Abstract
We investigated whether insulin-like growth factor-1 (IGF-1), an endogenous potent activator of skeletal muscle proliferation and differentiation, enhances L-type Ca2+ channel gene expression resulting in increased functional voltage sensors in single skeletal muscle cells.
Charge movement and inward Ca2+ current were recorded in primary cultured rat myoballs using the whole-cell configuration of the patch-clamp technique. Ca2+ current and maximum charge movement (Qmax) were potentiated in cells treated with IGF-1 without significant changes in their voltage dependence. Peak Ca2+ current in control and IGF-1-treated cells was -7·8 ± 0·44 and -10·5 ± 0·37 pA pF−1, respectively (P < 0·01), whilst Qmax was 12·9 ± 0·4 and 22·0 ± 0·3 nC μF−1, respectively (P < 0·01).
The number of L-type Ca2+ channels was found to increase in the same preparation. The maximum binding capacity (Bmax) of the high-affinity radioligand [3H]PN200-110 in control and IGF-1-treated cells was 1·21 ± 0·25 and 3·15 ± 0·5 pmol (mg protein)−1, respectively (P < 0·01). No significant change in the dissociation constant for [3H]PN200-110 was found.
Antisense RNA amplification showed a significant increase in the level of mRNA encoding the L-type Ca2+ channel α1-subunit in IGF-1-treated cells.
This study demonstrates that IGF-1 regulates charge movement and the level of L-type Ca2+ channel α1-subunits through activation of gene expression in skeletal muscle cells.
The dihydropyridine (DHP)-sensitive L-type Ca2+ channel plays a central role in intracellular Ca2+ homeostasis. In particular, this channel is essential in skeletal muscle excitation-contraction coupling (Melzer et al. 1995). When a muscle fibre is activated by a nerve impulse, an action potential spreads throughout the surface and T-tubular sarcolemma. The L-type Ca2+ channel α1-subunit senses changes in membrane voltage. Conformational changes of the channel associated with the movement of positively charged segments of α1-subunits (S4 segments) couple depolarization of the T-tubule and Ca2+ release (Schneider, 1994; Melzer et al. 1995). The L-type Ca2+ channel α1-subunit is the main source of intramembrane current together with the Na+ and T-type Ca2+ channel in skeletal muscle. The L-type Ca2+ channel by itself contributes more than 60 % of the total charge movement recorded in skeletal myotube (Adams et al. 1990). This voltage-dependent non-linear capacitive current is thought to be associated with the voltage sensor function and is termed gating current or charge movement (Schneider & Chandler, 1973). Because the integral of charge movement is an indication of the number of ion channels inserted in the membrane, the level of channel expression is associated with the amplitude of the charge movement recordings.
Insulin-like growth factor-1 (IGF-1) is a peptide structurally related to proinsulin and has a primary role in promoting skeletal muscle differentiation and growth in a relatively slow time scale (Florini et al. 1996). IGF-1, as well as some other growth factors, regulates the ion permeation function of various ion channels. Growth/trophic factor-mediated potentiation of Ca2+ permeation through different ion channels in clonal pituitary and neuroblastoma cell lines has been reported (Meza et al. 1994; Selinfreund & Blair, 1994; Delbono & Sonntag, 1996; Blair & Marshall, 1997). In skeletal muscle, we have demonstrated that IGF-1 potentiates Ca2+ current through L-type Ca2+ channels in adult fibres (Delbono et al. 1997), but not in muscles from ageing mice (Renganathan et al. 1997c) probably due to IGF-1 resistance (Dardevet et al. 1994). Using a transgenic mouse model overexpressing IGF-1 exclusively in skeletal muscle (Coleman et al. 1995) we found increased levels of binding sites for the dihydropyridine PN200-110 (Renganathan et al. 1997b).
In the present study we examined the influence of IGF-1 on charge movement in cultured muscle cells. The role of IGF-1 in the biochemical expression of the L-type Ca2+ channel α1-subunit was assessed by a high-affinity radioligand binding assay in skeletal muscle cells. The influence of IGF-1 on α1s gene expression was examined in myoballs by combining patch clamp and single cell antisense RNA amplification.
METHODS
Skeletal muscle primary culture
Sprague-Dawley newborn rats (days 1 and 2) were decapitated and hindlimb muscles were dissected. Rats were housed in a pathogen-free area at Wake Forest University School of Medicine (WFUSM). Animal handling and procedures followed an approved protocol by the Animal Care and Use Committee of WFUSM. Cell dissociation and culture followed described procedures (Neville et al. 1997) with some modifications. Briefly, hindlimb muscles from one to three pups were dissected in sterile cold Hanks' balanced salt solution (HBSS; Ca2+, Mg2+ free). Minced tissue was incubated in HBSS containing 0·25 % trypsin at 37°C for 10 min and then triturated with Pasteur pipettes of different tip sizes. Visible clumps were separated from dispersed cells using a nylon mesh cell strainer (Falcon no. 2360, Gibco). The collected flow-through was centrifuged (200 g) for 5 min and the pellet was resuspended in Dulbecco's modified Eagle's medium (DMEM) containing 10 % fetal calf serum (FCS), 200 mM glutamine and 12·5 u ml−1 penicillin-streptomycin (plating medium). Cells were plated on glass coverslips coated with 1 % gelatin and incubated in 5 % CO2. After 2 days, cells were transferred into DMEM containing 2 % horse serum (differentiation medium). Human recombinant IGF-1 (RBI; 20 ng ml−1) was added to the Petri dishes daily after the cells had been transferred to differentiation medium. Four days after cell plating 10 μM cytosine-β-D-arabino-furanoside (AraC; Sigma) was added to the cultured cells for 24 h. AraC was used to select against proliferating cell types (i.e. fibroblasts) and generated a pure myotube culture (Neville et al. 1997). Myotubes exhibited spontaneous contractions after 2-3 days in differentiation medium. The experiments were carried out 3 days after cell transfer to differentiation medium.
Charge movement and Ca2+ current recordings
Coverslips were mounted in a small flow-through Lucite chamber positioned on a microscope stage. Cells were continuously perfused with external solution (see below) using a push-pull syringe pump (WPI, Saratoga, FL, USA). Spontaneously formed myoballs were used for electrophysiological recordings. Plenty of myoballs for experimentation were usually found after 2-3 days in differentiation medium. Cells were voltage clamped in the whole-cell configuration of the patch-clamp technique (Hamill et al. 1981) using an Axopatch 200A amplifier (Axon Instruments). Micropipettes were pulled from borosilicate glass (Boralex) using a Flaming Brown micropipette puller (P97, Sutter Instrument Co.) to obtain electrode resistances ranging from 2 to 4 MΩ. The composition of the internal solution (pipette) was (mM): 140 caesium aspartate, 5 magnesium aspartate, 10 Cs2EGTA and 10 Hepes; pH was adjusted to 7·4 with CsOH. The high concentration of Mg2+ in the pipette solution helped to maintain the stability of the preparation for a longer time. The external solution used for Ca2+ current recording contained (mM): 145 TEA-Br, 10 CaCl2, 10 Hepes and 0·001 tetrodotoxin (Beam & Knudson, 1988). Solution pH was adjusted to 7·4 with CsOH. For charge movement recordings, Ca2+ current was blocked with a solution containing (mM): 145 TEA-Br, 2 CaCl2, 0·5 Cd2+, 0·3 La3+, 10 Hepes and 0·001-0·003 tetrodotoxin (Adams et al. 1990).
