Abstract
Although glutamate receptors have been shown to be involved in neuronal maturation, a developmental role for kainate-type receptors has not been described. In addition, the single-channel properties of native kainate receptors have not been studied in situ. We have characterized the electrophysiological properties of native kainate receptors of granule cell neurons at two distinct stages in postnatal development, using whole-cell and outside-out patch-clamp recordings in acute cerebellar slices.
Kainate-type currents were detected in both immature and mature granule cells. However, noise analysis showed that the apparent unitary conductance of kainate-type channels is significantly higher in proliferating than post-migratory granule cells. The conductance and rectification behaviour of the channels in immature granule cells indicate that they contain unedited GluR5 and GluR6 subunits and are likely to be calcium permeable.
Single-channel kainate-type currents were observed in outside-out patches from proliferating granule cells in the external germinal layer. The kinetic behaviour of kainate receptors in immature cells was complex. Openings to multiple conductance levels were observed, although our analysis indicates that the channels spend most of their open time in a 4 pS state.
Proper cerebellar development depends on a precisely choreographed sequence of postnatal events, some of which are mediated by glutamate receptors. For example, NMDA receptors have been implicated in granule cell migration (Komuro & Rakic, 1993) and synaptic pruning of climbing fibre inputs to Purkinje cells (Rabacchi et al. 1992). Although kainate receptors have recently been shown to be involved in synaptic transmission (Vignes & Collingridge, 1997; Cossart et al. 1998; Frerking et al. 1998; Mülle et al. 1998), little is known about their role in development. However, the expression of kainate-type glutamate receptor subunits in immature granule cells of the external germinal layer (EGL) of the developing cerebellum suggests that kainate receptors may also function in neuronal maturation (Ripellino et al. 1998).
Kainate-type glutamate receptors are assembled from the kainate-receptor subunits GluR5-7, and KA1 and KA2 (Bettler & Mulle, 1995). Diversity of kainate-type channel properties, such as unitary conductance, Ca2+ permeability, and rectification behaviour, arises from differences in receptor subunit composition and RNA editing of GluR5 and GluR6 (Sommer et al. 1991; Herb et al. 1992; Howe, 1996; Swanson et al. 1996). For example, studies of cloned GluR5 and GluR6 homomers have shown that RNA editing decreases both unitary conductance and Ca2+ permeability (Burnashev et al. 1995; Swanson et al. 1996), while incorporation of KA2 into heteromers increases channel conductance (Howe, 1996; Swanson et al. 1996). Our previous work showed that cultured cerebellar granule cells express functional kainate receptors (Pemberton et al. 1998) and that RNA editing of GluR5 and GluR6 increases, and KA2 expression decreases, as granule cells mature (Belcher & Howe, 1997; Ripellino et al. 1998). During postnatal days 7-14 of rat cerebellar development (P7-14), granule cells migrate from the EGL, where they proliferate, to the internal granular layer (IGL), where they receive synaptic input (Altman, 1972). The present study aimed to characterize the electrophysiological properties of native kainate-type channels of developing granule cells in acute cerebellar slices and to test the hypothesis that the developmental changes in RNA editing and subunit expression observed in vitro correlate with single-channel properties in situ.
METHODS
Slice preparation and patch-clamp recordings
Mice of ages P6-9 and P17-19 (C57-black-6, Charles River) were anaesthetized with Metofane (Pitman-Moore), decapitated, and the brains removed in ice-cold oxygenated artificial cerebrospinal fluid (ACSF). Parasagittal slices (150-200 μm thick) of cerebellum were cut with a vibratome. Slices were then incubated in ACSF at 37°C for 30-60 min, and at room temperature (20-22°C) thereafter. ACSF contained (mM): 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 10 glucose, 1.25 NaH2PO4H2O, 26 NaHCO3, 2 sodium pyruvate, 3 myo-inositol, 0.5 ascorbic acid; pH 7.4 when oxygenated. To reduce desensitization of kainate-type channels (Huettner, 1990; Partin et al. 1993), slices were incubated for at least 25 min in ACSF with concanavalin A (Con A; 10-25 μM) at room temperature before transfer to the recording chamber.
In the recording chamber, slices were continuously perfused (∼1-2 ml min−1) with control solution (ACSF with 10 mM tetraethylammonium chloride, 0.1 mM 4-aminopyridine, 20 μM 7-chlorokynurenate, and 20 μM DL-2-amino-5-phosphonovaleric acid (APV)). Kainate, domoate, and GYKI 53655 (1-(4-aminophenyl)-3-methylcarbamyl-4-methyl-7,8-methylenedioxy-3,4-dihydro-5H-2,3-benzodiazepine (200 μM, except for one kainate-evoked response in which 150 μM GYKI was used; Eli Lilly) were bath-applied in control solution. The pipette solution contained (mM): 97.5 potassium gluconate, 32.5 CsCl, 5 EGTA, 10 Hepes, 1 MgCl2, 0.1 spermine and 2 lidocaine n-ethyl bromide (QX314); pH 7.2. All recordings were performed at room temperature. Patch pipettes (5-14 MΩ) were made from borosilicate glass, coated with Sylgard and fire-polished. Granule cells were identified by their location, appearance and small capacitance (1-4 pF). To minimize distortion by resistance-capacitance (RC) filtering, recordings were discontinued if the membrane time constant, τ = RC, exceeded 200 μs. In most recordings, τ was less than 100 μs.
