Abstract
Development of the pacemaker system in the small intestine depends upon signalling via tyrosine kinase (Kit) receptors. The downstream pathways initiated by Kit in interstitial cells of Cajal (ICC) have not been investigated. Wortmannin and 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY 294002), inhibitors of phosphatidylinositol 3′-kinase (PI3-kinase), were used to test the involvement of this pathway in the development and maintenance of ICC and electrical rhythmicity in the murine small intestine.
ICC and electrical slow waves were present in the murine jejunum at birth. ICC and electrical rhythmicity continued to develop in neonates such that adult activity was recorded after 1 week. Development of ICC and rhythmicity were maintained in organ culture.
Wortmannin or LY 294002 inhibited the development of slow waves and blocked rhythmicity within 2-4 days. Loss of slow waves was preceded by disappearance of Kit-positive cells from the myenteric (IC-MY) and deep muscular plexus (IC-DMP) regions. Wortmannin or LY 294002 had no acute effect on slow waves.
Muscles from older animals (day 10-day 30) developed resistance to wortmannin treatment, but when the exposure to wortmannin was increased to 35 days, damage to ICC networks and electrical dysrhythmias were observed.
PI3-kinase appears to be a critical downstream signalling element linking Kit receptors to ICC development and maintenance of phenotype. ICC are more sensitive to Kit or PI3-kinase blockade at birth, but the importance of the PI3-kinase signalling in the maintenance of ICC persists into adulthood. Interference with PI3-kinase signalling in immature or adult animals could result in disruption of ICC and gastrointestinal dysrhythmias.
Interstitial cells of Cajal (ICC) are pacemaker cells in gastrointestinal (GI) muscles (see review by Sanders, 1996). ICC express the proto-oncogene, c-kit (Maeda et al. 1992; Ward et al. 1994; Huizinga et al. 1995; Torihashi et al. 1995), and signalling via the receptor tyrosine kinase gene product, Kit, is essential for development of the ICC phenotype and electrical rhythmicity (Torihashi et al. 1997). Although pacemaker ICC are present in the small bowel at birth, blocking Kit receptors immediately after birth results in loss of ICC and electrical dysrhythmias (Torihashi et al. 1995; Ward et al. 1997). Thus signalling via Kit continues well after ICC networks have formed and become functional. This suggests that along with development of ICC, signalling via Kit is important in the long-term maintenance of the ICC phenotype.
The pleiotropic function of receptor tyrosine kinases is regulated by their pattern of expression, the availability of ligand, and the downstream signal transduction molecules activated by receptor occupation. Activation of signal transduction molecules sets off a variety of tertiary cellular events that regulate multiple features of development, such as cell proliferation, migration, establishment and maintenance of phenotype, and cell survival. Any of a number of signalling molecules may be activated by Kit and other receptor tyrosine kinases, including phosphatidylinositol 3′-kinase (PI3-kinase), phospholipase C-γ1 (PLC-γ1), phospholipase D, p21ras GTPase-activating protein and mitogen-activated protein kinase (MAPK), JAK (Janus kinase) and STAT (signal transducer and activator of transcription), and Src family members (Rottapel et al. 1991; Margolis & Skolnik, 1994; Deberry et al. 1997; Kozawa et al. 1997; Linnekin et al. 1997). Each receptor tyrosine kinase shows unique specificities for the various signal transduction molecules, but PI3-kinase appears to be a common substrate for many receptor tyrosine kinases. Activation of Kit by its ligand, stem cell factor (SCF or steel factor), causes autophosphorylation of tyrosine residues and dimerization of Kit and the development of high-affinity binding sites for signalling molecules including PI3-kinase (Rottapel et al. 1991). Binding of PI3-kinase and other signalling molecules to the receptor may increase function by localizing the enzymes near the membrane where substrates are abundant or by enhancing catalytic activities by receptor-mediated tyrosine phosphorylation (e.g. Nishibe et al. 1990). Activation of PI3-kinase leads to phosphorylation of the 3-OH position of the inositol ring of phosphoinositides, producing phosphatidylinositol 3,4,5-trisphosphate and activation of diverse signalling pathways (see review by Duronio et al. 1998).
The nature of downstream signalling pathways coupled to Kit in ICC has not been examined. Because of the central role of PI3-kinase and the availability of pharmacological tools to block this pathway, we tested the PI3-kinase inhibitors, wortmannin, a fungal metabolite that irreversibly modifies the catalytic domain of PI3-kinase (Arcaro & Wymann, 1993; Yano et al. 1993), and a more specific, structurally unrelated PI3-kinase inhibitor, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY 294002; Vlahos et al. 1994), on the development of ICC in murine small intestinal muscles. We have also studied the ability of PI3-kinase blockers to disrupt ICC networks once the phenotype is established and ICC have matured and become fully functional.
METHODS
Animals
BALB/c mice (Harlan Sprague-Dawley) were killed at ages day (D) 0 through to D30. Animals were anaesthetized by chloroform inhalation and exsanguinated by decapitation following cervical dislocation. The Institutional Animal Use and Care Committee at the University of Nevada approved the use and treatment of the animals.
Electrophysiological studies
Segments of jejunum were isolated from animals and opened along the mesenteric border and the luminal contents were removed by washing with Krebs Ringer Buffer (KRB). After removing the mucosa by sharp dissection, muscle strips (4 mm × 10 mm) were cut and pinned to the Sylgard elastomere (Dow Corning) floor of a recording chamber with the mucosal side of the circular muscle facing upward. Alternatively tissues that were placed in organ culture (see below) were taken after different time periods and placed in the recording chamber. Parallel platinum electrodes were placed on either side of the muscle strips to evoke neural responses. Electrical recordings of jejunal tissues were made in the presence of nifedipine (10−6 M) to reduce muscle contraction. This compound did not affect slow wave activity from control recordings of animals from ages D0-D30.
Circular muscle cells were impaled with glass microelectrodes filled with 3 M KCl and having resistances between 40 and 80 MΩ. Transmembrane potential was measured using a high input impedance electrometer (WPI duo 773, Sarasota, FL, USA) and outputs were displayed on a Hameg oscilloscope (Frankfurt, Germany). Electrical signals were recorded onto videotape (A. R. Vetter Co., Rebersburg, PA, USA). Neural responses were elicited by square wave pulses of electrical field stimulation (EFS; Grass S48, Quincy, MA, USA), 1 pulse, 0.5 ms in duration at supramaximal voltage.
Data are expressed as means ±s.e.m. Differences in the data were evaluated by Student's t test, P values less than 0.05 being taken as a statistically significant difference. The number of cells from which recordings were made is denoted by n. The number of animals from which the n was obtained is also provided.
