Abstract
During its early ‘genomic’ phase of action (< 3 h), aldosterone activates pre-existing Na+ pumps (Na+,K+-ATPase) in epithelia formed by Xenopus laevis A6 kidney cells.
To test whether this action also applies to pumps containing mammalian α-subunits of different isoforms, we generated A6 cell lines expressing the naturally ouabain-resistant rat α1 subunit or the rat α2* and α3* subunits made ouabain resistant by site-directed mutagenesis.
Cell lines were obtained which expressed the exogenous α-subunits in active, basolateral Na+ pumps, such that ouabain-resistant pump current (Ip) could be measured following apical permeabilization with amphotericin B.
The inhibition constants (Ki) for ouabain of the current carried by the pumps containing exogenous rat α-subunits were similar to those reported previously for ATPase activity inhibition. The apparent Michaelis constant (Km) for Na+ (K+ replacement) was slightly higher for pumps containing the rat α1 than for those containing the α2* subunit (34.9 ± 1.9 versus 26.3 ± 2.6 mM).
At a Na+ concentration of 10 mM, aldosterone (2.5 h) increased the pump current carried by endogenous pumps as well as that carried by pumps containing the exogenous rat α1 subunit (by 1.8- to 2.2-fold). In contrast, the current carried by pumps containing the exogenous rat α2* subunit remained unchanged.
The fact that this early transcriptionally mediated activation of Na+ pumps by aldosterone is specific for pumps containing an α1 subunit should permit the identification in this subunit of structures involved in its regulation.
Aldosterone controls the final adjustment of urinary Na+ excretion by regulating its reabsorbtion across epithelial cells of the distal nephron segments. This Na+ transport across the two membrane barriers of principal cells involves apical influx via the epithelial Na+ channel (ENaC) and basolateral extrusion by Na+,K+-ATPase (the Na+ pump). In general the apical influx represents the rate-limiting step, providing that the functional reserve of the Na+ pump is sufficient (Rossier & Palmer, 1992; Verrey, 1995). Indeed, the intracellular Na+ concentration must stay within a certain range to maintain the driving force for Na+ entry and to allow the ENaC to respond to hormonal stimulation by increasing its activity. Otherwise, high intracellular concentrations of Na+ would inhibit channel activity due to a tight feedback regulation (Garty & Palmer, 1997). The functioning of the Na+ pump efficiently adapts to increasing Na+ influx due to its favourable kinetic properties (positive co-operativity for Na+, Km above [Na+]i). However, the fact that Na+ transport can be regulated over a very large range in these epithelia suggests that other mechanisms might be involved to co-ordinate the basolateral extrusion rate with the apical influx (Barlet-Bas et al. 1991; Verrey et al. 1996).
We have previously shown that aldosterone, during its early phase of action, increases the functioning of Na+ pumps independently of apical Na+ influx (Beron & Verrey, 1994; Beron et al. 1995). This effect was measured in epithelia formed by the Xenopus laevis kidney cell line A6 cultured on permeable supports. In such epithelia, which phenotypically resemble mammalian cortical collecting duct, the pump function was measured after apical permeabilization with amphotericin B as pump current (Ip) at fixed Na+ concentrations. Aldosterone produced a 1.5- to 2-fold increase in Ip only at low physiological intracellular Na+ concentrations. This transcription-dependent effect of aldosterone is considered early since it starts after a lag period of 30-60 min and reaches a maximum after 2.5 h. Mechanistically, it is thought to be mediated by the activation of a pump subpopulation presenting a higher Na+ affinity than the main population. The activated pumps contain an α1 isoform of the Na+,K+-ATPase catalytic subunit, since the stimulation has been shown to take place on pumps containing a transfected amphibian α1 subunit. This also confirms that this early aldosterone effect is not due to transcriptional induction of the α-subunit of the pump itself, an effect known to belong to the late phase of the aldosterone action (Verrey et al. 1989; Verrey et al. 1996).
We now ask the question whether this pump activation by aldosterone is specific for amphibian Na+,K+-ATPase or whether it also takes place with pumps containing a mammalian catalytic α-subunit and whether this effect is α-subunit isoform specific. There are at least three highly homologous α-subunits of the Na+ pump (Shull et al. 1986). The α1 subunit is ubiquitous and clearly the major or single α-isoform all along the kidney nephron (Lucking et al. 1996). The other isoforms, α2 and α3, are restricted to certain tissues, α2 mainly to brain, heart, skeletal muscle and fat cells, α3 to the brain. The differential function of these isoforms has not been studied extensively but differences have been described as regards the developmental expression pattern (Herrera et al. 1994; Betts et al. 1998), subcellular localization (Juhaszova & Blaustein, 1997; Slezak et al. 1997; Betts et al. 1998), regulation (CorthesyTheulaz et al. 1991; Lytton et al. 1994), ouabain sensitivity (Sweadner, 1989) and transport kinetics (Jewell & Lingrel, 1991; Munzer et al. 1994; Zahler et al. 1997).
Here we take advantage of the possibility of measuring the function of pumps containing a transfected ouabain-resistant α-subunit independent of the endogenous ones. We have established A6 cell lines expressing the rat α1 isoform, which is naturally resistant towards cardiotonic steroids, and others expressing the rat α2 and α3 isoforms rendered ouabain-resistant by point mutations at the level of the first extracellular loop (Jewell & Lingrel, 1991). We show that all these mammalian α-isoforms produce functional Na+ pumps localized at the basolateral membrane. However, only the pumps containing rat α1 subunits are stimulated by short-term application of aldosterone.
