Abstract
Membrane currents induced by noxious heat (Iheat) were studied in cultured dorsal root ganglion (DRG) neurones from newborn rats using ramps of increasing temperature of superfusing solutions.
Iheat was observed in about 70 % of small (< 25 μm) DRG neurones. At -60 mV, Iheat exhibited a threshold at about 43 °C and reached its maximum, sometimes exceeding 1 nA, at 52 °C (716 ± 121 pA; n = 39).
Iheat exhibited a strong temperature sensitivity (temperature coefficient over a 10 °C temperature range (Q10) = 17·8 ± 2·1, mean ± s.d., in the range 47-51 °C; n = 41), distinguishing it from the currents induced by capsaicin (1 μM), bradykinin (5 μM) and weak acid (pH 6·1 or 6·3), which exhibited Q10 values of 1·6-2·8 over the whole temperature range (23-52 °C). Repeated heat ramps resulted in a decrease of the maximum Iheat and the current was evoked at lower temperatures.
A single ramp exceeding 57 °C resulted in an irreversible change in Iheat. In a subsequent trial, maximum Iheat was decreased to less than 50 %, its threshold was lowered to a temperature just above that in the bath and its maximum Q10 was markedly lower (5·6 ± 0·8; n = 8).
DRG neurones that exhibited Iheat were sensitive to capsaicin. However, four capsaicin-sensitive neurones out of 41 were insensitive to noxious heat. There was no correlation between the amplitude of capsaicin-induced responses and Iheat.
In the absence of extracellular Ca2+, Q10 for Iheat was lowered from 25·3 ± 7·5 to 4·2 ± 0·4 (n = 7) in the range 41-50 °C. The tachyphylaxis, however, was still observed.
A high Q10 of Iheat suggests a profound, rapid and reversible change in a protein structure in the plasma membrane of heat-sensitive nociceptors. It is hypothesized that this protein complex possesses a high net free energy of stabilization (possibly due to ionic bonds) and undergoes disassembly when exposed to noxious heat. The liberated components activate distinct cationic channels to generate Iheat. Their affinity to form the complex at low temperatures irreversibly decreases after one exposure to excessive heat.
Acute pain represents the last warning to a living organism of imminent damage to the body tissue. Warning signals, of a physical or chemical nature, are transformed to impulses conveyed to the central nervous system by small-diameter afferent fibres whose peripheral endings are equipped with specialized receptors for detecting noxious stimuli (for review see Iggo, 1974; Zimmermann, 1979). These receptors are also functionally expressed on the cell body of cultured sensory neurones where they can be studied in vitro (Baccaglini & Hogan, 1983).
Heat of noxious intensity has been found to open cation permeable channels in a class of small sensory neurones (Cesare & McNaughton, 1996) that are sensitive to capsaicin, a pain-producing substance (Kirschstein et al. 1997). The membrane current induced by noxious heat (Iheat) can be sensitized by bradykinin (Cesare & McNaughton, 1996) and the identified capsaicin receptor (VR1) has been proposed to represent the protein structure activated by noxious heat (Caterina et al. 1997).
We have used cultured sensory neurones, isolated from newborn rats, to determine the temperature coefficient over a 10°C temperature range (Q10) of Iheat. This enables an estimation of the net free energy of stabilization of the structures responsible for generating Iheat. In addition, we examined the effects of algogens and of removing Ca2+o on Iheat. The aim was to better understand the mechanisms underlying nociception. Preliminary communications of some of these results have been published in abstract form (Vlachováet al. 1998; Vyklickýet al. 1998).
METHODS
Cell cultures
Primary cultures of dorsal root ganglion (DRG) neurones were prepared in two steps as described previously (Vlachová & Vyklický, 1993). The experiments were carried out according to the guidelines of the Institute of Physiology animal welfare committee. Two- to three-day-old Wistar rats were anaesthetized with ether and decapitated. Hippocampi were removed, dissociated with trypsin and plated on collagen-coated glass coverslips in a nutrient medium composed of 90 % Eagle's minimum essential medium (MEM) and 10 % fetal bovine serum. When the glial cultures became confluent, usually after 6-9 days, the medium was switched to nutrient-supplemented MEM with 5-fluoro-2-deoxyuridine (15 mg ml−1), uridine (35 mg ml−1) and 10 % horse serum to minimize cell division. In the second step, we took 2- to 3-day-old rats of the same breed and killed them according to the same protocol as mentioned above. Afterwards we dissected dorsal root ganglia and incubated them at 37°C in phosphate-buffered saline (PBS) containing 2 % collagenase for 45 min and then in PBS with 0.3 % trypsin for 10 min. The ganglia were then rinsed with calcium- and magnesium-free PBS and dissociated by trituration using a fire-polished Pasteur pipette. DRG cells were plated on the glial feeder layer cultures, grown in a medium composed of 90 % MEM and 10 % fetal calf serum and maintained at 37°C in a water-saturated atmosphere with 5 % CO2. The next day the medium was replaced by a nutrient-supplemented MEM containing 10 % horse serum and 5-fluoro-2-deoxyuridine (15 mg ml−1) and uridine (35 mg ml−1) (Guthrie et al. 1987). Nerve growth factor (mNGF 2.5S; Alomone Laboratories, Israel; 30 ng ml−1) was added to the nutrient medium, which was changed twice a week. Under these conditions, the neurones develop only short processes, if at all.
Recording and perfusion techniques
The single-electrode patch-clamp technique was used to record whole-cell membrane currents, employing an Axopatch-1D preamplifier and pCLAMP 6 programs (Axon Instruments) with a laboratory PC for storing and evaluating the data. Electrodes were pulled from borosilicate glass and after fire polishing and filling, they had a resistance of 2-4 MΩ. The series resistance was usually less than 10 MΩ and was not compensated.
For drug application, a system for fast superfusion of the neurones was used. It consisted of a manifold of 10 fused silica capillaries (0.36 mm inner diameter; Composite Metal Services Ltd, Worcester, UK) connected to a common outlet made from a glass capillary covered by a layer of platinum used for heating. The orifice of the outlet capillary was placed at a distance less than 100 μm from the soma of a selected neurone. Ramps of the heat application were 7 or 10 s. Each of the 10 tubes was connected to a reservoir containing solution separated from the tube by a valve that was closed under resting conditions. The opening and closing of the solenoid valves (General Valve Corp.) and heating was controlled by a microprocessor. The temperature was measured with a miniature thermocouple placed inside the common outlet capillary close to the orifice. The temperature of the solution applied was tested with another miniature thermocouple placed in front of the outlet at a distance from where the cell was examined in the experiment. There was less than 1°C difference between the temperatures measured by these two thermocouples, and the lag did not exceed 100 ms (Dittert et al. 1998). Before and after the test solutions, neurones were superfused with control extracellular solution (ECS) of the following composition (mM): NaCl, 160; KCl, 2.5; CaCl2, 1; MgCl2, 2; Hepes, 10; glucose, 10; pH adjusted to 7.3 with NaOH. In experiments when the effects of 0 Ca2+ were tested, CaCl2 was omitted and no EGTA was added. In acidic ECS, Mes was used instead of Hepes and the pH was adjusted as indicated. All experiments were performed at room temperature (22-24°C). The intracellular pipette solution (ICS) contained (mM): KCl, 140; CaCl2, 0.5; MgCl2, 2; EGTA, 10; Hepes, 10; pH adjusted to 7.3 with KOH. In some experiments ICS contained GTPγS (0.3 mM). No significant differences in the heat sensitivity of GTPγS-treated cells were observed, and therefore the data were pooled. Bradykinin was dissolved in the absence of oxygen and stored in aliquots at -20°C. Capsaicin was dissolved in 100 μl 96 % ethanol and diluted to 1 mM with 0.9 ml of distilled water. All drugs were purchased from Sigma. Data are given as means ±s.e.m. unless otherwise specified. For statistical comparisons Student's paired t test and Pearson's product moment correlation were performed using SigmaStat (Jandel Corporation). The Q10 distribution was compared by the Kolmogorov-Smirnov test (Stat::Fit; Geer Mountain Software Corp., South Kent, CT, USA). Differences were considered significant at P < 0.05.