Electrophysiological analysis
Data acquisition, spectral density analysis of steady-state agonist-evoked current noise, and the calculation of slope conductances have been described (Howe, 1996; Pemberton et al. 1998). The ratio of the slope conductances at +30 and -30 mV was used as an index of rectification. Estimates of channel open probability (popen) were obtained from analysis of current-associated variance during the relatively slow onset of the whole-cell currents. One-sided spectral densities (18 to 1000 Hz) were calculated from 55 ms segments of data (256 points). Even at high agonist concentrations, the mean current changed by much less than 1 % during each segment. After plotting variance as a function of the current, the data were fitted with the equation: var(t) = iI(t) - I(t)2/N, where i is the single-channel current, I(t) is the mean macroscopic current for a given data segment, and N is the number of channels. The maximal Popen of the channels underlying each kainate-evoked response was then calculated as Popen = Iss/Ni, where Iss is the steady-state kainate-evoked current. For the data shown (Fig. 3E), and for most responses, the fit did not deviate detectably from linearity, indicating a very low Popen.
Figure 3. Kainate receptors in immature granule cells have a larger apparent unitary conductance than those in mature cells.
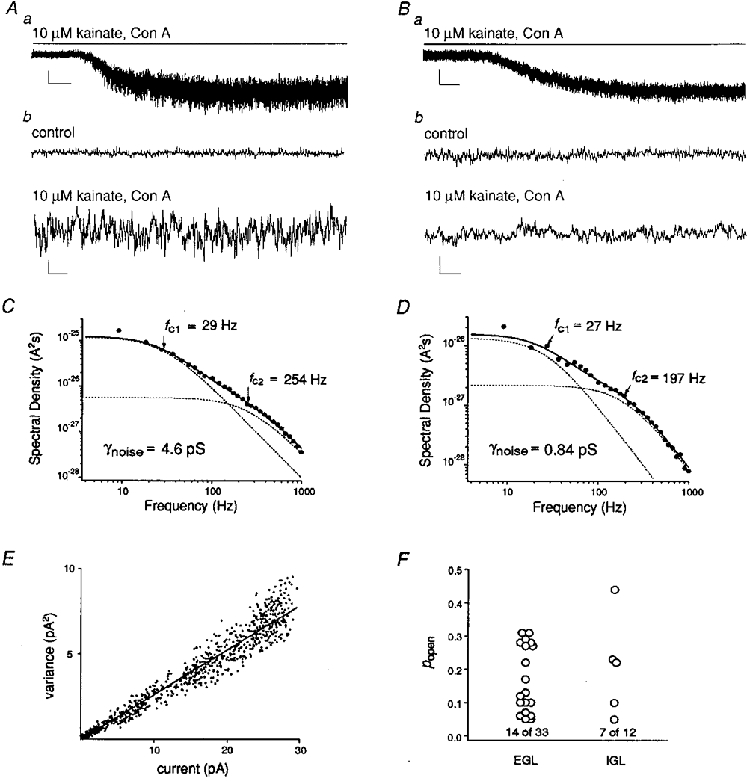
Aa, whole-cell current evoked by 10 μM kainate in an EGL cell (-60 mV). Scale, 20 pA, 5 s. b, portions of the record in a before (control) and during kainate at a larger gain and on a faster time scale. Scale, 10 pA, 50 ms. B, whole-cell current evoked by 10 μM kainate in an IGL cell (-60 mV). Note the smaller increase in kainate-evoked current noise compared with the current evoked in the EGL cell. C and D, power spectra of the currents in A and B, respectively. The data were fitted (continuous line) with the sum of two Lorentzian components (dashed lines). The half-power frequencies and γnoise values obtained from the fits are indicated. Data were sampled at 9.4 kHz and low-pass filtered at 2 kHz. E, current-variance plot from a response to 10 μM kainate in an EGL cell. F, plot of Popen values measured in EGL and IGL cells. The numbers represent how many cells gave a Popen value below 0.05. In these cases, the Popen was poorly defined (although very low), and conductance measurements were not corrected. The γnoise values were corrected for all cells that gave Popen > 0.05.
