Abstract
Climbing fibre field potentials evoked by low intensity (non-noxious) electrical stimulation of the ipsilateral superficial radial nerve have been recorded in the rostral paramedian lobule (PML) in awake cats. Chronically implanted microwires were used to monitor the responses at eight different C1 and C3 zone sites during quiet rest and during steady walking on a moving belt. The latency and other characteristics of the responses identified them as mediated mainly via the dorsal funiculus-spino-olivocerebellar path (DF-SOCP).
At each site, mean size of response (measured as the area under the field, in mV ms) varied systematically during the step cycle without parallel fluctuations in size of the peripheral nerve volley. Largest responses occurred overwhelmingly during the stance phase of the step cycle in the ipsilateral forelimb while smallest responses occurred most frequently during swing.
Simultaneous recording from pairs of C1 zone sites located in the anterior lobe (lobule V) and C1 or C3 zone sites in rostral PML revealed markedly different patterns of step-related modulation.
The findings shed light on the extent to which the SOCPs projecting to different parts of a given zone can be regarded as functionally uniform and have implications as to their reliability as channels for conveying peripheral signals to the cerebellum during locomotion.
Central regulation of afferent transmission or ‘gating’ has been demonstrated extensively in sensory paths leading to the cerebral cortex and it is now established that a similar phenomenon is also present in spino-olivocerebellar pathways (SOCPs) that terminate as climbing fibre afferents in the cerebellar cortex (for a review see Apps, 1999). However, precisely what information the climbing fibres and associated SOCPs signal during movements remains unclear (for a review see Simpson et al. 1996). Nonetheless, any regulation of afferent transmission will have important implications as to their function, since this will determine when information is forwarded to the cerebellum.
Although a large number of SOCPs are known to exist (for reviews see Oscarsson, 1980; Brodal & Kawamura, 1980; Voogd & Glickstein, 1998), the most thoroughly studied is the dorsal funiculus (DF)-SOCP. This is a compound system which includes climbing fibre paths with the shortest latency from the ipsilateral limbs to the cerebellum. Such paths involve relays at the level of the spinal cord, dorsal column nuclei and inferior olive (mainly in the rostral division of the dorsal accessory olive), before terminating as climbing fibres within a number of cerebellar cortical zones including the C1 and C3 zones in the paravermis (Oscarsson, 1969; Ekerot & Larson, 1979a, b; Groenewegen et al. 1979; Ekerot et al. 1991a, b; Trott & Apps, 1991, 1993). Furthermore, subpaths within the DF-SOCP relay climbing fibre input with similar receptive field characteristics to narrower subzones or ‘microzones’ within the broader zones (e.g. Ekerot et al. 1991a).
By comparison, the lateral funiculus (LF)-SOCP is synaptically more complex, conveys peripheral signals from all four limbs, has the rostral division of the medial accessory olive as its final pre-cerebellar relay, and provides longer latency climbing fibre input to the intervening paravermal C2 zone (Larson et al. 1969; Armstrong et al. 1973a; Groenewegen et al. 1979; Trott & Apps, 1991, 1993).
In light of these differences, previous studies have investigated the possibility that the C1, C2 and C3 zones might differ in the patterns of gating their SOCPs exhibit during rest and active movements (Apps et al. 1990, 1995, 1997; Lidierth & Apps, 1990; see also Horn et al. 1996). Low intensity electrical stimulation of the superficial radial nerve was used as a probe to assess changes in transmission in SOCPs targeting different paravermal recording sites. During locomotion, the phasing of step-related modulations in climbing fibre response size was found to be similar at C1 and C3 zone sites, with the largest responses (i.e. best transmission) consistently occurring during the swing phase of the step cycle in the ipsilateral forelimb (Lidierth & Apps, 1990; Apps et al. 1995). By marked contrast, at C2 zone sites, timing of best transmission occurred most frequently during the stance phase of the step cycle. However, the pattern of step-related modulation was more variable between C2 sites, suggesting the presence within the LF-SOCP of functionally distinct subpaths targeting different parts of that zone (Apps et al. 1990).
Given that the DF-SOCP is a compound system (cf. Ekerot et al. 1991a), the aim of the present study has been to investigate the possibility that intrazonal differences are also present within its projections to the C1 and C3 zones. To date, studies of gating in climbing fibre responses have been confined to sites located primarily within lobule V (a ‘forelimb’ receiving area of the anterior lobe) and it is not known whether the same zones located within the rostral paramedian lobule (a ‘forelimb’ receiving area within the posterior lobe) have similar or different patterns of gating.
The present findings reveal marked differences in step cycle-related timing of best transmission in DF-SOCP input to the C1 and C3 zones in the anterior and posterior lobes, implying that different rostrocaudal parts of the same zone monitor climbing fibre signals at different times during locomotion in the awake animal.
A preliminary report of this work has been published (Apps & Lee, 1997).
METHODS
Animals and implants
Experiments were performed on four purpose-bred adult male cats (4-5 kg) in accordance with UK Home Office guidelines. Following behavioural training (see below) implantations were carried out in an aseptic operation under sodium pentobarbitone anaesthesia (40 mg kg−1i.p.; Sagatal, Rhone Merieux, Harlow, UK; maintenance doses i.v. as required to abolish corneal and withdrawal reflexes). A single dose of atropine (0.5 ml s.c.) was given in order to prevent excessive secretion in respiratory passages and an antibiotic (Crystapen, Britannia Pharmaceuticals, UK) was administered pre- and post-operatively (4 spaced doses of 75 mg kg−1). Throughout the operation the temperature of the animal was kept within physiological limits and post-operative analgesia was maintained for 24 h with buprenorphine (Temgesic, 10 μg kg−1i.m.; Reckitt and Colman, Hull, UK). Recovery from the operation was rapid and uneventful and recording sessions were usually initiated 3 or 4 days later. No complications occurred post-operatively and the animals showed no signs of discomfort at any stage of the experiment. The techniques employed have been fully described in previous papers (see for example Apps et al. 1990).