Determination of Q10 and activation energy
The temperature coefficient Q10 and the activation energy Ea were used to characterize the temperature dependence of the membrane current (Hille, 1992a). Heat-induced currents were low-pass filtered at 0.5-1 kHz (4-pole Bessel filter) and digitized at 1-2 kHz.
Data sampled at the rising phase of the temperature ramp were pooled for every 0.25 or 0.5°C and the current was normalized to the value at the temperature of the bath (23-25°C). The common logarithm of normalized current was plotted against the reciprocal of the absolute temperature (Arrhenius plot). In all neurones under study the Arrhenius plot was curved, approximating asymptotically to a line above 47°C and in most cells (32/41, i.e. 78 %) to a line below 40°C. In the temperature range where the Arrhenius plot was linear (correlation coefficient r > 0.99 for temperatures above 47°C), Ea was expressed using the slope of the regression line:
| (1) |
where I1 is the value of normalized current at the lower absolute temperature T1, I2 is the value of normalized current at the higher absolute temperature T2 and R is the gas constant (8.314 J K−1 mol−1).
Q10 was determined using the formula:
| (2) |
An estimate of the threshold temperature for inducing the specific heat-evoked membrane current was determined from the point of intersection of the regression lines representing two different slopes.
General procedure
DRG neurones cultured on the monolayer of hippocampal glia survived at least 14 days provided that they were firmly attached to an underlying cell, probably an astrocyte. Signs of healthy appearance were a smooth plasma membrane, sharp boundary of the nucleus and one or two spot-like nucleoli. After several days some of the neurones exhibited one or several thin processes. Only small neurones (< 25 μm in diameter) without visible processes were selected for recordings. These neurones had a high probability of being capsaicin sensitive. Most of the experiments were performed between 2 and 6 days after plating. If any chemical was used, only one neurone was tested per coverslip.
RESULTS
Membrane currents induced by ramps of increasing temperature in small DRG neurones
Increasing the temperature of the superfusing solution induced a steeply increasing inward membrane current in the range between 43°C and 52°C in about 70 % of small DRG neurones in culture. This current is very likely to be identical with the heat-activated current discovered in the same type of DRG neurones by Cesare & McNaughton (1996). Figure 1A shows an example of the inward current evoked by a 7 s ramp of increasing temperature from 23°C to 51°C in a small DRG neurone at -60 mV. A distinct change in the slope of the membrane current in response to gradually elevating temperature can be seen in the record and in the graph in which the membrane current was expressed as a function of the temperature (Fig. 1B). Figure 1C is the Arrhenius plot, in which the reciprocal of the absolute temperature was plotted against the logarithm of the current. Two straight lines of different slopes can be distinguished and the Q10 and net free energies of activation (Ea) calculated (see Methods). In this record, the slowly rising phase in the temperature range 24-34°C exhibited a Q10 of 1.7 (corresponding to Ea= 40 kJ mol−1) and the fast rising phase calculated for the temperature range 44-50°C had a Q10 of 16 (Ea= 235 kJ mol−1). The threshold temperature for inducing the specific heat-evoked membrane current (see Methods) was 43°C. The steeply rising membrane current, sometimes exceeding 1 nA at 52°C (716 ± 121 pA; n = 39), was observed exclusively in DRG neurones that were sensitive to capsaicin.
Figure 1. Whole-cell membrane current induced by a ramp of temperature increase and its Q10 in cultured DRG neurones.
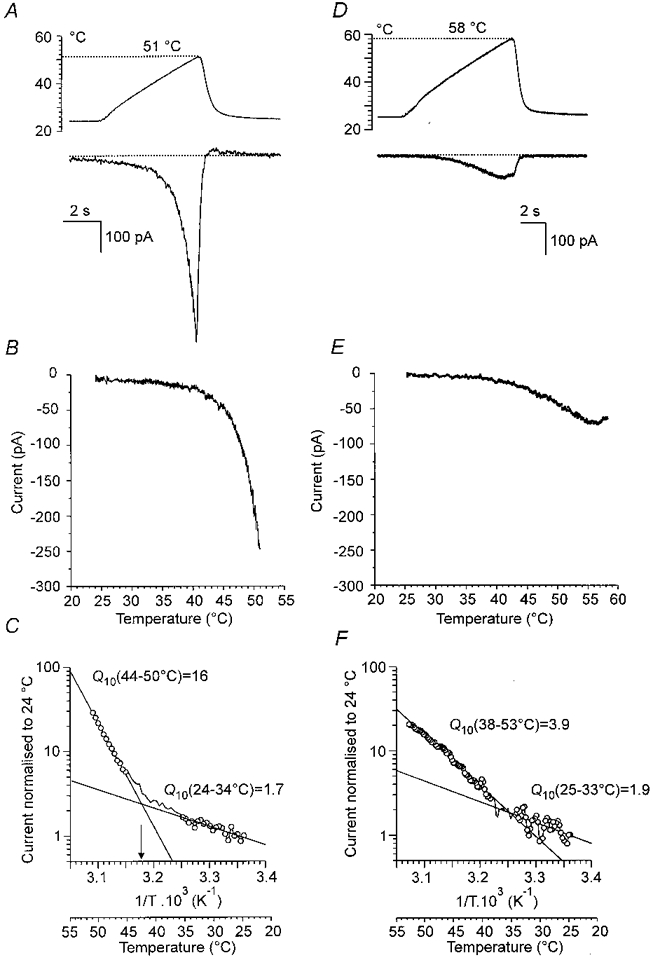
A (upper trace), temperature of the solution superfusing the neurone measured by a miniature thermocouple inserted in the orifice of the heated outlet capillary; maximum, 51 °C. Lower trace, current at a membrane potential of -60 mV recorded in the whole-cell mode of the single-electrode patch-clamp technique. The cell was capsaicin sensitive. B, relation between membrane current and temperature for the record in A. The current increased markedly with small increases in temperature above 43 °C. C, Arrhenius plot in which the reciprocal of the absolute temperature (abscissa) was related to the current normalized to that at 24 °C (ordinate, log scale). Two lines with different slopes were extrapolated. The lower slope exhibited Q10= 1.7; for the higher slope, Q10= 16. From the intersection of these two lines, the threshold temperature for Iheat was estimated to be 43 °C (arrow). The temperature scale in degrees centigrade is shown below the scale of the reciprocal of the absolute temperature. D (top trace), temperature ramp to 58 °C. Lower trace, membrane current from a capsaicin-insensitive neurone. E, relation between temperature and current for the cell in D. The current increased very slowly with the increase in temperature and actually decreased above 55 °C. F, Arrhenius plot for the cell in D. The Q10 for lower temperatures was 1.9 whereas that for the higher temperatures was 3.9.
