Abstract
Skeletal muscle is a major source of circulating insulin growth factor-1 (IGF-1), particularly during exercise. It expresses two main isoforms. One of the muscle IGF-1 isoforms (muscle L.IGF-1) is similar to the main liver IGF-1 and presumably has an endocrine action. The other muscle isoform as a result of alternative splicing has a different 3′ exon sequence and is apparently designed for an autocrine/paracrine action (mechano-growth factor, MGF). Using RNase protection assays with a probe that distinguishes these differently spliced forms of IGF-1, their expression and also the expression of two structural genes was measured in rabbit extensor digitorum longus muscles subjected to different mechanical signals.
Within 4 days, stretch using plaster cast immobilization with the limb in the plantar flexed position resulted in marked upregulation of both forms of IGF-1 mRNA. Electrical stimulation at 10 Hz combined with stretch (overload) resulted in an even greater increase of both types of IGF-1 transcript, whereas electrical stimulation alone, i.e. without stretch, resulted in no significant increase over muscle from sham-operated controls. Previously, it was shown that stretch combined with electrical stimulation of the dorsiflexor muscles in the adult rabbit results in a marked increase in muscle mass involving increases in both length and girth, within a few days. The expression of both systemic and autocrine IGF-1 growth factors provides a link between the mechanical signal and the marked increase in the structural gene expression involved in tissue remodelling and repair.
The expression of the β actin gene was seen to be markedly upregulated in the stretched and stretched/stimulated muscles. It was concluded that the increased expression of this cytoskeletal protein gene is an indication that the production of IGF-1 may initially be a response to local damage.
Switches in muscle fibre phenotype were studied using a specific gene probe for the 2X myosin heavy chain gene. Type 2X expression was found to decrease markedly with stimulation alone and when electrical stimulation was combined with stretch. Unlike the induction of IGF-1 and β actin, the decreased expression of the 2X myosin mRNA was less marked in the ‘stretch only’ muscles. This indicates that the interconversion of fibre type 2X to 2A may in some situations be commensurate with, but not under the control of IGF-1.
The adaptability of skeletal muscle, as seen in hypertrophy of exercised muscle, remodelling in response to increased functional length and repair following eccentric contraction damage, means that it must be able to respond quickly to local mechanical signals by locally increasing protein synthesis. It is well known that skeletal muscle has an inherent ability to alter its phenotypic characteristics (Booth & Thomason, 1991; Goldspink et al. 1992) in response to mechanical stimuli and that these lead to changes in nuclear DNA transcription (Jacobs et al. 1993). A great deal of work has been done to elucidate the mechanotransduction pathways at work but the critical links between mechanical activity and changes in tissue mass or cell phenotype are still elusive. It has been suggested that metabolic changes resulting from contractile activity and/or mechanosensitive ion channels may be part of the pathway. However, studies have revealed that these putative transducer systems may not really be needed (Sadoshima et al. 1992).
The study of the underlying mechanisms by which cells respond to mechanical stimuli, i.e. the link between the mechanical stimulus and gene expression, represents a new and important area of physiology (Goldspink & Booth, 1992). Muscle offers one of the best examples for studying this type of mechanotransduction as the mechanical activity generated by and imposed upon muscle tissue can be accurately controlled and measured in both in vitro and in vivo systems. Indeed, muscle is highly responsive to changes in functional demands. Overload leads to hypertrophy whilst decreased load force generation and immobilization of the muscle in the shortened position leads to atrophy (Goldspink et al. 1992). Increased frequency of use (Salmons & Vrbova, 1969; Pette & Vrbova, 1985; Barton-Davis et al. 1996), stretch and functional overload (Goldspink et al. 1992) result in increased expression of the slow muscle phenotype. As far as increase in mass is concerned, it has been shown that in adult muscles stretch is an important mechanical signal for the addition of new sarcomeres (Griffin et al. 1971; Williams & Goldspink, 1971, 1973; Goldspink, 1984). Stretch results in upregulation of protein synthesis (Goldspink & Goldspink, 1986; Loughna et al. 1990) as well as changes in gene transcription and muscle phenotype (Goldspink et al. 1992; Yang et al. 1997). In contrast, electrical stimulation at shorter muscle lengths results in atrophy (Tabary et al. 1981). For this reason it is important that the effects of stretch combined with electrical stimulation as well as stretch alone and electrical stimulation alone should be investigated.
The growth hormone/insulin growth factor-1 (GH/IGF-1) axis is the main regulator of tissue mass during early life and IGF-1 is one of the main growth factors that stimulates protein synthesis in muscle tissue (Stewart & Rotwein, 1996). In adult muscles, increasing evidence (LeRoith & Roberts, 1991; Yang et al. 1997) suggests that IGF-1, acting in an autocrine/paracrine fashion, may be an important link between mechanical activity and the resulting local cellular effects. Although the liver is usually thought of as the source of circulating IGF-1, it has recently been shown that during exercise skeletal muscle not only produces much of the circulating IGF-1 but the active musculature also utilizes most of the IGF-1 produced (Brahm et al. 1997).
It has long been appreciated that there is local control of growth because if a muscle is exercised it is that muscle that undergoes hypertrophy and not all the skeletal muscles of the body. As IGF-1 was a prime candidate for upregulating the expression of specific genes, it was felt that a study of the IGF-1 splice variants expressed in response to mechanical stress might provide the link between the mechanical stimulus and the altered expression of structural genes in skeletal muscle. The terminology for the IGF-1 splice variants is based on the liver isoforms (Chew et al. 1995) and has not fully evolved to take into account those produced by non-hepatic tissues. The latter are controlled to some extent by a different promoter (promoter 1) to the liver IGF-1 isoforms, which respond to hormones and are under the control of promoter 2 (Layall, 1996). Until the promoter and exon sequences have been fully determined we propose to call the two IGF-1 forms expressed in active muscle mechano-growth factor or MGF (autocrine/paracrine) and muscle liver-type L.IGF-1 (systemic type). It has been shown that MGF, which is not detectable in muscles unless they are subjected to exercise or stretch (Yang et al. 1996), has exons 4, 5 and 6 whilst the muscle L.IGF-1 has exons 4 and 6. Exon 5 in MGF has an insert of 52 bp which changes the 3′ reading frame and the carboxy end of the peptide.