METHODS
Cell culture
A6 cells from the A6-C1 subclone (Verrey et al. 1993) and transfected A6-C1 cells were cultured on polycarbonate filters (Transwell; 0.4 μm pore size, 4.7 cm2; Costar, Cambridge, MA, USA) coated with a thin collagen layer, as previously described (Beron & Verrey, 1994). Before the experiments, epithelia were in culture for 15-19 days, the first 10 days in medium buffered with bicarbonate and containing 10 % fetal bovine serum and then in serum-free Hepes-buffered medium (× 0.8 concentrated Dulbecco's modified Eagle's medium (DMEM); Life Technologies).
A6 cell lines expressing rat Na+,K+-ATPase subunits α1, α2* and α3* (rα1, rα2*, rα3*)
The cDNAs of rα1 (naturally cardiotonic steroid resistant), rα2* and rα3* (rendered resistant by point mutations, Jewell & Lingrel, (1991)) were obtained from J. Lingrel and transferred to the eukaryotic expression vector pcDNA3 (Invitrogen, Leek, Netherlands). A6-C1 cells were transfected by the Polybrene method (Brewer, 1994). Briefly, non-confluent A6-C1 cells were passaged at a density of 2 × 105 cells per 9.6 cm2 dish on the day before transfection. Plasmid DNA (5 μg) was mixed with 3.75 μg Polybrene (Aldrich) in 1 ml serum-free medium and given to pre-washed cells. After 6-8 h incubation, this medium was replaced by serum-free medium containing 30 % DMSO for exactly 5 min. Cells were then washed once and 2 ml of medium containing 10 % fetal bovine serum was given. The medium was changed the next day and after an additional 1-2 days, the cells were passaged to a 78 cm2 dish and selection for cardiotonic steroid-resistant cells was started by adding ouabain to a final concentration of 10, 20 or 50 μM. Every 3 days, the ouabain-containing medium was changed. After 2-3 weeks, ouabain-resistant colonies were isolated by ring cloning. The culture conditions were the same as for A6-C1 cells, except for the presence of ouabain (5 μM) for cells cultured on dishes. Experiments were performed between passages 4 and 40 after ring cloning. To enhance the expression of the transfected rα Na+,K+-ATPase subunits, sodium butyrate was given at a concentration of 2 mM for 3 days and 4 mM for 1 day before the experiment (Beron et al. 1995).
Electrical measurements
Transepithelial electrical measurements were performed as previously described on Transwell filter cups in a modified Ussing chamber using an automatic voltage clamp device (Verrey, 1994) at a temperature of 25-28°C. The measurements of transepithelial potential difference and short-circuit current (Isc) were done in serum-free Hepes-buffered culture medium (see above).
Measurements of Ip were performed as described in Beron et al. (1995). Briefly, epithelia were permeabilized apically to monovalent ions with 20 or 40 μg ml−1 amphotericin B for 30 min. During the measurements, epithelia were kept in the short-circuited state in symmetrical buffers containing 5 mM barium and the indicated Na+ concentration (Na++ K+ = 100 mM). The transepithelial resistance was 5000-17 000 Ω cm2 before permeabilization and 1500-5000 Ω cm2 after (Beron et al. 1995). Aldosterone (10−6 M) or diluent (ethanol) was given to cells starting 2.5 h before permeabilization. To measure the Ip carried by pumps containing the endogenous α1 subunit, 50 μM strophanthidin was given to the basolateral compartment. This concentration was previously shown to block 99.5 % of the endogenous pumps in untransfected cells (Beron et al. 1997). After an equilibration period of 1-3 min, the exogenous pumps were blocked by the basolateral addition of 3 mM ouabain from a 10 mM stock prepared in the same buffer with the same Na+ concentration. The difference in current before and 1 min after the addition of strophanthidin or ouabain was taken as Ip carried by the pumps containing the cardiotonic steroid-sensitive endogenous and the cardiotonic steroid-resistant exogenous α-subunit, respectively.
To determine the apparent Km for Na+ of the pumps containing endogenous and exogenous subunits, different preparations were used for measuring the IP values at five or six different Na+ concentrations ((5), 10, 20, 40, 60 and 90 mM). The Ip produced by pumps containing endogenous and exogenous α-subunits was measured in the same preparation, as described above. Current values obtained in independent experiments (5 and 8 for rat α1 and α2 subunit-expressing epithelia, respectively) were pooled after normalizing these values relative to the current measured at 90 mM Na+. Sigmoidal curves were fitted to these points using a non-linear regression analysis routine (GraphPad Prism 2.0; GraphPad, San Diego, CA, USA) and Na+ concentrations required for half-maximal activation were derived. The Hill coefficient was set to 2.8 based on the results of preliminary curve fittings in which the maximal fractional current was set to 1.1. Setting the Hill coefficient to 2.5 or 3.0 instead of 2.8 did not change the Km values significantly (0.3 % to 3.6 % change).
The Ki of cardiotonic steroids was measured at a near-maximal Ip induced by 90 mM Na+. For endogenous pumps strophanthidin was added stepwise at increasing concentrations and for hybrid pumps the endogenous pumps were first blocked with 50 μM strophanthidin and then ouabain was added stepwise at increasing concentrations. One site inhibition curves were fitted to the experimental points and the ouabain or strophanthidin concentrations required for half-maximal inhibition (Ki) were derived (GraphPad Prism).
Western blotting
Microsomal proteins were prepared from the different cell lines (Beron & Verrey, 1994). Equal amounts of protein were separated by SDS-polyacrylamide gel electrophoresis. Western blotting and signal detection were as previously described (Beron & Verrey, 1994).
