Abstract
Experiments were performed on anaesthetized cats to investigate the receptive properties of regenerated cutaneous tibial nerve nociceptors, and to obtain evidence for coupling between them and other afferent fibres as being possible peripheral mechanisms involved in neuropathic pain. These properties were studied 6-7 months after nerve section and repair.
Recordings were made from 25 regenerated nociceptors; 14 were A fibres and the remainder were C fibres. Their receptive field sizes and conduction velocities were similar to controls. There was no significant difference between their mechanical thresholds and those of a control population of nociceptors.
Regenerated nociceptors were significantly more responsive to suprathreshold mechanical stimuli than were uninjured control fibres. This increase in mechanical sensitivity occurred in both A and C fibres, although A fibres showed a greater increase in mechano-sensitivity than C fibres. Over half of the regenerated nociceptors (13/25) showed after-discharge to mechanical stimuli which was never seen in controls; the mean firing rate during this period of after-discharge was significantly related to both stimulus intensity and stimulus area.
There was no significant difference between the heat encoding properties of regenerated nociceptors and control nociceptors. Cold sensitivity was similarly unchanged. Thus, abnormal peripheral sprouting was unlikely to account for the increased mechanical sensitivity of the regenerated fibres. None of the regenerated nociceptors were found to be coupled to other fibres.
These results suggest that the clinical observation of mechanical hyperalgesia in patients after nerve injury may have a peripheral basis. Based on this model, other signs of neuropathic pain (i.e. tactile or thermal allodynia) are more likely to be due to altered central processing.
Under normal conditions the main function of the nociceptive system is to alert the organism to damaging or potentially damaging stimuli. Peripheral nerve injuries cause sensory loss which usually improves as regeneration occurs, but the protective function of the nociceptive system can become disordered as a consequence of injury. This can present clinically as the phenomena of hyperalgesia (an increased response to a stimulus that is normally painful) and allodynia (pain due to a stimulus that does not normally produce pain). Others have suggested that nerve injury-induced hyperalgesia and allodynia result from changes in central processing of otherwise normal nociceptor and mechanoreceptor inputs (Bennett, 1994). Our hypothesis was that peripheral mechanisms could account for some of the sensory abnormalities in the pain system that persist long after nerve injury.
This hypothesis was derived from several lines of evidence. The first was drawn from the effects of nerve injury on some of the receptive properties of cutaneous nerve fibres. The consensus of several studies is that the receptive properties of regenerated myelinated mechanoreceptors are virtually indistinguishable from their uninjured counterparts (Burgess & Horch, 1973; Terzis & Dykes, 1980; Horch & Lisney, 1981). Shea & Perl (1985) specifically studied regenerated unmyelinated units (C fibres) and again found that regenerated mechanoreceptors were similar to controls, but they also described alterations in the mechanical and heat thresholds of regenerated mechano-heat (polymodal) nociceptors. Mechanical thresholds were significantly higher 2 months after nerve injury but not at later time points, and 20 % of fibres had lower heat thresholds than normal units. The lowered heat thresholds could therefore account for the symptom of heat hyperalgesia, although the raised mechanical thresholds recorded after 2 months would favour reduced nociceptive sensitivity. Cooper et al. (1991) have described the phenomenon of inflammation-induced nociceptor sensitization to mechanical stimuli, without threshold reduction. We hypothesized that if this was a general property of nociceptors, rather than inflammation specific, then mechanical sensitization of nociceptors after nerve injury may have been missed in Shea and Perl's experiments due to reliance on threshold values.
The second line of evidence was based on the experiments of Lisney & Pover (1983) on ephaptic coupling between nerve fibres involved in an injury. This coupling, which is rare in normal uninjured nerves, (Lisney & Pover, 1983; Meyer et al. 1985) allows propagation of impulses in one fibre to one or more other fibres and is essentially ‘cross-talk’ between fibres. Lisney & Pover (1983) showed that after injury coupling occurred between pairs of unmyelinated or small myelinated fibres. We hypothesized that coupling between a mechanoreceptor and a nociceptor could account for the mechanical allodynia that some patients develop after nerve injuries.
Experiments were therefore performed to investigate the receptive properties of regenerated cutaneous nociceptors, particularly to suprathreshold mechanical stimulation. The possibility of ephaptic coupling between regenerated nociceptors and mechanoreceptors was also examined. Either of these mechanisms could be important peripheral components of the sensory abnormalities that arise after nerve injury. These experiments were performed on the tibial nerves of cats as our laboratory had previously described in detail their mechanical responses in normal animals (Garell et al. 1996).
METHODS
Experiments were carried out on nine young, adult cats (2.8-4.5 kg) of either sex. The experimental protocols used were approved by the Institutional Animal Care and Use Committee of the University of Maryland Dental School.
Nerve injury
The effects of nerve injury on the responses of cutaneous nociceptors were studied in four cats. Each was anaesthetized with Isoflurane (Abbott Labs, N. Chicago, IL, USA) in oxygen (induction 5 % v/v; maintenance 2-3 % v/v) and positioned prone. The skin over the dorsal surface of the right ankle and calf was shaved and disinfected with a 10 % solution of providone iodine. Under sterile conditions, a 4 cm incision was made proximal to the ankle and the tibial nerve identified by blunt dissection. The nerve was cut cleanly with fine scissors and the cut ends re-approximated with 2-5 epineurial sutures (Ethilon 6/0; Ethicon, Somerville, NJ, USA). The incision was closed in layers and the skin sutured with silk. The incision margins were infiltrated with local anaesthetic (lignocaine hydrochloride, (Abbott Labs) 2 % w/v) to minimize post-operative pain and an intra-muscular injection of penicillin G benzathine (Wyeth Ayerst, Philadelphia, PA, USA; 300 000 units) given. Anaesthesia was then discontinued and the animals allowed to recover consciousness before being returned to their cages. The skin sutures were removed 7-10 days later.
This simple model of nerve injury was chosen to maximize the number of regenerated units available for study. Such ‘clean’ injuries may cause post-operative neuropathic pains less frequently than more traumatic injuries, or those without repair. However, a study of 33 patients with complete transections of the median, ulnar or digital nerves showed that 62 % of those treated by epineurial suturing developed symptoms of neuropathic pain (dysaesthesia, allodynia or hyperpathia; Ochs et al. 1989). Behavioural testing in rats following tibial nerve section and epineurial suturing shows reflex changes indicative of hyperalgesia and allodynia (Timmermann et al. 1997). Also, sciatic nerve section and repair is known to cause self mutilation (autotomy) in rodents despite successful peripheral re-innervation (Wall et al. 1979).