Previous studies on the liver IGF-1 isoforms have shown that they have diverse functions depending on their exon sequences (Stewart & Rotwein, 1996). We hypothesize that in skeletal muscle the different IGF-1 isoforms also have specific functions and therefore it is important to know which type of mechanical signal activates the expression of the different transcripts. The recent cloning and sequencing of the cDNAs of the autocrine and systemic IGF-1 splice variants (Yang et al. 1996) made it possible to develop a probe to measure changes in their expression using RNase protection assays. Thus the expression of alternatively spliced IGF-1 transcripts as well as two structural genes could be quantitatively monitored in the rabbit extensor digitorum longus muscle after just 4 days of stretch, stretch combined with electrical stimulation and electrical stimulation alone. A preliminary account of this work has been presented to The Physiological Society (McKoy et al. 1996).
Muscle is the most abundant tissue in the body and myosin is the most abundant protein in this tissue. The intrinsic ability of muscle to adapt to different types of activity depends to a large extent on the ability to switch myosin gene expression. Recently, it has been shown by in situ hybridization studies that the fibres that commence to express embryonic myosins and then slow type 1 myosin are the ones that also express IGF-1. However, it is probable that in the model used, the upregulation of the slow type 1 (β cardiac) myosin as well as IGF-1 are responses to muscle cell damage (Yang et al. 1997). Therefore, it was felt that the change from 2X (2d) to 2A myosin gene expression (Schiaffino et al. 1989; Pette & Dusterhoft, 1992), together with the possible association between IGF-1 and another structural gene (β actin), should be established by making quantitative measurements of their mRNAs in stretch and/or electrically stimulated muscles.
METHODS
Animals and surgical procedure
New Zealand White rabbits were anaesthetized with diazepam (0·1 mg kg−1i.m.) and maintained on halothane (2 % in oxygen) before surgery. The extensor digitorum longus (EDL) was subjected to stretch and/or electrical stimulation continuously for 4, 6 and 10 days as previously described (Williams et al. 1986; Goldspink et al. 1992; McKoy et al. 1996). Briefly, muscle stretch was achieved by immobilizing the lower limb in a plaster cast with the ELD in its lengthened position. Electrical stimulation involved implanting Teflon-covered stainless steel electrodes near to the branch of the peroneal nerve as it emerges distally from the popliteal fossa. The electrode wires were externalized at the back of the neck and attached to a microstimulation circuit which was held in place with a Velcro saddle. Biphasic pulses of 1 ms duration were delivered at a frequency of 10 Hz. For sham-operated controls, the electrode wires were inserted but the electrical stimulation circuit was not switched on.
After 4, 6 and 10 days groups of rabbits in which the muscles had been subjected to stretch, stimulation, and stretch combined with stimulation, together with sham-operated controls were killed by intravenous injection with an overdose of pentobarbitone sodium (Sagatall, Merieux Ltd) into the marginal ear vein. These procedures were approved by the British Home Office and covered by the appropriate licences. The EDL muscle was immediately dissected from both hindlimbs of the experimental animals and the left hindlimb of the sham-operated rabbits. Each muscle was cut longitudinally into two halves and one half was rapidly frozen in liquid nitrogen and stored at -80°C for RNA isolation. This was always done in triplicate using three individual rabbits for each procedure. The other half was sectioned transversely and the blocks of tissue were fixed in 4 % paraformaldehyde fixative for 2 h then embedded in paraffin wax for in situ hybridization studies. As 4 days of mechanical stimulation gave the largest as well as the most rapid response only these data are presented.
RNA isolation
Total RNA was extracted from stretched and or electrically stimulated muscle essentially as described by Chomczynski & Saachi (1987). This was followed by further purification using RNATack resin (Ambion) to ensure complete removal of chromosomal DNA contamination. The integrity of the RNA preparations was checked by denaturing gel electrophoresis and the RNA concentration was determined by spectroscopy at 260 nm at a range of dilutions.
Probes
The IGF-1 probe used for RNase protection assays was an EcoNI/DraI subclone of the rabbit mechano-growth factor (MGF) splice variant, which is an IGF-1Eb isoform cDNA clone (Yang et al. 1996). This probe, which spans nucleotides 149-441 of the rabbit IGF-1Eb cDNA and covers exons 4-5-6 and part of the 3′ untranslated region (UTR), was labelled with 32P. It was designed to give full protection for transcripts with exons 4-5-6, which includes MGF, but not for transcripts with other exons, e.g. the muscle L.IGF-1, which would result in the appearance of more than one band. The IGF-1 probe for in situ hybridization studies was the 280 bp digoxigenin (DIG)-labelled antisense cRNA probe described previously (Yang et al. 1997). This probe spans exon 3 and part of the exon 4 region which is present in all alternatively spliced IGF-1 transcripts described to date.
The β actin probe used for RNase protection assays spans nucleotide 1483-polyA region of the rabbit actin cDNA. This was generated by RT-PCR using the actin specific primer 5′ GGGGCCACCCAGGGGAGG 3′ designed from the published rabbit β actin cDNA sequence (Harris et al. 1992) in conjunction with the anchored oligo-dT-derived primer RoRidT17 (Harvey & Darlison, 1991). The derived sequence from the RT-PCR clone was identical to corresponding regions in rabbit actin cDNA. The 2X myosin heavy chain probe was derived from the 2X myosin heavy chain clone described previously (McKoy et al. 1998). This probe covers part of exon 41 and the entire 3′ UTR of the rabbit 2X myosin heavy chain mRNA.