Whole-cell currents were acquired and filtered at 5 kHz with pCLAMP 6.04 software (Axon Instruments). A Digidata 1200 interface (Axon Instruments) was used for A-D conversion. Membrane current during a voltage pulse, P, was initially corrected by analog subtraction of linear components. The remaining linear components were digitally subtracted on-line using hyperpolarizing control pulses of one-quarter test pulse amplitude (-P/4 procedure) (Bezanilla, 1985; Delbono, 1992). Four control pulses were applied before the test pulse. Charge movements were evoked by 12·5 ms depolarizing voltage steps from the holding potential (-80 mV) to command potentials ranging from -70 to 70 mV. Intramembrane charge movement was calculated as the integral of the current in response to depolarizing pulses (charge on, Qon) and is expressed per membrane capacitance (coulombs per farad). The complete blockade of the inward Ca2+ current was verified by the Qon - Qoff linear relationship. Membrane capacitance was calculated as the integral of the transient current in response to a brief hyperpolarizing pulse from -80 mV (holding potential) to -90 mV. The mean of the membrane capacitance of the myoballs used for electrophysiological experiments was 151 ± 21 and 140 ± 18 pF for control and IGF-1-treated cells, respectively.
[3H]PN200-110 binding assay in muscle cells
Control and IGF-1-treated muscle cells were used for a [3H]PN200-110 binding assay after 3 days of incubation in differentiation medium or differentiation medium plus 20 ng ml−1 IGF-1. Protein concentration was determined by the Coomassie protein assay with bovine serum albumin as the protein calibration standard. PN200-110 receptor concentration was determined using the radioligand [3H]PN200-110. Control and IGF-1-treated cells (0·1 mg protein ml−1) were incubated with 0·05-5 nM [3H]PN200-110 for 1 h at 23°C in 50 mM Tris-HCl pH 7·5, 10 μM Ca2+, 1 mM diisopropyl fluorophosphate (DIFP) and 5 μM leupeptin. Membrane bound [3H]PN200-110 was determined by filtration through Whatman GF/B filters using a Millipore unit (XX2702550, Millipore Co., Bedford, MA, USA). Filters were rinsed three times with 5 ml of ice-cold 200 mM choline chloride and 20 mM Tris-HCl, pH 7·5. Non-specific [3H]PN200-110 binding was assessed in the presence of 10 μM unlabelled nifidepine (Sigma). The radioligand concentrations used resulted in occupancy of > 95 % of the high-affinity binding sites (Anderson et al. 1994). Linear regression and non-linear least square analysis were used to calculate non-specific and total binding of the radioligand to the receptors. Specific binding of [3H]PN200-110 at each concentration was calculated by subtracting the non-specific binding from the total binding obtained from the above analysis. The following equation was used to fit the binding isotherm:
| (1) |
where y is bound [3H]PN200-110, x is free [3H]PN200-110, a is the receptor number(maximum binding capacity, Bmax), b is the dissociation constant (Kd) and c is the non-specific binding or the low-affinity site. Data are also given in a graphical representation of the Scatchard plot.
Single cell antisense RNA amplification
After current recording in the whole-cell configuration of the patch-clamp technique, the cytoplasmic contents of the cell were aspirated and processed for mRNA amplification according to described procedures (Eberwine et al. 1992; Messi et al. 1997), as follows. The cell contents were incubated for 1 h at 42°C with avian myeloblastosis virus (AMV) reverse transcriptase (RT) (Seikagaku, Rockville, MD, USA) at a final concentration of 7 U μl−1, plus 0·5 mM each dNTP (dATP, dCTP, dGTP and dTTP), 3 ng μl−1 of an oligodeoxynucleotide containing an oligo-dT region and the T7 RNA polymerase promoter (AAACGACGGCCAGTGAATTGTAATACGACTCACTATAGGCGC(T)24; Genosys, Woodlands, TX, USA), 10 × RT buffer (500 mM Tris pH 8·2, 1·2 M KCl, 100 mM MgCl2 and 0·5 mM sodium pyrophosphate), 10 mM dithiothreitol (DTT) and 1·3 U μl−1 RNasin (Promega, Madison, WI, USA). The oligo-dT region primes the mRNA for subsequent cDNA synthesis. Single-strand cDNA synthesis was followed by phenol-chloroform extraction and ethanol precipitation. The precipitate was dissolved in distilled water and heated at 95°C for 3 min. Second-strand synthesis was accomplished using DNA hairpinning to produce a primer for DNA synthesis. Specifically, first-strand DNA-RNA hybrid, 5 × second-strand buffer (500 mM Tris pH 7·4, 100 mM KCl, 50 mM MgCl2, 25 mM DTT and 1·5 M (NH4)2SO4), 0·25 mM each dNTP, 0·2 U μl−1 Klenow fragment of DNA polymerase I and 0·2 U μl−1 T4 DNA polymerase were combined and incubated at 14°C overnight. The DNA was next treated with 0·1 U μl−1 S1 nuclease in S1 buffer (10 ×: 2 mM NaCl, 0·5 M sodium acetate pH 4·5 and 10 mM ZnSO4) for 5 min at 37°C to eliminate the hairpin formed in the second-strand synthesis reaction. After S1 nuclease treatment, the DNA was phenol-chloroform extracted, ethanol precipitated, and resuspended in distilled water. The cDNA was incubated at 37°C for 15 min in KFI buffer (10 ×: 200 mM Tris pH 7·5, 100 mM MgCl2, 50 mM DTT and 50 mM NaCl) plus 0·25 mM dNTPs and 0·2 U μl−1 Klenow. This incubation was followed by phenol-chloroform extraction and ethanol precipitation and the pellet was dissolved in 10 μl of TE buffer (10 mM Tris, 1 mM EDTA, pH 8·2). The cDNA was drop dialysed using a 0·025 μm pore filter (Millipore) against TE buffer for 4 h. This step removes the free dNTPs remaining from the prior reactions. This was followed by first round amplification using T7 RNA polymerase. The antisense RNA was synthesized from the cDNA template via an antisense oligonucleotide to the poly(A) RNA. For this RNA amplification, 10 × T7 RNA buffer (400 mM Tris pH 7·5, 7 mM MgCl2, 100 mM NaCl and 20 mM spermidine), 0·05 mM each DTT, 0·125 mM ATP, GTP, UTP and CTP, 1·3 U μl−1 RNasin and 1250 U μl−1 T7 RNA polymerase (Epicentre Technology, Madison, WI, USA) were added to the cDNA and incubated at 37°C for 4 h. Antisense RNA products were converted to cDNA using random primers and AMV reverse transcriptase in a second round of amplification. Single-stranded cDNA was primed with the oligo-dT-T7 RNA polymerase promoter oligonucleotide and another pool of double-stranded cDNA prepared as a template for amplification. In this amplification, radiolabelled CTP ([α-32P]CTP) was incorporated into the mRNA to generate a probe for hybridization (Eberwine et al. 1992). The contents of control and IGF-1-treated cells were labelled simultaneously with the same batch of [α-32P]CTP.