Preparation of animals for terminal experiments
Animals with previously injured tibial nerves were allowed to survive for 26-29 weeks after their initial injury; after this time no further significant regeneration occurs (Horch & Lisney, 1981). Control experiments were performed on five other cats to determine the properties of uninjured nociceptors. Ostensibly these experiments were necessary to investigate heat encoding by cat cutaneous nociceptors, but their mechanical properties were also studied. Although the mechanical response properties of cat cutaneous nociceptors have previously been described in control animals using the same protocols as in this series of experiments (Garell et al. 1996), we repeated these experiments for two reasons. Firstly, an improved mechanical stimulator was used for the current experiments which had a greater range of motion than the one previously employed and could therefore be used on more compliant tissues. Secondly, the current experiments were performed under anaesthesia, whereas those of Garell et al. (1996) were performed on decerebrate animals.
The animals were anaesthetized initially with sodium pentobarbitone (Nembutal, 42 mg kg−1i.p.; Abbott, N. Chicago, IL, USA). Cannulae were inserted into an external jugular vein for administration of drugs, and into a carotid artery to monitor arterial blood pressure. The trachea was also cannulated for subsequent artificial ventilation. Anaesthesia was maintained by additional doses of barbiturate (3-4 mg kg−1 h−1) given intravenously. A depth of anaesthesia was maintained so that the animals were areflexic to pinching a forepaw. The hair of the right hindlimb was clipped short. Body temperature was maintained at 38.0 ± 0.5°C with an electric blanket that was thermostatically controlled from a rectal thermistor. Intravenous lactated Ringer solution (10 ml kg−1 h−1; Baxter Healthcare, Deerfield, IL, USA) was given to prevent dehydration for experiments that lasted more than 24 h, and the bladder catheterized to prevent urine accumulation.
To study regenerated nociceptors the tibial nerve was exposed from the ankle to mid-thigh level, where it joined the sciatic nerve. The nerve was cut as centrally as possible, placed on a small plastic dissecting platform and a skin pool formed from the surrounding tissues. The pool was filled with warm mineral oil to prevent the exposed tissues from drying and to maintain electrical isolation. Pool temperature was maintained at 38°C with a heating coil. Three pairs of stimulating electrodes were placed on the nerve (Fig. 1A): a pair was placed either side of the injury site (S1 and S2, Fig. 1A) to identify regenerated fibres, and a third pair was placed on the cut end of the nerve (S3) to search for coupled units. In one experiment scar tissue prevented placing the distal stimulating electrodes (S1), and electrocutaneous stimulation was used to obtain conduction velocity measurements distal to the injury site. The connective tissue surrounding the nerve was slit with a chip of razor blade, fine filaments dissected from it and placed upon a pair of platinum wire electrodes (0.125 mm diameter). Differential recordings were made from pairs of filaments, one containing the unit under study. To prevent muscle contraction during electrical stimulation of the nerve, the animals were paralysed with pancuronium bromide (400 μg i.v.; Gensia Pharmaceuticals, Irvine, CA, USA) and artificially ventilated. Respiratory rate and tidal volume were adjusted to maintain end-tidal CO2 levels of 3.5-4.5 %. Three indicators of adequate anaesthetic depth were monitored during paralysis: (i) the pupils were constricted; (ii) heart rate and blood pressure did not fluctuate in response to noxious stimuli; (iii) when muscle relaxation wore off, as evidenced by muscle twitches during electrical stimulation, pinching a forepaw did not evoke a withdrawal reflex.
Figure 1. Diagram of the preparation and testing the receptive properties of regenerated nociceptors.
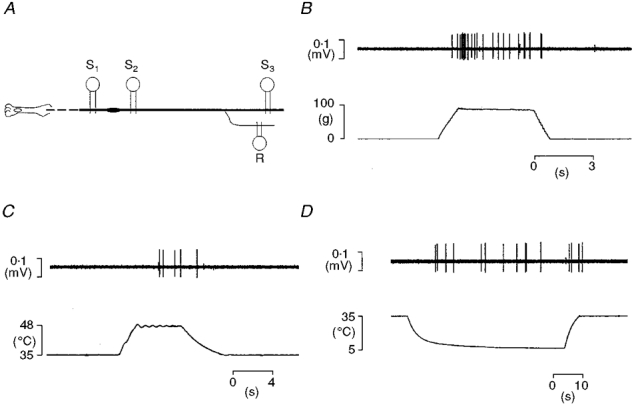
A, schematic representation of the preparation showing the sites of electrical stimulation and of recording of single units (S1-S3). B-D, examples of responses of a regenerated nociceptor (a mechano-heat-cold fibre, CV from S1 0.7 m s−1) to mechanical (B) and thermal (C and D) stimulation of its receptive field.
Recordings were also made from animals with uninjured nerves. These were performed on either the tibial nerve (n = 1) or the saphenous nerve (n = 4). The saphenous nerve experiments proved superior as they yielded a greater proportion of units suitable for testing (as the tibial nerve is a mixed nerve whereas the saphenous nerve is purely sensory). The tibial nerve was exposed as described above, placed on a pair of stimulating electrodes just proximal to the ankle, and fine filaments dissected from its cut central end. The saphenous nerve was exposed on the medial surface of the thigh and similarly prepared for single unit recording. Skin pools were formed as outlined above.
At the end of the experiment the animals were killed with an overdose of anaesthetic (50 mg kg−1 sodium pentobarbitone, i.v.).
