Abstract
The possibility was investigated that cortical excitation to human thigh motoneurones is relayed via lumbar premotoneurones.
Test responses were evoked by transcranial magnetic stimulation (TMS) in voluntarily contracting quadriceps (Q) and semitendinosus (ST) muscles: either a motor evoked potential (MEP) in surface recordings or a peak of cortical excitation in the post-stimulus time histogram (PSTH) of single motor units was used. These test responses were conditioned by stimuli to the common peroneal (CP) or gastrocnemius medialis (GM) nerves.
CP stimulation evoked a large biphasic facilitation of the Q MEP, with early, short-lasting, low-threshold (0·6-0·8 × motor threshold (MT)) and late, longer lasting and higher threshold (1·2-1·5 × MT) peaks separated by a period of depression. GM nerve stimulation evoked a similar early depression and late facilitation in the ST MEP.
CP-induced effects in the Q H reflex were different (smaller late facilitation not preceded by any depression), suggesting that CP and cortical volleys interact at a premotoneuronal level to modify the Q MEP.
Peaks of cortical excitation evoked by TMS in single motor unit PSTHs were modulated by the conditioning volley like the MEPs with, in Q motor units, early and late CP-induced facilitations separated by a depression, and in ST motor units a late GM-induced facilitation. Facilitations on combined stimulation (i) were greater than the sum of effects by separate stimuli and (ii) never affected the initial part of the cortical peak.
It is concluded that the features of the reported facilitatory interactions between cortical and peripheral volleys are consistent with interactions in a population of lumbar excitatory premotoneurones co-activated by group I and group II afferents. The potency of the effects suggests that a significant part of the cortical excitation to motoneurones of thigh muscles is relayed via these interneurones.
It is argued that the early depression in ST motoneurones and the separation of the two peaks of facilitation in Q motoneurones reflect a cortical facilitation of spinal inhibitory interneurones projecting on excitatory premotoneurones.
It has recently been shown that motoneurones of human forearm muscles receive a substantial disynaptic corticospinal excitation, which is relayed by cervical premotoneurones and acts in parallel with the monosynaptic component (Pauvert et al. 1998). Since, both in the monkey (Jankowska et al. 1975) and in man (Brouwer & Ashby, 1992), monosynaptic corticospinal excitatory projections have been shown to be significantly smaller on motoneurones of thigh muscles than on motoneurones of distal muscles, one would expect an even greater part of the cortical command to quadriceps (Q) and semitendinosus (ST) motoneurones to be transmitted through premotoneurones. In the cat, transmission in flexion reflex afferent (FRA) pathways is facilitated by stimulation of the sensorimotor cortex (Lundberg & Vorhooeve, 1962) and midlumbar premotoneurones co-excited by group I and group II afferents (Edgley & Jankowska, 1987; Cavallari et al. 1987) have been shown to receive monosynaptic corticospinal input (Davies & Edgley, 1994). Premotoneurones mediating the potent excitation of human Q and ST motoneurones by group II afferents described in the companion paper (Simonetta-Moreau et al. 1999) might therefore be good candidates for transmitting cortical excitation to these thigh motoneurones.
In the present investigation corticospinal volleys were elicited by transcranial magnetic stimulation (TMS) (Day et al. 1989; Brouwer & Ashby, 1992): the motor evoked potential (MEP) elicited in the ongoing voluntary EMG of Q and ST by TMS or the TMS-induced peak of excitation in single motor units of these muscles was used as a test response and conditioned by group I and group II volleys. It is shown that these peripheral volleys strongly modify, at a premotoneuronal level, the transmission of cortical effects to motoneurones of thigh muscles.
METHODS
Experiments were carried out on 16 healthy subjects (aged 25-63), all of whom had given informed consent to the experimental procedure, which was approved by the appropriate institutional ethics committees. Essentially the same methods as in the companion paper (Simonetta-Moreau et al. 1999) were used for (i) recording the EMG from two heads of the Q (vastus lateralis, VL, and rectus femoris, RF) and from the semitendinosus (ST), (ii) constructing post-stimulus time histograms (PSTHs) of single motor units, (iii) stimulating the common peroneal (CP), gastrocnemius medialis (GM), ST and femoral nerves, and (iv) eliciting and recording the Q H reflex. Only those methods that are specific to the present study are therefore described below.
Modulation of motor evoked potentials evoked by transcranial magnetic stimulation (TMS)
Transcranial magnetic stimulation (TMS) was applied over the motor cortex using a Magstim 200 (Magstim, Whitland, Dyfed, UK) through a double cone coil centred at the vertex. The intensity of the stimulator output was adjusted to obtain MEPs of about 10 % of maximal M wave (Mmax). EMG activity was filtered (100 Hz-1 kHz) and recorded using a sampling rate of 1 kHz. Because MEPs in thigh muscles often have a polyphasic shape, the MEPs were full-wave rectified and their whole area was assessed and expressed as a percentage of Mmax or of the unconditioned control MEP (vertical arrows indicate the window of analysis in the example illustrated in Fig. 1A).
Figure 1. CP-induced changes in the Q MEP during a voluntary weak tonic Q contraction.
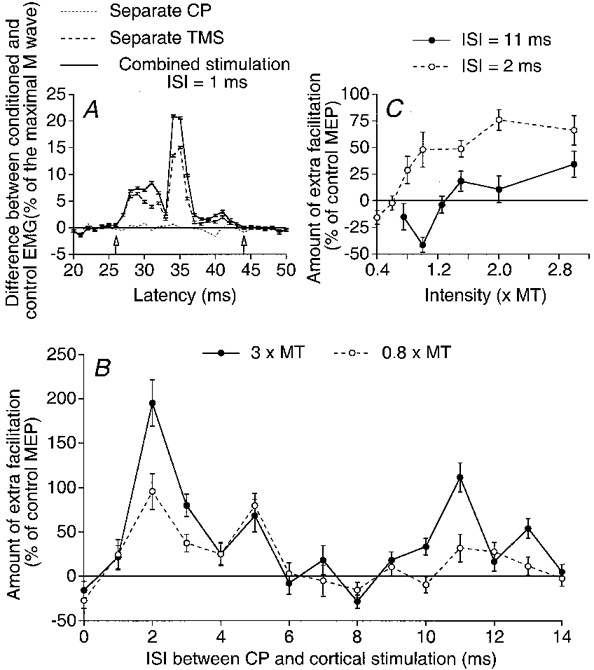
A, the difference between conditioned and control averaged (10 trials) EMGs, expressed as a percentage of the maximal M wave, is plotted against the latency after the conditioning stimulus; the EMG was conditioned by separate CP stimulation (3 × MT, dotted line), separate TMS (35 % of the maximal stimulator output, control MEP, dashed line) or combined TMS and CP stimulation at the 1 ms ISI (continuous line) (note that the abscissa is relative to TMS). Vertical arrows indicate the window of analysis including the whole area of the MEP. B, the amount of extra facilitation (i.e. the difference between the effect on combined stimulation and the algebraic sum of effects by separate stimuli, expressed as a percentage of the control MEP) is plotted against the interstimulus interval (ISI) between CP and cortical stimulations, the intensity of CP stimulation being 0.8 (○) or 3 (•) × MT. C, the amount of extra facilitation on combined stimulation is plotted against the CP stimulus intensity at ISIs of 2 ms (○) and 11 ms (•). Each symbol is the mean of 10 measurements. Vertical bars indicate 1 standard error of the mean (± 1 s.e.m.).
