Abstract
The effects of magnesium adenosine triphosphate (MgATP; also referred to as ‘substrate’) concentration on maximal force and shortening velocity have been studied at 5 °C in single and thin bundles of striated muscle myofibrils. The minute diameters of the preparations promote rapid diffusional equilibrium between the bathing medium and lattice space so that during contraction fine control of substrate and product concentrations is achieved.
Myofibrils from frog tibialis anterior and rabbit psoas fast skeletal muscles were activated maximally by rapidly (10 ms) exchanging a continuous flux of pCa 8.0 for one at pCa 4.75 at a range of substrate concentrations from 10 μM to 5 mM. At high substrate concentrations maximal isometric tension and shortening velocity of both frog and rabbit myofibrils were very close to those determined in whole fibre preparations from the same muscle types.
As in frog and rabbit skinned whole fibres, the maximal isometric force of the myofibril preparations decreases as MgATP concentration is increased. The maximal velocity of unloaded shortening (V0) depends hyperbolically on substrate concentration. V0 extrapolated to infinite MgATP (3.6 ± 0.2 and 0.8 ± 0.03 l0 s−1 in frog and rabbit myofibrils, respectively) is very close to that determined directly at high substrate concentration. The Km is 210 ± 20 μM for frog tibialis anterior and 120 ± 10 μM for rabbit psoas myofibrils, values about half those found in larger whole fibre preparations of the same muscle types. This implies that measurements in whole skinned fibres are perturbed by diffusional delays, even in the presence of MgATP regenerating systems.
In both frog and rabbit myofibrils, the Km for V0 is about one order of magnitude higher than the Km for myofibrillar MgATPase determined biochemically in the same experimental conditions. This confirms that the difference between the Km values for MgATPase and shortening velocity is a basic feature of the mechanism of chemomechanical transduction in muscle contraction.
Muscle contraction results from a cyclic interaction between myosin cross-bridges and sites on actin filament coupled with magnesium adenosine triphosphate (MgATP; also referred to as ‘substrate’) hydrolysis. A fundamental and still unresolved question is the correlation between the steps of the myosin MgATPase and the mechanical transitions of the cross-bridges leading to active force production and work (chemomechanical coupling). Several attempts have been made to correlate the extensive studies on the effects of substrate concentration and hydrolysis products on the mechanics of frog and rabbit fast skeletal muscle fibres with biochemical measurements on acto-myosin MgATPase in solution (for reviews see Ferenczi et al. 1982; Hibberd & Trentham 1986; Geeves, 1991; Cooke 1997) and various models of chemomechanical coupling in muscle have been proposed (Pate & Cooke, 1989; Smith & Geeves, 1995). Unfortunately the experimental models used for biochemical (isolated acto-myosin systems) and mechanical (demembranated or ‘skinned’ fibres) studies differ in the degree of structural organization, mechanical restraint and solvent accessibility, making any rigorous comparison problematic. In particular, with demembranated fibres, the presence of diffusional barriers introduces significant delays in the distribution of solutes throughout the specimen cross-section; these reduce control over the experimental conditions. Recently, it has been proposed that some of these limitations could be overcome by the use of single myofibrils, a preparation small enough for biochemical studies and also suitable for mechanical measurements (Colomo et al. 1997; Barman et al. 1998). Because of their extremely small diameters, it is possible to accurately estimate the dependence of mechanical parameters on substrate and product concentrations free of significant diffusional delays.
In this study, we investigated the dependence of isometric force and maximal shortening velocity on MgATP concentration in single myofibrils from frog and rabbit fast skeletal muscle, activated by rapid solution exchange. A major aim was to correlate the effect of substrate concentration on maximal velocity of unloaded shortening (V0) with recent biochemical measurements obtained on rabbit (Ma & Taylor, 1994; Lionne et al. 1996) and frog (Barman et al. 1998) myofibrils, freely shortening in solution.