Mouse monoclonal IgG antibodies McK1 (Felsenfeld & Sweadner, 1988) and McB2 (Urayama et al. 1989), kindly provided by K. Sweadner, and MA3-915 (Affinity BioReagents, Neshanic Station, NJ, USA) were used for the detection of rα1, rα2* and rα3*, respectively, and a goat anti-mouse IgG antibody coupled to horseradish peroxidase (A4416; Sigma) was used as the secondary antibody.
Statistics
The standard error of the mean (s.e.m.) of n experiments is indicated. Student's t tests were performed to estimate the significance of the differences.
RESULTS
Exogenous rat Na+,K+-ATPase α1, α2 and α3 subunit isoforms form functional basolateral pumps in A6 epithelia
A6 cells were transfected with rat Na+,K+-ATPase α1, α2* and α3* subunits. These α-subunits are known to associate with endogenous β1 subunits in heterologous expression systems and form pump units with a high resistance toward cardiotonic steroids (Jewell & Lingrel, 1991). Wild-type rat α1 subunit is naturally ouabain resistant and rat α2* and α3* subunits were made cardiotonic steroid resistant by site-directed mutagenesis (Jewell & Lingrel, 1991). Since endogenous A6 α1 subunit is a ouabain-sensitive form, the selection of transfected cells expressing the exogenous α-subunits could be performed with ouabain at concentrations of 10-50 μM. Cell lines (12 rα1, 26 rα2* and 30 rα3*) were isolated by ring cloning and grown on filter supports. The following electrophysiological criteria were considered in selecting those cell lines which were subsequently used: transepithelial resistance (RTE) > 5000 Ω cm2; regulation of transepithelial short-circuit current (Isc) by aldosterone (2.5 h, 10−6 M); and high cardiotonic steroid-resistant Ip. The highest level of cardiotonic steroid-resistant Ip was observed in cell lines containing an exogenous rα1 subunit (up to > 2 μA cm−2; mean of the 12 rα1-A6 cell lines, 0.75 ± 0.44 μA cm−2). Cell lines expressing rα2* and rα3* in which basolateral pump function could be measured as cardiotonic steroid-resistant Ip were also identified. Their cardiotonic steroid-resistant Ip was lower (mean, 0.44 ± 0.2 μA cm−2 for rα2* and 0.40 ± 0.18 μA cm−2 for rα3*) than that of cell lines expressing rα1. Nevertheless, the results show that a substantial amount of these subunits, which are atypical for epithelial cells, were expressed at the basolateral surface in functional Na+ pump units.
As a function-independent biochemical approach to test for the presence of the transfected rat α-subunits, Western blots with rat isoform-specific antibodies were performed. Figure 1 shows Western blots of microsomal proteins from A6 cells and A6 cells transfected with rα1, rα2* or rα3*. The isoform-specific bands were detected as expected at levels corresponding to a molecular weight of approximately 100 kD. There was no cross-reactivity with the endogenous X. laevisα-subunits. Lanes 3 and 4, 7 and 8, and 11 and 12 show two independent cell lines expressing rα1, rα2* and rα3*, respectively. Only cell lines showing a clear band for the exogenous rat α-subunits by Western blot expressed a cardiotonic steroid-resistant Ip, indicating that this current was indeed only carried by pumps containing an exogenous α-subunit. The total Ip (pumps with endogenous and exogenous α-subunits) was similar in A6 cells and transfected cell lines (α1 cell lines, 4.51 ± 1.43 μA cm−2; α2* cell lines, 4.87 ± 1.65 μA cm−2; and α3* cell lines, 5.04 ± 1.85 μA cm−2), suggesting that the total number of functional basolateral pumps is limited by another factor, possibly the availability of endogenous β-subunits.
Figure 1. Western blot showing the expression of rat Na+,K+-ATPase α-subunits in stably transfected A6 cells.
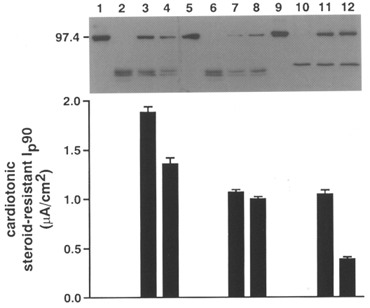
Equal amounts of microsomal proteins prepared from A6 cells (lanes 2, 6 and 10) and A6 cell lines expressing rat Na+,K+-ATPase α1 (lanes 3 and 4), α2* (lanes 7 and 8) and α3* (lanes 11 and 12) were separated by SDS-PAGE, transferred to nitrocellulose and probed with an anti-rat α1, α2* or α3* antibody, respectively. Lanes 1, 5 and 9 show proteins from a rat brain preparation. The cardiotonic steroid-resistant Na+ pump current at 90 mM Na+ (Ip90) of the corresponding cell lines is shown below.
For subsequent experiments, the two cell lines with the highest expression of rα1, rα2* and rα3* that fulfilled the electrophysiological criteria mentioned above were chosen (Fig. 1). To exclude the possibility that the results could be cell line specific, all experiments were performed with both cell lines of each isoform. Since there was no substantial difference between the cell lines expressing the same rat α-isoform, results were pooled.
Electrophysiological characterization of cell lines expressing rα1, rα2* and rα3* Na+,K+-ATPase subunits
For all electrophysiological measurements, cells were grown on filter supports. Ip was measured after apical permeabilization of the cells to monovalent ions with amphotericin B in Na+-free buffer. Na+ at the indicated concentration was added to the apical and basolateral sides of epithelia which were maintained at a voltage clamp of 0 mV to measure the Isc. Ip carried by endogenous pumps was then blocked by the addition of strophanthidin (50 μM). This concentration of strophanthidin blocked the endogenous pumps to > 99 % as expected and this is shown in wild-type A6 cells (Fig. 2A) (Beron et al. 1997). Ip carried by hybrid pumps containing a rat α-subunit was blocked by the addition of ouabain (3 mM final concentration).
