Abstract
The circadian timing system has been implicated in age-related changes in sleep structure, timing and consolidation in humans.
We investigated the circadian regulation of sleep in 13 older men and women and 11 young men by forced desynchrony of polysomnographically recorded sleep episodes (total, 482; 9 h 20 min each) and the circadian rhythms of plasma melatonin and core body temperature.
Stage 4 sleep was reduced in older people. Overall levels of rapid eye movement (REM) sleep were not significantly affected by age. The latencies to REM sleep were shorter in older people when sleep coincided with the melatonin rhythm. REM sleep was increased in the first quarter of the sleep episode and the increase of REM sleep in the course of sleep was diminished in older people.
Sleep propensity co-varied with the circadian rhythms of body temperature and plasma melatonin in both age groups. Sleep latencies were longest just before the onset of melatonin secretion and short sleep latencies were observed close to the temperature nadir. In older people sleep latencies were longer close to the crest of the melatonin rhythm.
In older people sleep duration was reduced at all circadian phases and sleep consolidation deteriorated more rapidly during the course of sleep, especially when the second half of the sleep episode occurred after the crest of the melatonin rhythm.
The data demonstrate age-related decrements in sleep consolidation and increased susceptibility to circadian phase misalignment in older people. These changes, and the associated internal phase advance of the propensity to awaken from sleep, appear to be related to the interaction between a reduction in the homeostatic drive for sleep and a reduced strength of the circadian signal promoting sleep in the early morning.
Sleep duration, sleep timing, sleep consolidation and sleep structure exhibit marked changes across the human life span (Miles & Dement, 1980; Prinz et al. 1990; Bliwise, 1993). The consolidation of the nocturnal sleep episode is typically very high in adolescents and young adults, but in older people consolidation of the nocturnal sleep episode is markedly reduced and awakening occurs at an earlier clock time.
In adolescents and young adults, the EEG during non-rapid eye movement (non-REM) sleep is characterized by high amplitude low frequency oscillations, which in the standard classification of sleep stages is represented by a high percentage (20-30 %) of stages 3 and 4 of non-REM sleep, otherwise known as slow wave sleep (SWS). In contrast, in older people, and in particular in older men, SWS is reduced (Webb & Agnew, 1971; Feinberg, 1974; Miles & Dement, 1980; Ehlers & Kupfer, 1989; Bliwise, 1993). In young adults, REM sleep exhibits nocturnal polarity, with short REM sleep episodes in the beginning of the nocturnal sleep episode and longer REM sleep episodes in the latter part of the night. This polarity is related to the sleep-dependent disinhibition of REM sleep as well as to the circadian rhythm of REM sleep (Dijk & Czeisler, 1995). Most studies show that in older people this polarity is less pronounced and the interval to the onset of the first REM sleep episode is reduced (Miles & Dement, 1980; Bliwise, 1993).
In mammals, including non-human primates and humans, the alternation between wakefulness and sleep and the structure of sleep itself are regulated by the interaction of outputs of the endogenous circadian pacemaker (Czeisler et al. 1980; Zulley et al. 1981; Åkerstedt & Gillberg, 1981), located in the suprachiasmatic nucleus (SCN) of the hypothalamus (Klein et al. 1991), and a homeostatic process (Daan et al. 1984). The homeostatic process is thought to reflect the need or pressure for sleep which builds up during sustained wakefulness and dissipates during sustained sleep. Slow wave sleep and slow wave activity (i.e. EEG power density in the 0·75-4·5 Hz range) are considered to be markers of this process (Daan et al. 1984) because they exhibit a predictive quantitative relationship with the duration of wakefulness and sleep (Webb & Agnew, 1971; Åkerstedt & Gillberg, 1986; Dijk et al. 1987).
The circadian pacemaker plays a crucial role in the timing and consolidation of wakefulness by providing a signal that becomes progressively stronger during the daytime hours, peaks at approximately 21.00-22.00 h and dissipates rapidly after the onset of nocturnal melatonin secretion (Dijk et al. 1997; Shochat et al. 1997). This signal opposes the wake-dependent (homeostatic) increase in sleep propensity, and in the absence of this signal, after SCN lesion or when wakefulness is scheduled out of phase with the endogenous circadian rhythm of wake propensity, wake episodes become fragmented and alertness is compromised (Dijk et al. 1992; Edgar et al. 1993). Nocturnal sleep consolidation is facilitated by a circadian signal that promotes sleep and peaks at, or shortly after, the nadir of the endogenous circadian temperature rhythm (Dijk & Czeisler, 1994), which is located at approximately 06.00 h in young subjects and 05.00 h in older subjects (Czeisler et al. 1992; Duffy et al. 1998). In the absence of this signal, after SCN lesion and when sleep is scheduled at adverse phases of the circadian rhythm of sleep propensity, sleep episodes become highly fragmented (Edgar et al. 1993; Dijk & Czeisler, 1994). The circadian variation in body temperature and plasma melatonin are closely associated with the circadian variation of sleep propensity in a wide variety of protocols (Dijk & Czeisler, 1995; Dijk et al. 1997; Shochat et al. 1997; Sack et al. 1997).
The demonstration that the circadian pacemaker plays a pivotal role in the regulation of sleep timing, sleep structure and sleep consolidation has led investigators to hypothesize that age-related changes in the characteristics of sleep are caused by age-related changes in the circadian timing system. In particular, it has been hypothesized that the earlier timing of awakening and reduced nocturnal sleep consolidation are linked to an age-related reduction in the intrinsic period of the circadian pacemaker, which results in an earlier phase of entrainment of the circadian pacemaker (see van Someren et al. 1993; Bliwise, 1993; Myers & Badia, 1995, for an overview of these hypotheses). Although we (Czeisler et al. 1992; Duffy et al. 1998) and others (Van Coevorden et al. 1991; Monk et al. 1995) have observed an earlier timing of circadian phase in older people even under carefully controlled conditions, results from recent experiments in our laboratory do not support the hypothesis that this is linked to an age-related reduction in the period of the circadian pacemaker (Czeisler et al. 1995).
Two alternative hypotheses that may be advanced to account for the age-related changes in sleep-wake timing, consolidation and structure focus on the amplitude of the circadian signal and the strength of the homeostatic drive for sleep. An age-related reduction in the amplitude of the circadian signal implies a reduction in the strength of the circadian drive for wakefulness during the daytime hours and/or a reduction of the circadian drive for sleep during the night-time hours, either of which could account for a reduced consolidation of vigilance states. The hypothesis that a reduction in the homeostatic drive for sleep can account for the age-related changes in sleep timing and consolidation is inferred from the demonstration that these sleep parameters result from the interaction of a circadian process and a sleep-wake-dependent homeostatic process. This hypothesis is further supported by the observation that there is a pronounced age-related reduction in SWS and by the notion that slow waves are major inhibitory events (Feinberg, 1974; Ehlers & Kupfer, 1989; Steriade et al. 1993).
Experimental protocols that have been applied to investigate the circadian and homeostatic regulation of sleep in older people include studies under baseline conditions (Campbell & Murphy, 1998), studies in which subjects lived in environments free of time cues and self-selected their sleep-wake schedule (Weitzman et al. 1982; Monk & Moline, 1988), acute sleep displacement/simulated jet lag protocols (Moline et al. 1992; Carrier et al. 1996), sleep deprivation (Webb, 1981; Reynolds et al. 1987), ultra-short sleep-wake cycles (Haimov & Lavie, 1997) and protocols in which the circadian pacemaker was phase shifted by light exposure (Campbell et al. 1993).
We and others have used the forced (or scheduled) desynchrony protocol to study the interaction between sleep homeostasis and circadian rhythmicity in young subjects (Hume & Mills, 1977; Dijk & Czeisler, 1995). Our version of this protocol, in which subjects are scheduled to rest- activity cycles (e.g. 28 h) that are outside the range of entrainment of the circadian pacemaker, which continues to oscillate at its intrinsic period of approximately 24·2 h in both young and older people (Czeisler et al. 1995), is particularly suited to investigation of the interaction of circadian and homeostatic processes. This is because subjects are scheduled to stay awake for two-thirds of the imposed rest-activity cycle and scheduled to sleep for one-third of the imposed rest-activity cycle. In this way, the duration of wakefulness preceding sleep episodes is similar even though the sleep episodes occur at many different circadian phases. Furthermore, sleep episodes are of sufficient length to study the sleep-dependent changes in sleep consolidation at all circadian phases. Because this protocol also allows for assessment of circadian phase it allows comparisons between young and older people while sleeping at identical circadian phases.
We recently conducted such a forced desynchrony protocol in healthy older people without sleep complaints (Duffy et al. 1998). We found that older people reported a longer duration of wakefulness at the end of their scheduled sleep episode, even when young and older subjects were scheduled to sleep at similar internal circadian phases. To assess whether these intriguing results from self reports could be confirmed and extended, we analysed the objective polysomnographic sleep recordings of these same older men and women during this forced desynchrony protocol. These data were compared with sleep recordings obtained in 11 young men (and in part previously reported in Dijk & Czeisler, 1994, 1995; Dijk et al. 1997), studied under identical conditions. The objectives of these analyses were to assess whether the reported subjectively perceived early awakenings were accompanied by polysomnographically verified wakefulness, to investigate whether age-related changes in the temporal organization of sleep persist when sleep occurs at all circadian phases, and to assess how sleep structure and sleep consolidation are affected by the interaction of circadian and homeostatic sleep processes in young and older people.
METHODS
Subjects
Volunteers were recruited through newspaper advertisements and flyers. Subjects underwent an extensive screening procedure consisting of a complete physical examination, including chest X-ray (only in the older subjects), an ECG and clinical biochemical tests on blood and urine. Psychological assessments consisted of the Minnesota Multiple Personality Inventory (MMPI), the Beck depression inventory and an interview by a clinical psychologist. All subjects were non-smokers, did not take any medication and were free of self-reported sleep complaints. All older subjects underwent sleep screening to assess sleep-related respiratory abnormalities and periodic limb movements (older subjects, n= 13; mean apnoea index = 2·8 h−1, s.d.= 2·4; periodic limb movements with EEG arousal index, mean = 4·8 h−1, s.d.= 2·9). No subject reported a history of rotating shift work or regular night work in the 3 years prior to the study, or travelled across more than one time zone in the 3 months prior to the study. Subjects were instructed to maintain a regular self-selected sleep-wake schedule, to maintain an 8 h time in bed each night for at least 3 weeks prior to entering the laboratory and to abstain from caffeine, nicotine, alcohol and all medications, including non-prescription medications, during the 3 weeks prior to the study. All subjects were drug free at the time of study as verified upon admission to the laboratory by toxicological analysis of their urine. Wrist-activity recordings were obtained for approximately 1 week prior to admission to the laboratory. This allowed verification of compliance with the requested regularity of the sleep-wake schedule. All subjects signed a consent form prior to admission to the research laboratory. Experiments were performed in accordance with the Declaration of Helsinki. The protocol, consent form and advertisements were approved by the Human Research Committee of the Brigham and Women's Hospital.
