Abstract
The process by which stretch of the external muscle of the intestine leads to excitation of myenteric neurons was investigated by intracellular recording from neurons in isolated longitudinal muscle-myenteric plexus preparations from the guinea-pig.
Intestinal muscle that was stretched by 40% beyond its resting size in either the longitudinal or circular direction contracted irregularly. Both multipolar, Dogiel type II, neurons and uniaxonal neurons generated action potentials in stretched tissue. Action potentials persisted when the membrane potential was hyperpolarized by passing current through the recording electrode for 10 of 14 Dogiel type II neurons and 1 of 18 uniaxonal neurons, indicating that the action potentials originated in the processes of these neurons. For the remaining four Dogiel type II and 17 uniaxonal neurons, the action potentials were abolished, suggesting that they were the result of synaptic activation of the cell bodies.
Neurons did not fire action potentials when the muscle was paralysed by nicardipine (3 μm), even when the preparations were simultaneously stretched by 50% beyond resting length in longitudinal and circular directions. Spontaneous action potentials were not recorded in unstretched (slack) tissue, but when the L-type calcium channel agonist (-)-Bay K 8644 (1 μm) was added, the muscle contracted and action potentials were observed in Dogiel type II neurons and uniaxonal neurons.
The proteolytic enzyme dispase (1 mg ml−1) added to preparations that were stretched 40% beyond slack width caused the myenteric plexus to lift away from the muscle, but did not prevent muscle contraction. In the presence of dispase, the neurons ceased firing action potentials spontaneously, although action potentials could still be evoked by intracellular current pulses. After the action of dispase, (-)-Bay K 8644 (1 μm) contracted the muscle but did not cause neurons to fire action potentials.
Gadolinium ions (1 μm), which block some stretch activated ion channels, stopped muscle contraction and prevented action potential firing in tissue stretched by 40%. However, when (-)-Bay K 8644 (1 μm) was added in the presence of gadolinium, the muscle again contracted and action potentials were recorded from myenteric neurons.
Stretching the tissue 40% beyond its slack width caused action potential firing in preparations that had been extrinsically denervated and in which time had been allowed for the cut axons to degenerate.
The present results lead to the following hypotheses. The neural response to stretching depends on the opening of stretch activated channels in the muscle, muscle contraction in response to this opening, and mechanical communication from the contracting muscle to myenteric neurons. Distortion of sensitive sites in the processes of the neurons opens channels to initiate action potentials that are propagated to the soma, where they are recorded. Neurons are also excited indirectly by slow synaptic transmission from neurons that respond directly to distortion.
Distension of the small intestine evokes patterned motor activity, including peristaltic movements, via activation of intrinsic neural circuits (see Furness & Costa, 1987; Hasler, 1994). Thus, the enteric nervous system must contain neurons that respond to distension or other stimuli that elicit the reflexes; these neurons are referred to as intrinsic primary afferent neurons (Kirchgessner & Gershon, 1988; Furness et al. 1998). Identification of intrinsic primary afferent neurons that respond to stretch was recently obtained by intracellular recording from myenteric nerve cell bodies in contracting segments of the guinea-pig ileum (Kunze et al. 1998). These were neurons with Dogiel type II morphology that fired action potentials when the tissue was stretched beyond its resting length. Dogiel type II neurons have large cell bodies and multiple processes which end around other myenteric neurons, and also project to the mucosa of the small intestine (Furness et al. 1998). The action potentials caused by tissue stretch arose in the cell processes and were conducted to the cell body. When the muscle was relaxed by adding isoprenaline (1 μm) or nicardipine (3 μm) to the superfusate, neurons with ongoing action potential firing in stretched tissue fell silent (Kunze et al. 1998). Thus, contractile activity of the muscle appears to be required for the neurons to be activated by stretch. The muscle cells themselves are excited by stretch (Bülbring, 1955) via stretch activated channels (SACs) in their membranes (Kirber et al. 1988). It was therefore proposed that the initial transduction of the stretch stimulus occurred in the muscle, from where it was communicated to the primary afferent neurons (Kunze et al. 1998).
The experiments reported here were designed to test the hypothesis that myenteric neurons respond to muscle tension and to determine how the change in the contractile state of the tissue is communicated to the myenteric neurons. A range of protocols was used to dissociate changes in tissue length from changes in muscle tension while intracellular records were taken from myenteric neurons. To test whether stretch related neuronal activity was initiated by axon reflexes in fibres of extrinsic origin (see for example Wood, 1994) experiments were performed using tissue from ileum that had been chronically denervated (Furness et al. 1995).
METHODS
Guinea-pigs (160-250 g) were killed by being stunned and having the carotid arteries and spinal cords severed. Segments of distal ileum, 2-3 cm long, were removed. The segments were placed in a recording dish and covered with physiological saline (composition, mM: NaCl, 118; KCl, 4.8; NaHCO3, 25; NaH2PO4, 1.0; MgSO4, 1.2; glucose, 11.1; CaCl2, 2.5; equilibrated with 95% O2-5% CO2) which was kept at room temperature (16-20°C) and frequently replaced during dissection. Segments of ileum were opened and pinned mucosal side up and the myenteric plexus was exposed by dissecting away the mucosa, the submucosa and the majority of the circular muscle. One edge of the tissue was pinned to a fixed block of silicone elastomer (Sylgard; Dow Corning) set into the base of a small tissue bath and the other edge was attached to a smaller block that could be moved laterally to stretch the tissue (Kunze et al. 1998). The recording dish was then placed on the stage of an inverted microscope and continuously superfused (4 ml min−1) with physiological saline solution that had been preheated to yield a bath temperature of 35-37°C.
Myenteric neurons lying over the fixed Sylgard base were impaled with flexibly mounted glass microelectrodes (Kunze, 1998), filled with 2% biocytin (Sigma-Aldrich) in 1 M KCl. Signals were stored on computer and analysed off-line. After recording was complete, neurons were injected with biocytin by passing negative current. The tissue was then fixed overnight in 2% formaldehyde plus 0.2% picric acid in 0.1 M sodium phosphate buffer, pH 7.0, cleared in three changes of dimethylsulphoxide, and placed in phosphate buffered saline. The cleared tissue was reacted with streptavidin coupled to the fluorescent dye Texas Red to reveal the biocytin, and processed for the immunohistochemical demonstration of calbindin immunoreactivity, using rabbit anti-calbindin antiserum, DEMLR1, at a dilution of 1:1600. After viewing the tissue to determine whether intracellularly labelled neurons were immunoreactive, the streptavidin was converted to a permanent deposit using goat anti-streptavidin antiserum coupled to biotin (Vector Laboratories, Burlingame, CA, USA), diluted 1:50, incubated with the tissue for 16-24 h at room temperature. The biotin was in turn localized using an avidin-biotin-horseradish peroxidase kit (Vectastain, Vector Laboratories). The horseradish peroxidase was reacted with diaminobenzidine and H2O2 to yield a permanent deposit.
Neurons were classified as rapidly adapting (RA) or slowly adapting (SA), according to their responses to the intracellular injection of depolarizing current through the recording electrode (Kunze et al. 1997). Neurons were RA if a current injection lasting 500 ms evoked one action potential at the beginning of the depolarization, or a series of action potentials that ceased before 250 ms. Neurons were SA if the current injection evoked a series of action potentials that lasted more than 250 ms. In myenteric neurons that are RA, increasing the intensity of current never causes the action potential train to extend beyond 250 ms (Kunze et al. 1997). Extracellular stimuli were delivered to internodal nerve strands located circumferential to the recording site via sharpened tungsten wires (10-50 μm in diameter), insulated except at the tip. Stimulus pulses were of 0.1 ms duration and 0.1-0.5 mA intensity.
Extrinsic denervation
Guinea-pigs were operated on under general anaesthesia and allowed to recover for 7-9 days. Anaesthesia was induced by a subcutaneous injection of sodium pentobarbitone (Boehringer-Ingelheim, NSW, Australia), 15 mg kg−1, followed 15-30 min later by an intramuscular injection of a mixture of fentanyl, 0.1 mg kg−1, and fluanisone, 5.0 mg kg−1 (Janssen-Cilag, Sydney, Australia). Animals were opened through a mid-line abdominal incision and part of the ileum was exteriorized. The vessels supplying a 6-10 cm length of ileum were stripped of paravascular nerves and the vessels at this site were swabbed with 80% phenol in distilled water. A loose ligature around the mesenteric vessels was used to mark the site of operation. After the operation, the ileum was returned to the abdominal cavity and the wound closed by sutures. Each animal received a 20 mg intramuscular injection of terramycin (Pfizer Agricare, Sydney, Australia). At the time the tissue was taken for electrophysiology, the region of operation was identified by the marking ligature and removed. This area was also identifiable by dilatation of its blood vessels. The region, generally about 6-10 cm in length, was removed. The two ends, about 1-2 cm long, were fixed for immunohistochemistry and the mid-part was taken for electrophysiological analysis. Each end segment was pinned tautly to a balsa wood sheet and fixed overnight by immersion in 2% formaldehyde plus 0.2% picric acid in 0.1 M sodium phosphate buffer, pH 7.0, and processed as described above. The primary antibody was a mouse monoclonal anti-tyrosine hydroxylase antibody (Boehringer-Mannheim).
