Abstract
Heteronymous group II effects were investigated in the human lower limb. Changes in firing probability of single motor units in quadriceps (Q), biceps (Bi), semitendinosus (ST), gastrocnemius medialis (GM) and tibialis anterior (TA) were studied after electrical stimuli between 1 and 3 times motor threshold (MT) applied to common peroneal (CP), superficial (SP) and deep (DP) peroneal, Bi and GM nerves in those nerve-muscle combinations without recurrent inhibition.
Stimulation of the CP and Bi nerves evoked in almost all of the explored Q motor units a biphasic excitation with a low-threshold early peak, attributable to non-monosynaptic group I excitation, and a higher threshold late peak. When the CP nerve was cooled (or the stimulation applied to a distal branch, DP), the increase in latency was greater for the late than for the early peak, indicating that the late excitation is due to stimulation of afferents with a slower conduction velocity than group I fibres, presumably in the group II range. In ST motor units the group II excitation elicited by stimulation of the GM and SP nerves was particularly large and frequent, and the non-monosynaptic group I excitation was often replaced by an inhibition.
A late group II-induced excitation from CP to Q motoneurones and from GM and SP to ST motoneurones was also observed when using the H reflex as a test.
The electrical threshold and conduction velocity of the largest diameter fibres evoking the group II excitation were estimated to be 2·1 and 0·65 times those of the fastest Ia afferents, respectively. In the combinations tested in the present investigation the group II input seemed to be primarily of muscle origin.
The potent heteronymous group II excitation of motoneurones of both flexors and extensors of the knee contrasted with the absence of a group II effect from DP to GM and from GM to TA. In none of the combinations explored was there any evidence for group II inhibition of motoneurones. The possible contribution to postural reactions of the potent group II excitation of thigh motoneurones is discussed.
It was long accepted that the monosynaptic excitation of motoneurones by Ia afferents was the main mechanism underlying the stretch reflex. Then it was found that the major response to stretching of a muscle during its voluntary contraction was a medium-latency response (MLR) occurring with a latency too long for the classical monosynaptic spinal reflex (Hammond, 1956; Melvill Jones & Watt, 1971). The question thus arose of whether the delay of this MLR was due to a slower conduction velocity in the peripheral afferent pathway or to a longer route in the central nervous system. Interestingly, observations in hand and lower limb muscles have led to opposite answers. In the lower limb, there are several lines of evidence showing that the MLR evoked by stretch in ankle and foot muscles is a spinal reflex originating in muscle spindle secondary endings and mediated by group II muscle afferents (see Dietz, 1992; Schieppati et al. 1995; Corna et al. 1995; Schieppati & Nardone, 1997).
The stretch reflex involves homonymous connections, but excitation from group II afferents is not confined to homonymous motoneurones and, in the cat, a given motoneurone receives potent excitation from group II afferents of various muscles (see Lundberg et al. 1987a). In man, evidence for excitation of quadriceps (Q) motoneurones by group II afferents in the common peroneal (CP) nerve was recently presented (Marque et al. 1996). In this preliminary report we showed that CP stimulation evoked in Q motoneurones two peaks of excitation: an early non-monosynaptic group I excitation (Forget et al. 1989) and a later excitation, the characteristics of which (4-8 ms longer latency, higher threshold) are consistent with a group II effect.
The aim of the present investigation was threefold: (i) to extend the evidence for the group II origin of this late excitation by eliminating the possible contribution of a long-latency Ia excitation either elicited by volleys in fusimotor efferents (β and/or γ) or mediated through a long-loop central pathway; (ii) to describe, as far as possible, the pattern of the heteronymous group II excitation of muscles in the human lower limb; (iii) to estimate the quantitative importance of this group II excitation compared with group I effects.
METHODS
The experiments were carried out on 12 healthy subjects (aged 25-63 years), all of whom had given informed consent to the experimental procedure, which was approved by the appropriate institutional ethics committees. The subjects were seated in an armchair and the leg to be examined was loosely fixed with the hip semi-flexed (120 deg), the knee slightly flexed (160 deg) and the ankle at 110 deg plantar flexion.
Recording
EMG was recorded by surface electrodes 2 cm apart secured to the skin over the muscle belly. The muscles from which recordings were made inluded: vastus lateralis (VL) and rectus femoris (RF) from the Q (25-30 cm above the patella on the lateral or anterior aspect of the thigh, respectively), the short head of the biceps femoris (Bi) and semitendinosus (ST) (10 cm above the patella on the lateral and medial part of the posterior aspect of the thigh, respectively), soleus (Sol), the medial part of the gastrocnemius medialis (GM), the lower part of the peroneus brevis (Per brev) and the medial part of the tibialis anterior (TA).
Conditioning stimulus
Electrical pulses (1 ms) were delivered to various nerves of the leg through bipolar surface electrodes (1 cm diameter silver plates or half-ball electrodes 2 cm apart, proximal cathode) placed as described in Meunier et al. (1993): the GM nerve was stimulated at the lower part of the popliteal fossa; the branches of the sciatic nerve to the Bi or the ST were stimulated on the posterior aspect of the thigh 30-35 cm above the electrodes stimulating the GM nerve; the inferior soleus (Inf Sol) nerve was stimulated on the posterior aspect of the leg; the CP nerve was stimulated 2-3 cm below the caput fibulae; the deep peroneal (DP, innervating pretibial flexors) nerve was stimulated on the upper part of the TA; the superficial peroneal (SP, innervating peroneal muscles) nerve was stimulated through the belly of the peroneus longus, about 10 cm more distally than the electrodes stimulating the CP nerve. The femoral nerve was stimulated through a unipolar electrode, the active cathode (half-ball) being in the femoral triangle. In each case, the site of stimulation was chosen such that increasing the stimulus above motor threshold (1 × MT) resulted in a steep increase in the motor response in the corresponding muscle. The current was measured by a current probe (Tektronix 6021) and expressed in multiples of the intensity for 1 × MT.
Absence of encroachment
It was verified that stimulation of each nerve at 3 × MT did not produce any contraction (revealed by tendon palpation) of muscles other than those innervated by the nerve. This was considered as evidence in favour of lack of activation of small-calibre afferent fibres (smaller than motor axons) from other muscles. It was usually possible to position the electrode stimulating the Bi nerve at 3 × MT so that no contraction of leg muscles was produced.
Cutaneous stimuli
The cutaneous sensation (weak local and/or radiating paraesthesia) evoked by mixed nerve stimulation was mimicked by pure cutaneous stimuli to estimate the contribution of cutaneous afferents. The local sensation was reproduced by plate electrodes placed 3 cm more laterally (or more medially) than the nerve trajectory and the radiating paraesthesia by plate electrodes placed over the nerve projection area (allowance was made for the extra peripheral conduction time). The stimulus intensity was adjusted to imitate the sensation evoked by mixed nerve stimulation. The mainly cutaneous sural nerve was also stimulated at the ankle and the saphenous nerve on the upper part of the leg.
Study of single motor units
Post-stimulus time histograms (PSTHs) of a voluntarily activated motor unit were constructed for the period following a conditioning stimulation. This process extracts from the naturally occurring spike train only those changes in firing probability that are time-locked to the stimulus (Stephens et al. 1976). Details of the PSTH technique used in this study were given elsewhere (Fournier et al. 1986), so it will be only briefly described here. PSTHs of motor units from various lower limb muscles (Q, ST, Bi, GM and TA) were constructed for the 25-65 ms following a conditioning stimulation (bin width: 0.2, 0.5 or 1 ms). The EMG potentials were converted into standard pulses by a spike discriminator and were then used to trigger a computer which subsequently triggered the stimulator about every 1 s. Stimuli were delivered in relation to the motor unit discharge so as to avoid the time when the motor unit was refractory. Histograms of the firing probability were constructed after the conditioning stimulus (open columns on the left of Figs 1–4 and 6) and in a control situation without stimulation (hatched columns on the left of Figs 1–4 and 6), both situations being randomly alternated in the same sequence. To clarify the differences between the results obtained in the two situations the control value in each bin was subtracted from that observed after conditioning stimulation (histograms on the right of Figs 1–4 and 6, in which the number of counts in each bin is expressed as a percentage of the total number of stimuli delivered during the sequence). The exceptional sequences in which a change in the control sequence significantly contributed to the differences seen between the two situations were not retained for further analysis. A χ2 test was used within different time-interval windows to determine the extent to which the distribution of firing probability after stimulation differed from that in the control situation. A peak of excitation was accepted if there was a significant (at least P < 0.05) increase in firing probability in one or more adjacent bins. The latency of the first bin of the increased firing probability was taken to be the latency of the excitation provided that the probability was significantly increased in the first group of two or three bins (probability was often significantly increased in the first bin itself). Although the relation between the amplitude of a peak in the PSTH and that of the underlying EPSP is complex (see Gustafsson & McCrea, 1984), the larger the EPSP the higher the peak. Thus, the size of the peak was estimated as the sum of the differences (conditioned - control counts) in the different consecutive bins, with increased firing probability contributing to a given peak, e.g. 45 % between 46 and 53 ms in Fig. 6B.