Nociceptor identification and characterization
Filaments were screened for regenerated fibres by electrical stimulation distal to the injury site (S1, Fig. 1A), and by stroking the skin with a camel's hair brush. Those fascicles that did not contain any identifiable units were discarded and not studied further. Filaments containing fibres that had regenerated to the periphery were repeatedly sub-divided until, ideally, only a single functional unit remained. Recordings were made when more than one fibre remained (usually 2-4) as long as their action potentials were clearly discriminable (different polarities and/or amplitudes), and their receptive fields were clearly separate. Receptor types of the units in a filament were identified by stroking the skin, and by gentle squeezing with a pair of blunt forceps. A unit was classified as a nociceptor if it fulfilled the following criteria: (i) it exhibited a sustained response to squeezing a skin fold; (ii) its mechanical threshold was ≥ 0.3 g; (iii) it did not respond to brushing (Burgess & Perl, 1967). With these criteria it is possible that regenerated nociceptors with abnormally low thresholds may have been discarded. To ensure that units did not become sensitized prior to evaluation of their receptive properties the following precautions were taken: (i) only gentle squeezing of skin folds was used as a search stimulus; (ii) heat was used sparingly, and only after nociceptor mechanical sensitivity was evaluated; (iii) a nociceptor was only studied if its receptive field did not overlap with that of a previously characterized fibre; (iv) during evaluation of a unit's responses stimulus blocks were temporally separated by 5-10 min. Once a nociceptor was isolated, its latency at threshold and twice threshold was determined for electrical stimuli delivered distal and proximal to the injury site. Confirmation of a unit's identity was always obtained by electrical stimulation of its receptive field (Garell et al. 1996). Each unit's mechanical threshold was determined using calibrated monofilaments (Semmes-Weinstein monofilaments; Stoelting Instruments, Chicago, IL, USA) and its receptive field mapped with fine forceps.
The intensity-coding properties of each unit were investigated using carefully controlled stimuli. Ramp-and-hold (ramp rate: 75 g s−1; hold time: 4.0 s; Fig. 1B) mechanical stimuli in the range 5-90 g were applied with a computer-controlled linear motor (Neurologic Inc., Bloomington, IN, USA) under force feedback regulation (model 501 motor controller; Biocommunication Electronics, Madison, WI, USA). The probe tip of the stimulator was interchangeable allowing stimuli to be delivered with probes of 5.0-0.1 mm2 contact area as previously described (Garell et al. 1996). Each unit was tested twice, first using the probes in sequence largest to smallest and then again in reverse. Thermal sensitivity was tested using a contact thermal stimulator (probe tip area 1.1 cm2; Taylor et al. 1993) that permitted either heating or cooling stimuli to be delivered by circulating either room temperature or pre-cooled ethylene glycol solution through the stimulator's probe (Fig. 1C and D). Heat sensitivity was tested with discrete ramp-and-hold (rise time 2.0 s, hold time 5.0 s) stimuli in the range 40-50°C delivered in 2°C steps from an adapting temperature of 35°C. Cold sensitivity was tested similarly but the lowest temperature that could be achieved was 5°C, and the rate of temperature change decreased progressively as the temperature fell below 20°C (Fig. 10). Temperature records were obtained from a thermocouple fixed to the surface of the probe.
Figure 10. Cold sensitivity of control C fibre nociceptors.
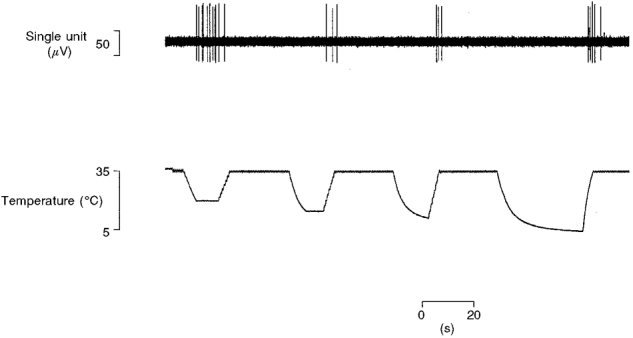
A typical example of cold-evoked responses from a saphenous nerve C fibre (CV 1.1 m s−1). The unit responded directly to a 20 °C stimulus from an adapting skin temperature of 35 °C but not to colder stimuli, it also responded reproducibly during rewarming.
After characterization of a unit's receptive properties, evidence was sought for ephaptic coupling between it and other tibial nerve fibres. Electrical stimuli suprathreshold for unmyelinated fibres (10 mA, 0.5 ms, 0.5 Hz) were applied to the whole nerve, but not to the unit being studied (S3, Fig. 1A), to determine if antidromic stimulation of the remainder of the nerve evoked an orthodromic impulse in the unit under study. Sudden jumps in latency to electrical stimuli of increasing intensity at either S1 or S2 were also indicative of coupled units (Matthews, 1977).
Data analysis
Data were displayed on an oscilloscope, recorded on magnetic tape and digitized with a computer interface (Cambridge Electronic Design, Cambridge, UK; type: 1401 plus). Neural records were sampled at 50 kHz, records of force, tissue displacement and temperature were sampled at 1 kHz. Action potentials of the unit under study were isolated using software capable of amplitude and time discrimination (Cambridge Electronic Design; Spike 2). Mean firing rates were determined by dividing the evoked discharge by the stimulus duration. Responses of spontaneously active units were corrected for background activity by subtracting the ongoing discharge present during the preceding intertrial interval. Statistical comparisons were made using commercially available software (Jandel Scientific, San Rafael, CA, USA; SigmaStat). Differences between the intensity-coding properties of regenerated and control units were tested using 2-factor analysis of variance, with repeated measures on one factor (RM ANOVA). For each test, one factor was stimulus intensity (force or temperature, the repeated measure) and the other factor was unit type (control or regenerated). A P value < 0.05 was considered significant.
RESULTS
All the animals recovered from the initial surgery without incident. At the time of the terminal experiment none displayed any signs of autotomy or trauma to the operated limb.
General properties of regenerated nociceptors
Recordings were made from 25 regenerated nociceptors, these comprised 14 A fibre nociceptors and 11 C fibre nociceptors. Seven, seven, six and five units were studied in each of the four terminal experiments. Their conduction velocities (CV), determined by electrical stimulation distal to the injury site (S1, Fig. 1A), ranged from 6.7-19.1 m s−1 (mean 12.8, s.d. 4.4) for the A fibres, and 0.6-1.4 m s−1 (mean 1.0, s.d. 0.2) for the C fibres. These values are within the limits of normal cat nociceptors (Burgess & Perl, 1967; Bessou & Perl, 1969; Beck et al. 1974; Fitzgerald & Lynn, 1977; Garell et al. 1996). Conduction velocities of the myelinated units proximal to the injury site (S2, Fig. 1A) were faster than those distal (mean 26.2 m s−1, range 10.5-28.2, s.d. 10.1; Fig. 2), similar to that reported for regenerated cutaneous mechanoreceptors (Horch & Lisney, 1981). In general, the receptive fields of regenerated nociceptors were similar in size and shape to control units (Fig. 3; Burgess & Perl, 1967; Bessou & Perl, 1969): the A fibre fields were composed of several (4-9) small sensitive spots separated by insensitive areas; C fibres had small, elliptical receptive fields 2-3 mm in diameter. Only one of the nociceptors studied had a split receptive field (a C-mechanical nociceptor, CV from S1 0.6 m s−1), similar to that reported for regenerated mechanoreceptors (Terzis & Dykes, 1980; Horch & Lisney, 1981).
