Abstract
Altered expression of proteins of the fibrinolytic and coagulation cascades in obesity may contribute to the cardiovascular risk associated with this condition. We previously reported that plasminogen activator inhibitor 1 (PAI-1) is dramatically up-regulated in the plasma and adipose tissues of genetically obese mice. This change may disturb normal hemostatic balance and create a severe hypofibrinolytic state. Here we show that tissue factor (TF) gene expression also is significantly elevated in the epididymal and subcutaneous fat pads from ob/ob mice compared with their lean counterparts, and that its level of expression in obese mice increases with age and the degree of obesity. Cell fractionation and in situ hybridization analysis of adipose tissues indicate that TF mRNA is increased in adipocytes and in unidentified stromal vascular cells. Transforming growth factor β (TGF-β) is known to be elevated in the adipose tissue of obese mice, and administration of TGF-β increased TF mRNA expression in adipocytes in vivo and in vitro. These observations raise the possibility that TF and TGF-β may contribute to the increased cardiovascular disease that accompanies obesity and related non-insulin-dependent diabetes mellitus, and that the adipocyte plays a key role in this process. The recent demonstration that TF also influences angiogenesis, cell adhesion, and signaling suggests that its exact role in adipose tissue physiology/pathology, may be complex.
Vascular hemostasis is achieved because of the exquisitely regulated interaction and balance between the coagulation and fibrinolytic systems, and imbalances in either system may lead to thrombotic or hemorrhagic problems (1–6). Obesity and related non-insulin-dependent diabetes mellitus (NIDDM) are among the most common health problems in industrialized societies and are associated with increased incidence of thrombosis and accelerated atherosclerosis (7, 8). Interestingly, a number of studies demonstrate dysregulation of both the coagulation and fibrinolytic systems in obesity/NIDDM (9–12) and suggest that these changes may contribute to the cardiovascular complications in these disorders. In this regard, several studies have shown an increase in tissue factor (TF)-mediated coagulation and/or in factor VII activity/antigen in obese and NIDDM patients (4, 10, 13–18). TF is the major cellular initiator of the coagulation cascade, and also serves as a cell-surface receptor for the activation of factor VII (5, 6). Activation of the coagulation cascade by aberrant expression of TF has been suggested to promote the thrombotic episodes in patients with a variety of clinical disorders, including Gram-negative sepsis (19) and atherosclerosis (20, 21), as well as adult respiratory distress syndrome, systemic lupus erythematosus, Crohn’s disease, rheumatoid arthritis, and various forms of cancer (22). TF is expressed in human atherosclerotic plaques and may play a significant role in the thrombotic complications associated with plaque rupture (20–22). These observations demonstrate that an increase in TF in obesity and associated NIDDM could promote the development of a hypercoagulable state and thus contribute to the cardiovascular complications associated with obesity. In spite of this, very little is known about TF expression in obesity or whether it is elevated in this condition.
Plasma plasminogen activator inhibitor 1 (PAI-1) is also elevated in human obesity (23–26), and we have made considerable progress in understanding the tissue and cellular origin of this increase by studying genetically obese (ob/ob) mice. The ob/ob mice cannot produce leptin (27), and as a consequence are associated with early onset obesity and severe insulin resistance (28). Our studies showed that plasma PAI-1 levels in obese mice were 5-fold higher than that of their lean counterparts (29), and that this increase seemed to reflect increased expression of PAI-1 by the adipocyte itself in response to chronically elevated levels of tumor necrosis factor α (TNF-α), insulin, and transforming growth factor-β [TGF-β (29–33)]. In this report, we employ this same model system to determine whether adipocytes also express TF, and if so, to ask whether TF gene expression is elevated in the adipose tissues from obese compared with lean mice. Our results demonstrate that TF gene expression is significantly elevated in the adipose tissue of obese mice, that it is localized to adipocytes and other unidentified stromal vascular cells, and that this increase may be regulated by TGF-β. The coordinate increase in TF and PAI-1 would be expected to increase coagulation and impair fibrinolysis thereby promoting a state that favors thrombosis.†
MATERIALS AND METHODS
Animals and Tissue Preparation.
Adult male CB6 mice (BALB/c/ByJ × C57B16/J), weighing 25–30 g, were obtained from the Scripps Rodent Breeding Colony (La Jolla, CA), whereas adult male obese mice (C57BL/6J ob/ob) aged 3–6 months and their lean counterparts (C57BL/6J +/?) were obtained from The Jackson Laboratories. CB6 mice were injected i.p. with the indicated amount of human recombinant TGF-β (Sigma) dissolved in 4 mM HCl containing 0.1% BSA or with recombinant murine TNF-α (4 μg/mouse in saline; kind gift of Richard Ulevitch, The Scripps Research Institute). Control mice were injected with an equivalent amount of 4 mM HCl/0.1% BSA or saline, respectively. For in vivo insulin experiments, lean mice (C57BL/6J +/?) were injected i.p. with 10 units of regular human insulin (Humulin R; Eli Lilly), whereas the controls were injected with an equivalent volume of saline alone. At the conclusion of each experiment, mice were anesthetized by metofane (Pitman-Moore, Mundelein, IL), and various tissues were removed and processed for in situ hybridization analysis and preparation of total RNA as described (30).
Quantitative Reverse Transcription–PCR (RT-PCR).
The concentrations of TF mRNA were determined by quantitative RT-PCR by using a competitor cRNA containing upstream and downstream primers for TF and β-actin (internal control) as described (34–36). After reverse transcription (by using 105 molecules of cRNA for TF and 107 molecules for β-actin, optimized in previous preliminary experiments) and PCR by using 32P end-labeled 5′ primers, 20 μl of the PCR products were electrophoresed on 2.5% agarose gels. The appropriate bands corresponding to the internal standard cRNA product and the target mRNA product were excised from the gel, and the incorporated radioactivity was quantified by using a scintillation counter. A standard curve for the internal control cRNA was constructed and used to determine the specific activity of the target mRNA as described (30, 35). Variations in sample loading were assessed by comparison to β-actin mRNA.