Figure 1. Changes in firing probability of one Q motor unit evoked by proximal and distal stimulation of the common peroneal (CP) nerve.
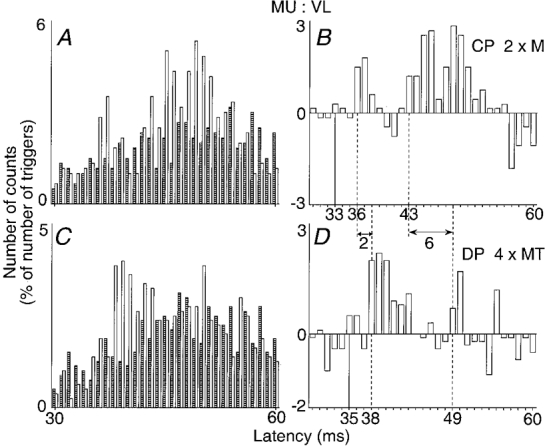
A and B, proximal stimulation of the CP nerve (2 × MT) at the upper part of the popliteal fossa. C and D, distal stimulation of the deep peroneal (DP) nerve (4 × MT). The histograms in A and C show discharges of the voluntary activated motor unit in control conditions (hatched columns) and after stimulation of the nerve (open columns). The differences between these two histograms are plotted in B and D. In both cases, the number of counts, expressed as a percentage of the number of the triggers (A and B, 600; C and D, 539) is plotted against the latency from the stimulation (bin width 1 ms). Continuous vertical lines, expected time of arrival of the conditioning Ia volley at the segmental spinal level of the motoneurone (latency of monosynaptic homonymous excitation after stimulation of the femoral nerve, 28 ms; distance between stimulation sites of femoral and CP nerves, 40 cm). Dashed vertical lines, latencies of non-monosynaptic group I and group II excitations. Double-headed horizontal arrows, difference between the latencies of the early peaks (2 ms) and of the late peaks (6 ms) in the two positions of the stimulation.
Figure 4. Changes in firing probability of semitendinosus (ST) motor units after stimulation of the gastrocnemius medialis (GM) and of the superficial peroneal (SP) nerves.
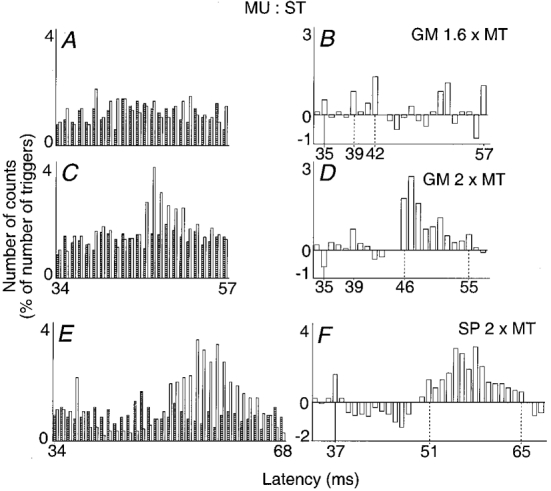
A-D, stimulation of the GM nerve at 1.6 (A and B) and 2 (C and D) × MT. E and F, stimulation of the SP nerve at 2 × MT (same subject but another motor unit). Left and right plots and vertical continuous lines as in Fig. 1 (bin width 1 ms). Vertical dotted lines, first and last bins of group I (B) or group II (D and F) excitation. Latency of homonymous monosynaptic excitation from the sciatic nerve, A-D, 31 ms; E and F, 32 ms; distances between stimulations sites of GM and sciatic, and of SP and sciatic nerves, 33 and 38 cm respectively. Number of triggers, A and B, 1105; C and D, 2252; E and F, 1085.
Figure 6. Absence of effect of a sural nerve stimulation.
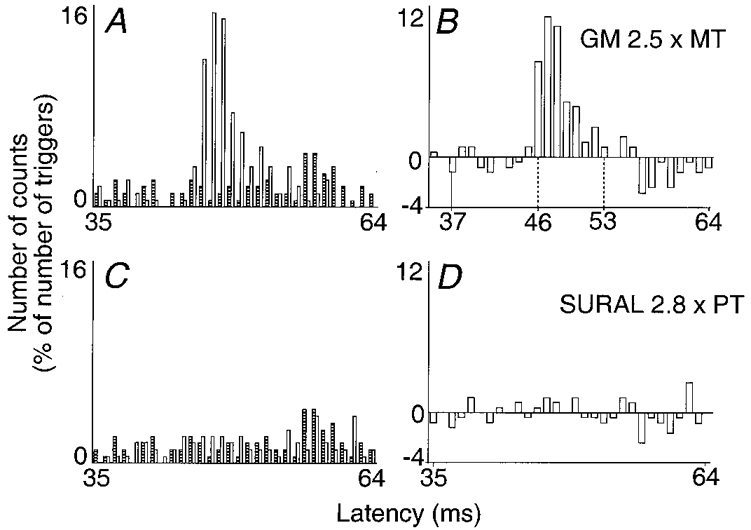
Changes in firing probability of a ST motor unit evoked by stimulation of the GM (A and B, 2.5 × MT) and of the sural (C and D, 2.8 × perception threshold, PT) nerves are compared. Left and right plots and vertical continuous line as in Fig. 1 (bin width 1 ms). Dotted vertical lines as in Fig. 3. Latency of homonymous monosynaptic excitation from the sciatic nerve, 31 ms; distance between the stimulation sites of the sciatic and GM nerves, 35 cm and between those of GM and sural nerves, 38 cm. Number of triggers, A-D, 1099.
Assessment of latencies
The latency of non-monosynaptic events was expressed as absolute values and with respect to the monosynaptic Ia latency in the corresponding heteronymous pathway. This was easy in pathways in which heteronymous monosynaptic Ia projections exist (Meunier et al. 1993). Otherwise, the time of arrival of the conditioning Ia volley at the motoneurone level was estimated from the conduction velocity (CV) in Ia afferents and from the distance between the stimulation site and the spinal cord. CV in Ia afferents from a given muscle was calculated from the latency of the monosynaptic Ia peaks measured in the PSTH of the same unit after stimulation of homonymous Ia afferents at two sites (Meunier et al. 1993). Distances were measured on the skin. Even though this can only be done approximately, it was calculated that, when comparing afferent conduction times (ACTs) of Ia and group II volleys on a common pathway, a 3 cm error on this common pathway would only alter the difference between ACTs of the two volleys by 0.2 ms.
H reflex study
Experiments using the H reflex in ST and Q (RF) muscles were performed to investigate whether results obtained with PSTH tests could also be obtained in the absence of voluntary contraction. The ST H reflex was obtained by stimulating the ST nerve through two half-ball electrodes placed on the posterior aspect of the thigh, and the Q H reflex by unipolar stimulation of the femoral nerve. The reflex responses were measured as the peak-to-peak amplitude of muscle action potentials. In each experimental run, 20 control and 20 conditioned reflexes were randomly alternated for each conditioning-test interval. The amount of reflex facilitation (or inhibition), i.e. conditioned minus control value expressed as a percentage of control value, was calculated. An F test (Scheffé's test, see Daniel & Lehmann, 1979) was used to determine whether the changes evoked by the conditioning stimulation were significant.