RNase protection assays
These assays resulted in a fully protected fragment of about 300 bp for MGF. As muscle L.IGF-1 has exons 4 and 6 and a different E domain the probe does not hybridize in this region. It is digested at this point by the RNase to yield two fragments of 140 and 100 bp. The general RNase protection assay procedures used have been described by McKoy et al. (1998). Briefly, pGEM-4Z (Promega) plasmid constructs containing the particular probe sequence were used as templates to generate RNA probes. Sense or antisense RNA was transcribed with T7 or SP6 RNA polymerase (Ambion) in the presence of [32P]CTP (800 Ci mmol−1, Amersham). The Promega RNase ONE Ribonuclease protection assay kit was used according to the manufacture's instructions. Either 10 or 5 μg of total RNA was used in the assay. Unhybridized probe was removed by digestion with 7-l0 U RNase ONE at 25°C for 75 min. The protected fragments were separated on a 5 % denaturing polyacrylamide gel and visualized by autoradiography. The size of the protected bands for each probe was determined by comigration with radiolabelled RNA marker generated by in vivo transcription of the RNA century marker template set (Ambion) with T7 RNA polymerase. The relative intensity of the protected bands was quantified from the 32P activity either directly from the gel using a PhosphoImager (Molecular Dynamics 425) or from the autoradiographs using a Densitometer (Molecular Dynamics). The experiments were designed so that the RNA extracted from the muscles of three rabbits that were subjected to stretch, three that were subjected to stimulation and three that were subjected to stretch combined with electrical stimulation, and three sham-operated and liver controls were run simultaneously on the same gel. Arbitrary units are used for expressing density, and by carrying out each experimental procedure simultaneously and in triplicate the expression levels could be compared. The statistical significance of the differences in the levels of specific RNA obtained in this way was determined using ANOVA and Mann-Whitney tests.
In situ hybridization and immunostaining
In situ hybridization was carried out as described previously (Yang et al. 1997) on 10 μm transverse sections of stretched muscles. Alternate sections from control and experimental muscles were mounted side by side and subjected to hybridization with DIG sense and antisense cRNA probes, and the alkaline phosphate method was used for detection of the probe annealed to the IGF-1 transcripts.
RESULTS
The results for EDL subjected to 4 days of stretch, stimulation, and stretch combined with stimulation are presented in Fig. lA. This shows that the cRNA probe used for the RNase protection assays protected several different sized fragments and that there are two main alternatively spliced IGF-1 transcripts expressed in skeletal muscle. As mentioned, the terminology of the various IGF-1 isoforms is unsatisfactory when describing IGF-1 in non-liver tissues. Also, evidence from the use of specific antibodies (S. Y. Yang & G. Goldspink, unpublished data) indicates that although they are of similar size the muscle IGF-1 isoforms are not completely homologous to the liver isoforms. In muscle, protected fragments included a fully protected 292 bp band which represents the MGF isoform. As in the liver, the predominantly expressed isoform in skeletal muscle (L.IGF-1) lacks the exon 5 region, and this is represented by two bands corresponding to partially protected 138 and 101 bp fragments. From the abundance of its transcript the muscle L.IGF-1 appears to be the main systemic type expressed by active muscle tissue. However, both this and MGF can be seen to be markedly upregulated by mechanical signals.
Figure 1. IGF-1 mRNA expression in skeletal muscle after 4 days of stretch only, electrical stimulation only, and stretch combined with electrical stimulation presented in triplicate together with controls.
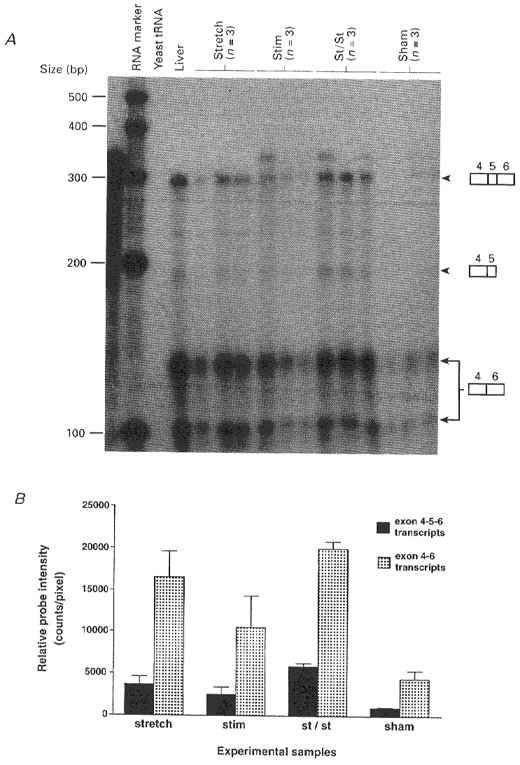
A, RNase protection assay after 2 days exposure of alternatively spliced IGF-1 mRNA transcripts expressed in rabbit EDL muscle after 4 days of stretch and/or electrical stimulation. The RNA probe used was complementary to exons 4-5-6. The probe fully protected the mRNA of the MGF isoform but resulted in two bands from the muscle L.IGF-1 mRNA. A 10 μg sample of total RNA from EDL muscle from each individual rabbit was used for each lane. Yeast tRNA was used to determine the specificity of the RNA probe. The radiolabelled RNA marker was generated by in vitro transcription using the RNA century marker template set (Ambion) and T7 RNA polymerase. Stretch, left hindlimb subjected to stretch in the plantar flexed position using a plaster cast; Stim, stimulation at 10 Hz continuously; St/St, stretch combined with electrical stimulation at 10 Hz continuously. B, quantification of RNase protection assay results by PhosphoImage analysis in triplicate. Data are expressed with s.e.m. and significance levels are given in the text.