Figure 2. Slowing of conduction velocity of regenerated myelinated nociceptors.
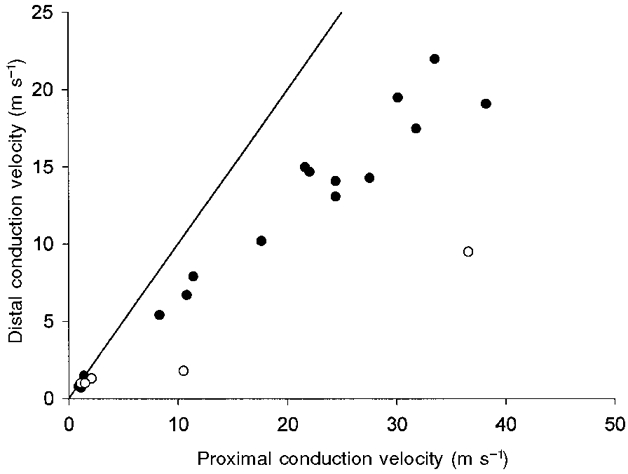
Myelinated, but not unmyelinated regenerated nociceptors conduct faster proximal to the injury site than through it. Proximal conduction velocities were obtained from latency measurements evoked by stimulation at S2 (Fig. 1A). Distal conduction velocities were obtained from stimulation at S1. For convenience faster conduction velocities have been plotted on the horizontal axis. ○, units whose distal conduction velocities were only determined by electrocutaneous stimulation (see Methods). The continuous line is the line of unity.
Figure 3. Distribution of receptive fields of regenerated nociceptors.
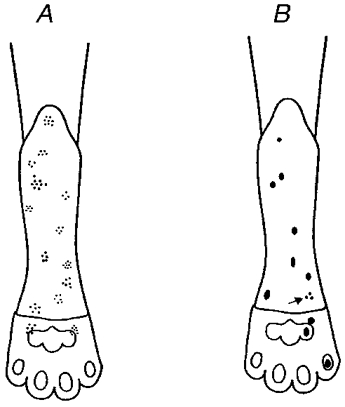
Receptive field distribution for regenerated A fibres (A) and regenerated C fibres (B). The arrow indicates a C fibre with a multi-spot receptor.
The mechanical thresholds of the A fibre nociceptors to monofilament stimulation were in the range 207-1639 kPa (mean 744, median 764, s.d. 345; 100 kPa = 1 bar = 10 g mm−2), those of the C fibres were 130-764 kPa, (mean 411, median 520, s.d. 225). The median threshold of the regenerated nociceptors (572 kPa) was not significantly different to that of control cat tibial nerve nociceptors (520 kPa, P > 0.66, Mann-Whitney U-test; Garell et al. 1996). Of the A fibres, 11 responded only to noxious mechanical stimuli and were classified as high-threshold mechanoreceptors (Burgess & Perl, 1967), two units responded to noxious heat as well as noxious mechanical stimuli and were considered mechano-heat nociceptors (type II) and one responded to cold stimulation as well as noxious mechanical stimuli - a mechano-cold nociceptor. Eight C fibres responded to mechanical and heat stimuli (mechano-heat or ‘polymodal’ units; Bessou & Perl, 1969) with three of them also responding to cold stimuli. Of the remaining three C nociceptors, one was a mechanical nociceptor and two were mechano-cold units. Due to the small size of individual groups of C fibre nociceptors they were treated as a single population for statistical purposes. Three units were spontaneously active when first isolated, two were C fibres (CV from S1 0.8 and 1.4 m s−1) and the third was an A fibre (CV from S1 15.0 m s−1). Their rates of ongoing activity were 0.7, 0.1 and 0.4 Hz respectively.
Intensity coding of mechanical stimuli
Recordings were made from 14 mechanically sensitive control C fibre nociceptors (CV range 0.6-1.4 m s−1, mean 1.0, s.d. 0.2) to test for differences in experimental protocols that may have influenced nociceptor responsivity, and therefore would have prevented statistical comparisons between the data reported here and those of Garell et al. (1996). Their mechanical response properties were compared with those of eight C fibre nociceptors reported by Garell et al. (1996). An example of the responses of a typical control C fibre nociceptor to increasing intensity mechanical stimulation of its receptive field is shown in Fig. 4, and mean intensity-coding properties of all units for all probes are shown in Fig. 5. There were no significant differences between the mechanical responses of control C fibre nociceptors recorded in the current experiments and those reported by Garell et al. (1996; (Fig. 5; P > 0.1 for each probe size, 2-factor RM ANOVA)). Statistical comparisons between control and regenerated nociceptors were made using the data reported for A fibre nociceptors by Garell et al. (1996), whereas data from both normal and regenerated C fibre nociceptors were obtained from the current experiments.
Figure 4. Typical response to mechanical stimuli of a control C fibre nociceptor.
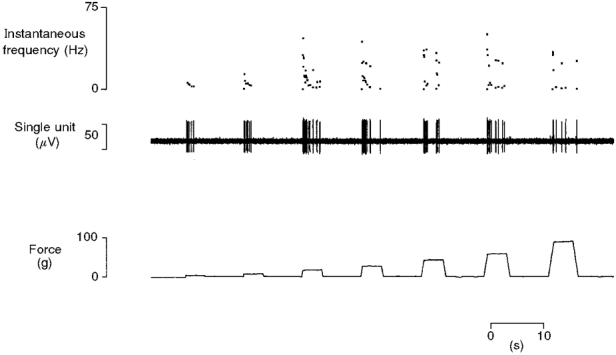
Mechanically evoked responses from a single C fibre (CV 1.1 m s−1) to progressively more intense stimuli delivered with a probe of tip area 0.25 mm2. The response of the unit increases with stimulus intensity. It is quiescent between stimuli and also shows the bursting typical of C fibres.
Figure 5. Intensity coding of mechanical stimuli by C fibre nociceptors in two different cutaneous fields.
