Abstract
The small GTP-binding Rho proteins are involved in the agonist-induced Ca2+ sensitization of smooth muscle. The action and the expression of Rnd1, a new member of the Rho protein family constitutively bound to GTP, has been studied in rat smooth muscle.
Recombinant prenylated Rnd1 (0.01-0.1 mg ml−1) dose dependently inhibited carbachol- and GTPγS-induced Ca2+ sensitization in β-escin-permeabilized ileal smooth muscle strips but had no effect on the tension at submaximal [Ca2+] (pCa 6.3). Rnd1 inhibited GTPγS-induced tension without shifting the dose-response curves to GTPγS.
pCa-tension relationships were not modified by Rnd1 and the rise in tension induced through the inhibition of myosin light chain phosphatase by calyculin A was not affected by Rnd1.
The Ca2+ sensitization induced by recombinant RhoA was completely abolished when RhoA and Rnd1 were applied together.
Rnd1 was expressed at a low level in membrane fractions prepared from intestinal or arterial smooth muscles. The expression of Rnd1 was strongly increased in ileal and aortic smooth muscle from rats treated with progesterone or oestrogen. Progesterone-treated ileal muscle strips showed a decrease in agonist-induced Ca2+ sensitization.
The present study shows that (i) Rnd1 inhibits agonist- and GTPγS-induced Ca2+ sensitization of smooth muscle by specifically interfering with a RhoA-dependent mechanism and (ii) an increase in Rnd1 expression may account, at least in part, for the steroid-induced decrease in agonist-induced Ca2+ sensitization.
The Rho protein family, which belongs to the Ras superfamily of small GTP-binding proteins, comprises Rho (A-C), Rac (1 and 2), Cdc42, TC10, RhoG and RhoE. These proteins are well accepted as regulators of the actin cytoskeleton and are involved in the formation of filopodia (Cdc42), lamellipodia (Rac), stress fibres and focal adhesion (Rho) in response to extracellular signals (Tapon & Hall, 1997). These effects have been ascribed to the interaction of the active GTP-bound form of the GTPase with specific target proteins. Several proteins have been defined as potential target proteins of Rho, including the serine/threonine kinases citron kinase (Madaule et al. 1998), PKN and the Rho-associated kinases (Rho-kinases) ROCK-I and ROCK-II (Van Aelst & D'Souza-Schorey, 1997). Myosin light chain (MLC) phosphatase is a substrate for Rho-kinases. Its phosphorylation leads to a decrease in its activity and, consequently, to an increased level of phosphorylation of MLC (Kimura et al. 1996). Increased MLC phosphorylation could be a major contributor to the effect of Rho on actin organization and perhaps focal adhesion assembly (Chrzanowskla-Wodnicka & Burridge, 1996).
In smooth muscle, contraction is primarily regulated by the level of phosphorylation of MLC by a Ca2+-calmodulin-dependent kinase. However, an increase in phosphorylation of MLC and tension can be induced at constant [Ca2+] by the activation of G-proteins by agonists or GTPγS through a mechanism that inhibits the MLC phosphatase (Kitazawa et al. 1991; Somlyo & Somlyo, 1994). It has been reported that rhoA p21 is involved in this Ca2+ sensitization of smooth muscle (Hirata et al. 1992; Fujita et al. 1995; Itagaki et al. 1995; Gong et al. 1996; Otto et al. 1996) and recently it has been shown that Ca2+-sensitizing agonists induce translocation of RhoA (Gong et al. 1997). In addition, direct phosphorylation of MLC by Rho-kinase (Amano et al. 1996) and Rho-kinase-induced contraction have been observed in smooth muscle (Kureishi et al. 1997). Agonist-induced Ca2+ sensitization thus appears to be linked to the activation of Rho proteins. The use of Y-27632, a new inhibitor of Rho-kinase, has shown that RhoA/Rho-kinase-mediated Ca2+ sensitization contributes to blood pressure regulation in vivo and is augmented in hypertension (Uehata et al. 1997).
Recently, new members of the Rho family which lack GTPase activity and are constitutively in the active GTP-bound form have been identified (Nobes et al. 1998). The involvement of these Rnd proteins in a signalling pathway is therefore related to their expression levels. Expression of Rnd1 in fibroblasts has been found to promote disassembly of actin filament structures and loss of cell adhesion. Since Ca2+ sensitization in smooth muscle and stress fibre formation in fibroblasts share the same signalling pathway involving RhoA and Rho-kinase, this study was designed to analyse the expression and action of Rnd1 in smooth muscle. We demonstrate that Rnd1 antagonizes the agonist- and GTPγS-induced Ca2+ sensitization by specifically inhibiting the RhoA-dependent pathways. We show that sex hormone steroids, known to decrease the contractility of vascular and intestinal smooth muscles (Gill et al. 1985; Jiang et al. 1991; Baron et al. 1993), increase the expression of Rnd1 in smooth muscles and decrease the agonist-induced Ca2+ sensitization. Preliminary results of some of the data presented in this paper have been published in abstract form (Loirand et al. 1999).
METHODS
Isometric tension measurement in skinned fibres
All experiments were conducted in accordance with institutional guidelines for the care and use of laboratory animals. Wistar rats (150 g) were stunned and then killed by cervical dislocation. The longitudinal muscle layer of ileum was peeled from the underlying circular muscle in physiological saline solution (PSS; composition given below). Small strips (approximately 200 μm wide and 4 mm long) of longitudinal muscle from rat ileum were dissected and tied at each end with a single silk thread to the tips of two needles, one of which was connected to a force transducer (AE 801, SensoNor, Norway). Strips were placed in a well on a bubble plate filled with PSS (Horiuti, 1988) and stretched to about 1.3 times the resting length. The solution was rapidly changed by sliding the plate to an adjacent well. After measuring contraction evoked by high-K+ solution, the strips were incubated in normal relaxing solution (Ca2+-free, 2 mM EGTA; composition below) for a few minutes, followed by treatment with β-escin (50-70 μM) in relaxing solution for 35 min at 25°C. The calcium ionophore A23187 was added to the skinning solution to deplete intracellular Ca2+ stores (Kobayashi et al. 1989). The skinned muscle strip was then washed several times with fresh relaxing solution containing 10 mM EGTA. The efficiency of the permeabilization was assessed by comparison of the amplitude of the high-K+ contraction in intact strips with the response to high-Ca2+ solution (-log[Ca2+]= 4.5; pCa 4.5) recorded after permeabilization. Only permeabilized strips showing responses to pCa 4.5 corresponding to 0.9-1.2 times the high-K+ response recorded before permeabilization were used in this study. Following a submaximal Ca2+-induced contraction, Ca2+ sensitization was induced by addition of agonist. The pretreatment with A23187 and the presence of 10 mM EGTA assured that the changes in force observed under these conditions were not due to changes in [Ca2+]i. Maximal force used to normalize the contractile response was determined by exposure to pCa 4.5 solution. To prevent the alteration of Ca2+-induced contraction, 1.5 μM calmodulin was added to the bathing solutions throughout the experiments. For each experimental condition, results were compared with those obtained in control strips taken from adjacent pieces of tissue and handled under similar conditions, using similar buffers at the same dilution. To evaluate the effect of the exoenzyme C3, which ADP-ribosylates and inhibits RhoA, permeabilized strips were pretreated by incubation with C3 (1.5 μg ml−1; or buffer without C3 for control strips) in the presence of 10 μM NAD for 20 min in pCa 6.3 solution.