Neurones that were not sensitive to capsaicin, and exceptionally also neurones found to be capsaicin sensitive, did not respond with a rapidly increasing inward current to temperature ramp above 43°C (Fig. 1D and E). In the Arrhenius plot they displayed a slowly rising current in the whole range of temperature increase between 23 and 52°C. Often two slopes could be determined (Fig. 1F), both with low Q10 (1.7 ± 0.2 and 4.3 ± 0.8; n = 4). The current maximum for these heat-insensitive cells was less than 150 pA at 52°C (65 ± 3 pA; n = 11).
Figure 2A shows the distribution of the Q10 values calculated for the temperature range 47-51°C in 41 neurones that were found to be sensitive to capsaicin. The value of Q10 ranged from 4 to 72 and log-normal distribution parameters had a mean and s.d. of 17.8 and 2.1, respectively (fit not shown, Kolmogorov-Smirnov test; n = 41). In nine of 41 neurones no significant regression of normalized current as a function of reciprocal absolute temperature at lower temperature ranges was detected. Examples of such records in which the slower component of the Arrhenius plot could not be elicited are shown in the control traces in Figs 4D and 6B. The distribution of Q10 derived for the innocuous range of temperatures between 26 and 40°C (1.9 ± 0.4, mean ±s.d.; n = 32) is shown in Fig. 2B.
Figure 2. Histograms of Q10 for membrane currents at noxious and innocuous temperatures.
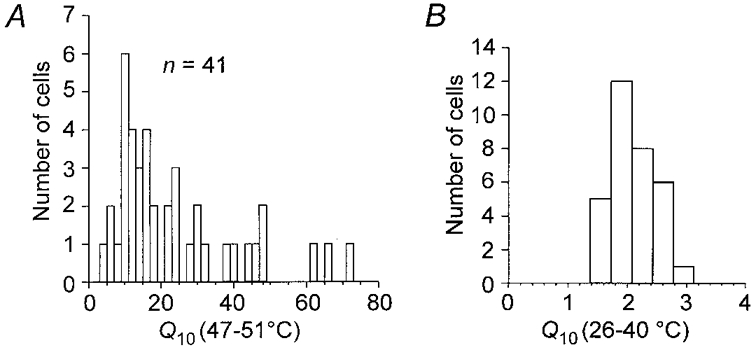
A, distribution of Q10 over a range of noxious heat, 47-51 °C, in 41 neurones found to be capsaicin sensitive. The diagram includes 4 neurones that were sensitive to capsaicin but in which no significant Iheat was detected. B, distribution of Q10 in the non-noxious range of temperature increase between 26 and 40 °C for 32 of the capsaicin-sensitive DRG neurones. In 9 neurones there was no significant effect of increasing the temperature over this range on the membrane current. Note the expansion of the abscissa in B.
Figure 4. Effect of excessive temperature on Iheat.
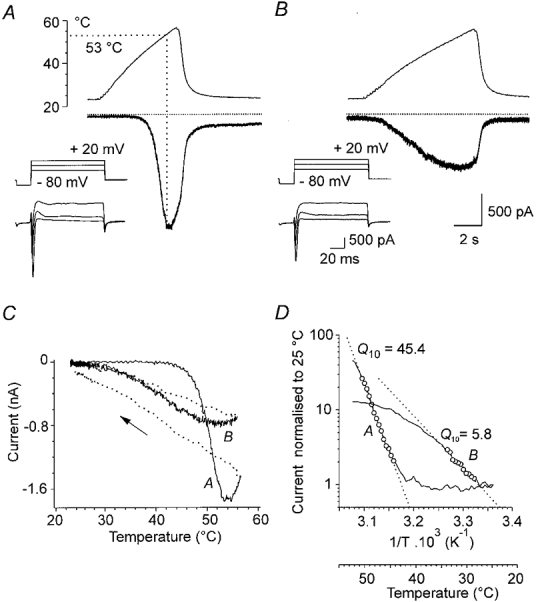
Two successive responses to ramps of increasing temperature to 57 °C in a DRG neurone at -60 mV. A, in the first trial, Iheat reached a maximum at 53 °C (dotted line). Increasing the temperature further resulted in a current plateau followed by a gradual decline before the ramp reached its peak. B, during the second trial, 3 min later, the maximum current produced by the same temperature increase was less than one-half of the control and was reached at about 48 °C. The threshold temperature for Iheat was shifted to a lower temperature. The insets in A and B illustrate the response of the neurone to 3 voltage steps from -80 to -20, 0 and +20 mV before the corresponding responses to the heat ramps. Calibrations: 500 pA, 2 s for heat currents; 500 pA, 20 ms for insets. C, plot of membrane currents versus temperature during the trials in A and B. The dotted lines and arrow indicate the current when the temperature is returning to control level. D, Arrhenius plots of the 2 responses during the ramp. The maximum Q10 was 45.4 (A) and, after the excessive heat, it was 5.8 (B).
Figure 6. The effects of removing extracellular Ca2+ on Iheat.
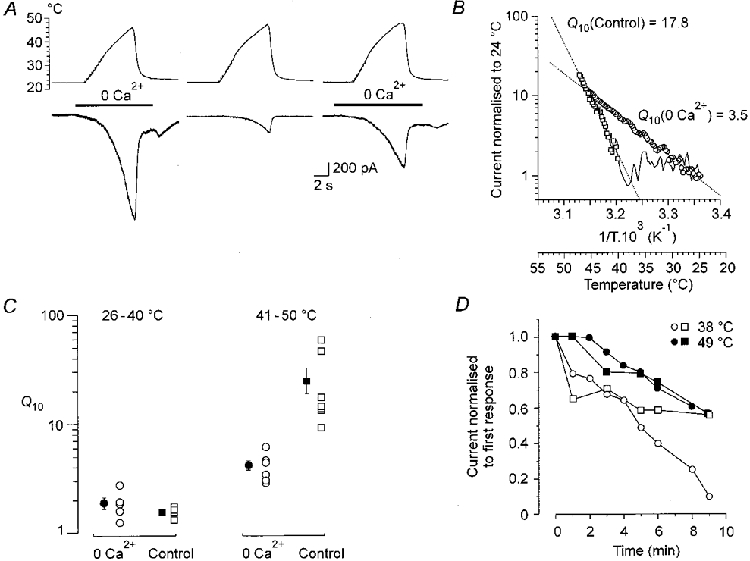
A, temperature (upper trace) and membrane current (lower trace) during superfusion of a neurone with Ca2+-free solution (left and right records) and with 1 mM Ca2+ (centre). Two minutes elapsed between the records. The Ca2+-free solution was superfused as indicated by the bar. It produced a small increase in resting current that reversed when the neurone was washed with control ECS. B, Arrhenius plot of currents illustrated in A. The 0 Ca2+Q10 (left response in A;○) was 3.5, while the control (middle response in A;□) was 17.8. C, graph of Q10 in 0 Ca2+ (○) and control (1 mM Ca; □) at innocuous (26-40 °C) and noxious (41-50 °C) temperatures. Filled circles or squares indicate means ±s.e.m. The Q10 at innocuous temperatures is the same with or without Ca2+; at noxious temperatures it is markedly reduced in 0 Ca2+. D, relative currents induced at 38 °C (open symbols) or 49 °C (filled symbols), in 2 cells, recorded in 0 Ca2+, repeated at intervals indicated on the abscissa. During the interval between application of the heat ramps the neurones were washed with ECS containing 1 mM Ca2+. The graph indicates that tachyphylaxis of Iheat persists in the absence of Ca2+.