Riboprobe Preparation and in Situ Hybridization.
A subclone containing 821 bp of the mouse TF cDNA (nt 229–1,049) cloned into the vector pGEM-3Z (37) was used to prepare a riboprobe for in situ hybridization (38). This vector was linearized and used as a template for in vitro transcription of radiolabeled antisense or sense riboprobes employing SP6 or T7 RNA polymerase, respectively, in the presence of [35S]UTP (>1200 Ci/mmol; Amersham). Both sense and antisense probes were routinely labeled to specific activities between 0.5 and 2 × 108 cpm/mg RNA. Slides were exposed in the dark at 4°C for 4–12 weeks.
Tissue Digestion and Cell Fractionation.
Epididymal fat pads were isolated from mice by dissection, washed in sterile PBS, minced, and washed in Krebs–Ringer bicarbonate (KRB) buffer (pH 7.4) containing 4% albumin and 5 mM glucose (39). The tissues were incubated with collagenase (2 mg/ml; Sigma) on a shaking platform at 37°C for 1 hour. Undigested tissue was removed with forceps, and the adipocytes were then separated from other cells by their ability to float upon low speed (200 × g) centrifugation. The medium below the adipocyte layer was centrifuged at 500 × g for 10 min to obtain the stromal vascular fraction, and the resulting pellet was washed three times with warm KRB buffer. Total RNA was extracted from the two fractions and the amount of TF, or β-actin mRNA associated with each, was determined by quantitative RT-PCR as described above.
Cell Culture.
Mouse 3T3-L1 cells were obtained from the American Type Culture Collection. The culturing of these cells and their differentiation from preadipocytes to mature adipocytes was carried out as described (31, 40). Total RNA was isolated from untreated mature adipocytes and from mature adipocytes treated with TGF-β (1 ng/ml) for 3 h.
RESULTS
TF mRNA Levels in Adipose Tissues from Lean and Obese Mice.
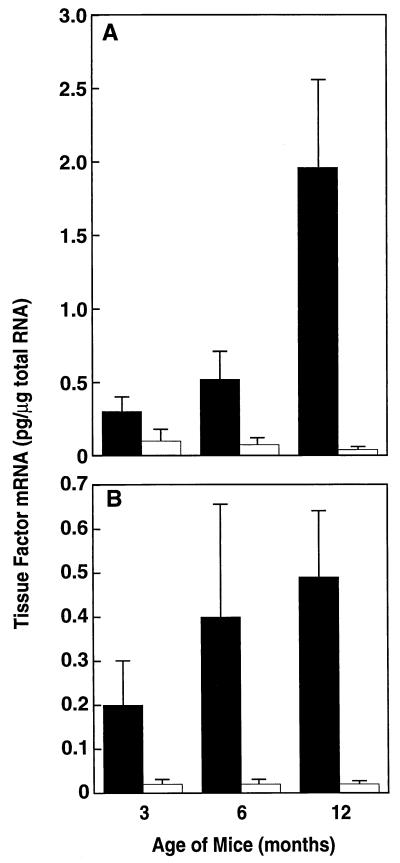
Experiments were performed to compare the level of TF gene expression in adipose tissues from lean and obese mice. Adipose tissues were removed from 3-, 6-, and 12-month-old lean and ob/ob mice, and total RNA was prepared and analyzed for TF mRNA by quantitative RT-PCR (Fig. 1). TF mRNA levels were elevated in both the epididymal (Fig. 1A) and subcutaneous (Fig. 1B) fat of ob/ob mice when compared with their lean counterparts (see legend for P values). The level of TF mRNA increased with age and the degree of obesity in the adipose tissue of ob/ob mice, but not in the adipose tissue from lean mice. For example, when compared with the 3-month-old mice, TF expression was significantly elevated in the adipose tissue of both 6-month-old (P < 0.01 for epididymal fat; P < 0.05 for subcutaneous fat) and 12-month-old (P < 0.003 for epididymal fat; P < 0.002 for subcutaneous fat) obese mice. TF expression was also significantly increased (P < 0.002) in the epididymal fat of 12-month-old obese mice when compared with that of the 6-month-old mice. However, TF mRNA was not significantly elevated in the subcutaneous fat of 12-month-old mice when compared with the 6-month-old mice. This specific elevation of TF mRNA in the epididymal fat may be physiologically important because in humans, cardiovascular risk is most often connected with android obesity (41).
Figure 1.
Expression of TF mRNA in adipose tissue from lean and obese mice. Total RNA was extracted from epididymal (A) or subcutaneous (B) fat of male lean (□) and obese (▪) animals of the indicated ages. TF mRNA was determined by using quantitative RT-PCR analysis. For each condition, n = 6 ± SD. Comparison of the epididymal fat from 3-month-old lean and obese mice by using the unpaired Student’s t test indicated P < 0.036; for 3-month-old lean vs. obese subcutaneous fat, P < 0.031; for 6-month-old lean vs. obese epididymal fat, P < 0.001; for 6-month-old lean vs. obese subcutaneous fat, P < 0.02; for 12-month-old lean vs. obese epididymal fat, P < 0.004; for 12-month-old lean vs. obese subcutaneous fat, P < 0.005.
Cellular Localization of TF mRNA in Tissues from Obese and Lean Mice.
