Abstract
The aim of this study was to inhibit specifically one type of gap junction channel in cells expressing multiple connexins (Cx) using synthetic oligopeptides.
A7r5 cells (an aortic smooth muscle cell line expressing Cx40 and Cx43) were incubated overnight with synthetic oligopeptides (P180-195) corresponding to a segment of the second extracellular loop of Cx43. This segment is different in sequence from the corresponding location in Cx40.
P180-195 (500 μM) decreased cell-to-cell coupling as assessed by dye coupling and dual whole-cell voltage clamp. The decrease in permeability and junctional conductance was caused by selective inhibition of Cx43 gap junction channels. In contrast, overnight incubation of A7r5 cells with oligopeptides corresponding to a segment of the intracellular cytoplasmic tail of Cx43 was without effect.
These results indicate that oligopeptides P180-195 may interact with the extracellular domain of the Cx43 protein, thereby possibly mimicking connexin-connexin binding. This apparently inhibits Cx43 channel activity without disturbing the activity of Cx40 channels.
Experiments with oligopeptides corresponding to the equivalent part of the second extracellular loop of Cx40 (P177-192) pointed towards a selective inhibition of Cx40 channel activity.
Competition assays using synthetic oligopeptides may help to resolve the regulatory properties of gap junction channels in primary cells expressing multiple Cx.
Gap junctions are specialized plasma membrane regions containing channels that provide low-resistance pathways for intercellular communication. Each gap junction channel is formed by the docking of two hemichannels (connexons) located in apposing cell membranes of neighbouring cells. These hemichannels are made up of a group of related proteins, commonly referred to as the connexin (Cx) family. Topological studies suggest that each connexin molecule consists of four transmembrane segments, three cytoplasmic domains and two extracellular loops. Members of the Cx family are differentially expressed in tissues, and one cell type can express several connexins. The diversity of Cx expression suggests that the cell-specific distribution of distinct types of gap junction channels may be an important factor for tissue function (e.g. Gros & Jongsma, 1996; Kumar & Gilula, 1996).
Studies on modulation of gap junctional coupling in primary cells are often complicated by their expression of multiple connexins. Indeed, parallel opposite regulation of the different types of gap junction channels may mask changes in cell coupling induced by physiological agonists. Expression systems for Cx have allowed the study of modulation of distinct gap junction channels (e.g. Bruzzone et al. 1996). However, the regulatory properties observed under these conditions may not reflect the actual gap junction channel behaviour in primary cells. An alternative approach is to block specifically Cx expression with antisense oligodeoxynucleotides (Moore & Burt, 1994). A limitation for applying this approach to primary cells, however, is the long incubation time before the antisense oligodeoxynucleotides exert their effects.
Antibodies raised against extracellular domains of Cx have been shown to inhibit gap junction assembly in Novikoff hepatoma cells (Meyer et al. 1992; Johnson & Meyer, 1993). More recently, it has been shown that synthetic peptides comprising parts of the extracellular loop sequences inhibit gap junction formation between oocytes, embryonic chick heart myoballs and smooth muscle in mesenteric arteries (Dahl et al. 1992, 1994; Warner et al. 1995, Chaytor et al. 1997). Whether these peptides specifically interact with only one type of gap junction channel in cells expressing multiple Cx has not been investigated. However, many of the peptides used in these studies contained highly conserved first or second extracellular loop motifs and are, therefore, expected to act against almost all connexins. To assess whether peptides could confer a selective inhibition of one type of gap junction channel over another, we have studied the effects of synthetic oligopeptides, which comprise a less conserved segment of the second extracellular loop, on Cx43- and Cx40-mediated intercellular communication.