In brief, a small craniotomy was made to expose the rostral folia of the paramedian lobule (PML) and an array of 12-24 flexible platinum-iridium microwires, Teflon insulated except at the tip and 35 μm in total diameter, were inserted to a depth of about 1-2 mm into the three most rostral folia of PML (see Fig. 1B). The microwires were purchased from several different manufacturers although best results were obtained from those supplied by A-M Systems (Carlsborg, WA, USA). The point of insertion of each microwire was indicated on a scale drawing. In three animals an additional craniotomy was made and 7-10 microwires were also implanted into the medial part of the paravermis of lobule V (Fig. 1A). The exposed cerebellar surface was covered with Sterispon (Allen and Hanburys, London, UK) and the microwires sealed in position with dental acrylic cement. For further details of microwire implants see Apps et al. (1995).
Figure 1. Cortical location of recording sites.
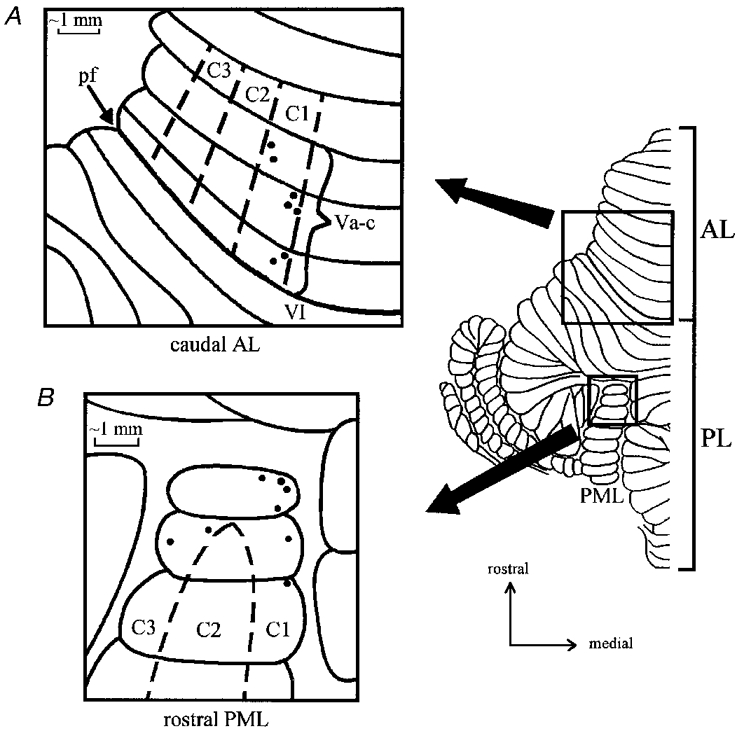
Dorsal view of the unfolded cat cerebellar cortex (left hemicerebellum only) with expanded views to show the approximate location of individual microwire recording sites. A, 7 sites within the C1 zone in lobule Va-c of the anterior lobe and B, 8 sites within the C1 and C3 zones in the rostral paramedian lobule of the posterior lobe. AL, anterior lobe; PL, posterior lobe; PML, paramedian lobule; pf, primary fissure.
In addition, two pairs of bipolar cuff electrodes, for stimulation and nerve volley recording, respectively, were implanted around the ipsilateral superficial radial (SR) nerve. A pair of recording leads were also implanted into the ipsilateral triceps brachii muscle (lateral head) to monitor EMG signals during rest and walking. All leads (multistranded stainless steel, Teflon insulated, diameter 0.3 mm, Cooner, Chatsworth, CA, USA) were fed subcutaneously to miniature connector sockets (Amphenol) attached to a small, light-weight Perspex headpiece.
Behavioural task and data collection
Cats were trained to walk on an exercise belt moving at a comfortable walking speed (ca 0.4 m s−1). During each recording session, low intensity electrical stimuli were applied to the ipsilateral SR nerve both during locomotion (usually about 400 stimuli) and during periods of rest (usually about 100 stimuli). Data during rest were obtained immediately before and after collection of walking data. Throughout the recording sessions the animals showed no signs of discomfort and at the end of each session they were returned to their pen (shared with other purpose-bred cats), where food was freely available.
Recordings and stimulation
Electrical stimuli to the ipsilateral SR nerve were delivered as single square pulses (100 μs) at an intensity of 2T, where T is the threshold for the most excitable fibres in the nerve as judged by monitoring the afferent nerve volley proximal to the site of stimulation. These stimulus parameters are inadequate to excite nociceptive afferents and the animals completely ignored the stimuli that were delivered at a frequency of 0.7 Hz. During locomotion, the stimulus was timed to drift with respect to different phases of the step cycle so that during a bout of walking (duration ca 10 min) cerebellar responses were evoked as a result of stimuli randomly delivered throughout the step cycle. The afferent volley in the nerve was monitored during rest and locomotion in all recording sessions. Filter settings for recording of evoked cerebellar field potentials and the nerve volley were, respectively, 30 Hz to 2.5 kHz and 300 Hz to 10 kHz. All data were stored on digital audio tape (DAT) for off-line analysis.
Data analysis
The biological signals were digitized with customized trigger-sampled software running a CED 1401 (Cambridge Electronic Design) A/D converter. The sampling rates for the cerebellar field and nerve responses were 2.5 kHz and 20 kHz, respectively. Two measures were used to assess the size of individual evoked climbing fibre field potentials, namely the total area of the response (duration typically ca 5 ms), and the area under the first 2 ms of the response (cf. Apps et al. 1990). The amplitude of individual nerve compound action potentials was measured peak-to-peak. For each recording session (n = 20), the step cycle was divided into ten equal epochs relative to onset of EMG activity in the ipsilateral triceps brachii muscle (taken by convention to coincide with the onset of the stance phase in the forelimb; cf. Apps et al. 1990, 1995). The individual responses (usually about 30-50) evoked by stimuli occurring during each tenth of the normalized step cycle were averaged to calculate mean size (total area of response ±s.e.m.) per step tenth and the results were displayed as histograms (e.g. Fig. 3A). To assess the depth of modulation in individual step histograms a modulation index was calculated using the formula: 1 - (response size in the smallest bin/response size in the largest bin). This index takes a value of zero when modulation is absent and a value of 1 when there is a part of the step when no response is evoked.