Drugs used
For some experiments, the smooth muscle was relaxed using either the L-type Ca2+ channel blocker nicardipine (Sigma-Aldrich; 3 μm) or isoprenaline (1 μm). Tissue was contracted using the L-type channel opener (-)-Bay K 8644 (1 μm) (Research Biochemicals International). To prevent its photo-degradation, experiments using Bay K were performed with a dull yellow (orthochromatic) photography light as room illumination. A blocker of stretch-activated channels, gadolinium, as Gd(NO3)3, was added to the superfusate in some experiments. To prevent Gd3+ precipitation, Hepes buffer (10 mM, pH = 7.4) was substituted for NaHCO3, and phosphate (Ward & White, 1994) was omitted from the physiological saline; the NaCl concentration was altered so that the total osmolality of the superfusate was not changed. In other experiments, the tissue was exposed for up to about 20 min to physiological saline (37°C) to which 1 mg ml−1 neutral dispase (Boehringer-Mannheim) had been added.
Statistics
Sample estimates of membrane potentials, input resistances (Rin) and firing frequencies are given as means ±s.d. (n); the numbers of action potentials discharged during test depolarizing pulses are given as ranges and medians (n). Comparisons of values from two independent samples were made using the Mann-Whitney U test, and comparisons between two proportions were made using Fisher's exact probability test (2 tailed). Two-tailed probabilities, denoted by P, were used for the null hypotheses of no difference. Two types of comparison have been made, paired and unpaired. In the case of paired comparisons, each neuron was recorded from in two conditions, for example in the presence and absence of a drug. For unpaired comparisons, different neurons were recorded from in the two conditions.
RESULTS
Tissue movements
The movements of the muscle were observed through the inverted microscope on which the recording dish was mounted. When the tissue was pinned out in physiological saline at 35-37°C without any tension being applied (referred to as slack width) a gentle swaying motion of low amplitude was observed. This consisted of irregular, localized contractions of muscle in regions about the size of individual ganglia; the effect was to pull ganglia in one or another direction without producing synchronized contractions that involved all of a field of view (which was 720 μm in diameter). The amplitudes of contractions and the region of synchronization became greater as the degree of stretch was increased. For preparations that were stretched to 40% beyond slack width, powerful rhythmic contractions occurred. Longitudinal contractions included areas that were several millimetres wide and usually involved the whole width of the tissue (∼7-15 mm). Periods of contraction ranged from about 5 to 60 s and were interspersed with periods of relaxation. Individual ganglia could move up to 1.4 mm, and the contractions continued until the applied stretch was removed. Continuously stretched preparations contracted for up to 10 h. When the tissue was relaxed by paralysing the muscle by adding isoprenaline (1 μm) or nicardipine (3 μm) to the superfusate, all movement ceased, even in tissue stretched 40% beyond its resting width.
Electrophysiological classification of neurons
The electrophysiological properties of neurons altered with the state of the tissue, which was varied from fully relaxed and immobile to stretched and contracting. In the absence of all muscle movement, neurons that were later identified as having Dogiel type II morphology all exhibited the after-hyperpolarization (AH) electrophysiological profile as previously described (Nishi & North, 1973; Hirst et al. 1974; Bornstein et al. 1994). There was a hump on the descending phase of the action potential, which could be revealed as a double inflection in the voltage-time differential (Fig. 1) (Wood, 1994; Clerc et al. 1998). Single action potentials were followed by both early after-hyperpolarizing potentials (AHPs) and delayed, slow AHPs that lasted more than 2 s. Fast EPSPs are rare in Dogiel type II neurons of the guinea-pig ileum (Bornstein et al. 1994), and were not observed in the present work (none of 25 neurons that were tested by stimulating internodal strands responded with fast EPSPs). In contrast, uniaxonal neurons all had S neuron electrophysiological characteristics: that is, they lacked a delayed, slow AHP, had prominent fast EPSPs in response to stimulation of internodal strands, and lacked a prominent hump of the descending phase of the action potential. A minority of S neurons, identified as filamentous descending interneurons, do have a small hump on the action potential which is, however, revealed as a single rather than double inflection in the differential of voltage-time traces (Clerc et al. 1998); in other respects they have the electrophysiological characteristics of S neurons.
Figure 1. Pattern of action potential discharge of a Dogiel type II neuron in tissue stretched by 40%.
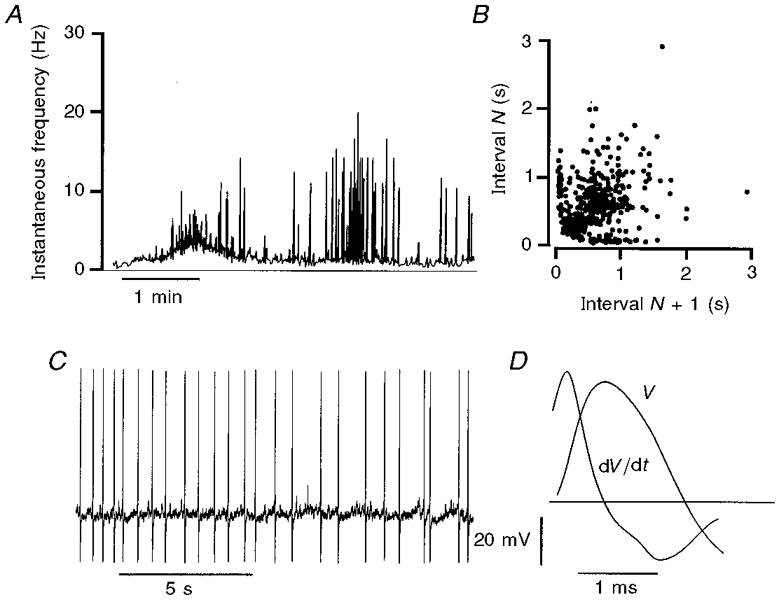
A, plot of the instantaneous frequency of firing (Hz), calculated as the reciprocal of the inter-spike interval, over a period of 5 min. The neuron fired action potentials consistently, but at varying frequencies, throughout the recording period. B, interval return map for action potential firing in this neuron. Each interval (N) between action potentials is plotted against the succeeding interval (N+ 1). The dispersion of the plot indicates that the neuron fired without obvious pattern for much of the time, while the alignment of points along the x- and y-axes indicates that there were interspersed bursts of firing. C, intracellular record of a period of action potential firing. Soma action potentials were followed by early AHPs, but not by late, slow AHPs. D, the shape of the action potential shown by a voltage trace (V) and its first time differential (dV/dt). The horizontal line marks zero for the time differential. The action potential has a hump on the descending phase which is seen as a double inflection in the differentiated record. The hump and double inflection are identifiers of Dogiel type II neurons.
In tissue that was contracting in response to 40% stretch, the delayed AHP was absent or of reduced duration (< 1 s) for all 22 morphologically identified Dogiel type II neurons. Nevertheless, the hump on the falling phase of the action potential remained, even when the delayed AHP was entirely absent. Thus a prominent hump, which is the calcium component of the action potential, is a robust electrophysiological identifier of Dogiel type II neurons whatever their state of excitability (Schutte et al. 1995; Clerc et al. 1998; Kunze et al. 1998). Because of this, neurons that were not successfully filled and processed for analysis of shape, but whose action potentials had pronounced humps, have been included with the Dogiel type II neurons. In 40% stretched tissue, three neurons were deduced to be Dogiel type II by electrophysiology alone.
Dogiel type II neurons in tissue stretched by 40%
The majority (20/25) of Dogiel type II neurons discharged spontaneous action potentials in tissue that was stretched by 40% (Fig. 1). Firing occurred at a steady rate in most neurons, but in some neurons the discharge consisted of bursts of firing separated by periods of low or absent activity (Figs 1 and 5). The patterns of firing could be represented for some neurons using Poincaré interval return maps (Dekhuijzen & Bagust, 1996) (Figs 1B, 7Ac, and 8B and D). For neurons from which a sufficiently large, uninterrupted sample was taken, firing patterns ranged from almost constant rate (Fig. 6Cb) to most action potentials occurring in bursts (Figs 5Bc and 8C). Other neurons fired in bursts but also had periods in which firing became irregular and others fired with no discernible pattern (Fig. 7Aa and Ac). Discharge which appeared steady when viewed over a short time (seconds) waxed and waned when examined over longer periods (Fig. 1), but did not vary in average frequency if the degree of stretch was not changed (Kunze et al. 1998). Neurons included in this cohort were held without apparent damage to the neuron for 3-40 min (median, 15 min), and they were impaled up to 2 h after the stretch was applied to the tissue. During stretch, the 25 Dogiel type II neurons had resting membrane potentials of -51 ± 8 mV and input resistances of 286 ± 85 MΩ. In slack tissue, where no stretch was applied (see below), membrane potential was -63 ± 7.6 mV (n = 8) and Rin was 219 ± 101 MΩ (n = 3). The action potentials in stretched tissue were usually of full amplitude, 40-70 mV (Fig. 1), but in some cases, the spontaneous potential that was recorded in the soma had an amplitude of 20 mV or less and was not followed by an AHP (Fig. 2). Such potentials are electrotonically conducted from the processes when the action potential fails to trigger a regenerative response in the part of the process adjacent to the soma or in the soma itself (Wood, 1989). We have used the term ‘process potential’ to describe these electrotonic potentials. They had fast rise times and decayed more rapidly than fast EPSPs (Fig. 2) and they were not increased in amplitude by hyperpolarizing current. Mean action potential discharge frequency (including both soma spikes and process potentials and taken as the reciprocal of the mean interspike intervals of individual neurons) ranged from 0.3 to 26 Hz (median, 5.2 Hz). A sample of these neurons was tested for the accommodation of their action potential discharge in response to intracellular current; 5 of 8 were slowly accommodating (SA) and the other three were rapidly accommodating (RA), during the application of stretch to the tissue. Rapidly accommodating neurons are those that fire briefly in response to a 500 ms depolarizing pulse, whereas SA neurons fire for more than 250 ms; see Methods.