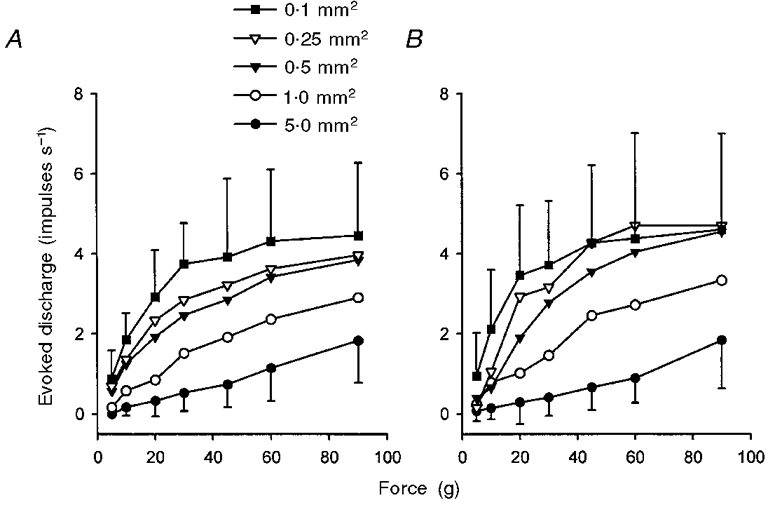
A, responses of 9 C fibres in the saphenous nerve to mechanical stimulation with probes of tip area 5.0-0.1 mm2. B, responses of 8 C fibres in the tibial nerve to identical stimulation (originally reported by Garell et al. 1996). Two-factor RM ANOVA revealed that there was no significant difference between the sensitivity of each population of fibres (P > 0.1 for each probe size). For clarity, error bars (± 1 s.d.) are shown only for the 5.0 and 0.1 mm2 probes.
The effects of nerve injury and regeneration on the mechanical responses of cat cutaneous nociceptors are shown in Fig. 6 and Fig. 7. Two main differences between their responses were observed: firstly, the regenerated units were significantly more responsive than the control fibres. That is, for a given force more impulses were evoked in the regenerated units than the control units. For the A fibres this was limited to the three smallest probes (0.5 mm2: P < 0.05, 0.25 mm2: P < 0.05, 0.1 mm2: P < 0.03; 2-factor RM ANOVA; Fig. 7A). For the C fibres, the regenerated units were significantly more responsive than controls for only the smallest sized probe (0.1 mm2: P < 0.05; 2-factor RM ANOVA; Fig. 7C). The regenerated C fibres were also more variable in their responses than controls (Fig. 7C), with some animals contributing to the enhanced mechano-sensitivity more than others (Fig. 7E). Secondly some, but not all (13/25) of the units demonstrated a sustained discharge which persisted after the stimulus had ceased (Fig. 6). This was never seen in normal nociceptors. Of these 13 units, 10 were A fibres (CV range from S1 6.7-19.1 m s−1, mean 12.4, s.d. 4.1) and 3 were C fibres (CV range from S1 0.6-1.4 m s−1, mean 1.0, s.d. 0.4). A fibres were more likely to exhibit after-discharge responses than C fibres (P < 0.05, Fisher exact test), but there was no tendency for such units to differ in terms of monofilament threshold (P > 0.8, Mann-Whitney U-test) or receptive field size from units that did not show after-discharge. The after-discharge was usually initiated following the first or second stimulus in a series, its frequency gradually increased, often peaking during the middle of the series then declining over time, sometimes ceasing altogether (Fig. 6 and Fig. 8). The after-discharge rate (or repetitive firing frequency) was significantly dependent on probe size (P < 0.01; 2-factor RM ANOVA) and also on the intensity of mechanical stimulation (P < 0.05; 2-factor RM ANOVA).
Figure 6. Typical response of a regenerated nociceptor to noxious mechanical stimulation.
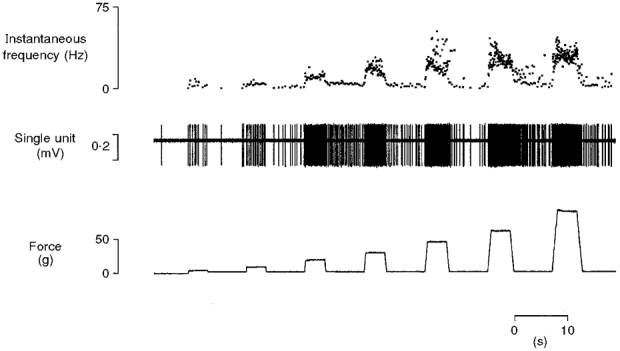
Response of an A fibre (CV from S1 14.7 m s−1) to mechanical stimulation (5-90 g) of its receptor with a probe of contact area 0.25 mm2. Both the increased sensitivity of the unit to mechanical stimuli and the development of repetitive firing are exhibited by this nociceptor.
Figure 7. Regenerated nociceptors are more responsive to mechanical stimulation than controls.
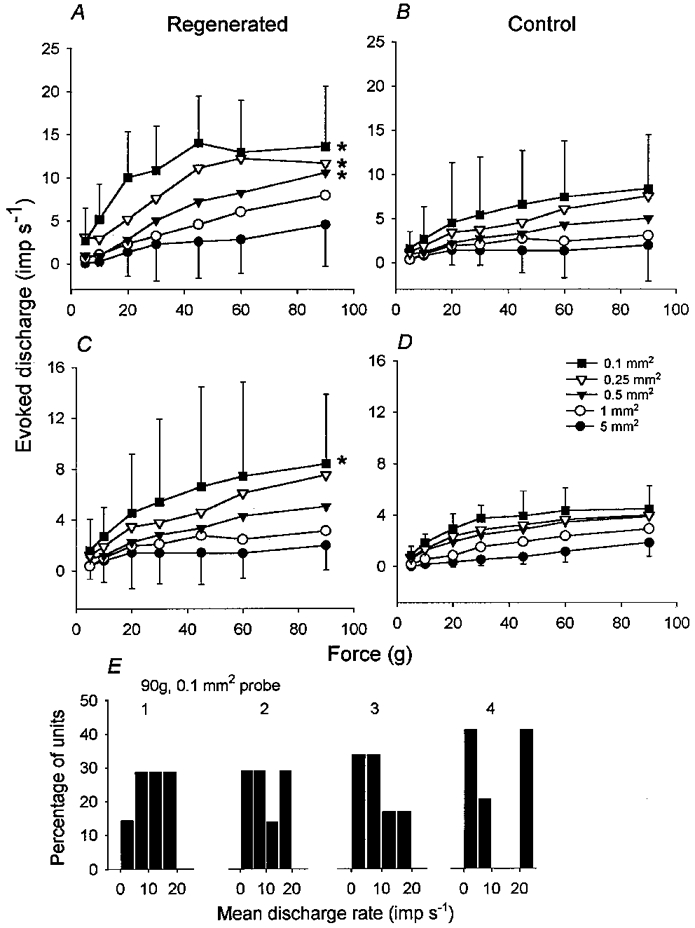
Intensity coding plots for regenerated A (A) and C (C) fibres and control A (B) and C (D) fibres. * Probe sizes that evoked significantly more impulses during stimulation in regenerated units than controls. Data in B originally reported by Garell et al. (1996). For clarity, error bars (± 1 s.d.) are shown only for the 5.0 and 0.1 mm2 probes. E, distributions of mean firing rates for all regenerated units studied during mechanical stimulation at 90 g intensity with a probe of 0.1 mm2 contact area. For each animal (1-4) unitary responses have been plotted in bins of width of 5 impulses s−1.
