Abstract
The degree of cell-to-cell coupling between ventricular myocytes of neonatal rats appeared well preserved when studied in the perforated version of the patch clamp technique or, in double whole-cell conditions, when ATP was present in the patch pipette solution. In contrast, when ATP was omitted, the amplitude of junctional current rapidly declined (rundown).
To examine the mechanism(s) of ATP action, an ‘internal perfusion technique’ was adapted to dual patch clamp conditions, and reintroduction of ATP partially reversed the rundown of junctional channels.
Cell-to-cell communication was not preserved by a non-hydrolysable ATP analogue (5′-adenylimidodiphosphate, AMP-PNP), indicating that the effect most probably did not involve direct interaction of ATP with the channel-forming proteins.
An ATP analogue supporting protein phosphorylation but not active transport processes (adenosine 5′-O-(3-thiotriphosphate), ATPγS) maintained normal intercellular communication, suggesting that the effect was due to kinase activity rather than to altered intracellular Ca2+.
A broad spectrum inhibitor of endogenous serine/threonine protein kinases (H7) reversibly reduced the intercellular coupling. A non-specific exogenous protein phosphatase (alkaline phosphatase) mimicked the effects of ATP deprivation. The non-specific inhibition of endogenous protein phosphatases resulted in the preservation of substantial cell-to-cell communication in ATP-free conditions.
The activity of gap junctional channels appears to require both the presence of ATP and protein kinase activity to counteract the tonic activity of endogenous phosphatase(s).
Gap junctions are specialized plasma membrane regions containing transmembrane channels that directly link the cytoplasms of adjoining cells and mediate the reciprocal exchange of ions and low molecular weight molecules (<1000 Da), including second messengers (such as cAMP, inositol trisphosphate and Ca2+) (for review, see Sáez et al. 1993). Structural studies have demonstrated that each gap junctional channel is formed by the extracellular interaction of two hemi-channels (connexons). Each connexon is a hexameric assembly of protein subunits (connexins) which delineate an aqueous pore. Connexins are homologous proteins encoded by a multigene family and are named according to their predicted molecular weight (Beyer et al. 1987). Connexin 43 (Cx43), widely distributed in different cell types, is the main gap junction protein expressed in ventricular myocytes, although Cx40 and Cx45 have been reported to be expressed as well (Kanter et al. 1992). Cx43 is a phosphoprotein, and one dephosphorylated (41 kDa) and two phosphorylated (43 and 47 kDa) forms of the protein have been identified (for review, see Sáez et al. 1993). In neonatal heart cells in primary culture, Cx43 is predominantly phosphorylated, and a large body of evidence suggests that changes in the connexin phosphorylation state could modulate the cell-to-cell communication (reviewed in Sáez et al. 1993). A nucleophilic agent, 2,3-butanedione monoxime (BDM), considered to have a ‘chemical phosphatase’ activity (Coulombe et al. 1990) or to enhance the activity of endogenous phosphatases (Zimmermann et al. 1996), interrupted cell-to-cell communication, and part of its action seemed to result from a dephosphorylation process (Verrecchia & Hervé, 1997a).
To sustain a sufficient phosphorylation state, high energy phosphates have to be present. In intact cardiac myocytes under physiological conditions, intracellular ATP concentration is kept at a level ranging from 3·0 to 7·5 mM (Allen et al. 1985). When conventional dual whole-cell conditions are used, ATP is commonly present in this concentration range in solutions used to fill patch clamp pipettes. When gap junctional conductance between paired cells is determined after excision of one of the cells, Sugiura et al. (1990) showed that ATP has to be present in the bath solution at a comparable concentration level to preserve cell-to-cell communication, but these authors suggested that junctional conductance was regulated through a specific ligand-receptor interaction between ATP and gap junctional proteins, rather than through the promotion of protein phosphorylation. It has also been suggested that intracellular Mg-ATP might be necessary to maintain a low Ca2+ concentration at the inner surface of the plasma membrane (Byerly & Yazejian, 1986), preventing channel inactivation by excessive cytosolic calcium concentration. The inhibition of intercellular communication which accompanied a mild depletion of endogenous ATP concentration, elicited by inhibition of the mitochondrial respiratory chain, was suggested to result from a cytosolic calcium accumulation (Vera et al. 1996).
The aim of the present study was to examine the possible mode(s) of action of ATP on cell-to-cell communication between rat ventricular myocytes.
METHODS
Cultures of newborn rat cardiomyocytes and solutions
Cardiomyocytes were obtained from neonatal (1-2 day old) Wistar rats, killed by cervical dislocation followed by decapitation. Heart ventricles were minced into small pieces (approximately 1 mm3), washed in a Ca2+- and Mg2+-free medium (Spinner's solution, containing (mM): NaCl, 116; KCl, 5·3; NaH2PO4, 8; NaHCO3, 0·3; Hepes, 10; and D-glucose, 5·6 (pH 7·4)) and incubated in the same solution, together with 0·02 % crude trypsin (Boehringer-Mannheim, Meylan, France). Five successive incubations at 37°C for 8 min with continuous stirring were carried out and the successive enzymatic releases, except the first, were cooled at 4°C and centrifuged at 500 g for 5 min. The cell pellets were resuspended in Ham's F10 culture medium (Gibco, Cergy-Pontoise, France) supplemented with 10 % fetal calf serum (Boehringer), 10 % heat-inactivated horse serum (Gibco), penicillin G (100 IU ml−1, Sigma) and streptomycin (50 IU ml−1, Sigma) and preplated in large polystyrene dishes (Nunclon, Roskilde, Denmark) to allow the attachment of non-muscle cells. The cardiac myocytes in the supernatants were counted, diluted with culture medium to reach a final concentration of 300000 cells ml−1 and seeded (about 55000 cells cm−2) in 35 mm disposable plastic Petri dishes. Finally, the cells were incubated at 37°C in a CO2 incubator (5 % CO2-95 % ambient air; pH 7·4). On the second day, the culture medium was replaced by a culture medium devoid of fetal calf serum. The experiments were performed after 2 or 3 days of culture.
Quantitative measurement of cell-to-cell dye transfer and evaluation of junctional conductance were made, at room temperature (22-24°C), after replacing the culture medium with a Tyrode solution (extracellular solution, Table 1). The dishes were then transferred onto the stage of an inverted microscope and the cells were observed by phase-contrast microscopy. The spontaneous synchronized mechanical activity was used as evidence to avoid confusion with non-muscle cells.
Table 1.
Composition (mM) of the extracellular bath solution and of the solutions used to fill the micropipettes
| Intracellular solutions | ||||||
|---|---|---|---|---|---|---|
| Extracellular solution | I | II | III | IV | V | |
| NaCl | 144 | — | — | — | — | — |
| KCl | 5.4 | 140 | 140 | 140 | 140 | 90 |
| MgCl2 | 1 | — | 0 or 1 | 0 | — | — |
| CaCl2 | 2.5 | — | — | — | — | — |
| Hepes | 5 | 10 | 10 | 10 | 10 | 10 |
| EGTA | — | 5 | 5 | 5 | 5 | 5 |
| Mg-ATP | — | 5 | — | 10 | 5 | — |
| GTP | — | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Glucose | 10 | 10 | 14 | 5 | 10 | 14 |
| NaH2PO4 | 0.3 | — | — | — | — | — |
| KF | — | — | — | — | — | 50 |
| Orthovanadate | — | — | — | — | — | 1 |
| Alkaline phosphatase | — | — | — | — | 1 or 10 IU ml−1 | — |
| pH | 7.4 | 7.2 | 7.2 | 7.2 | 7.2 | 7.2 |
The pH of the intracellular solutions was adjusted with NaOH.
Evaluation of the junctional conductance
Junctional conductance was measured using a dual whole-cell patch clamp technique (Neyton & Trautmann, 1985) applied to myocyte cell pairs. Low resistance (1·5-3 MΩ) patch pipettes backfilled with a filtered solution (see Table 1 for composition) connected the cells to their respective feedback amplifiers (Biologic RK 300, Grenoble, France). Macroscopic junctional conductance (Gj) was determined in dual voltage clamp conditions. Both cells of a pair were at first clamped at a common holding potential (Vh, close to -70 mV), then a pulse (10 to 30 mV) was applied to one cell while the other was maintained at Vh to generate a transjunctional voltage difference (Vj). Therefore, when cells in contact were connected by open junctional channels, this voltage gradient induced a junctional current (Ij) flowing through them from one cell to its neighbour, and was compensated by an opposite current supplied by the feedback amplifier connected to the cell maintained at Vh. Gj was then calculated by dividing Ij by the amplitude of the Vj pulse. Current and potential records were digitized, stored and analysed with a personal computer by means of the software package pCLAMP (Axon Instruments). Gj was expressed as means ± standard error of the mean (s.e.m.).