Steroid treatments and isometric tension measurements in intact fibres
Ovariectomized rats received one s.c. injection per day for 3 days, with vehicle (sesame oil; control), β-oestradiol 3-benzoate (200 μg kg−1) or progesterone (10 mg kg−1). Animals were killed by cervical dislocation on the morning of the third day of the treatment. The aorta and the longitudinal muscle layer of ileum were collected and used for membrane preparations or for contraction measurements in intact strips. Strips of longitudinal ileal muscle layer were suspended under isometric conditions in 3 ml organ baths filled with Krebs-Henseleit solution (mM: 118.4 NaCl, 4.7 KCl, 2 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 11 glucose) maintained at 31°C, and gassed with 95 % O2-5 % CO2. The preparations were initially placed under a resting tension of 500 mg, left to equilibrate for 1 h and washed at 20 min intervals. Successive applications of agonist were separated by a time interval of 20 min.
Recombinant protein expression
The Rnd1 and RhoA used were expressed in Escherichia coli, purified as described (Nobes et al. 1998) and loaded with GTP. Briefly, bacterial pellets were homogenized in buffer A (mM: 50 Hepes, 10 NaCl, 10 KCl, 1 dithiothreitol (DTT), 5 MgCl2 and a protease inhibitor cocktail (Complete, Boehringer; 1 tablet per 50 ml) and lysed on a French press at 1000 p.s.i. (7 kPa). The lysate was centrifuged and the supernatant applied to a S-sepharose fast flow column and eluted with a 10-500 mM linear NaCl gradient. Rnd1 eluted at about 300 mM NaCl and represented > 80 % of the total proteins in this peak. The purified protein was then frozen in liquid nitrogen. All steps were carried out at 4°C. Attachment of prenyl groups to cysteine near the C termini of the purified recombinant proteins is required to allow the targeting to the membrane. The linkage of geranylgeranyl groups from geranylgeranyl pyrophosphate to Rdn1 and RhoA was catalysed in vitro by the use of type 1 geranylgeranyltransferase (GGTase I) according to protocols previously described (Yokoyama & Gelb, 1993; Yokoyama et al. 1997). Although Rnd1 is normally farnesylated in vivo, it could be geranylgeranylated by GGTase I in these in vitro conditions.
Preparation of membrane pellets and Western blot analysis
The expression of Rnd1 was examined at the protein level with antibodies raised against Rnd1. Strips of smooth muscle from ileum or aorta were washed with ice-cold PSS and then homogenized with a polytron in ice-cold buffer containing (mM): 20 Hepes-NaOH, 10 KCl, 10 NaCl, 5 MgCl2, 1 DTT and Complete (1 tablet per 50 ml). Nuclei and unlysed cells were removed by low-speed centrifugation, the supernatant was centrifuged at 100 000 g, the pellet (P100) was resuspended in the same buffer, protein concentrations in the pellet or supernatant (S100) fractions were measured and adjusted, then Laemmli sample buffer was added. Similar amounts of proteins were loaded in each lane, electrophoresed on 12 % polyacrylamide-SDS gels and transferred to nitrocellulose. The amounts of proteins were checked by staining with Ponceau Red. Before immunoblotting, the membrane was blocked with 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1 % Tween 20 and 5 % non-fat milk for 2 h at room temperature and then probed with Anti-Rnd1 antibodies in the same solution for 2 h at room temperature. The Anti-Rnd1 antibodies do not recognize RhoA on Western blots, even when large amounts of RhoA were loaded (Nobes et al. 1998). After three washes, membranes were incubated for 1 h at room temperature (25°C) with horseradish peroxidase-conjugated anti-rabbit antibodies. The signal from immunoreactive bands was detected by enhanced chemiluminescence using a chemiluminescent detection system (Pierce), and quantified using ImageQuant (MolecularDynamics).
Statistics
All results are expressed as the means ±s.e.m. with n the sample size. Significance was tested by means of Student's t test. Probabilities less than 5 % (P < 0.05) were considered significant.
Solutions
The normal physiological saline solution (PSS) contained (mM): 130 NaCl, 5.6 KCl, 1 MgCl2, 2 CaCl2, 11 glucose, 10 Tris, pH 7.4 with HCl. High-K+ solution was prepared by replacing NaCl with the equivalent amount of KCl (60 mM). The normal relaxing solution contained (mM): 85 KCl, 5 MgCl2, 5 Na2ATP, 5 creatine phosphate, 2 EGTA and 20 Tris-maleate, brought to pH 7.1 at 25°C with KOH. In the activating solutions, 10 mM EGTA was used, and a specified amount of CaCl2 was added to give a desired concentration of free Ca2+. The pH of the solution was adjusted to 7.1. The ionic strength was kept constant at 0.2 M by adjusting the concentration of KCl.