Eleven young men (mean age, 23·7 years; range, 21-30 years), nine older men and four older women (mean age, 67·4 years; range, 64-74 years) were studied under near-identical conditions and were included in the present analysis. Data from some of the young men were included in previous publications (Dijk & Czeisler, 1994, 1995; Dijk et al. 1997) and data on the subjectively perceived duration of the terminal waking episode in scheduled sleep episodes from 10 of the 11 young men and all of the older subjects were included in a recent report (Duffy et al. 1998). Some of the melatonin data from the young and older subjects were included in a recent report submitted for publication (J. M. Zeitzer, unpublished observations). The polysomnographic sleep data of the older subjects have not been reported elsewhere.
Protocol
Sleep-wake schedule
Upon admission to the laboratory, subjects entered a sound-attenuated and windowless suite in which they had no access to any timepiece for the duration of the study. They were in frequent contact with staff members trained not to transmit any information on the time of day. During the first three baseline 24 h days of the protocol the 8 h sleep episodes (light intensity < 0·03 lx) and the 16 h episodes of wakefulness (light intensity approximately 150 lx) were scheduled according to each subject's habitual sleep times derived from the sleep log and actigraphy recordings obtained during the week prior to admission. These three baseline days were followed by 40 h of continuous wakefulness in a semi-recumbent posture during a constant routine, followed by 21-25 28 h cycles of forced desynchrony. There were deviations from this protocol in one young subject and one older subject. In the young subject, the initial constant routine was not conducted and the subject was scheduled to a 28 h cycle immediately following sleep episode 3. In the older subject, sleep episode 3 was followed by a 40 h constant routine that was then followed by four more 24 h days and another 40 h constant routine. After the baseline segment and initial constant routine all subjects were scheduled to 21-25 28 h rest- activity cycles that each consisted of 18 h 40 min of scheduled wakefulness in dim light (∼10-20 lx) and 9 h 20 min sleep episodes in darkness (< 0·03 lx). This scheduled desynchrony portion of the protocol was followed by a final constant routine and a recovery sleep episode. During all waking episodes, subjects were engaged in a variety of activities including filling out visual analog scales (approximately 3 times per hour) and calculation performance tests (approximately hourly). During the scheduled waking episodes, a technician entered the room prior to each of these assessments, to serve three meals and a snack, to collect the plasma samples and to complete the pre- and postsleep procedures. During the scheduled sleep episodes technicians did not enter the subject's room and blood samples were collected through a tubing assembly that extended into an adjacent room.
Recording procedures
Core body temperature was collected continuously and stored at 1 min intervals throughout each study using a rectal thermistor (YSI, Yellow Springs, OH, USA). During selected sections of the protocol (starting prior to the forced desynchrony part of the protocol and typically weekly thereafter), blood samples were drawn at hourly intervals through an indwelling intravenous catheter with side portholes (Deseret Medical Inc., Sandy, UT, USA) placed in a forearm vein. These blood sampling sessions were scheduled to include at least one circadian cycle each. Samples were centrifuged immediately, and the plasma was frozen at -20°C. During all scheduled sleep episodes EEG, electro-oculogram (EOG), EMG and ECG were recorded on Nihon Kohden-5208 or -4418 electroencephalographs or directly onto Nicolet Ultrasom Sun386I workstations (versions 1.1, 2.1 and 4·1). The EEGs were derived from electrodes placed on the mastoid and sensory motor areas (C3-A2 and C4-A1) as well as occipital areas (O1-A2 and O2-A1). The EEG signals were high-pass filtered with a time constant of 0·3 s and low-pass filtered at 35 Hz. Signals were either recorded on paper or on Nicolet Ultrasom Sun386I workstations. All sleep recordings were scored in 30 s epochs according to the criteria of Rechtschaffen & Kales (1968) either by an investigator or a Registered Polysomnographic Technologist. The sleep recordings obtained immediately prior to the forced desynchrony component of the protocol served as the baseline data. Four hundred and eighty-two sleep episodes were included in the analysis of sleep during the forced desynchrony portion of the protocol. Sleep episodes immediately following a constant routine were not included in the analysis.
Analysis procedures
All body temperature data were inspected and obvious artifacts related to sensor malfunction or improper insertion of the sensor were removed. Data (sampling frequency, 6 min) were subjected to non-orthogonal spectral analysis. The model applied in this analysis assumes that the body temperature data consist of a circadian component, modelled by a fundamental sinusoid function and its first harmonic, an evoked component, modelled by a 28 h fundamental and its seven harmonics, their interactions and correlated noise (Brown & Czeisler, 1992). Outputs of this analysis are the phase and period of the circadian component as well as the amplitude of the circadian and evoked components. In this paper, we present the estimated circadian component and the estimated evoked component. All data for temperature are expressed as deviations from the mean of core body temperature as estimated during the forced desynchrony segment of the protocol.
Plasma melatonin was assayed at Diagnos Tech (Osceola, WI, USA) by applying a radioimmunoassay with a sensitivity of detection of 5 pg ml−1 and intra-assay and inter-assay coefficients of variation of 8 and 13 %, respectively. Melatonin data were analysed in two ways: (1) the waveforms of the circadian and 28 h component were educed by folding the data at the circadian period of the core body temperature rhythm and the 28 h rest-activity schedule; (2) all melatonin data collected during the constant routines and the forced desynchrony part of the protocol were subjected to non-orthogonal spectral analysis. The model applied was similar to the model used for temperature data except that neither interactions between the circadian and evoked components nor serially correlated noise were included. The melatonin data were then folded at the circadian period of the melatonin rhythm. Because in the present analysis melatonin was used as a marker of the phase of the circadian pacemaker, and to account for differences in amplitude and baseline values, all melatonin data were expressed as z-scores within each subject. The total number of melatonin samples included in the present analyses was 5583. Reliable assessment of period using melatonin was not achieved in three older subjects. This was primarily due to problems related to the collection of a sufficient number of blood samples.
Definitions
Temperature nadir refers to the fitted minimum of the core body temperature rhythm and is assigned a circadian body temperature phase of 0 deg.
Melatonin maximum refers to the fitted maximum of the plasma melatonin rhythm and is assigned a circadian melatonin phase of 0 deg.
Sleep latency refers to the interval between lights out and first epoch of any sleep stage, i.e. sleep onset.
REM sleep latency refers to the interval between sleep onset and the first epoch of REM sleep.
Total recording time (TRT) refers to the total number of minutes between lights out and lights on that were recorded and could be classified as either wakefulness or any of the sleep stages.
Habitual bedtime refers to the average start of sleep episodes (i.e. lights out) as derived from wrist actigraphy and sleep logs recorded during the week prior to the laboratory segment of the study.
Total sleep time (TST) is the sum of sleep stages 1, 2, 3, 4 and REM sleep.
Statistics
The SAS statistical package (SAS Institute Inc., Cary, NC, USA) was used for all statistical tests. When possible, data were analysed with ANOVA with repeated measures or mixed ANOVA, as appropriate. To correct for the effects of sphericity, the Huynh- Feldt correction was applied but the original degrees of freedom are reported. Pair-wise comparisons were only made between age groups. The significance of selected contrasts was assessed using Student's t test. Unless otherwise indicated, the number of observations entering the statistical analyses was based on the number of subjects contributing to a particular estimate, i.e. we first computed a mean for each subject and these means were used in the ANOVA or contrasts. This was done to avoid inflation of the number of observations. Sleep latency data were log transformed prior to statistical tests. For assessment of the variation of REM latency, median REM latencies were computed in each 60 deg circadian bin for every subject separately. These median values were then entered into an ANOVA.
RESULTS
Sleep structure and sleep timing under baseline conditions
Baseline sleep parameters were assessed after at least two nights of fully instrumented sleep recordings in the laboratory (Table 1) with subjects sleeping at their habitual bedtimes. The habitual sleep episodes in the older people started at 266·1 deg (s.e.m.= 4·6) of the body temperature cycle (0 deg corresponds to the fitted nadir as derived from the temperature data during the scheduled desynchrony protocol), i.e. 6·3 circadian hours before the temperature nadir. In the young subjects habitual bedtimes were located significantly later in the circadian body temperature cycle at 282·7 deg (s.e.m.= 2·4) (P= 0·0050, Student's t test), i.e. 5·2 circadian hours before the temperature nadir. The between-subject variability of the circadian body temperature phase of habitual bedtimes was significantly larger in the older subjects (P= 0·0242). Relative to the fitted maximum of the plasma melatonin rhythm, habitual bedtimes were located at 298·2 deg (s.e.m.= 4·8), i.e. 4·1 h before the melatonin maximum, in the older subjects (n= 10) and 301·1 deg (s.e.m.= 3·2), i.e. 3·9 h before the melatonin maximum, in the young subjects (n= 11) (P= 0·62, Student's t test).
Table 1.
All night sleep parameters in older and young subjects while sleeping at their habitual bedtimes
| Variable | Older (mean ± s.e.m.) | Young (mean ± s.e.m.) | P |
|---|---|---|---|
| Sleep latency (min) | 11.2 ± 2.1 | 14.0 ± 4.4 | 0.4525 |
| REM latency (min) | 56.0 ± 6.9 | 77.4 ± 10.7 | 0.0970 |
| TRT (min) | 478.3 ± 2.3 | 479.2 ± 1.4 | 0.7369 |
| Wakefulness (min) | 107.3 ± 11.4 | 39.5 ± 4.8 | 0.0001 |
| TST (min) | 370.8 ± 11.6 | 433.1 ± 5.0 | 0.0002 |
| Sleep efficiency (%) | 77.5 ± 2.4 | 90.4 ± 0.9 | 0.0001 |
| Stage 1 (%) | 18.0 ± 2.8 | 7.7 ± 1.5 | 0.0046 |
| Stage 2 (%) | 49.6 ± 3.0 | 53.2 ± 2.2 | 0.3634 |
| Stage 3 (%) | 8.6 ± 1.7 | 6.4 ± 0.9 | 0.2421 |
| Stage 4 (%) | 4.6 ± 2.0 | 13.0 ± 1.7 | 0.0043 |
| SWS (%) | 13.2 ± 2.7 | 19.4 ± 1.8 | 0.0792 |
| REM sleep (%) | 19.1 ± 1.1 | 19.6 ± 1.6 | 0.8006 |
For definitions see Methods.