Experiments were performed during weak tonic contraction of the explored muscle. This (i) ascertained that the MEP reflected predominantly activity of the corresponding motoneurones, (ii) made the results easier to compare with data obtained in single voluntarily activated motor units, and (iii) enabled us to compare the CP-induced modulations of H reflex and MEP since, during voluntary contraction, the sequence of motoneurone recruitment (size principle) seems to be similar after stimulation of Ia afferents and of the motor cortex (J. Baumgarten, H. Morita, L. Christensen & J. Nielsen, personal communication). Rectified and integrated EMG activity was displayed on an oscilloscope so that the subjects could adjust the contraction to a constant level. Four combinations of stimuli were alternated in the same sequence: (a) the absence of stimulation to assess the background ongoing voluntary rectified EMG activity; (b) peripheral stimulation alone to assess the resulting modulation of the ongoing EMG; (c) TMS alone; (d) TMS and peripheral stimulation. Ten triggers were presented for each combination. A time course of the effect of the peripheral volley was constructed by repeating the process with various interstimulus intervals (ISIs). The background EMG activity (assessed during the same window of analysis as the MEP) was subtracted from the conditioned EMG responses to calculate the amount of facilitation (or inhibition) by separate ((b - a) and (c - a)) and combined (d - a) stimuli. The difference between the amount of facilitation on combined stimulation (d - a) and the sum of effects by separate stimuli ((b - a) + (c - a)) was then calculated to give the amount of extra facilitation on combined stimulation, which was expressed as a percentage of the control MEP (c - a). An F test (Scheffé's test; see Simonetta-Moreau et al. 1999) was used to test the statistical significance of results in individual subjects. The significance of mean values in the group was explored using the Student's paired t test.
Modulation of the peak of excitation evoked by TMS in single motor unit PSTHs
TMS intensity was set so that during voluntary activation of the motor unit cortical stimulation did not evoke any effect in the recorded motor unit other than a change in its firing probability. Motor unit firing was recorded using a sampling rate of 2 or 5 kHz. As with the MEPs, four combinations were alternated in the same sequence: (a) the absence of stimulation to assess the background firing probability of the motor unit (Fig. 3A); (b) stimulation of the peripheral nerve; (c) TMS; (d) combined stimulation (peripheral nerve and TMS). The background firing probability was subtracted from the conditioned histograms, which accounts for the negative values in A and B in Figs 4 and 5. Each experiment involved studying the time course of effects evoked at two nerve stimulus strengths. In order to complete the study in reasonable time, we chose to give only 50-60 stimuli of each type at each ISI. The resulting PSTHs are rather sparse, but nevertheless give reliable statistical results. A χ2 test was used to assess the difference between the response on combined stimulation and the sum of effects by separate stimuli in individual motor units. This was done during the whole duration of the cortical peak and within a window starting 0.6 ms after the onset of the cortical peak (upward arrows in Figs 4-5), since the conditioning stimulation did not affect the initial bins of this peak (see Pauvert et al. 1998 and Discussion). Group analyses were performed using the Student's paired t test. They concerned: (i) the amount of extra facilitation on combined stimulation in the window including the whole cortical peak; and (ii) the delay at which the facilitation of the cortical peak appeared on combined stimulation, assessed as the difference between the onset of the facilitation and that of the control peak (e.g. 30.2 - 29.4 = 0.8 ms in Fig. 5A).
Figure 3. CP-induced facilitation of the peak elicited by TMS in a Q (VL) motor unit.
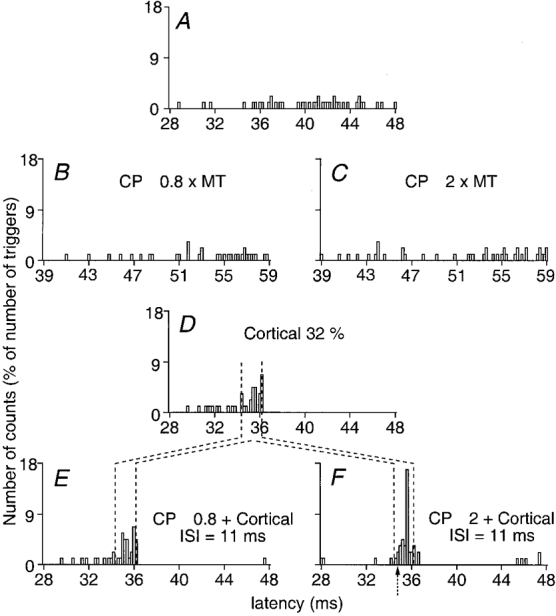
PSTHs from a Q motor unit (0.2 ms bins). The number of counts expressed as a percentage of the number of triggers is plotted against the latency after stimulation. A, background motor unit firing. B and C, effect of separate CP stimulus at 0.8 (B) and 2 (C) × MT. D, cortically evoked peak by separate TMS at 32 % of the maximal output. E and F, effect of combined (CP + TMS) stimulation with CP at 0.8 (E) and at 2 (F) × MT at the 11 ms ISI (note that the abscissa is relative to TMS). Vertical dotted lines show the window of analysis for the cortical peak. Arrow in F indicates the onset of the CP-induced facilitation of the cortically evoked peak.
Figure 4. Facilitation by a weak CP stimulation of the peak elicited by TMS in PSTHs from a Q (VL) motor unit.
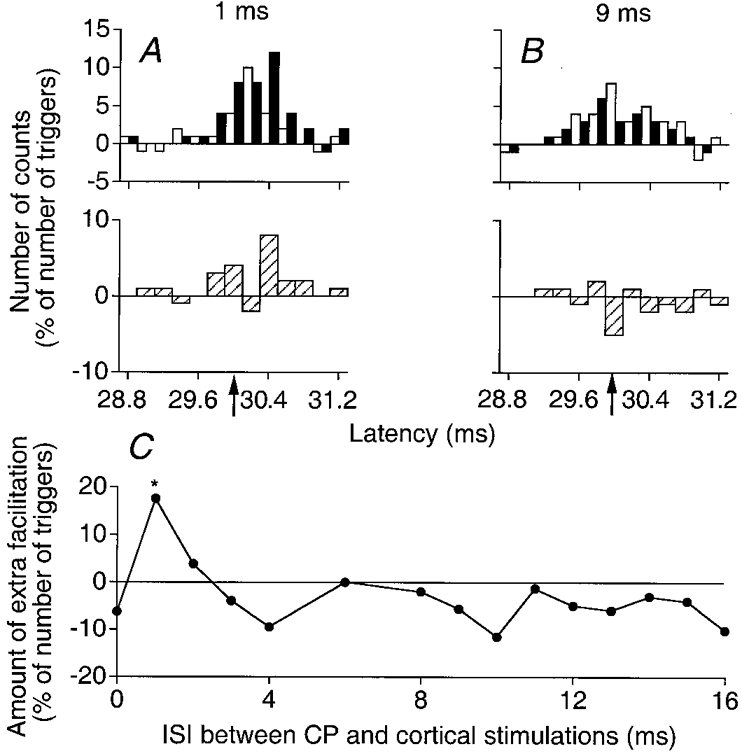
A and B, PSTHs from a Q motor unit (0.2 ms bins). The number of counts (after subtraction of the background firing probability), expressed as a percentage of number of triggers, is plotted against the latency after TMS. □, algebraic sum of effects by separate CP (0.8 × MT) and cortical (24 % of the maximal output) stimulations; ▪, the effect of combined (CP + TMS) stimulations at 1 ms (A) and 9 ms (B) ISIs. Subtraction histograms in the lower panel of each pair ( )represent the difference between filled and open columns. Filled upward arrows indicate the onset of the window of analysis starting 0.6 ms after the onset of the cortical peak. Note that the abscissa in A and B is relative to TMS. C, the amount of extra facilitation on combined stimulation (
)represent the difference between filled and open columns. Filled upward arrows indicate the onset of the window of analysis starting 0.6 ms after the onset of the cortical peak. Note that the abscissa in A and B is relative to TMS. C, the amount of extra facilitation on combined stimulation ( in A and B) is plotted against the ISI between CP and cortical stimulations. The asterisk indicates the result which is statistically significant (P < 0.05).
in A and B) is plotted against the ISI between CP and cortical stimulations. The asterisk indicates the result which is statistically significant (P < 0.05).
Figure 5. Facilitation by a strong CP stimulation of the peak elicited by TMS in PSTHs from a Q (VL) motor unit.