Figure 2. Cardiotonic steroid inhibition of Ip: original tracings and inhibition curves fitted to the experimental points.
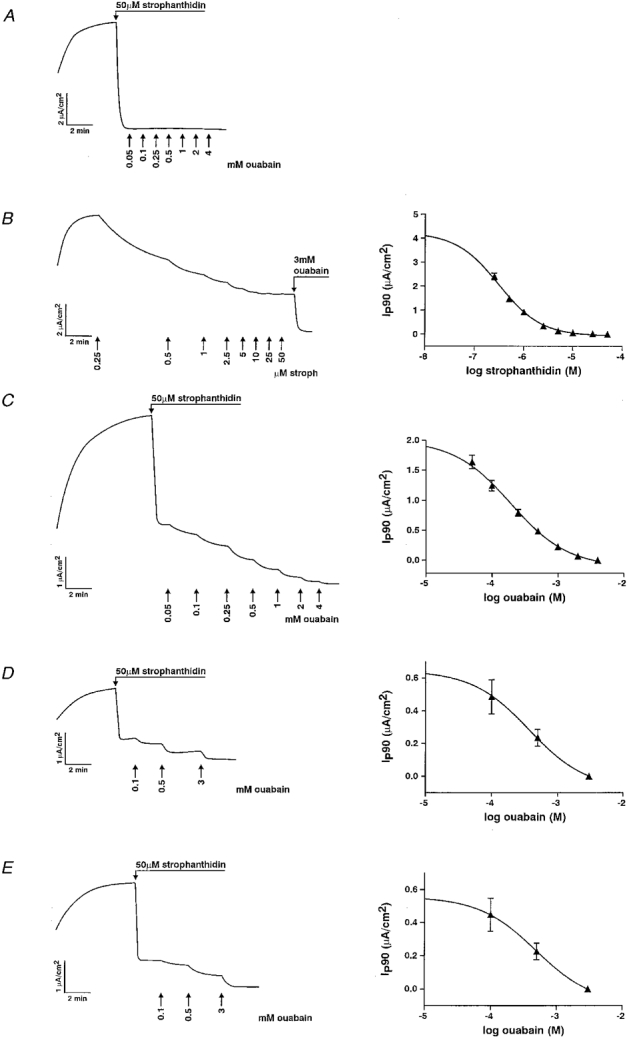
A, inhibition of endogenous pumps in A6 cells. Ip was completely blocked by 50 μM strophanthidin since addition of ouabain blocked no further Ip. B, inhibition of endogenous pumps by strophanthidin in a cell line expressing rat Na+,K+-ATPase α1 subunit. The Ip reaches a plateau at a strophanthidin concentration of 10 μM, indicating the full inhibition of pumps containing the endogenous α1 subunit. Pumps containing rα1 were then blocked by the addition of 3 mM ouabain. C-E, dose-dependent inhibition of pumps containing rα1 (C), rα2* (D) and rα3* (E) by ouabain.
To determine the Ki of strophanthidin on endogenous pumps and the Ki of ouabain on pumps containing rα1, the near-maximal Ip induced by 90 mM Na+ was inhibited by the basolateral addition of increasing concentrations of strophanthidin or ouabain, respectively. An example of inhibition of the endogenous pumps by strophanthidin is shown in Fig. 2B. The mean Ki of strophanthidin on endogenous pumps was 303 ± 26 nM (n = 6), similar to that determined previously (Beron et al. 1995). The Ki of ouabain on pumps containing rα1 measured after addition of 50 μM strophanthidin was 194 ± 14 μM (n = 6) (Fig. 2C). Hence it can be estimated that only approximately 5 % of the pumps containing rα1 were inhibited by 50 μM strophanthidin, considering that strophanthidin is 10-fold less potent than ouabain. For rα2* and rα3* subunits, which are ouabain insensitive due to point mutations introduced in their first extracellular loop (Jewell & Lingrel, 1991), fewer concentration steps were done. Half-maximal inhibition (Ki) was reached at ouabain concentrations of 372 ± 32 μM (n = 4) and 484 ± 47 μM (n = 5), respectively, similar to values obtained in HeLa cells by Jewell & Lingrel (1991) (Fig. 2D and E). Thus it can be estimated that even less of their function was inhibited by 50 μM strophanthidin than in the case of rα1.
To compare the Na+ activation kinetics of pumps containing the endogenous α1 subunit with those containing rα1 and rα2*, the cardiotonic steroid-inhibitable Ip values induced by five or six Na+ concentrations (K+ replacement) were measured and the apparent Km for Na+ derived (Fig. 3A and B). In cell lines expressing rα1, the apparent Km for Na+ of pumps with endogenous α1 was 36.0 ± 1.2 mM (n = 5) and 34.9 ± 1.9 mM (n = 5) for those with rα1. In cell lines expressing rα2* the apparent Km for Na+ of endogenous α1 was 33.8 ± 3.4 mM (n = 8); for rα2* it was 26.3 ± 2.6 mM (n = 8). Accurate values could not be obtained with cell lines expressing rα3* because of the low level of Ip carried by exogenous pumps.
Figure 3. Na+ activation of endogenous Na+ pumps (▴) and pumps containing exogenous subunits (▾) in cells expressing rα1 (A) and rα2* (B).