Chemicals and drugs
Carbachol (CCh), β-escin, Na2ATP, calmodulin, GTP, GTPγS, EGTA, creatine phosphate, calcium ionophore A23187, calyculin A, thapsigargin, methoxyverapamil, β-oestradiol 3-benzoate, progesterone, atropine and sesame oil were purchased from Sigma. The exoenzyme C3 was kindly provided by Dr P. Boquet (Inserm U452, Nice University Medical School, Nice, France). Geranylgeranyltransferase was a gift from Drs J. Basquin and M. Duchesnes (Rhone Poulenc Rorer, France). The Rho-kinase inhibitor Y-27632 was a gift from Yoshitomi Pharmaceutical Industries, Ltd, Saitama, Japan.
RESULTS
Ca2+ sensitization induced by carbachol or GTPγS is inhibited by C3 transferase
Following permeabilization, the strips were relaxed at pCa > 8 followed by submaximal activation at pCa 6.3, which increased the tension to 13.0 ± 1.5 % (n = 16) of the pCa 4.5-induced tension. This rise in tension corresponded to a force of 4.4 ± 0.6 mg, similar to previously published data (Otto et al. 1996). Addition of carbachol (CCh; 100 μM) in the presence of GTP (10 μM) at constant pCa (6.3) produced an increase in tension corresponding to 15.9 ± 1.8 % (n = 8) of the maximal Ca2+-induced contraction obtained at pCa 4.5 (Fig. 1Aa). GTPγS had similar properties. When added at pCa 6.3, GTPγS (10 μM) raised the tension to 36.7 ± 3.8 % (n = 8) of the pCa 4.5-induced force. Both the CCh- and GTPγS-induced Ca2+ sensitization were inhibited by a 20 min pretreatment with the exoenzyme C3 from Clostridium botulinum, known to ADP-ribosylate and inhibit RhoA (Machesky & Hall, 1996) (Fig. 1A and B).
Figure 1. Ca2+-sensitizing effect of CCh and GTPγS.

Aa,β-escin-permeabilized strips of ileum were submaximally activated with a pCa 6.3 solution then successively stimulated with CCh (100 μM in the presence of 10 μM GTP), GTPγS (10 μM) and pCa 4.5 to evoke maximal contraction. b, the Ca2+-sensitizing effects of CCh and GTPγS were inhibited by a 20 min pretreatment with the exoenzyme C3 (1.5 μg ml−1 in the presence of 10 μM NAD). B, the effects of C3 (1.5 μg ml−1 in the presence of 10 μM NAD for 20 min) on CCh- and GTPγS-induced Ca2+ sensitization. The tension responses induced by carbachol or GTPγS in control experiments (left two columns) and in the presence of C3 (right two columns) were expressed as the percentage of the pCa 4.5-induced force (n = 8 for each condition).
Effect of Rnd1 on CCh- and GTPγS-induced Ca2+ sensitization
Treatment of permeabilized muscle strips with Rnd1 at pCa 6.3 for 20 min inhibited the CCh- and GTPγS-induced Ca2+ sensitization (Figs 2 and 3). The inhibition was concentration dependent and the prenylated form of Rnd1 was considerably more potent than non-prenylated Rnd1. The CCh-induced Ca2+ sensitization was completely abolished in the presence of 0.1 mg ml−1 Rnd1, whereas the non-prenylated Rnd1 (nP-Rnd1) only (but significantly) reduced the CCh-induced responses to 7.54 ± 2.43 % (n = 8; P < 0.001) (Fig. 2B). This difference in the potency of the two forms of Rnd1 in inhibiting Ca2+ sensitization was much more pronounced on the GTPγS-induced response, as nP-Rnd1 (0.01 and 0.1 mg ml−1) was totally ineffective (Fig. 3). The same concentrations of prenylated Rnd1 produced half and complete inhibition of the GTPγS-induced Ca2+ sensitization, respectively. Concentration- response curves to GTPγS showed that the inhibitory effect of Rnd1 resulted from a decrease in the amplitude of the maximal response without a shift in the concentration of GTPγS producing half-maximal effect (EC50). EC50 values estimated from the mean curves corresponded to 0.47 μM under control conditions and in the presence of 0.1 mg ml−1 Rnd1. These results suggest that Rnd1 inhibited the GTPγS-induced Ca2+ sensitization in a non-competitive manner and that association of Rnd1 with the membrane was required for its inhibitory effect on Ca2+ sensitization.
Figure 2. Inhibition of CCh-induced Ca2+ sensitization by Rnd1.
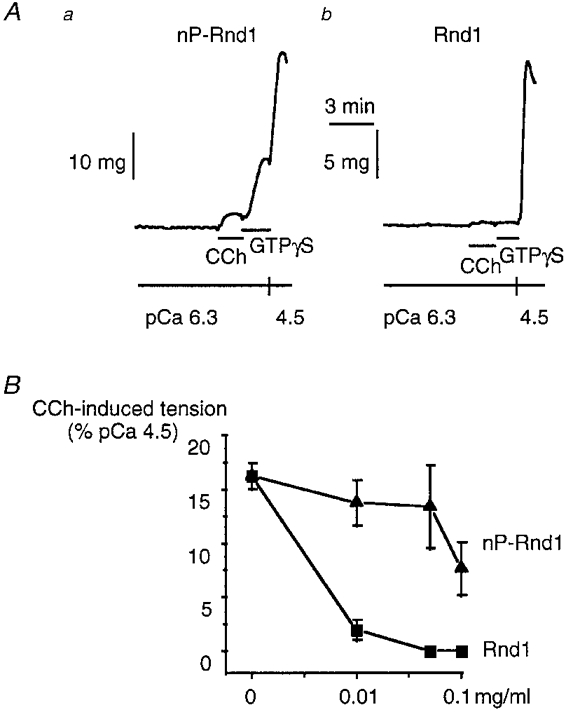
A, treatment of β-escin-permeabilized strips of ileum with Rnd1 (0.1 mg ml−1) for 20 min at pCa 6.3 inhibited the CCh (100 μM)-induced Ca2+ sensitization. The non-prenylated form of Rnd1 (nP-Rnd1; a) was less potent than the geranylgeranylated Rnd1 (b). nP-Rnd1 (0.1 mg ml−1) did not affect the GTPγS (10 μM)-induced Ca2+ sensitization (a) whereas it was completely inhibited by the same concentration of Rnd1 (b). B, concentration-response curve for the inhibitory effect of nP-Rnd1 (▴) and Rnd1 (▪) on the Ca2+-sensitizing effect of 100 μM CCh expressed as the percentage of the pCa 4.5-induced force. Each point represents the mean of 3-5 experiments.