Single-channel recordings were low-pass filtered at 10 kHz (-3 dB, Bessel-type) and stored on videotape. The replayed digitized signals were filtered with a low-pass Gaussian filter routine at 1 or 2 kHz (-3 dB), and compressed to sampling rates of 18.8 to 47.2 kHz. To measure the amplitudes of completely resolved single-channel openings, we used the mean low-variance method of Patlak (1988). First, a portion of the record was selected that contained no channel openings, and the mean amplitude and variance of the closed channel points were calculated. The closed level was set to zero and the entire record was then inspected. Slight corrections in the baseline level were made, if necessary, before the low-variance open-point analysis was performed. Sets of contiguous data points at least two filter rise times in duration were found for which the mean amplitude differed by more than two standard deviations from the closed level and for which the variance was not greater than one-quarter of the variance of the closed channel noise. This procedure reliably found events at least two filter rise times in duration. All of the open points found by the routine were visually inspected, and points that appeared to correspond to artefacts were deleted. Histograms of the so-edited low-variance open points were fitted with multiple Gaussians to obtain mean currents for the multiple open levels in the records. The mean currents were converted to conductances assuming that the single-channel currents reversed at 0 mV, which was close to the reversal potentials measured for whole-cell currents. The low-variance open point amplitudes and Popen values for the single-channel records were also used to calculate the mean current and current variance for individual data sets (closed point amplitudes were set to zero). This allowed calculation of predicted γnoise values for detectable single-channel activity in excised patches which could be compared with the corresponding values obtained from whole-cell current noise.
RESULTS
Whole-cell recordings of currents through kainate receptors
To determine whether immature granule cells express functional kainate receptors, whole-cell patch-clamp recordings were made from granule cells two to four cells deep from the pial surface of parasagittal slices of the cerebellar cortex of P6 to P9 mice. In mice of this age, the entire EGL is about six to ten granule cells across. By restricting our studies to superficial cells, our EGL results are likely to be from proliferating cells rather than from differentiated cells in the premigratory zone. Our results support the presence of kainate receptor channels in EGL granule cells. First, it is known that low concentrations of kainate selectively evoke desensitizing responses through kainate receptors, while higher concentrations produce incompletely desensitizing activation of AMPA receptors (Huettner, 1990; Lerma et al. 1993). Therefore the slow solution exchange in slices should prevent detection of kainate-type currents unless kainate-receptor desensitization is slowed. We did not observe EGL responses to low concentrations of kainate unless kainate-receptor desensitization was reduced with concanavalin A (Con A; Huettner, 1990; Partin et al. 1993). In Con A-treated EGL cells, however, 10 μM kainate routinely evoked currents (12.2 ± 1.4 pA, 34 of 36 cells). Kainate-evoked currents were completely reversible and, provided the recording remained stable, several reproducible responses could be elicited from a cell. Without Con A, concentrations of kainate sufficient to activate AMPA receptors (300-600 μM) failed to evoke currents in 5 of 9 EGL cells tested (Fig. 1Aa), and the currents in the other cells were small (5 to 8 pA). Second, in Con A-treated EGL cells, 300 μM kainate elicited only marginally larger currents than did 10 μM kainate (Fig. 1Ab and c), suggesting that the channels underlying the currents have a high affinity for kainate.
Figure 1. Kainate-evoked whole-cell currents from granule cells in acute cerebellar slices.
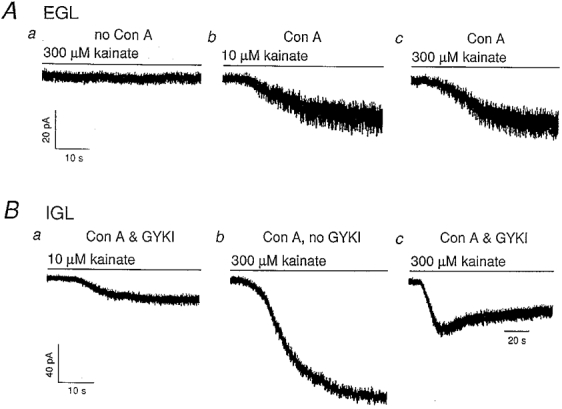
Holding potential, -60 mV. Aa, in an EGL cell, 300 μM kainate did not elicit a detectable inward current in the absence of Con A (P9 mouse); b and c, in the presence of Con A (25 μM), 10 μM and 300 μM kainate evoked responses of similar amplitudes in another EGL cell (P9 mouse). Ba, kainate-type current in an IGL cell from a P19 mouse in the presence of Con A (25 μM) and GYKI 53655 (200 μM); b, in the absence of GYKI, 300 μM kainate produced a large AMPA-type current in the same cell. c, the AMPA receptor response to 300 μM kainate is blocked by GYKI with a delay. The size of the GYKI-resistant current is similar to that evoked by 10 μM kainate. All currents shown were fully reversible. Data were sampled at 9.4 kHz and low-pass filtered at 2 kHz.