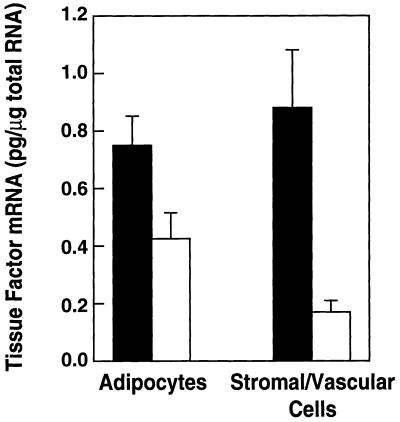
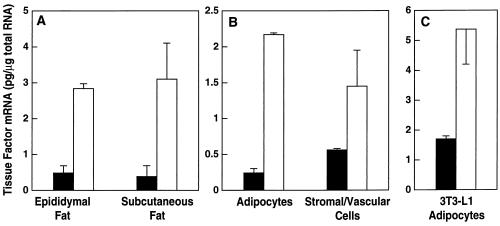
Cell fractionation and in situ hybridization experiments were performed to identify the cell types in the adipose tissue expressing TF mRNA. For the cell fractionation experiments, adipose tissues from lean and obese mice were digested with collagenase and then subjected to differential centrifugation to separate the mature adipocytes from the stromal vascular cells (39). The amount of TF mRNA associated with the two cell fractions was then determined by RT-PCR. Fig. 2 shows that TFmRNA was elevated in mature adipocytes (P < 0.04) from stromal vascular cells (P < 0.005) from obese mice. The stromal vascular cell fraction contains many cell types, including endothelial cells, smooth muscle cells, fibroblasts, mast cells, macrophages, and immature adipocytes. Analysis of the cells in the stromal vascular fraction by in situ hybridization together with Oil Red O staining for lipids shows that some of the TF hybridization signal in this fraction resulted from contaminating smaller adipocytes (data not shown).
Figure 2.
Expression of TF mRNA in the adipocyte and stromal vascular fraction of adipose tissue from lean and obese mice. Epididymal fat pads were isolated from 6-month-old male lean (□) and ob/ob (▪) mice. Adipocytes and stromal vascular cells were separated by collagenase digestion followed by differential centrifugation. Total RNA was extracted from each fraction and TF mRNA levels were quantified by RT-PCR (n = 3 ± SD). Comparison of the results of lean and obese adipocytes by using the unpaired Student’s t test reveal P < 0.04; for lean and obese stromal vascular cells, P < 0.05.
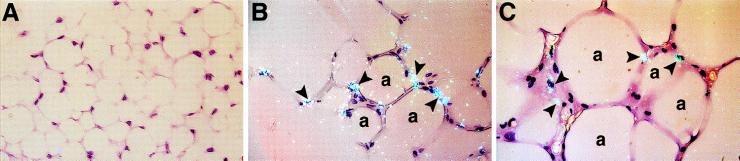
In situ hybridization analysis of paraffin embedded adipose tissues from lean mice showed no TF positive cells (Fig. 3A) or only occasional positive cells (for example, see Fig. 5A). Quantitation of these results by counting 1,000 cells on slides prepared from three different mice demonstrated that ≈2% of the cells expressed detectable TF mRNA. However, a significantly higher percentage of positive cells were detected in the 3-month-old (Fig. 3B) and 6-month-old (Fig. 3C) obese adipose tissues (16.3 ± 5% and 20 ± 6.2%, respectively). Many of the positive cells morphologically resemble adipocytes consistent with the cell fractionation (Fig. 2) and cell culture (Fig. 4C) data. Because of the relatively large signal in the stromal vascular fraction (Fig. 2), the possibility that TF may also be expressed by other cell types in the adipose tissue cannot be excluded. This question is currently under investigation.
Figure 3.
Cellular localization of TF mRNA in adipose tissue from lean and obese (ob/ob) mice. In situ hybridization was performed on paraffin embedded adipose tissues from lean mice (A), and from 3-month-old (B), and 6-month-old (C) obese mice by using 35S-labeled TF riboprobes as described. Representative sections are shown. Arrowheads indicate examples of positive hybridization signals. a, Adipocyte. Slides were exposed for 10 weeks at 4°C and stained with hematoxylin/eosin. (×400.)
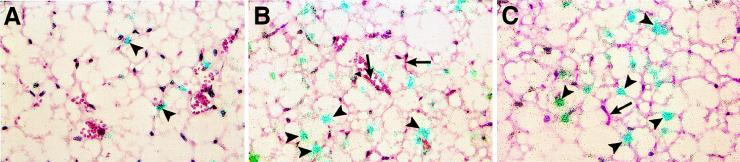
Figure 5.
Effect of TGF-β on the cellular distribution of TF mRNA in the adipose tissue of CB6 mice. In situ hybridization was performed on paraffin sections of adipose tissues from untreated mice (A) and from mice treated i.p. with TGF-β (2 μg) for 3 h (B and C). Slides were exposed for 10 weeks at 4°C and stained with hematoxylin/eosin. Arrowheads indicate positive cells, and arrows in B and C indicate endothelial cells in small vessels or capillaries. (×400.)
Figure 4.
Induction of TF mRNA expression in adipose tissue and adipocytes by TGF-β. (A) Six to 8-week-old male lean CB6 mice were injected i.p. with 2 μg of human recombinant TGF-β (□) or diluent (▪), and 3 h later, the adipose tissues were removed and analyzed for TFmRNA. Total RNA was prepared and analyzed for TF gene expression by quantitative RT-PCR (n = 3 ± SD). Comparison of TF mRNA levels in epididymal fat from control vs. TGF-β-treated mice using the unpaired Student’s t test reveal P < 0.01; for subcutaneous fat from control vs. TGF-β-treated mice, P < 0.01. (B) Mature adipocytes and stromal vascular cells were separated by differential centrifugation, total RNA was prepared, and TF mRNA levels were determined (n = 3 ± SD). Comparison of TF mRNA levels in adipocytes from control and TGF-β-treated mice reveal P < 0.0006; for control vs. TGF-β-treated stromal vascular cells, P < 0.1. (C) 3T3-L1 adipocytes were grown and differentiated in 6-well tissue culture plates as described (40). Total RNA was isolated from untreated cells (▪), and cells treated with 1 ng/ml TGF-β (□) for 3 h, and steady-state levels of TF mRNA were determined by using quantitative RT-PCR (n = 6 ± SD).