Figure 3. Inhibition of GTPγS-induced Ca2+ sensitization by Rnd1.

A, gradual tension response to cumulative applications of GTPγS (0.1, 0.3, 1 and 10 μM, as shown on the traces) at constant pCa 6.3 followed by a maximal response to pCa 4.5 under control conditions (a), in the presence of 0.1 mg ml−1 nP-Rnd1 (b) and in the presence of 0.01 mg ml−1 Rnd1 (c). B, concentration-response curve for the inhibitory effect of nP-Rnd1 (▴) and Rnd1 (▪) on the Ca2+-sensitizing effect of 10 μM GTPγS expressed as the percentage of the pCa 4.5-induced force (n = 3-5). C, cumulative dose-response curves for the Ca2+-sensitizing effect of GTPγS under control conditions (•), in the presence of nP-Rnd1 (0.1 mg ml−1, ▴) and in the presence of Rnd1 (0.01 mg ml−1, ▪; 0.1 mg ml−1, ▾). GTPγS-induced rise in tension was expressed as the percentage of the pCa 4.5-induced force. Each point represents the mean of 3-5 experiments.
Rnd1 did not modify the relationship between Ca2+ concentration and tension development
pCa-tension relationships were obtained by cumulatively increasing [Ca2+] (from pCa 8 to 4.5), and the amplitude of the tension responses was expressed as a percentage of the amplitude of the maximal tension obtained 30 min before in response to a single application of Ca2+ (pCa 4.5). Treatment of the strips with prenylated Rnd1 (0.1 mg ml−1) was started at pCa > 8, 20 min before the cumulative application of Ca2+. Compared with control strips, Rnd1 did not significantly modify either the maximal response or the pCa that induced the half-maximal tension (pCa50) (Fig. 4). The mean pCa50 values were 6.19 ± 0.01 (n = 4) and 6.12 ± 0.04 (n = 4, P > 0.2) under control conditions and in the presence of Rnd1, respectively, suggesting that Rnd1 did not directly affect the Ca2+-dependent phosphorylation of MLC responsible for the Ca2+-induced contractions. In addition, we did not detect a significant shift in the pCa-tension relationship in the presence of C3 (not shown), indicating that the RhoA-dependent pathway was not activated when this protocol was used.
Figure 4. Lack of effect of Rnd1 on the pCa-tension relationship.

Following permeabilization, the strips were relaxed at pCa > 8 followed by the activation of a maximal tension response with pCa 4.5. The strips were then washed for 10 min in pCa > 8, followed by treatment without (Aa) or with (Ab) 0.1 mg ml−1 prenylated Rnd1 for 20 min. After this time, pCa-tension relationships were obtained by cumulatively increasing [Ca2+] (pCa 8-4.5). B, pCa-tension relationships obtained in control strips (▪) and in the presence of Rnd1 (•). Tension responses were expressed as the percentage of the first pCa 4.5-induced response. Each point represents the mean of 4-6 experiments.
Lack of effect of Rnd1 on calyculin A-induced Ca2+ sensitization
We also wished to examine the effect of Rnd1 on the Ca2+ sensitization induced through a mechanism independent of receptor and trimeric G-protein activation. For this, we used calyculin A, a potent inhibitor of protein phosphatases 1 and 2A (Takai et al. 1987) known to produce smooth muscle contraction and to increase phosphorylation of MLC even in the absence of Ca2+ by inhibiting MLC phosphatase (Suzuki & Itoh, 1993). Permeabilized strips either under control conditions or treated with prenylated Rnd1 (0.1 mg ml−1) were stimulated by CCh at pCa 6.3, then maximal contraction was induced by pCa 4.5. After the tension returned to the resting level at pCa > 8, calyculin A (3 μM) was added (Fig. 5). It produced a slow rise in tension, the amplitude of which was similar (n = 4, P > 0.2) in control and in Rnd1-treated strips. Similarly, the rate of the rise in tension induced by calyculin A was not modified in Rnd1-treated strips (1.75 ± 0.17 mg min−1 (n = 4) and 1.79 ± 0.23 mg min−1 (n = 4, P > 0.5) in control and treated strips, respectively). This result is in agreement with the absence of effect of Rnd1 on the pCa-tension relationship, indicating that Rnd1 did not directly inhibit MLC phosphorylation.
Figure 5. Lack of effect of Rnd1 on calyculin A-induced rise in tension.

A, prenylated Rnd1 (0.1 mg ml−1) applied at pCa 6.3 20 min before stimulation with CCh (100 μM) completely inhibited the CCh-induced Ca2+ sensitization but did not affect the calyculin A (cal A; 3 μM)-induced rise in tension at pCa 8. B, comparison of the rise in tension induced by cal A in the absence and in the presence of 0.1 mg ml−1 Rnd1. Results are expressed as the percentage of the first pCa 4.5-induced response (n = 4 for each experimental condition).
Effect of Rnd1 on RhoA-induced Ca2+ sensitization
Geranylgeranylated RhoA (0.1 or 0.05 mg ml−1) applied at a submaximal free [Ca2+] (pCa 6.3) produced a maintained increase in tension of permeabilized muscle strips (n = 4, P < 0.001; Fig. 6A and D). This tension response to RhoA ranged between 8 and 14 % of the pCa 4.5 response (Fig. 6D). In agreement with a previous report (Gong et al. 1996), similar concentrations of recombinant RhoA that was not geranylgeranylated had no effect on tension. Addition of Rnd1 (0.05 mg ml−1) to permeabilized strips stimulated by RhoA (0.05 mg ml−1) at pCa 6.3 did not reverse the RhoA-induced increase in tension (Fig. 6B). However, RhoA did not produce a rise in tension when Rnd1 was simultaneously applied (0.05 mg ml−1 each; Fig. 6C). The tension measured under these conditions was not significantly different from that measured at pCa 6.3 (n = 4, P > 0.2; Fig. 6D). Responses to subsequent applications of CCh (100 μM) and GTPγS (10 μM) were also completely abolished.
Figure 6. Effect of Rnd1 on RhoA-induced tension.