Organ culture
Jejunal tissues were removed from animals from ages D0 to D30. Luminal contents were removed by washing with KRB and the mucosa was removed by sharp dissection. Muscle strips (2 mm × 5 mm) were cut and pinned to the base of a sterile tissue culture chamber slide (Corning Glass Works) lined with Sylgard elastomere, with the mucosal side of the circular muscle facing upward. Tissues were washed 4 times with KRB and placed in M199 media (Sigma) containing penicillin (200 U ml−1), streptomycin (200 mg ml−1) and amphotericin B (0.5 mg ml−1), washed another 4 times and incubated at 37°C (90 % humidity and 95 % O2-5 % CO2) for up to 35 days, with culture media being changed every second day. Some tissues were incubated in M199 media containing a monoclonal antibody raised against c-Kit protein (ACK2, 5 μg ml−1; Gibco BRL), wortmannin, or LY 294002. ACK2, wortmannin or LY 294002 were not added to chambers containing control tissues. Control experiments were also performed with the vehicles used to dissolve wortmannin and LY 294002 (DMSO and ethanol, respectively). Other control experiments were performed in which non-immune serum (Hyclone, Logan, UT, USA) was included in the incubation medium to control for possible effects of non-specific antibodies.
Immunohistochemistry
After various periods of culturing, jejunal tissues were fixed in acetone (4°C; 10 min). Following fixation, tissues were washed for 60 min in phosphate buffered saline (PBS; 0.1 M, pH 7.4). Non-specific antibody binding was reduced by incubation in 10 % rabbit serum for 1 h at room temperature. Tissues were incubated overnight at 4°C with ACK2 (5 μg ml−1 in PBS). Immunoreactivity was detected using FITC-conjugated secondary antibody (FITC-anti rat; Vector Laboratories, Burlingame, CA, USA; 1 : 100 in PBS, 1 h at room temperature, 22-24°C). Control tissues were prepared in a similar manner, either omitting ACK2 or the secondary antibody from the incubation solution. Tissues were examined with a Bio-Rad MRC 600 (Hercules, CA, USA) laser scanning confocal microscope with an excitation wavelength appropriate for FITC (488 nm). Confocal micrographs were made from digital composites of Z-series scans of 10-15 optical sections through a depth of 10-35 μm. Final images were constructed with Comos software (Bio-Rad).
Solutions and drugs
Muscles were maintained in KRB (37.5 ± 0.5°C; pH 7.3-7.4) containing (mM): Na+, 137.4; K+, 5.9; Ca2+, 2.5; Mg2+, 1.2; Cl−, 134; HCO3−, 15.5; H2PO4−, 1.2; dextrose, 11.5 and bubbled with 97 % O2-3 % CO2. Solutions of wortmannin and LY 294002 (Sigma) were prepared in DMSO and ethanol at 10−2 M, respectively, and diluted to a final concentration of 10−5 M where stated. A neutralizing antibody for Kit (ACK2; Nishikawa et al. 1991; 5 μg ml−1 in PBS) was tested in comparison to wortmannin and LY 294002 in some experiments. Atropine sulphate (Sigma) was prepared as a stock solution at 10−2 M in distilled water and diluted to a concentration of 10−6 M in KRB.
RESULTS
Development of electrical activity in organ culture
As previously reported, we found that circular muscle cells of the murine jejunum were electrically rhythmic at birth (D0), and this activity continued to develop for several days during the post-natal period (Torihashi et al. 1997). In the present study jejunal circular muscle cells had resting membrane potentials (Vm; maximum potential between slow waves) averaging -58 ± 3 mV and slow waves averaging 15 ± 1 mV in amplitude at birth. Slow waves occurred at an average frequency of 21 ± 1.5 cycles min−1 (n = 6). When the tunica muscularis was placed in organ culture, slow wave activity developed in a manner similar to natural development in vivo (Ward et al. 1997). With time in culture the electrical activity persisted and developed more adult-like characteristics (Fig. 1). After 6 days, Vm averaged -65 ± 3 mV, slow waves were 30 ± 2 mV in amplitude, and occurred at a frequency of 22.5 ± 2.1 cycles min−1 (n = 5; P < 0.05 for slow wave amplitude; other slow wave parameters were not significant from D0; see Table 1). As previously demonstrated (Ward et al. 1997), inclusion of a neutralizing anti-c-Kit antibody (ACK2) in the organ culture media inhibited the development of ICC and slow wave activity (data not shown). Further experiments were conducted to determine whether the PI3-kinase pathway is involved in the c-Kit-signalling pathway in ICC development.
Figure 1. Development of electrical activity of the circular muscle of the murine jejunum in organ culture.
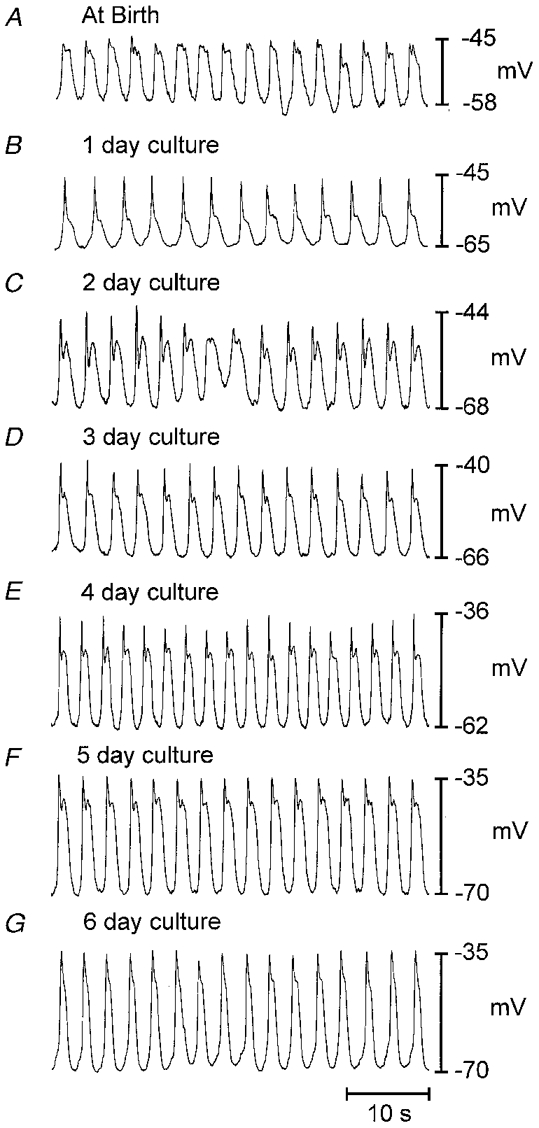
A shows slow wave activity of the jejunum at birth. B-G show electrical activity from tissues organ-cultured from birth for between 1 and 6 days. Resting membrane potential became slightly more polarized and slow wave activity became more pronounced as a function of time in culture. The development of electrical activity in organ culture mimicked development of activity in vivo (see Ward et al. 1997).
Table 1.