Figure 3. Step analysis for one rostral PML C1 zone site.
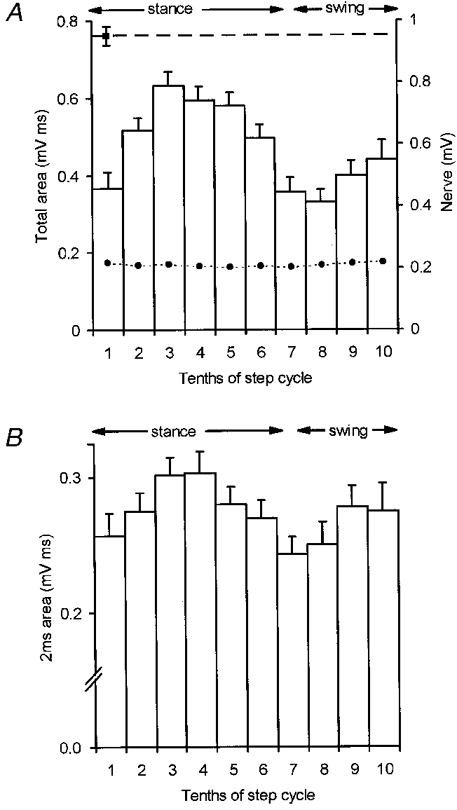
In A and B the step cycle has been divided into tenths with bin one coinciding with the onset of locomotor EMG in the ipsilateral triceps brachii muscle. Periods of stance and swing are approximate timings for trajectory of the ipsilateral forelimb in this and subsequent figures. A, step histogram to show the mean size of the climbing fibre field potential in terms of its total area (+s.e.m.). Dashed horizontal line, mean size of response at rest. •-•, mean peak-to-peak amplitude of the nerve volley. B, same data but mean size of the response in terms of the area under the initial 2 ms of the field.
For the purpose of statistical analysis, comparisons were made between the size of climbing fibre responses at rest and in each tenth of the step cycle. Multiple regression (analysis of variance) was used so that any fluctuations in the size of the nerve volley were taken into account. When cerebellar responses at two sites were recorded simultaneously, linear (least squares) regression analysis was used to assess the extent to which the fluctuations in size of individual responses occurred in parallel.
Histology
At the end of the experiment (ca 6 weeks after the initial operation) each animal was deeply anaesthetized with barbiturate and perfused transcardially with isotonic saline followed by 4 % paraformaldehyde. The cerebellum was removed and the folial location of the microwires verified by post-mortem inspection of the dorsal surface of the cerebellum. In one animal the depth of insertion of one of the microwires yielding responses in rostral PML was determined by producing an electrolytic lesion immediately prior to the perfusion. In this case the cerebellum was cut sagittally on a freezing microtome into 100 μm sections and one series collected. The sections were inspected microscopically and the lesion was located 2 mm below the cortical surface in folium 2 of pars anterior of rostral PML (folial identification according to Larsell, 1953).
RESULTS
Extracellular climbing fibre field potentials evoked by electrical stimulation of the ipsilateral superficial radial (SR) nerve were recorded in the cerebellar cortical C1 and C3 zones in rostral folia of the paramedian lobule (PML, see Fig. 1) in four awake cats during quiet rest and locomotion (see Methods for further details). The responses (see for example, Fig. 2A) were readily distinguishable from potentials related to mossy fibre input on the basis of their onset latencies (9-12 ms), waveform, fluctuations in amplitude between stimuli, and their pattern of response to a ‘paired pulse test’. When two stimuli were delivered at interstimulus intervals ranging from 10 to 100 ms, the second response exhibited a prolonged depression (see Fig. 2B; cf. Armstrong & Harvey, 1968; Eccles et al. 1966b).
Figure 2. Example climbing fibre field potentials in rostral PML.
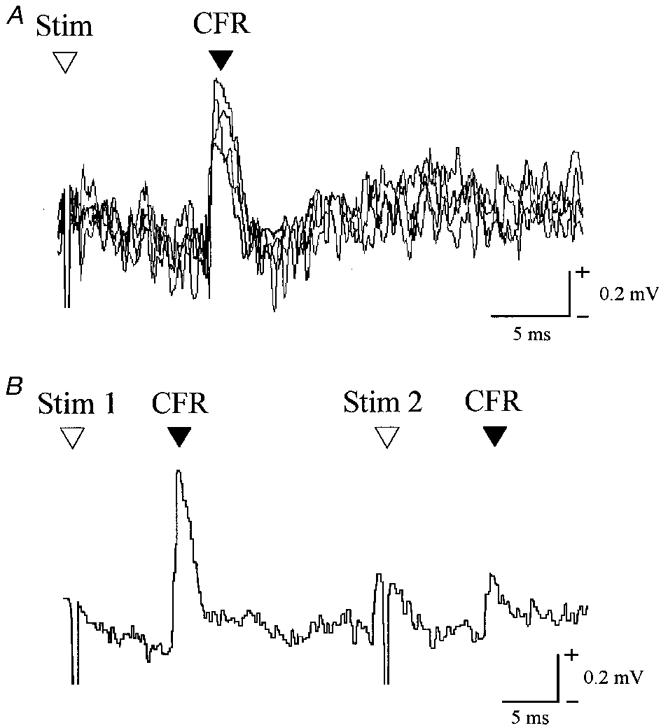
A, extracellular recording from the granular layer of the cerebellar cortex (4 consecutive sweeps superimposed) to show a C1 zone climbing fibre field potential evoked by ipsilateral superficial radial nerve stimulation (intensity, 2T). B, same site to show the effect of paired stimuli (interstimulus interval of 30 ms, average of 4 sweeps). Stim, stimulus; CFR, climbing fibre response.