METHODS
Frogs (Rana esculenta) were killed by decapitation followed by brain and spinal chord destruction; rabbits were killed with pentobarbitone (120 mg kg−1) administered through the marginal ear vein. All procedures were conducted in accordance with the official regulations of the European Community Council on use of laboratory animals (directive 86/609/EEC) and the study was approved by the Ethical Committee for Animal Experiments of the University of Florence. Myofibrils were isolated as described previously (Colomo et al. 1997). Frog tibialis anterior or rabbit psoas muscles were homogenized and a small volume of myofibril suspension was transferred to a temperature-controlled trough (5°C) filled with relaxing solution (pCa 8). The myofibrils selected for experiments were mounted horizontally between two glass microtools. One tool serves as a calibrated force probe (1-3 nm nN−1 compliance). The other tool is connected to the lever arm of a length control motor (Colomo et al. 1997). The preparations strongly adhered to the glass tools which were carefully micromanipulated to maximize the attachment area. Myofibrils were maximally activated and relaxed by rapidly translating (10 ms) the interface between two continuous streams of relaxing and activating (pCa 4.75) solutions (Colomo et al. 1998) containing varying MgATP concentrations. Maximal velocity of unloaded shortening (V0) was measured by the slack test method (Edman, 1979). To compare maximal isometric force at two different MgATP concentrations, myofibrils were sequentially activated using a translating holder carrying two double-barrelled injecting pipettes. Contractions were usually well reproduced over 5-10 activation cycles before there was a significant decline in the mechanical performance of myofibrils (i.e. more than 10 % decrease in isometric force and in speed of force development following activation). Frog myofibrils usually ran down faster than rabbit myofibrils.
All solutions, calculated as described previously (Brandt et al. 1998), contained an 1 mM excess of magnesium over ATP, were at pH 7.0 and had final ionic strengths of 130 mM or 200 mM for frog or rabbit, respectively. Although continuous solution flow minimizes alterations in the concentration of MgATP and its hydrolysis products in the myofibrillar space, all the present measurements were done in the presence of a MgATP regenerating system (10 mM creatine phosphate plus 200 units ml−1 creatine kinase). The regenerating system reduced the run-down of preparations, especially that of the frog. Whether the protective aspect of the regenerating system is related to the higher myofibrillar MgATPase of frog muscle is uncertain.
Mean slack sarcomere length was 1.94 ± 0.02 μm (mean ±s.e.m.; n = 55) in frog tibialis anterior muscle myofibrils and 2.47 ± 0.02 μm (mean ±s.e.m.; n = 57) in rabbit psoas muscle myofibrils. The initial length (l0) of the preparation was set at 5-10 % above the slack myofibril length.
RESULTS
Maximal isometric tension and V0 at high MgATP concentration
Frog tibialis anterior and rabbit psoas myofibrils, bathed in varying concentrations of MgATP (range 10 μM-5 mM; Fig. 1A and B) were subjected to cycles of activation and relaxation at 5°C. This was done by rapid switching of the perfusing solutions from pCa 8 to pCa 4.75. At high MgATP concentration (frog, 3 mM; rabbit, 5 mM), isometric tension and maximal velocity of unloaded shortening were 290 ± 30 mN mm−2 and 3.2 ± 0.2 l0 s−1 in frog myofibrils (mean ±s.e.m., n = 9) and 240 ± 30 mN mm−2 and 0.8 ± 0.1 l0 s−1 in rabbit myofibrils (mean ±s.e.m., n = 10). These values are in good agreement with previous observations in intact or skinned fibres from the same muscles at low temperature (Edman, 1979; Brenner, 1980; Ferenczi et al. 1984). Half-times for force development at high MgATP concentration were about three times smaller in frog tibialis anterior myofibrils (100 ± 10 ms, mean ±s.e.m., n = 9) than in rabbit psoas muscle myofibrils (310 ± 20 ms, mean ±s.e.m., n = 18).