Effects of wortmannin and LY 294002 on electrical slow wave parameters
| Control | Wortmannin (10 μM) | LY 294002 (10 μM) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Days in culture | Vm (mV) | Slow wave amplitude (mV) | Slow wave frequency (cycles min−1) | Vm (mV) | Slow wave amplitude (mV) | Slow wave frequency (cycles min−1) | Vm (mV) | Slow wave amplitude (mV) | Slow wave frequency (cycles min−1) |
| 0 | −58.2 ± 3.0 | 15.3 ± 1.0 | 21.5 ± 1.5 | — | — | — | — | — | — |
| 1 | −60.3 ± 1.9 | 21.9 ± 2.4 | 21.5 ± 1.3 | −55.1 ± 2.3 | 12.3 ± 4.0 | 14.4 ± 3.8 | −57.8 ± 0.9 | 16.2 ± 2.8 | 17.6 ± 1.3 |
| 2 | −61.3 ± 2.6 | 22.7 ± 2.1 | 19.1 ± 1.0 | −57.7 ± 2.2 | 7.2 ± 3.0* | 10.5 ± 2.7 | −59.0 ± 3.5 | 15.0 ± 2.1 | 17.8 ± 1.3 |
| 3 | −61.5 ± 2.7 | 26.0 ± 2.8* | 18.1 ± 1.9 | −53.1 ± 3.1 | 5.1 ± 2.0† | 9.3 ± 3.3 | −60.8 ± 7.8 | 14.5 ± 6.1 | 19.3 ± 1.9 |
| 4 | −64.6 ± 2.1 | 27.1 ± 1.6* | 18.1 ± 1.2 | −59.0 ± 4.0 | 2.7 ± 2.7† | 4.7 ± 4.7* | −61.0 ± 4.3 | 9.0 ± 2.8* | 16.2 ± 2.8 |
| 5 | −59.4 ± 2.4 | 26.9 ± 2.0* | 19.9 ± 1.5 | −58.1 ± 2.4 | 0.43 ± 0.43† | 0.86 ± 0.86† | −51.5 ± 2.1 | 6.8 ± 4.3† | 11.0 ± 2.6* |
| 6 | −64.8 ± 2.7 | 29.8 ± 2.1* | 22.5 ± 2.1 | n.d. | n.d. | n.d. | −58.8 ± 1.4 | 0.8 ± 0.5† | 5.6 ± 3.4* |
Vm, resting membrane potential. n.d., not determined.
P < 0.05
P < 0.01 when compared with control.
Effect of wortmannin treatment on development of slow waves and ICC networks
Strips of jejunum were cultured for up to 5 days in the absence and presence of wortmannin (10 μM dissolved in 0.1 % DMSO). Control experiments showed that DMSO at levels present in culture media had no effect on ICC or electrical rhythmicity (i.e. D0 muscles cultured for 5 days with DMSO (0.1 %) showed no difference in Vm, slow wave amplitude or frequency). At intervals of 24 h, muscles cultured with and without wortmannin were removed from the incubator and perfused for at least 1 h with KRB solution before beginning electrical recordings (wortmannin was not included in the perfusion solution). Exposure to wortmannin for 2 days caused significant reductions in slow wave amplitude and frequency. The average Vm of tissues treated with wortmannin for 2 days was -58 ± 2 mV and slow wave amplitude and frequency were reduced to 7 ± 3 mV and 11 ± 3 cycles min−1, respectively (Fig. 2; n = 14). After 4 days, wortmannin treatment, Vm was -59 ± 4 mV, and slow waves were absent in most preparations (4 of 5). Control muscles from the same animals cultured without wortmannin had an average Vm of -62 ± 2 mV and slow waves of 26 ± 2 mV, occurring at a frequency of 18 ± 1 cycles min−1 (P < 0.01; see Table 1). The single preparation with slow waves remaining displayed events of 8 mV amplitude at a frequency of 14 cycles min−1. Figure 3 shows a summary of the effects of wortmannin on electrical parameters as a function of time.
Figure 2. Effect of wortmannin on electrical slow wave activity.
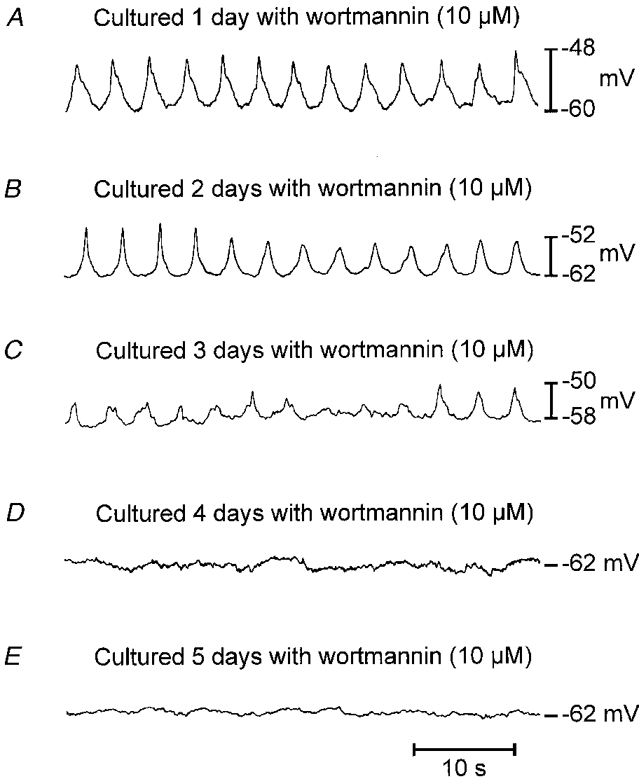
Jejunal muscle strips from newborn animals were cultured in the presence of wortmannin (10 μM) for up to 5 days (A-E). Wortmannin blocked the development of electrical activity, and inhibited activity observed in tissues of newborn animals. A 2 day exposure to wortmannin reduced the amplitude and frequency of slow waves, and activity was blocked in most tissues within 4 days. Resting membrane potential was also reduced by wortmannin treatment.
Figure 3. Summary of the effects of wortmannin on slow wave activity from the murine jejunum.
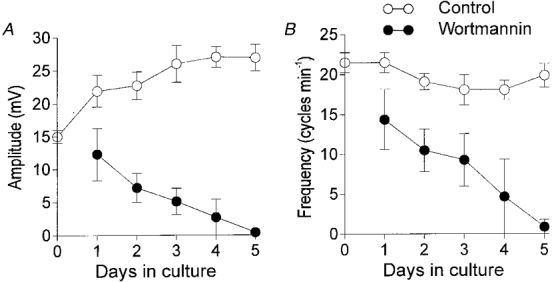
The amplitude of slow waves increased during the first 1-5 days in muscles cultured from birth (○ in A). Frequency remained relatively constant during this period (○ in B). Wortmannin (•) caused gradual reductions in slow wave amplitude and frequency of slow waves in cultured muscles. After 2 days, slow wave amplitude and frequency were greatly reduced and were abolished after 4-5 days in most muscles.
We also tested responses to electrical field stimulation (EFS; single pulse, 0.5 ms in duration, supramaximal voltage) in muscles made quiescent by treatment with wortmannin. Excitatory junction potentials with an average amplitude of 18.3 ± 5 mV and 1.9 ± 0.13 s in duration were elicited in these muscles after all slow wave activity was blocked (Fig. 4; n = 4). The excitatory junction potentials were substantially reduced by addition of atropine (1 μM), suggesting they resulted from the activation of muscarinic receptors.