The cDNA encoding the L-type Ca2+ channel α1-subunit (Tanabe et al. 1987; Sanford et al. 1991) was subcloned into the transcription competent vector pAGA2 (modified pGEM3) (Sanford et al. 1991; Wei et al. 1991), which was linearized with HindIII. A fragment of 1037 bp corresponding to the α1-subunit domain I was obtained with EcoRI digestion and used for hybridization onto nylon membranes (see below). This fragment has a very low homology with other clones expressed in skeletal muscle according to database sequence analyses using the Wisconsin package software (Wisconsin, Madison, WI, USA). The L-type Ca2+ channel α1s-subunit together with a 448 bp fragment of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) clone (Ambion Inc., Austin, TX, USA) were run on a 1 % agarose gel and transferred to Magnacharge nylon membranes (MSI, Westborough, MA, USA). Membranes were hybridized with the labelled probes in Church buffer (7 % of 20 % SDS, 0·25 M NaH2PO4, 0·25 M Na2HPO4 and 1 mM EDTA) overnight at 52°C in a hybridization oven. A Betascope blot analyser was used for beta-scanning of the membranes. Membranes resulting from antisense RNA/cDNA reverse Northern blots of control and IGF-1-treated cells were exposed simultaneously to the same X-ray film for 48 h and digitally scanned. The mean density of pixels across the band width was integrated over the band height using an optical scanner. For this analysis a Pdi imaging system together with Quantity One 2.6 software (Huntington Station, NY, USA) was used. The magnitude of hybridization to the 1037 bp band was normalized to the signal recorded from the 448 bp band corresponding to the GAPDH clone. Results are expressed as the integral of the optical density (OD × mm). The sequence of the L-type Ca2+ channel α1s fragment was determined by DNA sequencing performed on a Perkin Elmer (Norwark, CT, USA) ABI Prism 377.
Data values are given as means ±s.e.m. with the number of observations (n). Experimental groups were statistically analysed using Student's unpaired t test, and P < 0·05 was considered significant.
RESULTS
IGF-1 potentiates skeletal muscle charge movement
Charge movement was recorded after blocking the inward Ca2+ current with a solution containing a mixture of Cd2+ and La3+ (see Methods). The effects of IGF-1 on charge movement were studied simultaneously in treated and control cells at different days after cell transfer to differentiation medium. Figure 1 illustrates charge movement in both groups of cells in response to increasing depolarizing voltage pulses. Myoballs were clamped at a holding potential of -80 mV. Charge movement was evoked by 12·5 ms depolarizing voltage steps to command potentials ranging from -70 to 70 mV (Fig. 1A). Single traces of charge movement in control cells and in cells incubated for 3 days in 20 ng ml−1 IGF-1 are shown in Fig. 1B and C. Charge movement became measurable at -70 to -60 mV and saturated at 40 to 50 mV for control and test groups (see below). Only traces from -40 to 40 mV with a 20 mV interval are illustrated in Fig. 1. The IGF-1 concentration used in this study is about fourfold the EC50 for DHP-sensitive Ca2+ current potentiation in adult single skeletal muscle fibres (EC50= 5·6 ± 1·8 nM) (Delbono et al. 1997). IGF-1 potentiated charge movement in the whole range of potentials studied. These effects increased abruptly within the first 24 h of incubation in the growth factor and declined steeply after the interruption of IGF-1 administration (see below).
Figure 1. IGF-1 potentiates charge movement in muscle cells.
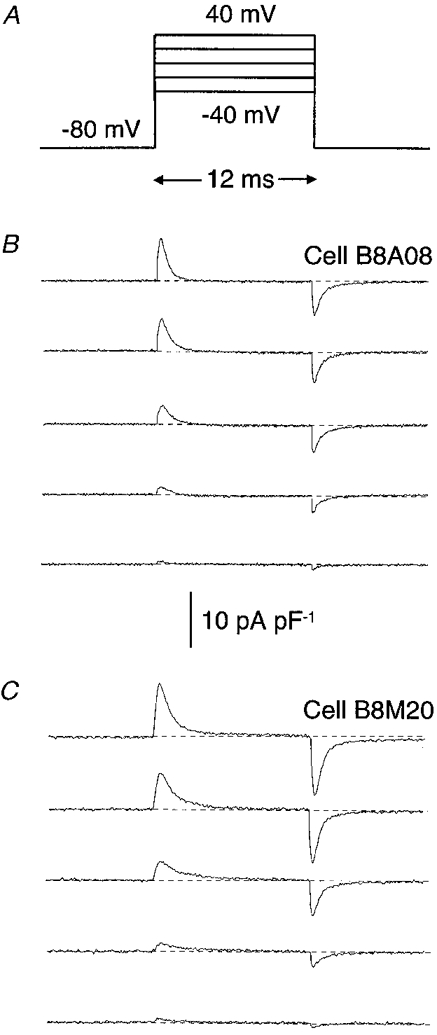
A, charge movement records in myoballs in response to 12 ms pulses from a holding potential of -80 mV to the voltages indicated. Single traces of charge movement in control conditions (B) are compared with recordings obtained from cells incubated for 3 days in 20 ng ml−1 IGF-1 (C). The baseline is shown as a dashed line. The cells used for the illustration exhibited similar linear capacitance: 128 and 123 pF for cells B8A08 and B8M20, respectively.
IGF-1 potentiates charge movement without changing the voltage distribution
IGF-1 did not significantly change the voltage distribution for charge movement. Figure 2 shows the Qon-membrane potential relationship for the range of potentials studied. Curves were fitted with a single Boltzmann equation of the form:
| (2) |
where Qmax is the maximum charge that can be moved, V is the test potential, V½Q is the potential at which half the charge has moved and K is a slope factor. IGF-1 potentiated charge movement. The fractional potentiation of charge movement elicited was 70 ± 5·1 % at 50 mV. The means of best-fit parameters describing the voltage dependence of charge movement in control cells (n= 41) were Qmax= 12·9 ± 0·4 nC μF−1, V½Q= 18·5 ± 0·7 mV and K= 14·0 ± 0·13. In IGF-1-treated cells (n= 52) the values were Qmax= 22·0 ± 0·3 nC μF−1 (P < 0·01), V½Q= 16·8 ± 0·8 mV (P > 0·5) and K= 13·3 ± 0·15 (P > 0·5).
Figure 2. IGF-1-dependent potentiation of charge movement.
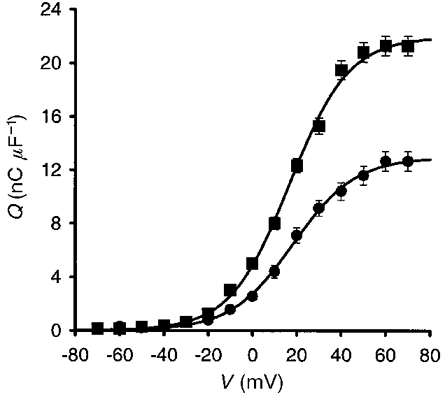
Charge movement-voltage relationship for control cells (•) and cells incubated in 20 ng ml−1 IGF-1 (▪). IGF-1 potentiates charge movement elicited by the pulse protocol illustrated in Fig. 1A. Experimental points were fitted to eqn (2). For the means of best-fit parameters describing the voltage dependence of charge movement, see text.
The significant increase in Qmax without significant changes in V½Q and K support the concept that IGF-1 increases the number of charges in the membrane and does not alter the voltage dependence of the channels.