Tachyphylaxis of the response to heat
In contrast to studies in which nociception was investigated in single nerve fibre preparations (Kumazawa & Perl, 1977; Lynn, 1979; Mizumura et al. 1992), no obvious changes in Iheat induced by repeated brief steps of temperature to the noxious range were observed by Cesare & McNaughton (1996). Using temperature ramps from 23 to 49°C, repeated at 30 s intervals, we found that the current maximum declined, while its threshold was shifted slightly to lower temperatures (Fig. 3A). The current-temperature plot of the first and second responses is shown in Fig. 3B, and the Arrhenius plots of the first and the fourth responses are shown in Fig. 3C. The Q10 of the steeply rising phase decreased from 48 to 4.5, while the slowly rising phase, reflecting increase of the temperature in the subnoxious range, remained largely unchanged (Q10= 1.4 for the first and 1.6 for the fourth response). There was considerable variation in the degree of tachyphylaxis in individual cells (Fig. 3D). The average value of the fourth heat-induced current measured at 49°C was 60 ± 5 % (n = 9) of the first response (Fig. 3E).
Figure 3. Tachyphylaxis of the heat-induced membrane current.
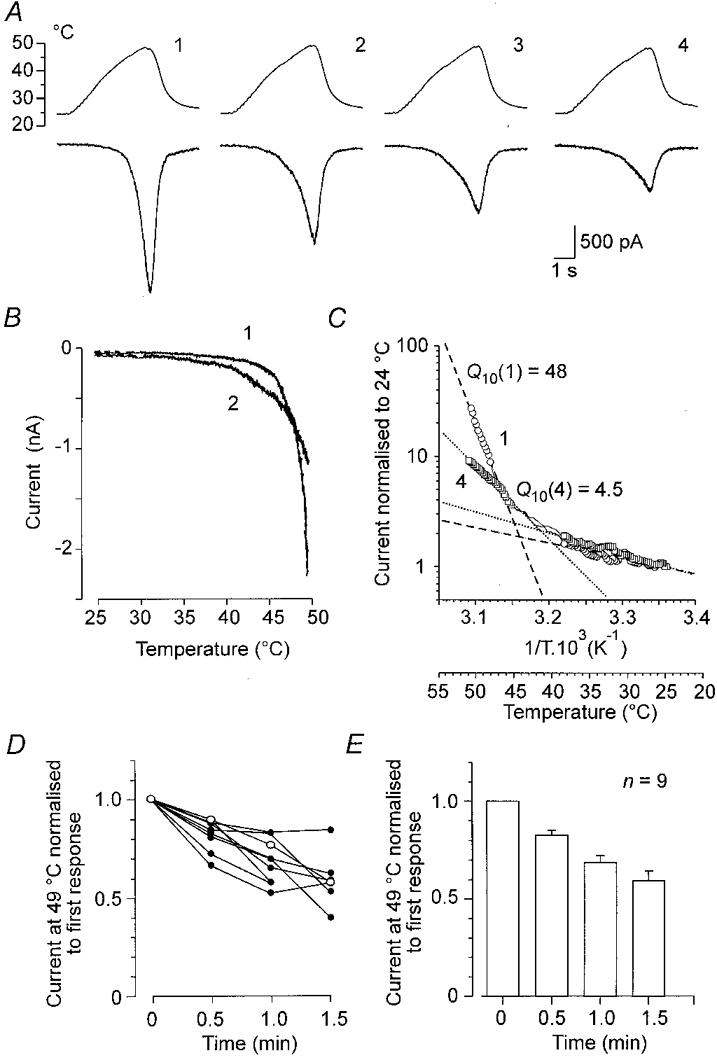
A,Iheat during 4 temperature ramps to 49 °C at 30 s intervals: upper trace, temperature; lower trace, whole-cell membrane currents; B, current-temperature plots of the first and second responses. Iheat decreased in repeated trials and its apparent threshold shifted to a lower temperature. C, superimposed Arrhenius plots for the first and fourth responses shown in B. The Q10 values for low temperatures, 1.4 in the first (○, dashed line) and 1.6 in the fourth (□, dotted line), were not significantly different. For noxious temperatures above 45 °C, the Q10 value decreased from 48 for the first response to 4.5 for the fourth. D, relative changes in membrane currents induced at 49 °C in 4 repeated trials separated by 30 s intervals in 9 neurones. Open circles are for the cell shown in A.E, bar graph indicating means ±s.e.m. for the same cells.
The effect of excessive temperature on Iheat
The membrane current induced by ramps of increasing temperature reached maximum between 52 and 53°C. If the temperature was increased further, the membrane current declined and in subsequent trials, Iheat was changed irreversibly. As shown in Fig. 4A, after one exposure of the neurone to 57°C, a slight increase of the resting membrane current (∼50 pA) was observed for several minutes after washing the neurone at the control temperature. The neurone exhibited only small changes in the responses to large voltage steps from -80 to +20 mV (insets). However, the subsequent response to the ramp of increasing temperature was profoundly changed. The current started to increase markedly at a temperature slightly above that in the bath and the maximum was 46 % of the initial response. Figure 4C is a graph of the currents expressed as a function of temperature for the two records in A and B. There was also a dramatic change of the Q10 of Iheat after a single exposure of the neurone to an excessive temperature (Fig. 4D). Maximum Q10 in the first trial was 45, while Q10 in the second trial was 5.8. Similar results were observed in eight cells (5.6 ± 0.8).
Small current responses of the cells which did not exhibit Iheat were largely unchanged after repeated exposures to the temperature up to 50°C. However, when the cells were exposed to an excessive temperature of ∼60°C or higher they exhibited a sudden inward membrane current from which they did not recover (Fig. 5A-C), or were lost completely. Much the same was observed in the cells that exhibited Iheat in the range of temperatures between 43 and 53°C (Fig. 5D and E). From this figure it is obvious that Iheat is an unique entity and that the sudden current resulting from an excessive temperature is likely to be due to damage to the lipid membrane from which only a partial recovery occasionally occurs.
Figure 5. Irreversible membrane currents induced by excessive heat in a heat-insensitive and a heat-sensitive neurone.