Figure 4. Electrical field stimulation (EFS) of tissue treated with wortmannin.
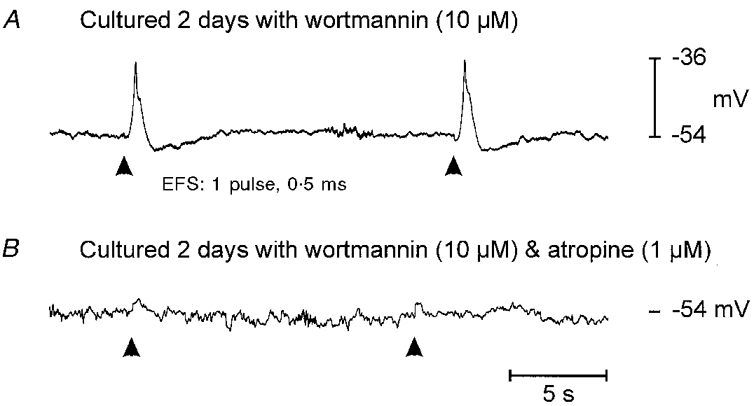
Wortmannin abolished slow wave activity in this preparation after 2 days of treatment. Although muscles were electrically quiescent, EFS (single pulses, 0.5 ms in duration with supramaximal voltage at arrowheads) caused excitatory junction potentials (18 mV in amplitude and approximately 1.5 s in duration; A). Atropine (1 μM; B) abolished the EJPs, suggesting they resulted from activation of muscarinic receptors.
Control experiments were performed to determine whether wortmannin had direct effects on ion channels or the slow wave rhythmicity mechanism. Muscles were removed from D0, D5 and D20-D30 animals, and prepared for electrophysiological recording. After an equilibration period of approximately 2 h, control recordings were made and the muscles were exposed to wortmannin (10 μM). This compound had no significant effect on Vm, slow wave amplitude or frequency. On D0, Vm averaged -55 ± 5 mV and slow waves were 14 ± 2 mV in amplitude, 2.1 ± 0.3 s in duration and occurred at 20.7 ± 3 cycles min−1. After wortmannin (for up to 2 h), Vm averaged -57 ± 5 mV and slow waves were 13 ± 2 mV in amplitude, 1.8 ± 0.14 s in duration and occurred at 18 ± 5 cycles min−1. These values were not significantly different from control (n = 3; P > 0.3). In D5 animals the circular muscle had an average Vm, slow wave amplitude duration and frequency of -63 ± 2 mV, 24 ± 5 mV, 2.1 ± 0.15 s and 25 ± 3 cycles min−1, respectively. After wortmannin, Vm was -66 ± 3 mV and slow waves were 24 ± 5 mV in amplitude, 2 ± 0.3 s in duration and occurred at 23 ± 3 cycles min−1. (n = 4; P > 0.4). Wortmannin also had no acute effect on muscles from D20-D30 animals (n = 3; P > 0.45; Fig. 5).
Figure 5. Effect of acute addition of wortmannin on electrical activity.
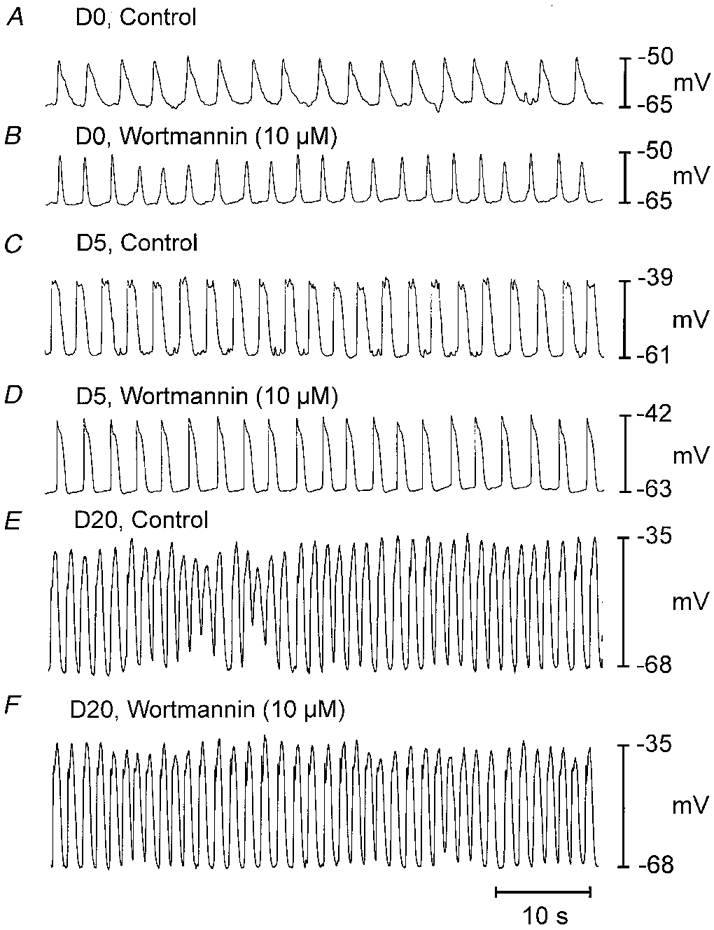
Electrical recordings were made from muscle strips taken from animals on D0 (A and B), D5 (C and D) and D20 (E and F). Wortmannin had no significant effects on slow waves or resting potentials when added acutely for periods up to 2 h.
Immunohistochemical techniques were used to examine the effects of wortmannin on ICC networks in 30 muscles cultured for 1-5 days (n = 3 for each day; 15 controls and 15 treated with wortmannin). ICC from the jejunum of D0 animals displayed Kit-like immunoreactivity (Kit-LI) at the level of the myenteric plexus (IC-MY) and occasional cells were observed at the level of the deep muscular plexus (IC-DMP). These two populations of cells retained ICC-like structure and Kit-LI in organ culture (Ward et al. 1997; and see Fig. 6A-C). Treatment with wortmannin caused loss in Kit-LI and disruption of ICC networks. Initially, ICC networks became patchy in whole mount preparations. After 3 days of exposure to wortmannin, Kit-LI was absent in most preparations (Fig. 6D-F). A few small patches of cells with Kit-LI were still observed after 4 days of incubation with wortmannin in two preparations. Kit-LI had however nearly disappeared after 5 days in culture in all three preparations examined.
Figure 6. Effects of wortmannin on ICC networks.