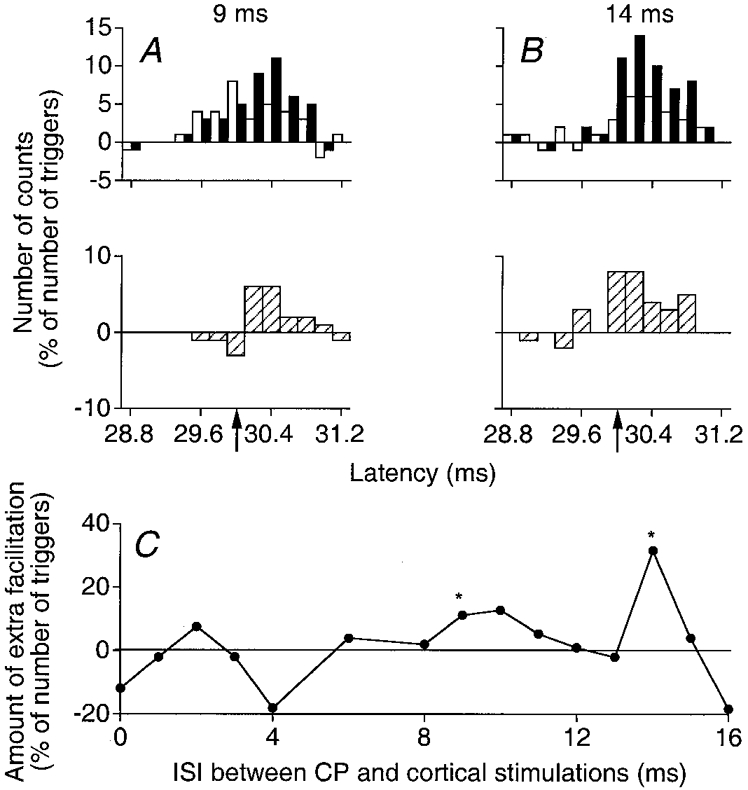
A and B, same Q motor unit and same protocol as in Fig. 4A and B but the CP stimulation was adjusted at 2 × MT and the results on combined stimulation were obtained at 9 ms (A) and 14 ms (B) ISIs. C, as in Fig. 4C.
RESULTS
Common peroneal-induced excitation of quadriceps motoneurones
Common peroneal-induced changes in the quadriceps (RF) MEP
Facilitation of the MEP induced by CP stimulation
Figure 1A illustrates the averaged (10 trials) rectified EMG activity observed in response to separate CP and cortical stimuli and to the combined stimuli in one subject. The additional activity elicited by separate CP stimulation (3 × MT) was less than 1 % of Mmax (dotted line). Separate TMS (35 % of the maximal output) evoked a MEP which was 15 % of Mmax (control MEP, dashed line). On combined stimulation, at the shortest effective interstimulus interval (ISI) of 1 ms, the MEP was facilitated (continuous line; a facilitation which spared the first 2 ms of the MEP). The amount of extra facilitation on combined stimulation (facilitation on combined stimulation minus sum of effects by separate stimuli, expressed as a percentage of the control MEP; see Methods) is the ordinate in Fig. 1B and C.
The time course of this extra facilitation, for the CP stimulus intensity of 3 × MT, is shown in Fig. 1B (•). The extra facilitation was biphasic, with early and late components peaking when CP stimulation preceded TMS by 2 and 11 ms, respectively. In this plot both components were particularly large (the early peak of extra facilitation reached 200 % of the control MEP, i.e. the MEP increased from 15 to 45 % of Mmax) and highly significant (P < 0.001). The finding that the facilitation on combined stimulation was much greater than the sum of effects by separate stimuli (obtained during background voluntary activity) and other observations (see below) supports the suggestion that the facilitation reflects an interaction between CP and cortical volleys at a premotoneuronal level.
It will be shown below (with the investigation of single motor units, which enables a more precise time resolution) that the shortest ISI at which the first cortical (D wave) and the fastest CP group I volleys may interact at the level of the relevant lumbar premotoneurones is when CP stimulation precedes TMS by about 3 ms. The finding that the facilitation begins at a shorter ISI (1 ms) in Fig. 1B might then indicate that the CP volley interacts with a later corticospinal volley. Indeed, TMS elicits multiple descending volleys (D, I1, I2, I3, etc. activating the same corticospinal neurones and separated by about 1.5 ms; see Day et al. 1989), and the minimum ISI at which a conditioning volley may modulate a complex test response corresponds to the moment when the postsynaptic actions of the conditioning stimulus coincide with the last component of the test response (Araki et al. 1960). We thus assume that the CP-induced facilitation of the MEP starts to manifest itself when the peripheral volley interacts with the last effective descending volley (D, or any I wave; see section below headed ‘Timing of the effects elicited by corticospinal volleys’).
A distinct MEP during Q contraction was evoked in each of the 16 subjects and a similar biphasic extra facilitation on combined stimulation was observed in all of them when the conditioning stimulation to the CP nerve was strong (≥ 2 × MT): the early peak reached statistical significance (P < 0.05) in 10/16 subjects and the late peak in 14/16. In the whole group mean values of the maximal amplitude of the extra facilitation were 25.4 ± 6.2 and 25.9 ± 5.8 % of the control MEP (P < 0.01 in both cases) for the early and late peaks, respectively. The mean values of the first ISI at which the early and late peaks appeared were 0.4 ± 0.5 and 9.6 ± 0.5 ms, respectively. The mean duration of the early peak was very brief (1.8 ± 0.2 ms), whereas that of the late peak was longer (3 ± 0.4 ms).
Afferents responsible for early and late facilitations
Open circles in Fig. 1B show that, when the conditioning stimulation to the CP nerve was at 0.8 × MT, i.e. below the threshold of group II afferents (see Marque et al. 1996), there was no significant late excitation, a result which was found in all six subjects tested. Figure 1C shows that the threshold of the early peak (2 ms ISI, ○) was 0.8 × MT and that of the late peak (11 ms ISI, •) between 1.2 and 1.5 × MT (data for the same subject as in B, but from another experiment). In four subjects in which a similar series of data were obtained, the threshold of the early peak was between 0.6 and 0.8 × MT and that of the late peak between 1.2 and 1.7 × MT, i.e. within the ranges found for group I and group II afferents, respectively (Marque et al. 1996; Simonetta-Moreau et al. 1999). It therefore appears likely that the early low-threshold and late high-threshold peaks of the extra facilitation observed here are of group I and group II origin, respectively, like the two peaks of facilitation elicited by these afferents in H reflexes and PSTHs (Marque et al. 1996; Simonetta-Moreau et al. 1999). Figure 1C also shows that, at the 11 ms ISI (•), CP stimuli at low intensities elicited a depression of the MEP, which was consistently found in the other subjects and in single motor units (Fig. 4C).
Facilitation of motor cortex excitability by the CP group I volley may be excluded as the cause of the late excitation because it takes at least 25 ms for the CP group I volley to reach the motor cortex: 11 ms of peripheral conduction time (Meunier et al. 1990) + 14 ms from L4 to cortex (Desmedt & Cheron, 1983). In fact, the study of the difference in latencies of the early and late peaks further suggests that the late peak is elicited by stimulation of afferents with smaller diameters than those of group I afferents.
Provided that the last effective descending volley induced by a constant TMS intensity (D, or any I wave) remains the same in the course of a given experiment, the shortest ISI at which each peak appeared should correspond to the interaction of the group I or group II CP volley with this last descending volley. If the excess delay of the late facilitation (over and above that of the early group I effect) is due to slower peripheral transmission by smaller afferents rather than to a longer central delay (e.g. via long-loop central pathways), one would expect this excess delay to increase with the height of the subject (see Nardone et al. 1996). In order to verify this possibility we compared the delays between the onsets of the early and late facilitations (ranging from 7 to 15 ms) and the heights of the 16 tested subjects (ranging from 153 to 183 cm). It was found that the taller the subject the larger the difference between the latencies of early and late facilitations and the correlation (ρ = 0.76, Spearman's test) was highly significant (P < 0.01).