Figure 5. The effects of the L-type calcium channel stimulant (-)-Bay K 8644 on action potential firing of neurons in unstretched tissue.
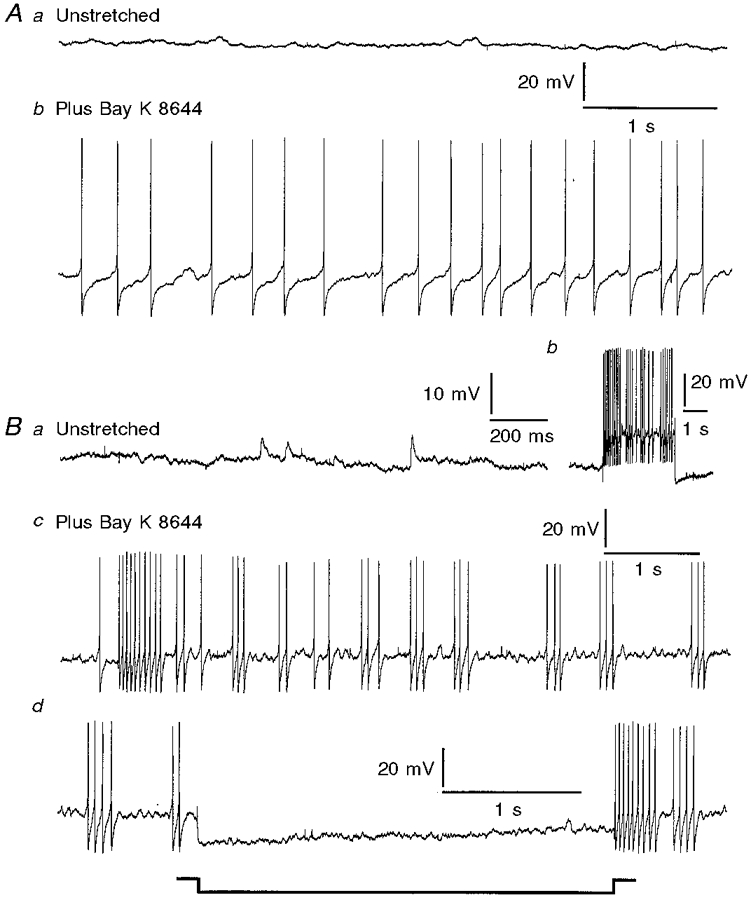
Aa, record from a Dogiel type II neuron in tissue that was pinned without stretch and not exposed to drug; no action potentials were recorded. Ab, record from the same neuron after the addition of (-)-Bay K 8644 (1 μm), showing maintained action potential firing. Ba, membrane activity in a Dogiel type I neuron in tissue that was unstretched. Small depolarizing potentials with time courses similar to fast EPSPs were observed, but the neuron did not fire action potentials. Bb, response of the neuron to a depolarizing current pulse, indicating that it was excitable although it did not fire action potentials spontaneously prior to the addition of (-)-Bay K 8644. Bc, action potential firing after the addition of (-)-Bay K 8644. Bd, action potential discharge in the presence of (-)-Bay K 8644 was abolished when the membrane was hyperpolarized in response to current passed through the recording electrode, during the period that is marked.
Figure 7. Effects of gadolinium, Bay K 8644 and dispase on action potential discharge of Dogiel type II neurons.
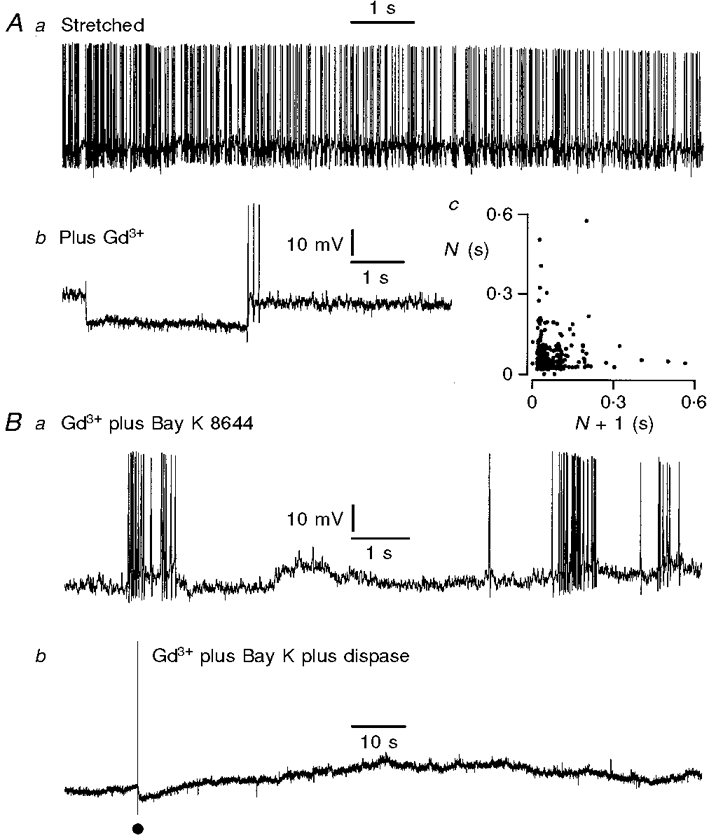
Aa, action potential firing while the tissue was stretched 40% beyond slack width, but not exposed to drug; membrane potential -55 mV. Ab, membrane potential record taken from the same neuron 5 min after exposure of the tissue to gadolinium (1 μm); membrane potential -58 mV. Although no spontaneous action potentials were recorded in this period, the cell was sufficiently excitable to respond with anode break action potentials following a hyperpolarizing current pulse. Ac, interval return map for this neuron, before the addition of Gd3+. The map shows that the neuron exhibited a mixture of bursting and irregular action potential discharge. Ba, spontaneous action potential discharge in a second neuron after Bay K 8644 (1 μm) was added in the presence of Gd3+. Despite the presence of Gd3+, contraction evoked by Bay K 8644 was accompanied by action potentials in Dogiel type II neurons. Bb, record from the same neuron after exposure of the tissue to dispase (1 mg ml−1). This abolished the action potentials in the neuron, although the muscle continued to contract. An intracellular current pulse, at the dot, evoked a full action potential, indicating that the cell had not lost its ability to be excited.
Figure 8. Stretch evoked activity of neurons in extrinsically denervated ileum.
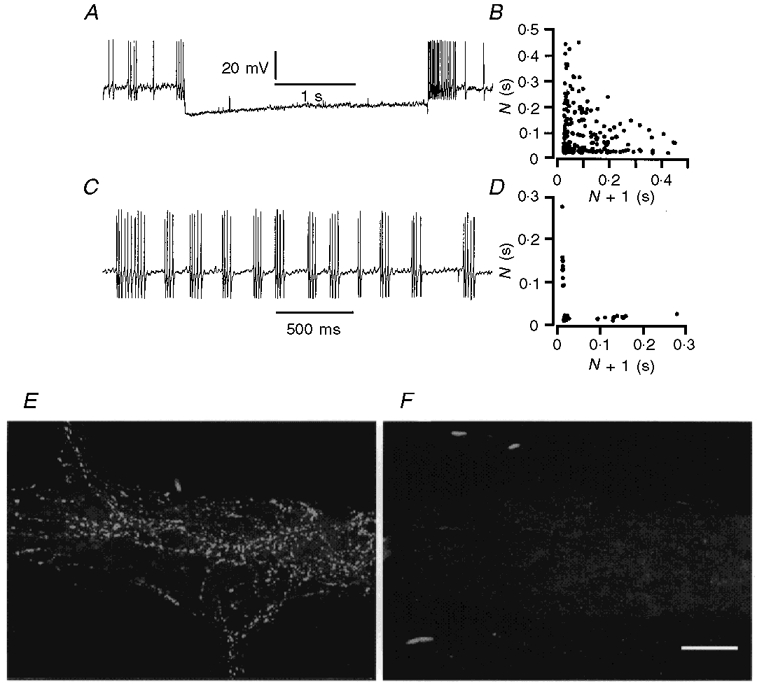
A, action potential discharge in a Dogiel type I neuron in tissue stretched by 40%. Action potentials ceased when the neuron was hyperpolarized by current passed through the recording electrode. B, interval return map for action potential firing in this neuron. The distribution of points along the x- and y-axes and in the central area indicates that there were bursts of firing in this neuron, superimposed on irregular activity. C, action potentials in a Dogiel type II neuron. D, interval return map illustrating that the neuron from which the record in C was obtained fired in bursts. E, tyrosine hydroxylase immunoreactivity of sympathetic nerve terminals in a myenteric ganglion from a segment of intestine remote from the area of denervation. F, myenteric ganglion from a denervated region that was tested for tyrosine hydroxylase immunoreactivity. All reactive nerve fibres degenerated following denervation. Scale bar, 50 μm.