METHODS
Cell culture
Embryonic rat aortic smooth muscle (A7r5) cells (ATCC CRL-1444) and human hepatocarcinoma (SKHep1) cells stably transfected with cDNA encoding either rat Cx43 (SKHep1-Cx43; Kwak et al. 1995) or human Cx40 (SKHep1-Cx40) were maintained in Dulbecco's modification of Eagle's medium supplemented with 10% fetal calf serum, 50 units ml−1 penicillin and 50 μg ml−1 streptomycin. The culture medium used for the SKHep1-Cx43 and SKHep1-Cx40 cells was also supplemented with 400 μg ml−1 G418. Media, sera and antibiotics were purchased from Gibco BRL.
Immunofluorescent labelling of A7r5 cells
Immunofluorescence studies were performed as previously described (Gros et al. 1994). Briefly, cells cultured on glass coverslips were fixed in methanol at -20°C for 5 min. After fixation, cells were rinsed and incubated in 0.2% Triton X-100 in PBS for 1 h and subsequently rinsed with PBS and incubated in 0.5 M NH4Cl in PBS for 15 min. Cells were preincubated with 2% bovine serum albumin (BSA) in PBS for 30 min and then incubated overnight with the primary antibody at the appropriate dilution (1 : 1000 for mouse monoclonal anti-Cx43 (Zymed Laboratories, San Francisco, CA, USA), 3 μg ml−1 for affinity-purified rabbit polyclonal anti-Cx40 (Gros et al. 1994) and 1 : 1000 for anti-smooth muscle actin (SMA) (Sigma)) and 10% normal donkey serum (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) in PBS. After this period, they were rinsed 3 times for 5 min with PBS and incubated for 4 h with secondary antibody (1 : 100 Texas Red-conjugated donkey-anti-rabbit IgG or 1 : 100 fluorescein isothiocyanate (FITC)-conjugated donkey-anti-mouse IgG (Jackson ImmunoResearch Laboratories)) followed by rinsing steps as described above. All steps were performed at room temperature (20-22°C). Coverslips were mounted on slides in Vectashield (Vector Laboratories, Burlingame, CA, USA) to reduce photobleaching. Cells were examined with a Nikon epifluorescence microscope equipped with appropriate filters.
Synthetic oligopeptides
The peptides used corresponded to a part of the second extracellular loop of rat Cx43 (amino acids 180-195, SLSAVYTCKRDPCPHQ) or to a corresponding segment of the second extracellular loop of rat Cx40 (amino acids 177-192, FLDTLHVCRRSPCPHP). As previously reported by Warner et al. (1995), maximal inhibitory effects of oligopeptides were reached on average at concentrations of 500 μM. We, therefore, used this concentration of Cx43 peptides in all our experiments. Control experiments with peptides comprising a segment of the intracellular cytoplasmic tail of rat Cx43 (amino acids 314-322, SAEQNRMGQ) were also performed at a concentration of 500 μM. Due to different chemical properties of the Cx40 peptides, the maximal concentration that could be dissolved in culture medium was 50 μM. We used this concentration of the Cx40 peptides in all our experiments.
To be certain that we measured dye and electrical coupling via de novo formed gap junction channels between A7r5 cells, we used a modification of the method previously published by Lampe (1994). One population of A7r5 cells was loaded with the lipophilic dye, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanide (Di-I, Molecular Probes) (0.2 μM final concentration, diluted × 3750 from 0.85 M stock solution in DMSO) for 30 min at 37°C. Using this procedure all cells were clearly labelled. A second population of A7r5 cells remained unlabelled. Thereafter, both populations of cells were trypsinized and mixed in Ham's F10 culture medium in the absence or presence of the different oligopeptides. Serum and antibiotics were left out of the culture medium because of their possible interaction with the oligopeptides. Cells were incubated overnight in a 5% CO2-95% air- and humidity-controlled incubator at 37°C.