Figure 8. Mechanically evoked after-discharges in regenerated nociceptors show dependence on the spatial and intensive aspects of the stimulus.
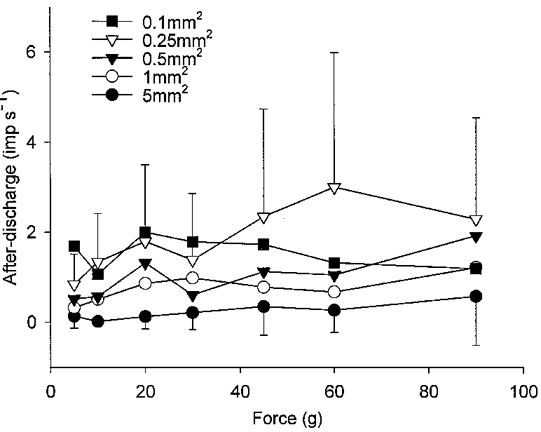
The number of impulses (imp s−1) between stimuli was counted, and mean firing rate determined and plotted as a function of probe size and stimulus intensity for all 13 units that showed after-discharge. For clarity, error bars (± 1 s.d.) are shown only for the 5.0 and 0.25 mm2 probes.
Responses to heat
Of the ten heat-sensitive regenerated nociceptors, two were A fibres (thresholds 43 and 49°C) and eight were C fibres (mean threshold 45°C, range 40-47, s.d. 4.1). The intensity coding properties of the C fibres are shown in Fig. 9. The responses of regenerated A fibres were similar to those of control A fibre nociceptors (Fitzgerald & Lynn, 1977), and there was no significant difference in the proportion of heat-sensitive A nociceptors in the regenerated population compared with controls (P > 0.05, χ2 test). The heat-evoked responses of regenerated C fibres were almost identical to those of 14 control C fibres recorded from the saphenous nerve (Fig. 9A). There were no significant differences between their stimulus-response properties whether considering the entire response period (P > 0.9, 2-factor RM ANOVA), or just the first second of the response (P > 0.7, 2-factor RM ANOVA; Fig. 9B).
Figure 9. Heat sensitivity of regenerated C fibre nociceptors is similar to controls.
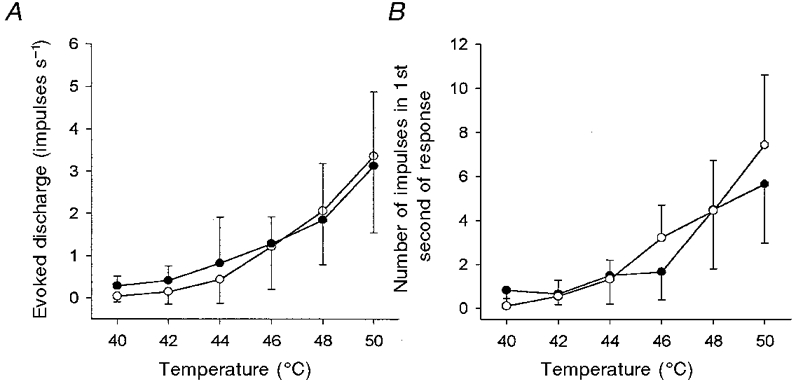
Neither the intensity coding properties (A) of regenerated C fibre nociceptors (•, n = 8) nor their firing frequency during the first second of a response (B) were significantly different to that of control C fibre nociceptors (○, n = 11; P > 0.9 for intensity coding, P > 0.7 for firing frequency, 2-factor RM ANOVA). Bars indicate ± 1 s.d.
Responses to cold
Six regenerated nociceptors were responsive to cold as well as heat and/or mechanical stimuli. Only one unit was an A fibre (CV from S1 10.5 m s−1), the remainder being C fibres (mean CV from S1 0.9 m s−1, range 0.6-1.2, s.d. 0.3). Probe surface temperature at the threshold of impulse generation ranged from 27-28°C (mean 20, s.d. 7.2). All these units were directly excited by cold (e.g. Fig. 1D), but two were also activated during the rewarming step of a cooling stimulus at lower (warmer) thresholds than that of the direct response. That is, impulse activity was recorded during the ascending phase of a cooling stimulus (rewarming back to 35°C), but not during the descending (active cooling from 35°C) or plateau phases. This rewarming response was also observed in 10 out of 14 control C fibres; a typical example is shown in Fig. 10. Interestingly, the control units that showed a rewarming response were usually (n = 7) excited by the initial 20°C stimulus but not by colder stimuli (Fig. 10).
Ephaptic coupling
Each nociceptor isolated was tested for coupling with other fibres within the remainder of the tibial nerve. Electrical stimuli were applied to all the remaining axons within the nerve, except for those in the filament containing the unit under study. None of the regenerated nociceptors were found to be coupled, although other units, that were inexcitable from the skin, were. With this method, one-way coupling would only be detectable if it occurred in the direction of nerve to single unit. This is unlikely to be the cause of our failure to find coupling between regenerated nociceptors and other fibres, as most coupling is bi-directional (Lisney & Pover, 1983).