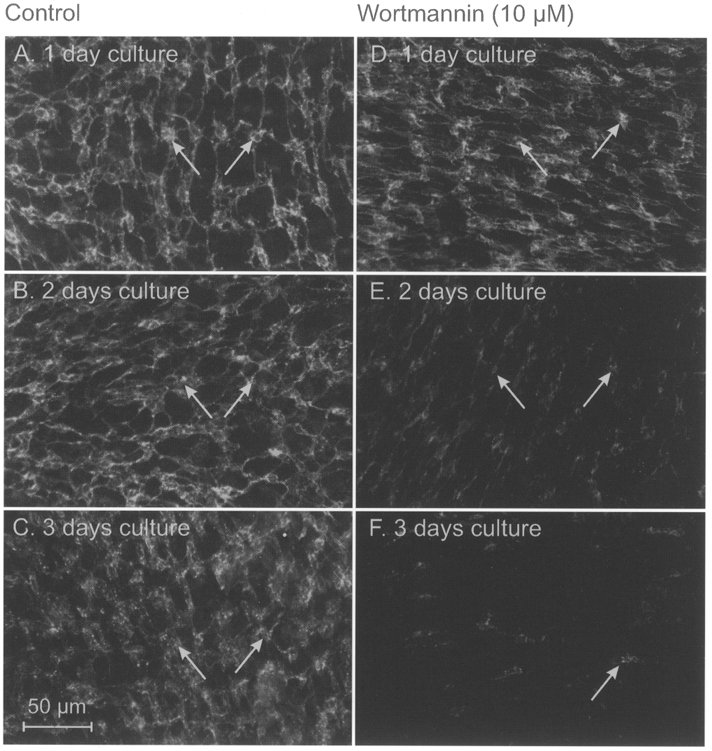
Jejunal muscles were placed into culture at birth (D0). A-C show ICC networks (via Kit-like immunoreactivity) after 1-3 days in culture. Normal distributions of ICC and ICC networks were observed in control cultured muscles. D-F show ICC in muscles cultured for the same time periods in the presence of wortmannin (10 μM). In this example Kit-positive cells were reduced after 2 days and only occasional cells were resolvable by 3 days. The scale bar of 50 μm in C applies to all panels.
Effect of LY 294002 treatment on slow waves and ICC networks
A more specific inhibitor of PI3-kinase, LY 294002 (Vlahos et al. 1994) was tested to reduce the possibility that the effects of wortmannin were non-specific. Muscles were exposed to LY 294002 (10 μM) for up to 6 days, and this caused a time-dependent reduction in the amplitude and frequency of slow waves. After 4 days in culture the Vm of tissues incubated with LY 294002 was not significantly different from that of control tissues (-58 ± 5 mV compared with -63 ± 2 mV). However, slow wave amplitude was significantly attenuated to 8 ± 3 mV compared with 27 ± 2 mV in control tissues (P < 0.005; n = 6). After 6 days in culture with LY 294002, slow waves were absent in three of five preparations (Fig. 7 and Fig. 8 and Table 1). In the two preparations in which slow waves were recorded, the average amplitude was 2 mV and these oscillations occurred at a frequency of 14 cycles min−1 compared with control tissues, which had slow waves with an average amplitude of 28 ± 4 mV and frequency of 22 ± 2 cycles min−1 (P < 0.01 and 0.05, respectively).
Figure 7. Effect of LY 294002 on electrical slow wave activity.
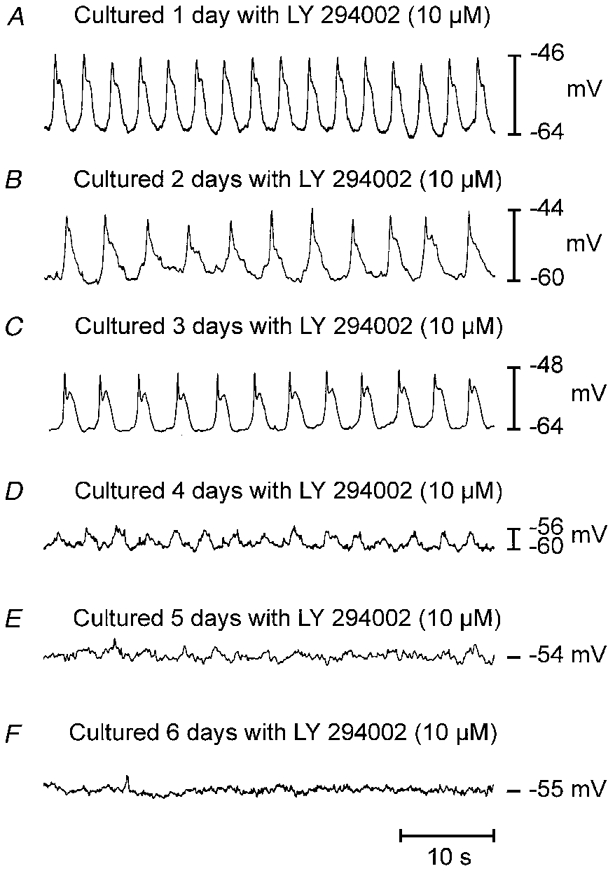
Muscle strips from newborn animals were cultured in the presence of LY 294002 (10 μM) for periods up to 6 days. LY 294002 abolished the development of electrical activity and blocked the activity present at birth. After 3-4 days, exposure to LY 294002 slow wave activity was greatly reduced in amplitude and frequency. After 6 days, electrical slow wave activity was abolished in most muscles.
Figure 8. Summary of the effects of LY 294002 on slow wave activity.
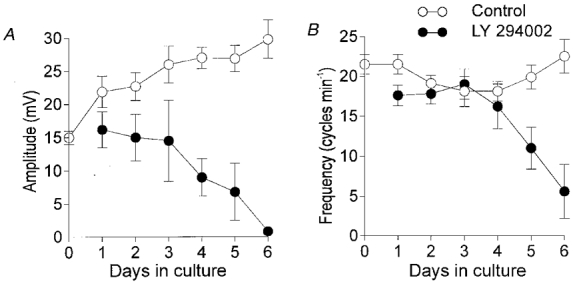
LY 294002 (10 μM; •) caused a time-dependent reduction in slow wave amplitude (A) and frequency (B). After 4 days of exposure to LY 294002, slow wave amplitude and frequency were greatly reduced. Activity was abolished in most muscles after 5-6 days in culture. Electrical slow waves from newborn control muscles (○) increased in amplitude and became more robust when cultured over a similar time period in the absence of LY 294002.
LY 294002 had no effect on Vm, upstroke amplitude or slow wave frequency when added acutely to D0, D5 or D30 animals. After addition of LY 294002 (10 μM) for 30 min, the average Vm of circular muscle cells from D0 animals was -62 ± 2 mV and slow waves were 14 ± 1 mV in amplitude, 2.1 ± 0.08 s in duration and occurred at a frequency of 23 ± 1 mV (P > 0.2 when compared with control conditions). D5 animals had an average Vm of -67 ± 4 mV and slow waves that were 19 ± 3 mV in amplitude, 1.75 s in duration and which occurred at a frequency of 24 ± 1 mV when exposed to LY 294002. These values were not significantly different from control slow wave activity (P > 0.3 when compared with control conditions).
The effects of LY 294002 on ICC networks were examined with Kit immunohistochemistry. ICC networks from newborn animals were similar to those described above and these networks were maintained in organ culture for at least 6 days (Fig. 9A-C; 5 days culture). In the presence of LY 294002 (10 μM), ICC networks at the level of the myenteric and deep muscular plexuses were disrupted within 2-3 days (Fig. 9D and E), and very few Kit-positive cells could be resolved after 5 days in culture with LY 294002 (Fig. 9F).