Time course of IGF-1 potentiation of charge movement
The time course of IGF-1 effects on charge movement is represented in Fig. 3. To examine the onset and reversibility of IGF-1 effects, cells were studied at 3, 6 and 12 h and then daily until 6 days after the addition of IGF-1 to the culture medium. At day 6, the administration of IGF-1 was discontinued and the cells were studied until day 10.
Figure 3. Time course of IGF-1 effects on charge movement.
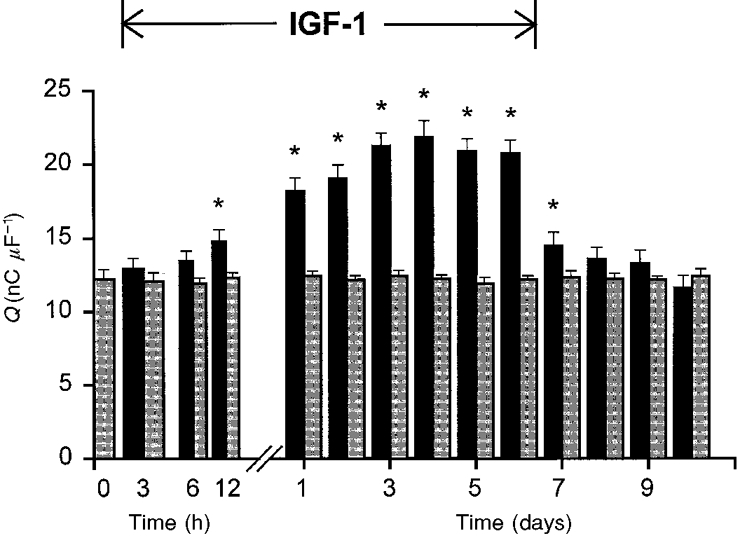
Charge movement (Qmax) was recorded in cells incubated for 6 days in 20 ng ml−1 IGF-1 (black bars) and in control cells (grey bars) studied at the same time of culture. The time of exposure to IGF-1 is shown on the x-axis. The 10 s intervals between pulses are not shown. Myoballs were also studied for 4 days after the interruption of IGF-1 administration. The number of cells studied for each group varied from 10 to 14. Asterisks indicate statistically significant differences between the two groups (P < 0·05).
A significant potentiation of charge movement was recorded within the first 24 h of incubation in IGF-1. Charge movement progressively increased up to day 3 to reach a plateau until day 6. A steep decline in the potentiation of charge movement was recorded 1 day after the suspension of IGF-1 administration. Charge movement was not significantly different from control from day 8 to day 10. The onset and recovery of the effect described in Fig. 3 is consistent with a modulatory action of IGF-1 on gene expression of the channels involved in charge movement.
Lack of acute modulatory effects of IGF-1 on charge movement
Because a significant increase in L-type Ca2+ channel expression was recorded within the first day of incubation in IGF-1 (Fig. 3), we investigated whether the growth factor has an acute modulatory effect on charge movement as demonstrated before for the DHP-sensitive Ca2+ current (Delbono et al. 1997). Figure 4 illustrates the lack of IGF-1 effects on charge movement. Figure 4A shows a compilation of 100 charge movement records obtained in a myoball in response to 12·5 ms pulses from a holding potential of -80 mV to 20 mV with 10 s intervals. The first four responses were recorded in control conditions. Between pulses 4 and 5 the bathing solution was completely replaced by a bathing solution containing 20 ng ml−1 IGF-1 and maintained for the remaining pulses (top bar). Exposure to the growth factor did not evoke upregulation of charge movement. The lack of IGF-1 effect on the integral of gating currents was compared with the spontaneous changes in charge movement recorded in control cells (Fig. 4B). Charge movement normalized to the mean of the first four pulses at 15 min of incubation in IGF-1 or in control solution (no IGF-1 added) was 0·81 ± 0·17 (n= 10) and 0·62 ± 0·15 (n= 14), respectively (P > 0·05). The progressive decline in charge movement density was associated with a decrease in cell capacitance. The ratio of cell capacitance at the beginning (mean of the first 4 pulses) to that at the end of the experiment (pulse 100) was 0·89 ± 0·16 and 0·84 ± 0·17 for control and IGF-1-treated cells, respectively (P > 0·5). This suggests that cell detubulation may account for the decline in the integral of charge movement.
Figure 4. Lack of acute effects of IGF-1 on charge movement.
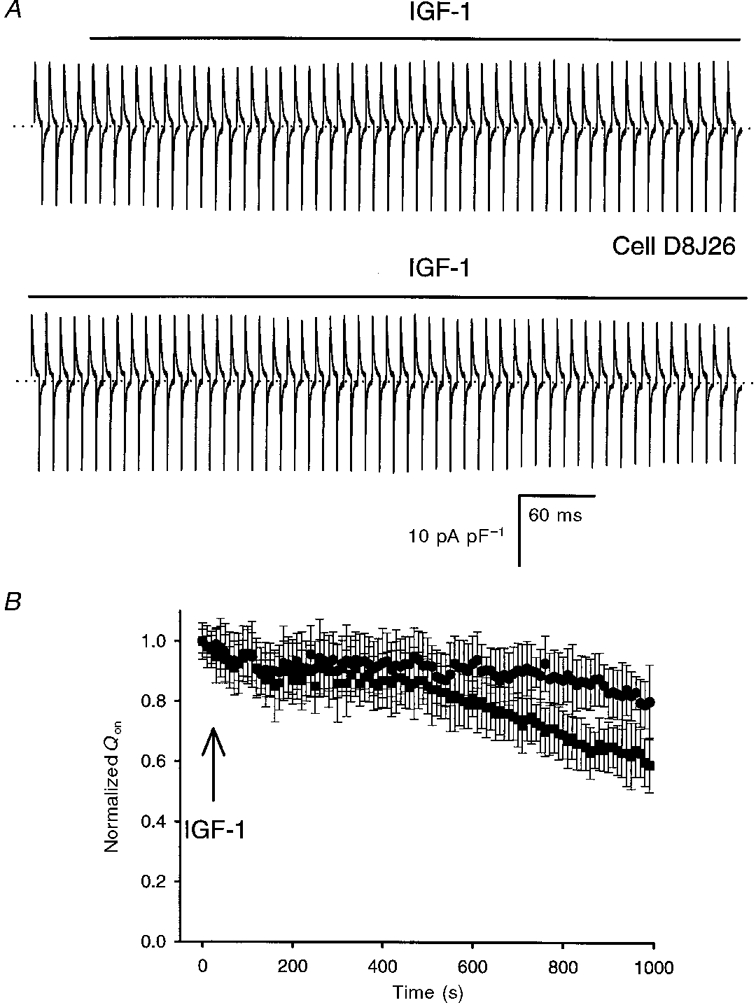
A, continuous recording of charge movement in response to 12 ms depolarizations from a holding potential of -80 mV to 20 mV with 10 s intervals (not shown; total recording time, 16 min). The bar above the traces indicates the presence of 20 ng ml−1 IGF-1 in the bathing solution. B, time dependence of normalized Qon in control (▪) and in cells exposed to 20 ng ml−1 IGF-1 (•). The arrow indicates the beginning of the exposure to IGF-1. The first 4 traces in control conditions (A) were averaged and used for normalization of the charge movement recorded after adding IGF-1.