Under the baseline conditions all subjects fell asleep readily and sleep latency did not differ significantly between the two age groups. Latency to REM sleep tended to be shorter in the older people although this difference did not reach statistical significance. REM sleep expressed as a percentage of TST was similar in the two groups but the percentage of stage 1 sleep was higher and stage 4 sleep was reduced in the older people. All night sleep efficiency averaged 77 % in the older people compared with 90 % in the younger subjects. Analysis of wakefulness per third of the baseline sleep episodes revealed a significant effect of the factor third (F2,44= 6·29, P= 0·0040), a significant effect of the factor age (F1,22= 26·51, P= 0·0001), but no significant interaction between these two factors (F2,44= 1·21, P= 0·3067). These significant differences (see also Fig. 1) probably reflect the increased wakefulness apparent in the last third of the sleep episode and the increased wakefulness present throughout the sleep episode in the older people (Fig. 1).
Figure 1. Sleep structure in older (•) and young (^) subjects while sleeping at their habitual bedtimes.
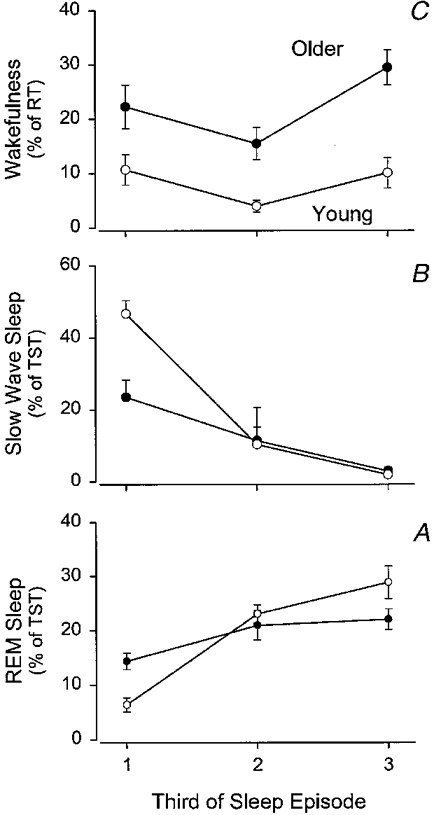
Data are plotted per third of the sleep episode. REM sleep (A) and SWS (B) are expressed as percentage of total sleep time (TST) per third of the sleep episode. Wakefulness (C) is expressed as percentage of recording time (RT) per third of the sleep episode. Vertical bars indicate s.e.m.
For REM sleep expressed as percentage of TST, the analysis per third of the sleep episode revealed no significant effect of the factor age (F1,22= 0·03, P= 0·8756), a significant effect of the factor third (F2,44= 29·39, P= 0·0001), and a significant interaction between these two factors (F2,44= 6·61, P= 0·0049). In the young subjects, REM sleep increased steeply as the sleep episode progressed, but such a steep increase was not observed in the older subjects. This was in particular related to the higher REM sleep percentage in the first third of the sleep episode in the older subjects (P= 0·006, young vs. older, Student's t test), whereas in the last third of the sleep episode REM sleep percentage tended to be higher in the young subjects (P= 0·0616). SWS was lower in the older people (factor age: F1,22= 4·30, P= 0·0501) and declined as sleep progressed (factor third: F2,44= 69·33, P= 0·0001). The interaction between the two factors was significant (F2,44= 11·78, P= 0·0001) and pair-wise comparisons revealed that SWS was significantly lower in the older people only in the first third of the sleep episode (P= 0·0013, Student's t test).
Forced desynchrony
Figure 2 illustrates the progression of the estimated circadian temperature nadir and the estimated circadian melatonin maximum in a young subject (left panel) and an older subject (right panel). The young subject fell asleep readily at all circadian phases. A substantial amount of wakefulness within the scheduled sleep episodes was observed only at the end of sleep episodes and only when the ends of these sleep episodes were scheduled several hours after the temperature nadir and melatonin maximum. The older subject also fell asleep readily at all circadian phases. However, in contrast to the young subject, a substantial amount of wakefulness was present throughout the sleep episodes. Close inspection of the data indicates that wakefulness was most abundant at the end of sleep episodes when these sleep episodes were scheduled several hours after the temperature nadir and melatonin maximum.
Figure 2. Double raster plot of the protocol in a young (1122, age 23 years) and an older subject (1215, age 64 years).
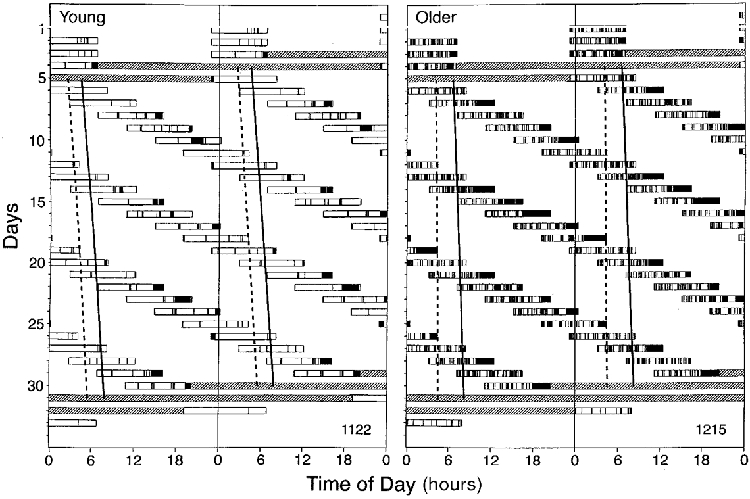
Successive days are plotted next to and beneath each other. Open bars indicate the timing of the scheduled sleep episodes. Filled bars within these open bars indicate wakefulness. The estimated progression of the temperature nadir and the melatonin crest are indicated by the solid and dashed line, respectively. Stippled areas indicate constant routines.
Core body temperature and plasma melatonin rhythms
The mean data (Fig. 3A and B) revealed that during the forced desynchrony section of the protocol both body temperature and plasma melatonin exhibited robust oscillations with a period that was on average slightly longer than 24 h in both age groups and similar for core body temperature and plasma melatonin, i.e. 24·2 h (Czeisler et al. 1995). Thus, on average, the minimum of the core body temperature and the maximum of the plasma melatonin rhythm drifted in synchrony to later clock hours in the course of the experiment but desynchronized from the scheduled sleep episodes that were scheduled to a 28 h period. The phase relationship between the core body temperature rhythm and the plasma melatonin rhythm was similar in the two age groups such that on average the crest of the melatonin rhythm preceded the body temperature nadir by 1-2 h. The amplitude of the circadian component of the core body temperature rhythm was 0·30°C (s.e.m.= 0·01) in the young men and 0·21°C (s.e.m.= 0·02) in the older subjects (P < 0·001, Student's t test).
Figure 3. Circadian variation of core body temperature (A), plasma melatonin (B), sleep efficiency (C), latency to sleep onset (D) and latency to REM sleep (E and F) in older (•) and young (^) subjects.
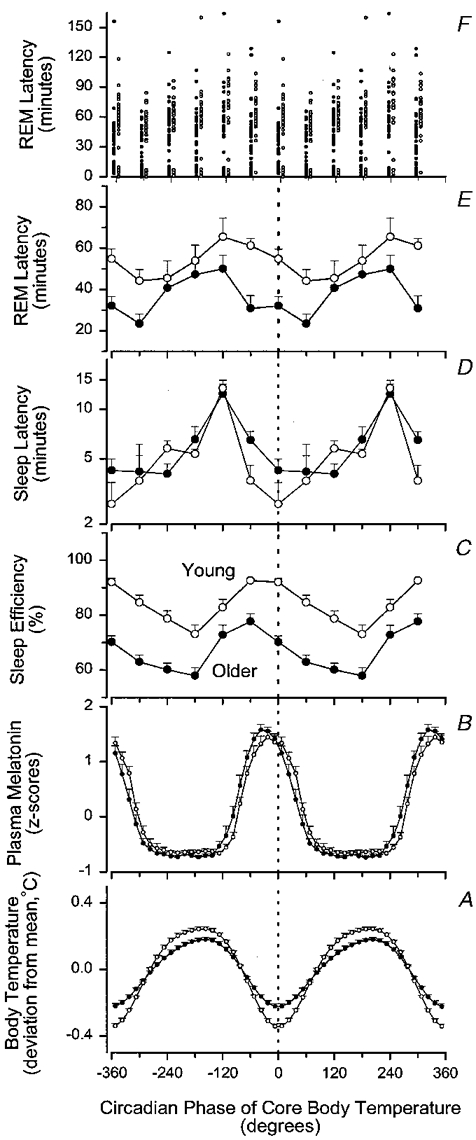
All data are plotted relative to the circadian phase of the core body temperature rhythm and are double plotted. Sleep latency, REM latency and sleep efficiency data are plotted with a 60 deg resolution at the mid-point of each bin. Mean REM latencies represent means of the median values in each subject (E). Individual REM sleep latencies are also represented (F) and the data from the older subjects are plotted to the left of the data from the young subjects in this panel. Plasma melatonin and core body temperature data are plotted with a 15 deg resolution at the mid-point of each bin. The waveform of plasma melatonin was derived by folding the data at the period of the core body temperature rhythm. Variation of plasma melatonin data was expressed as z-scores within each subject and then averaged across subjects. Circadian variation of core body temperature data was computed from the circadian waveform that was fitted to the raw temperature data, and expressed as deviation from the mean. All subjects contributed to every plotted data point for the temperature and sleep parameters, whereas 10 older and 11 young subjects contributed to the plasma melatonin data. Vertical bars indicate one s.e.m.
Sleep efficiency
Sleep efficiency during the 9 h 20 min sleep episodes is plotted at the estimated circadian phase in the middle of the sleep episode (Fig. 3C). During forced desynchrony between the sleep-wake cycle and the output of the circadian pacemaker, sleep efficiency was markedly and significantly lower in the older subjects than in the young subjects at all circadian phases (factor age: F1,22= 41·58, P= 0·0001). Nonetheless, sleep efficiency also varied with circadian phase (F5,110= 23·29, P= 0·0001), such that the highest average sleep efficiency was observed in both young and older subjects when the middle of the scheduled sleep episodes fell in the 270-330 deg interval and the 330-30 deg interval, i.e. close to the temperature nadir in the young subjects and in the preceding interval in the older people. Sleep efficiencies for the sleep episodes in the 270-330 deg interval were 77·7 and 92·6 % for the older and young subjects, respectively. Lowest sleep efficiencies were observed when the middle of the scheduled sleep episodes fell in the 150-210 deg bin. The sleep efficiency values were 57·9 and 73·0 % for the older and young subjects, respectively. Although the overall pattern was similar in the older and young subjects, ANOVA indicated a tendency for an interaction between the factors age and circadian phase (F5,110= 2·24, P= 0·0820). The trend for such an interaction may reflect the fact that although the difference in sleep efficiency between the age groups was significant at all circadian phases (P < 0·05 in all cases), it was smallest in the interval of 210-270 deg, i.e. the late evening hours, and largest in the 330-90 deg interval, i.e. the morning hours. Inspection of Fig. 3reveals that while sleep efficiency in the young subjects remained at high levels (92 %) when the mid-point of the sleep episode was shifted from 4 h before the temperature nadir to the temperature nadir, this shift markedly reduced sleep efficiency in the older subjects, from 78 to 70 %, indicating that in contrast to young subjects, sleep is less well consolidated in older subjects when sleep is centred at the temperature nadir, i.e. in the latter part of the phase during which melatonin is present in plasma.