Two recording configurations were used, either the whole-cell (Neyton & Trautmann, 1985) or the perforated patch (Takens-Kwak et al. 1992) configuration. In the latter situation, after the gigaseal formation, the membrane patch under the pipette tip was permeabilized with amphotericin B, a pore-forming antibiotic, instead of being ruptured. Membrane pores made by amphotericin B (or the related compound nystatin) are not voltage dependent, are permeable to monovalent ions (somewhat selective for monovalent cations over anions) and impermeable to multivalent ions and molecules > 0·8 nm in diameter. Therefore, this configuration allows the prevention of both the dilution of cytosolic components and the disruption of the normal intracellular Ca2+ buffering mechanisms.
From a 50 mg ml−1 stock solution of amphotericin B (Sigma) dissolved in DMSO, the antibiotic was added to the pipette filling solution to yield a final concentration of 150 μg ml−1. The solution was subjected to ultrasonication for 60 s to ensure homogeneous distribution of amphotericin B and remained usable for about 1-2 h, after which a fresh solution was prepared. Pipettes were filled by dipping the tip directly into amphotericin B-free solution for a few seconds and then backfilled with the amphotericin B-containing solution. After obtaining gigaohm seals, the access resistance dropped within 8 min to 20-40 MΩ, and experiments were started when the access resistance fell to 30 MΩ. On occasion, the perforated patch spontaneously converted into the whole-cell configuration; this change was evident by the rapid activation of a large leak background current in the related cell, together with a drop in junctional current in the second cell. A contracture of the first cell (and sometimes of both cells) was then observed, preceding cell death.
Internal perfusion technique
ATP was successively removed then reintroduced intracellularly via one of the pipettes by combining the ‘internal perfusion technique’ introduced by Irisawa & Kokubun (1983) with dual patch pipette conditions. In brief, a fine tube (diameter, 100 μm; Phymep, Paris), connected to a reservoir filled with the test solution, was inserted into one of the patch pipettes at a distance of 100-300 μm behind the tip. Exchange of the solution was performed by applying a negative pressure to the back end of the pipette, as illustrated in Fig. 1.
Figure 1. Recording arrangement for the exchange of the dialysing solution of cell 2.
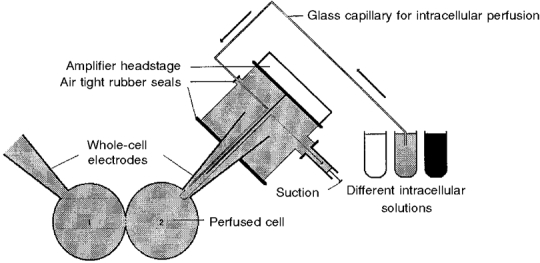
A glass capillary set inside patch pipette 2, 100-300 μm from the tip, allowed, by applying a negative pressure, the exchange of internal solution during the whole-cell recordings of gap junctional conductance between myocytes 1 and 2.
Cell-to-cell dye transfer estimated by fluorescence recovery after photobleaching
The permeability of the gap junctions of cultured ventricle myocytes for the fluorescent diffusion tracer 6-carboxyfluorescein (6-CF) was estimated by analysing the fluorescence recovery after photobleaching (gapFRAP; Wade et al. 1986), by means of the cytofluorimetric system ACAS 570 (Anchored Cell Analysis and Sorting, Meridian Instruments, Okemos, MI, USA). Cells were stained with 7 μg ml−1 6-carboxyfluorescein diacetate (Sigma) and scanned as described (Pluciennik et al. 1994). Briefly, a number of cardiac cells in a 180 μm × 180 μm field were selected for measurements and delineated by drawing a polygon into which the epifluorescence emission could be automatically integrated. In the selected cells, the fluorescence was photobleached by light pulses from an argon laser tuned at 488 nm, with an intensity chosen to induce a sufficient bleaching (remaining fluorescence emission 40 to 50 % of initial values) without causing cell damage. If the bleached cells were connected to their neighbours by permeable gap junctions, a fluorescence recovery occurred by diffusion of fluorescent molecules from adjacent unbleached cells. After bleaching, the fluorescence levels of the cells in the selected field were measured from sequential scans, beginning immediately post-bleach, then at intervals of 1 min.
From these data, the fluorescence redistribution from the unbleached cells to the bleached cells was monitored as a function of time. In a group of interconnected cells, the gap junctional membrane constitutes the rate-limiting boundary for the cell-to-cell diffusion; as expected for a diffusion process across a concentration gradient, the initial recovery of fluorescence in the bleached cells follows an exponential time course. In thin layers (up to 100 μm) and at low dye concentrations (range 10−8 to 10−3 M), the integrated fluorescence intensities vary in proportion to the dye concentration, and the relative permeability constant (k) of the exponential fluorescence recovery (the inverse value of the time constant) can be obtained from the equation:
| (1) |
where Fi, Fo and Ft are the integrated fluorescence intensities in the bleached cells before photobleaching, immediately after and at time t after photobleaching, respectively.
The relative permeability constant was determined in control conditions then after exposure to the drug in the same cells, and expressed as the mean ±s.e.m. unless stated otherwise. The differences between the means were compared using Student's t test for paired data and considered to be statistically significant when P < 0·01. A single unbleached cell served as control to monitor the spontaneous decay of background fluorescence and to detect any increase in fluorescence that would indicate the presence of uncleaved dye.
Solutions
The compositions of pipette and bath solutions are given in Table 1. Occasionally, as mentioned in the text, Mg-ATP was replaced in internal solution I with 5′-adenylimidodiphosphate (AMP-PNP, lithium salt) or adenosine 5′-O-(3-thiotriphosphate) (ATPγS, lithium salt); 1-(5-isoquinolinylsulphonyl)-2-methyl-piperazine (H7) was dissolved in dimethyl sulphoxide (DMSO) before addition to solution I. The alkaline phosphatase (from bovine intestinal mucosa, type I-S) was added as a solid to the patch pipette solution to the desired final concentration (1 or 10 IU ml−1, internal solution IV). A ‘protein phosphatase inhibitor cocktail’ (internal solution V) was prepared using agents known to inhibit protein phosphatases (PP): the F− ion is an inhibitor of serine/threonine phosphatases (Shenolikar & Nairn, 1991) and orthovanadate is a broad spectrum phosphatase inhibitor known to act on PP-1, PP-2A and PP-2C but not PP-2B (Shenolikar & Nairn, 1991). Free Mg2+ concentrations were calculated by means of the method described by Schoenmakers et al. (1992). All chemicals, unless otherwise stated, were obtained from Sigma.
RESULTS
Rundown of gap junctional currents
When recordings were carried out in double perforated patch conditions, currents elicited by a transjunctional difference remained stable over time as long as the gigaseal was preserved, frequently for 30 min (Fig. 2A), sometimes for a longer period (45-60 min). In double whole-cell conditions, when Mg-ATP was present (5 mM; intracellular medium I) in the solution filling the pipette, a similar stability was commonly observed (Fig. 2B).
Figure 2. Evolution with time of the gap junctional conductance measured in pairs of ventricular myocytes of neonatal rat in different conditions.