DISCUSSION
This study has documented the selective hypersensitivity of regenerated nociceptors to suprathreshold mechanical stimuli but not thermal stimuli. This mechanical sensitization of regenerated nociceptors occurred without a significant change in median mechanical threshold, when compared with a sample of uninjured units (Garell et al. 1996). Similar threshold and suprathreshold results were reported by Cooper et al. (1991) following inflammation-induced sensitization of goat palate mechanical nociceptors. Thus, previous studies that relied solely on threshold values to indicate peripheral sensitization of nociceptors are likely to have overlooked the type of suprathreshold sensitization reported here.
There could be several explanations for the mechanical sensitization of regenerated nociceptors. One possibility is a greater than normal peripheral sprouting of their terminals. This explanation is unlikely as the receptive fields of regenerated units were of a similar size to controls. Also, an increased peripheral sprouting, thus increasing the innervation density, would be expected to result in both enhanced thermal and mechanical responsiveness, whereas only mechanical hyper-responsiveness was evident. Our failure to observe increased heat responsiveness strongly suggests that the mechanical sensitization observed was not due to repetitive stimulation. It is possible that there was selective sprouting of terminals containing only mechanically sensitive transduction elements. Treede et al. 1990 have shown that the heat-sensitive zones of primate C mechano-heat nociceptors extend further than their zones of mechanical sensitivity, suggestive of parcellation of transduction elements within nociceptor terminals. Preferential sprouting of mechano-sensitive terminals but not heat-sensitive ones could therefore explain the current observations. A second explanation could be selective enhancement of the gating properties of mechanically sensitive transduction channels, such that the same stimulus would evoke more inward current in a regenerated axon terminal than in an uninjured one.
It is unlikely that changes in skin compliance after nerve injury could have accounted for the differences in mechano-sensitivity of the regenerated nociceptors. This is because the mechanical stimulator used by Garell et al. (1996) had a maximum travel of 2.5 mm and the tissue displacement measurements made in the current experiments were of similar magnitude. Also, the intensity coding properties of control C nociceptors in the saphenous nerve were not significantly different from those of control C fibre nociceptors in the tibial nerve (Garell et al. 1996), despite the saphenous nerve receptive fields being less compliant than those of the tibial nerve units.
The current experiments provide electrophysiological evidence for mechanical hyperalgesia, although we did not determine if this was evident behaviourally. Qualitative post-operative evaluation of the cats showed no evidence of tactile hypersensitivity on their operated hindlimb. However, we did not evaluate mechanical sensitivity with stimuli suprathreshold for nociceptor activation. Mechanical hyperalgesia certainly occurs in patients with neuropathic pain (Ochoa & Yarnitsky, 1993), and appears to be conducted by unmyelinated fibres. Interestingly, mechanical hyperalgesia is also observed in rats rendered diabetic with streptozotocin (Ahlgren & Levine, 1993), and appears to have a peripheral basis (Ahlgren et al. 1992). In diabetic rats the responses of C fibre nociceptors to mechanical stimuli were remarkably similar to those reported here for regenerated nociceptors: there was mechanical sensitization without significant alteration in threshold distribution; increased suprathreshold discharge; and increased after-discharge following stimulus cessation in units recorded from diabetic rats compared with controls.
We were unable to obtain convincing evidence for an increase in cold sensitivity of regenerated nociceptors, despite the fact that cold hyperalgesia is observed in patients with neuropathic pains of varied aetiology (Ochoa & Yarnitsky, 1994). This may be partly due to technical reasons: the lowest temperature that could be achieved with our stimulator was +5°C, which would only excite 15 % of rat A fibre nociceptors (Simone & Kajander, 1997). Also, the sensitivity of control C fibre nociceptors to cold was unexpected; 10 of 14 units were cold-sensitive in that they were directly excited by cold. However, such fibres displayed non-uniform sensitivity as they responded to cool (20°C) but not subsequent colder (10, 15 and 5°C) stimuli, yet they also responded during rewarming (Fig. 10). Perl and colleagues (Bessou & Perl, 1969; Kumazawa & Perl, 1977) have interpreted such cold-evoked responses as being due to receptor sensitization: they were seen after long duration (90 s) heat stimuli of increasing intensity up to 55°C, that were sufficient to cause background firing to develop (Kumazawa & Perl, 1977). Whilst we cannot rule out the possibility that the cold sensitivity of polymodal nociceptors was due to peripheral sensitization, the heat stimuli used in the current experiments were of much shorter duration than those used by Perl and colleagues, with the most intense stimulus tested being only 50°C. Also, none of the control units that were evaluated developed spontaneous activity as a consequence of repetitive stimulation. In addition, the sensation of burning pain evoked by skin cooling during compression block of the myelinated fibres (Yarnitsky & Ochoa, 1990) appears to be conducted by polymodal nociceptors with cold thresholds in the innocuous range. The current data would provide a physiological basis for this observation. The cold hyperalgesia of neuropathic pain is therefore probably due to a loss of central inhibition that unmasks the cold-evoked response of C mechano-heat nociceptors, as previously suggested (Ochoa & Yarnitsky, 1994).
None of the regenerated nociceptors were found to be electrically coupled to other fibres. In Lisney and Pover's (1983) experiments only 10 % of coupled pairs were associated with a regenerated cutaneous receptor. In all these pairs only one fibre had regenerated to the skin, and its end organ was always a mechanoreceptor. The other unit of the pair appeared to terminate in the neuroma formed at the injury site. It is not possible to exclude completely the possibility that regenerated nociceptors were coupled to other fibres within the nerve fascicle containing the unit being studied. However, the data reported by Meyer et al. (1985) show that coupling does occur between axons that are spatially separate within peripheral nerves.
Acknowledgments
This work was supported by the National Science Foundation (IBN 95-96205 and IBN 96-96127) and the University of Maryland Dental School Designated Research Initiative Fund. D.A. was a Senior International Scholar of the Fulbright Commission during this research. We thank Mr C. Cordes for his technical assistance.