A, original trace (upper trace) showing the effect of RhoA in permeabilized strips. A part of the original trace has been displayed on a larger scale (bottom). RhoA (0.1 mg ml−1) applied at a submaximal [Ca2+] (pCa 6.3) induced a rise in tension. CCh (100 μM) and GTPγS applied in the presence of RhoA further enhanced the tension. B, the rise in tension induced by 0.05 mg ml−1 RhoA was not reversed by the addition of Rnd1 (0.05 mg ml−1). C, simultaneous application of RhoA and Rnd1 (0.05 mg ml−1 each) had no effect on tension and successive applications of CCh (100 μM) and GTPγS (10 μM) were ineffective. D, results are expressed as the percentage of the pCa 4.5-induced tension (n = 4).
Expression of Rnd1 in rat smooth muscles
Rnd1 is constitutively in the active GTP-bound form, therefore its inhibitory action on Ca2+ sensitization would depend on its level of expression in smooth muscle cells. We determined whether Rnd1 was endogenously expressed in smooth muscle of rat ileum and aorta using Anti-Rnd1 antibodies (Nobes et al. 1998). Figure 7 shows that Rnd1 was expressed at a low level in membrane fractions prepared from intestinal or arterial smooth muscles. Two bands of the size of Rnd 1 protein were observed. These two bands were also observed when we transiently expressed Rnd 1 in Chinese hamster ovary (CHO) cells, and were absent in cells transfected with the empty vector as the negative control. These two bands could be due to differences in phosphorylation or in farnesylation, or other post-translational modifications. Rnd1 was not detected in the cytosolic fractions (not shown).
Figure 7. Expression of Rnd1.

Representative Western blots showing the increase in the expression of Rnd1 in the membrane fractions prepared from the longitudinal muscle layer of ileum or from aorta of control rats and of rats treated with oestradiol or progesterone.
Effect of gonadal steroid treatment
Sex hormone steroids are known to alter smooth muscle contractility, in particular receptor-dependent muscle contraction, through mechanisms that are not fully understood (Gill et al. 1985; Jiang et al. 1991; Baron et al. 1993). However, G-protein signalling has been shown to be altered by steroids. Coupling between receptors and heterotrimeric G-proteins as well as the expression of members and effectors of the Rho protein family have been shown to be modulated during pregnancy or by steroid treatments (Niiro et al. 1997; Chen et al. 1998). We therefore analysed the effect of progesterone or oestradiol treatment on the expression of Rnd1 and the contractile properties of smooth muscle. As steroid treatments are expected to cause a general increase in protein content, each lane of the gels was loaded with a similar amount of proteins (a protein, the expression of which was not modified by steroid treatments, would thus be detected as an immunoreactive band apparently less intensive than in the control). The expression of Rnd1 was strongly enhanced in both aorta and ileum from treated rats (Fig. 7). Treatment with oestradiol produced a 3-fold increase in Rnd1 protein levels in the aorta and ileum (n = 4). Treatment with progesterone led to a 6-fold increase in Rnd1 protein levels in both smooth muscle tissues (n = 4). The effect of steroid treatments on the agonist-induced Ca2+ sensitization was examined by measuring the CCh-induced contraction of intact longitudinal ileal muscle strips in the presence of the voltage-gated Ca2+ channel inhibitor methoxyverapamil (D600, 10 μM) and the Ca2+-releasing agent thapsigargin (TSG, 1 μM), added 20 min before. Under these conditions, CCh did not induce any change in intracellular Ca2+ concentration (Pacaud & Bolton, 1991) and CCh-induced contraction only depended on Ca2+-sensitizing mechanisms. This was confirmed by the reversible and concentration-dependent inhibitory action of the selective Rho-kinase inhibitor Y-27632 (Uehata et al. 1997). In the presence of TSG and D600, Y-27632 inhibited the CCh (10 μM)-induced rise in tension with an EC50 of 2.4 ± 0.1 μM (n = 4) and complete inhibition was obtained with 10 μM (Fig. 8). This suggests that the CCh-induced TSG- and D600-resistant tension was due to RhoA/Rho-kinase-dependent Ca2+ sensitization. The TSG- and D600-resistant rise in tension induced by 10 μM CCh was also completely abolished in the presence of the muscarinic receptor antagonist atropine (0.5 μM; Fig. 8B). When normalized to control values obtained before D600 and TSG application, the amplitude of the contraction evoked by 10 μM CCh in the presence of D600 and TSG was significantly smaller in muscle strips from progesterone-treated than from control rats (Fig. 8) (n = 12, P < 0.0001). This indicates that progesterone treatment decreased the RhoA/Rho-kinase-dependent Ca2+-sensitizing mechanisms in smooth muscle. Similar results were obtained for the phenylephrine-induced contraction in oestradiol-treated aorta (not shown).
Figure 8. Decrease in agonist-induced RhoA/Rho-kinase-dependent Ca2+ sensitization by progesterone treatment.

A, typical traces showing the contraction induced by CCh (10 μM) in intact ileal muscle strips from control rats (top) or from rats treated with progesterone (bottom) before (left) and 20 min after addition of thapsigargin (TSG, 1 μM) and D600 (10 μM). Under these conditions, the rise in tension induced by CCh was completely inhibited by the Rho-kinase inhibitor Y-27632 (10 μM). B, amplitude of the TSG and D600-resistant contraction induced by CCh (10 μM) in the absence or in the presence of atropine (0.5 μM) or Y-27632 (10 μM). Results obtained in control rats (□) or in progesterone-treated rats (▪) were normalized to the amplitude of the CCh-induced contraction in the absence of TSG and D600. Each column represents the mean of 6-12 experiments.
DISCUSSION
The present study demonstrates that the constitutively active member of the Rho family, Rnd1, inhibits agonist- and GTPγS-induced Ca2+ sensitization of smooth muscle by specifically interfering with a RhoA-dependent mechanism. We have shown that sex hormone steroids increased the expression of Rnd1 in both intestinal and aortic smooth muscles, and decreased agonist-induced Ca2+ sensitization.