IGF-1 potentiates Ca2+ current through L-type Ca2+ channels
We reported previously that acute exposure to IGF-1 enhances the amplitude of the inward Ca2+ current through DHP-sensitive L-type Ca2+ channels without affecting the voltage sensor in mature single mouse muscle fibre (Delbono et al. 1997). These results suggest that IGF-1 facilitates channel activation by exerting an acute modulatory effect on the L-type Ca2+ channel structures involved in calcium ion permeation, rather than via an effect on the voltage sensor (Delbono et al. 1997). In the present work we tested the hypothesis that the increase in charge movement is associated with a significant increase in the amplitude of the Ca2+ current. Ca2+ current was recorded in the whole-cell configuration of the patch-clamp technique. Ca2+ currents were evoked by 1·8 s depolarizing voltage steps to command potentials ranging from -70 to 50 mV. Figure 5A and B illustrates Ca2+ current traces recorded in control (Fig. 5A) and in cells treated with IGF-1 for 3 days (Fig. 5B). Figure 5C shows the current-voltage relationship for the complete voltage range (-70 to 80 mV). A total of 35 and 40 cells were studied for control and IGF-1-treated groups, respectively. A fast inactivating inward current that exhibited a maximum amplitude between -70 and -60 mV (Beam & Knudson, 1988) was recorded in control and IGF-1-treated cells. The slow Ca2+ current was significantly potentiated at positive potentials (10-50 mV) (Fig. 5A and B). In contrast to observations in cells acutely treated with IGF-1 (Delbono et al. 1997), no change in the Ca2+ current-voltage relationship was observed in cells chronically treated with the growth factor. Therefore, potentiation of the Ca2+ current density in cells chronically exposed to IGF-1 may result from a net increase in the number of Ca2+ channels (see below), in the fraction of channels that are available for opening, or in the open probability of each channel. These last two mechanisms mediate the IGF-1-dependent regulation of Ca2+ channel function described in a previous publication (Delbono et al. 1997). A group of experiments was performed to determine whether cultured myoballs respond to acute exposure to IGF-1 as demonstrated for mature muscle fibres (Delbono et al. 1997). The ratio between Ca2+ current amplitude after 30 min of incubation in 20 ng ml−1 IGF-1 and the control Ca2+ current amplitude (before exposure to IGF-1) was 1·5 ± 0·17 (n= 14). Control experiments showed a ratio between peak Ca2+ current amplitude after external solution exchange (no IGF-1 added) and peak Ca2+ current before solution exchange of 0·6 ± 0·08 (n= 15). These results show that IGF-1 not only prevents the spontaneous decrease, it also significantly potentiates the amplitude of Ca2+ flux through L-type Ca2+ channels in myoballs. We also examined the acute effects of IGF-1 on Ca2+ current in myoballs incubated in 20 ng ml−1 IGF-1 for 3 days. The growth factor was removed from the culture medium and 1 h later the myoballs were acutely perfused with 20 ng ml−1 IGF-1. The ratio between Ca2+ current amplitude after 30 min of acute exposure to 20 ng ml−1 IGF-1 and the control Ca2+ current amplitude (before acute exposure to IGF-1) was 1·4 ± 0·19 (n= 15). Although this acute and chronic exposure to IGF-1 results in Ca2+ current potentiation, the results are not significantly different from those recorded in myoballs either acutely exposed to IGF-1 or chronically incubated in the growth factor.
Figure 5. IGF-1 potentiates slow Ca2+ current.
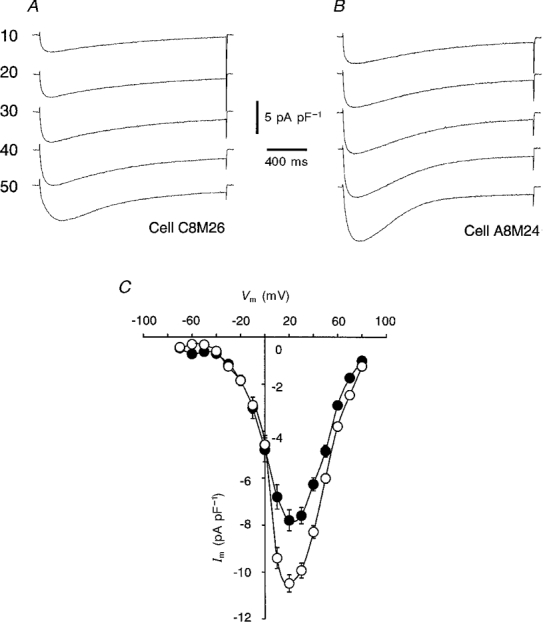
Slow Ca2+ current was recorded in control (A) and IGF-1-treated (B) cells. Pulses of 1·8 s duration from a holding potential of -80 mV to 10-50 mV were applied. The inward Ca2+ current-voltage relationship (Im, membrane current; Vm, membrane voltage) from -70 to 80 mV for both groups of cells is depicted in C (•, control; ^, IGF-1).
To determine whether the potentiation of the slow Ca2+ current results from the insufficient reversal of the acute effect of IGF-1 and/or from a net increase in the number of L-type Ca2+ channels, Ca2+ current density was studied in myoballs after brief or prolonged exposure to IGF-1. The Ca2+ current density was recorded 30-60 min after the suspension of growth factor administration to myoballs incubated for 30 min or 3 days in 20 ng ml−1 IGF-1. The recordings were performed 30-60 min after growth factor washout because this was the time at which the experiments shown in Fig. 5 were done. The ratio between Ca2+ current amplitude after 30 min or 3 days of exposure to IGF-1 and the control Ca2+ current amplitude (before acute exposure to IGF-1) was 1·03 ± 0·11 (n= 14) and 1·45 ± 0·12 (n= 15), respectively. These results show that the slow current density returns to control values after a brief incubation in IGF-1; however, the significant potentiation persists when the cells are incubated for 3 days.
IGF-1 increases the number of L-type Ca2+ channel α1-subunits
The concentration of L-type Ca2+ channel α1-subunits and their dissociation constant for the high-affinity radioligand [3H]PN200-110 were determined in control muscle cells and in myoballs treated with 20 ng ml−1 IGF-1 for 3 days (10 determinations in 3 different preparations). Control and treated cells were examined simultaneously 6 days after plating. The non-specific binding was quantified for both groups. The non-specific/specific binding ratio for control and IGF-1-treated cells was 0·28 ± 0·02 and 0·166 ± 0·01, respectively. Figure 6 shows [3H]PN200-110 binding to control (•) and IGF-1-treated cells (▪). The inset of Fig. 6 shows Scatchard analyses of ligand binding to the L-type Ca2+ channel α1-subunit. [3H]PN200-110 Bmax and Kd values were calculated from Scatchard analyses of the binding assay for ten determinations in three different preparations. [3H]PN200-110 Bmax values for control and IGF-1-treated cells were 1·21 ± 0·25 and 3·15 ± 0·5 pmol (mg protein)−1, respectively (P < 0·05). The difference in maximum binding capacity cannot be explained by changes in the dissociation constant of the receptor. The receptor Kd values for control and IGF-1-treated cells were 3·36 ± 1·04 and 2·37 ± 0·88 nM, respectively (P > 0·05). The values determined for the number of channels and the dissociation constant for [3H]PN200-110 in primary cultured muscle cells are in agreement with studies done in transgenic muscle fibres from adult mice overexpressing hIGF-1 (Renganathan et al. 1997b) (see below). These experiments demonstrate that IGF-1 significantly potentiates the number of L-type Ca2+ channel α1-subunits in muscle cells.