Comparison between CP-induced changes in the H reflex and in the MEP
The CP-induced facilitation of the MEP reflects an interaction between CP and cortical volleys. This interaction can occur at a motoneurone level (summation of conditioning and test EPSPs in Q motoneurones of the subliminal fringe of excitation created by the test corticospinal volley) and/or at a premotoneuronal level. To elucidate this point the effects of CP stimulation were compared (Fig. 2) on the MEP and on the H reflex elicited in the Q (RF) during a very weak (some motor units) voluntary tonic Q contraction (the sequence of motoneurone recruitment (size principle) is then similar after stimulation of Ia afferents and of the motor cortex; see Methods). Summation in motoneurones of conditioning and test EPSPs would be expected to produce similar (at least qualitatively) results, whether the test response is the Q H reflex or the MEP. In Fig. 2 the time courses of the changes evoked by CP stimulation (2 × MT) in the H reflex (○) and in the MEP (•) of the Q are shown in two subjects (A and B). Control responses were adjusted to be of the same size (about 10 % of Mmax) and CP-induced changes in the two test responses were investigated during the same experimental session.
Figure 2. Comparison between the changes induced by CP stimulation (2 × MT) in the Q MEP, in the Q H reflex and in the PSTH of an individual Q motor unit.
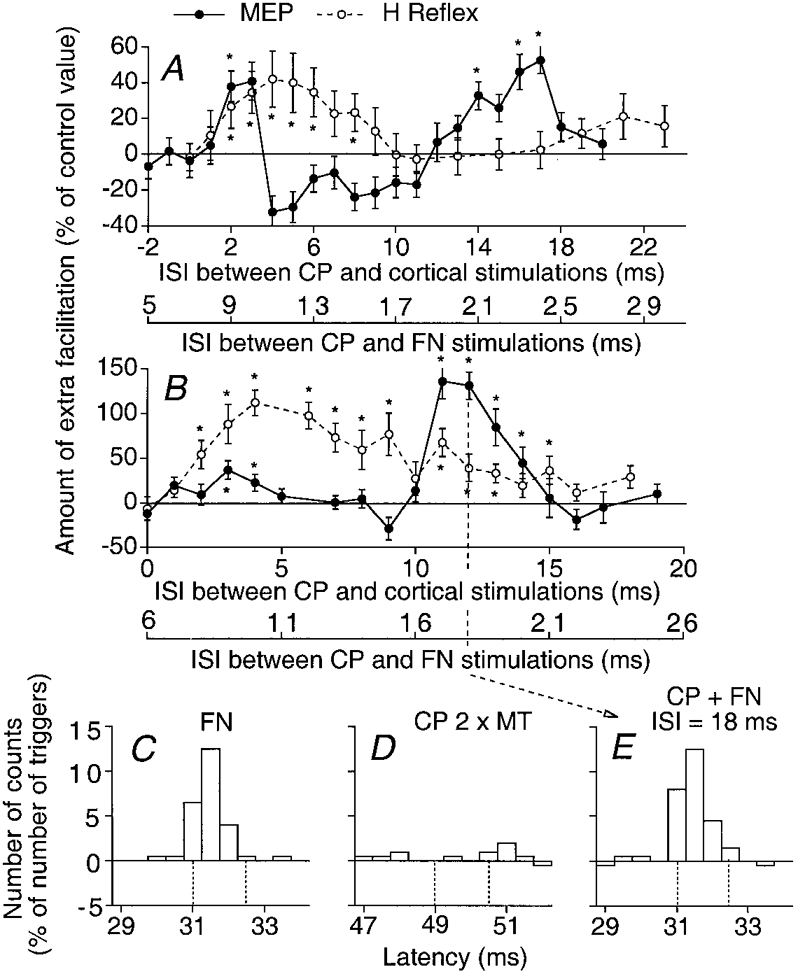
A and B, the amount of CP (2 × MT)-induced facilitation (difference between conditioned and control values, expressed as a percentage of the control value) of the MEP (•) and of the H reflex (○) is plotted against the ISI between CP and cortical stimulations (upper abscissa) and the ISI between CP and femoral nerve (FN) stimulations (lower abscissa) in two subjects (A and B). Each symbol represents the mean value of 20 measurements. Vertical bars indicate ±1 s.e.m. The asterisks indicate the results which are statistically significant (P < 0.05). C-E, PSTHs (0.5 ms bins) from a Q motor unit (same subject as in B). The background firing has been subtracted from each histogram and the difference (expressed as a percentage of the number of triggers) is plotted against the latency after stimulation. C and D, effects induced by separate FN (0.9 × MT, C) and CP (2 × MT, D) stimuli. E, effects induced by combined stimulation (CP + FN) at the 18 ms ISI (note that the abscissa is relative to FN stimulation). Vertical dotted lines in C-E indicate the window of analysis.
Since the conduction times of the test (femoral Ia and cortical) volleys to Q motoneurones are different (see below), the ISI corresponding to simultaneous arrival of CP and test volleys at the motoneurone level is different in the two experimental situations. Thus, to enable an easy comparison of the two time courses, the abscissae of the graphs illustrating CP-induced facilitations of the H reflex and of the MEP are set so that the shortest ISIs at which the CP group I volley elicits the earliest facilitation in the two test responses are aligned. These are 8 ms (A) and 7 ms (B) for the H reflex (lower abscissa), and 1 ms for the MEP (upper abscissa).
In both subjects the CP-induced modulation of the two test responses differed in two respects: (i) the early group I facilitation of the MEP (•), which was very brief, ceased abruptly and was separated from the late peak by a period of depression (particularly obvious in A); in contrast, as described by Marque et al. (1996), the early group I facilitation of the H reflex (○) progressively decreased; (ii) the late group II facilitation of the MEP was much larger than that of the H reflex. These two systematic differences between CP-induced alterations of H and MEP responses were found in all five subjects tested. Such opposite results indicate that the transmission of at least one of the test volleys is modified at a premotoneuronal level by the CP volley. This might alter either the femoral group I volley underlying the Q H reflex (e.g. by changing presynaptic inhibition of Ia terminals) and/or the excitability of premotoneurones mediating corticospinal excitation to motoneurones. Experiments described below eliminate the first of these possibilities.
In principle, presynaptic inhibition of Q Ia afferents by group I afferents from pretibial flexors (Iles & Roberts, 1987; Hultborn et al. 1987a) might reduce the efficiency of the femoral afferent volley in eliciting the Q H reflex. The CP volley might also modify the femoral-induced Ib inhibition of Q motoneurones, which may help determine the size of the H reflex (Burke et al. 1984). To determine whether CP volleys could modify the size of the homonymous group I EPSPs in Q motoneurones, these EPSPs were generated in single voluntarily activated Q motor units by stimulation of the femoral nerve, and PSTHs were compared with and without an appropriately timed CP volley. In Fig. 2C-E (same subject as in Fig. 2B), the effects of CP nerve stimulation (2 × MT) on the peak of monosynaptic Ia excitation evoked by femoral nerve stimulation (0.9 × MT) are shown at the 18 ms ISI, i.e. when the CP-induced facilitation of the Q H reflex was significantly smaller than that of the MEP in this subject (vertical dotted line in Fig. 2B). The effects of separate stimulations of the femoral nerve (C, 0.9 × MT, a large peak of monosynaptic Ia excitation starting at 31 ms), of the CP nerve (D, 2 × MT, a weak excitation) and of their combined stimulation (E) can be seen in this Q motor unit (0.5 ms bins). The excitation obtained on combined stimulation (28 counts within the four bins of the window 31-32.5 ms) was practically equal to the algebraic sum of the two separate effects (24 + 3 = 27 counts). A similar absence of depression on combined stimulation was observed in all nine motor units (3 subjects) for all ISIs tested between 10 and 30 ms. This absence of depression could be expected, given the reduction of both presynaptic inhibition of Ia terminals (Iles & Roberts, 1987; Hultborn et al. 1987b) and Ib inhibition (Fournier et al. 1983) directed on voluntarily activated motoneurones.