Ca2+ sensitization
Smooth muscle cells are involved in physiological functions (digestion, blood pressure regulation, respiration, urination, parturition) requiring slow and maintained contractions. Ca2+-sensitizing mechanisms which allow the development of maintained tension at low intracellular Ca2+ concentrations are a major mechanism regulating smooth muscle contraction. The participation of RhoA in agonist-induced Ca2+ sensitization has been suggested by the Ca2+-sensitizing effect of recombinant RhoA at constant pCa levels and the inhibition of agonist-induced Ca2+ sensitization by ADP-ribosylation of endogenous RhoA (Itagaki et al. 1995; Gong et al. 1996; Otto et al. 1996). In longitudinal muscle of rat ileum, the inhibitory effect of C3 on the CCh- and GTPγS-induced Ca2+ sensitization (Fig. 1) and the rise in tension induced by recombinant geranylgeranylated RhoA (Fig. 6) support the involvement of RhoA in the Ca2+ sensitization of smooth muscle. A demonstration that the RhoA-dependent pathway participates in agonist-induced contraction in intact preparations was recently provided by the use of chimeric toxin DC3B (Fujihara et al. 1997) or the Clostridium difficile toxin B (Lucius et al. 1998) in vascular and intestinal smooth muscle, respectively. The involvement of Rho-kinase was revealed by the use of a membrane permeable, Rho-kinase inhibitor Y-27632 (Uehata et al. 1997). This compound, at 10 μM, inhibited by 90 % the phenylephrine-induced contraction of aorta. We found that Y-27632 inhibited by 80 % the CCh-induced contraction of ileum under control conditions (not shown). Therefore, RhoA/Rho-kinase-mediated Ca2+ sensitization is a major mechanism of agonist-induced smooth muscle contraction.
Inhibitory action of Rnd1
Both the CCh- and GTPγS-induced Ca2+ sensitization were inhibited by recombinant Rnd1. The prenylated Rnd1 was considerably more potent than Rnd1 lacking the prenylated C-terminus suggesting that, like other small GTP-binding proteins (Boguski & McCormick, 1993; Casey & Seabra, 1996), and although constitutively in the active GTP-bound form, Rnd1 has to be targeted to the membrane to exert its effect. The absence of shift of the concentration-response relationships to GTPγS in the presence of Rnd1 suggests that Rnd1 does not act as a competitive inhibitor of GTP or GTPγS for a common site of action. By comparison of the inhibitory effect of Rnd1, it appears that the CCh-induced Ca2+ sensitization was ∼10 times more sensitive to Rnd1 than the GTPγS-induced sensitization (Figs 2 and 3). Contrary to CCh and GTPγS, the rise in tension induced by Ca2+ or by the MLC phosphatase inhibitor calyculin A was not affected by Rnd1, suggesting that Rnd1 does not directly interact with either the MLC phosphatase or actomyosin ATPase. Therefore, Rnd1 may act on a step of the Ca2+ sensitization pathway located upstream from inhibition of the MLC phosphatase. This hypothesis is confirmed by the action of Rnd1 on RhoA-induced Ca2+ sensitization. When applied simultaneously, Rnd1 prevented the RhoA-induced rise in tension (Fig. 6). We therefore suggest that the inhibitory effect of Rnd1 on the CCh and GTPγS-induced Ca2+ sensitization is due to the inhibition of RhoA or RhoA-dependent mechanisms upstream of MLC phosphatase inhibition. The fact that the CCh-induced Ca2+ sensitization was more sensitive to Rnd1 blockade than GTPγS supports this hypothesis, as the amount of RhoA translocated to the membrane in response to 10 μM GTPγS was 5 times greater than that induced in response to agonists (Gong et al. 1997). The agonist-induced translocation of RhoA was reported to be close to that induced by 0.3 μM GTPγS, which is in agreement with our results showing that CCh and 0.3 μM GTPγS caused a similar Ca2+ sensitization. In another way, the absence of action of Rnd1 on the calyculin A-induced tension, which is not associated with the translocation of RhoA (Gong et al. 1997), also supports the effect of Rnd1 on a RhoA-dependent pathway of Ca2+ sensitization.
RhoA has been shown to mediate the GTPγS-induced enhancement of MLC phosphorylation through inhibition of MLC phosphatase activity (Noda et al. 1995). This action involves the activation of Rho-kinase, which phosphorylates MLC phosphatase, thereby inhibiting its enzymatic activity (Amano et al. 1996) and, in addition, directly phosphorylating MLC (Kureishi et al. 1997). Both effects lead to an enhancement of force at constant [Ca2+]. Therefore, it is likely that Rnd1 interacts directly with RhoA and/or with Rho-kinase. The fact that Rnd1 was not able to relax the rise in tension induced by RhoA, when added in the continuous presence of RhoA, suggests that once the RhoA-dependent pathway of Ca2+ sensitization was activated, Rnd1 could no longer attain its target(s). The formation of complexes between RhoA and its effectors could be responsible for this observation by masking sites of interaction with Rnd1.
Expression of Rnd1
Sex steroid hormones are well known as decreasing the contractility of various smooth muscle types, including artery, vein, uterus and small intestine (Batra & Bengtsson, 1978; Gill et al. 1985; Jiang et al. 1991; Baron et al. 1993). This effect is responsible for a decrease in systemic vascular resistance and a reduction in gastrointestinal motility during pregnancy, when serum levels of progesterone and oestrogen are increased (Baron et al. 1993). The mechanisms underlying this reduction of contractility are not fully understood but clearly involve both non-genomic effects through direct inhibition of voltage-gated Ca2+ channels (Bielefeldt et al. 1996; Kitazawa et al. 1997) and genomic mechanisms. We show that treatment with progesterone leads to a decrease in the agonist-induced RhoA/Rho-kinase-dependent contraction. However, expression of Rho A and Rho-kinase was increased during pregnancy when levels of progesterone and oestrogen were high (Niiro et al. 1997), suggesting that inhibitory mechanisms were simultaneously upregulated. Steroids, through their genomic effects, lead to an increase in gene transcription and therefore an upregulation of numerous proteins. Potential candidates for the decrease in the agonist-dependent RhoA/Rho-kinase pathway are represented by factors known to decrease Ca2+ sensitivity in smooth muscle, including the cyclic nucleotide pathway (Wu et al. 1996) and Rnd1. Indeed, steroids have been shown to increase the cAMP and cGMP content in smooth muscle (Kuehl et al. 1974; Mugge et al. 1993). In this study, we have shown that steroid treatment also leads to an increase in Rnd1 expression. Since Rnd1, which is constitutively in the ‘active’ GTP-bound form, inhibits the RhoA/Rho kinase-mediated Ca2+ sensitization, a change in the expression of Rnd1, in addition to other mechanisms, might participate in the steroid-induced modulation of smooth muscle contractility.