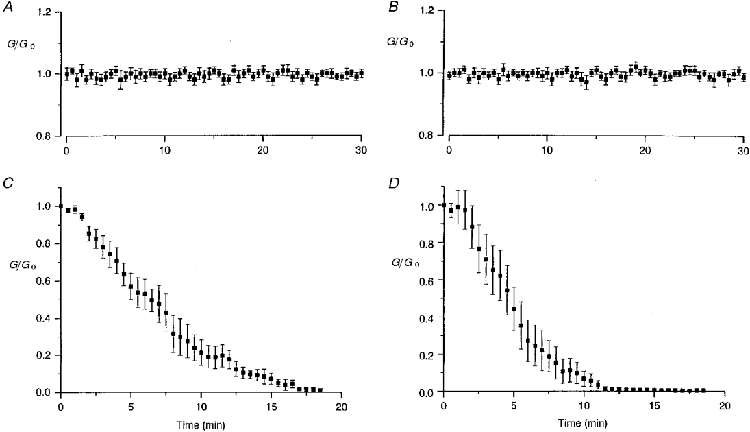
Both cells were clamped at -70 mV and one of them was stepped to -80 mV every 30 s while the second cell was maintained at the holding potential. Due to the transjunctional voltage difference, a current crossed the cell-to-cell junction, compensated by an opposite current supplied by the feedback amplifier connected to the cell maintained at -70 mV. Junctional conductances are presented in units of their original value (means ±s.e.m.). A, in perforated patch configuration (without Mg-ATP), the degree of cell-to-cell communication remained stable as long as tight seals were preserved (original Gj, 25·3 ± 7·8 nS; n= 12). B, in whole-cell conditions, when Mg-ATP (5 mM) was present in the patch pipette solution, intercellular electrical coupling was well preserved, even after 30 min (original Gj, 29·4 ± 6·3 nS; n= 25). C, in contrast, in the absence of Mg-ATP in the patch pipette solution, a progressive fading of junctional conductance was observed (original Gj, 31·2 ± 11·3 nS; n= 9). D, a channel rundown was similarly observed in the absence of ATP and presence of MgCl2 (1 mM) (original Gj, 73·9 ± 11·0 nS; n= 9).
In contrast, when recordings were carried with ATP-free internal solutions (intracellular medium II), the currents elicited by a transjunctional difference diminished with time, as shown in Fig. 2C and D, reflecting a progressive closure of junctional channels. This loss of channel activity was relatively rapid, leading to a complete interruption of the cell-to-cell communication within 12-20 min.
As the rundown of some membrane channels, for example inwardly rectifying, ATP-regulated K+ (ROMK1; McNicholas et al. 1994) or ATP-sensitive K+ (KATP; Kozlowski & Ashford, 1990) channels, was found to depend on the presence of cytosolic Mg2+, the experiments were carried in a nominally Mg2+-free solution (Fig. 2C) or in the presence of 1 mM MgCl2 in the pipette solution (Fig. 2D) where free [Mg2+], calculated using the program of Shoenmakers et al. (1992), was 0·75 mM, slightly higher than in intracellular medium I (5 mM Mg-ATP, Fig. 2B), estimated at 0·62 mM. A similar fading of the current was observed in the two conditions.
Reversibility of junctional current rundown
To obtain more direct evidence that channel rundown was dependent on ATP, the pipette solution was changed during junctional current recordings. As shown in Fig. 3, the amplitude of current responses evoked by a transjunctional voltage difference remained stable during the first minutes, when the control patch solution contained Mg-ATP (intracellular medium I), then decreased when it was unilaterally replaced with an ATP-free solution (intracellular medium II). When perfusion was carried on with 0 ATP, a complete electrical uncoupling was observed (Fig. 3, circles) whereas the reintroduction of ATP (intracellular medium III) partially restored the activity of junctional channels (Fig. 3, squares), demonstrating that the fading of the junctional current did not result from an irreversible loss of channel activity. In these experimental conditions, in the 15 pairs of cells investigated, the rundown of junctional current was observed while ATP was present in one of the cells and absent in the second one. Since ATP is expected to diffuse through gap junctional channels, the decline of junctional current showed that its diffusion to the cell exposed to the ATP-depleted solution was not sufficient to sustain channel activity.
Figure 3. Junctional channel rundown elicited by unilateral ATP removal was partially reversible after ATP reintroduction.
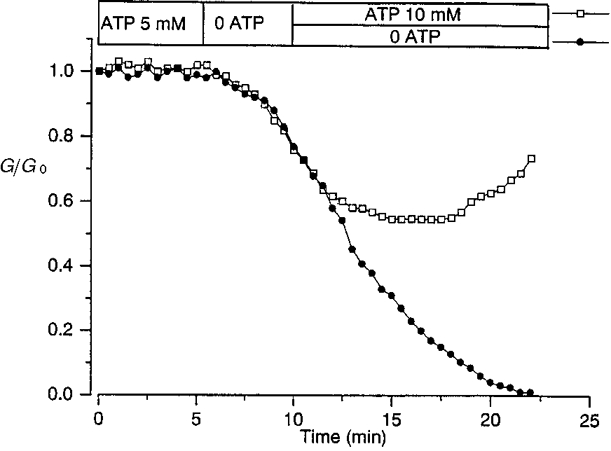
When the ATP-containing solution filling patch pipette 2 was replaced by an ATP-free medium, a progressive loss of channel activity was observed, leading to a complete interruption of cell-to-cell communication (•). When the ATP-free solution introduced near the pipette tip was replaced by an ATP-enriched solution (as indicated at the top), the decline in junctional conductance was stopped, then partially reversed (□). Conductances are presented in units of their original values, 28 (•) and 22 nS (□). Similar results were obtained in 6 (•) and 9 (□) myocyte pairs.
The prevention by ATP of the progressive wash-out of the junctional current may result from different mechanisms, such as a direct interaction of ATP with an intracellular binding side of the junctional channel, a facilitation of the clearance of calcium or the support of protein kinase activity.
Effect of a non-hydrolysable ATP analogue
In an effort to determine whether ATP hydrolysis is a requirement for gap junctional channel opening, ATP was replaced in the pipette filling solution by AMP-PNP, a non-hydrolysable ATP analogue, with an imido linkage between the β- and γ-phosphates. A similar substitution partially sustained the activity of some cardiac channels (e.g. L-type Ca2+ channel in guinea-pig ventricular myocytes; Yazawa et al. 1997). As shown in Fig. 4, AMP-PNP failed to support junctional channel activity, suggesting that ATP hydrolysis may be necessary to keep the junctional channels available.
Figure 4. A non-hydrolysable ATP analogue (AMP-PNP) failed to preserve cell-to-cell communication.
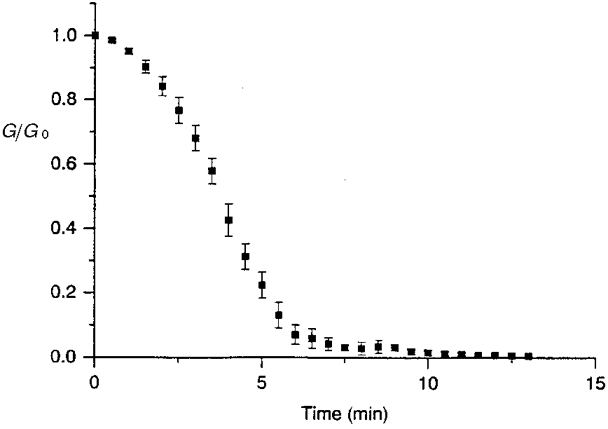
When, in dual whole-cell configuration, ATP was replaced in the pipette filling solution with AMP-PNP (5 mM), a progressive decline of the junctional conductance presented in units of its original value was observed. Original Gj, 35·2 ± 15·8 nS, n= 7.
Possible involvement of intracellular Ca2+ concentration in junctional current fading?
It has been suggested that the effect of intracellular Mg-ATP might be to maintain a low Ca2+ concentration near the inner surface of the plasma membrane (Byerly & Yazejian, 1986). High concentrations of ATP (EC50≈ 3 mM) stimulate the Na+-Ca2+ exchanger (which primarily controls the clearance of cytoplasmic calcium, indirectly via activation of the Na+-K+-ATPase) in cardiac myocytes (Collins et al. 1992) as well as the Ca2+ pump (Ca2+-ATPase) in the sarcoplasmic reticulum. Increases in intracellular calcium concentration up to high levels have been directly correlated with reduced gap junctional communication, but an important rise in bulk calcium concentration is made unlikely in the present experimental conditions (5 mM EGTA, 0 CaCl2 in the patch pipette solution). However, calcium greatly increased the rate of decay of several membrane currents, such as L-type Ca2+ (Chad & Eckert, 1986) or GABAAICl (Chen et al. 1990; Huang & Dillon, 1998). In the study of Huang & Dillon (1998), for example, 4 mM Mg-ATP failed to prevent channel rundown unless EGTA concentration in the pipette solution was increased from 4 to 10 mM.