As with most molecular biology analyses it was advisable to analyse the RNA samples simultaneously rather than pool data. Therefore, relative data for RNA were obtained by running experiments simultaneously and in triplicate, and expressing the results in arbitrary units. The relative levels of the two main classes of alternatively spliced IGF-1 transcripts expressed in skeletal muscle subjected to stretch and or electrical stimulation are shown in Fig. 1B. It can be seen that in sham-operated control muscles there is a low but detectable amount of both the L.IGF-1 and MGF transcripts. Sham-operated controls were used because in a previous study using RT-PCR (Yang et al. 1996) MGF was not detectable in normal resting muscle. It seems that the physical trauma involved in the sham operation did, however, result in detectable expression of both forms of IGF-1 even using RNase protection, which is a less sensitive but more quantitative and accurate technique than PCR. In the muscles that were subjected to stimulation alone, the MGF and L.IGF-1 mRNA levels were higher than those of the sham-operated muscle, but the increase in MGF level was not significant (confidence level less than 95 %) based on the triplicate analysis. In contrast, the results show that expression levels of MGF and L.IGF-1 in muscles that were subjected to stretch were significantly higher than those of the sham-operated controls (confidence level greater than 95 %). When stretch was combined with stimulation, the expression levels of both isoforms of IGF-1 were significantly increased compared with those in the sham-operated controls (confidence level greater than 99 %). Also, the stretch- stimulation IGF-1 mRNA levels for both forms were significant at the 95 % level when compared with the stimulation only results. Interestingly, the relative proportion of the MGF and the L.IGF-1 forms was the same. This presumably reflects the way the IGF-1 gene is alternatively spliced following expression in muscle induced by mechanical signals.
It was also important to determine the main source of the IGF-1 transcripts, as fibroblasts, satellite cells, macrophages and leukocytes, which are likely to infiltrate the damaged regions of muscles subjected to stretch and/or electrical stimulation, also express different classes of IGF-1 transcripts. In Fig. 2 the in situ hybridization studies show that the IGF-1 mRNAs were localized within the muscle fibres themselves. Interestingly, not all the muscle fibres were expressing IGF-1 equally which may relate to the different levels of local damage sustained by individual fibres.
Figure 2. In situ hybridization showing the localization of expression of the IGF-1 gene in muscles that were subjected to stretch.
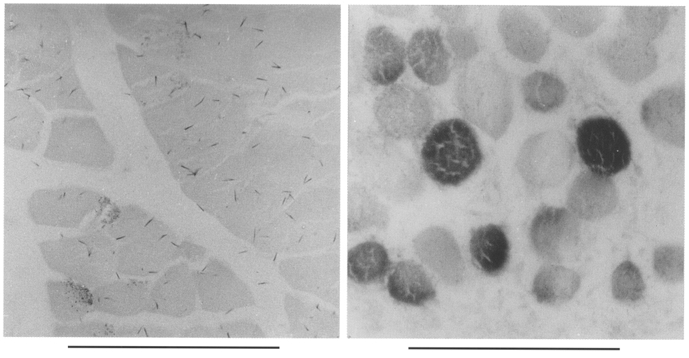
The probe used was DIG-labelled cRNA from a 280 bp that covers exon 4, which is common to the two forms of IGF-1 (Yang et al. 1996). Left, control muscle; right, muscle subjected to stretch by plastercast immobilization for 4 days. Scale bars, 100 μm.
It is shown in Fig. 3 that increased expression of the β actin mRNA occurred in parallel with the upregulation of both IGF-1 mRNAs. The stretch-stimulation muscles showed the greatest increase in β actin mRNA. This was statistically significant when compared with the sham-operated control muscles (99 % confidence level), and the ‘stretch alone’ (95 % confidence level) and ‘stimulation alone’ (95 % confidence level) muscles. Since the β actin gene encodes a cytoskeletal protein that is associated with the muscle membrane, its increased expression is probably a reflection of microdamage caused by overload resulting from stretch and particularly stretch combined with stimulation.
Figure 3. Results of RNase protection assays for β actin mRNA expression in rabbit muscle after 4 days of stretch and/or electrical stimulation.
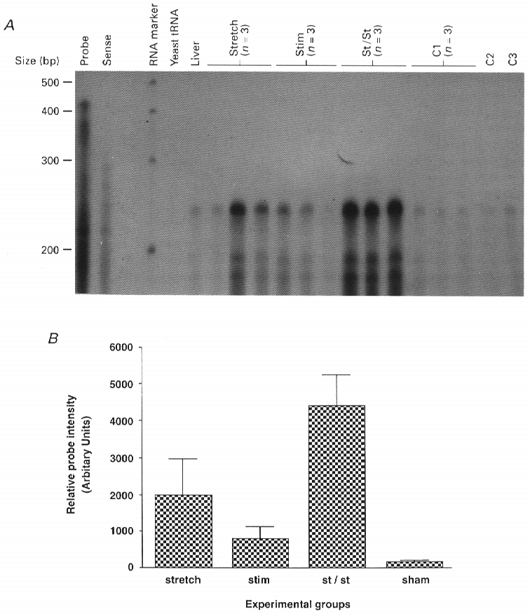
A, autoradiograph of the protected bands. Samples were separated on a 5 % denaturing polyacrylamide gel and the dried gel was exposed to X-ray film at room temperature for 30 min. B, quantification of the RNase protection assay results (in triplicate) by scanning densitometry. Data are expressed with s.e.m. Significance values are given in the text.
The mechanical stimuli were found to bring about a rapid change in phenotypic expression in that there was a marked drop in expression of 2X myosin heavy chain message (as seen in Fig. 4). The response was, however, somewhat different from the changes in IGF-1 and β actin gene expression. Muscles that were subjected to stretch alone showed some repression of 2X gene expression relative to the sham-operated control muscles (99 % confidence level). Electrical stimulation alone resulted in a marked decrease in expression of the 2X myosin heavy chain mRNA. However, stretch and stimulation combined resulted in the greatest suppression of this isoform (99 % confidence level). Both stimulation and stretch-stimulation muscles were significantly different in repression of this fast myosin mRNA compared with the ‘stretch only’ muscles (99 % confidence level).