Dye coupling
Subconfluent cultures of A7r5 or transfected SKHep1 cells were rinsed with a control solution containing (mM): 160 NaCl, 7 CsCl, 0.1 CaCl2, 1 MgCl2, 0.6 MgSO4, 10 Hepes (pH 7.4), and transferred to the stage of an inverted microscope (Nikon Diaphot TMD). In the case of A7r5 cells, clusters with approximately 50% Di-I-labelled cells were selected. Single cells within small clusters were then impaled with microelectrodes backfilled with a 3% Lucifer Yellow (LY)-3% Cascade Blue (CB) solution prepared in 150 mM LiCl (buffered to pH 7.2 with 10 mM Hepes). The fluorescent tracers were allowed to fill the cells by simple diffusion for 3 min. After the injection period, the electrode was removed and the fluorescent cells were counted. Cells were visualized using epifluorescent illumination provided by a 100 W mercury lamp and the appropriate filters. Lucifer Yellow was purchased from Sigma and Cascade Blue from Molecular Probes. Results are expressed as means±s.e.m. Dye coupling under different conditions was compared using an independent Student's t test.
Electrophysiology
Junctional conductance (gj) was monitored using the dual whole-cell voltage-clamp technique (Neyton & Trautmann, 1985) on newly formed pairs of cells of which only one cell was labelled with Di-I. Throughout the experiments, performed at room temperature, cells were superfused with control solution using a gravity-driven perfusion system. Patch electrodes had resistances of 2.5-7 MΩ when filled with a solution containing (mM): 135 CsCl, 0.5 CaCl2, 1 MgCl2, 5 Na2ATP, 10 EGTA, 10 Hepes (pH 7.2). CsCl was chosen to replace KCl in these experiments to reduce activity of membrane potassium channels. Both cells of a pair were voltage clamped at a common holding potential. To measure gap junctional currents (Ij), transjunctional potential differences (Vj) were elicited by changing the holding potential of one member of a cell pair for 1 s. Ij was defined as the current recorded in the cell kept at a constant potential. gj was then calculated from gj =Ij/Vj. Series resistances were compensated. gj values under different conditions were compared using an independent Student's t test.
In cell pairs in which gj was pharmacologically reduced with 2 mM halothane, gating of single gap junction channels could be detected. Current flowing through these channels was discriminated from non-junctional membrane current as step-like changes of opposite polarities but identical amplitudes recorded simultaneously in both current traces. All current and voltage signals were acquired at 2 kHz sampling rate using custom-made software (Scope; kindly provided by Drs J. Zegers and A. C. G. van Ginneken, Department of Physiology, Amsterdam, The Netherlands), and stored on the hard-disk of an Apple Macintosh computer (Quadra 660AV) equipped with data acquisition boards (National Instruments NB-MIO-16H9). Digitized current traces were filtered at 0.1-1 kHz for analysis and display of single channel activity using customized software (MacDAQ; kindly provided by Dr A. C. G. van Ginneken). To determine single gap junctional channel conductance, the amplitudes of single channel transitions were measured and divided by the applied Vj to obtain conductances associated with the current steps. Conductance values were converted into step-amplitude histograms with a bin width of 8 pS. To determine single channel conductance sizes, peak values of the single channel conductance distributions were determined by fitting the data to Gaussian distributions using KaleidaGraph software (Abelbeck Software, Reading, PA, USA). Results obtained from the fits are expressed as means±s.d., N is the number of experiments and n is the number of transitions measured. Calculated areas under the Gaussian distribution were used to obtain the relative contribution of the different channel populations.
RESULTS
Cx expression in A7r5 cells
A7r5 cells, a cell line derived from embryonic rat aorta smooth muscle cells, were incubated overnight in Ham's F10 culture medium without supplements. In order to test that this procedure did not affect proper Cx expression, we performed immunolabelling on these cultures. As can be observed in Fig. 1, A7r5 cells under these conditions express considerable amounts of SMA (A), Cx40 (B) and Cx43 (C).
Figure 1. Immunocytochemical characterization of the rat aortic smooth muscle cell line.