As in previous studies (e.g. Apps et al. 1997), only approximately 20 % of the implanted microwires (n = 8) yielded discriminable field potentials (see Methods). However, four of the responsive sites were recorded from on more than one occasion (on average, 4 recording sessions per site), yielding a total of 20 PML sessions (16 from 6 C1 zone sites and 4 from 2 C3 zone sites). As no systematic differences were found between C1 and C3 zone sites they will be considered together (cf. Apps et al. 1997). Note also that no attempt has been made to seek any differences between medial and lateral subzones.
In all sessions it was possible to compare step phase-related modulations in response size with mean size during rest (see Methods for further details) and the results were routinely presented in the form of step histograms (see for example, Fig. 3A, and cf. Apps et al. 1995). An overall impression of the extent to which response size was modulated during the step cycle may be gained from the finding that for all step histograms, the modulation index (see Methods) ranged from 0.15 to 0.61 (mean ±s.d., 0.37 ± 0.16, which corresponds to the smallest response during locomotion being 63 % of the largest). On average, response mean size during locomotion was 82 ± 19 % (mean ±s.d.) of rest mean size (range, 56-130 %). However, when mean size of response in the largest tenth in each step histogram was compared with rest, 3 out of 20 (15 %) were significantly larger (multiple regression, P < 0.05). By comparison, response mean size in the smallest step tenth was significantly smaller than rest in 17 out of 20 step histograms (85 %, multiple regression, P < 0.05).
The responses therefore resembled anterior lobe (lobule V) C1 and C3 zone sites in many respects, including the extent to which overall mean size of the response was reduced during locomotion, the depth of step-related modulation and the proportion of step histograms in which depressions were present (cf. Apps et al. 1995). One possible exception was the proportion of available step histograms in which facilitations were present, since a rather greater proportion was reported for C1 and C3 zone sites in the anterior lobe (ca 56 % of step histograms cf. Apps et al. 1995). The one parameter, however, that revealed the most striking difference between rostral PML C1/C3 sites and corresponding sites in the anterior lobe was the timing of step-related changes in response size.
Step cycle timing of the largest and smallest responses
Figure 3 displays step histogram data obtained from a C1 zone site in rostral PML and Fig. 3A shows that substantial variations in response size occurred in a step-phase-dependent manner. Typical of the material as a whole, the largest mean size of response occurred during the stance phase in the ipsilateral forelimb (step tenth 3) while the smallest mean size of response occurred during swing (step tenth 8). These variations in response size are not explicable through any parallel change in nerve volley size because the latter remained relatively constant throughout the step cycle (see dashed line connected by filled circles in Fig. 3A). Similar findings were obtained for the nerve volley in all other recording sessions.
The step cycle phasing of the modulation was strikingly different from the pattern for DF-SOCP responses recorded under similar experimental conditions but obtained at C1 and C3 zone sites in the anterior lobe (lobule V; Lidierth & Apps, 1990; Apps et al. 1995). Additional analysis was therefore carried out in which measurement was confined to the initial 2 ms of the response (which reflects the recruitment of Purkinje cells by the short latency DF-SOCP, excluding any possible contribution from longer latency paths; see Apps et al. 1990; Lidierth & Apps, 1990). Figure 3B shows the step-related variations in response size for the initial 2 ms of the response for the same data shown in Fig. 3A. Typical of the material as a whole the pattern is similar, providing evidence that the modulation was present in a single, functionally distinct SOCP.
Simultaneous recordings from lobule V and rostral PML
Additional evidence in favour of the fluctuations in response size at rostral PML sites exhibiting a different step-phase-dependent pattern from those at corresponding lobule V sites was gained by recording pairs of sites simultaneously. In such cases the responses at each site were evoked by the same peripheral nerve stimulation thereby controlling for any differences in intensity of the stimulation used in different experiments (a factor which has been shown to have a significant influence on the pattern of modulation at individual sites, cf. Apps et al. 1990, 1995).
Such a comparison was possible in 13 recording sessions and Fig. 4 shows one example pair of step histograms, typical of the material as a whole. At the rostral PML C1 zone site the mean size of response was largest in step tenth 5 (Fig. 4A) while, by contrast, at the anterior lobe C1 zone site the mean size of response was largest in step tenth 8 (Fig. 4B). The corresponding times of the smallest responses were step tenths 9 and 4, for the rostral PML and anterior lobe sites, respectively.
Figure 4. Simultaneous recording from a pair of rostral PML and lobule V sites.
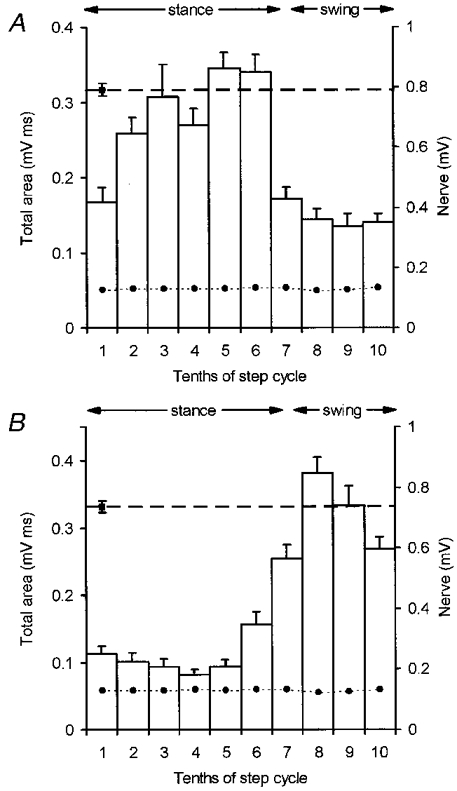
A and B, data obtained at 2 different recording sites evoked by the same ipsilateral superficial radial nerve stimulation (intensity, 2T). A, step histogram for a C1 zone site in rostral PML and B, step histogram for a C1 zone site in lobule V. Each histogram constructed according to the conventions of Fig. 3.