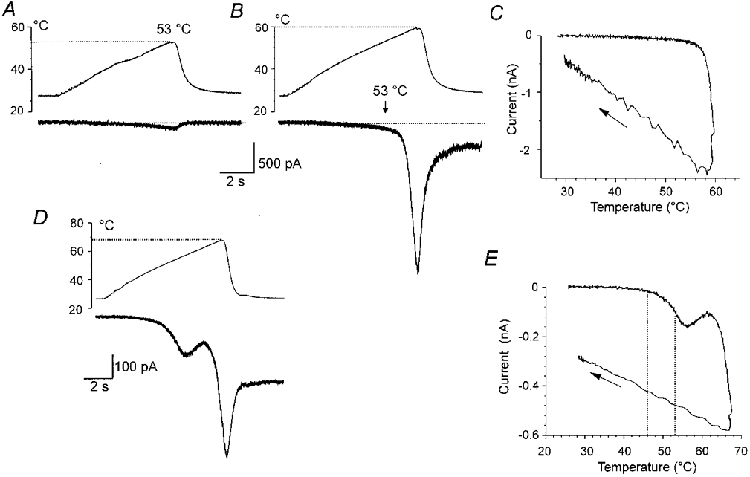
A, heat-insensitive neurone. A small membrane current (lower trace) was induced by a ramp of temperature increase to 53 °C (upper trace) in the first trial. B, the second trial, applied 2 min later, resulted in a current response of the same small magnitude at 53 °C (vertical arrow above current trace). Increasing the temperature further resulted in a steep current with a maximum of 2.4 nA at 60 °C. After washing the neurone by extracellular solution of control temperature, recovery was incomplete and leak membrane current (300 pA) persisted. Holding potential, -60 mV. C, plot of membrane current versus temperature in the trial shown in B. The arrow indicates the current when the temperature was returning to control level. D, heat-sensitive neurone. Membrane current was induced by a ramp of increasing temperature to 68 °C in the first trial. Iheat was followed by a steep inward current at 62 °C and reached maximum (580 pA) at 68 °C. After washing the neurone with extracellular solution of control temperature, an unrecoverable sustained inward membrane current (270 pA) was observed. E, plot of the membrane current versus temperature shown in D. The Q10 for Iheat estimated between 46 and 53 °C was 11.4 (the temperature interval is indicated by dotted lines in D).
Effects of the absence of extracellular Ca2+ on Iheat
Extracellular calcium plays an important role in the development of tachyphylaxis of chemically activated ionic currents (Docherty et al. 1996; Koplas et al. 1997). As Ca2+ was shown to participate in the generation of Iheat (Cesare & McNaughton, 1996), we examined whether removing [Ca2+]o could prevent development of tachyphylaxis of the membrane currents induced by ramps of noxious heat. In accord with Cesare & McNaughton (1996), we found that Iheat was greatly increased in the absence of extracellular Ca2+ (Fig. 6A). As can be seen from the Arrhenius plots in Fig. 6B, the Q10 of the responses in the absence of extracellular Ca2+ was reduced from 17.8 to 3.5. The diagrams in Fig. 6C show that the change in Q10 occurred only in the noxious range (41-50°C). In the innocuous range (26-40°C), Q10 was unaffected.
To determine if tachyphylaxis persists in Ca2+-free solution, Iheat was measured repeatedly at 30 s or 1 min intervals in 0 [Ca2+]o. Between tests, the neurone was superfused with control ECS containing 1 mM Ca2+. On average, the fourth response induced by raising the temperature measured to 38 and 49°C decreased with repeated applications of the heat ramps to 69 ± 2 % and 86 ± 6 %, respectively (n = 4). The results of two such experiments in which we were able to record for 9 min are shown in Fig. 6D. These experiments suggest that removing [Ca2+]o cannot completely prevent tachyphylaxis of the responses to noxious heat, but they do not exclude this possibility completely, as nominally 0 Ca2+ in ECS to which no EGTA has been added contains micromolar concentrations of Ca2+.
Interaction between membrane currents induced by capsaicin, bradykinin or weak acids and Iheat
The temperature threshold for inducing action potentials in nociceptors decreases in the presence of pain-producing agents (Lang et al. 1990; Kumazawa et al. 1991; Kress & Reeh, 1996). Capsaicin, bradykinin and weak acids are well recognized algogenic chemicals that can produce membrane currents and generate action potentials in nociceptors. The records in Fig. 7 demonstrate that the membrane current induced by these agents is greatly increased when applied in heated solutions. The responses are larger than the sum of the control Iheat and the current induced by any of the drugs in control temperature. The question arises whether this apparent facilitation is due to an increase in Iheat in the presence of the drug or whether the drug-induced responses are increased by heat. The diagrams attached to each row of the records show Arrhenius plots of the responses when control Iheat was subtracted. The Q10 calculated for 0.5 μM capsaicin was 2.7 (2.1 ± 0.2; n = 8). Similar results were found in three cells for 5 μM bradykinin (Q10= 1.9) and in seven cells for extracellular pH 6.1 (Q10= 2.8), respectively. The low Q10 of the increased current favours the idea that the chemically activated responses rather than Iheat are increased by raising the temperature. These experiments, however, are not contradictory to the experiments of Cesare & McNaughton (1996), who showed that the enhanced Iheat persisted after the bradykinin-induced membrane current had vanished and that the protein kinase C activator phorbol 12-myristate 13-acetate, which by itself does not induce any membrane current, can markedly increase Iheat. It is likely that absence of ATP in intracellular solution in our experiments resulted in an early inactivation of the G-protein transduction cascade.
Figure 7. Interaction of Iheat with responses to capsaicin, bradykinin and weak acids.
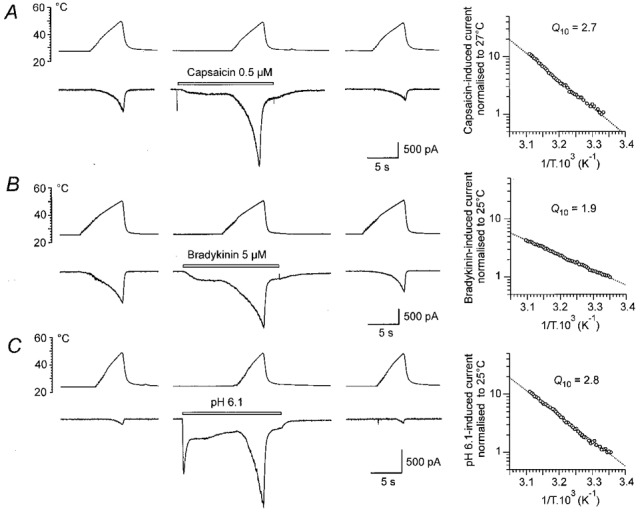
On the left are control responses to heat ramps. In the middle are responses to the heat ramps in the presence of 0.5 μM capsaicin (A), 5 μM bradykinin (B) and extracellular pH 6.1 (C). The records on the right were obtained 1 min after application of the drugs. All drugs produced inward current by themselves at control temperature (23-24 °C). Duration of drug application is indicated by the bars. The graphs to the right of the records are Arrhenius plots of responses in the presence of the drugs. Control responses (first response in A and C, last response in B) were subtracted from the response in the presence of the drug. The calculated Q10 is indicated on each graph. The first response in B is distorted by action potentials on the rising phase (filtered), apparently from an unclamped process which escaped attention in the microscope.