Figure 4. 2X myosin heavy chain mRNA expression in rabbit muscle after 4 days of stretch and/or electrical stimulation.
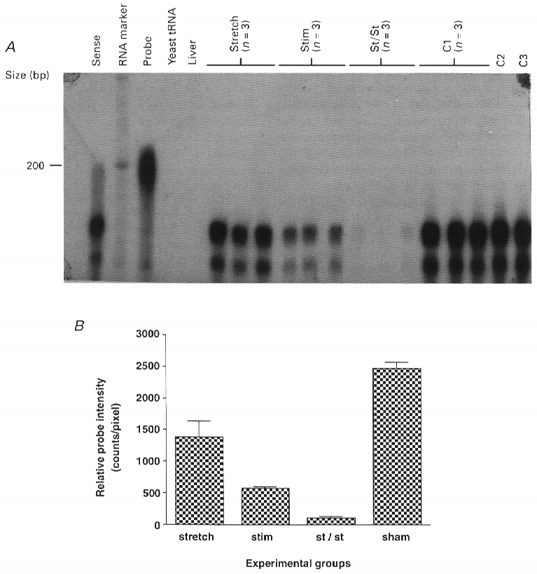
A, autoradiograph of the protected bands. Samples were separated on a 5 % denaturing polyacrylamide gel and the dried gel was exposed to X-ray film at room temperature for l h. B, quantification of the RNase assay results (in triplicate) by PhosphoImage analysis. Data are expressed with s.e.m. Significance values are given in the text.
DISCUSSION
By combining molecular biology and physiological methods it was possible to show that both of the main types of IGF-1 expressed in skeletal muscle are markedly upregulated by stretch and stretch combined with electrical stimulation but not by electrical stimulation alone. It was previously shown that IGF-1 induces synthesis of structural proteins (Ballard et al. 1986; Hill et al. 1986; Ewton et al. 1987) and, as shown here, IGF-1 upregulation is accompanied by increased expression of the β (cytoskeletal) actin gene. It appears therefore that mechanical induction of muscle IGF-1 is a physiological mechanism whereby local repair and adaptation of the tissue take place. In the liver, the IGF-1 gene is known to give rise to several isoforms by alternative splicing. Their mode of action and half-life are determined to a large extent by the specific proteins they bind to, the distribution of which determines their site of action. At the present time at least seven different binding proteins have been isolated from different tissues. The effects that the different liver IGF-1 splice variants elicit include muscle differentiation (Florini et al. 1994; Engert et al. 1996), nerve sprouting (D'Ercole et al. 1996), mitosis (Pietrzkowski et al. 1992), prevention of apoptosis and stimulation of protein synthesis and muscle fibre hypertrophy (Coleman et al. 1995) with or without any of the other effects. There is a growing appreciation that these diverse effects depend on the particular IGF-1 isoform expressed and the distribution of its binding protein(s).
It has been known for some time that muscle expresses IGF-1 and that expression is upregulated by physical activity (Czerwinski et al. 1994; Goldspink et al. 1995; Perrone et al. 1995). However, it was not appreciated until recently that there are two main muscle forms: one which is similar to the main IGF-1 isoform produced in liver and another that is apparently designed for an autocrine/paracrine mode of action (Yang et al. 1996). The latter form has only been detected in overloaded or damaged skeletal muscle (Goldspink et al. 1996) and cardiac muscle (Skarli et al. 1998). This putative autocrine/paracrine growth factor we previously named mechano-growth factor (MGF). From its sequence it can be seen that it is derived from the IGF-1 gene by alternative splicing but the exon forming the E domain of the IGF-1 peptide is different from that in the main liver isoform. Recently, we have generated a specific antibody to MGF and Western blots show it is also different from an isoform of similar size (2Eb), the transcript of which can be seen in Fig. 1A. Unlike the muscle L.IGF-1 and the main liver isoforms, MGF is not glycosylated and is therefore smaller and probably has a shorter half-life, and is thus suited for an autocrine/paracrine rather than a systemic mode of action. Also, a 52 bp insert in the E domain alters its reading frame and hence the carboxy-terminal end of the MGF peptide (Yang et al. 1996). This type of shift in the reading frame has been noted in another system that involves alternative splicing, in this case the NMDA gene, and this permits diversity of structure and function (Hollmann & Heinemann, 1994). Functional epitope mapping of IGF-1 using a battery of monoclonal antibodies (Manes et al. 1997) has shown that the carboxy terminus (3′ end) of IGF-1 is important in determining the affinity of the peptide for a particular receptor and/or binding protein. Therefore the MGF splice variant of IGF-1 is likely to bind to a different binding protein, e.g. BP5 which exists in the interstitial tissue spaces of bone, skeletal and cardiac muscle. The structure of MGF compared with that of L.IGF-1 is likely to localize its action as it would be unstable in the unbound form; this is important as its production would not be expected to disturb unduly the glucose homeostasis mechanism. In any event MGF has a different peptide structure from the liver and muscle L.IGF-1 forms and it is therefore expected to have a different action and to be more effective near its site of production.
The liver IGF-1 forms are induced by growth hormone (GH) and as shown here the muscle IGF-1 isoforms are induced by mechanical stress. Hence, it is possible that the induction of IGF-1 gene in muscle is via a different regulatory sequence to the one that includes the GH response elements (Layell, 1996). In a study on chickens that received recombinant GH, muscle, unlike liver IGF-1, was not upregulated by GH (Rosselot et al. 1995). As mentioned the IGF-1 gene has two promoters and these cause the IGF-1 gene to be spliced in different ways. Promoter 2 is activated by hormones and causes exon 2 but not exon 1 to be expressed (Jansen et al. 1992). Promoter 1 may be activated by mechanical signals resulting in transcripts with exon 1 and not exon 2, but this has yet to be demonstrated. However, it is possible that the same promoter could respond to mechanical signals as well as GH. Although the muscle isoforms have some of the same exons as the liver isoforms, they are not identical and the mode of action of the two types of muscle IGF-1 needs to be more clearly defined. Also, further physiological studies are required to define the nature of the mechanotransduction mechanism involved and the upstream regulation of the muscle forms of IGF-1.