In Vivo Regulation of TF mRNA in Adipose Tissue.
Because of the compensatory hyperinsulinemia that accompanies obesity (42), insulin is systemically increased in the plasma of ob/ob mice. Interestingly, TNF-α and TGF-β also appear to be elevated locally in the adipose tissues of these mice (31, 32). All three of the above mediators induce PAI-1 in the adipose tissue (29–31, 43), and each may thus contribute to the cardiovascular complications associated with obesity. We therefore determined the effect of these agents on TF gene expression in adipose tissue from lean mice. Lean mice (CB6 or C57BL/6J +/?) were injected i.p. with either vehicle alone or vehicle containing 4 μg of TNF-α, 2 μg of TGF-β, or 10 units of insulin. Tissues were removed 3 h later, and total RNA was prepared and analyzed for TF mRNA by quantitative RT-PCR. The dose of each mediator employed and the tissue harvesting times used in this experiment were based on the optimum doses and times obtained for the induction of PAI-1 in previous studies (30, 31, 44). In these experiments, TNF-α increased TF mRNA expression in the adipose tissue (maximum induction of 3-fold), and insulin showed a 1.5- to 2-fold increase (data not shown). However, TGF-β was the most potent inducer of TF mRNA in the adipose tissue, increasing it by 6- to 8-fold in both the epididymal as well as in the subcutaneous adipose tissue (Fig. 4A). Cell fractionation experiments were performed to begin to identify the cell type(s) expressing elevated TF levels in response to TGF-β treatment. Adipose tissue was again subjected to differential centrifugation to separate mature adipocytes from the stromal/vascular cells, and the concentration of TF mRNA in the two cell fractions was determined by RT-PCR. Fig. 4B shows that TGF-β induced TF expression primarily in mature adipocytes, although there was also induction in the stromal vascular cells. In this regard, TGF-β also induced TF gene expression in 3T3-L1 adipocytes cultured in vitro (Fig. 4C). In situ hybridization analysis (Fig. 5) revealed the presence of low numbers of TF-positive cells (6.1 ± 2.5%) in untreated control adipose tissues from normal CB6 mice (Fig. 5A). However, within 3 h after TGF-β treatment, a strong positive signal was observed in 40 ± 9% of cells that morphologically resembled adipocytes (Fig. 5 B and C). Endothelial cells (both large vessel and microvascular) did not appear to be induced by TGF-β to express TF (arrow in Fig. 5 B and C).
DISCUSSION
Obesity is frequently accompanied by related metabolic disorders such as hypertriglyceridemia, hyperinsulinemia, insulin resistance, and hypertension (8, 9, 45). It is also a pathological condition of the adipose tissue that is associated with increased risk for thrombosis (7, 8) and cardiovascular disease (7, 46, 47). In this regard, abnormalities in the coagulation and fibrinolytic systems of obese/NIDDM patients have been documented (13–18, 48), and a number of prospective studies implicate increases in plasma concentrations of factor VII, fibrinogen, and PAI-1 (49, 50). Although factor VII increases in the plasma of obese individuals, little information is available about whether TF, the cellular receptor for factor VII/VIIa and the primary initiator of the coagulation cascade (51), is also elevated. There is considerable precedent for this possibility because thrombotic episodes associated with other diseases (e.g., atherosclerosis, septic shock and cancer) are often correlated with increased expression of TF (22).
In previous studies, we demonstrated that the ob/ob mouse is a potentially useful model of human obesity because it provided insights into abnormal PAI-1 gene expression in this condition (29–31). The experiments described in the current study were undertaken to determine whether genetically obese mice could be used to determine whether TF gene expression is also altered in obesity/NIDDM. Our data show that the adipose tissue itself is a potent source of TF (Fig. 1), and that its expression is elevated in adipocytes and unidentified stromal vascular cells in adipose tissue of obese mice (Figs. 1–3). Whether elevated TF mRNA in the obese adipose tissues actually leads to a systemic hypercoagulable state in the blood remains to be determined. Although we have not systematically quantified TF protein levels in adipose tissues, the following considerations suggest that expression of murine TF mRNA is an indicator of TF activity. First, previous studies appear to correlate TF mRNA levels in various tissues with amounts of procoagulant activity (37). Moreover, in preliminary studies, clotting assays were performed by using human plasma and tissue extracts from murine adipose tissue. These studies demonstrated a 2- to 3-fold faster clotting time when adipose tissue extracts from obese mice were compared with those from the lean mice (data not shown). Finally, hypercoagulable states caused by shedding of TF-rich microvesicles from cell surfaces have been demonstrated in cancer (52), dessimionated intravascular coagulation (53, 54), collagen disease, diabetic microangiopathy, and chronic renal failure (55). Plasma TF activity was also observed in diabetes mellitus patients, with the concentrations being significantly higher in patients with retinopathy or nephropathy than in patients with no complications (56).