Summary of step cycle timing of largest and smallest responses
The frequency distribution of Fig. 5A emphasizes that the largest responses (i.e. best transmission), at rostral PML C1/C3 zone recording sites occurred overwhelmingly during the stance phase of the step cycle. In no fewer than 7 out of 8 sites (19/20 step histograms, 95 %), maximum response size occurred during the E2 and E3 phases of the step cycle (i.e. from foot contact until point of foot lift). The only exception was one site for which a single step histogram was available. However, in this case the step-related fluctuations in response size were not pronounced (modulation index = 0.27).
Figure 5. Frequency distributions for times of largest and smallest cerebellar responses.
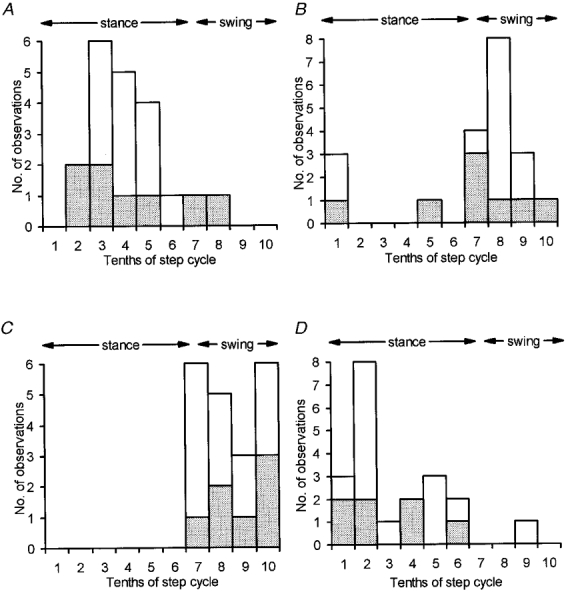
A shows distribution of the timing during the step cycle in the ipsilateral forelimb when mean size of response to SR stimulation was largest. Data obtained from 20 step histograms from 8 rostral PML recording sites. B, same as A, but for timing of smallest mean size of response. C, as A but for responses recorded in lobule V. Data obtained from 20 step histograms from 7 recording sites. D, as C but for timing of smallest mean size of response. Stippling selects for each recording site the timings from the step histogram with the least variation in nerve volley size.
The corresponding frequency distribution for the step timing of the smallest responses is presented in Fig. 5B, which shows that minimum response size occurred most frequently during the swing phase of the step cycle. For comparison, Fig. 5C and D shows the times of largest and smallest mean size of response for step histograms obtained in the present study for C1 zone sites located in the anterior lobe (n = 7 sites). In agreement with more extensive findings for C1 zone recording sites in lobule V (Apps et al. 1995), but in marked contrast to Fig. 5A and B, times of largest and smallest response occurred most frequently during the swing and stance phases of the step cycle, respectively.
The distributions in Fig. 5 are all based on the same intensity of stimulation (2T) but include different numbers of timings for the different individual sites. Nevertheless, if consideration was limited to one step histogram for each site (the one with the least step-related variation in nerve volley size) the distributions of timings were similar, as shown by the stippled areas in Fig. 5.
The frequency histograms of Fig. 5 do not, however, provide information about the variation in timing of maxima and minima for those sessions in which one PML site was recorded simultaneously with an anterior lobe site. This information can be gained from the frequency histograms of Fig. 6 which show the differences in timing between pairs of anterior and posterior lobe sites (in terms of tenths of a step), when the largest (Fig. 6A) and smallest (Fig. 6B) responses occurred. Clearly, the step tenth shifts were often substantial and for time of minima the greatest possible shift (5 tenths of a step cycle) occurred in 3 out of 13 pairs of comparisons (23 %).
Figure 6. Shifts in step timing between pairs of rostral PML and lobule V sites.
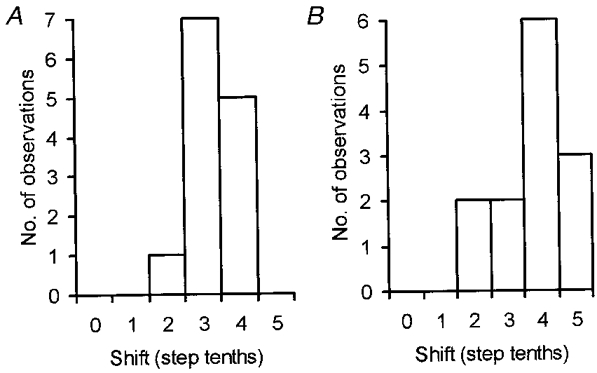
A, pairs of rostral PML and lobule V step histograms generated by the same nerve stimuli were compared as to the extent of any shift in the position of the step tenth in which response mean size was largest (n = 13 comparisons). B, same as A but for shifts in step tenth for timing of the smallest response mean size.
Comparison of individual response fluctuations at anterior lobe and PML recording sites
A characteristic feature of climbing fibre field potentials is their trial-by-trial fluctuations in size in response to a constant afferent stimulus (cf. Fig. 2A). These variations presumably reflect the action of a central influence on pathway excitability that is additional to any locomotor-related mechanism revealed by the step histograms. In the present study, the extent to which such fluctuations occur in parallel at rostrocaudally separated sites was studied in the 13 pairs of comparisons described above. However, in all such comparisons linear regression analysis failed to reveal any statistically significant correlation between fluctuations in response size (comparison based on total area of field measurements) either at rest (R2= 0.00-0.13, P > 0.05) or during locomotion (R2= 0.00-0.10, P > 0.05). Similar negative findings were obtained when the comparison was based on the size of the initial 2 ms of each response, implying that the fluctuations occur differentially in subcomponents of the DF-SOCP targeting different rostrocaudal sites within the C1/C3 zones.