Effects of cooling
In order to provide further support for the group II origin (see Schieppati & Nardone, 1997, and below) of the late excitation described here, the effects of cooling the CP nerve on the latencies of the early and late CP-induced excitations of Q motor units were compared. Ice packs were placed against the external aspect of the knee, along the route of the CP nerve, from the upper part of the popliteal fossa to the caput fibulae. The temperature of the skin of the popliteal fossa and the area around the caput fibulae, measured with a digital thermometer, was decreased from 28-30 to 18-20°C. Cooling was continued for up to 40 min. The temperature of the laboratory was 20-22°C and the subjects did not feel cold in themselves, so the deep body temperature is unlikely to have changed appreciably. Given that only six motor units were so explored, the non-parametric Wilcoxon test was used to compare differences in latencies of the early and late excitations before and during cooling.
RESULTS
In the cat, the threshold of group II afferents is about twice (2 × T) that of group Ia afferents (Jack, 1978) and sizeable group II EPSPs in motoneurones often require stimuli above 2.5-3 × T (see Lundberg et al. 1987a). Given an electrical threshold of Ia afferents in human lower limb nerves at about 0.5-0.6 × MT (Forget et al. 1989; Meunier et al. 1993; Chaix et al. 1997), and assuming a similar group II/group I threshold ratio to that in the cat, stimuli above 1 × MT should disclose group II effects. The resulting antidromically conducted volleys in motor axons activate Renshaw cells and evoke recurrent inhibition of motoneurones. Because in man recurrent inhibition is strong and much more widely distributed in heteronymous pathways than in the cat (Meunier et al. 1994), it might complicate interpretation of the effects evoked by stimulation of group II afferents. Group II effects were therefore studied only in nerve-muscle combinations where there is no recurrent inhibition.
Effects on quadriceps motoneurones
Common peroneal-induced excitation of quadriceps motoneurones
Changes in firing probability of a Q motor unit following stimulation of the CP nerve (2 × MT) are illustrated in Fig. 1A and B. Histograms obtained in the absence (hatched columns) and in the presence (open columns) of CP nerve stimulation are compared in A and the difference between the two histograms is shown in B. As shown previously (Marque et al. 1996), such CP nerve stimulation evokes a biphasic facilitation of the Q (VL) motor unit.
The early peak, occurring here at the 36 ms latency, has been shown to have a threshold of 0.6 × MT and to be of group I origin (see Forget et al. 1989). The latency of the homonymous monosynaptic Ia peak evoked by femoral nerve stimulation was 28 ms in this motor unit and the difference between the afferent conduction times of Ia volleys in the femoral and CP (more distal stimulation) nerves was 5 ms (for the particular position of the electrode stimulating the CP nerve in the upper part of the popliteal fossa in this experiment, see below). The expected time of arrival of the CP Ia volley at the motoneurone level (continuous vertical line) was therefore 33 ms (28 + 5), and the early facilitation occurred with a central delay of 3 ms (36 - 33), which is in keeping with an interneuronally mediated group I effect (Forget et al. 1989).
The second peak occurred here at the 43 ms latency, i.e. 7 ms later than the early peak. This late excitation has been shown to have a higher threshold (between 1 and 1.5 × MT), and its longer latency and higher threshold were taken to suggest an effect mediated by afferents with a smaller diameter than group I fibres (Marque et al. 1996). However, since the stimulation of the CP nerve evokes a group I excitation in Q motoneurones (see above), two issues, which were not considered in the previous report, had to be addressed: (i) since the late excitation is evoked by stimuli above 1 × MT, it would be conceivable that CP stimulation, in addition to the Ia volley elicited by electrical stimulation of afferent fibres, also elicits a later Ia discharge induced by the motor volley, ‘early discharge’ (Hunt & Kuffler, 1951) or Ia firing due to stimulation of β- and γ-fusimotor efferents; (ii) the longer latency of the late excitation could also be due to a longer central pathway only activated by strong Ia volleys (that, when evoked by surface electrodes, may not be maximal with stimulus intensities below 4 × MT; see Gracies et al. 1994). Two kinds of experiments were therefore performed to address these issues.
(i) Comparison of effects evoked by stimulation of the CP nerve at proximal and distal sites
The rationale behind this experiment was that distal stimulation should increase the latency of the late excitation if the late latency is due to electrical stimulation of slow afferent fibres. In contrast, if the late latency is secondary to Ia discharges induced by volleys in motor or fusimotor fibres, distal stimulation should decrease the latency because the conduction distance along motor axons (from stimulation site to muscle spindles) would then be decreased. In the experiment illustrated in Fig. 1, the proximal electrode stimulated the CP nerve in the upper and lateral part of the popliteal fossa (A and B, 63 cm from L4 spinal level) and another electrode stimulated its branch, the DP nerve, 12 cm more distally (C and D). To obtain a clear excitation from the DP branch, DP stimulation had to be stronger (4 × MT; see Forget et al. 1989) than that to the CP nerve (2 × MT). Figure 1 shows that the latencies of both peaks were longer after more distal stimulation: 38 vs. 36 ms for the early peak and 49 vs. 43 ms for the late peak. Longer latencies after distal stimulation were obtained in all five other experiments performed in three subjects (P < 0.05). The longer latency of the late responses evoked by distal stimulation argues against the possibility that the late excitation could reflect a motor-induced Ia discharge.
The increase in the latency was greater for the late than for the early excitation (49-43 = 6 ms vs. 38-36 = 2 ms in Fig. 1) in all experiments. This might also be taken to suggest that the late peak is not due to Ia excitation mediated through a longer central pathway (in which case the latencies of the two responses should be equally increased). An alternative possibility, however, arises from the finding that the early peak was larger after DP (D) than after CP (B) stimulation, whereas the late peak was smaller in D. This is in agreement with previous results (Chaix et al. 1997): the larger the early peak, the smaller the late excitation, an inverse relationship which may be explained by occlusion at the level of common interneurones (Chaix et al. 1997) and by the after-hyperpolarization and homonymous recurrent inhibition of the tested Q motoneurone following the first peak. This might not only reduce the size of the late excitation but also delay its appearance. Experiments using cooling of the CP nerve were therefore performed to exclude the possibility of a long-loop central transmission of Ia excitation.
(ii) Effects of cooling of the CP nerve
Experiments in animals have shown that cooling a nerve produces a greater slowing of conduction in group II than in group I afferents. One would therefore expect cooling to delay to a greater extent the late than the early excitation if early and late excitations are due to group I and group II afferents, respectively, but not if both are due to group I afferents where the later excitation involves a greater central delay (for references, see Matthews, 1991). The CP nerve was cooled by ice packs placed against the external aspect of the knee (see Methods). In the experiment illustrated in Fig. 2 (0.5 ms bins), in the control situation (A and B), CP nerve stimulation (2 × MT) evoked the early and late peaks at the 37 and 45 ms latencies, respectively, i.e. 3 and 11 ms after the expected arrival (34 ms) of the CP Ia volley at the motoneurone level. During cooling (20-40 min after its onset, C and D), while the skin temperature in the popliteal fossa had dropped from 31 to 19°C, the latency of the early peak (which had approximately the same size as in the control situation) was increased by 1 ms (from 37 to 38 ms), while the latency of the late excitation was increased by 2.5 ms (from 45 to 47.5 ms). After rewarming, when the skin temperature was back to 30°C, the latencies of both peaks returned to their control values. Similar results were obtained in five other experiments performed in two subjects. The finding that in all six motor units the latency of the late peak was significantly more delayed than the early Ia excitation (on average 4.1 ± 1.5 vs. 1.6 ± 0.8 ms, P < 0.05) provides evidence that the longer latency of the late peak is not due to a longer central pathway fed by Ia afferents, but mainly due to the activation of peripheral afferents of smaller diameter.
Figure 2. Effects of cooling on the changes in firing probability of a Q motor unit evoked by stimulation of the CP nerve (2 × MT).