High-affinity kainate-type channels were also detected in IGL cells from P17-19 mice, provided that the slices were pretreated with Con A. Under these conditions, 10 μM kainate evoked currents in 12 of 18 IGL cells (19.8 ± 4.4 pA). In each of five IGL cells tested, the currents evoked by 10 μM kainate were insensitive to the AMPA-receptor antagonist GYKI 53655 (150-200 μM; Paternain et al. 1995). In contrast, in IGL cells not exposed to Con A, 300 μM kainate evoked currents (50-400 pA at -60 mV) that were completely blocked by GYKI 53655 (200 μM). In Con A-treated IGL cells, 300 μM kainate evoked currents much larger than those evoked by 10 μM kainate, and GYKI 53655 only partially blocked responses to 300 μM kainate (Fig. 1B). We interpret the GYKI-insensitive currents in IGL cells to be mediated by kainate-type channels.
Further evidence supports the presence of kainate-type channels in developing granule cells. Concentration-response data (Fig. 2A) from three granule cells in the EGL gave a mean EC50 value of 8.4 ± 2.1 μM (≥ 4 concentrations of kainate), similar to values previously measured for native (Huettner, 1990; Lerma et al. 1993; Wilding & Huettner, 1997; Pemberton et al. 1998) and recombinant kainate-type channels (Howe, 1996). Similar analysis for kainate activation of AMPA receptors in IGL cells (no Con A) gave a mean EC50 value of 78 ± 10 μM (n = 5 cells, ≥ 4 concentrations of kainate). Currents in Con A-treated cells were also evoked by the kainate-receptor agonist domoate (2 to 10 μM elicited whole-cell currents of 2 to 22 pA, n = 9 of 9 cells), as well as by 100 μM glutamate in the presence of 200 μM GYKI 53655 and NMDA-receptor antagonists (3 to 6 pA, n = 5 of 5 cells). Kainate-evoked currents in EGL cells consistently showed inward rectification (Fig. 2B), characteristic of kainate receptors containing unedited GluR5 and GluR6 subunits (Bowie & Mayer, 1995; Kamboj et al. 1995). However, the mean rectification ratio (0.28 ± 0.07, n = 12 cells) also supports the presence of kainate-type channels containing edited subunits.
Figure 2. Native kainate receptors in immature cerebellar granule cells show a high affinity for kainate and display inward rectification.
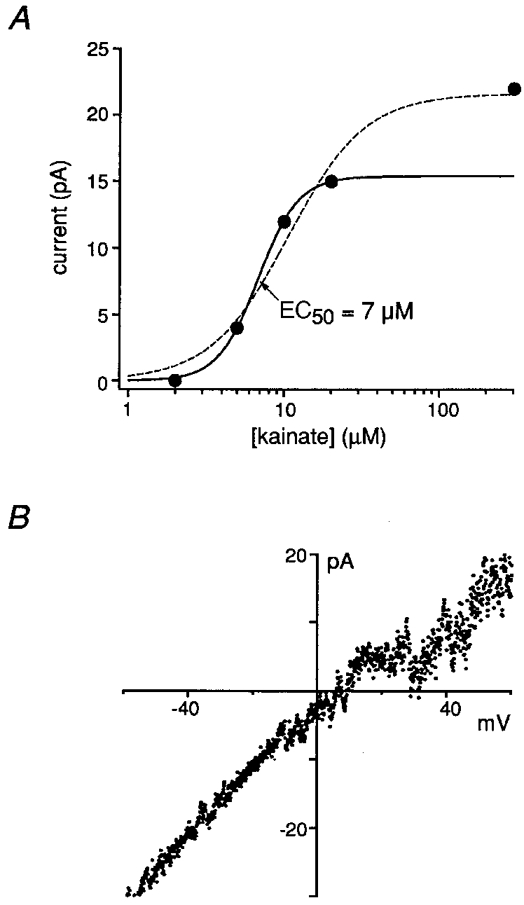
A, concentration-response data for steady-state kainate-evoked currents in an EGL cell (with Con A, 25 μM). The results were fitted with Hill-type equations, either including (dashed line) or excluding (continuous line) the 300 μM data point. The EC50 values obtained from the fits were similar (7 μM vs. 10 μM), suggesting that contamination of the currents by AMPA-type channels was minimal. B, current-voltage curve from voltage ramps (-60 to +60 mV) during a steady-state response to 10 μM kainate in an EGL cell. Despite the presence of potassium channel blockers, similar measurements could not be made for mature IGL cells due to residual potassium currents.