DISCUSSION
The present results provide evidence that different rostrocaudal loci within the cerebellar cortical C1 and C3 zones can display markedly different patterns of modulation of SOCP excitability during locomotion, implying functional heterogeneity between different parts of the same zones in the awake behaving animal. At rostral PML recording sites, peak excitability occurred overwhelmingly during the stance phase of the step cycle in the ipsilateral forelimb while peak excitability at corresponding sites in lobule V consistently occurred during swing (cf. Lidierth & Apps, 1990; Apps et al. 1995). This difference in phasing of the step-related modulation cannot be explained by any variation in the test volley in the SR nerve, nor by the use of different stimulus intensities in different experiments, because the same nerve stimulation evoked different patterns of modulation at pairs of sites recorded simultaneously within the anterior lobe and PML.
The modulatory influence(s) responsible may have been operating at the level of the cerebellar cortex and/or some pre-cerebellar site(s) in the SOCPs. However, changes in probability of evoking a climbing fibre response cannot be explained by changes in excitability at the level of the cerebellar cortex (due to the high security of the climbing fibre synapses on the Purkinje cells, e.g. Eccles et al. 1966a; see also Apps et al. 1990 for further details). It is relevant to note, therefore, that in 11 recording sessions the responses varied sufficiently in size from trial to trial that on occasion, the stimulus failed to evoke a detectable response. In such cases the probability of occurrence of a response was found to fluctuate essentially in parallel with the step-related variations in response mean size, implying that at least some (if not all) of the modulation was generated at a pre-cerebellar site.
However, step-related modulations were present in some sessions in which all stimuli successfully evoked a response, indicating that probability changes alone are not sufficient to account for the changes in mean response size. Since the patterns of step-related modulation were also obtained when measurement was restricted to the area under the first 2 ms of the climbing fibre field potential, variation in the number of olive cells discharging as a result of activity in the short-latency DF-SOCP is also likely to be an important factor in generating the observed variations in response size (see Apps et al. 1990 for a more detailed discussion).
Comparison with previous climbing fibre branching studies
The present results are unexpected because earlier studies in pentobarbitone anaesthetized cats have shown that climbing fibre field potentials generated as a result of activity in the DF-SOCP and recorded simultaneously from pairs of recording sites in the anterior lobe (lobule V) and rostral PML, displayed synchronous fluctuations in response size and latency, implying a close functional link between the olive cells that target these ‘corresponding’ regions of cerebellar cortex (Armstrong et al. 1973a).
Although speculative, one possible explanation for this apparent discrepancy is the use of barbiturate anaesthesia in these previous studies. Olive cells are known to be electrotonically coupled (e.g. Llinas et al. 1974; Llinas & Yarom, 1981) and can be dynamically linked even when they are located some distance from each other (De Zeeuw et al. 1996; Lang et al. 1996). It is possible therefore, that the use of pentobarbitone may have increased the coupling between neighbouring olive cells, resulting in more widespread synchronization of climbing fibre activity.
Nevertheless, other investigations in the barbiturate-anaesthetized cat have provided direct evidence that at least some olivary neurones have axons that branch to supply climbing fibre afferents to lobule V and rostral PML. Electrical stimulation of the paravermal anterior lobe was found to generate climbing fibre axon reflex responses in rostral PML, while single olivary neurones could be antidromically invaded by stimulation at loci within the paravermis of the anterior lobe (lobule V) and rostral PML (Armstrong et al. 1973b, c see also Armstrong et al. 1974). The apparent discrepancy between these findings and the present results may be the result of a combination of factors. In the axon reflex studies, for example, reasonably high stimulus intensities (typically ca 1 mA) were delivered to the surface of the anterior lobe. Such stimuli have been estimated to excite climbing fibre terminals over a fairly extensive region of cortex (to a depth of 2 mm immediately below the electrode tip and within a circle with a diameter of 4 mm on the cortical surface; Armstrong et al. 1973d), raising the possibility that climbing fibre branches were activated in regions of the anterior lobe not implanted with microwires in the awake animal.
An additional factor may be the spatial distribution in rostral PML of climbing fibres that arise from olivocerebellar axons that also supply climbing fibres to the anterior lobe. In the studies by Armstrong and colleagues it was noted that when the stimulation was applied to a fixed locus within the anterior lobe, the axon reflex responses generated on the surface of rostral PML showed a very restricted spatial distribution, implying a high degree of localization within the pattern of climbing fibre branching (Armstrong et al. 1973b). It is likely, therefore, that the responses in rostral PML were generated by a subset of climbing fibres, many of which were probably located close to the exposed surface of the folia. In the present experiments, it is possible that the microwire recording tips were located in deeper parts of the folia in which climbing fibre input from olive cells that also innervate lobule V may be less common or even absent. Consistent with this possibility are the histological findings for the cortical location of one of the microwires yielding rostral PML responses (see Methods). This was found to be implanted to a depth of 2 mm below the cortical surface.
It is also relevant to note that anatomical studies using double fluorescence retrograde tracing techniques have estimated that about one third of the cells in the rostro-medial part of the dorsal accessory olive (DAO, the main olivary relay for the forelimb component of the DF-SOCP) have axons that branch to supply climbing fibres to paravermal lobule V and rostral PML (Rosina & Provini, 1983, 1987; Apps & Trott, 1991). Thus branching within the olivocerebellar projection is likely to involve only a subgroup of the total population of olive cells that target the C1 and C3 zones in paravermal lobule V and rostral PML, providing ample scope for independent lines of communication.
Microzonal organization
A detailed representation of the ipsilateral forelimb has been described for the climbing fibre input to the C3 zone relayed via different subpaths of the DF-SOCP and it is likely that a similar organization is also present within the C1 zone (Ekerot & Larson, 1979a, b). Climbing fibres with similar receptive field characteristics and with multimodal input from cutaneous Aβ, Aδ and C fibres target the same narrow sagittal strips or ‘microzones’ and at least 30 microzones have been identified within the C3 zone (Ekerot et al. 1991a).