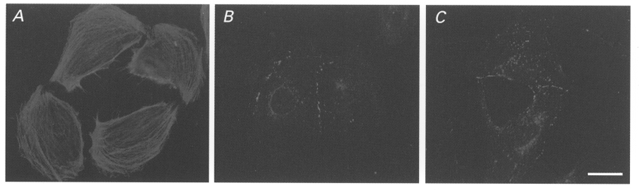
Photomicrographs showing abundance and distribution of immunoreactivity to anti-smooth muscle actin (A), anti-Cx40 (B) and anti-Cx43 (C) antibodies in A7r5 cells grown overnight under serum-free conditions. Bar, 15 μm.
Dye coupling
Permeability of gap junctions was assessed by diffusion of the fluorescent tracers Lucifer Yellow (LY, molecular weight (MW) 457, charge -2) and Cascade Blue (CB, MW 548, charge -2). In order to test for the specificity of these tracers, we used communication-deficient tumour cells which were stably transfected with the cDNAs coding for Cx43 or Cx40. As can be observed in Fig. 2, Cx43 channels transferred both LY (A) and CB (B) to several neighbouring cells in the cluster. On the other hand, Cx40 channels did not transfer molecules as large as the fluorescent dye CB (Fig. 2D) although the co-injected dye LY could readily pass to the neighbouring cells (Fig. 2C).
Figure 2. Specificity of fluorescent tracers.
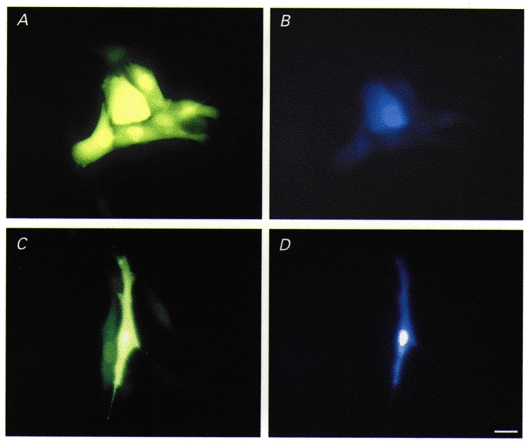
Diffusion of the fluorescent tracers Lucifer Yellow (LY) (A and C) and Cascade Blue (CB) (B and D) in SKHep1-Cx43 (A and B) and SKHep1-Cx40 (C and D) cells. LY passes through both Cx43 and Cx40 gap junction channels, whereas CB passes through only Cx43 channels. Bar, 20 μm.
Gap junctional permeability of clusters of A7r5 cells was measured by co-injection of LY and CB (Fig. 3). Under control conditions, LY diffused into 4.5± 0.3 cells (mean±s.e.m., 20 injections) and CB diffused into 2.9± 0.2 cells (20 injections). After overnight treatment with the synthetic oligopeptides corresponding to a segment of the second extracellular loop of Cx43 (P180-195), the diffusion of LY was decreased to 3.5± 0.3 cells (20 injections; P < 0.02) and that of CB was abolished (20 injections). Similar experiments using synthetic oligopeptides corresponding to an equivalent part of the extracellular loop of Cx40 (P177- 192) also decreased the diffusion of LY to 3.6± 0.2 cells (20 injections; P < 0.02). In contrast, incubation of A7r5 cells with oligopeptides corresponding to a segment of the intracellular cytoplasmic tail of Cx43 (P314-322) was without effect on LY diffusion (4.7± 0.3 cells, 20 injections).
Figure 3. Effects of synthetic oligopeptide P180-195 on dye transfer between A7r5 cells.