Latency to sleep onset
Both older and young subjects fell asleep readily during the forced desynchrony section of the protocol and no significant effect of the factor age was obtained for sleep latency (F1,22= 0·30, P= 0·5907). The latency to sleep onset varied significantly with the circadian phase at which the sleep episodes were scheduled to be initiated (F5,110= 23·73, P= 0·0001) and a significant interaction between the factors circadian phase and age was obtained (F5,110= 2·98, P= 0·0147), indicating a difference in the magnitude or waveform of the circadian variation in sleep propensity as indexed by sleep latency in the two age groups. The overall pattern of the circadian variation in sleep latency was quite similar in the two age groups (see Fig. 3D). In both age groups the longest latencies to sleep onset were observed in the interval of 210-270 deg, i.e. 6-10 h before the nadir of the temperature cycle and around the time of the nocturnal increase in plasma melatonin in both the older and young subjects. Thereafter sleep latencies became shorter in both the older and young subjects but more rapidly so in the young subjects. Pair-wise comparisons revealed that sleep latencies of sleep episodes initiated between 270 and 330 deg were significantly longer in the older subjects (P= 0·0112, Student's t test on log-transformed values). In both young and older subjects short sleep latencies were observed close to the nadir of the temperature cycle. Thereafter, i.e. on the rising limb of the temperature cycle, sleep latencies became gradually longer.
Latency to REM sleep
The interval between sleep onset and the first occurrence of REM sleep exhibited a bimodal distribution in both older and young subjects at most circadian phases (Fig. 3F) with sleep onset REM episodes in both age groups. Mean REM latency was significantly shorter in the older subjects (F1,22= 5·64, P= 0·0267) and varied significantly with circadian phase (F5,110= 7·89, P= 0·0001) (Fig. 3E). Shortest latencies to REM sleep were located in the 30-90 deg bin, i.e. 2-6 h after the temperature minimum. The interaction between the factors circadian phase and age was significant (F5,110= 2·97, P= 0·0149). This reflects the fact that REM latencies in older people were significantly shorter than in young people when sleep was initiated in the bins centred at -60, -30, 0 and 30 deg (P < 0·01 in all cases), i.e. from 6 h before the temperature nadir until 3 h after the temperature nadir. This part of the cycle corresponds to the falling limb of the core body temperature rhythm and the phase during which melatonin is present in plasma at high levels. In the remainder of the circadian cycle, REM latencies in older subjects were not significantly different from those in young subjects.
Main effects of age, circadian phase and time elapsed since start of sleep episode on sleep consolidation and sleep structure
Circadian and homeostatic contributions to sleep structure and sleep consolidation, as well as the interaction between these factors and age were investigated further by sorting all 30 s epochs of polysomnographically recorded sleep into a 32 cell matrix composed of eight circadian bins (45 deg each) and four sleep-dependent bins (140 min each) (Fig. 4).
Figure 4. Main effects of circadian phase of the core body temperature cycle (left-hand panels) and time elapsed since the start of the sleep episode (right-hand panels) in older (•) and young (^) subjects.
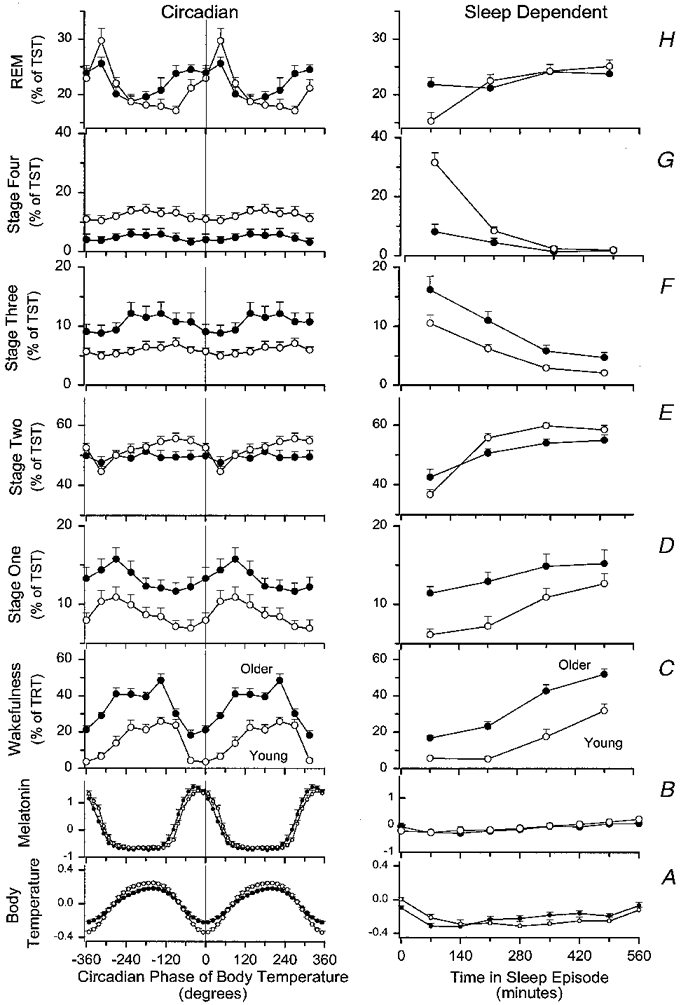
Results are presented for body temperature (A), plasma melatonin (B), wakefulness within scheduled sleep episodes (C), stage 1 sleep (D), stage 2 sleep (E), stage 3 sleep (F), stage 4 sleep (G) and REM sleep (H). Circadian waveforms are all double plotted with a resolution of 45 deg, except core body temperature and plasma melatonin waveforms, which are plotted with a resolution of 15 deg. Sleep-dependent main effects are plotted at quarters of the sleep episode (i.e. a resolution of 140 min). Sleep-dependent main effects of temperature and plasma melatonin are plotted with a resolution of 70 min. All parameters are plotted at the centre of the bins. Body temperature data (deviation from mean, °C) represent the fitted circadian component (left-hand panels) and the fitted sleep-dependent component (right-hand panels) as assessed by non-orthogonal spectral analysis. Plasma melatonin data are expressed as z-scores. Note that the first data point for the sleep-dependent modulation of body temperature and plasma melatonin represents the mean of these values during the first 35 min before lights out and the first 35 min thereafter. The s.e.m. is represented by the vertical bars and represents the between-subject standard error of the mean in each age group. Vigilance state parameters are expressed as percentage of total recording time (TRT) (wakefulness) or percentage of sleep time (stage 1, 2, 3, 4, REM).
Sleep duration and consolidation, as reflected by wakefulness within scheduled sleep episodes, was significantly affected by age (F1,22= 44·11, P= 0·0001), such that sleep consolidation was poorer in older people, and significantly affected by circadian phase (factor phase: F7,154= 36·35, P= 0·0001). Sleep consolidation was best around the temperature nadir, and significantly affected by time since start of sleep episode (factor sleep: F3,66= 81·12, P < 0·0001). Sleep consolidation deteriorated as the sleep episodes progressed in both older and young people (Fig. 4C). Significant interactions among the factors were observed (age-phase: F7,154= 4·05, P= 0·001; age-sleep: F3,66= 3·26, P= 0·0313; age-phase- sleep: F21,462= 3·01, P= 0·0003). Wakefulness was higher at all circadian phases in older people. In both age groups wakefulness within the scheduled sleep episodes was greatest 225 deg after the nadir of the temperature rhythm. In the next interval, i.e. 270 deg after the temperature nadir, wakefulness remained high in the young subjects but exhibited a steep decline in the older people. In the older people, minimum values of wakefulness were observed 45 deg before the temperature nadir, and wakefulness started to increase as soon as sleep coincided with the temperature nadir. In contrast, in young people this increase was not observed until 45 deg after the temperature nadir. Inspection of the main effect of time since start of sleep episode in older and young people revealed that wakefulness increased more rapidly as sleep progressed and was higher throughout the sleep episode in the older people.
Stage 1 sleep, expressed as percentage of total sleep time, was significantly (F1,20= 7·05, P= 0·0152) higher in older people while sleeping at all circadian phases, and significantly affected by circadian phase (F7,140= 6·55, P= 0·0003). Highest values of stage 1 sleep were observed 90 deg after the temperature nadir and increased as sleep progressed (F3,60= 18·58, P= 0·0001) in both age groups (Fig. 4D). No significant interaction between the factors age and either circadian phase or time since start of sleep episode were observed. (Note that because in some of the cells of the 8 × 4 matrix no sleep occurred, only 22 subjects could be included in the analysis of sleep stages expressed as percentage of total sleep time.)
Stage 2 sleep, expressed as percentage of total sleep time, was not significantly affected by age (F1,20= 2·05, P= 0·168), varied with circadian phase (F7,140= 4·60, P= 0·0001), such that the lowest values were observed shortly after the temperature nadir, and increased significantly (F3,60= 54·17, P= 0·0001) as sleep progressed. The sleep-dependent increase of stage 2 sleep was less steep in the older subjects than in the young adults (age-sleep: F3,60= 7·99, P= 0·0001) (Fig. 4E).
Stage 3 sleep, expressed as percentage of total sleep time, was significantly higher in the older subjects (F1,20= 5·20, P= 0·0337) and varied with circadian phase (F7,140= 4·16, P= 0·0006) such that the lowest values were observed at and shortly after the temperature nadir. Stage 3 declined as sleep progressed (F3,60= 41·88, P= 0·0001) and no significant interaction between the factors age and time since start of sleep episode was observed.
Stage 4 sleep, expressed as percentage of total sleep time, was markedly reduced in the older people (F1,20= 9·61, P= 0·0056) and exhibited a small but significant (F7,140= 2·49, P= 0·0295) circadian modulation, such that the lowest values were observed near the temperature nadir. Stage 4 sleep declined as sleep progressed (F3,60= 83·14, P= 0·0001) and a significant interaction between the factor sleep and the factor age (F3,60= 34·48, P= 0·0001) was obtained. This interaction reflects the fact that stage 4 sleep was much higher in the young subjects in the initial quarter of the sleep episodes only.