Figure 6. IGF-1 increases the number of [3H]PN200-110 binding sites.
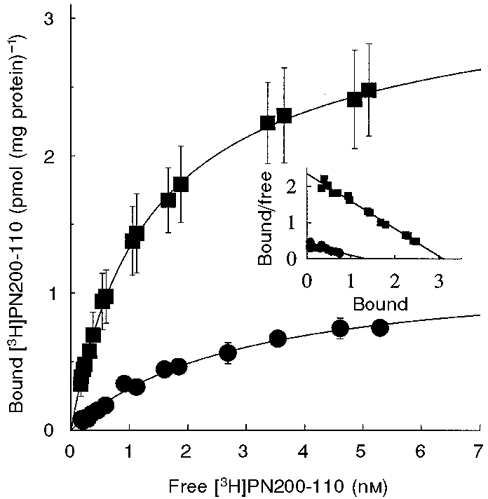
Determination of the number of L-type Ca2+channels and the dissociation constant for [3H]PN200-110 in primary cultured muscle cells in the absence (control, •) and presence of IGF-1 (IGF-1-treated cells, ▪). Inset, Scatchard analysis of ligand binding to the L-type Ca2+ channel α1-subunit. [3H]PN200-110 Bmax and Kd values were calculated from Scatchard analyses of the binding assay for 10 determinations in 3 different preparations. IGF-1-treated cells exhibit higher [3H]PN200-110 maximum binding capacity than control without significant changes in the dissociation constant of the receptor (see text).
IGF-1 promotes L-type Ca2+ channel expression by enhancing gene expression
To determine whether IGF-1 increases the number of L-type Ca2+ channel α1-subunits via the activation of α1s gene expression, we combined the whole-cell configuration of the patch-clamp technique with an antisense RNA amplification technique. The amplified mRNA from a group of control and IGF-1-treated cells was labelled with [α-32P]CTP and hybridized to a fragment of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA and L-type Ca2+ channel α1s cDNA that had been immobilized on nylon membranes. Figure 7A shows the 448 bp GADPH and 1037 bp α1s cDNA fragments on a 1 % agarose gel. A 1 kb cDNA ladder (1 kb DNA ladder, Promega) (Fig. 7Aa) together with analysis of the restriction map were used to determine the size of the α1s cDNA fragment. The magnitude of hybridization was measured by digital scanning of membranes exposed to X-ray films (see Methods). Figure 7 illustrates the magnitude of the hybridization to the 448 and 1037 bp bands for control (Fig. 7B and C) and IGF-1-treated cells (Fig. 7D and E). We found that the level of GAPDH mRNA did not change significantly in cells treated with IGF-1 for 3 days (cells treated with IGF-1/non-treated cells = 0·98 ± 0·05; n= 15). Therefore, the signal at the 448 bp band, corresponding to the GAPDH clone, was used for normalization of the signal recorded at the 1037 bp L-type Ca2+ channel α1-subunit band. It is apparent that the level of L-type Ca2+ channel α1s mRNA was higher in cells treated with IGF-1 than in control. This observation was confirmed in 12 control cells and 12 cells treated with 20 ng ml−1 IGF-1 for 3 days. The ratio of the IGF-1-treated cells to control cells resulting from optical scanning of the hybridization signal at the 1037 bp band was 2·01 ± 0·18. Because the level of [3H]PN200-110 binding sites and mRNA for the L-type Ca2+ channel α1-subunit were undetectable in muscle cells cultured for 1-2 days in plating medium (Kyselovic et al. 1994) these early cultures were used as a negative control for hybridization tests. No hybridization was detected in any of the ten cells studied at this time of culture. These results support the concept that IGF-1 increases the number of L-type Ca2+ channel α1-subunits by enhancing α1s gene expression.
Figure 7. IGF-1 enhances L-type Ca2+ channel α1-subunit mRNA levels in single muscle cells.
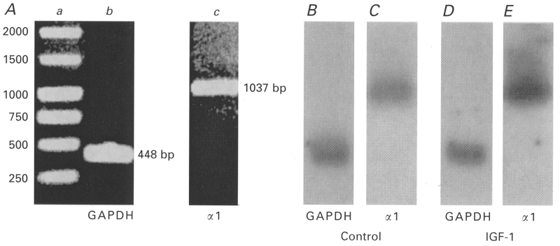
A, 1 % agarose gel showing DNA ladder (a), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA fragment (448 bp; b) and L-type Ca2+ channel α1s cDNA fragment (1037 bp; c). B-E, digital scanning of X-ray films illustrating the magnitude of the hybridization to the 448 and 1037 bp bands for control (B and C) and IGF-1-treated cells (D and E). Data were normalized to the hybridization signal at the GAPDH band. It is apparent that the level of L-type Ca2+ channel α1s mRNA is higher in IGF-1-treated cells than in control.
DISCUSSION
In this study we report that physiological concentrations of IGF-1 enhance α1s gene expression, leading to an absolute increase in the number of L-type Ca2+ channels and potentiation of charge movement and Ca2+ current in cultured rat skeletal muscle cells.
Charge movement and Ca2+ current in skeletal muscle primary culture
Charge movement recorded in the present work shows some similarities and discrepancies with the data recorded in mouse myotubes. K values in rat myoballs were within the range of values reported for mouse muscle cells, whereas Qmax was twofold higher and V½Q was shifted about 10 mV to more positive potentials (Adams et al. 1990; Garcia et al. 1994). Differences in the stimulation pulse protocol, animal species and stage of cell development at the time of electrophysiological recordings, and variations in the culture technique such as culture medium, coating materials and cell density, should be considered.
Qmax and K values were similar to those reported for adult rat cut muscle fibres recorded in a double Vaseline gap system (Lamb, 1986; Delbono, 1992; Delbono et al. 1997); however, the V½Q value was less negative in the present study. The voltage of the half-maximum value of charge movement recorded with the Vaseline gap technique is more negative than that recorded in mature muscle fibres using a microelectrode voltage-clamp technique (Hollingworth et al. 1990) and in cultured myotubes studied with the whole-cell configuration of the patch-clamp technique (Garcia et al. 1994). Although the stage of cell development and the Ca2+ concentration in the bathing solution account partially for the difference in V½Q, differences inherent to the methodology employed should be considered. A less polarized membrane under the Vaseline seals may contribute to the shift of the Q-V relationship in the voltage axis (Chandler & Hui, 1990).
Ca2+ currents recorded here show a similar voltage dependence to those reported for mouse (Beam & Knudson, 1988) and rat (Fleig & Penner, 1995) myotubes. The current density was in the same range as values recorded in mouse myotubes in response to increasing depolarizing voltage steps (Beam & Knudson, 1988), and was twofold higher than the values obtained in response to a ‘charge immobilizing’ protocol (Garcia et al. 1994).