Figure 9. Effects of LY 294002 on ICC networks cultured for 5 days.
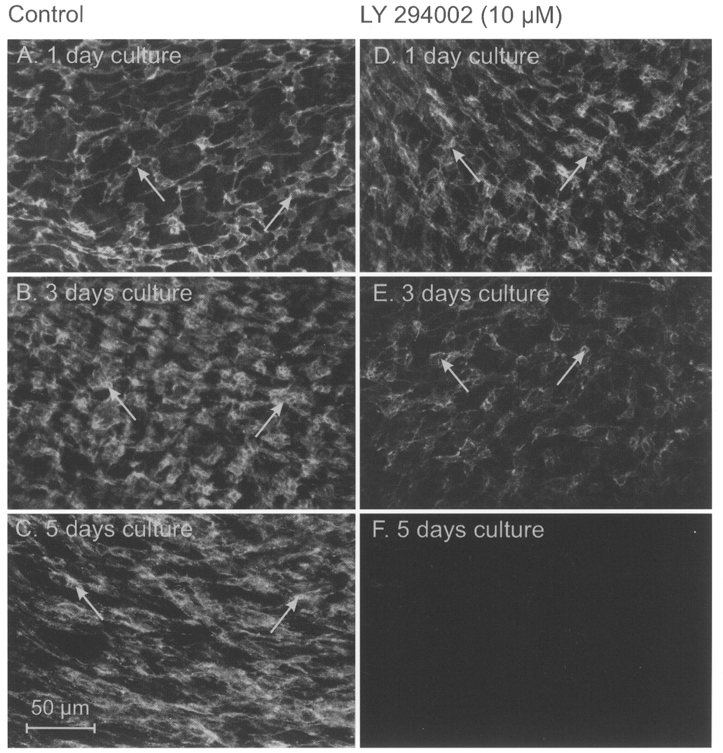
ICC networks from newborn animals were well preserved in organ culture as shown with Kit immunofluorescence on days 1, 3 and 5 (arrows; A-C, respectively). ICC networks were disrupted after 3-5 days in culture with LY 294002 (arrows; D-F). Very few Kit-positive cells were observed after 5 days of exposure to LY 294002 (F). Scale bar of 50 μm in C applies to all panels.
Loss of ICC from tissues of older animals when exposed to wortmannin
Previous studies have suggested that ICC are susceptible to block of Kit function for a short period after birth. Interference with Kit signalling after this period did not disrupt ICC networks and block electrical rhythmicity (Torihashi et al. 1995). We tested the effects of wortmannin and neutralizing Kit antibody (ACK2) on ICC and slow waves of jejunal tissues isolated from animals of different ages to determine if the effects of PI3-kinase are limited to a short developmental period immediately after birth. Jejunal muscles from animals aged D10 (n = 12), D20 (n = 10) and D30 (n = 12) days were incubated with wortmannin and ACK2 for 5-35 days. As previously reported, ACK2 had no effect on jejunal muscles placed into culture for 5 days after removal from D10-D30 animals (Ward et al. 1997). Wortmannin also had no significant effect on electrical slow wave properties when D10-D30 muscles were exposed to this compound for 5 days (not shown). Both ACK2 and wortmannin disrupted ICC networks when D10-D30 tissues were incubated for periods of 25-35 days. Under control conditions the Vm of cultured control D10-D30 jejunal muscles averaged -72 ± 2.5 mV and slow waves were 38 ± 3.5 mV in amplitude and occurred at a frequency of 21.3 ± 1.4 cycles min−1. After incubation with ACK2, Vm averaged -52 ± 2.5 mV and small oscillations in membrane potential were recorded in a single preparation (n = 12; average amplitude of 4 mV and frequency of 1 min−1). The Vm of tissues incubated for 25-35 days in the presence of wortmannin (10 μM) was -61.4 ± 3.8 mV and slow wave activity was completely inhibited in each preparation (n = 7; Fig. 10).
Figure 10. Effects of wortmannin and ACK2 on electrical slow waves.
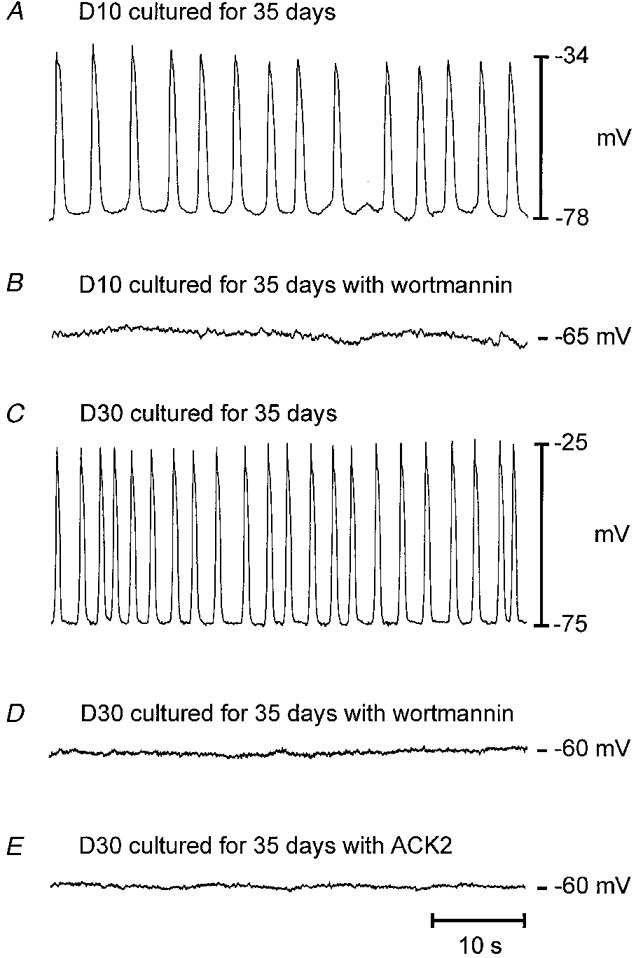
A shows control electrical activity from a D10 animal after 35 days in organ culture. B shows the lack of slow waves when an adjoining piece of tissue was incubated for a similar time period in the presence of wortmannin (10 μM). C-E show the electrical activity of tissues removed from a D30 animal, recorded under control conditions (C) and after culturing in the presence of wortmannin (D) or ACK2 (E).
DISCUSSION
Kit receptors are required for the development of ICC in the GI tract (Ward et al. 1994, 1995; Torihashi et al. 1995). The present study suggests that Kit receptors elicit intracellular signalling through activation of PI3-kinase. Blocking PI3-kinase results in effects similar to blocking Kit receptors with neutralizing Kit antibody (Torihashi et al. 1995; Ward et al. 1997). Neonatal muscles were relatively sensitive to inhibition of PI3-kinase, and ICC and electrical rhythmicity were lost within a few days of blocking this signalling molecule. As animals matured, however, the effectiveness of PI3-kinase inhibition waned, but did not vanish. Reduction in ICC and disruption of ICC networks was accomplished in mature GI muscles, but much longer periods of chronic exposure to PI3-kinase inhibitors or neutralizing Kit antibody were required. These findings suggest that dependence upon the Kit signalling pathway for maintenance of the ICC phenotype and rhythmicity extends into adulthood.