The ATP analogue ATPγS is regarded as being a relatively good substrate for protein kinases but is a poor substitute for ATP in active transport processes (Collins et al. 1992). Indeed, thio-substitution of a non-bridge oxygen on the γ-phosphate (ATPγS) results in a compound which can be used in some kinase catalysed reactions, but not in ATPase reactions. Thus, it fails to support reactions which require ATP hydrolysis as an energy source; if the sole effect of Mg-ATP were to remove Ca2+, it is expected that ATPγS would enhance the junctional current rundown. When myocytes, under the experimental conditions used for the present study (5 mM EGTA), were dialysed with an intracellular solution I containing ATPγS (3 mM) instead of Mg-ATP, the junctional conductance remained constant for at least 30 min (n= 7; data not shown)
Are ATP effects attributable to the promotion of protein phosphorylation?
Alkaline phosphatase-induced rundown of junctional currents
The foregoing results suggest that junctional channels or their closely associated proteins are under the influence of protein kinases and phosphatases and, thus, the lack of ATP inside the cells shifts the balance between phosphorylation and dephosphorylation over to the latter. The enhancement of phosphatase activity by adding alkaline phosphatase, a non-specific enzyme that hydrolyses many compounds containing phosphorus, regardless of the chemical nature of the moiety to which the phosphorus is bound (McComb et al. 1979), should induce a rundown of junctional current. The addition of alkaline phosphatase (1 U ml−1, data not shown, n= 8, or 10 U ml−1, n= 9, Fig. 5) to the Mg-ATP containing solution used to fill the patch pipettes (intracellular medium IV) led, after a baseline period, to a progressive fading of the junctional currents (Fig. 5, triangles)
Figure 5. A dephosphorylating treatment interrupted cell-to-cell communication.
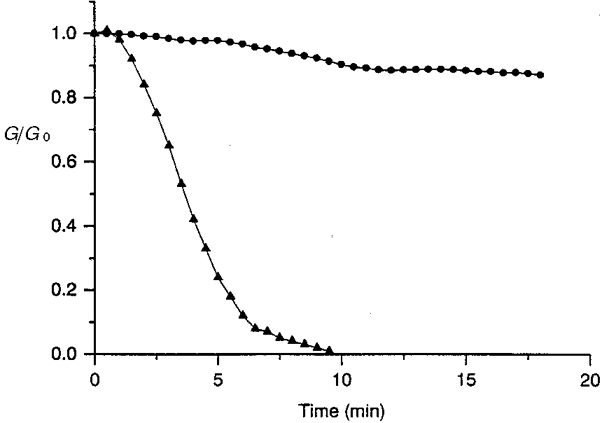
When a non-specific dephosphorylating enzyme (alkaline phosphatase, 10 U ml−1) was intracellularly applied, the junctional conductance, presented in units of the original conductance, 28 (•) and 32 nS (▴), progressively declined and led to complete interruption of intercellular communication (▴); in contrast, in the presence of an inhibitor of alkaline phosphatase activity (β-glycerophosphate, 50 mM, •), the main part of the intercellular coupling was preserved. Similar results were obtained in each of 9 (▴) and 8 (•) myocyte pairs.
In order to rule out other possible mechanisms of action of alkaline phosphatase (i.e. a deleterious effect on membranes or a possible contamination by proteases), the involvement of the phosphatase activity was verified by means of blockers of intestinal phosphatase activity. In preliminary experiments, the harmless action of these blockers on gap junctional communication was tested. L-Phenylalanine (10 mM), usually used as a relatively specific inhibitor of the intestinal isoenzyme of alkaline phosphatase, was found to rapidly abolish cell-to-cell communication (data not shown). In contrast, a less specific inhibitor of intestinal alkaline phosphatase, β-glycerophosphate (50 mM in both intrapipette solution and bath), was without effect on intercellular coupling. When the phosphatase activity of alkaline phosphatase was prevented by the presence of β-glycerophosphate, the peak amplitude of the response declined at first, then reached an approximately steady-state level (Fig. 5, circles). These results suggest that phosphorylation at some sites is critical for the normal activity of junctional channels to occur and that the dephosphorylation of junctional proteins by endogenous phosphatase(s) is responsible for the decline of the junctional current. In such cases, the inhibition of protein kinases should lead to a loss of channel activity.
Effects of a non-specific protein kinase inhibitor, H7
The influence of H7, a non-specific inhibitor of serine/threonine protein kinases, was examined by means of both the effect of gapFRAP on intact cells and double whole-cell patch clamp techniques. H7, although structurally unrelated to ATP, is considered to compete with ATP for free enzymes. In cell-free assays, the inhibition constant Ki has been reported to be (μM): 3 (cAMP-dependent kinase), 6 (cGMP-dependent kinase and protein kinase C), 100 (myosin light chain kinase and casein kinase I), 780 (casein kinase II) (Hidaka et al. 1984) and 20-32 (calmodulin-dependent kinase II; Malenka et al. 1989). In the present study on intact cells, H7 was used at a relatively high concentration (1 mM) with intent to inhibit a range of serine/threonine protein kinases.
A comparison of cell-to-cell dye transfers occurring in control conditions and after treatment with H7 (1 mM for 1 h) is illustrated in Fig. 6. After the cells were stained with 6-CF, the computer-generated images of the fluorescent dye distribution were obtained before (Fig. 6A) then just after photobleaching (Fig. 6B) and 8 min later (Fig. 6C), in Tyrode solution. The unequal fluorescence levels inside one cell and between different cells reflect variations in cell thickness. In the first approximation, the redistribution of 6-CF after photobleaching takes place in a system of two compartments, namely the photobleached cell and the set of unbleached adjacent cells, separated by gap junctional membranes acting as a diffusion barrier. Immediately after the photobleaching of the selected cell, its light emission was reduced to about 50 % of the initial level, then a rise in fluorescence emission took place with a monoexponential time course. The rate constant k obtained by fitting eqn (1) provides a measure of the relative permeability of the gap junction of the tested cell pair. Figure 6G shows a typical example of the evolution of the integrated fluorescence intensities with time after photobleaching, measured in ventricular myocytes in Tyrode solution.
Figure 6. Examples of quantification of the cell-to-cell dye diffusion in cardiac ventricle myocytes of newborn rat cultured for 3 days.
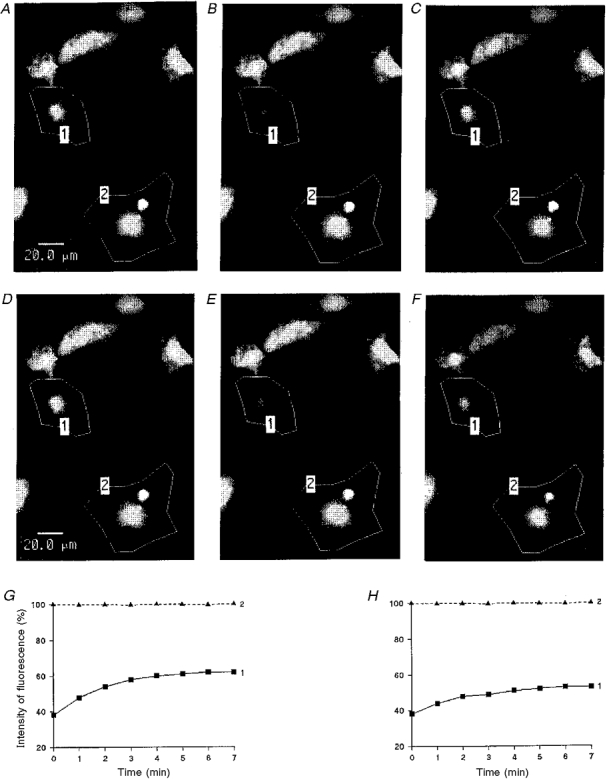
Grey density images of fluorescence intensities (A-C and D-F) were obtained by scanning a group of cells with low intensity light pulses. After a prebleach scan (A and D), 6-CF was photobleached in a selected area (polygon 1) by means of strong illumination. The evolution of the fluorescence levels in the selected cells was compared in the same set of cells, in control conditions (A-C) and after a 60 min exposure to H7 (able to penetrate into intact cells; 1 mM) (D-F), just after photobleaching (B and E) and 8 min later (C and F). Graphs: time courses of the fluorescence emission of the selected cell before (G) and after (H) H7 application. The fluorescence intensity is represented as a percentage of the prebleach emission versus the time after photobleaching. In control conditions (G), the fluorescence emission of the bleached cell increased progressively whereas after H7 treatment (H), the fluorescence level only slightly rose in the bleached cell (continuous lines). In both cases, the light emission of unbleached cell remained stable (dashed lines).