IGF-1 potentiates charge movement and Ca2+ current in skeletal muscle cells
IGF-1-evoked potentiation of charge movement was associated with a significant increase in the number of DHP receptors and an increase in the L-type Ca2+ current. Based on these findings we postulate that IGF-1 enhances the charge movement associated with L-type Ca2+ channels. However, due to the multiple sources of the charge movement, effects of IGF-1 on the number of Na+ and T-type Ca2+ channels, among others, cannot be ruled out. The onset, persistence and decline of IGF-1 effects on charge movement suggest that the number of functional channels expressed in the sarcolemma increases in the presence of the growth factor. This may happen by two basic mechanisms, changes in protein turnover and/or gene expression. High-affinity binding assays together with antisense RNA amplification studies support a gene-mediated process triggered by IGF-1.
Potentiation of Ca2+ current in IGF-1-pretreated or control myoballs acutely exposed to IGF-1 indicates that neonatal muscle cells respond to the trophic factor in a similar way to mature rat skeletal muscle fibres. The experiments of acute exposure to IGF-1 in control and in cells pretreated with IGF-1 show that cultured myoballs retain the ability to respond to the growth factor.
To determine whether the potentiation of the Ca2+ current results from insufficient reversal of the acute effect of IGF-1 and/or a net increase in the number of L-type Ca2+ channels, the current density was measured in myoballs exposed to the growth factor for brief or prolonged periods. The complete return to control values in myoballs exposed for 30 min to IGF-1 shows that the direct modulatory effect of the growth factor on this channel is transient, reversible and briefer than in adult mouse muscle fibres (Delbono et al. 1997). The persistence of the current potentiation in myoballs incubated for longer periods, together with the results of the radioligand binding assays, supports the concept that a net increase in the number of channels underlies the potentiation of the slow Ca2+ current shown in Fig. 5.
Acute exposure to IGF-1 enhances Ca2+ current through DHP-sensitive Ca2+ channels in mature skeletal muscle fibres. This effect has been recorded for 30 min and has been associated with a facilitation of current activation by shifting the current-voltage relationship to more negative potentials (Delbono et al. 1997). In the present work, a clear increase in the number of DHP receptors expressed in the sarcolemma was also associated with slow Ca2+ current potentiation; however, no change in the voltage dependence of the current activation was recorded. The increase in the number of channels without changes in their pharmacological properties for the high-affinity radioligand (PN200-110; see below) supports the view that the new population of channels was similar to that existing before IGF-1 treatment. Therefore, no changes in the current-voltage relationship are expected. These results may indicate that the activation of the tyrosine kinase-PKC cascade at the beginning of the cell exposure to IGF-1 is balanced by activation of phosphatases (LeRoith et al. 1995). Therefore, cells chronically exposed to IGF-1 manifest primarily the effect of the growth factor on gene expression. The differential IGF-1-evoked potentiation of Bmax (260 %), Ca2+ current (35 %) and Qmax (70 %) could be explained by the fact that not all the L-type Ca2+ channels pharmacologically detected function as voltage sensors and changes in unitary conductance and/or open probability may alter the macroscopic current.
Whether IGF-1-dependent potentiation of charge movement results in effective increases in SR Ca2+ release and more forceful muscle contractions remains to be assessed. Changes in cytosolic Ca2+ concentration in cultured mouse and rat myotubes have been measured (Bakker et al. 1996). Whether the new DHP receptors participate in the process of sarcolemmal-sarcoplasmic reticulum Ca2+ release coupling cannot be established at this moment. The fact that the number of DHP-sensitive L-type Ca2+ channels decreases with age (Renganathan et al. 1997a) suggests that the administration of IGF-1 may ameliorate this deficit. Age-related IGF-1 resistance is associated with decreases in the level of circulating IGF-1 (Sonntag et al. 1992), and therefore IGF-1 replacement can delay and/or decrease the age-related downregulation of this molecule at older ages (Delbono et al. 1995).
Mechanisms of IGF-1 potentiation of charge movement
This work demonstrates that IGF-1 is able to enhance charge movement significantly within the first day of exposure to the growth factor. In this study we also showed that IGF-1 induces α1s gene expression. However, the mechanism by which the trophic factor activates DNA transcription remains unknown. It has been shown that IGF-1 regulates the transcription of a number of genes encoding proteins involved in growth and metabolism (Florini & Magri, 1989). Immediate early genes such as c-fos and c-jun associated with muscle cell proliferation are activated by IGF-1 (Angel et al. 1988). These may be the early events leading to binding of the products of fos and jun protein dimerization to the DNA consensus sequence known as the tetradecanoylphorbol acetate (TPA) response element (Rosenzweig et al. 1994). Further studies on the activation of sarcolemma-nucleus signalling activated by IGF-1 might help to clarify the mechanism(s) by which this growth factor mediates increases in L-type Ca2+ channel α1-subunit expression.
Acknowledgments
This work was supported by National Institutes of Health/National Institute on Aging Grants AG00692, AG13934, AG10484 and AG15820 to Osvaldo Delbono. Dr L. Birnbaumer provided the L-type Ca2+ channel α1-subunit cDNA. We thank Elyse Jung for assistance in DNA sequencing.
References
- Adams BA, Tanabe T, Mikami A, Numa S, Beam KG. Intramembrane charge movement restored in dysgenic skeletal muscle by injection of dihydropyridine receptor cDNAs. Nature. 1990;346:569–572. doi: 10.1038/346569a0. 10.1038/346569a0. [DOI] [PubMed] [Google Scholar]
- Anderson K, Cohn AH, Meissner G. High-affinity [3H]PN200–110 and [3H]ryanodine binding to rabbit and frog skeletal muscle. American Journal of Physiology. 1994;266:C462–466. doi: 10.1152/ajpcell.1994.266.2.C462. [DOI] [PubMed] [Google Scholar]
- Angel P, Hattori K, Smeal T, Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988;55:875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- Bakker AJ, Head SI, Stephenson DG. Measurement of membrane potential and myoplasmic [Ca2+] in developing rat myotubes at rest and in response to stimulation. Cell Calcium. 1996;19:409–418. doi: 10.1016/s0143-4160(96)90114-1. [DOI] [PubMed] [Google Scholar]
- Beam KG, Knudson CM. Effect of postnatal development on calcium currents and slow charge movement in mammalian skeletal muscle. Journal of General Physiology. 1988;91:799–815. doi: 10.1085/jgp.91.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F. Gating of sodium and potassium channels. Journal of Membrane Biology. 1985;88:97–111. doi: 10.1007/BF01868424. [DOI] [PubMed] [Google Scholar]
- Blair LAC, Marshall J. Igf-1 modulates N and L calcium channels in a PI 3-kinase-dependent manner. Neuron. 1997;19:421–429. doi: 10.1016/s0896-6273(00)80950-2. [DOI] [PubMed] [Google Scholar]