Experiments were performed to identify potential mechanisms that contribute to the chronically elevated levels of TF associated with the adipose tissues of obese mice. The ob/ob mice are not only insulin resistant and hyperinsulinemic (42), but, as in human obesity, TNF-α (32) and TGF-β (31) are also chronically elevated in the adipose tissues of these mice. Each of these agents has been reported to induce TF expression in vivo and/or in vitro (51, 57–60). We thus asked whether TF expression in the adipose tissue could be induced by these cytokines. Intraperitoneal administration of insulin or TNF-α increased TF mRNA in adipose tissue by 1.5- and 3-fold, respectively (data not shown), whereas TGF-β increased it by 6- to 8-fold (Fig. 4A). Again, the major cell type responding to TGF-β was the adipocyte (Figs. 4B and 5). In this regard, 3T3-L1 adipocytes in culture, also responded to TGF-β (Fig. 4C), TNF-α and insulin (data not shown) with increased expression of TF mRNA. Induction of TF gene expression and TF procoagulant activity by TGF-β was previously reported in mouse fibroblasts (59). In that same study, insulin also was reported to cause a weak induction of TF gene expression and procoagulant activity (59). In previous studies from our laboratory, TGF-β was the major inducer of PAI-1 in adipocytes (31). Thus TGF-β may be a potent regulator of abnormal gene expression in adipocytes. TGF-β may also influence the normal physiology and disease of adipose tissue. For example, obese transgenic mice over expressing TGF-β develop a lipodystrophy-like syndrome with severe fibrosis in the white adipose tissue (61).
We did not observe expression of TF mRNA in large vessel endothelial cells in adipose tissues from control, insulin-, TNF-α-, or TGF-β-treated lean or obese mice (for example, see Fig. 5). Whether capillary endothelial cells express TF is currently under investigation. Although in vitro studies have demonstrated inducible expression of TF activity in cultured endothelial cells in response to a range of mediators (51, 58), most in vivo studies have failed to detect TF in endothelial cells (20, 37, 58, 62). One exception appears to occur in the baboon during lethal Escherichia coli sepsis (63). In this model, TF expression was observed in endothelial cells of the spleen but not in endothelial cells of the lung (63). These results suggest that TF expression by endothelial cells in vivo may be influenced by the local environment. In this respect, TF expression was also observed in vascular endothelial cells within tumors of patients with invasive breast cancer, but not in benign tumors (64).
At present, we can only speculate about the physiologic and/or pathologic significance of TF gene expression in adipose tissues. Tissue factor mRNA and/or antigen was previously localized to the epidermis, astroglia, bronchial epithelia, mucosal epithelial layers, and adventitia of large vessels (51). This pattern of distribution in vivo suggests that TF forms a hemostatic envelope that activates the coagulation system when vascular integrity is disrupted (6, 22, 65). The adipose tissue is a highly vascularized organ, and the adipocytes appear to be in intimate contact with capillary vascular beds (66, 67). Adequate blood flow to and from the adipocyte is a critical component for the storage of lipids and for its utilization as an energy source. Under these circumstances, the integrity of the microcirculation is important and TF may help maintain this integrity in the adipose tissue. This function may be more important in obese conditions, where the adipocytes tend to be more fragile because of the several-fold increase in their size and mass. In this regard and as already mentioned, the adipose tissues of obese mice express considerably higher levels of TF mRNA than their lean counterparts (Fig. 1).
Although TF may contribute to the hypercoagulable state observed in obese and insulin-resistant patients, a local nonhemostatic function for TF in adipose tissue physiology should also be considered. In this regard, several studies have suggested that TF may have other functions apart from its role in coagulation (3). For example, it has been implicated in cellular signaling (68, 69) and in the maintenance of vascular integrity and/or vascular development as demonstrated by the embryonic lethality in TF knockout mice (70–72). Unlike most other tissues, the adipose tissue has the ability to grow throughout most of adult life. In situations such as obesity, where pathological growth of the adipose tissue occurs, it is generally accepted that angiogenesis must also take place. Although adipose tissue angiogenic growth factors have been identified in adipose tissue biopsies from obese humans and in differentiating adipocytes (73, 74), the mechanism of angiogenesis in severe obesity is largely unknown. Interestingly, a role for TF in tumor angiogenesis was demonstrated recently (75). In these studies, overexpression of an antisense TF construct in meth-A sarcoma cells not only prevented the tumor cells from developing TF procoagulant activity but also caused a marked reduction in their ability to secrete vascular endothelial growth factor in vitro or in vivo. Moreover, the tumors appeared to have a markedly reduced angiogenic response when implanted in immunodeficient mice. These observations support the speculation that TF may play a role in angiogenesis in the adipose tissue of obese mice.
Finally, the potential role of the adipocyte in the control of hemostatic balance in obesity/NIDDM must be considered. Besides TF, these cells appear to synthesize and secrete abnormally high levels of PAI-1 (29, 31, 76–78). The elevated PAI-1 and TF may simultaneously compromise normal fibrin clearance mechanisms and lead to a procoagulant state. These observations raise the possibility that increased coagulation and impaired fibrinolysis may contribute to cardiovascular risk in this condition. The potential importance of these changes is emphasized even more when the dramatic expansion of the adipose tissue in obesity is considered. This situation is, in many respects, similar to the growth of large tumors that may eventually alter the composition of blood. In this regard, adipocytes appear to be true secretory cells. Besides PAI-1 and TF, they have been reported to secrete a number of molecules into blood, including TNF-α (32, 33), leptin (27), components of the complement system (79, 80), angiotensinogen (81), lipoprotein lipase (82, 83), apolipoprotein E (84), and cholesterol ester transfer protein (85). Thus, the adipocyte secretes a variety of proteins into blood that may influence normal hemostatic balance. The abnormally high levels of some of these proteins may directly contribute to the thrombotic and cardiovascular problems associated with obesity.
In summary, the mechanisms that promote hemostatic imbalance in obese and diabetic conditions are obviously complex and may involve the dysregulation of several genes of the coagulation and fibrinolytic cascades. Moreover, obesity itself, whether caused by the lack of leptin as in the ob/ob mouse or by other means such as diet, is associated with the derangement of multiple metabolic pathways. Leptin itself is known to alter a variety of metabolic processes (86). Thus, the observed changes in TF may be an epistatic effect caused by the absence of leptin rather than by obesity per se. The fact that the amount of TF mRNA increases as the animals become more obese (Fig. 1) supports the hypothesis that it is obesity per se that leads to elevated TF expression in this model. Obviously, additional studies of multiple obesity models will help to clarify this problem. Whatever the mechanism, our data clearly demonstrate that TF is made in the adipose tissue, that the adipocyte itself is one source of this protein, and that TF gene expression by adipocytes is stimulated by TGF-β and is elevated in adipose tissue of obese mice. Moreover, these results raise the possibility that TF may also play a complex role in the physiology and/or pathology of the adipose tissue itself.