In control experiments, no significant change in k was noticed when up to four consecutive photobleaches were performed on the same cells (Verrecchia & Hervé, 1997a). The mean rate constant k measured in Tyrode solution (control conditions) was evaluated as 0·22 ± 0·005 min−1 (n= 600). In other words, the fluorescence recovery after photobleaching followed an exponential time course with a mean time constant of 4·5 min.
In contrast, after exposure to H7 (1 mM for 60 min) the relative permeability constant k was reduced to very low values (from 0·254 ± 0·031 to 0·023 ± 0·013 min−1, n= 13) and the diffusion of fluorescent molecules through cell-to-cell channels was considerably slowed down (Fig. 6D-F and H) reflecting the closure of a number of junctional channels. The fluorescence decay in unbleached cells (dashed line in Fig. 6G and H), which is the sum of background photobleaching in the successive scans and of an outflux through non-junctional cell membranes, was very similar in control and H7-treated cultures (polygon 2 in both illustrations). This observation indicates that the permeability of the non-junctional cell membrane for organic molecules was not altered. As illustrated in Fig. 7, the cell-to-cell diffusion of the fluorescent dye was progressively and reversibly reduced. Gap junctional conductance was similarly reduced to approximately 13 % of its initial value within 10 min (Fig. 8).
Figure 7. Cell-to-cell communication between ventricular myocytes was progressively and reversibly hindered by H7.
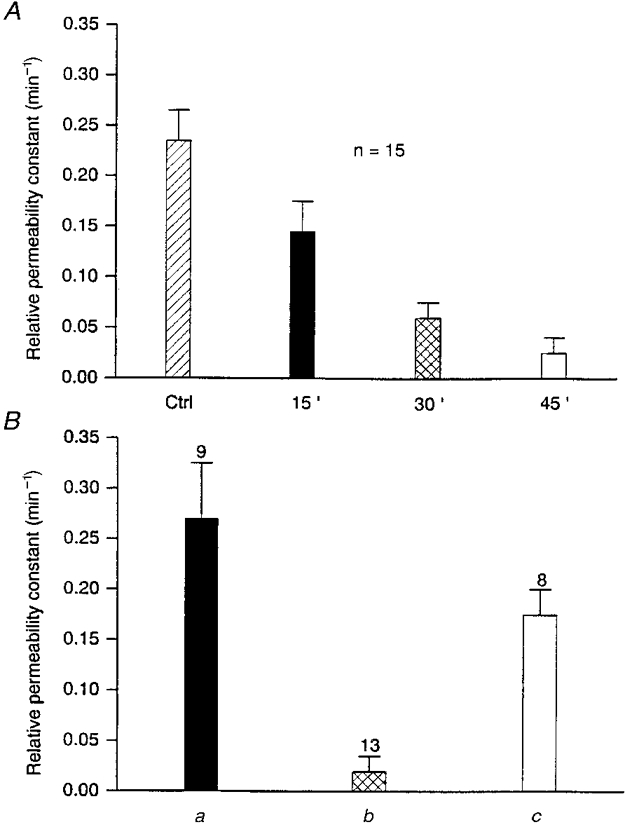
A, time-dependent reduction of the relative permeability constant (k) of dye transfer after 15, 30 and 45 min exposures to H7, a non-specific inhibitor of serine/threonine protein kinases. B, recovery of cell-to-cell dye transfer after washing off H7. k was determined in control conditions (a), after exposure to H7 (1 mM for 1 h; b), and then 30 min after washing with control medium (c). Bars indicate k values (min−1) as means and s.e.m., with values of n above.
Figure 8. Time course of the H7-induced reduction in gap junctional conductance.
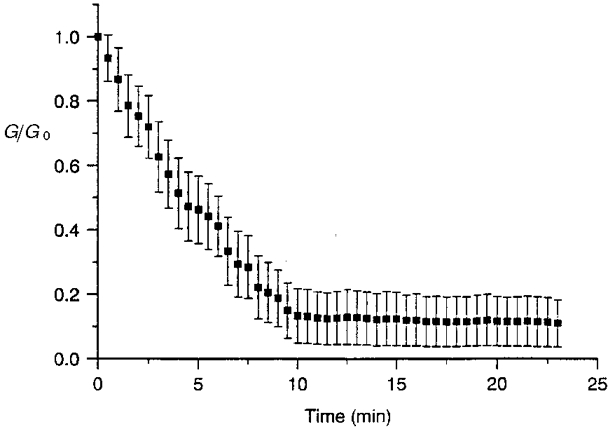
In the presence of H7 (1 mM) and ATP (5 mM) in the patch pipette solution, a progressive decline of junctional conductance (presented in units of its original value) was observed, reaching after approximately 10 min a stable plateau equivalent to about 13 % of its initial level (original Gj, 71·1 ± 10·1 nS; n= 6).
It should be noticed that the time course of H7 action was much quicker when studied in patch clamp conditions rather than with the gapFRAP method, even considering that the drug was directly introduced into the cytosol via the patch pipette in the first case, and added to the external bath in the second. A similar difference in time courses was previously observed, even when junctional uncouplers (e.g. 1-oleoyl-2-acetyl-sn-glycerol, testosterone, 17β-oestradiol, gossypol) were externally applied in both cases. Gossypol (5 μM), for example, elicited a complete electrical uncoupling between ventricular myocytes within only 4 min whereas a small but measurable dye diffusion still persisted after 15 min of exposure (Hervéet al. 1996). Whole-cell conditions might render the cells much more fragile than the non-invasive gapFRAP method.
H7 seems able to inactive the main part of the basal activity of protein serine/threonine kinase(s) responsible for the maintenance of junctional channels in an open state. In contrast, a protein tyrosine kinase inhibitor (genistein) had no effect on intercellular dye diffusion between cardiac myocytes (Verrecchia et al. 1998).
These results, consistent with the properties of Ij running down under ATP-depleted conditions, suggest that junctional channels are constantly under the influence of protein kinase(s) and phosphatase(s).
The activity of endogenous phosphatase(s) is responsible for the rundown of the junctional current
If the hypothesis is correct that endogenous phosphoprotein phosphatases are involved in the fading of junctional conductance observed in ATP-free solutions, the inhibition of protein phosphatases would be expected to prevent or to retard the rundown of junctional current under ATP-depleted conditions. This possibility was examined by adding a broad spectrum ‘protein phosphatase inhibitor cocktail’ to the ATP-free solution (intracellular medium V). As shown in Fig. 9, in these conditions, after a latency of approximately 2 min, the junctional conductance declined progressively for about 5 min before reaching a plateau corresponding to approximately 60 % of the initial conductance. A substantial cell-to-cell communication was then preserved (58 ± 7 % after 15 min; Fig. 9), and when whole-cell conditions were maintained for a longer period, 59 ± 7 % of the original conductance was still measured after 20 min (n= 3, data not shown). The stability of this plateau leads to the thought that protein phosphatase activity was stopped whereas the initial loss of channel activity might be due to a relatively slow kinetics of enzyme inhibition. Broad spectrum phosphatase inhibitors had approximately the same efficiency in preventing some other rundowns considered to be caused by protein dephosphorylation; for example they preserved about 57 % of ROMK1 currents (McNicholas et al. 1994) and 64 % of KATP currents (Kubokawa et al. 1995).
Figure 9. Non-selective inhibition of endogenous protein phosphatases preserved cell-to-cell communication in ATP-deprived conditions.
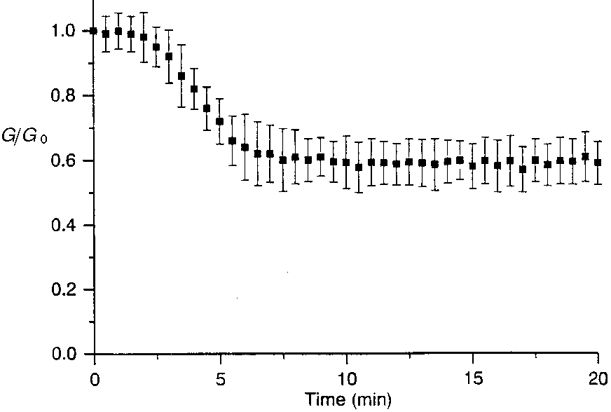
The loss of junctional channel activity, in whole-cell, ATP-free conditions, was interrupted in the presence of a ‘broad spectrum cocktail’ of protein phosphatase inhibitors (KF 50 mM + orthovanadate 1 mM), allowing preservation of a substantial cell-to-cell communication (original Gj, 33·2 ± 15·8 nS; n= 9).