The climbing fibre responses recorded in the present experiments were evoked by low intensity electrical stimulation of the superficial radial nerve. This is a purely cutaneous nerve which supplies most of the paw dorsum, digits and dorso-medial regions of the distal forelimb in the cat (Kitchell et al. 1982). Inspection of the receptive field characteristics of the different C3 microzones suggests that stimulation of the SR nerve could evoke climbing fibre activity in as many as 12 different cortical microzones (classes 1-3 of Ekerot et al. 1991a, see their Fig. 4). The different patterns of gating in the C1/C3 zones in rostral PML and lobule V may therefore reflect the presence of different microzones within these different cortical regions, each supplied by different subcomponents of the DF-SOCP. In the study by Apps et al. (1995) one out of a total of seven C3 zone recording sites in lobule V displayed peak excitability during stance in a similar fashion to the present results, implying that at least some microzones are common to both regions of cortex (although microzones with this timing pattern are clearly more prevalent in rostral PML).
Functional implications
It is widely thought that climbing fibres signal ‘errors’ to the cerebellum whenever there is a mismatch between intended and achieved movements (Oscarsson, 1979). Increased pathway excitability during the swing phase of the step cycle at C1 and C3 zone sites in the anterior lobe has been interpreted therefore as a ‘… temporally tuned transcerebellar mechanism, designed to intervene in the execution of the current step when an obstacle is contacted as the limb is manoeuvred towards footfall.’ (Apps et al. 1995). The present findings suggest that caudal parts of the same cortical zones may be concerned more with perturbations that arise during the stance phase of the step cycle in the ipsilateral forelimb. Different rostrocaudal regions of the same zone may therefore ‘sample’ climbing fibre inputs at different times so that, collectively, they monitor activity during all phases of the step cycle.
Acknowledgments
We thank Professor David Armstrong and Dr Martin Garwicz for their comments on the manuscript and Miss Rachel Bissett and Miss Clare Everard for their expert technical assistance. This work was funded by an MRC Senior Fellowship to R. A. S. L. was supported by a Wellcome Trust Prize Studentship.
References
- Apps R. Movement related gating of climbing fibre input to cerebellar cortical zones. Progress in Neurobiology. 1999;57:537–562. doi: 10.1016/s0301-0082(98)00068-9. 10.1016/S0301-0082(98)00068-9. [DOI] [PubMed] [Google Scholar]
- Apps R, Atkins MJ, Garwicz M. Gating of cutaneous input to cerebellar climbing fibres during a reaching task in the cat. The Journal of Physiology. 1997;502:203–214. doi: 10.1111/j.1469-7793.1997.203bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps R, Hartell NA, Armstrong DM. Step phase-related excitability changes in spino-olivocerebellar paths to the c1 and c3 zones in cat cerebellum. The Journal of Physiology. 1995;483:687–702. doi: 10.1113/jphysiol.1995.sp020614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps R, Lee S. Movement-related gating of spino-olivocerebellar paths in the awake cat. The Journal of Physiology. 1997;504.P:16–17S. [Google Scholar]
- Apps R, Lidierth M, Armstrong DM. Locomotion-related variations in excitability of spino-olivocerebellar paths to cat cerebellar cortical c2 zone. The Journal of Physiology. 1990;424:487–512. doi: 10.1113/jphysiol.1990.sp018079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps R, Trott JR. Branching in the rostrocaudal axis within the projection from the inferior olive to the C1 zone of the cat cerebellum. Society for Neuroscience Abstracts. 1991;17:627.8. [Google Scholar]
- Armstrong DM, Harvey RJ. Responses of a spino-olivo-cerebellar pathway in the cat. The Journal of Physiology. 1968;194:147–16. doi: 10.1113/jphysiol.1968.sp008399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DM, Harvey RJ, Schild RF. Spino-olivocerebellar pathways to the posterior lobe of the cat cerebellum. Experimental Brain Research. 1973a;18:1–18. doi: 10.1007/BF00236553. [DOI] [PubMed] [Google Scholar]
- Armstrong DM, Harvey RJ, Schild RF. Cerebello-cerebellar responses mediated via climbing fibres. Experimental Brain Research. 1973b;18:19–39. doi: 10.1007/BF00236554. [DOI] [PubMed] [Google Scholar]
- Armstrong DM, Harvey RJ, Schild RF. Branching of inferior olivary axons to terminate in different folia, lobules or lobes of the cerebellum. Brain Research. 1973c;54:365–371. doi: 10.1016/0006-8993(73)90062-0. 10.1016/0006-8993(73)90062-0. [DOI] [PubMed] [Google Scholar]
- Armstrong DM, Harvey RJ, Schild RF. The spatial organisation of climbing fibre branching in the cat cerebellum. Experimental Brain Research. 1973d;18:40–58. doi: 10.1007/BF00236555. [DOI] [PubMed] [Google Scholar]
- Armstrong DM, Harvey RJ, Schild RF. Topographical localization in the olivocerebellar projection: An electrophysiological study in the cat. Journal of Comparative Neurology. 1974;154:287–302. doi: 10.1002/cne.901540305. [DOI] [PubMed] [Google Scholar]
- Brodal A, Kawamura K. Olivocerebellar projection: A review. In: Brodal A, Hild W, van Limborgh J, editors. Advances in Anatomy, Embryology and Cell Biology. Berlin, Heidelberg, New York: Springer-Verlag; 1980. pp. 1–140. [PubMed] [Google Scholar]