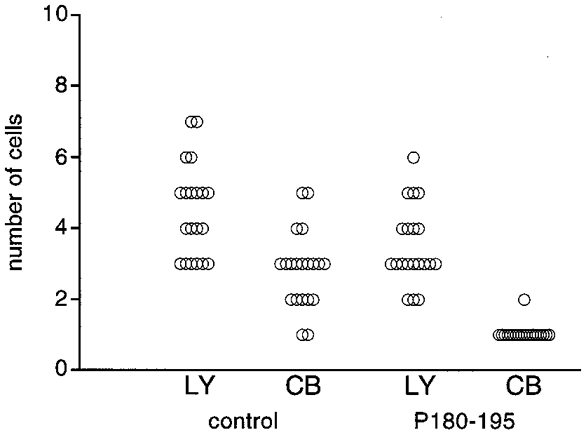
Under control conditions, A7r5 cells were found rather well coupled to each other as measured with LY or CB. Overnight treatment with 500 μM of P180-195 decreased LY diffusion, whereas CB did not pass out of the injected cells.
Gap junctional conductance
Newly formed A7r5 cell pairs were electrically well coupled with macroscopic gap junctional conductance (gj) averaging 29.8± 3.8 nS (mean±s.e.m., N = 8). Incubation with P180-195 decreased gj to 17.9± 5.8 nS (N = 6, P = 0.05) and with P177-192 to 19.0± 1.0 nS (N = 4, P < 0.05). In contrast, incubation of A7r5 cells with the control peptide P314-322 did not affect electrical coupling (gj = 28.6± 3.2 nS; N = 9).
To assess the selectivity of the oligopeptides, single gap junction channel conductances were obtained in A7r5 cells under reduced coupling conditions using bath application of halothane. As shown in Fig. 4A, A7r5 cell pairs exhibited channels of different sizes that could be separated in the frequency distribution of channel transitions as two major peaks around 80 and 150 pS. Under control conditions, the 80 pS channel population accounted for 56% of the total number of events (Fig. 4B, N = 5, n = 1145). This distribution was not affected by the control peptide P314-322 (Fig. 4C, N = 2, n = 275). In contrast, incubation with the Cx43 peptide P180-195 resulted in a relative decrease in the proportion of 80 pS events (Fig. 4D, N = 6, n = 886). The 80 pS peak under these conditions represented only 29% of the total number of events. Incubation with the Cx40 peptide P177-192, on the other hand, resulted in a relative decrease in the proportion of 150 pS events (Fig. 4E, N = 2, n = 275), the corresponding peak representing under these conditions only 13% of the total number of events.
Figure 4. Effects of the different peptides on single gap junctional channel conductance.
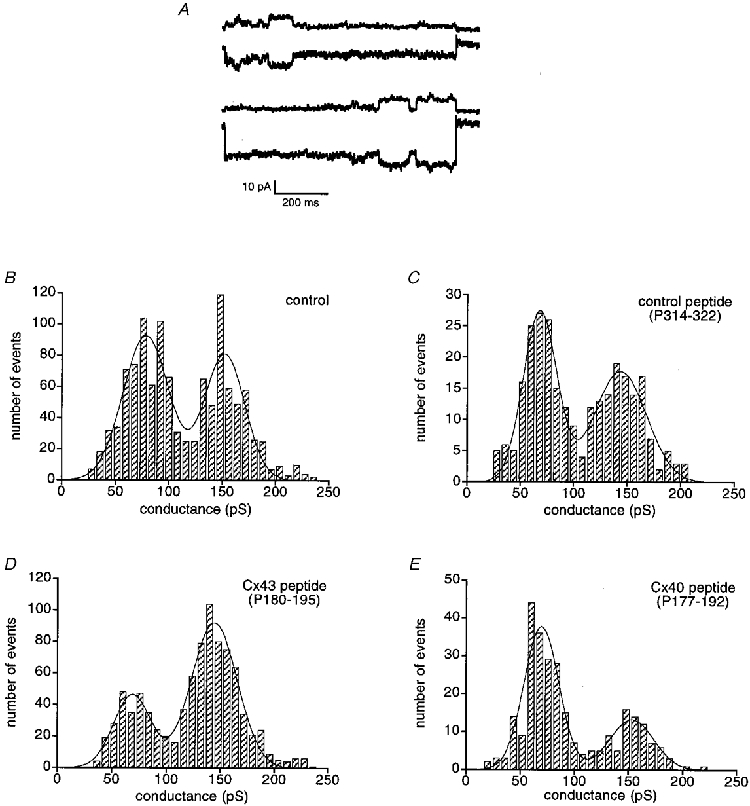
A, junctional current traces (Ij) recorded in response to Vj of 50 mV just before complete uncoupling induced by halothane treatment. Opening and subsequent closure of individual channels of different sizes can be discerned in A7r5 cell pairs. B-E, frequency histograms of conductance changes were determined from the amplitudes of single channel events in Ij recorded in response to Vj of 50 mV. Bin width, 8 pS. A7r5 cell pairs under control conditions (B), after incubation with P314-322 (C), after incubation with P180-195 (D), and after incubation with P177-192 (E).