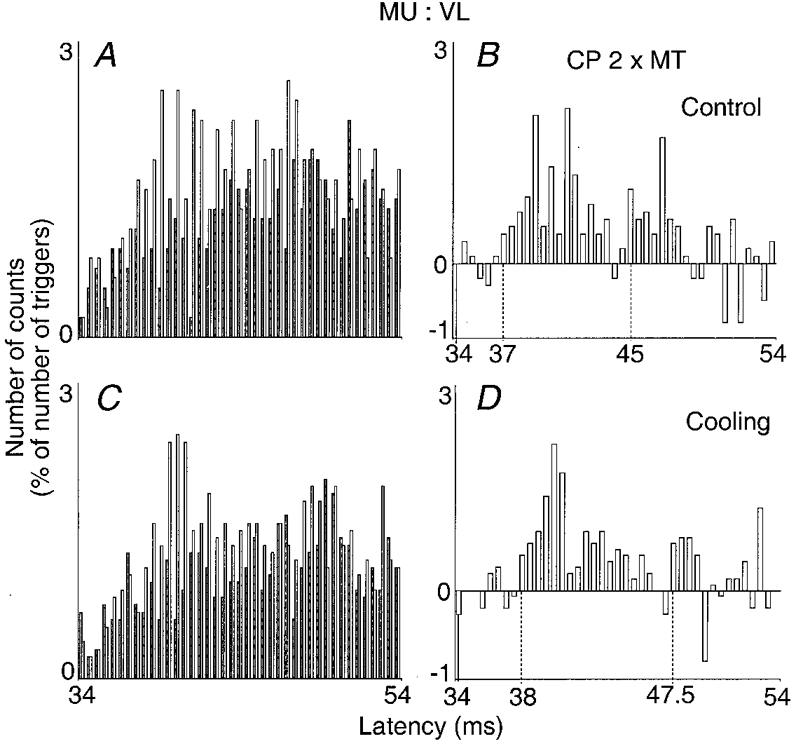
Results obtained before (A and B) and during (C and D) cooling (20-40 min) of the lower limb are compared (bin width 0.5 ms). Left and right plots and dotted vertical lines as in Fig. 1. Latency of homonymous monosynaptic Ia excitation after femoral nerve stimulation, 27.5 ms; distance between stimulation sites of CP and femoral nerves, 46 cm. Number of triggers: A and B, 1008; C and D, 904. Skin temperature in the popliteal fossa: A and B, 31 °C; C and D, 19 °C.
Frequency and strength of CP-induced excitation of Q motoneurones
A significant (P < 0.05) high-threshold-late excitation of Q motoneurones, attributable to group II afferents in the CP nerve, was found in 27/34 VL motor units (in 10/11 subjects).
In the only subject in whom it was not found, a depression was instead regularly (4/4 experiments) observed in all motor units tested, which lasted for 5-10 ms. Because of its relatively short central delay (4-5 ms) and low threshold (below 1 × MT), it was attributable to group I afferents. This depression contrasted with the early facilitation of the Q H reflex existing at rest as during voluntary tonic contraction in this subject (see Discussion).
With the exception of this one subject, the late excitation (occurring on average 10 ± 1.8 ms later than the expected time of arrival of the CP Ia volley at motoneurone level) was observed in 27/30 motor units and was most often highly significant (P < 0.001). The maximum late excitation, obtained with CP stimuli between 2 and 3 × MT and assessed as explained in Methods, was on average as high as 22 % of the number of triggers (Table 1). Yet, the size of the underlying group II EPSP was underestimated, since in 25/27 motor units the late excitation was preceded by a significant early group I excitation, which probably counteracted the expression of group II excitation in VL motoneurones (see above).
Table 1.
Distribution of group II excitation in the human lower limb
| 1 | 2 | 3 | 4 |
|---|---|---|---|
| Motor nucleus explored | Nerve stimulated | Frequency of occurrence | Magnitude (%) |
| Q | CP | 27/30 (90%) | 22 |
| DP | 10/14 (71%) | 4 | |
| SP | 2/8 (25%) | 3 | |
| Bi | 6/10 (60%) | 25 | |
| ST | GM | 21/21 (100%) | 39 |
| SP | 5/8 (63%) | 29 | |
| Inf Sol | 0/8 (0%) | 0 | |
| DP | 0/6 (0%) | 0 | |
| Bi | DP | 7/16 (44%) | 27 |
| GM | DP | 0/7 (0%) | 0 |
| TA | GM | 0/4 (0%) | 0 |
Column 3, frequency of occurrence is given as a ratio, with the number of motor units where group II excitation reached statistical significance (P < 0.05) as the numerator and the number of explored motor units as the divisor, and a percentage in parentheses, with the former as a percentage of the latter; column 4, mean magnitude of the effect (assessed in motor units in which it was statistically significant) assessed as the sum of the difference conditioned minus control counts in the different consecutive bins within the peak (expressed as a percentage of the number of triggers).
The mean difference between the latencies of the two peaks was 6.3 ± 0.5 ms. In H reflex experiments, the two peaks most often overlapped (e.g. Fig. 1 in Marque et al. (1996) and Fig. 2B in Marchand-Pauvert et al. (1999)). In contrast, in single motor units, the late peak never overlapped with the early peak. This relatively short duration of the early peak in single motor units might suggest that it is truncated by a subsequent depression (see Discussion).
The contribution of afferents in the branches (DP and SP) of the CP nerve to the group II excitation of Q motoneurones was investigated in seven subjects. In 71 % of the tested motor units, stimulation of the DP branch (at 3-4 × MT) evoked the biphasic effect with significant early group I and late group II excitations (see Fig. 1C and D), but the mean size of the late excitation was small (4 %, Table 1). Stimulation of the SP branch only evoked a significant late excitation in 25 % of the tested motor units (see Table 1).
Quadriceps motoneurone excitation from biceps nerve
In four subjects, stimulation of the Bi nerve remained selective at intensities between 1.5 and 2 × MT. Changes in firing probability of a VL motor unit induced by stimulation of the Bi nerve at 1.8 × MT are illustrated in Fig. 3A and B. At the 39 ms latency, i.e. 8 ms later than the expected time of arrival of the Bi Ia volley at the motoneurone level (31 ms in this motor unit), there was a significant peak of excitation (P < 0.001). Such a late excitation, occurring with a threshold above 1.5 × MT, reached significance in 6/10 tested VL motor units (mean size = 25 %, Table 1). In 4/6 of these units, the late peak was preceded by a significant early and low-threshold peak, which was, as in the case of the CP nerve, attributable to an interneuronally mediated group I effect.
Figure 3. Changes in firing probability of a Q (VL) motor unit after stimulation of the biceps (Bi) nerve (A and B, 1.8 × MT) and of a Bi motor unit after stimulation of the DP nerve (C and D, 2 × MT).
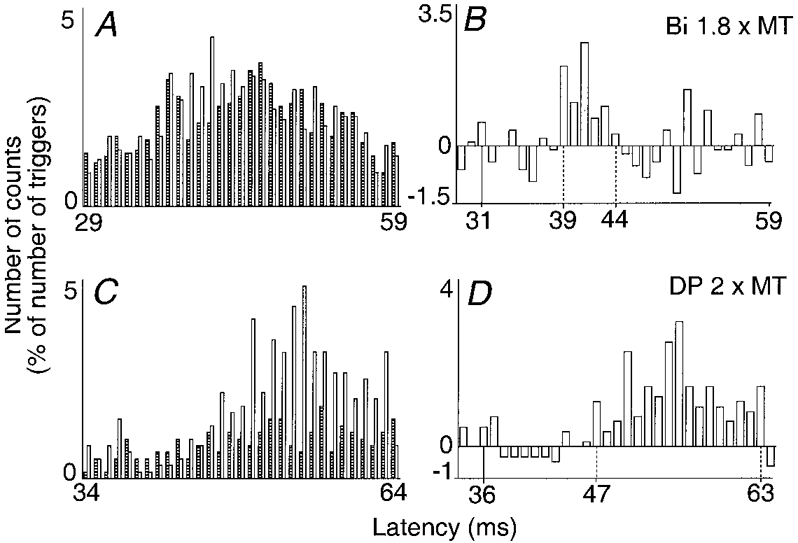
Left and right plots and vertical continuous lines as in Fig. 1 (bin width 1 ms). Vertical dotted lines, first and last bins of the group II excitation. A and B, latency of homonymous monosynaptic Ia excitation from the femoral nerve, 29 ms; distance between the sites of stimulation of the femoral and Bi nerves, 20 cm; number of triggers, 1205. C and D, latency of homonymous monosynaptic Ia excitation from the sciatic nerve, 31 ms; distance between the sites of stimulations of DP and sciatic nerves, 35 cm; number of triggers, 599.