Figure 1. Effect of MgATP concentration on isometric contraction of frog and rabbit skeletal myofibrils.
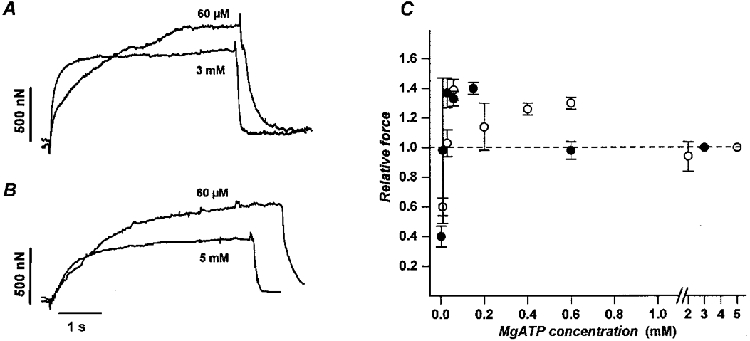
Tension records obtained from a frog tibialis anterior myofibril (A; l0 = 101 μm) and a rabbit psoas myofibril (B;l0 = 55 μm) activated at high (3 and 5 mM, respectively; control conditions) and low (60 μM) MgATP concentrations. At 60 μM MgATP, force was higher than in control conditions (37 % in A and 54 % in B). The half-times of force development were: in frog (A), 90 ms at 3 mM and 450 ms at 60 μM MgATP; in rabbit (B), 480 ms at 5 mM and 1100 ms at 60 μM MgATP. C, plot of isometric tension, expressed relative to the value obtained at high substrate, versus MgATP concentration for frog (•) and rabbit (○) myofibrils showing that in both cases relative force peaked at about 50-150 μM MgATP. The high scattering of experimental data obtained below 50 μM MgATP prevented resolution of the rising phase of the relation. Data points are average values (mean ± 1 s.e.m.) of relative force obtained in 7-10 myofibrils tested from two consecutive contractions at control and low MgATP. Temperature, 5 °C.
V0 and the rates of force rise and relaxation were highly sensitive to MgATP concentration. The effect of substrate concentration on maximal isometric force was less severe (Fig. 1A and B; Fig. 2).
Figure 2. Estimation of the maximal velocity of unloaded shortening by the slack test method in frog and rabbit myofibrils at high and low substrate.
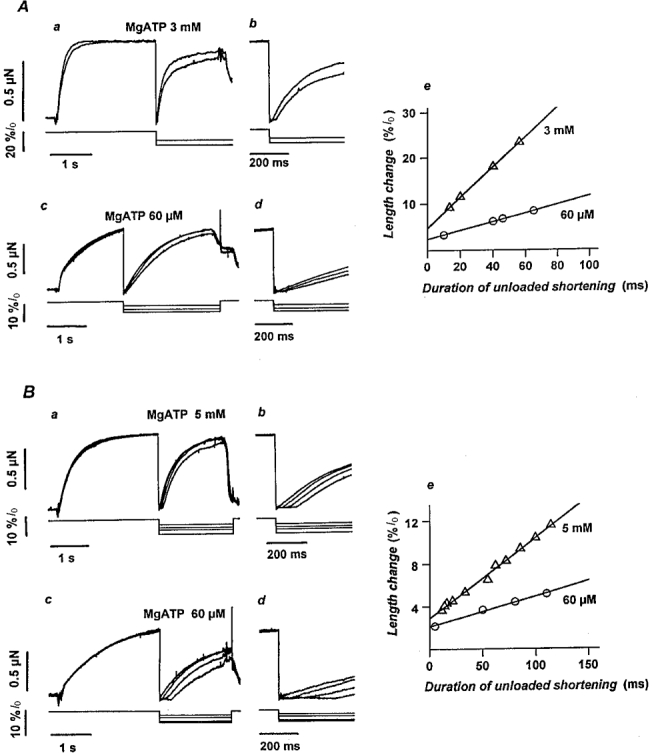
Superimposed force records (upper traces) from frog tibialis anterior (A) and rabbit psoas (B) myofibrils at high (Aa, 3 mM; Ba, 5 mM) and low (Ac and Bc, 60 μM) MgATP concentrations during the imposition of rapid (1 ms) shortening steps of different amplitude (lower traces; 2-20 %l0). Force falls to zero and the unloaded myofibril shortens at its maximum speed until the slack is taken up and force starts to redevelop at the new length. On an expanded time base, the duration of the unloaded shortening phase was clearly resolved at both high (Ab and Bb) and low (Ad and Bd) substrate concentrations. The maximal velocity of unloaded shortening (V0) in frog (Ae) and rabbit (Be) myofibrils was estimated from the slope of the relation between the amplitude of the length change and the duration of the unloaded shortening phase. Frog tibialis anterior, 3 mM MgATP: 3.3 l0 s−1 (l0 = 64 μm); 60 μM MgATP: 0.9 l0 s−1 (l0 = 44 μm). Rabbit psoas, 5 mM MgATP: 0.8 l0 s−1 (l0 = 91 μm); 60 μM MgATP 0.3 l0 s−1 (l0 = 84 μm). Temperature, 5 °C.
Isometric force