It is reasonable to question the specificity of the compounds used to inhibit PI3-kinase and the selectivity of the effects of these inhibitors on ICC. Wortmannin blocks other enyzmes in smooth muscle tissues (e.g. Duronio et al. 1998). LY 294002 is a more specific blocker of PI3-kinase that is structurally unrelated to wortmannin (Vlahos et al. 1994), but unrealized non-specific effects of this compound are also possible. We tested whether acute application of these compounds affected the electrical parameters used to assay ICC function and found that short-term treatments with wortmannin and LY 294004 were without significant effect on electrical rhythmicity. Other cells within the tunica muscularis might also be affected by chronic inhibition of PI3-kinase, since this is an important signalling molecule coupled to several receptor tyrosine kinases (Duronio et al. 1998). A thorough analysis of other functions of GI muscles was not performed, but the smooth muscle syncytium (which was used to assay the electrical activity generated by ICC) appeared to remain morphologically and functionally intact. Smooth muscle cells maintained resting membrane potentials close to normal levels, and the effects of PI3-kinase were strikingly similar to the effects of treatment with the neutralizing Kit antibody, which would be expected to provide a much more specific block of the Kit pathway than inhibitors of PI3-kinase. The similarities between the actions of PI3-kinase inhibitors and ACK2 suggest that the dominant actions of wortmannin and LY 294004 on ICC were to block downstream signalling via the Kit pathway. Possible secondary actions of PI3-kinases in the tunica muscularis appeared to be of lesser importance. The data are consistent with the hypothesis that Kit signalling, which employs PI3-kinase as a major secondary signalling molecule, is a critical pathway for the development and maintenance of ICC in GI muscles.
PI3-kinase couples to a variety of downstream effectors (see review by Duronio et al. 1998). There is relatively strong evidence that the product of PI3-kinase, phosphatidylinositol 3,4,5-trisphosphate, activates some isoforms of protein kinase C (e.g. Zhang et al. 1995; Moriya et al. 1996), and others have shown that PI3-kinase is associated with and co-immunoprecipitates with PKCδ and PKCε (Ettinger et al. 1996). PI3-kinase has also been shown to activate protein kinase B (PKB/Akt), a serine/threonine kinase, which may inhibit apoptosis by phosphorylating growth and survival factors (e.g. Dudek et al. 1997; Kauffmann-Zeh et al. 1997). PI3-kinase activation of PKB appears to be a major pathway mediating growth factor induced survival. Another protein activated by PI3-kinase is p70 ribosomal protein S6 kinase (p70S6K; Weng et al. 1995), but the role of this factor in PI3-kinase mediated events is unclear. The specific pathways that are important factors in ICC development and survival are unknown at the present time, but as new pharmacology and mutant animals are developed, it may be possible to further investigate the role of PI3-kinase in the regulation of ICC.
The present study expands our understanding of the duration over which Kit signalling is essential for development and maintenance of ICC. Original experiments suggested that Kit signalling was important for ICC development over a relatively narrow window of time (i.e. from late embryogenesis to about 10 days; see Maeda et al. 1992; Torihashi et al. 1995). There has been controversy about the duration of the Kit-dependent developmental window. One study, using transgenic mice in which the wild-type c-kit gene was rendered non-functional by replacement with a c-kit/lacZ construct, concluded that Kit was important only for post-natal ICC development (Bernax et al. 1996). These authors suggested that Kit receptors and signalling via the stem cell factor/Kit pathway is not necessary for the migration, proliferation and/or survival of ICC during embryogensis. Another study utilized Wbanded (Wbd) mutants, which possess a 2.5 kb genomic inversion within chromosome 5 that results in ectopic expression of c-kit (Kluppel et al. 1998). Wbd/Wbd mice do not express resolvable Kit in ICC. Networks of ICC were identified at D5 but not in adult mice. These experiments suggest that Kit was not necessary for lineage determination during embryogenesis, but that this pathway was essential in the post-natal period for proliferation and maintenance of the ICC phenotype. We have recently tested the role of Kit signalling in embryonic tissues by removing small intestinal muscles at E15 (before ICC lineage determination) and culturing the tissues with and without neutralizing antibody for Kit (ACK2; Ward et al. 1997). Control muscles developed functional ICC networks, but muscles cultured with ACK2 were devoid of ICC and electrically quiescent. Taken together, these studies suggest that Kit signalling was important for the emergence of the ICC lineage during embryogenesis in wild-type animals, but other pathways might be able to compensate for Kit signalling and support ICC development to the point of birth in mutants in which Kit is absent or levels are low. In the present study we have provided evidence that the developmental window necessary for Kit function should be expanded to include the period of adulthood. Neonatal ICC were strongly sensitive to block of Kit (Ward et al. 1997) and PI3-kinase (this study), and adult animals were more resistant to blockade of Kit and downstream signalling (Torihashi et al. 1995; and the present study). Thus, ICC develop resistance to blockade of the Kit signalling pathway with maturity, but these cells do not become independent of Kit function. Chronic treatments with ACK2 or wortmannin, over a period of several weeks, resulted in loss of Kit immunoreactive cells and electrical rhythmicity. Thus, our data suggest that dependence of ICC on Kit signalling extends from the time of lineage determination (i.e. about E15; Ward et al. 1997) into adulthood, but the relative sensitivity of ICC to signalling via the Kit pathway varies as a function of the developmental period.
We are only beginning to understand the various factors that affect ICC. The present study suggests that interference with Kit signalling causes ICC to disappear, but the fate of these cells is not clear at present. Recent studies suggest that ICC redifferentiate and assume a smooth muscle phenotype when Kit signalling is blocked (S. Torihashi, S. M. Ward and K. M. Sanders, unpublished observations). Several studies of human GI muscles have recently reported loss or damage to ICC in a variety of clinical disorders, such as chronic idiopathic pseudo-obstruction and inflammatory bowel disease (Isozaki et al. 1997; Lu et al. 1997). Processes leading to loss of ICC in motility disorders may or may not have much in common with the loss of ICC caused by blockade of Kit function, but the question needs more investigation. ICC serve critical functions in GI motility, so the effects of inflammatory mediators, environmental factors and disease conditions on these cells is an important new direction for gastrointestinal motility research.
Acknowledgments
The authors are grateful to Julia R. Bayguinov for excellent technical assistance. This work was supported by a Program Project Grant, DK41315 from NIH/NIDDK.