Effects on hamstring motoneurones
PSTHs constructed from activity in single voluntarily activated motor units
Semitendinosus motoneurone excitation from GM nerve
Figure 4A-D illustrates the changes in firing probability elicited in a ST motor unit by GM nerve stimulation. The expected latency of arrival of the GM Ia volley at motoneurone level was 35 ms. GM nerve stimulation at 1.6 × MT (A and B) evoked a peak of early excitation (39-42 ms), weak but significant (P < 0.05), occurring with a central delay of 4 ms (39-35), which was already present at 1 × MT and might therefore reflect the interneuronally evoked group I excitation. At 2 × MT (C and D), this early peak decreased. In contrast, there was a very large late excitation appearing at the 46 ms latency (i.e. 11 ms after the latency corresponding to the arrival at the spinal level of the GM Ia volley). It lasted for 10 ms and was highly significant (P < 0.001).
This was the largest and the most constant of any group II effect observed in this series of experiments: a high-threshold (above 1.2 × MT) and late (mean latency 10 ± 1.3 ms longer than that of the heteronymous monosynaptic Ia excitation) excitation was observed in all 21 ST motor units analysed (5 subjects); it was always highly significant (e.g. see Fig. 6A and B, another subject) and its mean size reached 39 % (Table 1). Increasing GM stimulus intensity above 2.5 × MT resulted in some decrease in this late effect.
A weak heteronymous monosynaptic Ia excitation was observed in 10/21 motor units but was significant in only two motor units. In contrast with what was observed in Q motor units, the early peak of non-monosynaptic group I excitation was rarely observed (4/21 motor units), was always weak (reaching statistical significance in only 2 motor units) and decreased when the stimulus intensity was increased above 1.6-1.8 × MT.
Semitendinosus motoneurone excitation from SP nerve
Figure 4E and F illustrates changes in firing probability evoked in a ST motor unit by stimulation of the SP nerve at 2 × MT. There was a significant (P < 0.01) peak of heteronymous monosynaptic Ia excitation at 37 ms, which was followed by a depression lasting for 10 ms. The depression was in turn followed by a huge (P < 0.001) and long-lasting (15 ms) facilitation occurring with a latency of 14 ms with respect to heteronymous monosynaptic Ia latency and with a threshold at 1.5 × MT. In 5/8 ST motor units the late facilitation was similarly large (mean = 29 %, Table 1). In four of them it was similarly preceded by both monosynaptic Ia excitation and early low threshold depression.
Effects evoked in semitendinosus motoneurones from other nerves
In contrast, the late and high-threshold excitation was never observed in ST motor units after stimulation of the Inf Sol and DP nerves (see Table 1). Stimuli to these nerves only evoked an early and low-threshold inhibition, which was preceded in half of the cases by a heteronymous monosynaptic Ia excitation (Meunier et al. 1993).
Biceps motoneurone excitation from DP nerve
The DP nerve is the only mixed nerve supplying ankle muscles whose stimulation does not evoke recurrent inhibition of Bi motoneurones (Meunier et al. 1994). Figure 3C and D illustrates the changes in firing probability elicited in a Bi motor unit by a DP nerve stimulation at 2 × MT. There was a weak excitation occurring at monosynaptic Ia latency (36 ms), as described in Meunier et al. (1993), and a highly significant (P < 0.001) late excitation appearing at a latency of 47 ms. This late (mean latency 11.6 ± 0.9 ms longer than monosynaptic Ia latency excitation) and high-threshold (always above 1.5 × MT) excitation reached significance in only 7/16 motor units (3 subjects) (mean size = 27 %, Table 1). It was never preceded by any significant interneuronally mediated group I excitation.
Investigations at rest using H reflex tests
The GM-induced excitation of ST motoneurones was also analysed at rest in four subjects using the H reflex technique. Figure 5A shows the time course of the amount of facilitation evoked in the ST H reflex (control reflex equal to 19 % of maximum M wave) by a GM nerve stimulation at 2 × MT. Conditioning and test Ia volleys should have arrived simultaneously at the spinal level at the 4 ms interstimulus interval (ISI) (0 ms central delay, vertical dashed line). There was a huge reflex facilitation starting at the 16 ms ISI, i.e. the 12 ms (16-4) central delay, peaking at the 22 ms ISI (18 ms central delay), and lasting for 16 ms, thus closely matching the late excitation found in individual motor units. No earlier significant effect preceded this late facilitation. In the other three subjects, GM stimulation at 2 × MT evoked a similarly large facilitation of the H reflex, which appeared at 10-12 ms central delays and was not preceded by any sizeable early facilitation or inhibition.
Figure 5. Facilitation of the ST H reflex elicited by stimulation of the GM and SP nerves.
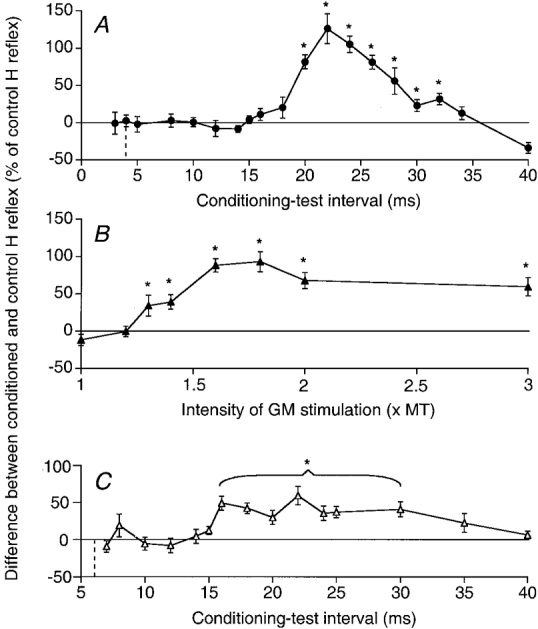
The difference between the amplitude of conditioned and control H reflexes (expressed as a percentage of the control H reflex) is plotted against the conditioning-test interval (A, GM nerve stimulation = 2 × MT, and C, SP stimulation = 2 × MT) or the GM stimulus intensity (B, 22 ms conditioning-test interval). Data from two different subjects (A, B and C). The vertical dotted lines in A and C indicate the ISI corresponding to the simultaneous arrival at the segmental spinal level of the conditioning and test Ia volleys. Each symbol represents the mean of 20 measurements. Vertical bars, 1 standard error of the mean. Asterisks indicate the results which are statistically significant (P < 0.05; note that in C all values between 16 and 30 ms were tested together).
Figure 5B shows that the threshold of the late facilitation (at the 22 ms ISI) was between 1.2 and 1.3 × MT, i.e. about 2.1 times the threshold of Ia afferents (0.6 × MT; Meunier et al. 1993). Maximum facilitation was reached at 1.8 × MT, and, as in PSTH experiments, it declined with further increases in the GM stimulus intensity.
Figure 5C shows the time course of the changes induced in the ST H reflex by SP stimulation (2 × MT, same subject as in Fig. 4E and F): the late and significant facilitation of the reflex was present at 16-35 ms ISIs, but the early depression seen in PSTHs had no equivalent in reflex changes.
Effects evoked in motoneurones of ankle muscles
The absence of recurrent inhibition in two nerve-muscle combinations (GM nerve on TA motoneurones and DP nerve on GM motoneurones; 7 and 4 motor units, respectively; 4 subjects) made it possible to investigate effects of stimulation of group II afferents in motoneurones of ankle muscles. In both cases there was never any late high-threshold excitation but only a low-threshold early (2-3 ms central delay) inhibition.
Afferents responsible for the late excitation
Evidence for group II origin of the late excitation
Experiments described in above (‘Effects on quadriceps motoneurones’) have shown that the late CP-induced excitation of Q motoneurones is elicited by stimulation of afferents with a smaller diameter than Ia afferents. The late excitation of hamstring motoneurones may be similarly attributed to smaller diameter afferents in view of the weakness, or even the absence, of early non-monosynaptic excitation of these motoneurones after stimulation of Ia afferents from ankle muscles both in PSTH (Figs 3C and D, 4C-F, 6A and B) and H reflex (Fig. 5) experiments. The absence of the late facilitation of the ST H reflex at a GM intensity of 1.2 × MT (Fig. 5B), i.e. twice the threshold of Ia afferents (see above), also makes it very unlikely that the late excitation is due to Ia afferents (either activated secondarily to stimulation of β- and/or γ-fusimotor fibres or through a long-loop central pathway). The electrical threshold of the late excitation (about 2.1 times that of Ia excitation; see above) and the estimated CV of the afferents responsible (see below) strongly suggest that they belong to the group II range.