DISCUSSION
The present experiments have demonstrated for the first time that peptides homologous to a specific region of the second extracellular loop of connexins can selectively inhibit the activity of one type of gap junction channels in cells containing multiple connexins.
Extracellular loops of connexins are involved in the docking process of connexons. The first and second extracellular loops each contain three conserved cysteine residues that form intramolecular disulphide bridges (Dahl et al. 1991, 1992). In a recent study, Foote et al. (1998) moved the first and third cysteines pairwise to various positions in each of the extracellular loops. Based on the periodicity of the movements, they suggested a model in which the extracellular loops of each Cx could interdigitate with the corresponding loops on the apposed connexon during docking of hemichannels, probably forming a β-barrel motif within the extracellular gap. This model would imply that any peptide disturbing this β-barrel motif can reduce gap junction formation. In addition, it has been demonstrated that the second extracellular domain is a determinant for Cx compatibility in heterotypic connexon interaction (White et al. 1994). In this study, we have generated synthetic oligopeptides (P180-195) homologous to amino acids 180 to 195 in the second extracellular loop of Cx43. This sequence contains two conserved cysteine residues and shows only 44% homology to the sequence of Cx40 in this region. Because Cx43 and Cx40 connexons do not form functional gap junction channels (Bruzzone et al. 1993), we investigated the selective inhibitory properties of P180-195 on gap junction channel activity in a smooth muscle cell line (A7r5) expressing both Cxs (Moore & Burt, 1994).
The expression of Cx43 and Cx40 in A7r5 cells was confirmed by immunocytochemistry. Moreover, we demonstrated that the expression of these connexins is not affected by maintaining cells in culture medium without serum and antibiotics, a condition that was used for peptide incubation. To assess the selective effects of P180-195 on coupling, we used two different approaches. First, we took advantage of the differential diffusion properties of two fluorescent dyes (LY and CB) through Cx43- and Cx40-built gap junction channels. Indeed, both fluorescent tracers were found to spread through Cx43 channels in SKHep1-Cx43 cells. In contrast, no intercellular diffusion of CB was detected in SKHep1-Cx40 cells, indicating that Cx40 channels are impermeant to this tracer. Under control conditions, both LY and CB readily spread within clusters of A7r5 cells. In the presence of P180-195, the intercellular diffusion of LY was reduced (P < 0.02) and that of CB was blocked (P < 0.001). This suggests that the peptides interfered with the formation of Cx43 channels, resulting in A7r5 cells communicating mainly through Cx40 channels.