If the efficiency of the femoral group I volley in eliciting the Q H reflex is not significantly altered by the CP volley, the differential effect seen in the H and MEP test responses must then be attributed to a CP-induced change in the transmission of the corticospinal volley at the level of premotoneurones. This view is further supported by results obtained in single motor units.
Changes induced in single motor units
Changes evoked by a conditioning stimulus in responses exploring an entire motoneurone pool (such as MEP and H reflex) depend on how evenly the conditioning PSPs are distributed in the motoneurones of this pool (see Discussion). Since this factor does not need to be considered while studying single motor units, CP-induced modulation of cortical excitation in single motor units was also analysed.
CP-induced facilitation of the peak of cortical excitation in single Q motor units
Figure 3 illustrates the basic finding that CP stimulation facilitates the peak of cortical excitation elicited by TMS in Q motor units. The background firing probability (A), the changes in firing probability induced by separate CP stimulation (0.8 × MT, B; 2 × MT, C), separate TMS (32 % of the maximal output, D) and combined stimulation (ISI = 11 ms, E and F) were explored, the different triggers being alternated in the same round. With the small number of triggers used (60) there was no evidence for facilitation on separate CP stimulation (B and C). Separate TMS evoked a cortical peak of excitation within the analysis window of 34.4-36.2 ms (D, vertical dotted lines). Note that in this motor unit, as well as in another 14 motor units analysed, separate TMS did not evoke any late excitation (about 10 ms later than the early peak), as described in PSTHs from proximal arm muscles (Colebatch et al. 1990).
When a strong (2 × MT) CP stimulus was used, the cortical peak was significantly (P < 0.05) facilitated on combined stimulation (F). The extra facilitation was very short-lasting since it was only present at the 11 ms ISI, and even replaced by a depression at ISIs 10 and 12 ms. It had a high threshold since it was not present with the weak (0.8 × MT) CP stimulus at this 11 ms ISI (E). With this weak CP stimulus, however, an early facilitation appeared at the 3 ms ISI (not illustrated).
Figures 4 and 5 illustrate results obtained at various ISIs with 0.8 (Fig. 4) and 2 (Fig. 5) × MT CP nerve stimulus intensities in one Q motor unit (from another subject). In A and B, changes in firing probability evoked by combined cortical and CP stimuli (filled columns) are compared with the sum of effects elicited by the two stimuli when applied alone (open columns); subtraction histograms (hatched columns in the lower panel of each pair) show the difference between these two values. The total number of counts in each subtraction histogram was then calculated within the window of analysis, including the whole cortical peak (29.4-30.8 ms). This value, expressed as a percentage of the number of triggers, is the ordinate in the time courses of Figs 4C and 5C. The significance (P < 0.05) of the extra facilitation on combined stimulation was tested within the more restricted window starting 0.6 ms after the onset of the cortical peak (upward arrows; see Methods). Here again, there was a significant early (1 ms ISI) and low-threshold (present when 0.8 × MT CP stimuli were used) and a late (9 and 14 ms ISIs) and higher threshold (only present when 2 × MT CP stimuli were used; compare Figs 4B and 5A) extra facilitation on combined stimulation. Note also that the early and late extra facilitations were separated by a depression at the 4 ms ISI where the peak of cortical excitation became smaller on combined stimulation (Figs 4 and 5C).
Plots in A and B of Figs 4 and 5 show also that the initial part of the cortical peak was spared by the extra facilitation on combined stimulation: thus, when 0.8 × MT stimuli were used, the facilitation on combined stimulation at the 1 ms ISI spared the initial 0.4 ms of the cortical peak (Fig. 4A); similarly, when 2 × MT CP stimuli were used (Fig. 5), the first 0.8 ms (A, ISI = 9 ms) or 0.6 ms (B, ISI = 14 ms) of the cortical peak were not affected by the extra facilitation.
Group analyses
Similar results were obtained in all 15 Q motor units (13 from VL, 2 from RF) so investigated in five subjects: as in the CP-induced modulation of the MEP, there was always a biphasic facilitation on combined stimulation with an early low-threshold peak and a late high-threshold peak separated by a period of depression.
CP stimulation at intensity 0.8 × MT
The facilitation evoked on combined stimulation was greater than the sum of effects by separate stimuli at short ISIs in all motor units, but the difference reached statistical significance only in six motor units. The mean value of the facilitation, assessed as in Fig. 4C within a window including the whole cortical peak, was only 5.5 ± 2.8 % of the number of triggers. Its mean latency (shortest ISI at which it occurred) was 1.7 ± 0.4 ms. This early excitation was constantly followed by a depression, and there was never any late facilitation.
CP stimulation at intensity 2 × MT
Such stimuli also evoked an early (mean latency 1.7 ± 0.4 ms) facilitation, which was followed by a 2-6 ms depression. The early facilitation was on average larger (7.5 ± 2.1 %) than at 0.8 × MT and significant in the group (P < 0.01) (which might reflect the increase in the group I volley with the increased CP stimulus intensity; Gracies et al. 1994). A late facilitation regularly appeared at a mean latency of 10.3 ± 0.5, i.e. 8.6 ± 0.4 ms later than the early facilitation. This late facilitation on combined stimulation was significant in 12/15 motor units. Its mean value reached 17 ± 2.6 % of the number of triggers (P < 0.0001) when assessed within the window including the whole cortical peak.
Sparing of the initial part of the cortically evoked PSTH peak
Figures 4 and 5 show that the extra facilitation on combined stimulation spared the initial part of the cortical peak in both the early low-threshold and the late high-threshold peaks. Such an initial sparing was found in 13/15 motor units. Its mean value was 0.6 ± 0.1 ms for both peaks (P < 0.0001).
Timing of the effects elicited by corticospinal volleys
Contrary to what happens in the case of upper limb muscles (Day et al. 1989), the onset of responses evoked by TMS at threshold intensity in tibialis anterior (TA) motor units is due to the direct (D) wave (Priori et al. 1993; Awiszus & Feistner, 1994). Estimates based on the conduction velocities (CV) of fastest corticospinal fibres (Inghilleri et al. 1989) and femoral Ia afferents (Hultborn et al. 1987a), and the distances from motor cortex (Herdmann et al. 1991) and from the femoral triangle (Hultborn et al. 1987a) to L2-L4 spinal levels (upper and lower levels of the Q motoneurone column) show that the conduction time of the fastest cortical volley to Q motoneurones is about 3.5 ms longer than that of the volley running along the fastest femoral Ia afferents.
In motor units of the subject illustrated in Fig. 3 the difference between latencies of cortical- and femoral Ia-induced peaks varied between 3.3 and 3.6 ms, i.e. it was entirely explained by the differences in conduction times of the two volleys. This is in accord with the view that, in this subject, the onset of the cortical peak to Q motoneurones is due to a monosynaptic EPSP elicited by the most direct descending volley (D wave), as in the TA. Under these conditions one would expect that the shortest ISI with CP-induced facilitation should correspond to the simultaneous arrival of the fastest CP group I and corticospinal D volleys at the level of the relevant premotoneurones (see Discussion for why the convergence is considered at a premotoneuronal level and not at the level of the motoneurone pool). That appears to be so, since, in motor units of this subject, the shortest ISI with facilitation was regularly 3 ms, which matches almost exactly the difference in conduction times of CP group I (Simonetta-Moreau et al. 1999) and corticospinal (see above) volleys to premotoneurones (11-7.7 = 3.3 ms).
In motor units of the subject illustrated in Figs 4 and 5 the difference between latencies of cortical- and femoral Ia-induced peaks varied between 4 and 4.8 ms, i.e. was 1-1.5 ms longer than the estimated difference between conduction times of the two volleys to the lumbar spinal level. This suggests that the corticospinal D wave was then insufficient to fire Q motoneurones and that summation of D and I waves was required to discharge them. Accordingly, in this subject, the shortest ISI with CP-induced facilitation was 1 ms (Fig. 4A and C), i.e. 2 ms shorter than the difference in conduction times of CP group I and cortical volleys, reflecting the fact that the CP volley then interacted in premotoneurones with a late corticospinal volley (activating the same corticospinal neurones; for references, see Day et al. 1989).