Figure 6. The effect of enzymatic weakening of connective tissue with dispase on neuronal activity.
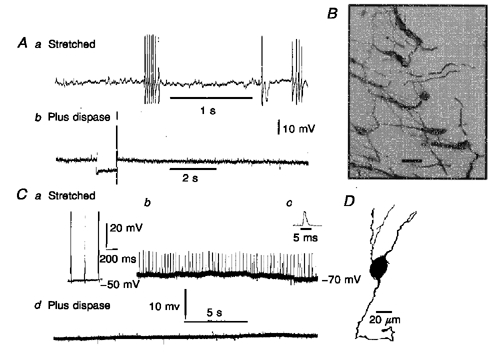
Aa, spontaneous action potential discharge in a neuron in a preparation that was stretched by 40%. Ab, membrane potential recorded in the same neuron after 15 min exposure of the tissue to dispase (1 mg ml−1). An anode break action potential follows a hyperpolarizing current pulse, indicating that the neuron was still excitable, although no spontaneous action potential discharge was recorded after this time of exposure to dispase. B, the appearance of a region of myenteric plexus near the edge of the preparation after exposure to dispase. The plexus lifted away from the preparation and no longer had its rectilinear form. Scale bar, 100 μm. Ca, spontaneous action potentials in a Dogiel type II neuron in tissue stretched by 40%. Cb, discharge of process potentials in the same neuron while the membrane potential was hyperpolarized, by passing current through the recording electrode. This neuron showed very regular firing. Cc, a process potential on an expanded time base. Cd, membrane potential record from the neuron 10 min after the application of dispase to the tissue. D, camera lucida drawing of the Dogiel type II neuron from which records Ca-d were taken.
Figure 2. Comparison of a spontaneous process potential and action potential in a Dogiel type II neuron and fast excitatory postsynaptic potentials in an S neuron.
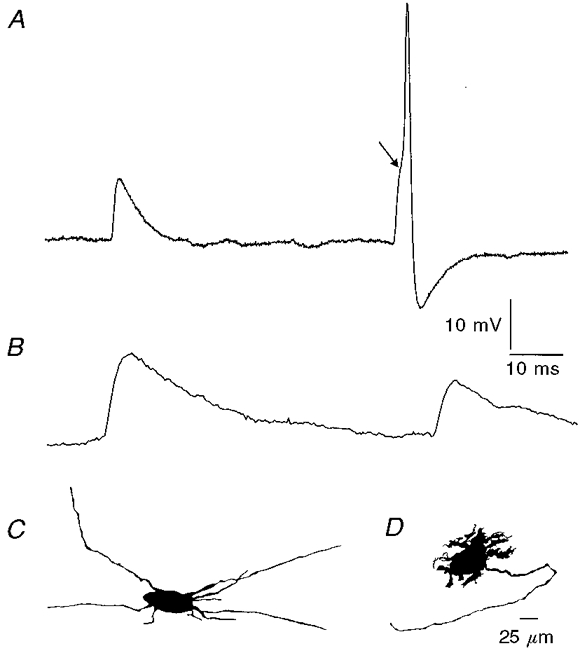
These records were from neurons in tissue stretched by 40%. A, record from a Dogiel type II neuron (camera lucida drawing in C). A process potential, which occurs when action potential regeneration fails in the nerve cell process and an electrotonic potential is conducted to the soma, and a full action potential are shown. Note the inflection on the rising phase of the action potential (arrow), indicating that it arose from a process potential. Process potentials have half-decay times of less than about 5 ms. B, spontaneous fast EPSPs recorded from an S neuron (camera lucida drawing in D). Fast EPSPs have slower rise and decay times than process potentials. C, camera lucida drawing of the Dogiel type II neuron from which record A was taken. D, camera lucida drawing of the nerve cell from which record B was taken. This is an ascending interneuron with Dogiel type I morphology.
For 14 of the 20 spontaneously active neurons, hyperpolarizing current was injected through the recording electrode to determine whether the action potentials were generated at the soma (Kunze et al. 1998). Spontaneous firing persisted in 10 of these neurons; for the remaining four, action potentials were abolished by the hyperpolarization. Although there was little reduction in spike frequency in 10 neurons, the spikes were changed from full action potentials to process potentials. Conversion of action potentials to process potentials during hyperpolarization indicates that action potentials were conducted towards the cell body from its processes (Wood, 1989; Kunze et al. 1998).
Uniaxonal neurons in tissue stretched by 40%
Spontaneous firing occurred in 16 of 32 uniaxonal neurons at 40% stretch while muscle was contracting. Three patterns of discharge were discerned: some neurons fired continuously (n = 3) with only small variations in frequency, others fired in bursts (n = 5, e.g. Figs 5Bc, and 8A and B), some were irregular showing neither obvious bursting nor steady firing (n = 4, e.g. Fig. 3) and the remainder fired too slowly for a pattern to be discernible. The pattern of bursting behaviour in regularly bursting neurons was similar in Dogiel type II and uniaxonal neurons, and was similar to the patterns described previously in the guinea-pig small intestine (Wood, 1973; Wood & Mayer, 1978). In three neurons (2 uniaxonal and 1 Dogiel type II), interburst intervals were 1.1 ± 0.3, 0.83 ± 0.06 and 1.8 ± 0.7 s and the frequencies of firing during bursts were 21, 8 and 59 Hz. Spontaneous firing was quenched by hyperpolarization in all but 1 of 18 uniaxonal neurons tested (Figs 3B and 8A), although fast EPSPs, in neurons where they occurred, were of greater amplitude during hyperpolarization. During stretch, resting membrane potentials were -48 ± 7 mV (n = 32) and input resistances were 313 ± 118 MΩ (n = 27), compared with -45.5 ± 6.3 mV (n = 9) and 159 ± 75 MΩ (n = 4) in unstretched tissue.
Figure 3. Spontaneous action potential firing in a Dogiel type I neuron in tissue stretched by 40%.
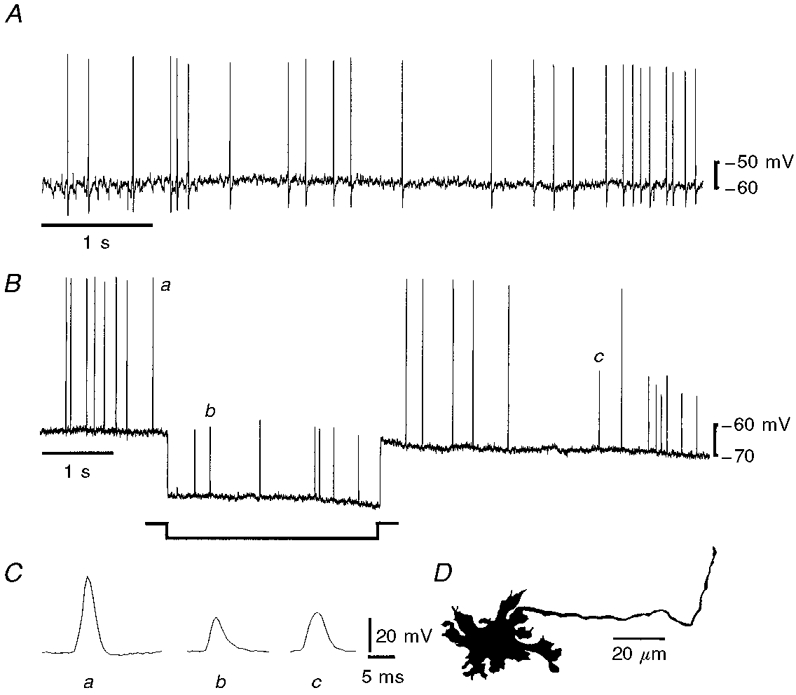
A, irregular firing of action potentials during tissue stretch. B, hyperpolarization of the membrane, by passing current through the recording electrode during the period marked on the record, reduced action potentials to process potentials, but did not change the firing rate in the neuron. C, expanded records of an action potential (a) and two process potentials, one recorded during the hyperpolarization (b) and one following the hyperpolarization (c) taken from the trace in B, as indicated by the labelling. It can be seen that the action and process potentials have the same time course. There was no hump on the action potential. D, camera lucida drawing of the neuron from which these records were taken. The neuron had type I morphology and an axon that projected anally.