Acknowledgments
We thank T. Thinnes for her excellent technical assistance and M. McRae for her expert secretarial skills. This work was supported in part by National Institutes of Health Grant HL 47819, and in part by a grant from Norvatis. This is manuscript number 10891 VB from the Department of Vascular Biology.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: PAI-1, plasminogen activator inhibitor 1; TF, tissue factor; TGF-β, transforming growth factor β; NIDDM, non-insulin-dependent diabetes mellitus; TNF-α, tumor necrosis factor α; RT-PCR, reverse transcription–PCR.
This work was presented in part at the 1997 Gordon Conference on Atherosclerosis, June 15–20, Kimball Union Academy, Meriden, NH.
References
- 1.Loskutoff D J, Samad F. Arterioscler Thromb Vasc Biol. 1998;18:1–6. doi: 10.1161/01.atv.18.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P, Stassen J M, Schoonjans L, Ream B, Van den Oord J J, De Mol M, Mulligan R C, Collen D. J Clin Invest. 1993;92:2756–2760. doi: 10.1172/JCI116893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osterud B. Thromb Haemostasis. 1997;78:755–758. [PubMed] [Google Scholar]
- 4.Meade T W, Ruddock V, Stirling Y, Chakrabarti T, Miller G J. Lancet. 1993;342:1076–1079. doi: 10.1016/0140-6736(93)92062-x. [DOI] [PubMed] [Google Scholar]
- 5.Bach R R. CRC Crit Rev Biochem. 1988;23:339–368. doi: 10.3109/10409238809082548. [DOI] [PubMed] [Google Scholar]
- 6.Edgington T S, Mackman N, Brand K, Ruf W. Thromb Haemostasis. 1991;66:67–79. [PubMed] [Google Scholar]
- 7.Larson B. Int J Obes. 1991;15:53–57. [PubMed] [Google Scholar]
- 8.Björntorp P. Ann Med. 1992;24:15–18. doi: 10.3109/07853899209164140. [DOI] [PubMed] [Google Scholar]
- 9.Juhan-Vague I, Alessi M C. Thromb Haemostasis. 1997;78:656–660. [PubMed] [Google Scholar]
- 10.Juhan-Vague I, Alessi M C, Vague P. Ann Med. 1996;28:371–380. doi: 10.3109/07853899608999095. [DOI] [PubMed] [Google Scholar]
- 11.Ceriello A. Diabetologia. 1993;36:1119–1125. doi: 10.1007/BF00401055. [DOI] [PubMed] [Google Scholar]
- 12.Juhan-Vague I, Vague P. Am J Obstet Gynecol. 1990;163:313–315. doi: 10.1016/0002-9378(90)90573-p. [DOI] [PubMed] [Google Scholar]
- 13.Chan P, Lin T H, Pan W H, Lee Y H. Int J Obes Related Metab Disorders. 1995;19:756–759. [PubMed] [Google Scholar]
- 14.Licata G, Scaglione R, Avellone G, Ganguzza A, Corrao S, Arnone S, Di Chiara T. Metabolism Clin Exp. 1995;44:1417–1421. doi: 10.1016/0026-0495(95)90140-x. [DOI] [PubMed] [Google Scholar]
- 15.Avellone G, Di Garbo V, Cordova R, Rotolo G, Abruzzese G, Raneli G, De Simone R, Bompiani G D. Diabetes Res. 1994;25:85–92. [PubMed] [Google Scholar]
- 16.Matsuda T, Morishita E, Jokaji H, Asakura H, Saito M, Yoshida T, Takemoto K. Diabetes. 1996;45:S109–S110. doi: 10.2337/diab.45.3.s109. [DOI] [PubMed] [Google Scholar]
- 17.Mansfield M W, Heywood D M, Grant P J. Arterioscler Thromb Vasc Biol. 1996;16:160–164. doi: 10.1161/01.atv.16.1.160. [DOI] [PubMed] [Google Scholar]
- 18.Kario K, Matsuo T, Kobayashi H, Matsuo M, Sakata T, Miyata T. Arterioscler Thromb Vasc Biol. 1995;15:1114–1120. doi: 10.1161/01.atv.15.8.1114. [DOI] [PubMed] [Google Scholar]
- 19.Osterud B, Flaegstad T. Thromb Haemostasis. 1983;49:5–7. [PubMed] [Google Scholar]
- 20.Wilcox J N, Smith K M, Schwartz S M, Gordon D. Proc Natl Acad Sci USA. 1989;86:2839–2843. doi: 10.1073/pnas.86.8.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taubman M B, Fallon J T, Schecter A D, Giesen P, Mendlowitz M, Fyfe B S, Marmur J D, Nemerson Y. Thromb Haemostasis. 1997;78:200–204. [PubMed] [Google Scholar]
- 22.Semeraro N, Colucci M. Thromb Haemostasis. 1997;78:759–764. [PubMed] [Google Scholar]
- 23.McGill J B, Schneider D J, Arfken C L, Lucore C L, Sobel B E. Diabetes. 1994;43:104–109. doi: 10.2337/diab.43.1.104. [DOI] [PubMed] [Google Scholar]
- 24.Potter van Loon B J, Kluft C, Radder J K, Blankenstein M A, Meinders A E. Metabolism. 1993;42:945–949. doi: 10.1016/0026-0495(93)90005-9. [DOI] [PubMed] [Google Scholar]
- 25.Legnani C, Maccaferri M, Tonini P, Cassio A, Cacciari E, Coccheri S. Fibrinolysis. 1988;2:211–214. [Google Scholar]
- 26.Vague P, Juhan-Vague I, Chabert V, Alessi M C, Atlan C. Metabolism. 1989;38:913–915. doi: 10.1016/0026-0495(89)90241-2. [DOI] [PubMed] [Google Scholar]