Single-channel properties of kainate receptors
Although both immature and mature granule cells express kainate receptors, we found that the single-channel properties of their receptors differ. Because some kainate-type channels have conductances that are too small to measure single-channel currents directly, we initially estimated channel conductances with spectral density analysis of whole-cell currents. Figure 3A-D compares the whole-cell current responses to 10 μM kainate from an EGL cell and an IGL cell. We consistently found that kainate-type currents in immature granule cells were accompanied by larger increases in noise than the currents in mature granule cells. Noise analysis of steady-state whole-cell currents evoked by 10 μM kainate in immature and mature granule cells revealed a significant reduction in apparent unitary conductance (γnoise) during development (mean ± s.e.m.: EGL, γnoise = 5.4 ± 0.4 pS, n = 33 cells; IGL, γnoise = 1.8 ± 0.2 pS, n = 12 cells; P < 0.000001, Student's unpaired t test). The γnoise values for EGL cells were obtained in the absence of GYKI 53655, but are unlikely to be contaminated by AMPA-type currents. Without Con A, 10 μM kainate did not elicit currents in EGL cells, and in IGL cells, where AMPA-type currents were much more prominent, 10 μM kainate failed to elicit detectable responses in 6 of 6 cells. In IGL cells, the γnoise values measured with 10 μM kainate in the absence and presence of 200 μM GYKI 53655 were similar (1.9 ± 0.2 pS and 1.8 ± 0.3 pS, n = 7 and 5 cells, respectively), and the values from the two groups were pooled.
Channel open probability (Popen) was estimated for each kainate-evoked response, and γnoise values were calculated according to the expression:
where var is the variance associated with the agonist-evoked current noise, Iss is the mean steady-state macroscopic current, and V is the membrane voltage. To estimate Popen, we constructed current-variance plots by calculating one-sided spectral densities from short data segments during the onset of the currents, during which the mean current changed by much less than 1 % even at high agonist concentrations. In many cases the fit of the current-variance plot did not deviate detectably from linearity (Fig. 3E). In other cases the fit converged and gave values ranging from 0.05 to 0.4. Thus the maximal Popen for kainate-type channels in EGL and IGL cells was low (Fig. 3F), as shown for recombinant kainate receptors under similar conditions (Howe, 1996), and the difference in the amplitude of the noise increases evoked in EGL and IGL cells is not due to differences in channel open probabilities. We also observed that the half-power frequencies for the two Lorentzian components (fc1 and fc2) in the steady-state noise spectra were, on average, significantly smaller for IGL cells, suggesting an increase in the mean burst length of kainate-type channels in mature granule cells (EGL, fc1 = 32 ± 1 Hz, fc2 = 366 ± 19 Hz, n = 33 cells; IGL, fc1 = 25 ± 2 Hz, fc2 = 249 ± 38 Hz, n = 12 cells; both fc values were significantly lower in IGL cells, P < 0.002, Student's unpaired t test).
The γnoise value from EGL cells suggested that it should be possible to resolve openings of single kainate-type channels. We therefore pulled outside-out patches from Con A-treated EGL cells in an effort to study these channels more directly. Single-channel currents were evoked by domoate (0.5 to 10 μM) and kainate (1 to 10 μM) in 13 patches from EGL cells. In each of our three best patches, the most frequent events were small isolated openings (Fig. 4A, top two traces). Analysis of these events with the mean low-variance method of Patlak (1988) gave histograms with single peaks corresponding to a unitary conductance of 4 pS. We also observed short periods of high Popen activity that included openings to larger levels (Fig. 4A, bottom two traces). A typical histogram of low-variance open point amplitudes obtained from one of these high Popen periods is shown in Fig. 4B. The fit to the results gave conductance levels of 4, 9 and 15 pS. These values agree well with levels found for cloned homomers of unedited GluR5, or heteromers of unedited GluR5 with KA2 (Swanson et al. 1996), and are close to open levels reported for native kainate receptors in cultured dorsal root ganglion neurons (Huettner, 1990). Similar analysis of long continuous records confirmed that openings to the 4 pS level were most common, although discrete peaks at the larger, less frequently visited levels were obscured (Fig. 4C). The lack of discrete peaks at larger open levels appeared to reflect the presence of multiple channel types with slightly different conductance levels. Although defined peaks could not be resolved at larger open levels, the predicted γnoise values calculated from the single-channel records (5-6 pS, see Methods), were close to the γnoise value calculated from whole-cell current noise (5.4 pS).
Figure 4. Single-channel currents through native kainate receptors.