DISCUSSION
The gap junctional conductance measured between neonatal rat cardiomyocytes was well maintained when cell dialysis was prevented in perforated cell patch clamp conditions or, in conventional whole-cell mode, when ATP was present in the patch pipette solution, whereas Ij rapidly vanished when an ATP-free solution was used to fill the patch electrodes or at least one of them. It should be noticed, however, that the activity of junctional channels was remarkably well preserved in the presence of ATP. The absence of rundown in these conditions was similarly observed, for example, with N-methyl-D-aspartate (NMDA) channels (Rosenmund & Westbrook, 1993) or inwardly rectifying K+ (KIR) channels (Inoue & Imanaga, 1995), contrasting with the slow and irreversible losses of activity frequently observed in similar conditions with several other, either voltage- or ligand-activated, membrane channels, such as L-type calcium channels (ascribed to an endogenous, Ca2+-activated, proteolytic activity; Chad & Eckert, 1986) and ROMK1 K+ channels (McNicholas et al. 1994), or GABAA responses (Chen et al. 1990).
When the non-junctional membrane of one of the cells of a pair of guinea-pig ventricular myocytes was mechanically ruptured, gap junctional conductance remained stable when ATP was present in the bath solution (Noma & Tsuboi, 1987; Sugiura et al. 1990) but was rapidly and reversibly reduced when ATP concentration was lowered (Sugiura et al. 1990). Considering the relatively low Q10 (1·29) of gap junctional conductance in the range 24-35°C, much lower than that of enzyme reactions such as phosphorylation regulated by high-energy phosphates (Q10= 3), Sugiura et al. (1990) suggested that ATP regulation was mediated by a ligand-receptor interaction between ATP and channel proteins. For several membrane channels, it has also been suggested that ATP might bind to a site within or close to the channel, produce a conformation change and keep the channel in a state of activity without involving energy-consuming processes; for example ATP might directly bind to the intracellular side of GABAA receptors (Shirasaki et al. 1992) or L-type Ca2+ channels in guinea-pig ventricular myocytes (Yazawa et al. 1997). The latter authors observed that substituting a non-hydrolysable analogue (AMP-PNP) for Mg-ATP partially sustained the channel activity. In contrast, this substitution failed to prevent the rundown of junctional current.
ATP might also prevent rundown by stabilizating cellular metabolism, since metabolic disorders lead to a lower pH and an increase in [Ca2+]i, and both changes are well known inhibitors of gap junctional communication (for review, see Sáez et al. 1993). The inhibition of intercellular communication between astrocytes resulting from ATP depletion was ascribed to a calcium-mediated mechanism (Vera et al. 1996). Similarly, the prevention of NMDA channel rundown by ATP was primarily attributed to a facilitation of the calcium clearance (Rosenmund & Westbrook, 1993). In the present study, the rundown prevention by ATPγS, a poor substitute for ATP in active transport processes, makes an accumulation of intracellular calcium unlikely.
Our results rather suggest that intracellular Mg-ATP sustained the junctional channel function via a phosphorylation process of either the channel itself or an associated regulatory protein and that dephosphorylation of junctional channels by endogenous, membrane-bound, protein phosphatase(s) is responsible for the loss of activity of junctional channels. The fact that the effects of ATP deprivation can be (at least qualitatively) reproduced by a non-selective inhibition of endogenous protein kinases or by a non-specific exogenous phosphatase, together with the antagonizing action of non-specific protein phosphatase inhibitors, gives support to the protein phosphorylation theory for the basal activity of junctional channels.
The enhancement of phosphatase activity by adding alkaline phosphatase also induced a rundown of junctional currents in SKHep1 human hepatocarcinoma cells transfected with human connexin43 (Moreno et al. 1994). Similarly, the addition of alkaline phosphatase to the patch pipette solution was also able to facilitate the loss of activity of several other membrane channels whose activity is considered to be under the influence of protein kinase(s) and phosphatase(s), such as KIR channels (Inoue & Imanaga, 1995) and GABAA chloride channels (Chen et al. 1990). H7 caused a reversible slump of the cell coupling level, which seems to result from the inactivation of the main part of the basal activity of protein serine/threonine kinase(s). A residual part of this activity might have subsisted because H7, considered to be a competitive inhibitor with respect to ATP, was present in the intracellular medium at lower concentration than ATP (e.g. in whole-cell conditions, 1 versus 5 mM) and/or because involved protein kinase(s) might have relatively high H7 inhibition constants. The reversible inhibition of IK(IR) by H7 was also interpreted as being due to suppression of the basal activity of protein kinase(s) responsible for the maintenance of these currents (Inoue & Imanaga, 1995). The persistence of a substantial junctional current in ATP-depleted conditions when the phosphatase activity is simultaneously depressed is also consistent with the notion that cell-to-cell communication is under the influence of phosphorylation and dephosphorylation processes. Phosphatase inhibitors were also able to prevent the rundown of several membrane channels, including, for example, CFTR Cl− (Becq et al. 1994), ROMK1 K+ (McNicholas et al. 1994), KATP (Kubokawa et al. 1995) and KIR (Inoue & Imanaga, 1995).
Conversely, for membrane channels whose activity is maintained by ATP by processes other than protein phosphorylation: (i) protein kinase inhibitors failed to affect the open probability of the channels, for example of KATP in guinea-pig ventricular myocytes (Furukawa et al. 1994); (ii) the rundown of membrane currents was not facilitated by alkaline phosphatase application, as for example for GABA-gated ICl in rat neurones (Shirasaki et al. 1992) or NMDA channels (Rosemund & Westbrook, 1993); and (iii) protein phosphatase inhibitors were not able to prevent the rundown, as, for example, for skeletal muscle KATP (see Light, 1996) or NMDA channels (Rosemund & Westbrook, 1993), or for GABA responses in rat neurones (Shirasaki et al. 1992).
Gap junctional channels, as with several other membrane channels, appear to be under the influence of the activities of cellular protein kinases and phosphatases; thus the energy status of the cell might regulate intercellular communication. This is consistent with results showing that Cx43 was both rapidly phosphorylated and dephosphorylated (Crow et al. 1990); the turnover rate of phosphate on Cx43 in neonatal rat heart myocytes under resting conditions was found to be rapid (Sáez et al. 1997), similar to the half-life of the protein (1 to 2 h; Laird et al. 1991). Taken together, these results suggest the need for a continuous endogenous supply of ATP for protein kinase activity.
There are many possible enzymes that could be responsible for the phosphorylation/dephosphorylation processes and we are currently examining several possibilities.
Up to now, little was known about protein phosphatase(s) plausibly involved in the regulation of junctional communication. A part of the junctional uncoupling action of BDM seemed to result from a dephosphorylation process (Verrecchia & Hervé, 1997a) and it was suggested (Zimmermann et al. 1996) that this compound could enhance the activity of endogenous phosphatases (PP-2A and, to a lesser extent, PP-1). In the present study, the lack of influence of Mg2+ on the loss of channel activity does not favour the involvement of Mg2+-dependent protein phosphatase-2C (PP-2C) in the rundown of junctional current.
In contrast, numerous studies have examined the changes in channel activity that could result from phosphorylation events. Junctional permeability between cardiac ventricular myocytes remained unmodified when cells were exposed to the following two conditions.
(i) Factors that promote cyclic AMP-dependent protein phosphorylation (internally applied cAMP as well as externally applied 8-bromo cyclic AMP) (Sugiura et al. 1990; Kwak & Jongsma, 1996; Verrecchia et al. 1998). This result is consistent with the fact that ATP alone is sufficient to prevent the rundown of junctional current, in contrast with many other membrane channels, both voltage or ligand activated, where Mg-ATP failed to prevent the loss of activity of several membrane channels unless purified catalytic subunit of protein kinase A (PKA) was co-added (for review, see for example Ismailov & Benos, 1995; Becq, 1996). In this case, a membrane-bound protein kinase involved in the phosphorylation of the channel (or at least its catalytic subunit) might have been lost after patch excision. In excised cardiac myocytes, the addition of the catalytic subunit of PKA had no effect on junctional conductance (Sugiura et al. 1990).