The dependence of maximal isometric force on MgATP concentration was determined by sequentially activating the same myofibril at a low and at fully saturating (reference condition, 3-5 mM) substrate concentration. This procedure was necessary in order to normalize the data to the highly variable maximal developed force observed among different myofibrils. Figure 1C shows the relations obtained between relative isometric force and MgATP concentration in frog (•) and rabbit (○) skeletal muscle myofibrils. As previously observed in skinned fibres (Reuben et al. 1971; Brandt et al. 1972; Ferenczi et al. 1984; Cooke & Pate, 1985), this relation was biphasic with a maximum that in both preparations was at about 50-150 μM MgATP concentration. No significant effect of MgATP on resting tension was present, even at the lowest concentration tested (results not shown).
Maximal velocity of unloaded shortening
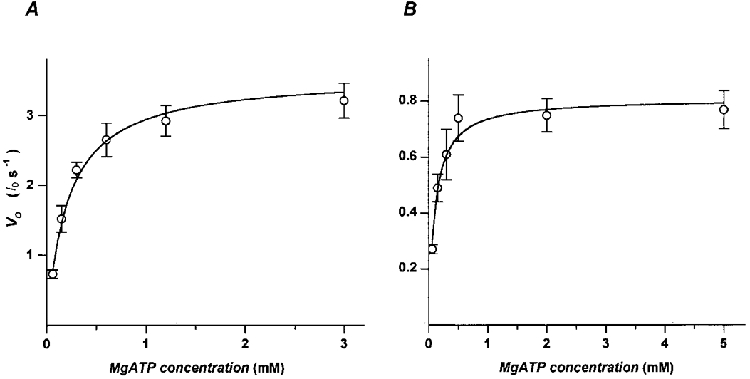
V0 was determined by the slack test method on myofibrils briefly (4-5 s) activated at intervals of about 3 min. During each contraction, myofibrils were rapidly (1 ms) brought below the slack length by the application of length changes of different amplitude and then restretched back to their original length during or after relaxation. Each myofibril was tested at a single MgATP concentration. Sample records from experiments on frog and rabbit skeletal muscle myofibrils at the highest and lowest MgATP concentration used for these experiments are shown in Fig. 2A and B. V0 was obtained from the slope of the relation between the amplitude of applied length changes and the duration of the corresponding unloaded shortening phase, i.e. the time taken by the myofibrils to take up the slack and start to redevelop force (Fig. 2Ae and Be). In all frog and rabbit myofibrils tested, V0 and myofibril series compliance (represented by the intercept on the y-axis) are reduced as the substrate concentration is lowered. At high substrate concentration, the mean values (±s.e.m.) of series compliance are 8.5 ± 0.8 %l0 (n = 9) in frog and 5.2 ± 0.5 %lo (n = 10) in rabbit myofibrils. At 60 μM MgATP these values reduce to 4.0 ± 0.5 %l0 (n = 9) and 2.5 ± 0.5 %l0 (n = 9), respectively. In Fig. 3, mean V0 for frog (A) and rabbit (B) myofibrils at full activation are plotted versus the MgATP concentrations. The relation is hyperbolic and can be described by a Michaelis-Menten kinetics with a Km of 210 ± 20 μM for frog tibialis anterior muscle and 120 ± 10 μM for rabbit psoas muscle myofibrils. The V0 values of the fitted hyperbola extrapolated to infinitely high substrate concentration are 3.6 ± 0.2 and 0.8 ± 0.03 l0 s−1 in frog and rabbit, respectively. These values are 5-10 % higher than the mean V0 values found at the highest ATP concentrations tested.