- De Zeeuw CI, Lang EJ, Sugihara I, Ruigrok TJH, Eisenman LM, Mugnaini E, Llinas R. Morphological correlates of bilateral synchrony in the rat cerebellar cortex. Journal of Neuroscience. 1996;16:3412–3426. doi: 10.1523/JNEUROSCI.16-10-03412.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Llinas R, Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. The Journal of Physiology. 1966a;182:268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Llinas R, Sasaki K, Voorhoeve PE. Interaction experiments on the responses evoked in Purkinje cells by climbing fibres. The Journal of Physiology. 1966b;182:297–315. doi: 10.1113/jphysiol.1966.sp007825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekerot C-F, Garwicz M, Schouenborg J. Topography and nociceptive receptive fields of climbing fibres projecting to the cerebellar anterior lobe in the cat. The Journal of Physiology. 1991a;441:257–274. doi: 10.1113/jphysiol.1991.sp018750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekerot C-F, Garwicz M, Schouenborg J. The postsynaptic dorsal column pathway mediates cutaneous nociceptive information to cerebellar climbing fibres in the cat. The Journal of Physiology. 1991b;441:275–284. doi: 10.1113/jphysiol.1991.sp018751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekerot C-F, Larson B. The dorsal spino-olivo-cerebellar system in the cat. I. Functional organization and termination in the anterior lobe. Experimental Brain Research. 1979a;36:201–217. doi: 10.1007/BF00238905. [DOI] [PubMed] [Google Scholar]
- Ekerot C-F, Larson B. The dorsal spino-olivo-cerebellar system in the cat. II. Somatotopical organization. Experimental Brain Research. 1979b;36:219–232. doi: 10.1007/BF00238906. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Voogd J, Freedman SL. The parasagittal zonation within the olivocerebellar projection. II. Climbing fibre distribution in the intermediate and hemispheric parts of the cat cerebellum. Journal of Comparative Neurology. 1979;183:551–602. doi: 10.1002/cne.901830307. [DOI] [PubMed] [Google Scholar]
- Horn KM, Van Kan PLE, Gibson AR. Reduction of rostral dorsal accessory olive responses during reaching. Journal of Neurophysiology. 1996;76:4140–4151. doi: 10.1152/jn.1996.76.6.4140. [DOI] [PubMed] [Google Scholar]
- Kitchell RL, Canton DD, Johnson RD, Maxwell SA. Electrophysiologic studies of cutaneous nerves of the forelimb of the cat. Journal of Comparative Neurology. 1982;210:400–410. doi: 10.1002/cne.902100406. [DOI] [PubMed] [Google Scholar]
- Lang EJ, Sugihara I, Llinas R. GABAergic modulation of complex spike activity by the cerebellar nucleoolivary pathway in rat. Journal of Neurophysiology. 1996;76:255–275. doi: 10.1152/jn.1996.76.1.255. [DOI] [PubMed] [Google Scholar]
- Larsell O. The cerebellum of the cat and monkey. Journal of Comparative Neurology. 1953;99:135–200. doi: 10.1002/cne.900990110. [DOI] [PubMed] [Google Scholar]
- Larson B, Miller S, Oscarsson O. A spinocerebellar climbing fibre path activated by the flexor reflex afferents from all four limbs. The Journal of Physiology. 1969;203:641–649. doi: 10.1113/jphysiol.1969.sp008883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidierth M, Apps R. Gating in the spino-olivocerebellar pathways to the c1 zone of the cerebellar cortex during locomotion in the cat. The Journal of Physiology. 1990;430:453–469. doi: 10.1113/jphysiol.1990.sp018301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Baker R, Sotelo C. Electrotonic coupling between neurons in the cat inferior olive. Journal of Neurophysiology. 1974;37:560–571. doi: 10.1152/jn.1974.37.3.560. [DOI] [PubMed] [Google Scholar]
- Llinas R, Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro: different types of voltage-dependent ionic conductances. The Journal of Physiology. 1981;315:549–567. doi: 10.1113/jphysiol.1981.sp013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscarsson O. Termination and functional organization of the dorsal spino-olivocerebellar path. The Journal of Physiology. 1969;200:129–149. doi: 10.1113/jphysiol.1969.sp008685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscarsson O. Functional units of the cerebellum. Sagittal zones and microzones. Trends in Neurosciences. 1979;2:143–145. [Google Scholar]
- Oscarsson O. Functional organization of olivary projection to the cerebellar anterior lobe. In: Courville J, De Montigny C, Lamarre Y, editors. The Inferior Olivary Nucleus: Anatomy and Physiology. New York: Raven Press; 1980. pp. 279–289. [Google Scholar]
- Rosina A, Provini L. Somatotopy of climbing fiber branching to the cerebellar cortex in the cat. Brain Research. 1983;289:45–63. doi: 10.1016/0006-8993(83)90005-7. 10.1016/0006-8993(83)90005-7. [DOI] [PubMed] [Google Scholar]
- Rosina A, Provini L. Spatial distribution of axon collaterals of single inferior olive neurones. Journal of Comparative Neurology. 1987;256:317–328. doi: 10.1002/cne.902560302. [DOI] [PubMed] [Google Scholar]
- Simpson JI, Wylie DR, De Zeeuw CI. On climbing fiber signals and their consequence(s) Behaviour and Brain Science. 1996;19:384–398. [Google Scholar]
- Trott JR, Apps R. Lateral and medial subdivisions within the olivocerebellar zones of the paravermal cortex in lobule Vb/c of the cat anterior lobe. Experimental Brain Research. 1991;87:126–140. doi: 10.1007/BF00228514. [DOI] [PubMed] [Google Scholar]
- Trott JR, Apps R. Zonal organization within the projection from the inferior olive to the rostral paramedian lobule of the cat cerebellum. European Journal of Neuroscience. 1993;5:162–173. doi: 10.1111/j.1460-9568.1993.tb00482.x. [DOI] [PubMed] [Google Scholar]
- Voogd J, Glickstein M. The anatomy of the cerebellum. Trends in Neurosciences. 1998;21:370–375. doi: 10.1016/s0166-2236(98)01318-6. 10.1016/S0166-2236(98)01318-6. [DOI] [PubMed] [Google Scholar]