Origin of group II afferents
Electrical stimuli exciting secondary spindle afferents will also stimulate non-spindle afferents (cutaneous, joint) in the group II range. It has already been shown that stimulation of the skin close to the caput fibulae or on the dorsum of the foot mimicking the sensation evoked by strong CP stimulation does not contribute to the late excitation of Q motoneurones (Marque et al. 1996). Similarly, pure cutaneous stimuli mimicking the sensation evoked by DP or GM nerve stimulation were never seen to evoke in Q, Bi or ST motoneurones a late excitation similar to that elicited in these motoneurones by mixed nerve stimulations. Any significant contribution from cutaneous afferents to the group II excitation elicited by the stimuli used in the present investigation was therefore unlikely.
In addition, changes in firing probability of Q motor units were investigated in four subjects after stimulation (2-3 × perceptual threshold (PT)) of the saphenous nerve, which supplies sensation to the skin of the anterior and medial aspect of the leg: at the latency of group II excitation, either there was a significant inhibition (3 motor units) or no effect (6 motor units). Finally, as illustrated in Fig. 6, which shows the results of an experiment in which sural (2.8 × PT) and GM (2.5 × MT) nerve stimuli were alternated, it was verified in three subjects that stimulation of the sural nerve at the ankle (C and D) did not evoke in ST motor units any excitation similar to that elicited by GM nerve stimulation (A and B). This absence of cutaneous excitation is further discussed below (see Discussion).
Estimation of the conduction velocity of group II muscle afferents inducing late excitation of motoneurones
The afferent conduction time of group II afferent volleys may be estimated from the extra latency of group II excitation over and above the expected time of arrival of the Ia volley in the same nerve at the spinal level of the analysed motoneurone. Table 2 shows results obtained in different muscle-nerve combinations in one of the subjects (1.68 m tall). The Ia afferent conduction time (ACT, column 4) was calculated from the distance from the site of stimulation to the segmental spinal level of the motoneurone (measured on the skin; see Methods; column 2) and the previously determined CV of Ia afferents (column 3). The extra latency of group II excitation over and above the time of arrival of the Ia volley at motoneurone level is shown in column 5 (mean value for different motor units in this subject; note that, as in Fig. 1, the more distal the peripheral stimulation the longer this extra latency of group II excitation). Then the latency of excitation of motoneurones by group II afferents is the sum of the conduction time in Ia afferents plus the extra latency of group II excitation of the motoneurone shown in column 5 (column 6 = column 4 + column 5).
Table 2.
Estimate of the conduction velocity in group II afferents in one subject
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|
| Nerve-muscle combination | Stim. site-to- motoneurone distance (cm) | I a CV (m s−1) | I a ACT | Extra time: II vs. mono- synaptic I a | I a ACT + extra time | Group I central delay | Group II central delay | IIACT | CV | II CV/I CV |
| Bi→VL | 40 | 68 | 5.9 | 8.2 | 14.1 | 3.9 | 4.9 | 9.2 | 43 | 0.64 |
| CP→VL | 70 | 68 | 10.3 | 9.0 | 19.3 | 3.9 | 4.9 | 14.4 | 48 | 0.68 |
| GM→ST | 70 | 65 | 10.8 | 9.7 | 20.5 | 5.0 | 6.0 | 15.5 | 45 | 0.64 |
| DP→VL | 75 | 68 | 11.0 | 11.7 | 22.7 | 3.9 | 4.9 | 17.8 | 42 | 0.62 |
| DP→Bi | 75 | 68 | 11.0 | 11.5 | 22.5 | 5.0 | 6.0 | 16.5 | 45 | 0.66 |
| SP→ST | 80 | 68 | 11.7 | 12.7 | 24.5 | 5.0 | 6.0 | 18.5 | 43 | 0.63 |
Column 2, distance between site of stimulation and segmental spinal level for the motoneurone; column 3, conduction velocity in I a afferents; column 4, afferent conduction time (ACT) in I a afferents (column 2/column 3); column 5, mean values from different motoneurones for the extra times of group II excitation (over that of heteronymous monosynaptic I a excitation); column 6, delay taken by group II volley to excite motoneurones (column 4 + column 5); column 7, central delay of non-monosynaptic group I excitation (Chaix et al. 1997); column 8, central delay of group II excitation estimated by adding 1 ms to the central delay of non-monosynaptic group I excitation; column 9, ACT of group II volley obtained by subtracting the central delay of group II excitation from the delay taken by group II volley to excite motoneurones (column 6 - column 8); column 10, conduction velocity (CV) in group II afferents (column 2/column 9); column 11, group II CV to group I CV ratio.
Subtracting from this value the central delay of group II actions provides an estimate of the conduction time along group II afferents. Assuming that non-monosynaptic group I and group II excitations are mediated through a common interneuronal pathway (see Discussion), the group II central delay (column 8) may be deduced from the previously calculated central delays of group I non-monosynaptic actions (column 7): 3.9 and 5 ms for Q and Bi, respectively (Chaix et al. 1997). Taking into account a longer conduction time along intraspinal collaterals of group II afferents (see Jankowska, 1992), the group II central delay may be estimated by adding 1 ms to this value (column 8). Note that this estimated central delay (6 ms in sacral motoneurones) is very close to that found by Nardone & Schieppati (1998) for the homonymous group II excitation in the flexor digitorum brevis (FDB; 6.7 ms). From the estimated conduction time of the group II volley (column 9: column 6 minus column 8) the CV of group II afferents may be easily calculated (column 10). Note the very similar values (42-48 m s−1) found for the different nerve- motoneurone combinations explored. Similar values were also found in a second subject.
The values found here for the CV of both Ia and group II fibres are higher than those found by Nardone & Schieppati (1998) in afferents mediating FDB stretch responses travelling in the distal part of the leg (51 and 21 m s−1, respectively). This is not surprising, given that (i) the CV of afferents travelling in proximal nerve trunks are presumably higher due to diameter and temperature changes (Nardone & Schieppati, 1998), and (ii) the electrical stimulation activates preferentially the fastest fibres within both Ia and group II ranges, whereas this is not necessarily true with natural muscle stretch.
DISCUSSION
Excitatory effects
This study shows that stimulation of afferents from some ankle muscles by electrical stimuli above twice the threshold of Ia afferents evokes a potent late excitation in motoneurones of both flexors and extensors of the knee. In Q motoneurones the late excitation is regularly preceded by a substantial, early and lower-threshold, non-monosynaptic group I excitation, whereas in hamstring motoneurones the early excitation is rare, weak, and often replaced by a depression. When the conditioning stimulation was applied at more distal sites and when the leg was cooled, the latency of the late excitation increased more than that of the early excitation. This provides evidence against mediation of the former by Ia afferents either activated secondarily to stimulation of β- and/or γ-fusimotor fibres or through a long-loop central pathway. Hence, the long latency and high threshold of the late excitation investigated in this study are attributed to afferent fibres with smaller diameters than those of Ia afferents.
Peripheral pathway
The CV of the fastest afferents evoking the late excitation was about 42-48 m s−1 (column 10 in Table 2) vs. 68 m s−1 for the fastest Ia afferents (Meunier et al. 1993). This indicates that the CV of these afferents is about 65 % of that of group I afferents in the nerves investigated (column 11 in Table 2). The electrical threshold of the late excitation was about 2.1 times higher than that of Ia afferents. These ratios, which are very close to those found for group II/Ia afferents in the cat (see Matthews, 1972), strongly suggest that the late excitation investigated here is of group II origin. Any significant contribution from cutaneous afferents to the group II excitation elicited by the stimuli used in the present investigation was eliminated by control experiments. Although a contribution from other (e.g. joint) non-spindle group II afferents is possible, we suggest that the group II excitation studied here is evoked primarily from secondary muscle spindle afferents.
Central pathway
Convergence of group II and group I afferents has been shown in the feline lumbar enlargement onto common intermediate zone/ventral horn interneurones mediating disynaptic excitation to motoneurones (Edgley & Jankowska, 1987; Cavallari et al. 1987; Jankowska et al. 1996). Indirect arguments suggest that group I and group II excitations might also be mediated through common interneurones in man: (i) as previously argued (Chaix et al. 1997), this might explain the highly significant negative correlation found between early group I- and late group II-induced facilitations of the Q H reflex at rest: a large recruitment of common interneurones by the CP group I volley would make them unresponsive to the following group II volley (occlusion); (ii) similarly, the finding that the onset of the group II-induced excitation of Q motoneurones is delayed when the interneurones activated by group I fibres are inhibited (on the combined actions of cortical and group I volleys; see Marchand-Pauvert et al. 1999) is consistent with a mediation of excitatory effects of group I and group II afferents via common interneurones.