Is Iheat carried by capsaicin receptors?
The suggestion that the capsaicin receptor, identified as VR1, is responsible for generating Iheat (Caterina et al. 1997; Tominaga et al. 1998) was supported by the finding that only capsaicin-sensitive DRG neurones in culture exhibit Iheat (Kirschstein et al. 1997). If this hypothesis is correct, a positive correlation would be expected between the current induced by a supramaximal dose of capsaicin and maximal Iheat.
To test this hypothesis, we measured the response to capsaicin (3 μM) and subsequently the maximum Iheat induced by a ramp of increasing temperature to 51-59°C. The graph in Fig. 8A reveals no correlation between Iheat and capsaicin responses in 25 neurones. Two examples are shown in Fig. 8Ba and b.
Figure 8. Relation between the currents produced by capsaicin and noxious heat.
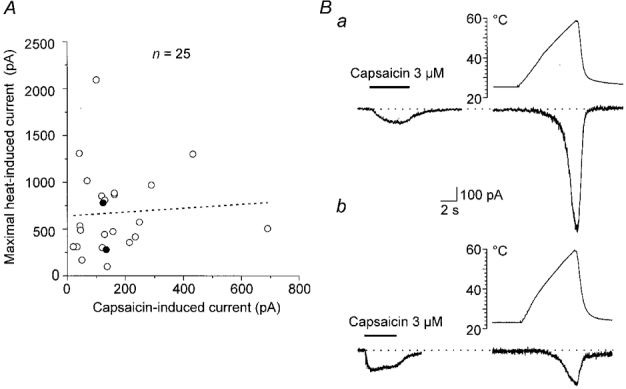
A, graph of the relation between maximal membrane current produced by heating to 51-59 °C in the first trial (ordinate) and current produced solely by superfusion with 3 μM capsaicin. Filled symbols are the experiments in Ba and b. The calculated regression line (dotted) with correlation coefficient r of 0.06, P = 0.75 (n = 25) indicates that the two variables are not correlated. Ba, responses of a neurone to 3 μM capsaicin and to a heat ramp from 25 to 58 °C in the first trial. b, responses of another neurone to 3 μM capsaicin and to a heat ramp from 23 to 59 °C.
In agreement with the study of Kirschstein et al. (1997) we found that DRG neurones sensitive to capsaicin also exhibited Iheat. However, in four out of 41 capsaicin-sensitive neurones, no Iheat was induced with temperature ramps exceeding 49°C. This suggests that the capsaicin receptor may not be the membrane protein generating Iheat in DRG neurones or at least not the only one.
DISCUSSION
Using ramps of increasing temperature, we found an unusually high net free energy of stabilization of the structures involved in generation of the membrane current produced by noxious heat in small DRG neurones in culture. The average Q10= 18 found for Iheat in our experiments corresponds to an Ea value of ∼250 kJ mol−1 (41-50°C). In general, the activation energies for ion channels are low and correspond to Q10 < 2.5. This suggests that the ‘heat receptor’ is different from voltage or chemically gated ion channels and its operation requires a profound and rapidly reversible change in the conformation of a protein sensing noxious heat.
The membrane current induced by noxious heat was discovered in cultured small DRG neurones of the rat by Cesare & McNaughton (1996). They found that this current is carried by cations, including Ca2+, and sensitized by activation of protein kinase C. The range of temperature in which Iheat can be induced, and the finding that it is observed only in small DRG neurones, is in agreement with numerous studies in which nociception induced by heat was examined in single nerve preparations in vivo or in nerve-organ preparations (Kumazawa & Mizumura, 1980; Lynn, 1984; Handwerker & Kobal, 1993; Treede et al. 1995; Handwerker, 1996). The high Q10 of Iheat found in our experiments can explain the exponential increase of firing frequency observed in polymodal nociceptors exposed to raising temperature in the noxious range (Lynn, 1979).
In agreement with Cesare & McNaughton (1996), we found that the processes of activation and deactivation of Iheat are rapid and highly reversible. However, in contrast to the studies on nociception in single fibre preparations (Kumazawa & Perl, 1977; Lynn, 1979; Mizumura et al. 1992), Cesare & McNaughton (1996) did not observe sensitization of Iheat after successive stimuli. Our experiments indicate that after repeated trials not only do the peak responses of Iheat decrease but also the threshold of heat-induced current shifts slightly to lower temperatures. The likely explanation for this apparent discrepancy is that only brief heat pulses (∼500 ms) were used in the study of Cesare & McNaughton (1996), while in our experiments the cells were exposed to noxious heat for several seconds, similar to that used in single-fibre preparations.
Striking effects of heating were observed when temperature ramps exceeded 52°C (Fig. 3). Only one application of the heat to 57°C could irreversibly change the properties of Iheat without producing a marked change in the excitability of the neurone. After such treatment, peak Iheat decreased to less than 50 %, and the threshold for evoking Iheat decreased to a temperature slightly above that in the bath. Together with the dramatic fall of Q10 to 4.5, this suggests a profound and irreversible change of the protein structure responsible for generating Iheat for the period available for recording.
The question arises whether an increase in [Ca2+]i during Iheat contributes to the tachyphylaxis, as it was shown after repeated applications of capsaicin (Docherty et al. 1996; Koplas et al. 1997). In agreement with Cesare & McNaughton (1996) we found that in nominally zero [Ca2+]o, Iheat is markedly larger, which was explained by removal of the partial block exerted by calcium ions that participate in carrying Iheat. Our results demonstrate that Iheat can be detected at lower temperatures and that the apparent Q10 of Iheat in the absence of [Ca2+]o is markedly lower than in the control ECS containing 1 mM Ca2+. It seems likely that removal of the neutralization of the surface charges exerted by external Ca2+ (Ascher & Nowak, 1988), which results in a slight increase of the resting current, may also be involved in the mechanisms that contribute to the stability of the protein responsible for the generation of Iheat. Because nominally 0 Ca2+ was used in ECS that contains micromolar concentrations of Ca2+, our experiments do not completely rule out that influx of Ca2+ contributes to the development of tachyphylaxis.
Our results demonstrate that the membrane currents induced by capsaicin, bradykinin and acidic extracellular pH are markedly increased by raising the temperature to the noxious range. This may explain the potentiating effects of algogenic chemicals on the firing of nociceptors in single nerve fibre experiments (Kumazawa et al. 1987; Lang et al. 1990; Kumazawa et al. 1991; Kress & Reeh, 1996). The low Q10 that characterizes this process favours the idea that an increased conductance and/or mean opening time of ligand-gated channels at higher temperatures is the major factor involved in the mechanisms of this facilitation. However, it has to be expected that other inflammatory mediators and algogenic substances can also trigger a cascade of intracellular processes leading to facilitation of Iheat, because it has been demonstrated that the potentiating effect of bradykinin on Iheat outlasts the membrane current induced and that activation of protein kinase C is involved in this process (Cesare & McNaughton, 1996).