GM-induced effects on semitendinosus motoneurones
Stimulation of the GM nerve evokes a high-threshold late excitation of ST motoneurones, which has been shown to be of group II origin (Simonetta-Moreau et al. 1999). Figure 6 illustrates the GM-induced modulation of the MEP in ST motoneurones during weak ST tonic voluntary contraction. Figure 6A shows the averaged (10 trials) EMG activity observed in response to separate and combined stimuli. Separate GM nerve stimulation (1.3 × MT) evoked an early depression of the ongoing EMG activity (dotted line). Separate TMS evoked a MEP (12 % of Mmax, dashed line). On combined stimulation (ISI = 9 ms) the MEP was facilitated (continuous line). The amount of the extra facilitation on combined stimulation (see Methods) is plotted against the ISI (Fig. 6B) and GM stimulus intensity (Fig. 6C).
Figure 6. GM-induced changes in the semitendinosus MEP.
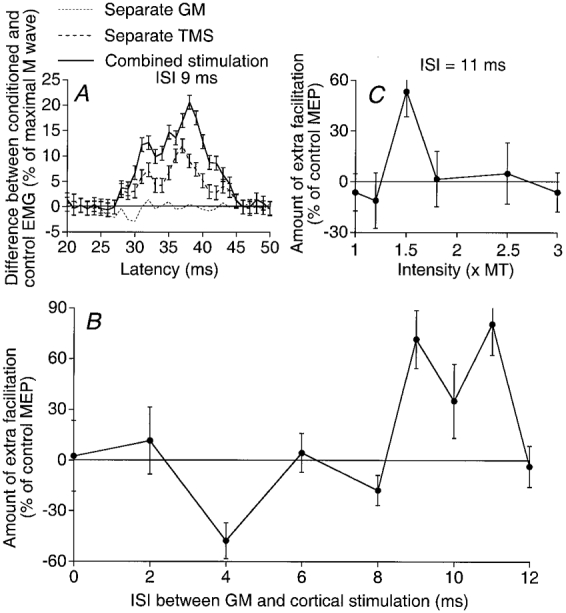
A, same legend as in Fig. 1A, but GM stimulation = 1.3 × MT, TMS = 33 %, ISI between GM and cortical stimulations = 9 ms. B, same legend as in Fig. 1B, but GM stimulus = 1.3 × MT. C, same legend as in Fig. 1C, but ISI between GM and cortical stimulations = 11 ms.
In contrast to the results obtained with Q motoneurones, Fig. 6B shows that there was no early facilitation. However, as in the Q, there was an early depression at the 4 ms ISI followed by a significant extra facilitation at 9-11 ms ISIs. Similar results were obtained in the seven subjects tested. There was never any early significant extra facilitation, but in 6/7 subjects there was an early depression, already existing at 1 × MT and thus attributable to group I afferents. The late facilitation, obtained with stimuli between 1.3 and 1.8 × MT, was significant (P < 0.05) in 6/7 subjects (mean value = 28 %, P < 0.01), and appeared at ISIs varying from 7 to 13 ms (the taller the subject the greater the latency; mean = 9.7 ± 0.7 ms).
Depression of the late extra facilitation at high stimulus intensities
Figure 6C shows that, at the 11 ms ISI, the threshold of the facilitation was between 1.2 and 1.5 × MT and that increasing the GM stimulus intensity above 1.5 × MT caused the extra facilitation to disappear. This depression at intensities above 1.5-1.8 × MT was observed in 7/7 experiments (4 subjects). Such a depression at high stimulus intensities (about 3 times the threshold of Ia afferents) suggests that the recruitment of group II afferents within the high-threshold group II range (whether of spindle origin or not) and/or of group III afferents results in an inhibition counteracting the group II excitation.
Investigation of single motor units
PSTH experiments were performed in five ST motor units (2 subjects). In all these units, stimulation of the GM at 1.5 × MT evoked a significant (P < 0.05) facilitation of the cortical peak at long ISIs (10 to 12 ms). This facilitation was larger than the algebraic sum of the effects by separate stimuli and spared the initial (0.4-0.8 ms) bins of the cortical peak. In the only motor unit, which was kept long enough to explore early ISIs, a significant early inhibition was also observed.
DISCUSSION
The main finding in the present study is that electrical stimulation of the CP nerve evoked a very large facilitation of the cortical excitation elicited by TMS in single and multi-unit Q recordings. The facilitation is biphasic with an early low-threshold and a late high-threshold component, which have thresholds and latencies similar to those of excitations of Q motoneurones by CP group I and group II afferents, respectively (Marque et al. 1996; Simonetta-Moreau et al. 1999). GM stimulation evokes an early group I depression and a late group II excitation in the ST MEP.
Evidence for interaction of peripheral and corticospinal volleys at a premotoneuronal level
The very large CP-induced facilitation of the MEP elicited in Q motoneurones might be due to a summation at the motoneurone level of EPSPs evoked by conditioning and test stimuli that are not sufficiently large to fire the motoneurones by themselves. However, several lines of evidence indicate that the facilitation occurs primarily at a premotoneuronal level.
Differential effect of CP stimulation on MEP and H reflex responses
The late CP-induced facilitation of the MEP is much larger than that of the H reflex (see Fig. 2A and B). The sequence of motoneurone recruitment (size principle) in a voluntarily activated motoneurone pool seems to be the same after Ia and TMS (see Methods), and control experiments (see Fig. 2C-E) have shown that CP volleys never depressed the homonymous group I EPSPs underlying the Q H reflex. This larger effect with TMS therefore suggests a CP-induced facilitation of the premotoneuronal transmission of corticospinal excitation to Q motoneurones.
Extra facilitation on combined stimulation
The spatial facilitation technique used to demonstrate convergence from two different fibre systems (A and B) onto common interneurones while recording EPSPs in one motoneurone in animals (see Lundberg, 1975) rests on a comparison of effects of stimulation of these fibre systems separately and jointly. Spatial summation at premotoneuronal level is inferred when the EPSP on combined stimulation (A + B) is larger than the algebraic sum of EPSPs evoked by separate stimulation of A and B systems. The same principle can be used when the synaptic effects are assessed in the motoneurone pool by using the H reflex (Fournier et al. 1986) or the modulation of ongoing rectified EMG activity (Figs 1 and 6). The large extra facilitation found on combined peripheral and cortical stimulations might thus be due to the convergence of peripheral and cortical volleys onto common premotoneurones. However, when the excitability of the whole motoneurone pool is investigated, the possibility must also be considered that the extra facilitation on combined stimulation results from a skewed distribution of cortical and/or CP-induced effects within the motoneurone pool (see Kernell & Hultborn, 1990; Nielsen & Kagamihara, 1993). Experiments were therefore performed in single motor units in which this factor does not need to be considered.
Facilitation of cortical excitation in single motor units
As previously shown (Pauvert et al. 1998), summation of two excitatory inputs in a motoneurone may give rise to little more than algebraic summation of their effects in the PSTH. In the present experiments, however, CP stimulation almost always evoked a significant extra facilitation of the peak of excitation elicited by TMS in individual Q motor units, over and above that expected from summation of effects from separate stimuli. The simplest explanation for this is that the two inputs converge onto a population of interneurones, which then project onto the tested motoneurone. Within this interneuronal population some inactive cells will fail to fire in response to either input alone and will discharge only on their simultaneous actions. The net result of this is that the response of the interneuronal population to two inputs will be more than the algebraic sum of the response to each input alone (spatial facilitation). This in turn will be reflected in motoneurone discharge.
