JD, Marsh DR. Effects of atropine, tetrodotoxin and lidocaine on rebound excitation of guinea-pig small intestine. Journal of Pharmacology and Experimental Therapeutics. 1973;184:590–598. [PubMed] [Google Scholar]
- Wood JD, Perkins WE. Mechanical interaction between longitudinal and circular axes of the small intestine. American Journal of Physiology. 1970;218:762–768. doi: 10.1152/ajplegacy.1970.218.3.762. [DOI] [PubMed] [Google Scholar]
- Yokoyama S, Ozaki T. Contractions of the longitudinal and circular muscle of the small intestine. Progress in Clinical and Biological Research. 1990;327:483–492. [PubMed] [Google Scholar]
- Yuan SY, Furness JB, Bornstein JC, Smith TK. Mucosal distortion by compression elicits polarized reflexes and enhances responses of the circular muscle to distension in the small intestine. Journal of the Autonomic Nervous System. 1991;35:219–226. doi: 10.1016/0165-1838(91)90100-h. [DOI] [PubMed] [Google Scholar]