Interestingly, the alternatively spliced putative autocrine form of IGF-1 mRNA is not detected in dystrophic muscle even when it is subjected to stretch. The inability of muscle in both the autosomal and dystrophin deficient dystrophies to respond to overload by stretch (Goldspink et al. 1996) indicates that the cytoskeleton may be involved in the transduction mechanism. The dystrophin complex, which includes a tyrosine kinase and nitric oxide synthase subunits as well as at least six different sarcoglycans, might be expected to do more than merely stabilize the membrane. Indeed, it is probable that there is a basic mechanism that detects overload and which results in the expression of both variant forms of IGF-1 acting in an autocrine/paracrine manner to induce local tissue repair and prevent apoptosis. In skeletal muscle, which is a mechanical tissue and in which there is no cell replacement, it is essential to prevent cell death resulting from day-to-day wear and tear.
Systemic IGF-1 is required for the general wellbeing of the body as a whole. It may be speculated that these growth factors produced by active skeletal muscles become much more important with age as the systemic GH-induced liver IGF-1 levels decline (Rudman et al. 1981). Resting levels of muscle IGF-1 in ageing rats were found not to change significantly (Hamilton et al. 1995). This work did not involve exercise studies and probably relates to L.IGF-1. This is interesting and it emphasizes that it is the upregulation of both forms of muscle IGF-1 that provides the rationale for maintaining a relatively active lifestyle into old age.
Although it is difficult to establish the cause and effect, the possibility that IGF-1 is directly responsible for the switch in muscle myosin heavy chain phenotype is not very convincing. A previous study (Yang et al. 1997) has shown that in muscle subjected to stretch there was an increase in slow-type myosin in the same muscle fibres that were showing IGF-1 expression. However, this might be explained by both being a response to damage. Certainly, in the case of the conversion of type 2X to type 2A myosin it was found here that stimulation alone resulted in a decrease in 2X RNA, whereas stretch and overload appear to be the main stimulants for IGF-1 upregulation, not electrical activity and frequency of use. Therefore, the sort of qualitative gene switching resulting in fibre phenotypic change appears to be a different process from the quantitative increase in muscle IGF-1 expression, although the two processes may occur in a commensurate way. Nevertheless, the possibility still exists that IGF-1 upregulates the expression of some genes more than others. In this way it may be involved in the regulation of tissue phenotype as well as tissue mass. This work is now being extended to producing the peptides for the autocrine and systemic forms of muscle IGF-1 so that their receptors and binding proteins can be characterized.
Acknowledgments
This work was funded by a grant from The Wellcome Trust. William Ashley is an MD/PhD student at the University of Illinois in Chicago and received his research stipend via Professor Brenda Russell's laboratory in Chicago. Dr Shi Yu Yang was supported by a grant from Action Research.
References
- Ballard FJ, Read LC, Francis GL, Bagley CJ, Wallace JC. Binding properties and biological potencies of insulin-like growth factors in L6 myoblasts. Biochemical Journal. 1986;233:223–230. doi: 10.1042/bj2330223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton-Davis ER, LaFramoise WA, Kushmerick MJ. Activity-dependent induction of slow myosin gene expression in isolated fast-twitch mouse muscle. American Journal of Physiology. 1996;271:C1409–1414. doi: 10.1152/ajpcell.1996.271.4.C1409. [DOI] [PubMed] [Google Scholar]
- Booth FW, Thomason DB. Molecular and cellular adaptation of muscle in response to exercise: perspectives of various models. Physiological Reviews. 1991;71:541–585. doi: 10.1152/physrev.1991.71.2.541. [DOI] [PubMed] [Google Scholar]
- Brahm H, Piehl-Aulin K, Saltin B, Ljunghall S. Net fluxes over working thigh of hormones, growth factors and biomarkers of bone metabolism during lasting dynamic exercise. Calcified Tissue. 1997;60:175–180. doi: 10.1007/s002239900210. [DOI] [PubMed] [Google Scholar]
- Chew SL, Lavender P, Clark AJL, Ross RJM. An alternative spliced human insulin-like growth transcript with hepatic tissue expression that diverts away from the mitogenic IBPE1 peptide. Endocrinology. 1995;136:1939–1944. doi: 10.1210/endo.136.5.7720641. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocynate-phenol-chloroform extraction. Annals of Biochemistry. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coleman ME, DeMayo F, Yin KC, Lee HM, Geske R, Montgomery C, Schwartz RJ. Myogenic vector expression of insulin-like growth factor 1 stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. Journal of Biological Chemistry. 1995;270:12109–12116. doi: 10.1074/jbc.270.20.12109. 10.1074/jbc.270.20.12109. [DOI] [PubMed] [Google Scholar]
- Czerwinski SM, Martin JM, Bechtel PJ. Modulation of IGF mRNA abundance during stretch-induced skeletal muscle hypertrophy and regression. Journal of Applied Physiology. 1994;76:2026–2030. doi: 10.1152/jappl.1994.76.5.2026. [DOI] [PubMed] [Google Scholar]