References
- Arcaro A, Wymann MP. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochemical Journal. 1993;296:297–301. doi: 10.1042/bj2960297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernax F, De Sepulveda P, Kress C, Elbaz C, Delouis C, Panthier J-J. Spatial and temporal patterns of c-kit-expressing cells in WlacZ/+ and WlacZ/WlacZ mouse embryos. Development. 1996;122:3023–3033. doi: 10.1242/dev.122.10.3023. [DOI] [PubMed] [Google Scholar]
- Deberry C, Mou S, Linnekin D. Stat1 associates with c-kit and is activated in response to stem cell factor. Biochemical Journal. 1997;327:73–80. doi: 10.1042/bj3270073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- Duronio V, Scheid MP, Ettinger S. Downstream signalling events regulated by phosphatidylinositol 3-kinase activity. Cell Signalling. 1998;10:233–239. doi: 10.1016/s0898-6568(97)00129-0. 10.1016/S0898-6568(97)00129-0. [DOI] [PubMed] [Google Scholar]
- Ettinger SL, Lauener RW, Duronio V. Protein kinase Cδ specifically associates with phosphatidylinositol 3-kinase following cytokine stimulation. Journal of Biological Chemistry. 1996;271:14514–14518. doi: 10.1074/jbc.271.24.14514. 10.1074/jbc.271.24.14514. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Isozaki K, Hirota S, Miyagawa J, Taniguchi M, Shinomura Y, Matsuzawa Y. Deficiency of c-kit+ cells in patients with a myopathic form of chronic idiopathic intestinal pseudo-obstruction. American Journal of Gastroenterology. 1997;92:332–334. [PubMed] [Google Scholar]
- Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- Kluppel M, Huizinga JD, Malysz J, Bernstein A. Developmental origin and Kit-dependent development of the interstitial cells of Cajal in the mammalian small intestine. Developmental Dynamics. 1998;211:60–71. doi: 10.1002/(SICI)1097-0177(199801)211:1<60::AID-AJA6>3.0.CO;2-5. 10.1002/(SICI)1097-0177(199801)211:1<60::AID-AJA6>3.3.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Kozawa O, Blume-Jensen P, Heldin CH, Ronnstrand L. Involvement of phosphatidylinositol 3′-kinase in stem-cell-factor-induced phospholipase D activation and arachidonic acid release. European Journal of Biochemistry. 1997;248:149–155. doi: 10.1111/j.1432-1033.1997.00149.x. 10.1111/j.1432-1033.1997.00149.x. [DOI] [PubMed] [Google Scholar]
- Linnekin D, DeBerry CS, Mou S. Lyn associates with the juxtamembrane region of c-Kit and is activated by stem cell factor in hematopoietic cell lines and normal progenitor cells. Journal of Biological Chemistry. 1997;272:27450–27455. doi: 10.1074/jbc.272.43.27450. 10.1074/jbc.272.43.27450. [DOI] [PubMed] [Google Scholar]
- Lu G, Qian X, Berezin I, Telford GL, Huizinga JD, Sarna SK. Inflammation modulates in vitro colonic myoelectric and contractile activity and interstitial cells of Cajal. American Journal of Physiology. 1997;273:G1233–1245. doi: 10.1152/ajpgi.1997.273.6.G1233. [DOI] [PubMed] [Google Scholar]
- Maeda H, Yamagata A, Nishikawa S, Yoshinaga K, Kobayashi S, Nishi K, Nishikawa S. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992;116:369–375. doi: 10.1242/dev.116.2.369. [DOI] [PubMed] [Google Scholar]
- Margolis B, Skolnik EY. Activation of Ras by receptor tyrosine kinases. Journal of the American Society of Nephrology. 1994;5:1288–1299. doi: 10.1681/ASN.V561288. [DOI] [PubMed] [Google Scholar]
- Moriya S, Kazlauskas A, Akimoto K, Hirai S, Mizuno K, Takenawa T, Fukui Y, Watanabe Y, Ozaki S, Ohno S. Platelet-derived growth factor activates protein kinase Cɛ through redundant and independent signalling pathways involving phospholipase Cγ or phosphatidylinositol 3-kinase. Proceedings of the National Academy of Sciences of the USA. 1996;93:151–155. doi: 10.1073/pnas.93.1.151. 10.1073/pnas.93.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibe S, Wahl MI, Hernandez-Sotomayor SM, Tonks NK, Rhee SG, Carpenter G. Increase of the catalytic activity of phospholipase C-γ1 by tyrosine phosphorylation. Science. 1990;250:1253–1256. doi: 10.1126/science.1700866. [DOI] [PubMed] [Google Scholar]
- Nishikawa S, Kusakabe M, Yoshinaga K, Ogawa M, Hayashi S-I, Kunisada T, Era T, Sakakura T, Nishikawa S-I. In utero manipulation of coat color formation by a monoclonal anti-c-kit antibody: two distinct waves of c-kit dependency during melanocyte development. EMBO Journal. 1991;10:2111–2118. doi: 10.1002/j.1460-2075.1991.tb07744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottapel R, Reedijk M, Williams DE, Lyman SD, Anderson DM, Pawson T, Bernstein A. The Steel/W transduction pathway: kit autophosphorylation and its association with a unique subset of cytoplasmic signalling proteins is induced by the Steel factor. Molecular and Cellular Biology. 1991;11:3043–3051. doi: 10.1128/mcb.11.6.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- Torihashi S, Ward SM, Nishikawa S-I, Nishi K, Kobayashi S, Sanders KM. c-kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell and Tissue Research. 1995;280:97–111. doi: 10.1007/BF00304515. 10.1007/s004410050334. [DOI] [PubMed] [Google Scholar]
- Torihashi S, Ward SM, Sanders KM. Development of c-Kit positive cells and the onset of electrical rhythmicity in murine small intestine. Gastroenterology. 1997;112:144–155. doi: 10.1016/s0016-5085(97)70229-4. [DOI] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)- 8-phenyl-4H-1-benzopyran-4-one (LY 294002) Journal of Biological Chemistry. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. The Journal of Physiology. 1994;480:91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Harney SC, Bayguinov JR, McLaren GJ, Sanders KM. Development of electrical rhythmicity in the murine gastrointestinal tract is specifically encoded in the tunica muscularis. The Journal of Physiology. 1997;505:241–258. doi: 10.1111/j.1469-7793.1997.241bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng QP, Andrabi K, Klippel A, Kozlowski MT, Williams LT, Avruch J. Phosphatidylinositol 3-kinase signals activation of p70 S6 kinase in situ through site-specific p70 phosphorylation. Proceedings of the National Academy of Sciences of the USA. 1995;92:5744–5748. doi: 10.1073/pnas.92.12.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano H, Nakanishi S, Kimura K, Hanai N, Saitoh Y, Fukui Y, Nonomura Y, Matsuda Y. Inhibition of histamine secretion by wortmannin through the blockade of phosphatidylinositol 3-kinase in RBL-2H3 cells. Journal of Biological Chemistry. 1993;268:25846–25856. [PubMed] [Google Scholar]
- Zhang J, Falck JR, Reddy KK, Abrams CS, Zhao W, Rittenhouse SE. Phosphatidylinositol (3,4,5)-trisphosphate stimulates phosphorylation of pleckstrin in human platelets. Journal of Biological Chemistry. 1995;270:22807–22810. doi: 10.1074/jbc.270.39.22807. 10.1074/jbc.270.39.22807. [DOI] [PubMed] [Google Scholar]