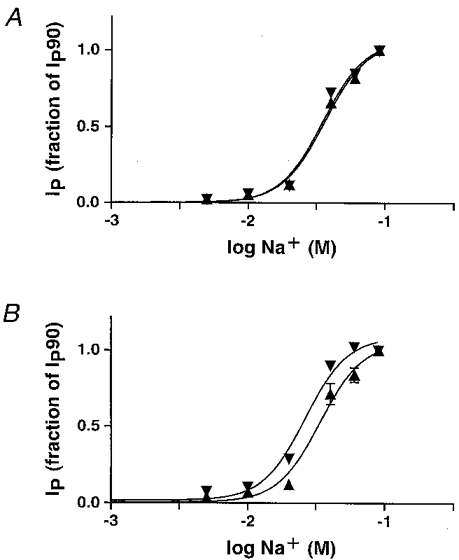
Ip was induced with six different Na+ concentrations and blocked by the addition of cardiotonic steroids. The Ip is given as fraction of the current measured at 90 mM Na+ (fraction of Ip90). Mean Ip90 in rα1-expressing epithelia (A) was 4.62 ± 0.19 μA cm−2 for pumps containing the endogenous α1 subunit and 1.94 ± 0.13 μA cm−2 for pumps containing the exogenous rat α1 subunit (n = 5). In rα2*-expressing epithelia (B), mean IP90 was 3.99 ± 0.39 μA cm−2 for pumps containing the endogenous α1 subunit and 0.75 ± 0.05 μA cm−2 for pumps containing the exogenous rα2* subunit(n = 8).
Aldosterone acts on pumps containing rα1 subunits but not on pumps containing rα2* subunits
Aldosterone induced an increase in amiloride-inhibitable transepithelial Na+ transport in the transfected cell lines as well as in the A6 cell line. A treatment of 2.5 h aldosterone (early effect) induced a 2.42 ± 0.15-fold (n = 5) increase in Isc across epithelia of untransfected A6 cells and a 3.30 ± 0.96-fold (n = 8), 3.33 ± 0.95-fold (n = 9) and 3.32 ± 0.53-fold (n = 9) increase across those formed by cells expressing rα1, rα2* and rα3*, respectively, compared with cells treated with diluent (Fig. 4). Incubation with diluent (EtOH) induced no increase in Isc. To investigate the effect of aldosterone on Na+,K+-ATPase at a physiological intracellular Na+ concentration, Ip at 10 mM Na+ was measured in epithelia pretreated with aldosterone (2.5 h, 10−6 M) or diluent (EtOH). The fractional change of Ip blocked by the addition of 50 μM strophanthidin (Ip carried by pumps containing endogenous α1) after 2.5 h aldosterone was 2.2 ± 0.3 and 1.8 ± 0.1 in rα1 and rα2* cell lines, respectively (Fig. 5A and B), similar to the effect on the Ip of A6 cells (Beron et al. 1995). The Ip carried by pumps containing an exogenous subunit was measured as the current subsequently blocked by the addition of 3 mM ouabain. Similar to its effect on endogenous pumps, aldosterone induced a fractional change of Ip carried by pumps containing rα1 of 1.8 ± 0.2. In contrast, the Ip carried by pumps containing rα2* was not increased (fractional change, 1.1 ± 0.1). As expected, there was no ouabain-inhibitable current in untransfected A6 cells after the addition of strophanthidin. In the case of cell lines expressing the rα3* subunit, there was also no visible effect of aldosterone (2.5 h) on the Ip carried by pumps containing this exogenous subunit. However, this current was too small to permit reliable quantification (data not shown). As previously shown in A6 cells, this stimulatory effect of aldosterone on Ip was only significant at physiologically low Na+ concentrations. Indeed, aldosterone did not significantly increase the Ip of endogenous or exogenous pumps measured at high Na+ concentrations (90 mM) (Fig. 5C and D).
Figure 4. Stimulation of transepithelial short-circuit current by aldosterone.
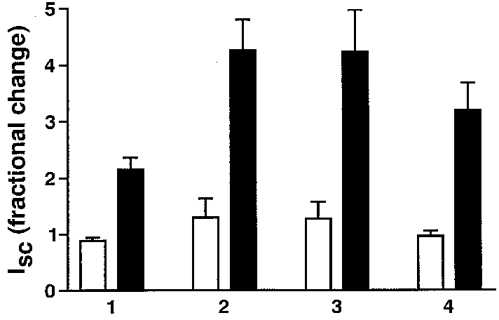
Effect of aldosterone (2.5 h) (▪) on amiloride-inhibitable short-circuit current (Isc) in epithelia formed by A6 cells (1) and A6 cell lines expressing rα1 (2), rα2* (3) and rα3* (4) Na+,K+-ATPase subunits. Diluent (EtOH; □) induced no increase in Isc.
Figure 5. Stimulation by aldosterone (2.5 h) of Ip produced by pumps containing different α-subunits at 10 mM (IP10) and 90 mM (IP90) Na+.