Initial sparing
In individual motor units, it was regularly found that CP nerve stimulation never affected the earliest bins of the cortical response. This is what would be expected if cortical and CP volleys converged onto common interneurones rather than directly onto the motoneurones (see Pauvert et al. 1998). Due to the synaptic delay at the interneurone, this cortical input would arrive at the motoneurone after the direct fast-conducting monosynaptic corticomotoneuronal input. Thus interneuronal facilitation would be unable to affect the onset of the cortical response and initial sparing would occur. Possible reasons accounting for the relative brevity of this initial sparing (mean 0.6 ms) have been discussed in connection with the recently explored peripherally induced facilitation of disynaptically evoked cortical excitation of forearm motoneurones (Pauvert et al. 1998).
The characteristics of the extra facilitation on combined stimulation found in single and multi-unit ST recordings at long latencies after GM stimulation at intensities above 1.2 × MT were very similar to those of the CP-induced facilitation of Q motoneurones. This likewise suggests a premotoneuronal facilitation by the GM group II volley of the transmission of corticospinal excitation to ST motoneurones.
Which interneurones?
It is argued in the companion paper (Simonetta-Moreau et al. 1999) that group I and group II non-monosynaptic excitations from CP nerve to Q motoneurones are mediated by common interneurones, probably homologues of the feline intermediate zone/ventral horn midlumbar interneurones co-excited by group I and group II afferents (Edgley & Jankowska, 1987). In the cat, these midlumbar interneurones have been shown to be monosynaptically excited by descending tract neurones with a separate subpopulation that is co-excited by rubro- and, to a lesser extent, by corticospinal tract neurones (Davies & Edgley, 1994). The potent excitation observed in the present study suggests that corticospinal neurones might provide much stronger input to these premotoneurones in high primates.
Inhibitory effects of cortical stimulation
There is an early group I-induced depression of the ST MEP (Fig. 6B) and the total suppression, when increasing GM stimulus intensity, of the late group II-induced facilitation of the ST MEP (Fig. 6C) also indicates that inhibitory pathways were then activated. On the other hand, the group I-induced facilitation of cortical excitation in Q motoneurones was followed by a period of group I-induced depression (Figs 1, 2, 4 and 5). The very brief duration (1-2 ms) and the abrupt termination of the preceding facilitation suggest that this depression is evoked with only a slightly longer central delay than the early facilitation, the initial cortical EPSP being truncated by IPSPs mediated through pathways involving only one more synapse.
The truncation of EPSPs by IPSPs may occur at the motoneurone level (IPSPs in motoneurones) or at the level of the excitatory premotoneurones themselves (producing a disfacilitation of the motoneurones; see the tentative wiring diagram in Fig. 7). Arguments in favour of a group I-induced and a group II (or group III)-induced inhibition of the premotoneurones rather than of the motoneurones have been presented in the companion paper (Simonetta-Moreau et al. 1999). This view is further supported by the finding that the group I-evoked depression of the cortical excitation of Q motoneurones occurred 1 ms later than the group I-induced facilitation: this would fit a trisynaptic disfacilitation better than a disynaptic inhibition of motoneurones (both pathways having been described in the cat; see Jankowska, 1992).
Figure 7. Diagram sketching the possible connections discussed in the present investigation.
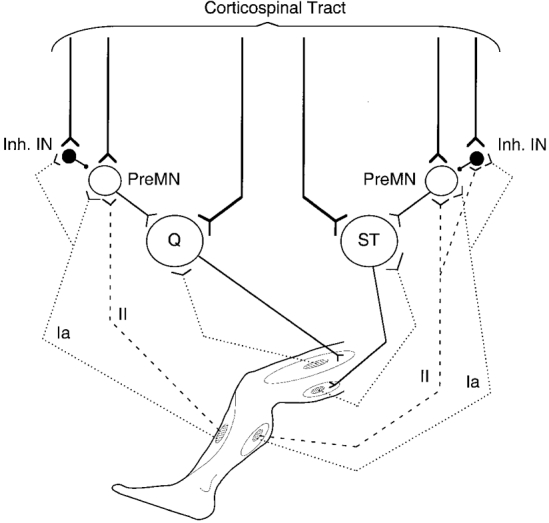
Possible pathways mediating non-monosynaptic Ia and group II excitations from tibialis anterior (TA) to quadriceps (Q) motoneurones and from gastrocnemius medialis (GM) to semitendinosus (ST) motoneurones are sketched. Y-shaped bars and small filled circles represent excitatory and inhibitory synapses, respectively. The medium-sized open circles represent excitatory premotoneurones (PreMN) onto which Ia and group II fibres are supposed to converge. Medium-sized filled circles represent inhibitory interneurones (inh. IN) activated by group I afferents (TA to Q) and by group I and group II afferents (GM to ST; for simplicity's sake, activation of inhibitory INs is shown from muscle group II afferents, but a contribution from non-spindle group II and/or group III afferents is not excluded; see text). The corticospinal tract is shown activating motoneurones through a monosynaptic line and a disynaptic connection with interposition of group I-group II premotoneurones. Corticospinal excitatory projections to inhibitory INs synapsing with premotoneurones are also represented.
The early group I-induced depression of the Q MEP has no equivalent in H reflex changes (Fig. 2A and B) and, when increasing GM stimulus intensity, the total group II-induced suppression of the MEP (Fig. 6C) contrasts with the very weak decrease in the group II-induced facilitation of the H reflex in the ST (Simonetta-Moreau et al. 1999). Thus peripheral volleys, which cannot activate the inhibitory interneurones in the absence of TMS, become very efficient when their synaptic actions are combined with actions of the TMS. This suggests that inhibitory interneurones, as excitatory premotoneurones, would receive corticospinal excitation (Fig. 7).
If excitation evoked by group I and group II afferents is mediated to Q motoneurones through common neurones, one would expect the onset of the group II-induced excitation to be delayed in the presence of TMS when the inhibition of these neurones is enhanced by the combined actions of cortical and group I volleys (see above). Therefore our conclusion on mediation of excitatory effects of group I and group II afferents by the same neurones (see Simonetta-Moreau et al. 1999) is supported by the finding that the mean difference in the latencies of group I and group II excitations (assessed in single motor units) was greater in the presence of TMS (8.7 ms) than in its absence (6.3 ms; see Simonetta-Moreau et al. 1999).
Functional implications
The very large extra facilitation found on combined stimulation of the motor cortex and peripheral afferents (see above) suggests that a significant part of the cortical command to motoneurones of thigh muscles is mediated via premotoneurones co-activated by group I and group II afferents. Lundberg et al. (1987) have postulated that excitatory premotoneurones with input from group II afferents may servoassist movements commanded from the brain which might be initiated by descending activation of these neurones, but maintained in a later phase by discharges in muscle spindle secondary endings activated by descending co-activation of static γ-motoneurones. They also assumed that a parallel descending excitation of the group II interneurones mediating inhibition of motoneurones (which is strong in the cat; see Jankowska, 1992) might then be used to prevent contraction of muscles not required in a given movement. In intact awake man, where there is no evidence for group II inhibition of motoneurones (Simonetta-Moreau et al. 1999), this selective function might be taken over by the potent corticospinal excitation of interneurones inhibiting premotoneurones (see Fig. 7): combined actions of peripheral and corticospinal volleys on these inhibitory interneurones might select the subsets of excitatory premotoneurones appropriate for each given motor task (postural adjustments, locomotion or voluntary movement).
Acknowledgments
The authors wish to express their gratitude to Dr L. Jami, Professors E. Jankowska and A. Lundberg, Drs L. Mazières and S. Meunier and Professor J. Nielsen for reading and commenting upon the manuscript. Our thanks are also due to Annie Rigaudie and Michèle Dodo for excellent technical assistance. This work was supported by grants from Assistance Publique-Hôpitaux de Paris (AP-HP, PHRC AOM 95078), Institut National de la Santé et de la Recherche Médicale (INSERM, CRI 96037), Ministère de l'Enseignement Supérieur et de la Recherche (EA 2393) and Institut pour la Recherche sur la Moelle Épinière (IRME).