(ii) PKC inhibitors (staurosporine or calphostin C) had no effect on the degree of cell-to-cell dye coupling between neonatal rat cardiomyocytes (Verrecchia & Hervé, 1997b; Verrecchia et al. 1998). However, PKC activation was seen to have various effects on cells of different types. In neonatal rat cardiomyocytes, a TPA-induced increase of macroscopic gap junctional conductance was ascribed to PKC activation (Kwak et al. 1995; Kwak & Jongsma, 1996; F. Verrecchia, K. Lallouche and J. C. Hervé, unpublished observations). In this preparation, PKC might be involved in the upregulation of the channel rather than in determining the basal degree of phosphorylation of the junctional channel proteins or of a closely associated regulatory molecule, which governs channel activity.
However, in contrast, some decreases in the degree of cell-to-cell communication were also ascribed to the activation of some protein kinases, among which are the following two.
(i) cGMP-activated protein kinase (PKG), whose activation led to a decrease (-24 %) in gap junctional conductance in SKHep1 cells transfected with cardiac rat Cx43 whereas no effect was observed in the same cells transfected with cardiac human Cx43 (Kwak et al. 1995)
(ii) Mitogen-activated protein (MAP) kinases. The disruption of gap junctional communication caused by some growth factors would result from an activation of the MAP kinase cascade, leading to Cx43 phosphorylation, for example junctional uncouplings caused by epidermal growth factor (EGF; for review, see Lau et al. 1996) or by platelet-derived growth factor (PDGF; Hossain et al. 1998). According to Hossain et al. (1998), PDGF-induced phosphorylation of Cx43 could trigger the activation of a proteolytic pathway and cause Cx43 degradation.
These observations suggest that at least two pathways involving phosphorylation and dephosphorylation of Cx43 might be entailed in the regulation of gap junctional intercellular communication, which may be dependent upon the extent and specificity of phosphorylation.
In conclusion, phosphorylation processes are essential for activity of the junctional channels to be maintained. It seems likely that protein kinase(s) and phosphatase(s) involved in the regulation must be located in the vicinity of the channel within the membrane; plausibly they are membrane- or cytoskeleton-associated enzymes. The presence of Mg2+ is not essential for both the phosphorylating and dephosphorylating processes to occur. The rundown reflects the tonic activity of an endogenous phosphatase(s) and its rapidity points to a relatively high basal dephosphorylation rate.
Acknowledgments
The authors are grateful to Dr K. Yamaoka (Department of Physiology, School of Medicine, Hiroshima University, Japan) who performed the calculations of free Mg2+ concentrations, to Dr J. Délèze (Laboratoire de Physiologie Cellulaire, Poitiers) and Dr F. Becq (Laboratoire de Physiologie Animale, Poitiers) for their valuable comments on this study, and to Dr E. T. MacKenzie and Mrs H. Tomlinson, for careful reading of the manuscript. This study was supported in part by grants from the Concerted Action programme (no BMH4-CT 96-1427) under the BIOMED2 programme of the European Community, from the Fondation Langlois and from the Fondation pour la Recherche Médicale.
References
- Allen DG, Morris PG, Orchard CH, Pirolo JS. A nuclear magnetic resonance study of metabolism in the ferret heart during hypoxia and inhibition of glycolysis. The Journal of Physiology. 1985;361:185–204. doi: 10.1113/jphysiol.1985.sp015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becq F. Ionic channel rundown in excised membrane patches. Biochimica et Biophysica Acta. 1996;1286:53–63. doi: 10.1016/0304-4157(96)00002-0. [DOI] [PubMed] [Google Scholar]
- Becq F, Jensen TJ, Chang XB, Savoia A, Rommens JM, Tsui LC, Buchwald M, Riordan JR, Hanrahan JW. Phosphatase inhibitors activate normal and defective CFTR chloride channels. Proceedings of National Academy of Sciences of the USA. 1994;91:9160–9164. doi: 10.1073/pnas.91.19.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer EC, Paul DL, Goodenough DA. Connexin43: a protein from rat heart homologous to a gap junction protein from liver. Journal of Cell Biology. 1987;105:2621–2629. doi: 10.1083/jcb.105.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L, Yazejian B. Intracellular factors for the maintenance of calcium currents in perfused neurones from the snail. The Journal of Physiology. 1986;370:631–650. doi: 10.1113/jphysiol.1986.sp015955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chad JE, Eckert R. An enzymatic mechanism for calcium current inactivation in dialysed Helix neurones. The Journal of Physiology. 1986;378:31–51. doi: 10.1113/jphysiol.1986.sp016206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QX, Stelzer A, Kay AR, Wong RKS. GABAA-receptor function is regulated by phosphorylation in acutely dissociated guinea-pig hippocampal neurones. The Journal of Physiology. 1990;420:207–221. doi: 10.1113/jphysiol.1990.sp017908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A, Somly AV, Hilgemann DW. The giant cardiac membrane patch method: stimulation of outward Na+-Ca2+ exchange current by MgATP. The Journal of Physiology. 1992;454:27–57. doi: 10.1113/jphysiol.1992.sp019253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe A, Lefèvre IA, Deroubaix E, Thuringer D, Coraboeuf E. Effects of 2,3-butanedione monoxime on the slow inward and transient outward currents in rat ventricular myocytes. Journal of Molecular and Cellular Cardiology. 1990;22:921–932. doi: 10.1016/0022-2828(90)90123-j. [DOI] [PubMed] [Google Scholar]
- Crow DS, Beyer EC, Paul DL, Kobe SS, Lau AF. Phosphorylation of connexin43 gap junction protein in uninfected and Rous sarcoma virus-transformed mammalian fibroblasts. Molecular and Cellular Biology. 1990;10:1754–1763. doi: 10.1128/mcb.10.4.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T, Virág L, Furukawa N, Sawanobori T, Hiraoka M. Mechanism for reactivation of the ATP-sensitive K+ channel by MgATP complexes in guinea-pig ventricular myocytes. The Journal of Physiology. 1994;479:95–107. doi: 10.1113/jphysiol.1994.sp020280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervé JC, Pluciennick F, Bastide B, Cronier L, Verrecchia F, Malassiné A, Joffre M, Délèze J. Contraceptive gossypol blocks cell-to-cell communication in human and rat cells. European Journal of Pharmacology. 1996;313:243–255. doi: 10.1016/0014-2999(96)00476-1. 10.1016/0014-2999(96)00476-1. [DOI] [PubMed] [Google Scholar]
- Hidaka H, Inagaki M, Kawamoto S, Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984;23:5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hossain MZ, Ao P, Boynton AL. Rapid disruption of gap junctional communication and phosphorylation of connexin43 by platelet-derived growth factor in T51B rat liver epithelial cells expressing platelet-derived growth factor receptor. Journal of Cell Physiology. 1998;174:66–77. doi: 10.1002/(SICI)1097-4652(199801)174:1<66::AID-JCP8>3.0.CO;2-E. 10.1002/(SICI)1097-4652(199801)174:1<66::AID-JCP8>3.3.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Huang RQ, Dillon GH. Maintenance of recombinant type A γ-aminobutyric acid receptor function: role of protein tyrosine phosphorylation and calcineurin. Journal of Pharmacology and Experimental Therapeutics. 1998;286:243–255. [PubMed] [Google Scholar]
- Inoue M, Imanaga I. Phosphatase is responsible for run down, and probably G protein-mediated inhibition of inwardly rectifying K+ currents in guinea pig chromaffin cells. Journal of General Physiology. 1995;105:249–266. doi: 10.1085/jgp.105.2.249. 10.1085/jgp.105.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irisawa H, Kokubun S. Modulation by intracellular ATP and cyclic AMP of the slow current in isolated single ventricular cells of the guinea-pig. The Journal of Physiology. 1983;338:321–337. doi: 10.1113/jphysiol.1983.sp014675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismailov II, Benos DJ. Effects of phosphorylation on ion channel function. Kidney International. 1995;48:1167–1179. doi: 10.1038/ki.1995.400. [DOI] [PubMed] [Google Scholar]