Figure 3. Relation between the maximal shortening velocity (V0) and the MgATP concentration in frog and rabbit myofibrils.
DISCUSSION
Rapid exchange of steady fluxes bathing single myofibrils offers important advantages in the study of maximal velocity of unloaded shortening as a function of the substrate concentration. The small diameter of the preparation essentially eliminates diffusional delays so that the concentration of substrate inside the myofilament lattice matches that of the perfusing solution. At physiological MgATP concentration (3-5 mM), single frog or rabbit skeletal myofibrils closely reproduce the contractile properties of larger preparations (Edman, 1979; Brenner, 1980; Ferenczi et al. 1984). A good agreement exists also between our data obtained in rabbit psoas myofibrils and previous estimations of maximal isometric tension (Bartoo et al. 1993; Friedman & Goldman, 1996) and V0 (Friedman & Goldman, 1996) in the same preparation, but at higher temperature (18-24°C). As with skinned fibres, the mechanical behaviour of myofibrils is highly sensitive to MgATP concentration: a decrease in substrate increases the amplitude of the isometric force plateau while force rise and relaxation are prolonged.
As previously observed in skinned fibres, the relationships here reported between the velocity of unloaded shortening and MgATP concentration are hyperbolic although the quantitative aspects of the relation in frog and rabbit myofibrils are significantly different from those in larger preparations from the same muscle type. The substrate concentrations for half the maximal V0 (Km) were 210 ± 20 μM and 120 ± 10 μM, for frog and rabbit myofibrils, respectively. These values are about half of the values obtained from mechanical experiments with frog (450-550 μM; Ferenczi et al. 1984; Stienen et al. 1988) and rabbit (150-250 μM; Cooke & Bialek, 1979; Cooke & Pate, 1985; Regnier et al. 1998) skinned fibres. Based on these observations we suggest that diffusion gradients perturb substrate concentrations in skinned fibres, leading to overestimation of the Km values.
It is interesting to note that for rabbit psoas myofibrils, our estimation of the Km for unloaded shortening velocity (5°C, 200 mM ionic strength) is very close to the Km (80-130 μM) estimated from the MgATP dependence of the maximal velocity of F-actin filaments propelled by rabbit fast skeletal muscle heavy meromyosin (25°C, 50 mM ionic strength; Homsher et al. 1992; Regnier et al. 1998). This similarity in Km could be fortuitous, due to the widely different conditions used in myofibril and in vitro motility experiments and the lack of information about the temperature sensitivity of the MgATP dependence of V0. Unfortunately, no motility assay information is available for frog skeletal myosin, presumably due to its widely reported instability.