REM sleep, expressed as percentage of total sleep time, was at near identical levels in the older and young people (age: F1,20= 0·31, P= 0·5847) and varied markedly as a function of circadian phase (F7,140= 10·16, P= 0·0001). The highest percentages of REM sleep were observed 45 deg after the temperature nadir in both young and older subjects. The waveform of the circadian rhythm of REM sleep was somewhat different in the older and young people (age- phase: F7,140= 3·07, P= 0·0135). The percentage of REM sleep was higher in older people when sleep was located on the falling limb of the circadian temperature cycle, i.e. the rising limb of the plasma melatonin rhythm. This was particularly evident 90 deg before the temperature nadir. Analysis of the sleep-dependent modulation of REM sleep yielded a significant main effect of time since start of sleep episode (F3,60= 9·93, P= 0·0001) and a significant interaction between this factor and the factor age (F3,60= 4·57, P= 0·0060). Whereas in the young people REM sleep increased as sleep progressed, this increase was less pronounced in the older people, in particular because in older people REM sleep was already at high levels in the initial quarter of sleep.
Interaction of circadian phase and time since start of sleep episode: effect on the propensity for awakening
The interaction between circadian phase and sleep-dependent modulation is illustrated in Fig. 5. In this quasi-3-D plot, the simultaneous effects of circadian phase and sleep homoestasis on wakefulness are plotted separately for the young and older people. In both age groups sleep consolidation was poorest when the end of the sleep episode coincided with the rising limb of the core body temperature rhythm. Sleep consolidation remained quite high in both age groups when the end of the sleep episode coincided with the temperature nadir, which on average occurs at 06.00 h in young subjects and 05.15 h in older subjects. This representation also indicates that young subjects can maintain high sleep efficiency at the end of the sleep episode, even when the end of the sleep episode occurs several hours after the temperature nadir. In contrast, sleep efficiency in older subjects deteriorates rapidly when the second half of the sleep episode occurs after the temperature nadir, i.e. after approximately 05.00 h during entrainment.
Figure 5. Quasi-3-D representation of wakefulness in scheduled sleep episodes as a function of circadian phase of the core body temperature cycle and time in sleep episode in older and young subjects.
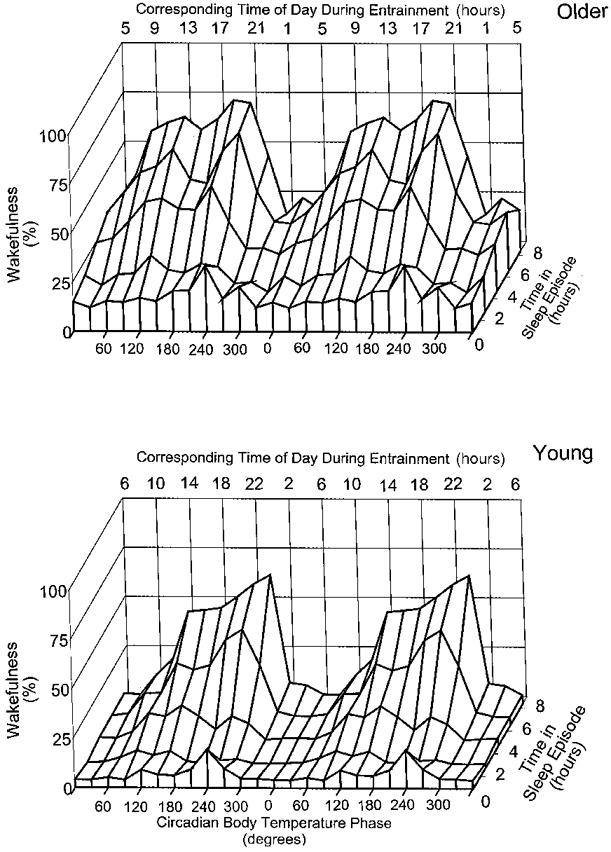
Data are plotted per 30 circadian deg and per fifth of the sleep episode (112 min). All data are plotted at the mid-point of the bins. The mean approximate corresponding times during entrainment in young and older people (according to Duffy et al. 1998) are also indicated.
We analysed families of trajectories on this 3-D representation by computing wakefulness per quarter of the sleep episode for sleep episodes grouped according to the circadian phase of the core body temperature rhythm at the start of the sleep episode (Fig. 6). During sleep episodes that were initiated 10-14 h before the temperature nadir, sleep was moderately disrupted throughout the sleep episode, but especially so in the third quarter (Fig. 6A). This disruption was less pronounced in sleep episodes initiated 6-10 h before the temperature nadir, although in the older people more wakefulness was present throughout the sleep episode than in the young people (Fig. 6B). The pattern of wakefulness in sleep episodes was markedly different between young and older subjects for sleep episodes initiated 2-6 h before the temperature nadir, i.e. where sleep usually occurs during entrainment (Fig. 6C). In the young people, wakefulness remained at low levels throughout the sleep episode, whereas in the older people a steep increase occurred in the third and fourth quarter, with wakefulness reaching 60 % in the last quarter. Sleep episodes initiated on the rising limb of the endogenous circadian temperature cycle were all severely disrupted, in both older and young people, and in particular in the latter half of the sleep episode (Fig. 6D-F).
Figure 6. Effect of interaction between circadian phase of the start of the sleep episode and time since the start of the sleep episode on wakefulness within quarters of the scheduled sleep episodes in older (•) and young (^) subjects.
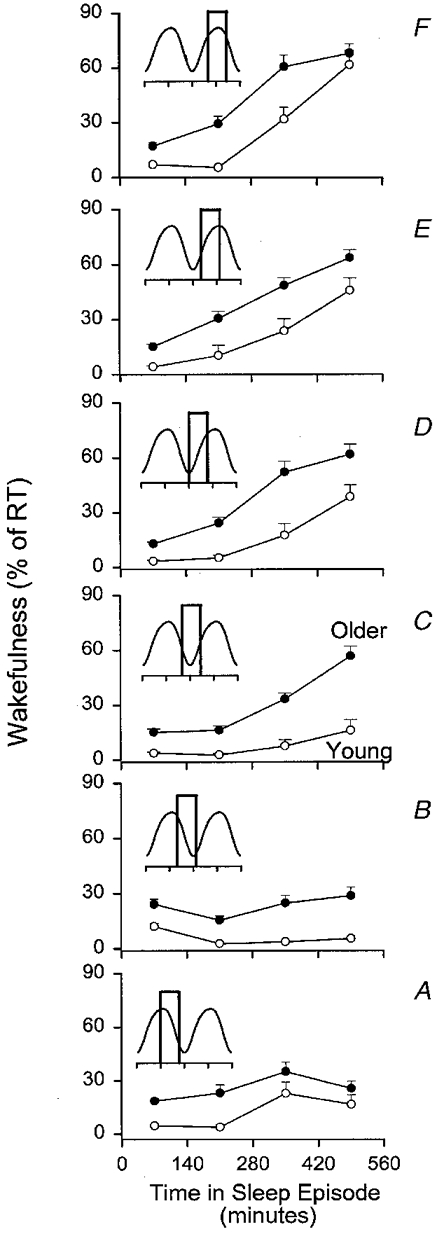
Sleep episodes were sorted according to the circadian phase of the core body temperature rhythm at lights out (resolution, 60 deg). A, sleep episodes starting at 180 deg (range, 150-210 deg); B, 240 deg (range, 210-270 deg); C, 300 deg (range, 270-330 deg); D, 360 deg (range, 270-390 (i.e. 30) deg; E, 60 deg (range, 30-90 deg) F, 120 deg (range, 90-150 deg). Wakefulness is expressed as percentage of recording time. Insets reflect the circadian component of the body temperature rhythm in young and older subjects and boxes indicate (approximately) the part of the circadian cycle traversed from start of sleep to end of sleep.
Next we investigated whether this differential interaction between the sleep-dependent and circadian regulation of wakefulness in the two age groups was also present when circadian phase was determined on the basis of the plasma melatonin rhythm in the 11 young and 10 older subjects for whom sufficient plasma melatonin data were available. The circadian variation of wakefulness within sleep was computed per quarter of the sleep episode. In older people more wakefulness was observed in all quarters of the sleep episode (age: F1,19= 42·40, P < 0·0001). Wakefulness increased from the first to the fourth quarter (F3,57= 74·91, P < 0·0001) in both age groups, and was modulated by the circadian phase of the melatonin rhythm (F11,209= 23·74, P < 0·0001). The interaction between the factors quarter of sleep episode and age was significant (F3,57= 3·40, P= 0·0309) indicating a more rapid deterioration of sleep consolidation in older people. Although the interaction between the factors age and circadian phase was not significant (F11,209= 1·02, P= 0·424) the three-way interaction (age-quarter of sleep episode-circadian phase) was significant (F33,627= 1·89, P= 0·0134). This three-way interaction may indicate that when circadian phase and the time since start of sleep episode change simultaneously sleep efficiency of young and older people is affected differently. In the first quarter of the sleep episode the circadian modulation of wakefulness was minor in both age groups and sleep was more disrupted in older people at all circadian phases. Most wakefulness was observed just prior to the onset of nocturnal melatonin secretion in both age groups (Fig. 7, I). In the second quarter of the scheduled sleep episode wakefulness increased, especially in the older people when the second quarter occurred outside the phase during which melatonin is elevated in plasma. In the young people the circadian variation of wakefulness was very small in this second quarter (Fig. 7, II). In the third quarter, a robust circadian modulation of wakefulness was observed in both the young and the older people. In both age groups lowest values of wakefulness were observed when this third quarter coincided with the phase during which melatonin was present, but more wakefulness was observed in the older people. After the offset of the melatonin rhythm, wakefulness gradually increased and reached a maximum shortly before or at the onset of nocturnal melatonin secretion in the two age groups. Wakefulness in the third quarter of the sleep episodes dropped precipitously when it occurred after the onset of nocturnal melatonin secretion (Fig. 7, III). In the last quarter of the sleep episode a further increase of wakefulness was observed, especially in the sleep episodes of the young subjects occurring outside that part of the circadian cycle during which melatonin was elevated in plasma. In the young subjects wakefulness remained at very low levels in the last quarter of scheduled sleep episodes that coincided with the phase during which melatonin was elevated. In contrast, in the older subjects wakefulness was at 32 % when the last quarter coincided with the melatonin maximum and reached 62 % when the last quarter coincided with the end of the nocturnal melatonin peak, i.e. 90 deg. Corresponding values of wakefulness in the young subjects were 6 and 15 %, respectively (Fig. 7, IV).