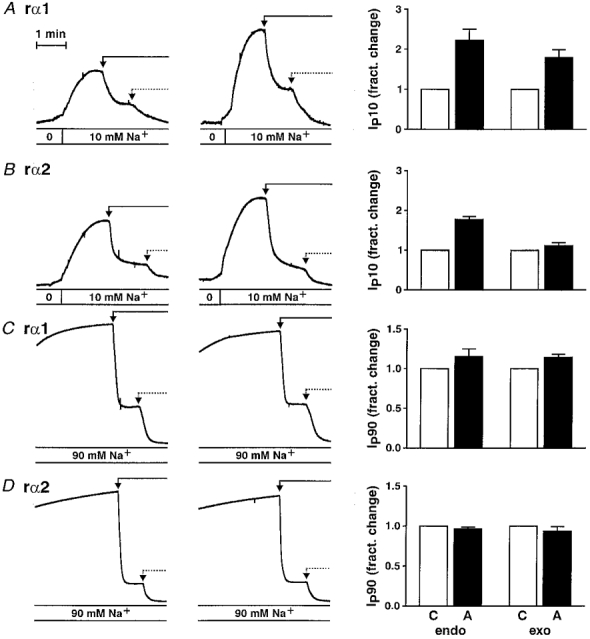
Original recordings are shown in the left panels. Ip was induced by the addition of 10 mM (A and B) and 90 mM (C and D) Na+. Ip carried by endogenous pumps was then blocked by the addition of 50 μM strophanthidin (continuous arrow). Ip carried by pumps containing an exogenous subunit was subsequently blocked by the addition of 3 mM ouabain (dotted arrow). Cell lines expressing rα1 are shown in A and C and cell lines expressing rα2* are shown in B and D. The right-hand panels show the mean fractional changes in Ip (aldosterone (▪) vs. control (□)) carried by pumps containing endogenous (endo) and exogenous (exo) α-subunits, respectively, measured in 8, 12, 7 and 4 paired experiments (A-D). Mean total IP10 (IP90) in cell lines expressing rα1 and rα2* was 0.38 ± 0.06 μA cm−2 (5.17 ± 0.32 μA cm−2) and 0.30 ± 0.02 μA cm−2 (5.02 ± 0.78 μA cm−2), respectively, in control epithelia and 0.71 ± 0.09 μA cm−2 (5.83 ± 0.50 μA cm−2) and 0.46 ± 0.03 μA cm−2 (4.84 ± 0.86 μA cm−2) in aldosterone-treated epithelia.
DISCUSSION
Expression of rat α1, α2* and α3* Na+,K+-ATPase subunits in A6 cells
Subcellular localization as well as transcriptional and post-transcriptional regulation of Na+ pumps containing different α-subunit isoforms have been shown to differ in cells naturally expressing more than one isoform (CorthesyTheulaz et al. 1991; Herrera et al. 1994; Juhaszova & Blaustein, 1997; Slezak et al. 1997). For instance, with respect to the polarity of surface expression in epithelial cells, α1 and α3 isoforms have been shown to localize mainly to basolateral and apical membrane domains of bovine trophectoderm, respectively (Betts et al. 1998). Therefore it may be inferred that the differentially composed Na+ pump hetero-oligomers play differential physiological roles. Kinetically some differences, in particular in apparent Km for Na+, have been noticed between pumps containing the three major α-isoforms upon expression in the same cell type (Jewell & Lingrel, 1991; Munzer et al. 1994; Zahler et al. 1997). Kinetic differences have also been observed with pumps of same αβ-isoform composition expressed in different cells (Therien et al. 1996). As yet, little is known about determinants of differential localization, regulation and/or kinetics of the different α-subunit isoforms. Concerning potential subcellular localization determinants, a basolateral sorting signal has been localized to the N-terminal half of the α1 subunit, and determinants for the apical sorting of the related gastric H+,K+-ATPase α-subunit have been localized to the fourth transmembrane domain (a segment which is fully conserved in the three major Na+,K+-ATPase α-subunit isoforms) (Dunbar et al. 1997; Muth et al. 1998). Furthermore, the minimal ankyrin-binding domain has been localized to the second cytoplasmic domain and found to be necessary for polarized membrane localization in MDCK cells. However, this sequence is also fully conserved in the different isoforms (Devarajan et al. 1994, 1997).
In the present study, the three major rat α-subunit isoforms were expressed in the amphibian cell line A6, which is a widely used model to study transepithelial transport in aldosterone target cells. These three mammalian α-subunit isoforms, which are naturally ouabain resistant (rα1) or rendered ouabain resistant by point mutations in the first extracellular loop (rα2* and rα3*), form functional pumps together with endogenous β-subunit (mainly of the β1 isoform) such that cell lines can be selected using ouabain. Interestingly, transfection with rα3* repetitively led to a higher number of resistant colonies than transfection with the two other isoforms. However, the ouabain-resistant Ip measured in differentiated epithelia formed by cell lines expressing rα3* was mostly very low and those rα3*-expressing cell lines with the highest level of ouabain-resistant Ip showed currents clearly lower than those obtained in rat rα1-expressing cells. The level of Ip obtained with rα2* expression was also lower than with rα1. Among possible reasons to explain this observation are that rα2* and rα3* subunit isoforms may be less strictly polarized to the basolateral membrane in A6 cells than the rα1 subunit, as suggested for α3 by its apical localization in trophectoderm mentioned above, or that the half-life at the basolateral membrane of pumps containing rα2* or rα3* may be shorter than that of pumps containing rα1. This would probably not be due to a deficient anchoring via ankyrin, since the binding domain appears to be fully conserved. Concerning the pumps containing the rα3* isoform, it might be that the apparent affinity of these pumps for Na+ is lower, as suggested by other studies (Jewell & Lingrel, 1994; Munzer et al. 1994; Zahler et al. 1997), preventing the measurement of near-maximal Ip values in our system. In any case, it is remarkable that pumps containing any of the three major rat Na+,K+-ATPase α-subunit isoforms are expressed as functional units at the basolateral membrane of a differentiated epithelium, as demonstrated by the fact that a specific Ip could be measured upon apical permeabilization with amphotericin B.
Interestingly, the Na+ activation curves obtained with rα1- and rα2*-containing pumps were quite similar (Fig. 3). The slightly higher Na+ sensitivity of the pumps containing the rα2* subunit parallels the observations reported by Jewell & Lingrel (1991) but contradicts the results of Munzer et al. 1994 and Zahler et al. 1997. These discrepancies confirm that the kinetic differences due to the differential structure of the pump isoforms are, to a major extent, codetermined by other cellular factors and/or by the experimental approach.