Although the Km values for the MgATP dependencies of V0 reported here are definitively lower than those previously estimated in skinned fibres, they are still about one order of magnitude higher than Km values for maximally activated MgATPase estimated under similar experimental conditions from suspensions of frog and rabbit myofibrils (9.2 ± 2 μM in rabbit psoas myofibrils: Lionne et al. 1996; 33 ± 2 μM in frog tibialis anterior myofibrils: Barman et al. 1998). Cross-bridge detachment rates calculated from V0 (extrapolated to saturating MgATP) and referenced to slack sarcomere length (Ferenczi et al. 1984) are about 350 s−1 for frog and about 100 s−1 for rabbit myofibrils (assuming a power stroke of 10 nm). These values are about 60-80 times greater than the maximal MgATPase rates measured during the initial constant phase of shortening of myofibrils in suspension (4.6 ± 0.1 s−1 for frog and 1.7 ± 0.1 s−1 for rabbit myofibrils; Barman et al. 1998). These results support the hypothesis that different rate limiting steps govern MgATPase and mechanical shortening: MgADP release and/or MgATP binding for shortening velocity and MgATP hydrolysis and/or Pi dissociation for MgATPase (Hibberd & Trentham, 1986). Recently, this simplistic view has been challenged by Pi release measurements obtained with MgATP analogues, known to strongly affect V0 (White et al. 1997).
From the ratio between maximal shortening velocity (as reported here) and the maximal MgATPase (determined by Barman and collaborators; Barman et al. 1998), and assuming a power-stroke length of 10 nm, it is possible to estimate the fraction of the myosin ATPase cycle that is spent in a force-generating state (duty cycle) and subsequently the fraction of cross-bridges strongly attached (Cooke, 1997). In agreement with energy balance studies on rapidly shortening frog muscle fibres (Homsher et al. 1981) and with most of the estimates in unloaded rabbit preparations (Ma & Taylor, 1994; Cooke 1997), the duty cycle values we obtained were very low: 0.013 and 0.017 for frog and rabbit myofibrils, respectively. The similarity of the duty cycle in the two preparations implies to us that the duty cycle may be a constant that is independent of fast skeletal muscle types. Recently, higher values for MgATPase have been reported in rabbit and frog muscle fibres using a fluorescent dye-labelled Pi-binding protein, (He et al. 1997). This results in a 3 to 5 times larger duty cycle than that estimated here but kinetic data obtained with this technique from unloaded shortening experiments are needed to further refine this estimate.
It is interesting to note that the difference between the rates of cross-bridge detachment of frog and rabbit myofibrils, obtained here following the Ferenczi interpretation (Ferenczi et al. 1984) is about the same as the difference between the rates of force development observed here (about 6.9 s−1 for frog tibialis anterior and 2.2 s−1 for rabbit psoas myofibrils). The strong dependence of the rate of force development and relaxation on MgATP concentration confirms the early observations of Ferenczi and collaborators (Ferenczi et al. 1984) on skinned fibres activated and relaxed by slow solution change.
From the ratio between detachment rate and Km, the apparent second-order rate constant for cross-bridges detachment (or MgATP binding; Ferenczi et al. 1984) can be estimated. The values we obtain are about 1.7 × 106 M−1 s−1 and 0.8 × 106 M−1 s−1 in frog and rabbit myofibrils, respectively. These are at the upper limit of the values obtained in the past for skeletal muscle from mechanical and biochemical measurements (Homsher & Morris, 1998). Our estimations of the apparent second-order binding constants are somewhat uncertain because of experimental and fitting error, and we do not believe that frog and rabbit values are significantly different. This is based not only on the potential errors but also on the comparison of our numbers with estimations of the same parameter obtained from suspensions of frog and rabbit fast skeletal muscle myofibrils (Barman et al. 1998). There may be similar kinetics for the substrate binding process in different muscle types, obtained from different animal genera. This supports the hypothesis advanced by Stienen and collaborators (Stienen et al. 1988) that maximum cycling rate (or V0) and Km are proportionate to each other, as expected from Michaelian kinetics where the kinetic of substrate binding is constant.