Figure 7. Variation of wakefulness within quarters of sleep episodes (I-IV) as a function of the circadian phase of the plasma melatonin rhythm in older (•) and young (^) subjects.
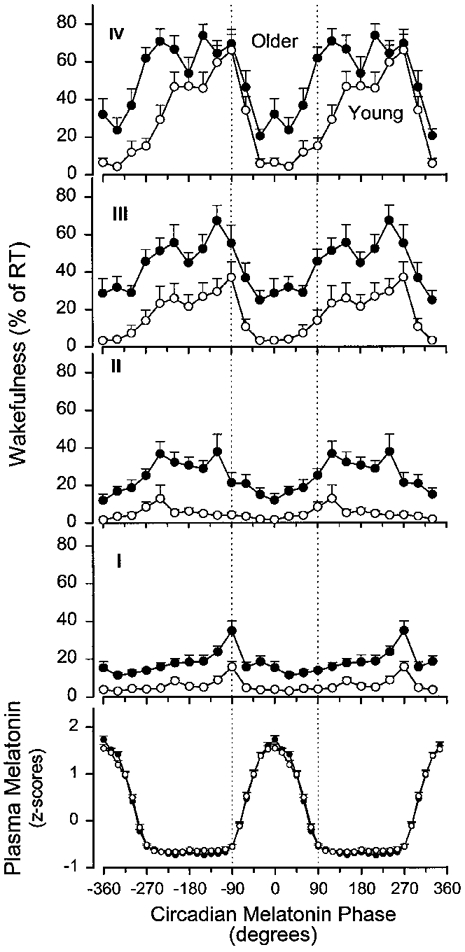
The fitted maximum of the melatonin rhythm is represented by 0 deg. Wakefulness is expressed as percentage of recording time obtained in each bin. Bin width is 30 deg for wakefulness and data are plotted at the mid-points of each bin. Melatonin data are expressed as z-scores and plotted with a resolution of 15 deg. Vertical dotted lines are plotted at the onset of melatonin secretion (-90 deg) and at the end of that part of the circadian cycle during which melatonin was elevated in plasma (90 deg). Vertical bars represent the between-subject s.e.m. within each age group (•: older people, n= 10; ^: young people, n= 11). Data are double plotted.
DISCUSSION
The data confirm the profound effect of age on sleep structure, sleep duration and sleep consolidation in healthy people without sleep complaints. These changes persist when sleep is scheduled to occur at many circadian phases and the duration of wakefulness preceding sleep is similar in the young and older age groups. This suggests that changes in sleep consolidation and sleep structure are an intrinsic characteristic of the ageing process in healthy people. The circadian modulation of sleep structure and sleep consolidation both co-vary with the circadian rhythm of plasma melatonin and body temperature in young and older people. The strong effects of the sleep process itself on these parameters are qualitatively similar in older and young people. Comparison of the interaction of the circadian and sleep-dependent, i.e. homeostatic, processes in young and older subjects indicates that quantitative age-related changes in both the homeostatic process and the circadian process, and in their (non-additive) interaction, are present and that these changes have a major impact on sleep structure and consolidation in older people. In particular, the interaction between an age-related reduction in the strength of the circadian drive for sleep in the morning hours and the sleep-dependent decline in sleep consolidation results in an apparent internal circadian advance, i.e. relative to the body temperature and melatonin rhythm, of the propensity to awaken from sleep in older people.
Sleep during entrainment and the rhythms of body temperature and plasma melatonin
The baseline sleep parameters obtained in our older subjects are in good agreement with previous reports confirming the age-related reduction in SWS and sleep efficiency, the increase in stage 1 sleep and the reduced polarity of REM sleep (Webb & Agnew, 1971; Miles & Dement, 1980; Webb, 1981; Hirshkowitz et al. 1992). These data also confirm the fact that, when sleeping at their habitual bedtimes, the latency to sleep initiation is similar in healthy young and older people. During entrainment to the 24 h day, the phase relationship between the sleep-wake cycle and the endogenous circadian temperature rhythm exhibited larger variability in this sample of older subjects compared with the young subjects, and the timing of the habitual sleep episodes was slightly earlier relative to the temperature cycle, in accordance with a recent analysis in a much larger group that included these subjects (Duffy et al. 1998). This indicates that in older people the sleep-wake cycle is not only entrained to an earlier clock time but also to an earlier endogenous circadian phase of the temperature cycle. In both older and young people, the fitted melatonin maximum occurred shortly before the temperature nadir, confirming the earlier reported persistent phase relationship between these two markers of the circadian pacemaker (Shanahan & Czeisler, 1991), although minor changes in this phase relationship between melatonin and temperature may occur with age (see Fig. 3A and B). Our assessment of the endogenous amplitude of the core body temperature rhythm indicates that the age-related reduction of the endogenous amplitude, previously observed during constant routine conditions and associated sustained wakefulness (Czeisler et al. 1992), is also present during desynchrony of the sleep- wake cycle and endogenous circadian systems. In the present analysis both melatonin and body temperature served primarily as phase markers of the circadian timing system. More extensive discussions on age-related changes - or the absence thereof - in the endogenous amplitude of these variables and the problems associated with the assessment of these parameters can be found elsewhere (Van Coevorden et al. 1991; Czeisler et al. 1992; Monk et al. 1995; Duffy et al. 1998).
Sleep during desynchrony of the rest-activity cycle and the endogenous rhythms of body temperature and plasma melatonin
Age-related changes in sleep structure, as observed during entrainment, persisted during desynchrony of the sleep- wake cycle and the rhythms of body temperature and melatonin. This general conclusion extends a previous report in which sleep structure was assessed in older people living in an environment without time cues (Weitzman et al. 1982). In that study the sleep-wake cycle remained synchronized with the free running rhythm of body temperature, and sleep structure was therefore not assessed at many different circadian phases. In the present study sleep episodes occurred at virtually all phases of the circadian cycle. At no circadian phase was sleep efficiency and slow wave sleep in the older people restored to the values obtained in the young subjects during entrainment. This held for both the older women and older men. In general the effects of circadian phase and the effects of ageing were qualitatively similar in older men and women (data not shown) although some indication of sex differences was obtained in particular for SWS, in accordance with previous reports. Due to the small number of older women in our study population, we were not able assess the statistical significance of these effects of sex. However, since in most studies on ageing, sleep and circadian rhythms it is reported that the effects of ageing on sleep are less pronounced in women (Rediehs et al. 1990), it is unlikely that the inclusion of women in our group of older people led to an overestimation of the effects of ageing.
Non-REM sleep
The marked increase of stage 1 sleep in older people while sleeping at all circadian phases, and its sleep-dependent increase are indicative of the shallower sleep of older people. The preservation of stage 2 sleep should not be interpreted as indicating that this sleep stage is not significantly affected by either age or circadian phase. Computerized analysis of sleep spindle characteristics in stage 2 sleep in a subset of the subjects reported here demonstrated significant reductions of spindle incidence and amplitude, and an increase in their frequency associated with ageing (Wei et al. 1999). Furthermore, the amplitude of the circadian modulation of spindle characteristics was markedly reduced in older people. The reduction of stage 4 sleep in older people, the lack of substantial circadian variation of this sleep stage as well as the pronounced sleep-dependent decline of this sleep stage are all in accordance with previous observations in young and older people. In the context of the proposed role of SWS in sleep consolidation, i.e. SWS being an indicator of the homeostatic drive for sleep (Webb & Agnew, 1971; Daan et al. 1984), and the observation that the reduction of SWS and sleep efficiency was observed at all circadian phases, it may be hypothesized that the age-related changes in SWS play a primary role in the age-related changes in sleep consolidation. However, whereas the largest age-related differences in stage 4 sleep were observed in the initial part of the sleep episode, the largest differences in sleep consolidation were located in the second half of sleep. In this latter part of the sleep episode, stage 4 sleep was at similar levels in the two age groups. Quantitative analyses of low frequency components of the EEG, which are more sensitive than visual scoring of the sleep EEG, may provide further insights in the relationships between these parameters.
REM sleep
REM sleep was preserved in the older age group and exhibited the well-known circadian variation with a crest located shortly after the temperature nadir in both the older and young subjects (Czeisler et al. 1980; Zulley, 1980; Dijk & Czeisler, 1995). The amplitude of the circadian variation appeared somewhat diminished in the older subjects, with slightly higher values before and lower values at the crest, but these effects were small. The observed bimodal distribution of REM latencies in both young and older people is somewhat surprising. The observation that REM latencies were significantly shorter in the older subjects especially when sleep coincided with the falling limb of the temperature cycle, during the part of the circadian cycle in which melatonin levels were high, points to the importance of interactions between the effects of ageing and circadian phase. This observation also indicates that the shorter latencies often observed under baseline conditions are not necessarily related to a phase advance of the circadian timing system but may reflect an apparent age-related internal phase advance of the REM sleep propensity rhythm. Similar arguments apply to the sleep-dependent regulation of REM sleep. Whereas in the young subjects a sleep-dependent disinhibition of REM sleep was observed, i.e. an increase of REM sleep in the course of sleep, this was virtually absent in the older people. This effect was primarily related to the increase of REM sleep in the first quarter of the sleep episode in the older people. This lack of polarity and the increase of REM sleep in the beginning of sleep may indeed reflect a diminished inhibition of REM sleep by SWS in older people as has been hypothesized (Bliwise, 1993).
Sleep initiation
The scheduled duration of wakefulness preceding each sleep episode was 18 h 40 min in both the older and young subjects. Although we did not continuously monitor the EEG during scheduled wake episodes, it is unlikely that the older subjects napped extensively or more so than the young subjects. This is because subjects were instructed not to sit or lie on the bed, no easy chairs were available in the suites and, most importantly, technical staff video-monitored the subjects and entered the suites frequently to administer visual analog scales for mood and sleepiness assessments (frequency, 3 times per hour) or to draw blood samples (frequency, 1 per hour). Thus we are confident that all subjects remained awake throughout most of each scheduled waking episode.
Sleep duration varies as a function of circadian phase and thereby the homeostatic drive preceding each sleep episode varies slightly as a function of circadian phase. This potentially confounds the assessment of the circadian variation of the propensity to initiate sleep. However, this factor is unlikely to have had a major impact because of the long duration of the scheduled wake episodes and the relatively small variation in total sleep time, in conjunction with the presumed exponential saturating increase in sleep propensity as a function of the duration of wakefulness (Daan et al. 1984).