Differential regulation of Na+ pumps containing mammalian α1 and α2 subunits by aldosterone in A6 epithelia
We have previously shown that within the time frame of the early transcriptional response (2.5 h), aldosterone increases the Na+ pump function at physiologically low intracellular Na+ concentrations by a factor of approximately 2 in A6 epithelia. This observation suggested that a relatively small subpopulation of pumps with a higher apparent affinity for Na+ was activated or that a fraction of the already active pumps was modified in its apparent affinity for Na+. However, the mechanism underlying this effect is not known. It was, however, shown that pumps containing an exogenous amphibian α1 subunit could be the target of the aldosterone action (Beron et al. 1995).
In the present study, we show that this aldosterone effect is not limited to pumps containing amphibian α1 subunits, since pumps containing the rat α1 subunit were also stimulated. In contrast, aldosterone did not act on pumps containing the rat α2* subunit isoform. In the case of pumps containing rat α3* subunit, no stimulation was observed either, but the level of Ip was too low to draw a definitive conclusion.
The difference in regulation between pumps containing the rα1 or rα2* (and rα3*) subunit suggests that some structure(s) within the α1 subunits are necessary to allow aldosterone to produce its regulatory action. Furthermore, it shows that this α1-specific structure is conserved between amphibians and mammals. The present experiments do not, however, permit us to reach a conclusion about the nature of the regulatory action of aldosterone on Na+ pumps. As discussed above, this aldosterone-induced increase in pump function observed at physiological intracellular Na+ concentrations has been suggested to be due to the modification of the apparent Na+ affinity of a fraction of the already functional pumps or, alternatively, to the activation of a pool of pre-existing, previously silent high affinity Na+ pumps. Such regulating effects on Na+ pumps could be mediated by their interaction with: (i) an aldosterone-induced protein; (ii) a protein activated by an aldosterone-induced protein; or (iii) by their covalent modification via the action of (an) aldosterone-induced protein(s). Whichever mechanism applies, the differential effect of aldosterone on Na+ pumps containing highly similar α-subunit isoforms (87 % identity) opens the possibility of identifying the structures within the α1 subunit which are the target of this regulatory action.
Acknowledgments
The authors thank Kathleen Sweadner for her kind gift of two monoclonal antibodies, Jerry Lingrel for the rat α-subunit cDNAs and Christian Gasser for the artwork. This study was supported by the Swiss NSF grant 31-49727.96 and the Olga Mayenfisch Stiftung.
References
- Barlet-Bas C, Cheval L, Féraille E, Marsy S, Doucet A. Regulation of tubular Na-K-ATPase. In: Hatano M, editor. Nephrology. Tokyo: Springer-Verlag; 1991. pp. 419–434. [Google Scholar]
- Beron J, Forster I, Beguin P, Geering K, Verrey F. Phorbol 12-myristate 13-acetate down-regulates Na,K-ATPase independent of its protein kinase C site - decrease in basolateral cell surface area. Molecular Biology of the Cell. 1997;8:387–398. doi: 10.1091/mbc.8.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beron J, Mastroberardino L, Spillmann A, Verrey F. Aldosterone modulates sodium kinetics of Na,K-ATPase containing an α1 subunit in A6 kidney cell epithelia. Molecular Biology of the Cell. 1995;6:261–271. doi: 10.1091/mbc.6.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beron J, Verrey F. Aldosterone induces early activation and late accumulation of Na-K-ATPase at surface of A6 cells. American Journal of Physiology. 1994;266:C1278–1290. doi: 10.1152/ajpcell.1994.266.5.C1278. [DOI] [PubMed] [Google Scholar]
- Betts DH, Barcroft LC, Watson AJ. Na/K-ATPase-mediated Rb-86(+) uptake and asymmetrical trophectoderm localization of α-1 and α-3 Na/K-ATPase isoforms during bovine preattachment development. Developmental Biology. 1998;197:77–92. doi: 10.1006/dbio.1998.8874. [DOI] [PubMed] [Google Scholar]
- Brewer CB. Cytomegalovirus plasmid vectors for permanent lines of polarized epithelial cells. Methods in Cell Biology. 1994;43:233–245. doi: 10.1016/s0091-679x(08)60606-8. [DOI] [PubMed] [Google Scholar]
- CorthesyTheulaz I, Merillat AM, Honegger P, Rossier BC. Differential regulation of Na-K-ATPase isoform gene expression by T3 during rat brain development. American Journal of Physiology. 1991;261:C124–131. doi: 10.1152/ajpcell.1991.261.1.C124. [DOI] [PubMed] [Google Scholar]
- Devarajan P, Liu M, Stabach PR, Morrow JS. Ankyrin binding is required for polarized distribution of Na,K-ATPase. Journal of the American Society of Nephrology. 1997;8:60A. [Google Scholar]
- Devarajan P, Scaramuzzino DA, Morrow JS. Ankyrin binds to two distinct cytoplasmic domains of Na,K-ATPase α-subunit. Proceedings of the National Academy of Sciences of the USA. 1994;91:2965–2969. doi: 10.1073/pnas.91.8.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar LA, Aronson PL, Scanzano R, Blostein R, Caplan MJ. The 4th transmembrane span of the H,K-ATPase contributes to sorting and cation selectivity. Journal of the American Society of Nephrology. 1997;8:60A. [Google Scholar]