Although we did not observe any obvious differences in the Iheat values when using whole-cell patch electrodes with or without GTPγS, we realize that this is not sufficient evidence to exclude the possibility that G-proteins are involved in the generation or modulation of Iheat. In view of the prominent role of G-proteins in other sensory functions e.g. vision and olfaction (see Hille, 1992b), this has to be further explored.
Raising the temperature increases the opening probability of voltage-dependent and chemically activated channels. The Q10 for these effects is mostly less than 2.5 (ffrench-Mullen et al. 1988; Nobile et al. 1990; van Lunteren et al. 1993). It has been suggested that a low Q10 reflects the effects of heating on weak, non-covalent bonds in globular proteins (Jaenicke, 1991; Somero, 1996). In a study of presumed ‘primary’ warm-sensitive neurones, Kiyohara et al. (1990) found a Q10 of 4.3-7.0 for a non-inactivating TTX-sensitive inward current to an increase in temperature in the hyperthermic range 35-40°C. This suggests that these neurones have a specific heat-sensitive detector related to their function. The finding of unusually high Q10 values (∼18) in heat-sensitive nociceptors suggests that a more profound change in protein structure, e.g. disassembly of a protein complex, is involved in the generation of Iheat.
It has been suggested that the capsaicin receptor VR1 is the protein structure which is responsible for generating Iheat (Caterina et al. 1997; Tominaga et al. 1998). One piece of evidence in support of this hypothesis is the correlation between current produced by heat and capsaicin in HEK293 cells transiently transfected with VR1. Although there is no doubt about the heat sensitivity of VR1, our results suggest caution in accepting the hypothesis that VR1 is the physiological heat sensor in DRG neurones. First, we found no correlation between the size of the maximal capsaicin response and maximal Iheat in the first trial, when it is likely that all receptors are available. Second, we found four neurones out of 41 which were highly capsaicin sensitive and exhibited no detectable Iheat. Although these discrepancies might be explained by a variance of the capsaicin-induced and heat-induced current distributions or by different subpopulations of capsaicin-sensitive neurones (Dedov & Roufogalis, 1998), other mechanisms involved in Iheat generation have to be considered.
The relative strengths of the different types of chemical bond that determine folding of globular proteins are indicated by their bond energies (or, more strictly, bond enthalpies). The enthalpy of a bond is the energy released on the formation of the bond or the energy required to break it. For different types of bonds, the following values of net free energies of stabilization (Ea) are given (in kJ mol−1): covalent, 200-800; ionic, 40-400; hydrogen, 10-30; hydrophobic, 10-30; van der Waals, 3-10; ion-dipole, 3-10; dipole-dipole, 0.5-3 (Freifelder, 1985). In the range of temperature between 40 and 50°C, Q10 values of 10, 35, 40 and 60 correspond to ∼195, 300, 310 and 345 kJ mol−1, respectively.
Ion channels are complex proteins which usually consist of several subunits. Measurements of the effects of temperature changes on the kinetics of channel opening or on the whole-cell membrane currents can give additional information on the function of these structures. It is not practical to measure the net free energies in the patch-clamp studies. However, it is experimentally feasible to measure the membrane currents under different temperatures and express the changes by means of the temperature coefficient (Q10) provided that reliable techniques for producing temperature changes and membrane current measurements are available. In physiological ranges of temperatures between 20 and 40°C the Q10 of 1.5 would correspond to an Ea of 30 kJ mol−1, which suggests weak bonds, including ion-dipole bonds, van der Waals forces, hydrophobic bonds and hydrogen bonds. The Q10 of 10-35 observed for Iheat would correspond to an Ea of 195-300 kJ mol−1, suggesting that much stronger bonds are involved in the protein structures responsible for its generation. Such a high value of Ea is compatible with a reversible weakening of ionic bonds. However, caution has to be exercised in accepting such a simple interpretation until we learn more about the protein structure responsible for generating Iheat.
Experimental data suggest that the heat sensor in DRG neurones is a specific protein complex incorporated in the plasma membrane of the heat-sensitive nociceptors. One can speculate that the complex consists at least of two components, of which one may be a G-protein. This protein structure is endowed with a high net free energy of stabilization and undergoes disassembly when exposed to noxious heat (exceeding 43°C). Due to the high affinity to make a complex, the components reassemble after returning to low temperature. The affinity to make a complex is decreased or lost if the neurone is exposed to high temperature (∼57°C). The liberated components, however, remain available in the membrane, and bind to the channels at lower temperatures in a process that exhibits a low Q10 (∼4-5). This idea is in agreement with the findings of primary hyperalgesia following mild injury produced by heat (LaMotte et al. 1982, 1984; Lynn, 1984; Mizumura et al. 1992). It is likely that the stability of the protein complex unique for heat sensitive nociceptors is regulated by a number of intracellular messengers. Convincing evidence on this has been demonstrated for the products of protein kinase C (Cesare & McNaughton, 1996).
Acknowledgments
This work was supported by the Fogarty Foundation, the National Institute of Neurological Diseases and Stroke of the NIH, the Grant Agency of the Czech Republic (305/97/0519) and the EU CIPA.