The observation that at all circadian phases older subjects slept less than the young people constitutes another potential confounding factor in the assessment of the effects of age on the ability to initiate sleep. A reduction in sleep time preceding an assessment of sleep latency results in a reduction of sleep latency (Carskadon & Dement, 1981). In view of this observation it is remarkable that the circadian variation of sleep initiation was very similar in the two age groups during the desynchrony portion of the experiment. The circadian variation in the propensity to initiate sleep as obtained in the older people exhibited the paradoxical pattern first observed by Weitzman et al. (1974) and reported thereafter (see Dijk & Czeisler, 1994, for references), i.e. sleep latencies are longest shortly before the onset of melatonin secretion, which under entrained conditions occurs at approximately 21.00-23.00 h, and then suddenly become shorter after the onset of melatonin secretion (Shochat et al. 1997). Sleep latencies in the older people were not shorter than sleep latencies in the young people, even though the older people obtained less sleep in the prior sleep episodes. One implication of this finding is that the often inferred reduced amplitude of the output of the circadian pacemaker does not appear to apply to the circadian rhythm of sleep initiation (i.e. the circadian variation in wake maintenance). This conclusion is in accordance with an analysis of daytime sleepiness in healthy ‘old old’ subjects from which it was concluded that healthy people in old age may have a level of daytime sleepiness no greater, and perhaps even less, than that seen in healthy young subjects (Reynolds et al. 1991). Our data are similar to those in a recent report on the circadian variation in sleep propensity in older people as assessed by the ultra-short sleep-wake schedule (Haimov & Lavie, 1997). In that study, in which sleep propensity was analysed as a function of clock time and information on circadian phase was not available, shorter sleep latencies in older people were only observed in the evening hours. During the night, sleep latencies in older people were actually longer than in young subjects. These findings lead us to conclude that in healthy older people, the circadian variation in the drive for wakefulness (which becomes progressively stronger on the rising limb of the core body temperature rhythm and which peaks shortly before the onset of the nocturnal melatonin secretion and declines abruptly thereafter) is not markedly diminished. Further studies in which the homeostatic drive preceding the assessment of sleep latency is lower may provide more accurate assessment of the circadian variation in sleep propensity as indexed by sleep latency.
Sleep consolidation
Wakefulness in scheduled sleep episodes was higher in older people but not just at the very end of sleep episodes. This observation is in accordance with data demonstrating an increased frequency of sleep interruption in the older population and points to the importance of continuous assessment of sleep. Consolidation of sleep deteriorated as sleep progressed in both age groups, but more rapidly so in the older people. This may indicate a diminished homeostatic drive for sleep or a faster dissipation of this drive in older people. The circadian modulation of sleep consolidation indicated maximum disruption of sleep shortly before the onset of melatonin secretion and a sudden diminishment of this disruption after the onset of melatonin secretion. The latter phenomenon was even observed when sleep pressure was relatively low, i.e. in the second half of the sleep episode. This is in accordance with the proposed role of endogenous melatonin secretion in the silencing of the waking signal (Lavie, 1997; Sack et al. 1997). This waking signal - thought to be generated by the SCN - becomes progressively stronger on the rising limb of the temperature cycle. Recent data indicate that the binding of melatonin to melatonin receptors may inhibit this wake-promoting signal (Liu et al. 1997). The significant interactions between age, circadian phase and time since start of sleep episode provide direct evidence that sleep consolidation in older people is more susceptible to misalignment between the sleep-wake cycle and the endogenous circadian cycles than sleep consolidation in young people. In particular, sleep consolidation in the older subjects became very low when the last quarter of the sleep episodes was located at or after the temperature nadir, i.e. shortly after the melatonin peak, the time at which the endogenous secretion of melatonin subsides (Brown et al. 1997). This observation is in accordance with the analysis of self-reported awakening in these same subjects in this protocol (Duffy et al. 1998), and also with reports on age-related differences in recovery from simulated jet lag (Moline et al. 1992).
In our study the changes in sleep consolidation were observed while the older healthy subjects had no knowledge of clock time and had no access to light sources. Furthermore, all comparisons between young and older subjects were made with respect to internal circadian phase. This demonstrates that these age-related changes are likely to be linked to age-related changes in the neurobiology of sleep regulation rather than to environmental or psychological factors, the clock time of entrainment of the circadian pacemaker or to ailments associated with ageing.
In view of the near-equal contribution of sleep homeostasis and circadian rhythmicity to sleep consolidation (Dijk & Czeisler, 1994), their non-additive interaction (Dijk & Czeisler, 1995) and the lack of experiments in which homeostatic drive for sleep has been experimentally manipulated in older people, we can only speculate about the potential mechanisms involved in this apparent internal phase advance of the propensity to wake up from sleep. Under the assumption that the circadian pacemaker actively promotes both wakefulness during the biological day (Edgar et al. 1993; Dijk & Czeisler, 1994) and sleep during the biological night (Dijk et al. 1997) - perhaps via SCN activation of sleep-active neurons in the ventrolateral preoptic neurons (Sherin et al. 1996) - we posit that age-related disruption of nocturnal sleep consolidation is related to a reduction of the homeostatic drive for sleep in conjunction with the diminished strength of the circadian promotion of sleep. Our current analyses provide near-direct evidence for a more rapid dissipation of sleep consolidation in older people. Data supporting the circadian component of this hypothesis include the observation that during sustained wakefulness subjective activation and performance in older people exhibit a more linear decline associated with time awake whereas in young subjects this decline contains a more pronounced circadian component especially in the morning hours (Monk et al. 1992). Our current data, as well as the recent data by Haimov & Lavie (1997), show that the diurnal and circadian variation of the ability to initiate sleep is very similar in older and young people except for during the early morning hours - a phase at which the circadian pacemaker is thought to maximally promote sleep. It is at this phase that the older people are less sleepy.
The physiological mechanisms of this reduced homeostatic drive as well as the reduced circadian drive for sleep are not known, and accepted pharmacological interventions are not available. Pharmacological manipulation of homeostatic sleep pressure appears a promising avenue in particular because the neurobiological mechanisms of sleep homeostasis and those responsible for generating mechanisms of low frequency oscillations in the EEG have been in part elucidated (Steriade et al. 1993). A scheduled increase in the duration of wakefulness preceding sleep - an obvious non-pharmacological approach - to enhance sleep consolidation is unlikely to become a popular therapy. Furthermore, enhancement of SWS is not a characteristic effect of hypnotics currently prescribed for older people.
It may be possible to manipulate the circadian component of sleep consolidation by strengthening the circadian variation of sleep propensity via administration of exogenous melatonin. Some promising results have been reported, although these effects appear to be most effective in promoting sleep consolidation in sleep episodes scheduled outside the phase of endogenous melatonin secretion (Haimov et al. 1994; Garfinkel et al. 1995; Hughes & Badia, 1997).
In view of the observed increased sensitivity of sleep duration and consolidation to phase misalignment in older people and the observation that under baseline conditions the phase relationship between circadian rhythms and self-selected sleep-wake time is more variable, it seems reasonable to theorize that accurate control of this phase relationship - for instance by scheduled light exposure - may contribute to the treatment of sleep disturbances in older people. Some positive results from such an approach have been reported (Campbell et al. 1993).
In conclusion, age-related changes in sleep duration and consolidation appear to be mediated in part by changes in the homeostatic and circadian regulation of sleep and their interaction. A better characterization of the neurophysiological substrates of these components and their interaction will be needed to develop new approaches for alleviating age-related sleep disturbances.
Acknowledgments
We wish to thank the subject volunteers for their participation, the subject recruiters of our laboratory, the staff of the General Clinical Research Center and the staff of the Environmental Scheduling Facility and Intensive Physiological Monitoring Unit. This research was supported in part by a grant (P01 AG09975) from the National Institute on Aging. The experiments were conducted in a General Clinical Research Center supported by grant M01 RR02635 from the National Center for Research Resources.