In the cat, however, the main excitation mediated to motoneurones through intermediate zone/ventral horn interneurones is from group II afferents (Cavallari et al. 1987), which fits the present results in hamstring motoneurones but not those in Q motoneurones where group I and group II excitations seem to be similarly strong. In this respect, it is worth noting that strong heteronymous monosynaptic Ia connections between muscles operating at the ankle and Q, not found in felines, have been described in humans (Meunier et al. 1993). This probably reflects a phylogenetic evolution related to the particular role played by the Q during the stance phase of bipedal gait, where it supports all the body weight.
The strong cutaneous excitation found to group II interneurones in spinal cats also contrasts with the present absence of evidence for excitation from cutaneous afferents. A possible explanation for this discrepancy might arise from the strong descending control exerted on transmission in cutaneous pathways, which may completely abolish the effects of cutaneous stimuli observed in the spinal animal (see Holmqvist & Lundberg, 1961). It may also be pointed out that in the awake intact man cutaneous facilitation of transmission in spinal pathways to a given motoneurone pool has only been observed from the very specific skin field which can meet the target (or an obstacle) during the corresponding movement: this holds for both Ib pathways (Pierrot-Deseilligny et al. 1982) and cervical premotoneurones mediating disynaptic cortical excitation to forearm motoneurones (see Pierrot-Deseilligny, 1996). A cutaneous excitation of group II interneurones by afferents originating from skin fields of particular relevance in stance and gait (foot sole and/or toes), which were not explored here, is thus, by no means, excluded in humans.
Comparison with the distribution of group II excitation in the cat
Results seen in man differ from those described in the cat in two main ways. (i) The similar strength of group II excitation in extensor and flexor motoneurones of thigh muscles described here in man seems to contradict the classical asymmetry of group II actions with dominating flexor excitation and extensor inhibition found in anaesthetized low spinal cats (see Lundberg et al. 1987a). In fact, there are alternative pathways which are disclosed by a low pontine lesion in the decerebrate animal (Holmqvist & Lundberg, 1961), and large group II EPSPs are more common in extensor motoneurones in unanaesthetized high (Wilson & Kato, 1965) and low (Hongo & Petterson, 1988) spinal cats. (ii) Group II excitation, which is constant from GM to ST motoneurones and frequent from DP to VL motoneurones, was never found in combinations from the same nerves to muscles operating at the ankle (GM to TA and DP to GM). Such a discrepancy between the group II excitatory projections from ankle muscles to motoneurones of muscles operating at knee and ankle levels, which has not be described in the cat (see Lundberg et al. 1987a), could reflect a phylogenetic evolution related to bipedal stance and gait.
However, these differences between the pattern of group II excitation, as the absence of evidence for group II inhibition of motoneurones in man (see below), must be treated with some caution, given the relatively limited number of nerve-muscle combinations that were investigated (only those without recurrent inhibition).
Inhibitory effects
Group I-induced depression
When investigating CP-induced effects in single Q motor units, an early depression elicited by stimuli around 1 × MT, and therefore probably of group I origin, was repeatedly seen in one subject. A depression truncating the early excitation, suggested by the brief duration of this group I-induced peak in the other subjects (see Results), is very marked when CP and cortical stimulations are combined (Marchand-Pauvert et al. 1999). This depression may reflect a direct inhibition of motoneurones (IPSPs in motoneurones) or their disfacilitation (i.e. group I-induced inhibition of premotoneurones mediating excitation to the motoneurone maintaining the voluntary level of firing during PSTH experiments). Two arguments are in favour of disfacilitation: (i) the absence of early group I-induced depression of the Q H reflex, at rest (Marque et al. 1996) as well as during tonic Q contraction (Marchand-Pauvert et al. 1999), argues against an IPSP evoked in motoneurones, since such an IPSP would be expected to depress the H reflex as well (see the Discussion in Burke et al. 1994); (ii) experiments using cortical stimulation suggest that this depression is evoked via a pathway involving one more synapse than the pathway mediating group I non-monosynaptic excitation (see Marchand-Pauvert et al. 1999), which would fit a trisynaptic disfacilitation (described in the cat; see Jankowska, 1992) better than a disynaptic inhibition of motoneurones.
Group II-induced depression
In contrast to the ease with which heteronymous group II excitation was disclosed, the only evidence for a depressive effect induced by group II muscle afferents was the small decrease in the late GM-induced excitation of ST motoneurones observed in both PSTH and H reflex (Fig. 5B) experiments with GM stimulus intensity > 1.8-2 × MT. This depression was indeed very weak in the present experiments, but it will be shown in the companion paper (Marchand-Pauvert et al. 1999) that, when combined with the actions of cortical stimulation, it is strong enough to annihilate the very large facilitation of cortical excitation induced by group II afferents in ST motoneurones. The finding that this depression was never found in combinations without group II excitation (Inf Sol and DP onto ST motoneurones, or projections onto motoneurones of ankle muscles) but only appeared as a suppression of this excitation (Fig. 5B, and Marchand-Pauvert et al. 1999) suggests, as previously argued for another pathway in the cervical spinal cord (see Pierrot-Deseilligny, 1996), that it reflects a disfacilitation of premotoneurones mediating group II excitation.
Although Rymer et al. (1979) have suggested that the group II IPSPs elicited in feline motoneurones by electrical stimuli in the group II range are produced by non-spindle group II afferents, there is conclusive evidence for such spindle group II IPSPs in cat motoneurones (see Lundberg et al. 1987a). The absence of evidence for group II inhibition of motoneurones in humans is therefore the most striking difference with animal data. However, it is possible that the pathway exists but is not open in awake intact man. Under these conditions, the selective function proposed for group II inhibition of motoneurones in the cat (Lundberg et al. 1987b) might be taken over by an inhibition of the excitatory premotoneurones co-activated by group I and group II afferents (see the companion paper, Marchand-Pauvert et al. 1999).
Functional implications
Excitation evoked by heteronymous group II afferents in human motoneurones is far more potent than excitation in corresponding monosynaptic Ia pathways: e.g. in ST motoneurones, the GM-induced group II excitation was found in all tested motor units and had a mean size of 39 %, whereas the monosynaptic Ia excitation reached statistical significance in only 10 % of the motor units. The ease with which monosynaptic Ia excitation can be investigated in man (because it is the first effect to appear in motoneurones after peripheral stimulation and with the lowest threshold) has probably led to an overestimation of its role. In fact the potency of the heteronymous group II excitation described here, like that of homonymous group II excitation (see Dietz, 1992; Schieppati & Nardone, 1997), suggests that group II excitatory pathways may be very important for fast and co-ordinated stretch-induced postural adjustments during bipedal stance and gait, especially since the relevant premotoneurones (at least those projecting onto Q motoneurones) also receive potent excitatory input from Ia spindle afferents. With respect to heteronymous projections, it is worth noting that the maintenance of bipedal stance when leaning forwards is due to co-contraction of GM and ST, and when leaning backwards to co-contraction of TA and Q, i.e. precisely the combinations in which the strongest group II excitations have been found. In addition, the potent cortical control to interneurones co-activated by group I and group II afferents described in the companion paper (Marchand-Pauvert et al. 1999) suggests that a significant part of the cortical excitation to motoneurones of thigh muscles is mediated through these neurones in man.
Acknowledgments
The authors wish to express their gratitude to Dr L. Jami, Professors. E. Jankowska, Y. Laporte and A. Lundberg, Drs L. Mazières and S. Meunier and Professor J. Nielsen for reading and commenting upon the manuscript. Our thanks are also due to Annie Rigaudie and Michèle Dodo for excellent technical assistance. This work was supported by grants from Assistance Publique-Hôpitaux de Paris (PHRC AOM 95078), Institut National de la Santé et de la Recherche Médicale (INSERM, CRI 96037), Ministère de l'Enseignement Supérieur et de la Recherche (EA 2393) and Institut pour la Recherche sur la Moelle Épinière (IRME).