References
- Ahlgren SC, Levine JD. Mechanical hyperalgesia in streptozotocin-diabetic rats. Neuroscience. 1993;52:1049–1055. doi: 10.1016/0306-4522(93)90551-p. 10.1016/0306-4522(93)90551-P. [DOI] [PubMed] [Google Scholar]
- Ahlgren SC, White DM, Levine JD. Increased responsiveness of sensory neurones in the saphenous nerve of the streptozotocin-diabetic rat. Journal of Neurophysiology. 1992;68:2077–2085. doi: 10.1152/jn.1992.68.6.2077. [DOI] [PubMed] [Google Scholar]
- Beck PW, Handwerker HO, Zimmerman M. Nervous outflow from the cat's foot during noxious radiant heat stimulation. Brain Research. 1974;67:373–386. doi: 10.1016/0006-8993(74)90488-0. [DOI] [PubMed] [Google Scholar]
- Bennett GJ. Neuropathic Pain. In: Wall PD, Melzack R, editors. Textbook of Pain. New York: Churchill Livingstone; 1994. pp. 201–224. [Google Scholar]
- Bessou P, Perl ER. Responses of cutaneous sensory units with unmyelinated fibres to noxious stimuli. Journal of Neurophysiology. 1969;32:1025–1043. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- Burgess PR, Horch KW. Specific regeneration of cutaneous fibres in the cat. Journal of Neurophysiology. 1973;36:101–114. doi: 10.1152/jn.1973.36.1.101. [DOI] [PubMed] [Google Scholar]
- Burgess PR, Perl ER. Myelinated afferent fibres responding specifically to noxious stimulation of the skin. The Journal of Physiology. 1967;190:541–562. doi: 10.1113/jphysiol.1967.sp008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper B, Ahlquist M, Friedman RM, Labanc J. Properties of high threshold mechanoreceptors in the goat oral mucosa. II. Dynamic and static reactivity in carrageenan-inflamed mucosa. Journal of Neurophysiology. 1991;66:1280–1290. doi: 10.1152/jn.1991.66.4.1280. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Lynn B. The sensitization of high threshold mechanoreceptors with myelinated axons by repeated heating. The Journal of Physiology. 1977;265:549–563. doi: 10.1113/jphysiol.1977.sp011730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garell PC, McGillis SLB, Greenspan JD. Mechanical response properties of nociceptors innervating feline hairy skin. Journal of Neurophysiology. 1996;75:1177–1189. doi: 10.1152/jn.1996.75.3.1177. [DOI] [PubMed] [Google Scholar]
- Horch KW, Lisney SJW. On the number and nature of regenerating myelinated axons after lesions of cutaneous nerves in the cat. The Journal of Physiology. 1981;313:275–286. doi: 10.1113/jphysiol.1981.sp013664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazawa T, Perl ER. Primate cutaneous sensory units with unmyelinated (C) afferent fibres. Journal of Neurophysiology. 1977;40:1325–1338. doi: 10.1152/jn.1977.40.6.1325. [DOI] [PubMed] [Google Scholar]
- Lisney SJW, Pover CM. Coupling between fibres involved in sensory nerve neuromata in cats. Journal of the Neurological Sciences. 1983;59:255–264. doi: 10.1016/0022-510x(83)90043-6. 10.1016/0022-510X(83)90043-6. [DOI] [PubMed] [Google Scholar]
- Matthews B. Responses of intradental nerves to electrical and thermal stimulation of teeth in dogs. The Journal of Physiology. 1977;264:641–664. doi: 10.1113/jphysiol.1977.sp011687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RA, Raja SN, Campbell JN. Coupling of action potential activity between unmyelinated fibres in peripheral nerve of monkey. Science. 1985;227:184–187. doi: 10.1126/science.3966152. [DOI] [PubMed] [Google Scholar]
- Ochoa JL, Yarnitsky D. Mechanical hyperalgesias in neuropathic pain patients: Dynamic and static subtypes. Annals of Neurology. 1993;33:465–472. doi: 10.1002/ana.410330509. [DOI] [PubMed] [Google Scholar]
- Ochoa JL, Yarnitsky D. The triple cold syndrome. Cold hyperalgesia, cold hypoaesthesia and cold skin in peripheral nerve disease. Brain. 1994;117:185–197. doi: 10.1093/brain/117.1.185. [DOI] [PubMed] [Google Scholar]
- Ochs G, Schenk M, Struppler A. Painful dysaesthesias following peripheral nerve injury: A clinical and electrophysiological study. Brain Research. 1989;496:228–240. doi: 10.1016/0006-8993(89)91070-6. 10.1016/0006-8993(89)91070-6. [DOI] [PubMed] [Google Scholar]
- Shea VK, Perl ER. Regeneration of cutaneous unmyelinated (C) fibres after transection. Journal of Neurophysiology. 1985;54:502–512. doi: 10.1152/jn.1985.54.3.502. [DOI] [PubMed] [Google Scholar]
- Simone DA, Kajander KC. Responses of cutaneous A fibre nociceptors to noxious cold. Journal of Neurophysiology. 1997;77:2049–2060. doi: 10.1152/jn.1997.77.4.2049. [DOI] [PubMed] [Google Scholar]
- Timmermann L, Stegmann J-U, Häbler H-J, Michaelis M, Jänig W. Behavioural evidence for mechanical hyperalgesia and allodynia after tibial nerve lesions in rats. Society for Neuroscience Abstracts. 1997;23:1004. [Google Scholar]
- Taylor DJ, McGillis SLB, Greenspan JD. Body site variation of heat pain sensitivity. Somatosensory and Motor Research. 1993;10:455–465. doi: 10.3109/08990229309028850. [DOI] [PubMed] [Google Scholar]
- Terzis JK, Dykes RW. Reinnervation of glabrous skin in baboons: Properties of cutaneous mechanoreceptors subsequent to nerve transection. Journal of Neurophysiology. 1980;44:1214–1225. doi: 10.1152/jn.1980.44.6.1214. [DOI] [PubMed] [Google Scholar]
- Treede R-D, Meyer RA, Campbell JN. Comparison of heat and mechanical receptive fields of cutaneous C fibre nociceptors in monkey. Journal of Neurophysiology. 1990;64:1502–1513. doi: 10.1152/jn.1990.64.5.1502. [DOI] [PubMed] [Google Scholar]
- Wall PD, Devor M, Inbal R, Scadding JW, Schonfeld D, Seltzer Z, Tomkiewicz MM. Autotomy following peripheral nerve lesions: experimental anaesthesia dolorosa. Pain. 1979;7:103–113. doi: 10.1016/0304-3959(79)90002-2. 10.1016/0304-3959(79)90002-2. [DOI] [PubMed] [Google Scholar]
- Yarnitsky D, Ochoa JL. Release of cold-induced burning pain by block of cold-specific afferent input. Brain. 1990;113:893–902. doi: 10.1093/brain/113.4.893. [DOI] [PubMed] [Google Scholar]