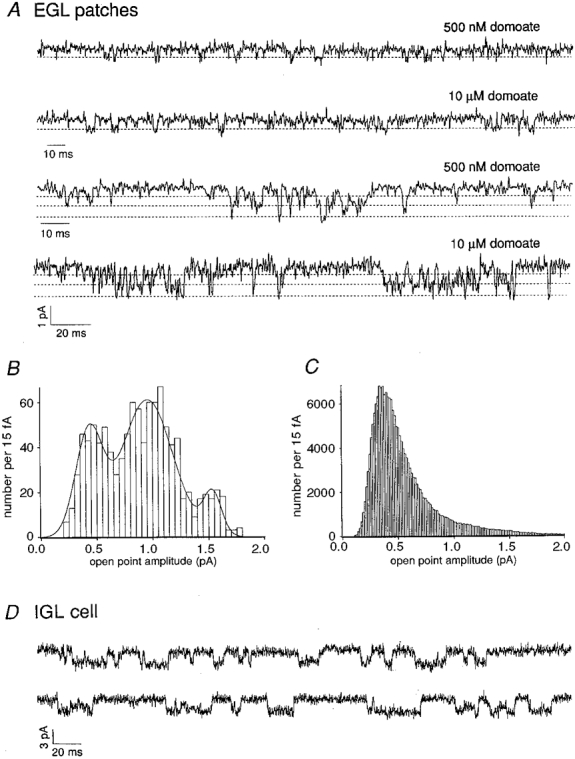
A, unitary currents in outside-out patches from two EGL cells (-100 mV, Con A). Data were sampled at 18.8 kHz and filtered at 1 kHz. B, analysis of the bottom trace in A gave a low-variance open point histogram with three visible peaks. The three-Gaussian fit to the results gave mean conductance values of 4, 9 and 15 pS (dashed lines in A), levels frequently observed in other portions of the record. C, low-variance open point histogram for 2.5 min of the recording during application of 500 nM domoate. The peak corresponds to a conductance of 4 pS. D, whole-cell recording at -100 mV from a Con A-treated IGL cell during application of 10 μM kainate in the presence of 200 μM GYKI 53655, 20 μM APV, 20 μM 7-chlorokynurenate and 10 μM bicuculline. Note the long duration of the channel openings (downwards).
The smaller γnoise value measured for IGL cells (1.8 pS) is intermediate between values measured for kainate-type channels formed from edited and unedited subunits (Howe, 1996; Swanson et al. 1996). Because γnoise is a weighted mean conductance of the population of channels in a given neuron, femtosiemens conductance channels are likely to predominate, but some larger-conductance channels should also be present. Indeed, single-channel currents were detected occasionally in whole-cell recordings from IGL neurons, examples of which are shown in Fig. 4D. These events (about 20 pS) were quite long, in contrast to the briefer openings in patches from EGL cells (openings smaller than about 10 pS could not be resolved directly in the whole-cell recordings due to increased background noise). The large-conductance openings seen in whole-cell mode, although infrequent, would contribute substantially to the current variance. In addition, the long duration of these events may contribute to the decrease in half-power frequencies we measured with noise analysis for IGL cells.
DISCUSSION
We have shown that cerebellar granule cells express functional kainate receptors in situ. These receptors are present in both proliferating granule cells and granule cells that have reached the IGL and received synaptic inputs. Although AMPA-type currents were detected in some EGL cells, they were much smaller than those detected in IGL cells. The paucity of AMPA-receptor channels in EGL cells, and their relatively low affinity for kainate, ensure that the currents evoked by 10 μM kainate in EGL cells were through kainate-type channels. The resistance of similar currents in Con A-treated IGL cells to AMPA-receptor blockade likewise confirms the presence of kainate-type channels in mature granule cells.
We also provide evidence that the single-channel conductance of kainate receptors declines as granule cells mature. Taken together, our noise and single-channel results indicate that most kainate receptors in immature granule cells contain the unedited forms of GluR5 and GluR6, perhaps in combination with KA2. It is likely that the edited forms of GluR5 and GluR6 are also expressed in EGL cells, but direct openings of these channels would be undetectable in outside-out patches (Howe, 1996; Swanson et al. 1996). While our results do not exclude the presence of channels containing edited subunits in immature granule cells, the predominance of single-channel openings to the 4 pS level, together with the mean γnoise value (5.4 pS) obtained from EGL cells with similar agonist concentrations, suggests that channels with conductances much smaller than 4 pS are unlikely to be numerous in immature granule cells. Conversely, the decline in γnoise for mature granule cells is likely to reflect the increased contribution of femtosiemens conductance channels containing edited subunits, although the mean γnoise value (1.8 pS) indicates that some unedited channels are also likely to be expressed. Both the conductance and the longer duration of the openings shown in the IGL cell in Fig. 4D are reminiscent of single-channel currents through unedited GluR5 homomers (Swanson et al. 1996). Although the γnoise for kainate receptors declines during development, GluR5 remains predominantly unedited (Belcher & Howe, 1997) and all the KA2 protein in the cerebella of mature animals associates with GluR6 (Ripellino et al. 1998).