- D'Ercole AJ, Ye P, Calikoglu AS, Gutierrez-Ospina G. The role of the insulin-like growth factors in the central nervous system. Molecular Neurobiology. 1996;13:227–255. doi: 10.1007/BF02740625. [DOI] [PubMed] [Google Scholar]
- Engert JC, Berglund EB, Rosenthal N. Proliferation precedes differentiation in IGF-1 stimulated myogenesis. Journal of Cell Biology. 1996;135:431–440. doi: 10.1083/jcb.135.2.431. 10.1083/jcb.135.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewton DZ, Falen SL, Florini JR. The type II insulin-like growth factor (IGF) receptor has low affinity for IGF-1 analogs: pleiotypic actions of IGFs on myoblasts are apparently mediated by the type I receptor. Endocrinology. 1987;120:115–123. doi: 10.1210/endo-120-1-115. [DOI] [PubMed] [Google Scholar]
- Florini JR, Ewton DZ, Magri KA, Mangiacapra FJ. IGFs and muscle differentiation. In: LeRoith D, Raizada MK, editors. Current Directions in Insulin-Like Growth Factor Research. New York: Plenum Press; 1994. pp. 319–326. [Google Scholar]
- Goldspink DF, Cox VM, Smith SK, Eavues LA, Osbaldeston NJ, Lee DM, Mantle D. Muscle growth in response to mechanical stimuli. American Journal of Physiology. 1995;268:E288–297. doi: 10.1152/ajpendo.1995.268.2.E288. [DOI] [PubMed] [Google Scholar]
- Goldspink DF, Goldspink G. The role of passive stretch in retarding muscle atrophy. In: Nix WA, Vrbova G, editors. Electrical Stimulation and Neuromuscular Disorders. Berlin and Heidelberg: Springer Verlag; 1986. pp. 91–100. [Google Scholar]
- Goldspink G. Alterations in myofibril size and structure during growth, exercise and changes in environmental temperature. In: Peachy LD, Adrian RH, Geiger SR, editors. Handbook of Physiology, Skeletal Muscle. Bethesda, MD, USA: The American Physiological Society; 1984. pp. 539–554. [Google Scholar]
- Goldspink G, Booth F. General remarks for editorial issue - mechanical signals and gene expression in muscle. American Journal of Physiology. 1992;262:R327–328. [Google Scholar]
- Goldspink G, Scutt A, Loughna P, Wells D, Jaenicke T, Gerlach GF. Gene expression in skeletal muscle in response to mechanical signals. American Journal of Physiology. 1992;262:R356–363. doi: 10.1152/ajpregu.1992.262.3.R356. [DOI] [PubMed] [Google Scholar]
- Goldspink G, Yang SY, Skarli M, Vrbova G. Local growth regulation is associated with an isoform of IGF-1 that is expressed in normal muscles but not in dystrophic muscles when subjected to stretch. The Journal of Physiology. 1996;496.P:162. P. [Google Scholar]
- Griffin G, Williams P, Goldspink G. Region of longitudinal growth in striated muscle fibres. Nature New Biology. 1971;232:28–29. doi: 10.1038/newbio232028a0. [DOI] [PubMed] [Google Scholar]
- Hamilton MT, Marsh DR, Criswell DS, Lou W, Booth FR. No effect of aging on skeletal muscle insulin-like grown factor mRNAs. American Journal of Physiology. 1995;269:R1183–1188. doi: 10.1152/ajpregu.1995.269.5.R1183. [DOI] [PubMed] [Google Scholar]
- Harris DE, Warshaw DM, Periasamy M. Nucleotide sequences of the rabbit alpha-smooth-muscle and beta-non-muscle actin mRNAs. Gene. 1992;112:265–266. doi: 10.1016/0378-1119(92)90388-6. 10.1016/0378-1119(92)90388-6. [DOI] [PubMed] [Google Scholar]
- Harvey RJ, Darlison MG. Random-primed cDNA synthesis facilitates the isolation of multiple 5′-cDNA ends by RACE. Nucleic Acids Research. 1991;19:4002. doi: 10.1093/nar/19.14.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DJ, Crace CJ, Strain AJ, Milner RD. Regulation of amino acid uptake and deoxyribonucleic acid synthesis in isolated human fetal fibroblasts and myoblasts: effect of human placental lactogen, somatomedin-C, multiplication-stimulating activity, and insulin. Journal of Clinical Endocrinology and Metabolism. 1986;62:753–760. doi: 10.1210/jcem-62-4-753. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annual Review of Neuroscience. 1994;17:89–90. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Jacobs EL, Ashley W, Russell B. Iix and slow myosin expression follow mitochondrial increases in transforming muscle fibers. American Journal of Physiology. 1993;265:C79–84. doi: 10.1152/ajpcell.1993.265.1.C79. [DOI] [PubMed] [Google Scholar]
- Jansen E, Steenbergh PH, Van Schaik FM, Sussenbach JS. The human IGF-1 gene contains two cell type-specifically regulated promoters. Biochemical and Biophysical Research Communications. 1992;187:1219–1226. doi: 10.1016/0006-291x(92)90433-l. [DOI] [PubMed] [Google Scholar]
- Layall JEW. University of Cambridge; 1996. Transcriptional regulation of the ovine IGF-1 gene. PhD thesis. [Google Scholar]
- LeRoth D, Roberts CT. Insulin-like growth factor 1 (IGF-1): a molecular basis for endocrine versus local action. Molecular and Cellular Endocrinology. 1991;77:C57–61. doi: 10.1016/0303-7207(91)90054-v. [DOI] [PubMed] [Google Scholar]
- Loughna PT, Izumo S, Goldspink G, Nadal-Ginard B. Disuse and passive stretch cause rapid alterations in expression of development and adult contractile protein genes in adult skeletal muscle. Development. 1990;109:217–223. doi: 10.1242/dev.109.1.217. [DOI] [PubMed] [Google Scholar]