The accommodation of action potential firing in response to intracellular depolarizing current was evaluated in stretched tissue for 23 of the uniaxonal neurons; 19 were slowly accommodating (SA), that is, they fired for ≥250 ms from the beginning of a depolarizing current passed through the recording electrode (see Methods). The maximum number of action potentials fired ranged from five to 48 (median, 22). Four neurons were rapidly accommodating (RA) when they were artificially depolarized; the maximum number of action potentials fired ranged from one to 11 (median, 5) and the action potential trains lasted less than 250 ms.
Maximum stretch with muscle paralysis
To determine whether extreme distension can cause myenteric neurons to fire action potentials when the muscle is not actively contracting, nine experiments were done in which nicardipine (3 μm) was added to the superfusing saline. The nicardipine was added after measuring the slack circumference and length, and the tissue was then stretched to 50-55% beyond its slack width in both dimensions by re-pinning the tissue. In the absence of mucosa, submucosa and circular muscle, this degree of stretch is almost to the point of tearing the tissue. When maximally stretched preparations exposed to nicardipine were viewed through the inverted microscope, no movement could be detected. Notwithstanding the extreme stretch that was applied during continuous recording periods up to 3 h, each of 14 neurons (11 with Dogiel type II morphology, 3 uniaxonal neurons) was silent, spiking only after internodal strand stimulation or depolarization through the recording electrode. This contrasts with the 36/57 spontaneously active neurons detected in the unparalysed, 40% stretched, contracting preparations (see above). Resting membrane potentials and input resistances of the neurons were -59 ± 6 mV (n = 14) and 180 ± 145 MΩ (n = 12). The input resistances were significantly smaller than those of neurons in contracting tissue stretched by 40% (P < 0.05).
Firing accommodation was tested in 11 of the 14 neurons; 8/8 Dogiel type II and 2/3 uniaxonal neurons were in the RA state. Maximum numbers of action potentials discharged ranged from 1 to 40 (median, 2). Pooling the results for both types of neuron, 10/11 were RA when tissue was stretched and paralysed, compared with 7/31 in stretched but unparalysed tissue.
Rapid stretch with muscle paralysis
In 26 experiments, the muscle was paralysed with nicardipine (3 μm) and neurons were impaled. The tissue was then rapidly stretched (0.7 to 1 mm s−1) to 20-40% beyond slack width. In most cases the electrode was almost immediately dislodged from the neuron, but in seven recordings it was maintained long enough for responses to be seen. Action potentials were elicited in four Dogiel type II neurons and three uniaxonal neurons (Fig. 4). In each experiment in which the impalement was maintained, the stretch was removed after about 30 s. In one case the impalement was retained after removal of stretch and the neuron responded to a second stretch stimulus.
Figure 4. Action potential discharge in response to rapid stretch in the presence of nicardipine.
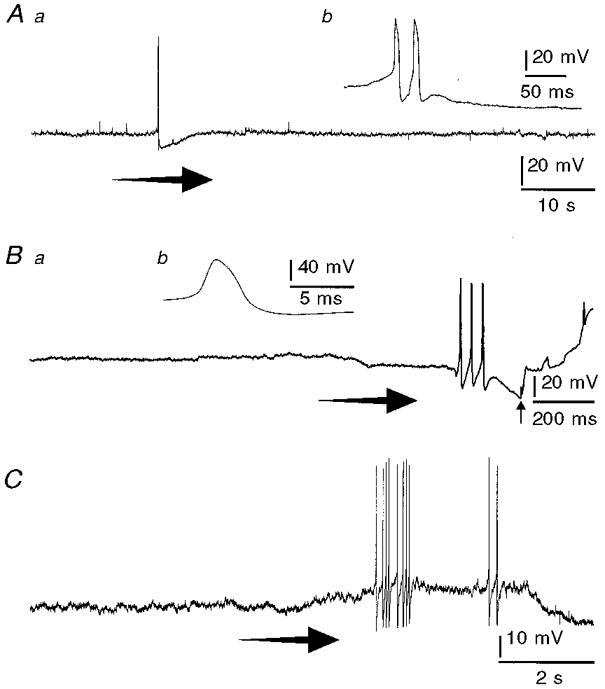
Records from 3 neurons, 2 of which were Dogiel type II (A and B) and one of which was uniaxonal (C), in tissue that was superfused with nicardipine (3 μm). The tissue was initially unstretched and the neurons did not display action potentials. Rapid stretch applied at the arrows caused action potentials to discharge. Aa, a Dogiel type II neuron that responded with a pair of action potentials (expanded record in Ab shows the action potential hump), followed by an early and a late after-hyperpolarizing potential. Ba, a Dogiel type II neuron that responded with 3 action potentials (expanded record in Bb shows the action potential hump). Soon after the action potentials, the impalement was lost (arrow). C, action potentials and slight membrane potential depolarization in response to stretch recorded from a uniaxonal neuron.
Muscle contraction in slack tissue
Neurons were impaled in tissue stretched minimally (about 5% beyond slack width) to prevent buckling and allow impalement. Soon after impalement the tissue was returned to slack by removing all applied stretch. For succeeding impalements, the tissue was again slightly stretched and the above steps repeated. When slack, the tissue swayed gently but did not contract strongly in any direction. The muscle was caused to contract by superfusing the tissue with saline to which the L-type calcium channel opener (-)-Bay K 8644 (Bay K; 1 μm) had been added, but was prevented from overall shortening by being pinned. Vigorous muscle contractions were observed within 2-5 min and these persisted for more than 30 min after washout with normal saline. All neurons that were tested by the addition of Bay K were silent for at least 5 min before the drug was added. Introduction of Bay K to the organ bath caused the neurons to discharge action potentials (Fig. 5). The neuron in Fig. 5 fired action potentials for about 1 min before the impalement was lost. The firing rate was 11.0 ± 2.1 Hz (621 action potentials). Other neurons also fired throughout the period of exposure to Bay K. The violence of contractions induced by Bay K often caused loss of impalement within 30 s of their onset. Switching the superfusate to a saline solution containing 3 μm nicardipine and no Bay K stopped the contractions allowing further impalements of neurons to be made. Switching the superfusate back to one containing Bay K restarted the contractions.
Paired and unpaired records were made (see Methods) before and in the presence of Bay K. In unpaired records from 25 neurons (12 Dogiel type II, 13 uniaxonal), 2/10 (both uniaxonal) showed action potential discharge in slack, minimally contracting preparations, but in contracting tissue, 14/15 spiked spontaneously. Paired recordings were made in 10 neurons (7 Dogiel type II and 3 uniaxonal). All 10 neurons were silent before Bay K application, and 9/10 fired spontaneous action potentials as muscle contractions began in response to Bay K. The unresponsive neuron had Dogiel type II morphology. For three preparations, nicardipine was added to the bath after the tissue had been contracted by Bay K; within about 10 min the tissue stopped contracting and spontaneous action potentials were reduced in frequency and then abolished.
Nine of the 14 neurons (unpaired records) that generated full action potentials at the soma in the presence of Bay K were Dogiel type II neurons, and spontaneous firing of six of these was examined after membrane hyperpolarization through the recording electrode. Firing in four persisted in the form of process potentials although the membrane was hyperpolarized to more than 20 mV negative to resting potential, and was abolished in the other two neurons. Spontaneous action potentials in the five active uniaxonal neurons was tested by hyperpolarization, which abolished the spiking in each case (Fig. 5). Of the two uniaxonal neurons that were spontaneously active without Bay K, spiking in one was quenched by hyperpolarization, but the other was not tested.
For Dogiel type II neurons before Bay K application, resting membrane potential was -63 ± 7.6 mV (n = 8) and Rin was 219 ± 101 MΩ (n = 3), and in Bay K activated, contracting tissue these values were -58 ± 8.9 mV (n = 9) and 313 ± 61 MΩ (n = 6). For uniaxonal neurons the corresponding values were: -45.5 ± 6.3 mV (n = 9) and 159 ± 75 MΩ (n = 4) before Bay K application and -38 ± 9.7 mV (n = 9) and 493 ± 82 MΩ (n = 4) in its presence. Taking both classes of neurons together, input resistances were greater in Bay K contracted tissue than in resting tissue (P = 0.002). Membrane potentials, however, were not significantly different between the two treatments.
Neurons were examined for action potential accommodation by injection of depolarizing current. In slack tissue 13/15 of neurons (5 Dogiel type II, 10 uniaxonal) were in the RA state; this contrasts to the case for contracting tissue, in which 0/6 neurons (4 Dogiel type II, 2 uniaxonal) were RA (P < 0.001).