Furthermore, Rnd1 proteins are normally farnesylated at their C-terminus and therefore represent potential targets for farnesyl transferase inhibitors (Nobes et al. 1998). Some of these compounds are currently in phase 1 trials as anti-cancer drugs. Our results suggest that they could also be of interest in pathophysiological processes associated with a loss of smooth muscle contractility, such as varicose veins or inflammatory bowel diseases.
Acknowledgments
This work was supported by the CNRS, grants from the Association Recherche et Partage, the Fondation pour la Recherche Médicale and the Region Pays de Loire. Gervaise Loirand and Pierre Chardin were supported by INSERM, and Pierre Pacaud was supported by the Ministére de l'Education Nationale. We thank Drs J. Basquin and M. Duchesnes for the gift of geranylgeranyltransferase, Dr P. Boquet for the gift of the exoenzyme C3 and Yoshitomi Pharmaceutical Industries, Ltd for the gift of the p160ROCK inhibitor Y-27632.
References
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) Journal of Biological Chemistry. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Baron TH, Ramirez B, Richter JE. Gastrointestinal motility disorders during pregnancy. Annals of Internal Medicine. 1993;118:366–375. doi: 10.7326/0003-4819-118-5-199303010-00008. [DOI] [PubMed] [Google Scholar]
- Batra S, Bengtsson B. Effects of diethylstilboestrol and ovarian steroids on the contractile responses and calcium movements in rat uterine smooth muscle. The Journal of Physiology. 1978;276:329–342. doi: 10.1113/jphysiol.1978.sp012237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeldt K, Waite L, Abboud FM, Conklin JL. Nongenomic effects of progesterone on human intestinal smooth muscle cells. American Journal of Physiology. 1996;271:G370–376. doi: 10.1152/ajpgi.1996.271.2.G370. [DOI] [PubMed] [Google Scholar]
- Boguski MS, McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366:643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- Casey PJ, Seabra MC. Protein prenyltransferases. Journal of Biological Chemistry. 1996;271:5289–5292. doi: 10.1074/jbc.271.10.5289. 10.1074/jbc.271.10.5289. [DOI] [PubMed] [Google Scholar]
- Chen Q, Chitinavis V, Xiao Z, Yu P, Oh S, Biancani P, Behar J. Impaired G protein function in gallbladder muscle from progesterone-treated guinea pigs. American Journal of Physiology. 1998;274:G283–289. doi: 10.1152/ajpgi.1998.274.2.G283. [DOI] [PubMed] [Google Scholar]
- Chrzanowskla-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. Journal of Cellular Biology. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita A, Takeuchi T, Nakajima H, Nishio H, Hata F. Involvement of heterotrimeric GTP-binding protein and Rho protein, but not protein kinase C, in agonist-induced Ca2+ sensitization of skinned smooth muscle in guinea pig vas deferens. Journal of Pharmacology and Experimental Therapeutics. 1995;274:555–561. [PubMed] [Google Scholar]
- Gill RC, Bowes KL, Kingma YJ. Effect of progesterone on canine colonic smooth muscle. Gastroenterology. 1985;88:1941–1947. doi: 10.1016/0016-5085(85)90023-x. [DOI] [PubMed] [Google Scholar]
- Gong MC, Fujihara H, Somlyo AV, Somlyo AP. Translocation of rhoA associated with Ca2+ sensitization of smooth muscle. Journal of Biological Chemistry. 1997;272:10704–10709. doi: 10.1074/jbc.272.16.10704. 10.1074/jbc.272.16.10704. [DOI] [PubMed] [Google Scholar]
- Gong MC, Iizuka K, Nixon G, Browne JP, Hall A, Eccleston JF, Sugai M, Kobayashi S, Somlyo AV, Somlyo AP. Role of guanine nucleotide-binding proteins - ras-family or trimeric proteins or both - in Ca2+ sensitization of smooth muscle. Proceedings of the National Academy of Sciences of the USA. 1996;93:1340–1345. doi: 10.1073/pnas.93.3.1340. 10.1073/pnas.93.3.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihara H, Walker LA, Gong MC, Lemichez E, Boquet P, Somlyo AV, Somlyo AP. Inhibition of RhoA translocation and calcium sensitization by In Vivo ADP-ribosylation with the chimeric toxin DC3B. Molecular Biology of the Cell. 1997;8:2437–2447. doi: 10.1091/mbc.8.12.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata K, Kikuchi A, Sasaki T, Kuroda S, Kaibuchi K, Matsuura Y, Seki H, Saida K, Takai Y. Involvement of rho p21 in the GTP-enhanced calcium ion sensitivity of smooth muscle contraction. Journal of Biological Chemistry. 1992;267:8719–8722. [PubMed] [Google Scholar]
- Horiuti K. Mechanism of contracture on cooling of caffeine-treated frog skeletal muscle fibres. The Journal of Physiology. 1988;398:131–148. doi: 10.1113/jphysiol.1988.sp017034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itagaki M, Komori S, Unno T, Syuto B, Ohashi H. Possible involvement of a small G-protein sensitive to exoenzyme C3 of Clostridium botulinum in the regulation of myofilament Ca2+ sensitivity in β-escin skinned smooth muscle of guinea pig ileum. Japanese Journal of Pharmacology. 1995;67:1–7. doi: 10.1254/jjp.67.1. [DOI] [PubMed] [Google Scholar]
- Jiang C, Sarrel PM, Lindsay DC, Poole-Wilson PA, Collins P. Endothelium-independent relaxation of rabbit coronary artery by 17β-oestradiol in vitro. British Journal of Pharmacology. 1991;104:1033–1037. doi: 10.1111/j.1476-5381.1991.tb12545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kitazawa T, Hamada E, Kitazawa K, Gaznabi AKM. Non-genomic mechanism of 17β-oestradiol-induced inhibition of contraction in mammalian vascular smooth muscle. The Journal of Physiology. 1997;499:497–511. doi: 10.1113/jphysiol.1997.sp021944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa T, Masuo M, Somlyo AP. G-protein mediated inhibition of myosin light-chain phosphatase in vascular smooth muscle. Proceedings of the National Academy of Sciences of the USA. 1991;88:9307–9310. doi: 10.1073/pnas.88.20.9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Kitazawa T, Somlyo AV, Somlyo AP. Cytosolic heparin inhibits muscarinic and α-adrenergic Ca2+ release in smooth muscle. Journal of Biological Chemistry. 1989;264:17997–18004. [PubMed] [Google Scholar]