As a second approach, we resolved single gap junction channel activity in pairs of A7r5 cells monitored under dual whole-cell voltage-clamp conditions. It has been shown that Cx43 gap junction channels exhibit unitary conductances ranging between 30 and 90 pS, whereas Cx40 channels display larger conductances of 120-160 pS (Moreno et al. 1994; Beblo et al. 1995; Bukauskas et al. 1995; Kwak et al. 1995). Under control conditions, two major populations of gap junction channels with peak conductances of about 80 pS and 150 pS, respectively, were detected in A7r5 cell pairs. The 80 pS channel population contributes for 56% of the total number of events. Incubation with peptides corresponding to a segment of the intracellular C-terminus of Cx43 (P314-322) did not affect the relative contribution of the two channel populations. In contrast, the contribution of the 80 pS channel population decreased to 29% in the presence of P180-195. Accordingly, the macroscopic junctional conductance was reduced by 40%. This suggests again that the peptides markedly altered Cx43-mediated gap junctional coupling in A7r5 cell pairs.
To further assess for selectivity of gap junction channel inhibition, we used synthetic oligopeptides corresponding to the equivalent part of the second extracellular loop of rat Cx40 (P177-192). In the presence of P177-192, dye and electrical coupling were significantly reduced. In the distribution of single channel events, the contribution of the 150 pS channel population decreased from 44% under control conditions to 13% in the presence of P177-192. This suggests that these peptides markedly altered Cx40-mediated gap junctional coupling in A7r5 cell pairs. Together with the data obtained with P180-195, these results indicate that peptides originating from this location in the second extracellular loop display selectivity in their blocking properties on gap junction channel types.
Although large changes were observed in the distribution of single channel events, the selective inhibition of gap junction channels was not complete with either the Cx43 peptides or the Cx40 peptides. This might imply that docking of some hemichannels remained possible even though the cells were incubated with an excess of oligopeptides. Alternatively, the peptides may alter the structural conformation of the gap junction channels, which may lead to reduced assembly or increased degradation of hemichannels. Finally, the possibility that the peptides interact with other membrane proteins, which in turn interact with gap junction channels, cannot be completely ruled out. Irrespective of the mechanism of action, our results point to a selective inhibition of gap junction channel activity by these peptides. Thus, competition assays using synthetic oligopeptides may help to reveal the regulatory properties of gap junction channels in primary cells expressing multiple connexins.
Acknowledgments
We thank Dr Monique Hermans for providing us with the Cx43- and Cx40-transfected SKHep1 cells, Dr Daniel Gros for providing us with Cx40 antibodies, and Dr Marc Chanson for critical reading of the manuscript. This work was supported by the Netherlands Organization for Scientific Research (902-16-093).
References
- Beblo DA, Wang H-Z, Beyer EC, Westphale EM, Veenstra RD. Unique conductance, gating, and selective permeability properties of gap junction channels formed by connexin40. Circulation Research. 1995;77:813–822. doi: 10.1161/01.res.77.4.813. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Haefliger J-A, Gimlich RL, Paul DL. Connexin40, a component of gap junctions in vascular endothelium, is restricted in its ability to interact with other connexins. Molecular Biology of the Cell. 1993;4:7–20. doi: 10.1091/mbc.4.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone R, White TW, Paul DL. Connections with connexins: the molecular basis of intercellular signaling. European Journal of Biochemistry. 1996;238:1–27. doi: 10.1111/j.1432-1033.1996.0001q.x. [DOI] [PubMed] [Google Scholar]