Disconnecting the myenteric plexus from the muscle
Active muscle contraction appears to cause spontaneous action potential firing in myenteric neurons; however, the manner by which the stretch sensitive myenteric neurons detect the contraction is unclear (see Introduction). Because the most direct connection between the muscle and the myenteric plexus is by connective tissue anchoring the one to the other, we attempted to weaken and/or sever this connection with the proteolytic enzyme dispase (1 mg ml−1) and to determine the effect on spontaneous discharge in contracting segments. The effectiveness of enzyme treatment was verified on histological examination under fluorescein isothiocyanate (FITC) fluorescence after staining the myenteric plexus using antiserum against the calcium binding protein calbindin. About 15 min of exposure to the enzyme resulted in a separation between the longitudinal muscle layer and the network of myenteric ganglia. The myenteric plexus layer appeared to peel off the muscle layer with separation being most evident near the edges of the tissue (Fig. 6).
Unparalysed segments were stretched 40% and myenteric neurons impaled. When a stable, spontaneously firing neuron was impaled, the tissue was exposed for up to 30 min to saline to which 1 mg ml−1 neutral dispase had been added. Eight spontaneously spiking neurons in eight separate preparations were examined; before the dispase was added, the spontaneous activity was quenched by membrane hyperpolarization for 1/2 Dogiel type II neurons and 2/2 uniaxonal neurons. For all eight preparations, spontaneous action potential discharge in the impaled neurons (5 Dogiel type II, 3 uniaxonal) was abolished within 6-20 min of exposure to dispase; nonetheless the neurons could be made to fire by injection of current (Fig. 6). Although neurons that were previously firing action potentials were now silent, the rhythmic muscle contractions, as observed via the inverted microscope, were not changed even after 30 min exposure to dispase. Before adding dispase, the resting membrane potentials and Rin for Dogiel type II and uniaxonal neurons were -49 ± 8 mV (n = 8) and 323 ± 63 MΩ (n = 5). After treatment they were -55 ± 7 mV and 242 ± 39 MΩ. Taking neurons of both types together, input resistances were greater before than after treatment with dispase (P = 0.02), but no difference was statistically discernible between resting membrane potentials (P = 0.08).
Effects of gadolinium
The trivalent cation gadolinium has been frequently used as a blocker of stretch sensitive channels (SACs) in the membranes of mechanosensitive cells (Sackin, 1995). We tested whether Gd3+ at a SAC-blocking concentration (1 μm) would arrest neuronal and/or muscular activity in the small intestine. Addition of Gd3+ caused a rapid cessation of contractile activity of tissue stretched by 40%. In three Dogiel type II and two unipolar neurons in tissue stretched by 40%, for which impalement was maintained during change to Gd3+, spontaneous action potential discharge stopped within 30 s (Fig. 7). In total, including both these three Dogiel type II neurons and other neurons that were only recorded in the absence or presence of Gd3+, 18 of 23 neurons fired spontaneous action potentials without Gd3+ present and 1 of 6 fired in its presence. Of unipolar neurons, 6/22 were active in the absence of Gd3+ and 0/2 fired spontaneous action potentials in its presence. Addition of Gd3+ was associated with a small hyperpolarization of resting membrane potential from -50 ± 5 to -55 ± 4 mV and decreased input resistance from 313 ± 72 to 267 ± 50 MΩ (P = 0.04).
Depolarizing current injection evoked action potentials after the spontaneous firing was suspended and movement stilled by gadolinium. Washout with normal physiological saline restarted the movement and neuronal discharge after about 15-40 min. For Dogiel type II neurons, action potential humps and late AHPs were manifest with Gd3+ present, indicating that, at the concentration used, neither N-type calcium currents nor BK calcium dependent potassium currents were blocked by the cation.
Sites of action of (-)-Bay K 8466 and gadolinium
Because muscle contraction and neuronal discharge were both antagonized by Gd3+, the ion could have blocked stretch sensitive channels on both muscle cells and neurons. To distinguish between these possibilities we switched from a superfusate containing Gd3+ (1 μm) to one containing Bay K 8466 plus Gd3+ (both 1 μm). Bay K 8466 in the presence of Gd3+ caused rhythmic muscle contraction and action potential discharge in neurons. After the addition of Bay K, each of five neurons (3 Dogiel type II and 2 unipolar neurons) from which records were taken were spontaneously active. Two Dogiel type II neurons were recorded from both before and after addition of Bay K to the Gd3+ containing solution; in both cases addition of Bay K caused the previously silent neurons to spike. These results suggest that Gd3+ did not block stretch sensitive channels on neurons. However, the results could imply that Bay K 8466 acted on L-type calcium channels on the neurons as well as on muscle. We tested this supposition by disconnecting the muscle from the myenteric plexus by addition of dispase to tissue already exposed to Gd3+ plus Bay K (Fig. 7Ba and Bb). In unpaired comparisons, three Dogiel type II and two unipolar neurons spiked spontaneously in the presence of Bay K and Gd3+, but after dispase (1 mg ml−1) was added to the superfusate none of five neurons (4 Dogiel type II, 1 unipolar) was active (P = 0.008), although Bay K still contracted the muscle. Records from a neuron before and after dispase are shown in Fig. 7Ba and Bb. Within 15 min of exposure to the same superfusate to which dispase had been added the neuron no longer discharged action potentials. However, injection of a brief (15 ms) depolarizing current pulse evoked an action potential that was followed by a slow AHP (Fig. 7Bb); the presence of the AHP suggests that synaptic drive to this myenteric intrinsic primary afferent neuron had ceased.
Chronic extrinsic denervation
Seven to ten days before the recording sessions, operations were performed on the animals to deprive an intact segment of the distal ileum of extrinsic axons by severing nerves in the mesentery (Furness et al. 1995). The effectiveness of extrinsic denervation was evaluated by immunohistochemistry for tyrosine hydroxylase. In the operated region, there were no tyrosine hydroxylase immunoreactive fibres, whereas such fibres were plentiful in the adjacent, non-operated regions (Fig. 8). In eight experiments, 22 neurons (7 Dogiel type II and 15 uniaxonal neurons) were recorded from in chronically denervated segments of ileum that were stretched by 40%. Three of the seven Dogiel type II and 8/15 uniaxonal neurons fired spontaneous action potentials (Fig. 8).
Correlation of responsiveness, immunohistochemistry and neuronal shape
Calbindin immunoreactivity was tested for in 28 Dogiel type II neurons from which recordings were obtained in actively contracting tissue; 21 had spontaneous action potentials and 11 of these were calbindin positive. This proportion (11/21) did not differ significantly (P = 0.5) from the proportion of those Dogiel type II neurons that were silent in contracting tissue and had calbindin immunoreactivity (8/12).
Twenty-six Dogiel type II neurons were sufficiently well filled to identify their projection patterns; all possessed two or more processes projecting circumferentially beyond the source ganglion and most had one or more retraction bulbs marking severed collaterals projecting toward the mucosa. Of the 26 neurons, 17 fired action potentials in contracting tissue and five of these had a process that projected anally for three or more rows of ganglia (range = 3-8), but none of the nine Dogiel type II neurons that were not active in stretched tissue projected anally. Comparison of the proportions of spontaneously active and silent neurons with anally projecting processes yielded a value of P = 0.06. Of eight Dogiel type II neurons whose spontaneous spiking was not abolished by membrane hyperpolarization, two had neurites that projected anally.
The axonal projections and somatic shapes of 26 unipolar neurons in contracting tissue were identified. Fourteen of the neurons spiked spontaneously; the axons of 11 of these projected anally, one projected orally and two projected circumferentially before ending in a retraction bulb. The somatic shapes of the anally projecting neurons were Dogiel type I (n = 8) and filamentous (n = 3), the orally projecting neuron had Dogiel type I morphology, and the two circumferentially projecting neurons were of simple morphology, having small cell bodies with few short processes and one axon (Clerc et al. 1998). Twelve of the filled neurons were silent, of which eight projected anally, three orally (one ran orally for 4 ganglia before being lost and was immunoreactive for calretinin, and two had process that ended in retraction bulbs and were calretinin negative), and one did not project beyond its ganglion but ended in a local retraction bulb. Seven of the anally projecting neurons were Dogiel type I and one had filamentous dendrites. For the orally projecting neurons two, including the calretinin positive one, were Dogiel type I and one was filamentous. The remaining neuron whose axon did not project beyond its own ganglion in the myenteric plexus had a large soma with tapering filamentous dendrites, similar to secretomotor neuron morphology.
DISCUSSION
The nature of the responses of intrinsic primary afferent neurons to stretch
The present work was undertaken to investigate how tension in the longitudinal layer of the external muscle is communicated to responsive neurons in the guinea-pig small intestine, the majority of which have Dogiel type II morphology (Kunze et al. 1998). Distending the intestine generates both passive and active tension; the active tension arises because distension opens stretch activated channels (SACs) in the muscle cells, causing them to contract (Bülbring, 1955; Kirber et al. 1988). Because both excitation of the Dogiel type II neurons by tissue stretch and synaptic activation of neurons that are their targets were prevented by drugs that relax the muscle, it was suggested that the tension generated by the muscle was essential to activate the mechanosensitive intrinsic primary afferent neurons (Kunze et al. 1998). That is, it was proposed that these neurons fire in response to increases in tension rather than in length.