References
- Åkerstedt T, Gillberg M. The circadian variation of experimentally displaced sleep. Sleep. 1981;4:159–169. doi: 10.1093/sleep/4.2.159. [DOI] [PubMed] [Google Scholar]
- Åkerstedt T, Gillberg M. Sleep duration and the power spectral density of the EEG. Electroencephalography and Clinical Neurophysiology. 1986;64:119–122. doi: 10.1016/0013-4694(86)90106-9. 10.1016/0013-4694(86)90106-9. [DOI] [PubMed] [Google Scholar]
- Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- Brown EN, Choe Y, Shanahan TL, Czeisler CA. A mathematical model of diurnal variations in human plasma melatonin levels. American Journal of Physiology. 1997;272:E506–516. doi: 10.1152/ajpendo.1997.272.3.E506. [DOI] [PubMed] [Google Scholar]
- Brown EN, Czeisler CA. The statistical analysis of circadian phase and amplitude in constant-routine core-temperature data. Journal of Biological Rhythms. 1992;7:177–202. doi: 10.1177/074873049200700301. [DOI] [PubMed] [Google Scholar]
- Campbell SS, Dawson D, Anderson MW. Alleviation of sleep maintenance insomnia with timed exposure to bright light. Journal of the American Geriatrics Society. 1993;41:829–836. doi: 10.1111/j.1532-5415.1993.tb06179.x. [DOI] [PubMed] [Google Scholar]
- Campbell SS, Murphy PJ. Relationships between sleep and body temperature in middle-aged and older subjects. Journal of the American Geriatrics Society. 1998;46:458–462. doi: 10.1111/j.1532-5415.1998.tb02466.x. [DOI] [PubMed] [Google Scholar]
- Carrier J, Monk TH, Buysse DJ, Kupfer DJ. Inducing a 6-hour phase advance in the elderly: effects on sleep and temperature rhythms. Journal of Sleep Research. 1996;5:99–105. doi: 10.1046/j.1365-2869.1996.00015.x. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107–113. doi: 10.1111/j.1469-8986.1981.tb02921.x. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Dijk D-J, Rimmer DW, Ronda JM, Allan JS, Emens JS, Kronauer RE. Reassessment of the intrinsic period (τ) of the human circadian pacemaker in young and older subjects. Sleep Research. 1995;24A:505. [Google Scholar]
- Czeisler CA, Dumont M, Duffy JF, Steinberg JD, Richardson GS, Brown EN, Sánchez R, Ríos CD, Ronda JM. Association of sleep-wake habits in older people with changes in output of circadian pacemaker. Lancet. 1992;340:933–936. doi: 10.1016/0140-6736(92)92817-y. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Weitzman ED, Moore-Ede MC, Zimmerman JC, Knauer RS. Human sleep: Its duration and organization depend on its circadian phase. Science. 1980;210:1264–1267. doi: 10.1126/science.7434029. [DOI] [PubMed] [Google Scholar]
- Daan S, Beersma DGM, Borbély AA. Timing of human sleep: Recovery process gated by a circadian pacemaker. American Journal of Physiology. 1984;246:R161–178. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- Dijk D-J, Beersma DGM, Daan S. EEG power density during nap sleep: reflection of an hourglass measuring the duration of prior wakefulness. Journal of Biological Rhythms. 1987;2:207–219. doi: 10.1177/074873048700200304. [DOI] [PubMed] [Google Scholar]
- Dijk D-J, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neuroscience Letters. 1994;166:63–68. doi: 10.1016/0304-3940(94)90841-9. 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- Dijk D-J, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves and sleep spindle activity in humans. Journal of Neuroscience. 1995;15:3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk D-J, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. Journal of Sleep Research. 1992;1:112–117. doi: 10.1111/j.1365-2869.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]
- Dijk D-J, Shanahan TL, Duffy JF, Ronda JM, Czeisler CA. Variation of electroencephalographic activity during non-rapid eye movement and rapid eye movement sleep with phase circadian melatonin rhythm in humans. The Journal of Physiology. 1997;505:851–858. doi: 10.1111/j.1469-7793.1997.851ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Dijk D-J, Klerman EB, Czeisler CA. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. American Journal of Physiology. 1998;275:R1478–1487. doi: 10.1152/ajpregu.1998.275.5.r1478. [DOI] [PubMed] [Google Scholar]
- Edgar DM, Dement WC, Fuller CA. Effect of SCN lesions on sleep in squirrel monkeys: Evidence for opponent processes in sleep-wake regulation. Journal of Neuroscience. 1993;13:1065–1079. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Kupfer DJ. Effects of age on delta and REM sleep parameters. Electroencephalography and Clinical Neurophysiology. 1989;72:118–125. doi: 10.1016/0013-4694(89)90172-7. 10.1016/0013-4694(89)90172-7. [DOI] [PubMed] [Google Scholar]
- Feinberg I. Changes in sleep cycle patterns with age. Journal of Psychiatric Research. 1974;10:283–306. doi: 10.1016/0022-3956(74)90011-9. 10.1016/0022-3956(74)90011-9. [DOI] [PubMed] [Google Scholar]
- Garfinkel D, Laudon M, Nof D, Zisapel N. Improvement of sleep quality in elderly people by controlled-release melatonin. Lancet. 1995;346:541–544. doi: 10.1016/s0140-6736(95)91382-3. 10.1016/S0140-6736(95)91382-3. [DOI] [PubMed] [Google Scholar]
- Haimov I, Laudon M, Zisapel N, Souroujon M, Nof D, Shlitner A, Herer P, Tzischinsky O, Lavie P. Sleep disorders and melatonin rhythms in elderly people. British Medical Journal. 1994;309:167. doi: 10.1136/bmj.309.6948.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimov I, Lavie P. Circadian characteristics of sleep propensity function in healthy elderly: A comparison with young adults. Sleep. 1997;20:294–300. doi: 10.1093/sleep/20.4.294. [DOI] [PubMed] [Google Scholar]
- Hirshkowitz M, Moore CA, Hamilton CR, III, Rando KC, Karacan I. Polysomnography of adults and elderly: Sleep architecture, respiration, and leg movement. Journal of Clinical Neurophysiology. 1992;9:56–62. [PubMed] [Google Scholar]
- Hughes RJ, Badia P. Sleep-promoting and hypothermic effects of daytime melatonin administration in humans. Sleep. 1997;20:124–131. [PubMed] [Google Scholar]
- Hume K, Mills J. Rhythms of REM and slow-wave sleep in subjects living on abnormal time schedules. Waking and Sleeping. 1977;1:291–296. [Google Scholar]
- Klein DC, Moore RY, Reppert SM. Suprachiasmatic Nucleus: The Mind's Clock. New York: Oxford University Press; 1991. [Google Scholar]
- Lavie P. Melatonin: Role in gating nocturnal rise in sleep propensity. Journal of Biological Rhythms. 1997;12:657–665. doi: 10.1177/074873049701200622. [DOI] [PubMed] [Google Scholar]
- Liu C, Weaver DR, Jin X, Shearman LP, Pieschl RL, Gribkoff VK, Reppert SM. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron. 1997;19:91–102. doi: 10.1016/s0896-6273(00)80350-5. 10.1016/S0896-6273(00)80350-5. [DOI] [PubMed] [Google Scholar]
- Miles LE, Dement WC. Sleep and aging. Sleep. 1980;3:119–220. [PubMed] [Google Scholar]
- Moline ML, Pollak CP, Monk TH, Lester LS, Wagner DR, Zendell SM, Graeber RC, Salter CA, Hirsch E. Age-related differences in recovery from simulated jet lag. Sleep. 1992;15:28–40. doi: 10.1093/sleep/15.1.28. [DOI] [PubMed] [Google Scholar]
- Monk TH, Buysse DJ, Reynolds CF, III, Jarrett DB, Kupfer DJ. Rhythmic vs. homeostatic influences on mood, activation, and performance in young and old men. Journal of Gerontology. 1992;47:P221–227. doi: 10.1093/geronj/47.4.p221. [DOI] [PubMed] [Google Scholar]
- Monk TH, Buysse DJ, Reynolds CF, III, Kupfer DJ, Houck PR. Circadian temperature rhythms of older people. Experimental Gerontology. 1995;30:455–474. doi: 10.1016/0531-5565(95)00007-4. 10.1016/0531-5565(95)00007-4. [DOI] [PubMed] [Google Scholar]
- Monk TH, Moline ML. Removal of temporal constraints in the middle-aged and elderly: effects on sleep and sleepiness. Sleep. 1988;11:513–520. doi: 10.1093/sleep/11.6.513. [DOI] [PubMed] [Google Scholar]
- Myers BL, Badia P. Changes in circadian rhythms and sleep quality with aging: Mechanisms and interventions. Neuroscience and Biobehavioral Reviews. 1995;19:553–571. doi: 10.1016/0149-7634(95)00018-6. 10.1016/0149-7634(95)00018-6. [DOI] [PubMed] [Google Scholar]
- Prinz PN, Vitiello MV, Raskind MA, Thorpy MJ. Geriatrics: Sleep disorders and aging. New England Journal of Medicine. 1990;323:520–526. doi: 10.1056/NEJM199008233230805. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Washington, DC: US Government Printing Office; 1968. [Google Scholar]
- Rediehs MH, Reis JS, Creason NS. Sleep in old age: Focus on gender differences. Sleep. 1990;13:410–424. [PubMed] [Google Scholar]
- Reynolds CF, III, Jennings JR, Hoch CC, Monk TH, Berman SR, Hall FT, Matzzie JV, Buysse DJ, Kupfer DJ. Daytime sleepiness in healthy ‘old old’: A comparison with young adults. American Geriatric Association. 1991;39:957–962. doi: 10.1111/j.1532-5415.1991.tb04041.x. [DOI] [PubMed] [Google Scholar]
- Reynolds CF, III, Kupfer DJ, Hoch CC, Houck PR, Stack JA, Berman SR, Campbell PI, Zimmer B. Sleep deprivation as a probe in the elderly. Archives of General Psychiatry. 1987;44:982–990. doi: 10.1001/archpsyc.1987.01800230062011. [DOI] [PubMed] [Google Scholar]
- Sack RL, Hughes RJ, Edgar DM, Lewy AJ. Sleep-promoting effects of melatonin: At what dose, in whom, under what conditions, and by what mechanisms? Sleep. 1997;20:908–915. doi: 10.1093/sleep/20.10.908. [DOI] [PubMed] [Google Scholar]
- Shanahan TL, Czeisler CA. Light exposure induces equivalent phase shifts of the endogenous circadian rhythms of circulating plasma melatonin and core body temperature in men. Journal of Clinical Endocrinology and Metabolism. 1991;73:227–235. doi: 10.1210/jcem-73-2-227. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- Shochat T, Luboshitzky R, Lavie P. Nocturnal melatonin onset is phase locked to the primary sleep gate. American Journal of Physiology. 1997;273:R364–370. doi: 10.1152/ajpregu.1997.273.1.R364. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Van Coevorden A, Mockel J, Laurent E, Kerkhofs M, L'Hermite-Balériaux M, Decoster C, Néve P, Van Cauter E. Neuroendocrine rhythms and sleep in aging men. American Journal of Physiology. 1991;260:E651–661. doi: 10.1152/ajpendo.1991.260.4.E651. [DOI] [PubMed] [Google Scholar]
- van Someren EJW, Mirmiran M, Swaab DF. Non-pharmacological treatment of sleep and wake disturbances in aging and Alzheimer's disease: Chronobiological perspectives. Behavioural Brain Research. 1993;57:235–253. doi: 10.1016/0166-4328(93)90140-l. 10.1016/0166-4328(93)90140-L. [DOI] [PubMed] [Google Scholar]
- Webb WB. Sleep stage responses of older and younger subjects after sleep deprivation. Electroencephalography and Clinical Neurophysiology. 1981;52:368–371. doi: 10.1016/0013-4694(81)90065-1. 10.1016/0013-4694(81)90065-1. [DOI] [PubMed] [Google Scholar]
- Webb WB, Agnew HW. Stage 4 sleep: Influence of time course variables. Science. 1971;174:1354–1356. doi: 10.1126/science.174.4016.1354. [DOI] [PubMed] [Google Scholar]
- Wei HG, Riel E, Czeisler CA, Dijk D-J. Attenuated amplitude of circadian and sleep-dependent modulation of sleep spindle characteristics in elderly human subjects. Neuroscience Letters. 1999;260:29–32. doi: 10.1016/s0304-3940(98)00851-9. 10.1016/S0304-3940(98)00851-9. [DOI] [PubMed] [Google Scholar]
- Weitzman ED, Moline ML, Czeisler CA, Zimmerman JC. Chronobiology of aging: Temperature, sleep-wake rhythms and entrainment. Neurobiology of Aging. 1982;3:299–309. doi: 10.1016/0197-4580(82)90018-5. 10.1016/0197-4580(82)90018-5. [DOI] [PubMed] [Google Scholar]
- Weitzman ED, Nogeire C, Perlow M, Fukushima D, Sassin J, McGregor P, Gallagher TF, Hellman L. Effects of a prolonged 3-hour sleep-wake cycle on sleep stages, plasma cortisol, growth hormone, and body temperature in man. Journal of Clinical Endocrinology and Metabolism. 1974;38:1018–1030. doi: 10.1210/jcem-38-6-1018. [DOI] [PubMed] [Google Scholar]
- Zulley J. Distribution of REM sleep in entrained 24 h and free-running sleep-wake cycles. Sleep. 1980;2:377–389. [PubMed] [Google Scholar]
- Zulley J, Wever R, Aschoff J. The dependence of onset and duration of sleep on the circadian rhythm of rectal temperature. Pflügers Archiv. 1981;391:314–318. doi: 10.1007/BF00581514. [DOI] [PubMed] [Google Scholar]