- McKoy G, Leger M-E, Bacou F, Goldspink G. Differential expression of myosin mRNA and protein isoforms in four functional diverse rabbit skeletal muscles during pre- and post-natal development. Developmental Dynamics. 1998;211:193–203. doi: 10.1002/(SICI)1097-0177(199803)211:3<193::AID-AJA1>3.0.CO;2-C. 10.1002/(SICI)1097-0177(199803)211:3<193::AID-AJA1>3.3.CO;2-N. [DOI] [PubMed] [Google Scholar]
- McKoy G, Mander J, Williams N, Goldspink G. The effect of stretch and electrical stimulation on the expression of the type 2X myosin heavy chain genes in rabbit tibialis anterior muscle. The Journal of Physiology. 1996;491.P:98. P. [Google Scholar]
- Manes S, Kremer L, Albar JP, Mark C, Llopis R, Martinez A. Functional epitope mapping of insulin-like growth factor I (IGF-1) by anti-IGF-1 monoclonal antibodies. Endocrinology. 1997;138:905–915. doi: 10.1210/endo.138.3.4965. 10.1210/en.138.3.905. [DOI] [PubMed] [Google Scholar]
- Perrone CE, Fenwick-Smith D, Vandenburgh HH. Collagen and stretch modulate autocrine secretion of insulin-like growth factor-1 and insulin-like growth factor binding proteins from differentiated skeletal muscle cells. Journal of Biological Chemistry. 1995;270:2099–2106. doi: 10.1074/jbc.270.5.2099. 10.1074/jbc.270.5.2099. [DOI] [PubMed] [Google Scholar]
- Pette D, Dusterhoft S. Altered gene expression in fast-twitch muscle induced by chronic stimulation. American Journal of Physiology. 1992;262:R333–338. doi: 10.1152/ajpregu.1992.262.3.R333. [DOI] [PubMed] [Google Scholar]
- Pette D, Vrbova G. Neural control of phenotypic expression in mammalian muscle fibres. Muscle and Nerve. 1985;8:676–689. doi: 10.1002/mus.880080810. [DOI] [PubMed] [Google Scholar]
- Pietrzkowski Z, Wernicke D, Porcu P, Jameson BR, Baserga R. Inhibition of cellular proliferation by peptide analogues of insulin-like growth factor 1. Cancer Research. 1992;52:6647–6451. [PubMed] [Google Scholar]
- Rosselot G, McMurtry JP, Vasilatos-Younken R, Czerwinski S. Effect of exogenous chicken growth hormone (cGH) administration on insulin-like growth factor-1 (IGF-1) gene expression in domestic fowl. Molecular and Cellular Endocrinology. 1995;114:157–166. doi: 10.1016/0303-7207(95)96796-k. 10.1016/0303-7207(95)96796-K. [DOI] [PubMed] [Google Scholar]
- Rudman DM, Kutner MH, Rogers CM, Lubin MF, Fleming GA, Bain RP. Impaired growth hormone secretin in the adult population. Journal of Clinical Investigation. 1981;67:1361–1369. doi: 10.1172/JCI110164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoshima J, Jahn L, Takahashi T, Kulik TJ, Izumo S. Molecular characterization of the stretch-induced adaptation of the cultured cardiac cells. An in vitro model of load-induced cardiac hypertrophy. Journal of Biological Chemistry. 1992;267:10551–10560. [PubMed] [Google Scholar]
- Salmons S, Vrbova G. The influence of activity on some contractile characteristics of mammalian fast and slow muscles. The Journal of Physiology. 1969;210:535–549. doi: 10.1113/jphysiol.1969.sp008771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S, Gorza L, Sartore S, Saggin L, Ausoni S, Vianello M, Lomo T. Three myosin heavy chain isoforms in type 2 skeletal muscle fibers. Journal of Muscle Research and Cell Motility. 1989;10:197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- Skarli M, Yang SY, Bouloux P, Yellon DM, Goldspink G. Upregulation and alternative splicing of the IGF-1 gene in the rabbit heart following a brief pressure/volume overload. The Journal of Physiology. 1998;509.P:192. P. [Google Scholar]
- Stewart CEH, Rotwein P. Growth, differentiation and survival: Multiple physiological functions for the insulin-like growth factors. Physiological Reviews. 1996;76:1005–10024. doi: 10.1152/physrev.1996.76.4.1005. [DOI] [PubMed] [Google Scholar]
- Tabary JC, Tardieu G, Tabary C. Experimental rapid sarcomere loss with concomitant hypoextensibility. Muscle and Nerve. 1981;43:198–203. doi: 10.1002/mus.880040305. [DOI] [PubMed] [Google Scholar]
- Williams PE, Bicik V, Goldspink G. Effect of stretch combined with electrical stimulation on the type of sarcomeres produced at the ends of muscle fibers. Experimental Neurology. 1986;93:500–509. doi: 10.1016/0014-4886(86)90170-6. 10.1016/0014-4886(86)90170-6. [DOI] [PubMed] [Google Scholar]
- Williams PE, Goldspink G. Longitudinal growth of striated muscle fibres. Journal of Cell Science. 1971;9:751–767. doi: 10.1242/jcs.9.3.751. [DOI] [PubMed] [Google Scholar]
- Williams PE, Goldspink G. The effect of immobilization on the longitudinal growth of striated muscle. Journal of Anatomy. 1973;116:45–55. [PMC free article] [PubMed] [Google Scholar]
- Yang SY, Alnaqeeb M, Simpson H, Goldspink G. Cloning and characterisation of an IGF-1 isoform expressed in skeletal muscle subjected to stretch. Journal of Muscle Research and Cell Motility. 1996;17:487–495. doi: 10.1007/BF00123364. [DOI] [PubMed] [Google Scholar]
- Yang SY, Alnaqeeb M, Simpson H, Goldspink G. Changes in muscle fibre type, muscle mass and IGF-1 gene expression in rabbit skeletal muscle subjected to stretch. Journal of Anatomy. 1997;190:613–622. doi: 10.1046/j.1469-7580.1997.19040613.x. 10.1046/j.1469-7580.1997.19040613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