- Bukauskas FF, Elfgang C, Willecke K, Weingart R. Biophysical properties of gap junction channels formed by mouse connexin40 in induced pairs of transfected human HeLa cells. Biophysical Journal. 1995;68:2289–2298. doi: 10.1016/S0006-3495(95)80411-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaytor RT, Evans WH, Griffith TM. Peptides homologous to extracellular loop motifs of connexin 43 reversibly abolish rhythmic contractile activity in rabbit arteries. The Journal of Physiology. 1997;503:99–110. doi: 10.1111/j.1469-7793.1997.099bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl G, Levine E, Rabadan-Diehl G, Werner R. Cell/cell channel formation involves disulfide exchange. European Journal of Biochemistry. 1991;197:141–144. doi: 10.1111/j.1432-1033.1991.tb15892.x. [DOI] [PubMed] [Google Scholar]
- Dahl G, Nonner W, Werner R. Attempts to define functional domains of gap junction proteins with synthetic peptides. Biophysical Journal. 1994;67:1816–1822. doi: 10.1016/S0006-3495(94)80663-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl G, Werner R, Levine E, Rabadan-Diehl G. Mutational analysis of gap junction formation. Biophysical Journal. 1992;62:172–182. doi: 10.1016/S0006-3495(92)81803-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote CI, Zhou L, Zhu X, Nicholson BJ. The pattern of disulfide linkages in the extracellular loop regions of connexin 32 suggests a model for the docking interface of gap junctions. Journal of Cell Biology. 1998;140:1187–1197. doi: 10.1083/jcb.140.5.1187. 10.1083/jcb.140.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros D, Jarry-Guichard T, Ten Velde I, De Mazière A, Van Kempen MJA, Davoust J, Briand JP, Moorman AFM, Jongsma HJ. Restricted distribution of connexin40, a gap junction protein, in mammalian heart. Circulation Research. 1994;74:839–851. doi: 10.1161/01.res.74.5.839. [DOI] [PubMed] [Google Scholar]
- Gros DB, Jongsma HJ. Connexins in mammalian heart function. BioEssays. 1996;18:719–730. doi: 10.1002/bies.950180907. [DOI] [PubMed] [Google Scholar]
- Johnson RG, Meyer RA. Gap junction assembly: the external domains in the connexins fulfill an essential function. In: Hall JE, Zampighi GA, Davis RM, editors. Progress in Cell Research. Vol. 3. Amsterdam: Elsevier Science Publishers B.V.; 1993. pp. 283–289. [Google Scholar]
- Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84:381–388. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- Kwak BR, Hermans MMP, DeJonge HR, Lohmann SM, Jongsma HJ, Chanson M. Differential regulation of distinct types of gap junction channels by similar phosphorylating conditions. Molecular Biology of the Cell. 1995;6:1707–1719. doi: 10.1091/mbc.6.12.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe PD. Analyzing phorbol ester effects on gap junctional communication: a dramatic inhibition of assembly. Journal of Cell Biology. 1994;127:1895–1905. doi: 10.1083/jcb.127.6.1895. 10.1083/jcb.127.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RA, Laird DW, Revel JP, Johnson RG. Inhibition of gap junction and adherens junction assembly by connexin and A-CAM antibodies. Journal of Cell Biology. 1992;119:179–189. doi: 10.1083/jcb.119.1.179. 10.1083/jcb.119.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore LK, Burt JM. Selective block of gap junction channel expression with connexin-specific antisense oligodeoxy nucleotides. American Journal of Physiology. 1994;267:C1371–1380. doi: 10.1152/ajpcell.1994.267.5.C1371. [DOI] [PubMed] [Google Scholar]
- Moreno AP, Saez JC, Fishman GI, Spray DC. Human connexin43 channels: regulation of unitary conductances by phosphorylation. Circulation Research. 1994;74:1050–1057. doi: 10.1161/01.res.74.6.1050. [DOI] [PubMed] [Google Scholar]
- Neyton J, Trautmann A. Single-channel currents of an intercellular junction. Nature. 1985;317:331–335. doi: 10.1038/317331a0. [DOI] [PubMed] [Google Scholar]
- Warner A, Clements DK, Parikh S, Evans WH, DeHaan RL. Specific motifs in the external loops of connexin proteins can determine gap junction formation between chick heart myocytes. The Journal of Physiology. 1995;488:721–728. doi: 10.1113/jphysiol.1995.sp021003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TW, Bruzzone R, Wolfram S, Paul DL, Goodenough DA. Selective interactions among multiple connexin proteins expressed in the vertebrate lens: the second extracellular domain is a determinant of compatibility between connexins. Journal of Cell Biology. 1994;125:879–892. doi: 10.1083/jcb.125.4.879. 10.1083/jcb.125.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]