- 27.Spiegelman B M, Flier J S. Cell. 1996;87:377–389. doi: 10.1016/s0092-8674(00)81359-8. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J M. Nature (London) 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 29.Samad F, Loskutoff D J. Mol Med. 1996;2:568–582. [PMC free article] [PubMed] [Google Scholar]
- 30.Samad F, Yamamoto K, Loskutoff D J. J Clin Invest. 1996;97:37–46. doi: 10.1172/JCI118404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samad F, Yamamoto K, Pandey M, Loskutoff D. Mol Med. 1997;3:37–48. [PMC free article] [PubMed] [Google Scholar]
- 32.Hotamisligil G S, Shargill N S, Spiegelman B M. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 33.Hotamisligil G S, Arner P, Caro J F, Atkinson R L, Spiegelman B M. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang A M, Doyle M V, Mark D F. Proc Natl Acad Sci USA. 1989;86:9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto K, Loskutoff D J. J Clin Invest. 1996;97:2440–2451. doi: 10.1172/JCI118691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanden Heuvel J P, Tyson F L, Bell D A. BioTechniques. 1993;14:395–398. [PubMed] [Google Scholar]
- 37.Mackman N, Sawdey M S, Keeton M R, Loskutoff D J. Am J Pathol. 1993;143:76–84. [PMC free article] [PubMed] [Google Scholar]
- 38.Keeton M, Eguchi Y, Sawdey M, Ahn C, Loskutoff D J. Am J Pathol. 1993;142:59–70. [PMC free article] [PubMed] [Google Scholar]
- 39.Rodbell M. J Biol Chem. 1964;239:375–380. [PubMed] [Google Scholar]
- 40.Green H, Kehinde O. Cell. 1975;5:19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- 41.Finer N. Br Med Bull. 1997;53:229–450. doi: 10.1093/oxfordjournals.bmb.a011620. [DOI] [PubMed] [Google Scholar]
- 42.Shafrir E. Diabetes Metab. 1996;22:122–131. [PubMed] [Google Scholar]
- 43.Sawdey M S, Loskutoff D J. J Clin Invest. 1991;88:1346–1353. doi: 10.1172/JCI115440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfeiffer A, Schatz H. Exp Clin Endocrinol Diabetes. 1995;103:7–14. doi: 10.1055/s-0029-1211323. [DOI] [PubMed] [Google Scholar]
- 45.DeFronzo R A, Ferrannini E. Diabetes Care. 1991;14:173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 46.Björntorp P. Ann Med. 1992;24:15–18. doi: 10.3109/07853899209164140. [DOI] [PubMed] [Google Scholar]
- 47.Larsson B. Int J Obes. 1991;15:53–57. [PubMed] [Google Scholar]
- 48.Jude B, Watel A, Fontaine O, Fontaine P, Cosson A. Haemostasis. 1989;19:65–73. doi: 10.1159/000215891. [DOI] [PubMed] [Google Scholar]
- 49.Asplund-Carlson A, Hamsten A, Wiman B, Carlson L A. Diabetologia. 1993;36:817–825. doi: 10.1007/BF00400356. [DOI] [PubMed] [Google Scholar]
- 50.Juhan-Vague I, Roul C, Alessi M C, Ardissone J P, Heim M, Vague P. Thromb Haemostasis. 1989;61:370–373. [PubMed] [Google Scholar]
- 51.Camerer E, Kolsto A B, Prydz H. Thromb Res. 1996;81:1–41. doi: 10.1016/0049-3848(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 52.Kakkar A K, De Ruvo N, Chinswangwatanakul V, Tebbutt S, Williamson R C N. Lancet. 1995;346:1004–1005. doi: 10.1016/s0140-6736(95)91690-3. [DOI] [PubMed] [Google Scholar]
- 53.Lijima K, Fukuda C, Nakamura K. Thromb Res. 1991;61:29–38. doi: 10.1016/0049-3848(91)90166-t. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi H, Satoh N, Wada K, Takakuwa E, Seki Y, Shibata A. Am J Hematol. 1994;46:333–337. doi: 10.1002/ajh.2830460414. [DOI] [PubMed] [Google Scholar]
- 55.Koyama T, Nishida K, Ohdama S, Sawada M, Murakami N, Hirosawa S, Kuriyama R, Matsuzawa K, Hasegawa R, Aoki N. Br J Haematol. 1994;87:343–347. doi: 10.1111/j.1365-2141.1994.tb04919.x. [DOI] [PubMed] [Google Scholar]
- 56.Saito M, Morishita E, Asakura H, Jokaji H, Uotani C, Kumabashiri I, Yamazaki M, Aoshima K, Matsuda T. Jpn J Clin Hematol. 1996;37:794–798. [PubMed] [Google Scholar]
- 57.Mackman N. Thromb Haemostasis. 1997;78:747–754. [PubMed] [Google Scholar]
- 58.Mackman N. FASEB J. 1995;9:883–889. doi: 10.1096/fasebj.9.10.7615158. [DOI] [PubMed] [Google Scholar]
- 59.Ranganathan G, Blatti S P, Subramaniam M, Fass D N, Maihle N J, Getz M J. J Biol Chem. 1991;266:496–501. [PubMed] [Google Scholar]