- Chandler WK, Hui CS. Membrane capacitance in frog cut twitch fibers mounted in a double Vaseline-gap chamber. Journal of General Physiology. 1990;96:225–256. doi: 10.1085/jgp.96.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman ME, DeMayo F, Yin KC, Lee HM, Geske R, Montgomery C, Schwartz RJ. Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. Journal of Biological Chemistry. 1995;270:12109–12116. doi: 10.1074/jbc.270.20.12109. 10.1074/jbc.270.20.12109. [DOI] [PubMed] [Google Scholar]
- Dardevet D, Sornet C, Attaix D, Baracos VE, Grizard J. Insulin-like growth factor-1 and insulin resistance in skeletal muscles of adult and old rats. Endocrinology. 1994;134:1475–1484. doi: 10.1210/endo.134.3.8119189. 10.1210/en.134.3.1475. [DOI] [PubMed] [Google Scholar]
- Delbono O. Calcium current activation and charge movement in denervated mammalian skeletal muscle fibres. The Journal of Physiology. 1992;451:187–203. doi: 10.1113/jphysiol.1992.sp019160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbono O, O'Rourke K, Ettinger WH. Excitation-contraction uncoupling in aged single human skeletal muscle fibers. Journal of Membrane Biology. 1995;148:211–222. doi: 10.1007/BF00235039. [DOI] [PubMed] [Google Scholar]
- Delbono O, Renganathan M, Messi ML. Regulation of mouse skeletal L-type Ca2+ channel by activation of the insulin-like growth factor-1 receptor. Journal of Neuroscience. 1997;17:6918–6928. doi: 10.1523/JNEUROSCI.17-18-06918.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbono O, Sonntag WE. Influence of insulin like growth factor-1 (IGF-1) on dihydropyridine receptor (DHPR) function and SR calcium release on rat skeletal muscle fibers at different ages. Biophysical Journal. 1996;70:A38. [Google Scholar]
- Eberwine J, Yeh H, Miyashiro K, Cao Y, Nair S, Finnell R, Zettel M, Coleman P. Analysis of gene expression in single live neurons. Proceedings of the National Academy of Sciences of the USA. 1992;89:3010–3014. doi: 10.1073/pnas.89.7.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleig A, Penner R. Excessive repolarization-dependent calcium currents induced by strong depolarizations in rat skeletal myoballs. The Journal of Physiology. 1995;489:41–53. doi: 10.1113/jphysiol.1995.sp021028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florini JR, Ewton DZ, Coolican SA. Growth hormone and the insulin-like growth factor system in myogenesis. Endocrine Reviews. 1996;17:481–517. doi: 10.1210/edrv-17-5-481. 10.1210/er.17.5.481. [DOI] [PubMed] [Google Scholar]
- Florini JR, Magri KA. Effects of growth factors on myogenic differentiation. American Journal of Physiology. 1989;256:C701–711. doi: 10.1152/ajpcell.1989.256.4.C701. [DOI] [PubMed] [Google Scholar]
- Garcia J, Tanabe T, Beam KG. Relationship of calcium transients to calcium currents and charge movements in myotubes expressing skeletal and cardiac dihydropyridine receptors. Journal of General Physiology. 1994;103:125–147. doi: 10.1085/jgp.103.1.125. 10.1085/jgp.103.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hollingworth S, Marshall MW, Robson E. The effects of tetracaine on charge movement in fast twitch rat skeletal muscle fibres. The Journal of Physiology. 1990;421:633–644. doi: 10.1113/jphysiol.1990.sp017966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyselovic J, Leddy JJ, Ray A, Wigle J, Tuana BS. Temporal differences in the induction of dihydropyridine receptor subunits and ryanodine receptors during skeletal muscle development. Journal of Biological Chemistry. 1994;269:21770–21777. [PubMed] [Google Scholar]
- Lamb GD. Asymmetric charge movement in contracting muscle fibres in the rabbit. The Journal of Physiology. 1986;376:63–83. doi: 10.1113/jphysiol.1986.sp016142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRoith D, Werner H, Beitner-Johnson D, Roberts CT. Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocrine Reviews. 1995;16:143–160. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- Melzer W, Herrmann-Frank A, Lüttgau HC. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochimica et Biophysica Acta. 1995;1241:59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- Messi ML, Renganathan M, Grigorenko E, Delbono O. Activation of a7 nicotinic acetylcholine receptor promotes survival of spinal cord motoneurons. FEBS Letters. 1997;411:32–38. doi: 10.1016/s0014-5793(97)00600-5. [DOI] [PubMed] [Google Scholar]
- Meza U, Avila G, Felix R, Gomora JC, Cota G. Long-term regulation of calcium channels in clonal pituitary cells by epidermal growth factor, insulin, and glucocorticoids. Journal of General Physiology. 1994;104:1019–1038. doi: 10.1085/jgp.104.6.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville C, Rosenthal N, McGrew M, Bogdanova N, Hauschka S. Skeletal muscle cultures. In: Emerson CP, Sweeney HL, editors. Methods in Muscle Biology. Vol. 52. San Diego: Academic Press; 1997. pp. 85–116. [PubMed] [Google Scholar]
- Renganathan M, Messi ML, Delbono O. Dihydropyridine receptor-ryanodine receptor uncoupling in aged skeletal muscle. Journal of Membrane Biology. 1997a;157:247–253. doi: 10.1007/s002329900233. [DOI] [PubMed] [Google Scholar]
- Renganathan M, Messi ML, Schwartz R, Delbono O. Overexpression of hIGF-1 exclusively in skeletal muscle increases the number of dihydropyridine receptors in adult transgenic mice. FEBS Letters. 1997b;417:13–16. doi: 10.1016/s0014-5793(97)01225-8. [DOI] [PubMed] [Google Scholar]
- Renganathan M, Sonntag WE, Delbono O. L-type Ca2+ channel-insulin-like growth factor-1 receptor signaling impairment in aging rat skeletal muscle. Biochemical and Biophysical Research Communications. 1997c;235:784–789. doi: 10.1006/bbrc.1997.6881. [DOI] [PubMed] [Google Scholar]
- Rosenzweig SA, Oemar BS, Law NM, Shankavaram UT, Miller BS. Insulin like growth factor 1 receptor signal transduction to the nucleus. In: LeRoith D, Raizada MK, editors. Current Directions in Insulin-Like Growth Factor Research. New York: Plenum Press; 1994. pp. 159–168. [Google Scholar]
- Sanford J, Codina J, Birnbaumer L. Gamma-subunits of G proteins, but not their alpha- or beta-.s, are polyisoprenylated. Studies on post-translational modifications using invitro translation with rabbit reticulocyte lysates. Journal of Biological Chemistry. 1991;266:9570–9579. [PubMed] [Google Scholar]
- Schneider MF. Control of calcium release in functioning skeletal muscle fibers. Annual Review of Physiology. 1994;56:463–484. doi: 10.1146/annurev.ph.56.030194.002335. [DOI] [PubMed] [Google Scholar]
- Schneider MF, Chandler WK. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973;242:244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Selinfreund RH, Blair LA. Insulin-like growth factor-I induces a rapid increase in calcium currents and spontaneous membrane activity in clonal pituitary cells. Molecular Pharmacology. 1994;45:1215–1220. [PubMed] [Google Scholar]
- Sonntag WE, Lenham JE, Ingram RL. Effects of aging and dietary restriction on tissue protein synthesis: relationship to plasma insulin-like growth factor-1. Journal of Gerontology. 1992;47:B159–163. doi: 10.1093/geronj/47.5.b159. [DOI] [PubMed] [Google Scholar]
- Tanabe T, Takeshima H, Mikami A, Flockerzi V, Takahashi H, Kangawa K, Kojima M, Matsuo H, Hirose T, Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987;328:313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- Wei XY, Perez-Reyes E, Lacerda AE, Schuster G, Brown AM, Birnbaumer L. Heterologous regulation of the cardiac Ca2+ channel alpha 1 subunit by skeletal muscle beta and gamma subunits. Implications for the structure of cardiac L-type Ca2+ channels. Journal of Biological Chemistry. 1991;266:21943–21947. [PubMed] [Google Scholar]