- Felsenfeld DP, Sweadner KJ. Fine specificity mapping and topography of an isozyme-specific epitope of the Na,K-ATPase catalytic subunit. Journal of Biological Chemistry. 1988;263:10932–10942. [PubMed] [Google Scholar]
- Garty H, Palmer LG. Epithelial sodium channels - function, structure, and regulation. Physiological Reviews. 1997;77:359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- Herrera VLM, Cova T, Sassoon D, Ruizopazo N. Developmental cell-specific regulation of Na+-K+-ATPase α(1)-, α(2)-, and α(3)-isoform gene expression. American Journal of Physiology. 1994;266:C1301–1312. doi: 10.1152/ajpcell.1994.266.5.C1301. [DOI] [PubMed] [Google Scholar]
- Jewell EA, Lingrel JB. Comparison of the substrate dependence properties of the rat Na,K-ATPase α1, α2, and α3 isoforms expressed in HeLa cells. Journal of Biological Chemistry. 1991;266:16925–16930. [PubMed] [Google Scholar]
- Juhaszova M, Blaustein MP. Na+ pump low and high ouabain affinity α subunit isoforms are differently distributed in cells. Proceedings of the National Academy of Sciences of the USA. 1997;94:1800–1805. doi: 10.1073/pnas.94.5.1800. 10.1073/pnas.94.5.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucking K, Nielsen JM, Pedersen PA, Jorgensen PL. Na-K-ATPase isoform (α(3), α(2), α(1)) abundance in rat kidney estimated by competitive RT-PCR and ouabain binding. American Journal of Physiology. 1996;40:F253–260. doi: 10.1152/ajprenal.1996.271.2.F253. [DOI] [PubMed] [Google Scholar]
- Lytton J, Lin J, DiAntonio L, Brodsky J, McGeoch J, McGill D, Guidotti G. Regulation of the Na+, K+-pump by insulin. In: Bamberg E, Schoner W, editors. The Sodium Pump. Darmstadt: Steinkopf; 1994. pp. 670–681. [Google Scholar]
- Munzer JS, Daly SE, Jewell-Motz EA, Lingrel JB, Blostein R. Tissue- and isoform-specific kinetic behavior of the Na,K-ATPase. Journal of Biological Chemistry. 1994;269:16668–16676. [PubMed] [Google Scholar]
- Muth TR, Gottardi CJ, Roush DL, Caplan MJ. A basolateral sorting signal is encoded in the α-subunit of Na-K-ATPase. American Journal of Physiology. 1998;43:C688–696. doi: 10.1152/ajpcell.1998.274.3.C688. [DOI] [PubMed] [Google Scholar]
- Rossier BC, Palmer LG. Mechanisms of aldosterone action on sodium and potassium transport. In: Seldin DW, Giebisch G, editors. The Kidney: Physiology and Pathophysiology. 2. New York: Raven Press; 1992. pp. 1373–1409. [Google Scholar]
- Shull GE, Greeb J, Lingrel JB. Molecular cloning of three distinct forms of the Na+,K+-ATPase α-subunit from rat brain. Biochemistry. 1986;25:8125–8132. doi: 10.1021/bi00373a001. [DOI] [PubMed] [Google Scholar]
- Slezak J, Schulze W, Okruhlicova L, Tribulova N, Singal PK. Cytochemical and immunocytochemical localization of Na,K-ATPase α subunit isoenzymes in the rat heart. Molecular and Cellular Biochemistry. 1997;176:107–112. 10.1023/A:1006883130242. [PubMed] [Google Scholar]
- Sweadner KJ. Isozymes of the Na+/K+-ATPase. Biochimica et Biophysica Acta. 1989;988:185–220. doi: 10.1016/0304-4157(89)90019-1. [DOI] [PubMed] [Google Scholar]
- Therien AG, Nestor NB, Ball WJ, Blostein R. Tissue-specific versus isoform-specific differences in cation activation kinetics of the Na,K-ATPase. Journal of Biological Chemistry. 1996;271:7104–7112. doi: 10.1074/jbc.271.12.7104. 10.1074/jbc.271.12.7104. [DOI] [PubMed] [Google Scholar]
- Urayama O, Shutt H, Sweadner KJ. Identification of three isozyme proteins of the catalytic subunit of the Na,K-ATPase in rat brain. Journal of Biological Chemistry. 1989;264:8271–8280. [PubMed] [Google Scholar]
- Verrey F. Antidiuretic hormone action in A6 cells: effect on apical Cl and Na conductances and synergism with aldosterone for NaCl reabsorption. Journal of Membrane Biology. 1994;138:65–76. doi: 10.1007/BF00211070. [DOI] [PubMed] [Google Scholar]
- Verrey F. Transcriptional control of sodium transport in tight epithelia by adrenal steroids. Journal of Membrane Biology. 1995;144:93–110. doi: 10.1007/BF00232796. [DOI] [PubMed] [Google Scholar]
- Verrey F, Beron J, Spindler B. Corticosteroid regulation of renal Na,K-ATPase. Mineral and Electrolyte Metabolism. 1996;22:279–292. [PubMed] [Google Scholar]
- Verrey F, Digicaylioglu M, Bolliger U. Polarized membrane movements in A6 kidney cells are regulated by aldosterone and vasopressin/vasotocin. Journal of Membrane Biology. 1993;133:213–226. doi: 10.1007/BF00232021. [DOI] [PubMed] [Google Scholar]
- Verrey F, Kraehenbuhl JP, Rossier BC. Aldosterone induces a rapid increase in the rate of Na,K-ATPase gene transcription in cultured kidney cells. Molecular Endocrinology. 1989;3:1369–1376. doi: 10.1210/mend-3-9-1369. [DOI] [PubMed] [Google Scholar]
- Zahler R, Zhang ZT, Manor M, Boron WF. Sodium kinetics of Na,K-ATPase α isoforms in intact transfected Hela cells. Journal of General Physiology. 1997;110:201–213. doi: 10.1085/jgp.110.2.201. 10.1085/jgp.110.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]