- 60.Kirchhofer D, Tschopp T B, Hadváry P, Baumgartner H R. J Clin Invest. 1994;93:2073–2083. doi: 10.1172/JCI117202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clouthier D E, Comerford S A, Hammer R E. J Clin Invest. 1997;100:2697–2713. doi: 10.1172/JCI119815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Solberg S, Osterud B, Larsen T, Sorlie D. Blood Coagul Fibrinol. 1990;1:595–600. [PubMed] [Google Scholar]
- 63.Drake T A, Cheng J, Chang A, Taylor F B., Jr Am J Pathol. 1993;142:1458–1470. [PMC free article] [PubMed] [Google Scholar]
- 64.Contrino J, Hair G, Kreutzer D L, Rickles F R. Nat Med. 1996;2:209–215. doi: 10.1038/nm0296-209. [DOI] [PubMed] [Google Scholar]
- 65.Luther T, Flössel C, Mackman N, Bierhaus A, Kasper M, Albrecht S, Sage E H, Iruela-Arispe L, Grossmann H, Ströhlein A, et al. Am J Pathol. 1996;149:101–113. [PMC free article] [PubMed] [Google Scholar]
- 66.Rosell S, Belfrage E. Physiol Rev. 1979;59:1078–1104. doi: 10.1152/physrev.1979.59.4.1078. [DOI] [PubMed] [Google Scholar]
- 67.DiGirolamo M, Espositio J. Am J Physiol. 1975;229:107–112. doi: 10.1152/ajplegacy.1975.229.1.107. [DOI] [PubMed] [Google Scholar]
- 68.Rottingen J A, Enedn T, Camerer E, Iversen J G. J Biol Chem. 1995;270:4650–4660. doi: 10.1074/jbc.270.9.4650. [DOI] [PubMed] [Google Scholar]
- 69.Cuatrecasas P, Wilchek M, Anfinsen C B. Proc Natl Acad Sci USA. 1968;61:636–643. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Toomey J R, Kratzer K E, Lasky N M, Stanton J J, Broze G J., Jr Blood. 1996;88:1583–1587. [PubMed] [Google Scholar]
- 71.Carmeliet P, Mackman N, Moons L, Luther T, Gressens P, Van Vlaenderen I, Demunck H, Kasper M, Breier G, Evrard P, et al. Nature (London) 1996;383:73–75. doi: 10.1038/383073a0. [DOI] [PubMed] [Google Scholar]
- 72.Bugge T H, Xiao Q, Lombrinck K W, Holmback K, Danton M J S, Colbert M C, Witte D P, Fujikawa K, Davie E N, Degen J L. Proc Natl Acad Sci USA. 1996;93:6258–6261. doi: 10.1073/pnas.93.13.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Crandall D L, Herzlinger H E, Cervoni P, Scalea T M, Kral J G. In: Vasoactive Substances in Human Adipose Tissue Biopsies. Ailhaud G, Guy-Grand B, Lafontan M, Riquier D, editors. London: John Libby; 1991. pp. 405–409. [Google Scholar]
- 74.Castellot J J, Karnovsky M J, Spiegelman B M. Proc Natl Acad Sci USA. 1980;77:6007–6011. doi: 10.1073/pnas.77.10.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Y, Deng Y, Luther T, Müller M, Ziegler R, Waldherr R, Stern D M, Nawroth P P. J Clin Invest. 1994;94:1320–1327. doi: 10.1172/JCI117451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shimomura I, Funahashi T, Takahashi M, Maeda K, Kotani K, Nakamura T, Yamashita S, Miura M, Fukuda Y, Takemura K, et al. Nat Med. 1996;2:800–803. doi: 10.1038/nm0796-800. [DOI] [PubMed] [Google Scholar]
- 77.Lundgren C H, Brown S L, Nordt T K, Sobel B E, Fujii S. Circulation. 1996;93:106–110. doi: 10.1161/01.cir.93.1.106. [DOI] [PubMed] [Google Scholar]
- 78.Alessi M C, Peiretti F, Morange P, Henry M, Nalbone G, Juhan-Vague I. Diabetes. 1997;46:860–867. doi: 10.2337/diab.46.5.860. [DOI] [PubMed] [Google Scholar]
- 79.Rosen B S, Cook K S, Yaglom J, Groves D L, Volanakis J E, Damm D, White T, Spiegelman B M. Science. 1989;244:1483–1487. doi: 10.1126/science.2734615. [DOI] [PubMed] [Google Scholar]
- 80.Flier J S, Cook K S, Usher P, Spiegelman B M. Science. 1987;237:405–408. doi: 10.1126/science.3299706. [DOI] [PubMed] [Google Scholar]
- 81.Frederich R C, Jr, Kahn B B, Peach M J, Flier J S. Hypertension. 1995;19:339–344. doi: 10.1161/01.hyp.19.4.339. [DOI] [PubMed] [Google Scholar]
- 82.Ailhaud G, Grimaldi P, Négrel R. Int J Obes. 1992;16:S17–S21. [PubMed] [Google Scholar]
- 83.Olivecrona T, Bergo M, Hultin M, Olivecrona G. Can J Cardiol. 1995;11:73G–78G. [PubMed] [Google Scholar]
- 84.Zechner R, Moser R, Newman T C, Fried S K, Breslow J L. J Biol Chem. 1991;266:10583–10588. [PubMed] [Google Scholar]
- 85.Jiang X C, Moulin P, Quinet E, Goldberg I J, Yacoub L K, Agellon L B, Compton D, Schnitzer-Polokoff R, Tall A R. J Biol Chem. 1991;266:4631–4639. [PubMed] [Google Scholar]
- 86.Kulkarni R N, Wang Z-L, Wang R-M, Hurley J D, Smith D M, Ghatei M A, Withers D J, Gardiner J V, Bailey C J, Bloom S R. J Clin Invest. 1997;100:2729–2736. doi: 10.1172/JCI119818. [DOI] [PMC free article] [PubMed] [Google Scholar]