References
- Burke D, Gracies JM, Mazevet D, Meunier S, Pierrot-Deseilligny E. Non-monosynaptic transmission of the cortical command for voluntary movement in man. The Journal of Physiology. 1994;480:191–207. doi: 10.1113/jphysiol.1994.sp020352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallari P, Edgley SA, Jankowska E. Postsynaptic actions of midlumbar interneurones on motoneurones of hind limb muscles in the cat. The Journal of Physiology. 1987;389:675–690. doi: 10.1113/jphysiol.1987.sp016677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix Y, Marque Ph, Meunier S, Pierrot-Deseilligny E, Simonetta-Moreau M. Further evidence for non-monosynaptic group I excitation of motoneurones in the human lower limb. Experimental Brain Research. 1997;115:35–46. doi: 10.1007/pl00005683. [DOI] [PubMed] [Google Scholar]
- Corna S, Grasso M, Nardone A, Schieppati M. Selective depression of medium-latency leg and foot muscle responses to stretch by an α2-agonist in humans. The Journal of Physiology. 1995;484:803–809. doi: 10.1113/jphysiol.1995.sp020705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel C, Lehmann EL. Henry Scheffé. Annals of Statistics. 1979;7:1149–1161. [Google Scholar]
- Dietz V. Human neuronal control of automatic functional movements: interaction between central programs and afferent input. Physiological Reviews. 1992;72:33–69. doi: 10.1152/physrev.1992.72.1.33. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. An interneuronal relay for group I and II muscle afferents in the midlumbar segments of the cat spinal cord. The Journal of Physiology. 1987;399:675–690. doi: 10.1113/jphysiol.1987.sp016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget R, Pantieri R, Pierrot-Deseilligny E, Shindo M, Tanaka R. Facilitation of quadriceps motoneurones by group I afferents from pretibial flexors in man. 1. Possible interneuronal pathway. Experimental Brain Research. 1989;78:10–20. doi: 10.1007/BF00230681. [DOI] [PubMed] [Google Scholar]
- Fournier E, Meunier S, Pierrot-Deseilligny E, Shindo M. Evidence for interneuronally mediated Ia excitatory effects to human quadriceps motoneurones. The Journal of Physiology. 1986;377:143–169. doi: 10.1113/jphysiol.1986.sp016179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracies JM, Pierrot-Deseilligny E, Robain G. Evidence for further recruitment of group I fibres with high stimulus intensities when using surface electrodes in man. Electroencephalography and Clinical Neurophysiology. 1994;93:353–357. doi: 10.1016/0168-5597(94)90123-6. 10.1016/0168-5597(94)90123-6. [DOI] [PubMed] [Google Scholar]
- Gustafsson B, McCrea D. Influence of stretch-evoked synaptic potentials on firing probability of cat spinal motoneurones. The Journal of Physiology. 1984;347:431–451. doi: 10.1113/jphysiol.1984.sp015074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond PH. The influence of prior instruction to the subject on an apparently neuromuscular response. The Journal of Physiology. 1956;132:17–18. P. [PubMed] [Google Scholar]
- Holmqvist B, Lundberg A. Differential supraspinal control of synaptic actions evoked by volleys in the flexion reflex afferents in alpha motoneurones. Acta Physiologica Scandinavica. 1961;54(suppl. 186):1–51. [PubMed] [Google Scholar]
- Hongo T, Pettersson LG. Comments on group II excitation in hindlimb motoneurones in high and low spinal cats. Neuroscience Research. 1988;5:563–566. doi: 10.1016/0168-0102(88)90043-0. 10.1016/0168-0102(88)90043-0. [DOI] [PubMed] [Google Scholar]
- Hunt CC, Kuffler SW. Stretch receptor discharges during muscle contraction. The Journal of Physiology. 1951;113:298–315. doi: 10.1113/jphysiol.1951.sp004573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack JJB. Some methods for selective activation of muscle afferent fibres. In: Porter R, editor. Studies in Neurophysiology. Cambridge, UK: Cambridge University Press; 1978. pp. 155–176. [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Progress in Neurobiology. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Perfilieva EV, Riddell JS. How effective is integration of information from muscle afferents in spinal pathways? NeuroReport. 1996;7:2337–2340. doi: 10.1097/00001756-199610020-00012. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents. 1. Distribution and linkage of reflex actions to alpha-motoneurones. Experimental Brain Research. 1987a;65:271–281. doi: 10.1007/BF00236299. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents. 3. Secondary spindle afferents and the FRA: a new hypothesis. Experimental Brain Research. 1987b;65:294–306. doi: 10.1007/BF00236301. [DOI] [PubMed] [Google Scholar]
- Marchand-Pauvert V, Simonetta-Moreau M, Pierrot-Deseilligny E. Cortical control of spinal pathways mediating group II excitation to thigh motoneurones. The Journal of Physiology. 1999;517:301–313. doi: 10.1111/j.1469-7793.1999.0301z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marque Ph, Pierrot-Deseilligny E, Simonetta-Moreau M. Evidence for excitation of the human lower limb motoneurones by group II muscle afferents. Experimental Brain Research. 1996;109:357–360. doi: 10.1007/BF00231793. [DOI] [PubMed] [Google Scholar]
- Matthews PBC. Mammalian Muscle Spindles and their Central Action. London: Arnold; 1972. p. 630. [Google Scholar]
- Matthews PBC. The human stretch and the motor cortex. Trends in Neurosciences. 1991;14:87–91. doi: 10.1016/0166-2236(91)90064-2. 10.1016/0166-2236(91)90064-2. [DOI] [PubMed] [Google Scholar]
- Melvill Jones G, Watt DGD. Muscular control of landing from unexpected falls in man. The Journal of Physiology. 1971;219:729–737. doi: 10.1113/jphysiol.1971.sp009685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier S, Pierrot-Deseilligny E, Simonetta-Moreau M. Pattern of monosynaptic heteronymous Ia connections in the human lower limb. Experimental Brain Research. 1993;96:533–544. doi: 10.1007/BF00234121. [DOI] [PubMed] [Google Scholar]
- Meunier S, Pierrot-Deseilligny E, Simonetta-Moreau M. Pattern of heteronymous recurrent inhibition in the human lower limb. Experimental Brain Research. 1994;102:149–159. doi: 10.1007/BF00232447. [DOI] [PubMed] [Google Scholar]
- Nardone A, Schieppati M. Medium-latency response to muscle stretch in human lower limb: estimation of conduction velocity of group II fibres and central delay. Neuroscience Letters. 1998;249:29–32. doi: 10.1016/s0304-3940(98)00383-8. 10.1016/S0304-3940(98)00383-8. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E. Transmission of the cortical command for human voluntary movement through cervical premotoneurones. Progress in Neurobiology. 1996;48:489–517. doi: 10.1016/0301-0082(96)00002-0. 10.1016/0301-0082(96)00002-0. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Bergego C, Katz R. Reversal in cutaneous control of Ib pathways during human voluntary contraction. Brain Research. 1982;233:400–403. doi: 10.1016/0006-8993(82)91213-6. 10.1016/0006-8993(82)91213-6. [DOI] [PubMed] [Google Scholar]
- Rymer WZ, Houk JC, Crago PE. Mechanisms of the clasp-knife reflex studied in an animal model. Experimental Brain Research. 1979;37:93–113. doi: 10.1007/BF01474257. [DOI] [PubMed] [Google Scholar]
- Schieppati M, Nardone A. Medium-latency stretch reflexes of foot and leg muscles analysed by cooling the lower limb in standing humans. The Journal of Physiology. 1997;503:691–698. doi: 10.1111/j.1469-7793.1997.691bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieppati M, Nardone A, Siliotto R, Grasso M. Early and late stretch responses of human foot muscles induced by perturbation of stance. Experimental Brain Research. 1995;105:411–422. doi: 10.1007/BF00233041. [DOI] [PubMed] [Google Scholar]
- Stephens JA, Usherwood TP, Garnett R. Technique for studying synaptic connections of single motoneurones in man. Nature. 1976;263:343–344. doi: 10.1038/263343a0. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Kato M. Excitation of extensor motoneurons by group II afferent fibers in ipsilateral muscle nerves. Journal of Neurophysiology. 1965;28:545–554. doi: 10.1152/jn.1965.28.3.545. [DOI] [PubMed] [Google Scholar]