The present observations support the hypothesis that mechanosensitive neurons with cell bodies in the myenteric plexus respond to increased tension that is generated by contraction of the muscle cells, but not to the length of the tissue, per se. When tissue was pinned out with no applied tension (slack width), or was extended by up to 5%, to make it flat for impalement, very few neurons showed spontaneous activity. Moreover, when the muscle cells were rendered inexcitable, by the application of nicardipine, no neurons exhibited action potentials, even when the tissue was extended 50% beyond its slack dimensions in both the longitudinal and circular directions, which takes it almost to breaking point. In contrast, many neurons fired action potentials when (-)-Bay K 8644 was used to contract muscle cells in slack tissue which was pinned so that its overall width remained unchanged. Spontaneous action potentials in neurons also occurred when the tissue was stretched by 40% without muscle paralysis. It could be that Bay K activated the neurons in unstretched tissue because the neurons themselves have L-type channels. However, in tissue to which Bay K was applied, but mechanical connections between the muscle and the neurons were disrupted enzymatically by dispase, spontaneous action potentials were not elicited in the neurons although the muscle was contracted by Bay K. The calcium component of action potentials in myenteric primary afferent neurons is not blocked by nicardipine but appears to be mediated by N-type Ca2+ channel opening (Furness et al. 1998). The absence of neural activity in stretched tissue superfused with nicardipine could be explained by there being L-type channels on neuronal processes, through which stretch activates the neurons, if dispase disrupts the L-type channels on the neurites, but not on the muscle. Therefore we further tested the possibility that L-type channels were necessary for activation of neurons by stretch, by applying rapid stretch in the presence of nicardipine. Neurons responded by discharging action potentials under these circumstances. These observations imply that when stretch is maintained in the presence of nicardipine compliant elements are extended and no effective stimulus is applied to SACs on neurons, whereas rapid stretch applies effective forces to the neurons.
Due to the complexity of structure and connections of the smooth muscle and its associated connective tissue, it is not possible to deduce what internal, local, changes in length and tension occur when the intestine is stretched. However, the relation between neuronal excitation and behaviour of the whole muscle sheet was as if the neural tension detectors were ‘in series’ rather than ‘in parallel’ with the muscle (Grundy, 1988; Gordon & Ghez, 1991). Extrinsic, vagal, gut mechanoreceptors have also been concluded to be in-series receptors, with no evidence for in-parallel receptors (Andrews, 1986; Grundy, 1988). Consistent with the receptors being in series, contraction activated the intrinsic receptors in the present work, as it does for vagal in-series mechanoreceptors whose endings are in the stomach (Andrews, 1986; Grundy, 1988). The present experiments did not reveal any direction selectivity of the receptors: stretch in the longitudinal direction activated the neurons, even though the Dogiel type II neurons project primarily circumferentially, and they are also activated by circumferential stretch (Kunze et al. 1998). Whether there is in fact a preferred direction of stretch for the neurons to be activated will need to be investigated through the use of different protocols, for example, recordings with both muscle layers intact.
Action potentials occurred in bursts in some of the neurons. This was previously reported for the guinea-pig small intestine by Wood (1973), who used extracellular recording, and by Wood & Mayer (1978), who recorded with intracellular electrodes. Only brief segments of record were published from intracellular recording, presumably because of the difficulty, also found in the present work, of recording intracellularly from the neurons for extended periods. Wood (1973) found that bursts occurred at intervals from 0.7 to 5.7 s (n = 9), in comparison to 0.8 to 1.8 s (n = 3) in the present work. What causes the neurons to fire in bursts is unknown. It could be related to patterns of contractile activity in the muscle, or to properties of the neurons themselves. Analysis of the mechanism might be best undertaken with methods that allow prolonged recording from single neurons, such as the extracellular studies by Wood (1973), combined with protocols to determine the classes of neurons (e.g. Dogiel type II neuron, interneuron or motor neuron) from which records are obtained.
Stretch sensitive channels on muscle compared with intrinsic primary afferent neurons
We tested the effects of the lanthanide gadolinium (Gd3+), which has been previously used to block SACs in contractile tissues (Sackin, 1995) and has recently been shown to specifically block stretch activated non-selective cation channels on single, dissociated guinea-pig gastric smooth muscle cells (Yamamoto & Suzuki, 1996). Gd3+ (1 μm) blocked the muscle contraction that occurred in response to stretch, and also blocked the action potentials that are elicited in neurons by stretch. Based on this result alone, it is possible that Gd3+ blocked SACs on both the muscle and neurons. However, because the spontaneous action potential discharge in myenteric neurons occurred in the presence of Gd3+ when Bay K was used to contract the muscle, and Bay K does not excite the neurons directly (see preceding discussion), it can be concluded that SACs on the neurons are not significantly affected by the concentration of Gd3+ (1 μm) that blocked muscle cell SACs.
Mechanism of excitation of intrinsic primary afferent neurons
We have shown that excitation of intrinsic primary afferent neurons was dependent on muscle cell contraction, not on overall tissue length. At least three mechanisms could link the contraction of the muscle to excitation of intrinsic primary afferent neurons: (i) the connection could be mechanical, the contracting muscle pulling on tissue elements that in turn connect with mechanosensitive neurons, thus causing the opening of SACs in the neurons; (ii) the active muscle cells could release metabolites that cause neuronal excitation, or (iii) mechanosensitive endings of extrinsic afferent neurons could release excitatory transmitters onto the intrinsic neurons. If the Dogiel type II neurons were activated indirectly by axon reflexes in the endings of extrinsic afferent neurons, then the Dogiel type II neurons could not be responsible for the initiation of distension evoked reflexes in isolated intestine, because such reflexes occur after degenerative section of the extrinsic nerves (Langley & Magnus, 1905; Furness et al. 1995). We tested this possibility by examining responses to stretch after chronic extrinsic denervation and we found that intrinsic neurons, including the Dogiel type II neurons, were activated by stretch after extrinsic nerves were severed and their endings had degenerated. However, the operations would not have severed vagal afferent fibres which supply very few myenteric ganglia in the ileum (Berthoud et al. 1997). Activation of the neurons by release of metabolites from the contracting muscle seems unlikely, because uncoupling the connections between neurons and muscle by enzymatic degradation with dispase did not prevent muscle cells contracting, but it did prevent stretch causing neuronal action potential firing. The silencing of the neurons after dispase treatment is presumably because the contracting muscle could no longer pull on connective tissue elements to distort the neurons.
A high proportion (20/25) of Dogiel type II neurons fired action potentials when muscle cells were caused to contract by stretch of 40% beyond slack width, and a similarly high proportion (10/10) fired when contraction was induced by (-)-Bay K 8644. However, there is both direct and synaptic excitation of these neurons. Because the intrinsic primary afferent neurons are connected to each other through synapses at which slow excitation occurs, when they are activated they cause slow depolarization and excitation of other Dogiel type II neurons (Furness et al. 1998). During maintained sensory input, including stretching, the level of excitation of myenteric neurons is heightened (Kunze et al. 1997; Furness et al. 1998). This can be seen as increased spontaneous discharge of action potentials and decreased accommodation of spike firing during prolonged depolarization. These changes have been attributed to ongoing slow postsynaptic excitation of neurons. Thus, intrinsic primary afferent neurons would be excited both directly by opening of SACs on their processes and indirectly by slow postsynaptic input. Excitation by conduction of action potentials from processes and synaptically mediated excitation of the soma can be distinguished by applying hyperpolarizing current through the recording electrode; hyperpolarizing current restricts the ability of the neurons to fire action potentials in response to excitatory synaptic input, but does not block the electrotonic conduction of action potentials into the cell bodies, which may be recorded as process potentials in the cells (Wood, 1989; Kunze et al. 1998). In the present work, action potentials were not blocked by hyperpolarization in 14/20 Dogiel type II neurons, but were blocked in 17/18 S neurons. Thus about 70% of responding Dogiel type II neurons were tension detectors. Overall, 30/35 Dogiel type II neurons responded to tension in the muscle. Thus a minimum of 60% of Dogiel type II neurons appear to be tension detectors. The responses to tension are graded (Kunze et al. 1998), and the unresponsive neurons might have had a response threshold that was greater than the tension that occurred in the present experiments. The Dogiel type II neurons are multipolar, and there could be differences in sensitivity of the processes of individual neurons that contribute to the grading of responses. A factor that might alter the sensitivity of the neurons to mechanical stimuli is the formation of retraction bulbs on severed neurites. It is feasible that the retraction bulbs express greater numbers of channels and become sensitized to the stretch stimulus. Conversely, the retraction bulbs may lose their local connections to the tissue and thus be less susceptible to mechanical distortion.
It is likely that the neurons in which discharge was abolished by hyperpolarization were activated synaptically from other Dogiel type II neurons, although it is possible that these were directly activated by tension, but that the stretch activated channels were in or close to the cell bodies and that current through these SACs was insufficient to generate active responses when the cells were hyperpolarized. All of the Dogiel type II neurons have neurites that project to the intestinal mucosa, and about 60% of these neurons respond to chemicals (acid, base or fatty acid) applied to the mucosa (Bertrand et al. 1997). It is possible that adequate chemical stimuli would activate more Dogiel type II neurons. The Dogiel type II neurons synapse with and excite interneurons and motor neurons, which are S neurons electrophysiologically and uniaxonal neurons morphologically. Nevertheless, some of the S neurons also seem to react directly to stretch, 1 of 18 in the present experiments and 2 of 9 in the experiments of Kunze et al. (1998). Thus some uniaxonal neurons could also function as intrinsic primary afferent neurons.