- Kuehl FA, Jr, Ham EA, Zanetti ME, Stanford CH, Nicol SE, Goldberg ND. Estrogen-related increases in uterine guanosine 3′,5′-cyclic monophosphate levels. Proceedings of the National Academy of Sciences of the USA. 1974;71:1866–1870. doi: 10.1073/pnas.71.5.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kureishi Y, Kobayashi S, Amano M, Kimura K, Kanaide H, Nakano T, Kaibuchi K, Ito M. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. Journal of Biological Chemistry. 1997;272:12257–12260. doi: 10.1074/jbc.272.19.12257. 10.1074/jbc.272.19.12257. [DOI] [PubMed] [Google Scholar]
- Loirand G, Cario-Toumaniantz C, Ferrier L, Galmiche JP, Chardin P, Pacaud P. The Rho-related protein Rnd1 inhibits Ca2+ sensitization of rat intestinal smooth muscle. The Journal of Physiology. 1999;515.P:24P. doi: 10.1111/j.1469-7793.1999.0825u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucius C, Arner A, Steusloff A, Troschka M, Hofmann F, Aktories K, Pfitzer G. Clostridium difficile toxin B inhibits carbachol-induced force and myosin light chain phosphorylation in guinea-pig smooth muscle: role of Rho proteins. The Journal of Physiology. 1998;506:83–93. doi: 10.1111/j.1469-7793.1998.083bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky LM, Hall A. Rho: a connection between membrane receptor signalling and the cytoskeleton. Trends in Cellular Biology. 1996;6:304–310. doi: 10.1016/0962-8924(96)10026-x. 10.1016/0962-8924(96)10026-X. [DOI] [PubMed] [Google Scholar]
- Madaule P, Eda M, Watanabe N, Fujisawa, Matsuoka T, Bito H, Ishizaki T, Narumiya S. Role of citron kinase as a target of the small GTPase Rho in cytokinesis. Nature. 1998;394:491–494. doi: 10.1038/28873. 10.1038/28873. [DOI] [PubMed] [Google Scholar]
- Mugge A, Riedel M, Barton M, Kuhn M, Lichten PR. Endothelium independent relaxation of human coronary arteries by 17β-oestradiol in vitro. Cardiovascular Research. 1993;27:1939–1942. doi: 10.1093/cvr/27.11.1939. [DOI] [PubMed] [Google Scholar]
- Niiro N, Nishimura J, Sakihara C, Nakano H, Kanaide H. Upregulation of rho A and rho-kinase mRNAs in the rat myometrium during pregnancy. Biochemical and Biophysical Research Communications. 1997;230:356–359. doi: 10.1006/bbrc.1996.5960. 10.1006/bbrc.1996.5960. [DOI] [PubMed] [Google Scholar]
- Nobes K, Lauritzen I, Mattei MG, Paris S, Hall A, Chardin P. A new member of the Rho family, Rnd1, promotes disassembly of actin filament structures and loss of cell adhesion. Journal of Cell Biology. 1998;141:187–197. doi: 10.1083/jcb.141.1.187. 10.1083/jcb.141.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M, Yasuda-Fukazawa C, Moriishi K, Kato T, Okuda T, Kurokawa K, Takuwa Y. Involvement of rho in GTPγS-induced enhancement of phosphorylation of 20 kDa myosin light chain in vascular smooth muscle cells: inhibition of phosphatase activity. FEBS Letters. 1995;367:246–250. doi: 10.1016/0014-5793(95)00573-r. 10.1016/0014-5793(95)00573-R. [DOI] [PubMed] [Google Scholar]
- Otto B, Steusloff A, Just I, Aktories K, Pfitzer G. Role of Rho proteins in carbachol-induced contractions in intact and permeabilized guinea-pig intestinal smooth muscle. The Journal of Physiology. 1996;496:317–329. doi: 10.1113/jphysiol.1996.sp021687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacaud P, Bolton TB. Relation between muscarinic receptor cationic current and internal calcium in guinea-pig jejunal smooth muscle cells. The Journal of Physiology. 1991;441:477–499. doi: 10.1113/jphysiol.1991.sp018763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Itoh T. Effect of calyculin A on tension and myosin phosphorylation in skinned smooth muscle of the rabbit mesenteric artery. British Journal of Pharmacology. 1993;109:703–712. doi: 10.1111/j.1476-5381.1993.tb13631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai A, Bialojan C, Troschka M, Rüegg JC. Smooth muscle myosin phosphatase inhibition and force enhancement by sponge toxin. FEBS Letters. 1987;217:81–84. doi: 10.1016/0014-5793(87)81247-4. 10.1016/0014-5793(87)81247-4. [DOI] [PubMed] [Google Scholar]
- Tapon N, Hall A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Current Opinion in Cell Biology. 1997;9:86–92. doi: 10.1016/s0955-0674(97)80156-1. 10.1016/S0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes and Development. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- Wu X, Somlyo AV, Somlyo AP. Cyclic GMP-dependent stimulation reverses G-protein-coupled inhibition of smooth muscle myosin light chain phosphatase. Biochemical and Biophysical Research Communications. 1996;220:658–663. doi: 10.1006/bbrc.1996.0460. 10.1006/bbrc.1996.0460. [DOI] [PubMed] [Google Scholar]
- Yokoyama K, Gelb MH. Purification of a mammalian protein geranylgeranyltransferase. Journal of Biological Chemistry. 1993;268:4055–4060. [PubMed] [Google Scholar]
- Yokoyama K, Zimmerman K, Scholten J, Gelb MH. Differential prenyl pyrophosphate binding to mammalian protein geranyltransferase-I and protein farnesyltransferase and its consequence on the specificity of protein prenylation. Journal of Biological Chemistry. 1997;272:3944–3952. doi: 10.1074/jbc.272.7.3944. 10.1074/jbc.272.7.3944. [DOI] [PubMed] [Google Scholar]