Acknowledgments
The authors thank Dr P. W. Brandt for helpful comments on the manuscript, Dr P. Caini for collaboration with the experiments and Mr Alessandro Aiazzi, Mr Mario Dolfi and Mr Adrio Vannucchi for technical assistance. The present research was financed by Ministero dell'Università e della Ricerca Scientifica e Tecnologica (40 % funds) and Università degli Studi di Firenze (60 % funds). The financial support of Telethon-Italy to the present work is also acknowledged.
References
- Barman T, Brune M, Lionne C, Piroddi N, Poggesi C, Sthele R, Tesi C, Travers F, Webb M. ATPase and shortening rates in frog fast skeletal myofibrils by time-resolved measurements of protein-bound and free Pi. Biophysical Journal. 1998;74:3120–3130. doi: 10.1016/S0006-3495(98)78018-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoo ML, Popov VI, Fearn LA, Pollack GH. Active tension generation in isolated skeletal myofibrils. Journal of Muscle Research and Cell Motility. 1993;14:498–510. doi: 10.1007/BF00297212. [DOI] [PubMed] [Google Scholar]
- Brandt PW, Colomo F, Piroddi N, Poggesi C, Tesi C. Force regulation by Ca2+ in skinned cardiac myocytes of frog. Biophysical Journal. 1998;74:1994–2004. doi: 10.1016/S0006-3495(98)77906-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt PW, Reuben JP, Grundfest H. Regulation of tension in the skinned crayfish muscle fibre. II. Role of calcium. Journal of General Physiology. 1972;59:305–317. doi: 10.1085/jgp.59.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. Effect of free sarcoplasmic Ca2+ concentration on maximum unloaded shortening velocity: measurements on single glycerinated rabbit psoas muscle fibres. Journal of Muscle Research and Cell Motility. 1980;1:409–428. [Google Scholar]
- Colomo F, Nencini S, Piroddi N, Poggesi C, Tesi C. Calcium dependence of the apparent rate of force generation in single myofibrils from striated muscle activated by rapid solution changes. In: Sugi H, Pollack G, editors. Mechanism of Work Production and Work Absorption in Muscle. New York, NY, USA: Plenum Publishing Corporation; 1998. pp. 372–382. [Google Scholar]
- Colomo F, Piroddi N, Poggesi C, Te Kronnie G, Tesi C. Active and passive forces of isolated myofibrils from cardiac and fast skeletal muscle of the frog. The Journal of Physiology. 1997;500:535–548. doi: 10.1113/jphysiol.1997.sp022039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R. Actomyosin interactions in striated muscle. Physiological Reviews. 1997;77:671–697. doi: 10.1152/physrev.1997.77.3.671. [DOI] [PubMed] [Google Scholar]
- Cooke R, Bialek W. Contraction of glycerinated muscle fibres as a function of the ATP concentration. Biophysical Journal. 1979;28:241–258. doi: 10.1016/S0006-3495(79)85174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R, Pate E. The effects of ADP and phosphate on the contraction of muscle fibres. Biophysical Journal. 1985;48:789–798. doi: 10.1016/S0006-3495(85)83837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman KAP. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. The Journal of Physiology. 1979;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi MA, Goldman YE, Simmons RM. The dependence of force and shortening velocity on substrate concentration in skinned muscle fibres from Rana temporaria. The Journal of Physiology. 1984;350:519–543. doi: 10.1113/jphysiol.1984.sp015216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi MA, Simmons RM, Sleep JA. General considerations of cross-bridge models in relation to the dependence on MgATP concentration of mechanical parameters of skinned fibres from frog muscles. In: Twarog BM, Levine RJC, Dewey M, editors. Basic Biology of Muscles: a Comparative Approach. New York, NY, USA: Raven Press; 1982. pp. 91–107. [PubMed] [Google Scholar]