Our findings suggest that cerebellar granule cells regulate the subunit composition of their kainate receptors during development, as was shown previously for NMDA receptors in these cells (Farrant et al. 1994). It is possible that kainate-type channels have distinct functions at different postnatal times. Although the kainate receptors we observe in mature, innervated granule cells in the IGL may be involved in synaptic transmission (Vignes & Collingridge, 1997; Cossart et al. 1998; Frerking et al. 1998; Mülle et al. 1998), immature granule cells in the EGL are not known to receive synaptic input. Indeed, while miniature excitatory postsynaptic currents were not unusual in IGL cells, we did not observe synaptic currents in EGL cells.
Without synaptic input to activate voltage-gated Ca2+ channels, immature neurons may use small-conductance kainate receptors as a Ca2+ source, as unedited GluR6 homomers have been shown to conduct Ca2+ (Burnashev et al. 1995). The small conductance of kainate receptors may render them especially suited for calcium signalling in small granule cells (diameter, < 10 μm), without risking excitotoxicity. The mean currents seen in EGL cells, together with the γnoise and Popen values we estimated, indicate that immature granule cells express several hundred kainate receptors. Immature neurons may cluster channels where microdomains of glutamate-activated Ca2+ influx are needed to sustain Ca2+-dependent developmental activities, such as growth cone extension and navigation (Gitler et al. 1998; Kuhn et al. 1998). In addition, expressing Ca2+-permeable kainate receptors may prepare immature neurons for impending synaptic input by altering gene expression. Calcium influx is known to stimulate expression of ion channels (Bading et al. 1993; Bessho et al. 1994; Muller et al. 1998), and we have observed that potassium conductances in granule cells rise dramatically during P6-19. Although the advantage of developmentally regulating receptor subunit composition remains to be established, understanding the unitary properties of kainate receptors may speed the discovery of their multiple functions.
Acknowledgments
We thank Stacey Irizarry, Stephen Traynelis and Stephen Arch for helpful comments on the manuscript.
References
- Altman J. Postnatal development of the cerebellar cortex in the rat. I. The external germinal layer and the transitional layer. Journal of Comparative Neurology. 1972;145:353–398. doi: 10.1002/cne.901450305. [DOI] [PubMed] [Google Scholar]
- Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science. 1993;260:181–186. doi: 10.1126/science.8097060. [DOI] [PubMed] [Google Scholar]
- Belcher SM, Howe JR. Characterization of RNA editing of the glutamate-receptor subunits GluR5 and GluR6 in granule cells during cerebellar development. Molecular Brain Research. 1997;52:130–138. doi: 10.1016/s0169-328x(97)00252-0. [DOI] [PubMed] [Google Scholar]
- Bessho Y, Nawa H, Nakanishi S. Selective upregulation of an NMDA receptor subunit mRNA in cultured cerebellar granule cells by K+-induced depolarization and NMDA treatment. Neuron. 1994;12:87–95. doi: 10.1016/0896-6273(94)90154-6. [DOI] [PubMed] [Google Scholar]
- Bettler B, Mulle C. Review: Neurotransmitter receptors II. AMPA and kainate receptors. Neuropharmacology. 1995;34:123–139. doi: 10.1016/0028-3908(94)00141-e. [DOI] [PubMed] [Google Scholar]
- Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995;15:453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Zhou Z, Neher E, Sakmann B. Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. The Journal of Physiology. 1995;485:403–418. doi: 10.1113/jphysiol.1995.sp020738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart R, Esclapez M, Hirsch JC, Bernard C, Ben-Ari Y. GluR5 kainate receptor activation in interneurons increases tonic inhibition of pyramidal cells. Nature Neuroscience. 1998;1:470–478. doi: 10.1038/2185. [DOI] [PubMed] [Google Scholar]
- Farrant M, Feldmeyer D, Takahashi T, Cull-Candy SG. NMDA-receptor channel diversity in the developing cerebellum. Nature. 1994;368:335–339. doi: 10.1038/368335a0. 10.1038/368335a0. [DOI] [PubMed] [Google Scholar]
- Frerking M, Malenka RC, Nicoll RA. Synaptic activation of kainate receptors on hippocampal neurons. Nature Neuroscience. 1998;1:479–486. doi: 10.1038/2194. 10.1038/2194. [DOI] [PubMed] [Google Scholar]
- Gitler D, Spira ME. Real time imaging of calcium-induced localized proteolytic activity after axotomy and its relation to growth cone formation. Neuron. 1998;20:1123–1135. doi: 10.1016/s0896-6273(00)80494-8. 10.1016/S0896-6273(00)80494-8. [DOI] [PubMed] [Google Scholar]