References
- Araki T, Eccles JC, Ito M. Correlation of the inhibitory postsynaptic potential of motoneurones with the latency and time course of inhibition of monosynaptic reflexes. The Journal of Physiology. 1960;154:354–377. doi: 10.1113/jphysiol.1960.sp006584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awiszus F, Feistner H. Quantification of D- and I-wave effects evoked by transcranial magnetic brain stimulation on the tibialis anterior motoneuron pool in man. Experimental Brain Research. 1994;104:153–158. doi: 10.1007/BF00243225. [DOI] [PubMed] [Google Scholar]
- Brouwer B, Ashby P. Corticospinal projections to lower limb motoneurons in man. Experimental Brain Research. 1992;89:649–654. doi: 10.1007/BF00229889. [DOI] [PubMed] [Google Scholar]
- Burke D, Gandevia SC, McKeon B. Monosynaptic and oligosynaptic contributions to human ankle jerk and H-reflex. Journal of Neurophysiology. 1984;52:435–448. doi: 10.1152/jn.1984.52.3.435. [DOI] [PubMed] [Google Scholar]
- Cavallari P, Edgley SA, Jankowska E. Postsynaptic actions of midlumbar interneurones on motoneurones of hind limb muscles in the cat. The Journal of Physiology. 1987;389:675–690. doi: 10.1113/jphysiol.1987.sp016677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebatch JG, Rothwell JC, Day BL, Thompson PD, Marsden CD. Cortical outflow to proximal arm muscles in man. Brain. 1990;113:1843–1856. doi: 10.1093/brain/113.6.1843. [DOI] [PubMed] [Google Scholar]
- Davies HE, Edgley SA. Inputs to group II-activated midlumbar interneurones from descending motor pathways in the cat. The Journal of Physiology. 1994;479:463–473. doi: 10.1113/jphysiol.1994.sp020310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Dressler D, Hess CW, M aertens de Noordhout A, Marsden CD, Nakashima K, Rothwell JC, Thompson PD. Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. The Journal of Physiology. 1989;412:449–473. doi: 10.1113/jphysiol.1989.sp017626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt JE, Cheron G. Spinal and far-field components of human somatosensory evoked potentials to posterior tibial nerve stimulation analysed with oesophageal derivations and non-cephalic reference recording. Electroencephalography and Clinical Neurophysiology. 1983;56:635–651. doi: 10.1016/0013-4694(83)90031-7. 10.1016/0013-4694(83)90031-7. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. An interneuronal relay for group I and II muscle afferents in the midlumbar segments of the cat spinal cord. The Journal of Physiology. 1987;399:675–690. doi: 10.1113/jphysiol.1987.sp016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier E, Katz R, Pierrot-Deseilligny E. Descending control of reflex pathways in the production of voluntary isolated movements in man. Brain Research. 1983;288:375–377. doi: 10.1016/0006-8993(83)90122-1. 10.1016/0006-8993(83)90122-1. [DOI] [PubMed] [Google Scholar]
- Fournier E, Meunier S, Pierrot-Deseilligny E, Shindo M. Evidence for interneuronally mediated I a excitatory effects to human quadriceps motoneurones. The Journal of Physiology. 1986;377:143–169. doi: 10.1113/jphysiol.1986.sp016179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracies JM, Pierrot-Deseilligny E, Robain G. Evidence for further recruitment of group I fibres with high stimulus intensities when using surface electrodes in man. Electroencephalography and Clinical Neurophysiology. 1994;93:353–357. doi: 10.1016/0168-5597(94)90123-6. 10.1016/0168-5597(94)90123-6. [DOI] [PubMed] [Google Scholar]
- Herdmann J, Dvorak J, Rathmer L, Theiler R, Peuschel K, Zenker W, Lumenta CB. Conduction velocities of pyramidal tract fibres and lumbar motor nerve roots: normal values. Zentralblatt für Neurochirurgie. 1991;52:197–199. [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Morin C, Pierrot-Deseilligny E. Assessing changes in presynaptic inhibition of Ia fibres: a study in man and the cat. The Journal of Physiology. 1987a;389:729–756. doi: 10.1113/jphysiol.1987.sp016680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Pierrot-Deseilligny E, Shindo M. Changes in presynaptic inhibition of I a fibres at the onset of voluntary contraction in man. The Journal of Physiology. 1987b;389:757–772. doi: 10.1113/jphysiol.1987.sp016681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles JF, Roberts RC. Inhibition of monosynaptic reflexes in the human lower limb. The Journal of Physiology. 1987;385:69–87. doi: 10.1113/jphysiol.1987.sp016484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Priori A, Manfredi M. Corticospinal potentials after transcranial stimulation in humans. Journal of Neurology, Neurosurgery and Psychiatry. 1989;52:970–974. doi: 10.1136/jnnp.52.8.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Progress in Neurobiology. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Padel Y, Tanaka R. The mode of activation of pyramidal cells by intracortical stimuli. The Journal of Physiology. 1975;249:617–636. doi: 10.1113/jphysiol.1975.sp011034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernell D, Hultborn H. Synaptic effects on recruitment gain: a mechanism of importance for the input-output relations of motoneurone pools? Brain Research. 1990;507:176–179. doi: 10.1016/0006-8993(90)90542-j. [DOI] [PubMed] [Google Scholar]
- Lundberg A. The control of spinal mechanisms from the brain. In: Tower DB, editor. The Nervous System, The Basic Neurosciences. Vol. 1. New York: Raven Press; 1975. pp. 253–265. [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents. 3. Secondary spindle afferents and the FRA: a new hypothesis. Experimental Brain Research. 1987;65:294–306. doi: 10.1007/BF00236301. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Voorhoeve P. Effects from the pyramidal tract on spinal reflex arcs. Acta Physiologica Scandinavica. 1962;56:201–219. doi: 10.1111/j.1748-1716.1962.tb02498.x. [DOI] [PubMed] [Google Scholar]
- Marque Ph, Pierrot-Deseilligny E, Simonetta-Moreau M. Evidence for excitation of the human lower limb motoneurones by group II muscle afferents. Experimental Brain Research. 1996;109:357–360. doi: 10.1007/BF00231793. [DOI] [PubMed] [Google Scholar]
- Meunier S, Pénicaud A, Pierrot-Deseilligny E, Rossi A. Monosynaptic Ia excitation and recurrent inhibition from quadriceps to ankle flexors and extensors in man. The Journal of Physiology. 1990;423:661–675. doi: 10.1113/jphysiol.1990.sp018046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone A, Grasso M, Giordano A, Schieppati M. Different effect of height on latency of leg and foot short- and medium-latency EMG responses to perturbation of stance in humans. Neuroscience Letters. 1996;206:89–92. doi: 10.1016/s0304-3940(96)12430-7. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Kagamihara Y. Differential projection of the sural nerve on early and late recruited human tibialis anterior motor units: change of recruitment gain. Acta Physiologica Scandinavica. 1993;147:385–401. doi: 10.1111/j.1748-1716.1993.tb09515.x. [DOI] [PubMed] [Google Scholar]
- Pauvert V, Pierrot-Deseilligny E, Rothwell JC. Role of spinal premotoneurones in mediating corticospinal input to forearm motoneurones in humans. The Journal of Physiology. 1998;508:301–312. doi: 10.1111/j.1469-7793.1998.301br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori A, Bertolasi L, Dressler D, Rothwell JC, Day BL, Thompson PD, Marsden CD. Transcranial electric and magnetic stimulation of the leg area of the human motor cortex: single motor unit and surface EMG responses in the tibialis anterior muscle. Electroencephalography and Clinical Neurophysiology. 1993;89:131–137. doi: 10.1016/0168-5597(93)90095-7. [DOI] [PubMed] [Google Scholar]
- Simonetta-Moreau M, Marque P, Marchand-Pauvert V, Pierrot-Deseilligny E. The pattern of excitation of human lower limb motoneurones by probable group II muscle afferents. The Journal of Physiology. 1999;517:287–300. doi: 10.1111/j.1469-7793.1999.0287z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