References
- Ascher P, Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. The Journal of Physiology. 1988;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccaglini PI, Hogan PG. Some rat sensory neurons in culture express characteristics of differentiated pain sensory cells. Proceedings of the National Academy of Sciences of the USA. 1983;80:594–598. doi: 10.1073/pnas.80.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cesare P, McNaughton P. A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proceedings of the National Academy of Sciences of the USA. 1996;93:15435–15439. doi: 10.1073/pnas.93.26.15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedov VN, Roufogalis BD. Rat dorsal root ganglion neurones express different capsaicin-evoked Ca2+ transients and permeabilities to Mn2+ Neuroscience Letters. 1998;248:151–154. doi: 10.1016/s0304-3940(98)00351-6. 10.1016/S0304-3940(98)00351-6. [DOI] [PubMed] [Google Scholar]
- Dittert I, Vlachová V, Knotková H, Vitásková Z, Vyklický L, Kress M, Reeh PW. A technique for fast application of heated solutions of different composition to cultured neurones. Journal of Neuroscience Methods. 1998;82:195–201. doi: 10.1016/s0165-0270(98)00051-x. 10.1016/S0165-0270(98)00051-X. [DOI] [PubMed] [Google Scholar]
- Docherty RJ, Yeats JC, Bevan S, Boddeke HW. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflügers Archiv. 1996;431:828–837. doi: 10.1007/s004240050074. [DOI] [PubMed] [Google Scholar]
- Ffrench-Mullen JM, Tokutomi N, Akaike N. The effect of temperature on the GABA-induced chloride current in isolated sensory neurones of the frog. British Journal of Pharmacology. 1988;95:753–762. doi: 10.1111/j.1476-5381.1988.tb11701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D. Principles of Physical Chemistry with Applications to the Biological Sciences. Boston, MA, USA: Jones & Barlett; 1985. [Google Scholar]
- Guthrie PB, Brenneman DE, Neale EA. Morphological and biochemical differences expressed in separate dissociated cell cultures of dorsal and ventral halves of the mouse spinal cord. Brain Research. 1987;420:313–323. doi: 10.1016/0006-8993(87)91252-2. [DOI] [PubMed] [Google Scholar]
- Handwerker HO. Sixty years of C-fiber recordings from animal and human skin nerves: historical notes. Progress in Brain Research. 1996;113:39–51. doi: 10.1016/s0079-6123(08)61080-8. [DOI] [PubMed] [Google Scholar]
- Handwerker HO, Kobal G. Psychophysiology of experimentally induced pain. Physiological Reviews. 1993;73:639–671. doi: 10.1152/physrev.1993.73.3.639. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. 2. Sunderland, MA, USA.: Sinauer Associates, Inc.; 1992a. Elementary properties of ions in solution; pp. 261–290. [Google Scholar]
- Hille B. G protein-coupled mechanisms and nervous signaling. Neuron. 1992b;9:187–195. doi: 10.1016/0896-6273(92)90158-a. [DOI] [PubMed] [Google Scholar]
- Iggo A. Activation of cutaneous nociceptors and their actions on dorsal horn neurons. In: Bonica JJ, editor. Advances in Neurology. 4. New York: Raven Press; 1974. pp. 1–9. [Google Scholar]
- Jaenicke R. Protein stability and molecular adaptation to extreme conditions. European Journal of Biochemistry. 1991;202:715–728. doi: 10.1111/j.1432-1033.1991.tb16426.x. [DOI] [PubMed] [Google Scholar]
- Kirschstein T, Busselberg D, Treede RD. Coexpression of heat-evoked and capsaicin-evoked inward currents in acutely dissociated rat dorsal root ganglion neurons. Neuroscience Letters. 1997;231:33–36. doi: 10.1016/s0304-3940(97)00533-8. [DOI] [PubMed] [Google Scholar]
- Kiyohara T, Hirata M, Hori T, Akaike N. Hypothalamic warm-sensitive neurons possess a tetrodotoxin-sensitive sodium channel with a high Q10. Neuroscience Research. 1990;8:48–53. doi: 10.1016/0168-0102(90)90056-k. [DOI] [PubMed] [Google Scholar]
- Koplas PA, Rosenberg RL, Oxford GS. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. Journal of Neuroscience. 1997;17:3525–3537. doi: 10.1523/JNEUROSCI.17-10-03525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress M, Reeh PW. Chemical excitation and sensitization in nociceptors. In: Belmonte C, Cervero F, editors. Neurobiology of Nociceptors. New York: Oxford University Press; 1996. pp. 258–297. [Google Scholar]
- Kumazawa T, Mizumura K. Mechanical and thermal responses of polymodal receptors recorded from the superior spermatic nerve of dogs. The Journal of Physiology. 1980;299:233–245. doi: 10.1113/jphysiol.1980.sp013122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazawa T, Mizumura K, Minagawa M, Tsujii Y. Sensitizing effects of bradykinin on the heat responses of the visceral nociceptor. Journal of Neurophysiology. 1991;66:1819–1824. doi: 10.1152/jn.1991.66.6.1819. [DOI] [PubMed] [Google Scholar]
- Kumazawa T, Mizumura K, Sato J. Thermally potentiated responses to algesic substances of visceral nociceptors. Pain. 1987;28:255–264. doi: 10.1016/0304-3959(87)90120-5. [DOI] [PubMed] [Google Scholar]
- Kumazawa T, Perl ER. Primate cutaneous sensory units with unmyelinated (C) afferent fibers. Journal of Neurophysiology. 1977;40:1325–1338. doi: 10.1152/jn.1977.40.6.1325. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Thalhammer JG, Torebjörk HE, Robinson CJ. Peripheral neural mechanisms of cutaneous hyperalgesia following mild injury by heat. Journal of Neuroscience. 1982;2:765–781. doi: 10.1523/JNEUROSCI.02-06-00765.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte RH, Torebjörk HE, Robinson CJ, Thalhammer JG. Time-intensity profiles of cutaneous pain in normal and hyperalgesic skin: a comparison with C-fiber nociceptor activities in monkey and human. Journal of Neurophysiology. 1984;51:1434–1450. doi: 10.1152/jn.1984.51.6.1434. [DOI] [PubMed] [Google Scholar]
- Lang E, Novak A, Reeh PW, Handwerker HO. Chemosensitivity of fine afferents from rat skin in vitro. Journal of Neurophysiology. 1990;63:887–901. doi: 10.1152/jn.1990.63.4.887. [DOI] [PubMed] [Google Scholar]
- Lynn B. The heat sensitization of polymodal nociceptors in the rabbit and its independence of the local blood flow. The Journal of Physiology. 1979;287:493–507. doi: 10.1113/jphysiol.1979.sp012672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn B. Cutaneous nociceptors. In: Holden AV, Winlow W, editors. The Neurobiology of Pain. Manchester University Press; 1984. pp. 97–107. [Google Scholar]
- Mizumura K, Sato J, Kumazawa T. Strong heat stimulation sensitizes the heat response as well as the bradykinin response of visceral polymodal receptors. Journal of Neurophysiology. 1992;68:1209–1215. doi: 10.1152/jn.1992.68.4.1209. [DOI] [PubMed] [Google Scholar]
- Nobile M, Carbone E, Lux HD, Zucker H. Temperature sensitivity of Ca currents in chick sensory neurones. Pflügers Archiv. 1990;415:658–663. doi: 10.1007/BF02584002. [DOI] [PubMed] [Google Scholar]
- Somero GN. Temperature and proteins: little things can mean a lot. News in Physiological Science. 1996;11:72–77. [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Treede RD, Meyer RA, Raja SN, Campbell JN. Evidence for two different heat transduction mechanisms in nociceptive primary afferents innervating monkey skin. The Journal of Physiology. 1995;483:747–758. doi: 10.1113/jphysiol.1995.sp020619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lunteren E, Elmslie KS, Jones SW. Effects of temperature on calcium current of bullfrog sympathetic neurons. The Journal of Physiology. 1993;466:81–93. [PMC free article] [PubMed] [Google Scholar]
- Vlachová V, Vitásková Z, Kabát M, Vyklický L. Heat-induced membrane currents in small sensory neurones. European Journal of Neuroscience. 1998;10:80. [Google Scholar]
- Vlachová V, Vyklický L. Capsaicin-induced membrane currents in cultured sensory neurons of the rat. Physiological Research. 1993;42:301–311. [PubMed] [Google Scholar]
- Vyklický L, Vlachová V, Vitásková Z, Kabát M. The effects of algogens on membrane currents induced by noxious heat in sensory neurones from newborn rats. The Journal of Physiology. 1998;511.P:125. doi: 10.1111/j.1469-7793.1999.0181z.x. P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Peripheral and central nervous mechanism of nociception, pain, and pain therapy: Facts and hypotheses. In: Bonica JJ, Liebeskind JC, Albe-Fessard DB, editors. Advances in Pain Research and Therapy 3. New York: Raven Press; 1979. pp. 3–32. [Google Scholar]