- Herb A, Burnashev N, Werner P, Sakmann B, Wisden W, Seeburg PH. The KA-2 subunit of excitatory amino acid receptors shows widespread expression in brain and forms ion channels with distantly related subunits. Neuron. 1992;8:775–785. doi: 10.1016/0896-6273(92)90098-x. [DOI] [PubMed] [Google Scholar]
- Howe JR. Homomeric and heteromeric ion channels formed from the kainate-type subunits GluR6 and KA2 have very small, but different, unitary conductances. Journal of Neurophysiology. 1996;76:510–519. doi: 10.1152/jn.1996.76.1.510. [DOI] [PubMed] [Google Scholar]
- Huettner JE. Glutamate receptor channels in rat DRG neurons: activation by kainate and quisqualate and blockade of desensitization by Con A. Neuron. 1990;5:255–266. doi: 10.1016/0896-6273(90)90163-a. 10.1016/0896-6273(90)90163-A. [DOI] [PubMed] [Google Scholar]
- Kamboj SK, Swanson GT, Cull-Candy SG. Intracellular spermine confers rectification on rat calcium-permeable AMPA and kainate receptors. The Journal of Physiology. 1995;486:297–303. doi: 10.1113/jphysiol.1995.sp020812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Modulation of neuronal migration by NMDA receptors. Science. 1993;260:95–97. doi: 10.1126/science.8096653. [DOI] [PubMed] [Google Scholar]
- Kuhn TB, Williams CV, Dou P, Kater SB. Laminin directs growth cone navigation via two temporally and functionally distinct calcium signals. Journal of Neuroscience. 1998;18:184–194. doi: 10.1523/JNEUROSCI.18-01-00184.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J, Paternain AV, Naranjo JR, Mellström B. Functional kainate-selective glutamate receptors in cultured hippocampal neurons. Proceedings of the National Academy of Sciences of the USA. 1993;90:11688–11692. doi: 10.1073/pnas.90.24.11688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mülle C, Sailer A, Pérez-Otaño I, Dickenson-Anson H, Castillo PE, Bureau I, Maron C, Gage FH, Mann JR, Bettler B, Heinemann SF. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR-deficient mice. Nature. 1998;392:601–605. doi: 10.1038/33408. 10.1038/33408. [DOI] [PubMed] [Google Scholar]
- Muller YL, Reitstetter R, Yool AJ. Regulation of Ca2+-dependent K+ channel expression in rat cerebellum during postnatal development. Journal of Neuroscience. 1998;18:16–25. doi: 10.1523/JNEUROSCI.18-01-00016.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partin KM, Patneau DK, Winters CA, Mayer M L, Buonanno A. Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron. 1993;11:1069–1082. doi: 10.1016/0896-6273(93)90220-l. 10.1016/0896-6273(93)90220-L. [DOI] [PubMed] [Google Scholar]
- Paternain AV, Morales M, Lerma J. Selective antagonism of AMPA receptors unmasks kainate receptor-mediated responses in hippocampal neurons. Neuron. 1995;14:185–189. doi: 10.1016/0896-6273(95)90253-8. 10.1016/0896-6273(95)90253-8. [DOI] [PubMed] [Google Scholar]
- Patlak JB. Sodium channel subconductance levels measured with a new variance-mean analysis. Journal of General Physiology. 1988;92:413–430. doi: 10.1085/jgp.92.4.413. 10.1085/jgp.92.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton KE, Belcher SM, Ripellino JA, Howe JR. High-affinity kainate-type ion channels in rat cerebellar granule cells. The Journal of Physiology. 1998;510:401–420. doi: 10.1111/j.1469-7793.1998.401bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabacchi S, Bailly Y, Delhaye-Bouchard N, Mariani J. Involvement of the N-methyl-D-aspartate (NMDA) receptor in synapse elimination during cerebellar development. Science. 1992;256:1823–1825. doi: 10.1126/science.1352066. [DOI] [PubMed] [Google Scholar]
- Ripellino JA, Neve RL, Howe JR. Expression and heteromeric interactions of non-N-methyl-D-aspartate glutamate receptor subunits in the developing and adult cerebellum. Neuroscience. 1998;82:485–497. doi: 10.1016/s0306-4522(97)00296-0. 10.1016/S0306-4522(97)00296-0. [DOI] [PubMed] [Google Scholar]
- Sommer B, Köhler M, Sprengel R, Seeburg PH. RNA editing controls a determinant of ion flow in glutamate-gated channels. Cell. 1991;67:11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- Swanson GT, Feldmeyer D, Kaneda M, Cull-Candy SG. Effect of editing and subunit co-assembly on single-channel properties of recombinant kainate receptors. The Journal of Physiology. 1996;492:129–142. doi: 10.1113/jphysiol.1996.sp021295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignes M, Collingridge GL. The synaptic activation of kainate receptors. Nature. 1997;388:179–182. doi: 10.1038/40639. [DOI] [PubMed] [Google Scholar]
- Wilding TJ, Huettner JE. Activation and desensitization of hippocampal kainate receptors. Journal of Neuroscience. 1997;17:2713–2721. doi: 10.1523/JNEUROSCI.17-08-02713.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