- Friedman AL, Goldman YE. Force and force transients in bundles of 1–3 myofibrils from rabbit psoas muscle. Biophysical Journal. 1996;71:2774–2785. doi: 10.1016/S0006-3495(96)79470-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeves MA. The dynamics of actin and myosin association and the cross-bridge model of muscle contraction. Biochemical Journal. 1991;274:1–14. doi: 10.1042/bj2740001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z-H, Chillingworth RK, Brune M, Corrie JET, Trentham DR, Weeb MR, Ferenczi MA. ATPase kinetics on activation of rabbit and frog permeabilized isometric muscle fibres: a real time phosphate assay. The Journal of Physiology. 1997;501:125–148. doi: 10.1111/j.1469-7793.1997.125bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibberd MG, Trentham DR. Relationships between chemical and mechanical events during muscular contraction. Annual Review of Biophysics and Biophysical Chemistry. 1986;15:119–161. doi: 10.1146/annurev.bb.15.060186.001003. 10.1146/annurev.bb.15.060186.001003. [DOI] [PubMed] [Google Scholar]
- Homsher E, Irving M, Wallner A. High-energy phosphate metabolism and energy liberation associated with rapid shortening in frog skeletal muscle. The Journal of Physiology. 1981;321:423–436. doi: 10.1113/jphysiol.1981.sp013994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsher E, Morris C. The use of caged compounds. In: Sugi H, editor. Current Methods in Muscle Physiology: Advantages, Problems, and Limitations. Oxford, UK: Oxford University Press; 1998. pp. 71–89. [Google Scholar]
- Homsher E, Wang F, Sellers JR. Factors affecting movement of F-actin filaments propelled by skeletal muscle heavy meromyosin. American Journal of Physiology. 1992;262:C714–723. doi: 10.1152/ajpcell.1992.262.3.C714. [DOI] [PubMed] [Google Scholar]
- Lionne C, Travers F, Barman T. Mechanochemical coupling in muscle: attempts to measure simultaneously shortening and ATPase rates in myofibrils. Biophysical Journal. 1996;70:887–895. doi: 10.1016/S0006-3495(96)79632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YZ, Taylor EW. Kinetic mechanism of myofibril ATPase. Biophysical Journal. 1994;66:1542–1553. doi: 10.1016/S0006-3495(94)80945-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate E, Cooke R. A model of cross-bridge action: the effects of ATP, ADP and Pi. Journal of Muscle Research and Cell Motility. 1989;10:181–196. doi: 10.1007/BF01739809. [DOI] [PubMed] [Google Scholar]
- Regnier M, Lee DM, Homsher E. ATP analogs and muscle contraction: mechanics and kinetics of nucleoside triphosphate binding and hydrolysis. Biophysical Journal. 1998;74:3044–3058. doi: 10.1016/S0006-3495(98)78012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben JP, Brandt PW, Berman M, Grundfest H. Regulation of tension in the skinned crayfish muscle fibre. I. Contraction and relaxation in the absence of Ca (pCa > 9) Journal of General Physiology. 1971;57:385–407. doi: 10.1085/jgp.57.4.385. 10.1085/jgp.57.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DA, Geeves MA. Strain-dependent cross-bridge cycle for muscle. Biophysical Journal. 1995;69:524–537. doi: 10.1016/S0006-3495(95)79926-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienen GJM, Van Der Laarse WJ, Elzinga G. Dependency of the force-velocity relationships on MgATP in different types of muscle fibres from Xenopus laevis. Biophysical Journal. 1988;53:849–855. doi: 10.1016/S0006-3495(88)83165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HD, Belknap B, Webb MR. Kinetics of nucleoside triphosphate cleavage and phosphate release steps by associated rabbit skeletal actomyosin, measured using a novel fluorescent probe for phosphate. Biochemistry. 1997;36:11828–11836. doi: 10.1021/bi970540h. [DOI] [PubMed] [Google Scholar]