The primary afferent neurons with cell bodies in the gut wall have a number of properties that distinguish them from spinal and vagal primary afferent neurons with cell bodies in dorsal root and nodose ganglia (Furness et al. 1998). The intrinsic primary afferent neurons respond both to changes in the tissue environment and to transmission from other primary afferent neurons, unlike spinal and vagal afferent neurons that respond to the environment only. Thus the intrinsic primary afferent neurons can be viewed as both interneurons and afferent neurons and their activity when the intestine is stretched has two components, one direct, and one indirect. It is conceivable that individual neurons may be activated only indirectly, and thus it is uncertain whether all Dogiel type II neurons are in fact intrinsic primary afferent neurons. Even if all are intrinsic primary afferent neurons, they may not be directly activated in any particular experiment.
Conclusion
The present study indicates that the contraction that occurs when intestinal muscle is stretched pulls on connective tissue elements through which force is communicated to intrinsic primary afferent neurons, thus causing their excitation. The outcome of activation of these neurons in the intestine of the living animal is complicated by the concomitant activation of other neurons by distortion of the mucosa and by chemicals in the lumen, as well as by activation of the stretch responsive neurons by nerve-mediated contraction of the muscle. Further studies of these and other interactions will be required for a more complete understanding of intestinal motility control.
Acknowledgments
This work was supported by a grant from the National Health & Medical Research Council of Australia (grant no. 963213). We are grateful to Alan Lomax for his constructive comments. Siobhan Lavin and Clare Delaney are thanked for excellent assistance in preparing the manuscript and figures, and in experiments.
References
- Andrews PLR. Vagal afferent innervation of the gastrointestinal tract. Progress in Brain Research. 1986;67:65–86. doi: 10.1016/s0079-6123(08)62757-0. [DOI] [PubMed] [Google Scholar]
- Berthoud LM, Patterson F, Neumann F, Neuhuber WL. Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anatomy and Embryology. 1997;195:183–191. doi: 10.1007/s004290050037. [DOI] [PubMed] [Google Scholar]
- Bertrand PP, Kunze WAA, Bornstein JC, Furness JB, Smith ML. Analysis of the responses of myenteric neurons in the small intestine to chemical stimulation of the mucosa. American Journal of Physiology. 1997;273:G422–435. doi: 10.1152/ajpgi.1997.273.2.G422. [DOI] [PubMed] [Google Scholar]
- Bornstein JC, Furness JB, Kunze WA A. Electrophysiological characterization of myenteric neurons: how do classification schemes relate? Journal of the Autonomic Nervous System. 1994;48:1–15. doi: 10.1016/0165-1838(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Bülbring E. Correlation between membrane potential, spike discharge and tension in smooth muscle. The Journal of Physiology. 1955;128:200–221. doi: 10.1113/jphysiol.1955.sp005299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerc N, Furness JB, Bornstein JC, Kunze WA A. Correlation of electrophysiological and morphological characteristics of myenteric neurons of the duodenum in the guinea-pig. Neuroscience. 1998;82:899–914. doi: 10.1016/s0306-4522(97)00318-7. [DOI] [PubMed] [Google Scholar]
- Dekhuijzen AJ, Bagust J. Analysis of neural bursting: nonrhythmic and rhythmic activity in isolated cord. Journal of Neuroscience Methods. 1996;67:141–147. 10.1016/S0165-0270(96)00033-7. [PubMed] [Google Scholar]
- Furness JB, Costa M. The Enteric Nervous System. Edinburgh: Churchill Livingstone; 1987. [Google Scholar]
- Furness JB, Johnson PJ, Pompolo S, Bornstein JC. Evidence that enteric motility reflexes can be initiated through entirely intrinsic mechanisms in the guinea-pig small intestine. Neurogastroenterology and Motility. 1995;7:89–96. doi: 10.1111/j.1365-2982.1995.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Furness JB, Kunze WAA, Bertrand PP, Clerc N, Bornstein JC. Intrinsic primary afferent neurons of the intestine. Progress in Neurobiology. 1998;54:1–18. doi: 10.1016/s0301-0082(97)00051-8. 10.1016/S0301-0082(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Gordon J, Ghez C. Muscle receptors and spinal reflexes: The stretch reflex. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. 3. New York, Amsterdam, London, Tokyo: Elsevier; 1991. pp. 564–580. [Google Scholar]
- Grundy D. Speculations on the structure/function relationship for vagal and splanchnic afferent endings supplying the gastrointestinal tract. Journal of the Autonomic Nervous System. 1988;22:175–180. doi: 10.1016/0165-1838(88)90104-x. 10.1016/0165-1838(88)90104-X. [DOI] [PubMed] [Google Scholar]
- Hasler WL. Motility of the small intestine. In: Yamada T, editor. Textbook of Gastroenterology. Philadelphia: Lippincott; 1994. pp. 207–233. [Google Scholar]
- Hirst GDS, Holman ME, Spence I. Two types of neurones in the myenteric plexus of duodenum in the guinea-pig. The Journal of Physiology. 1974;236:303–326. doi: 10.1113/jphysiol.1974.sp010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirber MT, Walsh JV, Jr, Singer JJ. Stretch-activated ion channels in smooth muscle: a mechanism for the initiation of stretch-induced contraction. Pflügers Archiv. 1988;412:339–345. doi: 10.1007/BF01907549. [DOI] [PubMed] [Google Scholar]
- Kirchgessner AL, Gershon MD. Projections of submucosal neurons to the myenteric plexus of the guinea pig intestine: in vitro tracing of microcircuits by retrograde and anterograde transport. Journal of Comparative Neurology. 1988;277:487–498. doi: 10.1002/cne.902770403. [DOI] [PubMed] [Google Scholar]
- Kunze WAA. A mobile intracellular microelectrode designed to record from neurons in contracting tissue. Brain Research Protocols. 1998;3:94–99. doi: 10.1016/s1385-299x(98)00029-4. [DOI] [PubMed] [Google Scholar]
- Kunze WAA, Bertrand PP, Furness JB, Bornstein JC. Influence of the mucosa on the excitability of myenteric neurons. Neuroscience. 1997;76:619–634. doi: 10.1016/s0306-4522(96)00408-3. [DOI] [PubMed] [Google Scholar]
- Kunze WAA, Furness JB, Bertrand PP, Bornstein JC. Intracellular recording from myenteric neurons of the guinea-pig ileum that respond to stretch. The Journal of Physiology. 1998;506:827–842. doi: 10.1111/j.1469-7793.1998.827bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley JN, Magnus R. Some observations of the movements of the intestine before and after degenerative section of the mesenteric nerves. The Journal of Physiology. 1905;33:34–51. doi: 10.1113/jphysiol.1905.sp001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi S, North RA. Intracellular recording from the myenteric plexus of the guinea-pig ileum. The Journal of Physiology. 1973;231:471–491. doi: 10.1113/jphysiol.1973.sp010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackin H. Mechanosensitive channels. Annual Review of Physiology. 1995;57:333–353. doi: 10.1146/annurev.ph.57.030195.002001. [DOI] [PubMed] [Google Scholar]
- Schutte IWM, Kroese ABA, Akkermans LMA. Somal size and location within the ganglia for electrophysiologically identified myenteric neurons of the guinea pig ileum. Journal of Comparative Neurology. 1995;355:563–572. doi: 10.1002/cne.903550406. [DOI] [PubMed] [Google Scholar]
- Ward H, White E. Reduction in the contraction and intracellular calcium transient of single rat ventricular myocytes by gadolinium and the attenuation of these effects by extracellular NaH2PO4. Experimental Physiology. 1994;79:107–110. doi: 10.1113/expphysiol.1994.sp003737. [DOI] [PubMed] [Google Scholar]
- Wood JD. Electrical discharge of single enteric neurons of guinea pig small intestine. American Journal of Physiology. 1973;225:1107–1113. doi: 10.1152/ajplegacy.1973.225.5.1107. [DOI] [PubMed] [Google Scholar]
- Wood JD. Physiology of the enteric nervous system. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1994. pp. 423–482. [Google Scholar]
- Wood JD. Electrical and synaptic behavior of enteric neurons. In: Schultz SG, Wood JD, Ranner BB, editors. Handbook of Physiology. Bethesda MD, USA: American Physiological Society; 1989. pp. 465–516. [Google Scholar]
- Wood JD, Mayer CJ. Intracellular study of electrical activity of Auerbach's plexus in guinea-pig small intestine. Pflügers Archiv. 1978;374:265–275. doi: 10.1007/BF00585604. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Suzuki H. Two types of stretch-activated channel activities in guinea-pig gastric smooth muscle cells. Japanese The Journal of Physiology. 1996;46:337–345. doi: 10.2170/jjphysiol.46.337. [DOI] [PubMed] [Google Scholar]