- Kanter HL, Saffitz JE, Beyer EC. Cardiac myocytes express multiple gap junction proteins. Circulation Research. 1992;70:438–444. doi: 10.1161/01.res.70.2.438. [DOI] [PubMed] [Google Scholar]
- Kozlowski RZ, Ashford MLJ. ATP-sensitive K+-channel run-down is Mg2+ dependent. Proceedings of the Royal Society. 1990;B240:397–410. doi: 10.1098/rspb.1990.0044. [DOI] [PubMed] [Google Scholar]
- Kubokawa M, McNicholas CM, Higgins MA, Wang W, Giebisch G. Regulation of ATP-sensitive K+ channel by membrane-bound protein phosphatases in rat tubule cell. American Journal of Physiology. 1995;269:F355–362. doi: 10.1152/ajprenal.1995.269.3.F355. [DOI] [PubMed] [Google Scholar]
- Kwak BR, Jongsma HJ. Regulation of cardiac gap junction channel permeability and conductance by several phosphorylating conditions. Molecular and Cellular Biochemistry. 1996;157:93–99. doi: 10.1007/BF00227885. [DOI] [PubMed] [Google Scholar]
- Kwak BR, Sáez JC, Wilders R, Chanson M, Fishman GI, Hertzberg EL, Spray DC, Jongsma HJ. Effects of cGMP-dependent phosphorylation on rat and human connexin43 gap junction channels. Pflügers Archiv. 1995;430:770–778. doi: 10.1007/BF00386175. [DOI] [PubMed] [Google Scholar]
- Kwak BR, van 4Veen TAB, Analbers LJS, Jongsma HJ. TPA increases conductance but decreases permeability in neonatal rat cardiomyocyte gap junctional channels. Experimental Cell Research. 1995;220:456–463. doi: 10.1006/excr.1995.1337. [DOI] [PubMed] [Google Scholar]
- Laird DW, Puranam KL, Revel JP. Turnover and phosphorylation dynamics of connexin43 gap junction protein in cultured cardiac myocytes. Biochemical Journal. 1991;273:67–72. doi: 10.1042/bj2730067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau AF, Kurata WE, Kanemitsu MY, Loo WM, Warn-Cramer BJ, Eckart W, Lampe PD. Regulation of connexin43 function by activated tyrosine protein kinases. Journal of Bioenergetics and Biomembranes. 1996;28:359–368. doi: 10.1007/BF02110112. [DOI] [PubMed] [Google Scholar]
- Light P. Regulation of ATP-sensitive potassium channels by phosphorylation. Biochimica et Biophysica Acta. 1996;1286:65–73. doi: 10.1016/0304-4157(96)00004-4. [DOI] [PubMed] [Google Scholar]
- McComb RB, Bowers GN, Posen S. Measurement of alkaline phosphatase activity. In: McComb RB, Bowers GN, Posen S, editors. Alkaline Phosphatase. New York and London: Plenum Press; 1979. pp. 289–355. [Google Scholar]
- McNicholas CM, Wang W, Ho K, Hebert SC, Giebisch G. Regulation of ROMK1 K+ channel activity involves phosphorylation processes. Proceedings of the National Academy of Sciences of the USA. 1994;91:8077–8081. doi: 10.1073/pnas.91.17.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Kauer JA, Perkel DJ, Mauk MD, Kelly PT, Nicoll RA, Waxham MN. An essential role for post synaptic calmodulin and protein kinase activity in long-term potentiation. Nature. 1989;340:554–557. doi: 10.1038/340554a0. [DOI] [PubMed] [Google Scholar]
- Moreno AP, Sáez JC, Fishman GI, Spray DC. Human connexin43 gap junction channels. Regulation of unitary conductances by phosphorylation. Circulation Research. 1994;74:1050–1057. doi: 10.1161/01.res.74.6.1050. [DOI] [PubMed] [Google Scholar]
- Neyton J, Trautmann A. Single-channel currents of an intercellular junction. Nature. 1985;317:331–335. doi: 10.1038/317331a0. [DOI] [PubMed] [Google Scholar]
- Noma A, Tsuboi N. Dependence of junctional conductance on proton, calcium and magnesium ions in cardiac paired cells of guinea-pig. The Journal of Physiology. 1987;382:193–211. doi: 10.1113/jphysiol.1987.sp016363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluciennik F, Joffre M, Délèze J. Follicle-stimulating hormone increases gap junction communication in Sertoli cells from immature rat testis in primary culture. Journal of Membrane Biology. 1994;139:81–96. doi: 10.1007/BF00232427. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Westbrook GL. Rundown of N-methyl-D-aspartate channels during whole-cell recording in rat hippocampal neurones: role of Ca2+ and ATP. The Journal of Physiology. 1993;470:705–729. doi: 10.1113/jphysiol.1993.sp019884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáez JC, Berthoud VM, Moreno AP, Spray DC. Gap junctions. Multiplicity of controls in differentiated and undifferentiated cells and possible functional implications. In: Shenolikar S, Nairn AC, editors. Advances in Second Messenger and Phosphoprotein Research. Vol. 27. New York: Raven Press; 1993. pp. 163–198. [PubMed] [Google Scholar]
- Sáez JC, Nairn AC, Czernik AJ, Fishman GI, Spray DC, Hertzberg EL. Phosphorylation of Connexin43 and the regulation of neonatal rat cardiac myocyte gap junction. Journal of Molecular and Cellular Cardiology. 1997;29:2131–2145. doi: 10.1006/jmcc.1997.0447. [DOI] [PubMed] [Google Scholar]
- Shenolikar S, Nairn AC. Protein phosphatases: recent progress. In: Greengard P, Robinson GA, editors. Advances in Second Messenger and Phosphoprotein Research. Vol. 23. New York: Raven Press; 1991. pp. 1–119. [PubMed] [Google Scholar]
- Shirasaki T, Aibara K, Akaike N. Direct modulation of GABAA receptor by intracellular ATP in dissociated nucleus tractus solitarii neurones of rat. The Journal of Physiology. 1992;449:551–572. doi: 10.1113/jphysiol.1992.sp019101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmakers TJ, Visser GJ, Flik G, Theuvenet AP. CHELATOR: an improved method for computing metal ion concentrations in physiological solutions. Biotechniques. 1992;12:870–874. [PubMed] [Google Scholar]
- Sugiura H, Toyama J, Tsuboi N, Kamiya K, Kodama I. ATP directly affects gap junctional conductance between paired ventricular myocytes isolated from guinea pig heart. Circulation Research. 1990;66:1095–1102. doi: 10.1161/01.res.66.4.1095. [DOI] [PubMed] [Google Scholar]
- Takens-Kwak BR, Jongsma HJ, Rook MB, van Ginneken ACG. Mechanism of heptanol-induced uncoupling of cardiac gap junctions: a perforated patch-clamp study. American Journal of Physiology. 1992;262:C1531–1538. doi: 10.1152/ajpcell.1992.262.6.C1531. [DOI] [PubMed] [Google Scholar]
- Vera B, Sánchez-Abarca LI, Bolaños JP, Medina JM. Inhibition of astrocyte gap junctional communication by ATP depletion is reversed by calcium sequestration. FEBS Letters. 1996;392:225–228. doi: 10.1016/0014-5793(96)00794-6. [DOI] [PubMed] [Google Scholar]
- Verrecchia F, Hervé JC. Reversible blockade of gap junctional communication by 2,3-butanedione monoxime in rat cardiac myocytes. American Journal of Physiology. 1997a;272:C875–885. doi: 10.1152/ajpcell.1997.272.3.C875. [DOI] [PubMed] [Google Scholar]
- Verrecchia F, Hervé JC. Reversible inhibition of gap junctional communication by tamoxifen in cultured cardiac myocytes. Pflügers Archiv. 1997b;434:113–116. doi: 10.1007/s004240050370. [DOI] [PubMed] [Google Scholar]
- Verrecchia F, Kwak BR, Hervé JC. 17β-Estradiol-induced blockade of cell-to-cell communication in neonatal rat cardiomyocytes is not mediated by activation of tyrosine kinase, protein kinases A or C. In: Werner E, editor. Gap Junctions. Amsterdam: IOS Press; 1998. pp. 220–224. [Google Scholar]
- Wade MH, Trosko JE, Schindler M. A fluorescence photobleaching assay of gap junction-mediated communication between human cells. Science. 1986;232:525–528. doi: 10.1126/science.3961495. [DOI] [PubMed] [Google Scholar]
- Yazawa K, Kameyama A, Yasui K, Li JM, Kameyama M. ATP regulates cardiac Ca2+ channel activity via a mechanism independent of protein phosphorylation. Pflügers Archiv. 1997;433:557–562. doi: 10.1007/s004240050314. [DOI] [PubMed] [Google Scholar]
- Zimmermann N, Boknik P, Gams E, Gsell S, Jones LR, Maas R, Neumann J, Scholz H. Mechanisms of the contractile effects of 2,3-butanedione-monoxime in the mammalian heart. Naunym-Schmiedeberg's Archives of Pharmacology. 1996;354:431–436. doi: 10.1007/BF00168433. [DOI] [PubMed] [Google Scholar]


