Abstract
Using retrograde tracing with 1,1′-didodecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) in combination with electrophysiological and immunohistochemical techniques we determined the properties of the putative intrinsic primary afferent myenteric neurones with mucosal projections in the guinea-pig proximal colon.
Eighty-four out of eighty-five DiI-labelled myenteric neurones were AH neurones with a late after-hyperpolarization. Thirty-three per cent of them exhibited atropine- and tetrodotoxin-resistant spontaneously occurring hyperpolarizing potentials (SHPs) during which the membrane resistance and excitability decreased.
DiI-labelled AH neurones had multipolar Dogiel type II morphology, primarily of the dendritic type. Sixty-one per cent of the neurones were immunoreactive for choline acetyltransferase (ChAT) and calbindin (Calb) and 23% were ChAT positive but Calb negative.
DiI-labelled neurones did not receive fast excitatory postsynaptic potentials but 94% (34/36) received slow excitatory postsynaptic potentials (sEPSPs). The neurokinin-3 (NK-3) agonist (MePhe7)-NKB but not the NK-1 agonist [(SAR9,Met(O2)11]-SP mimicked this response. The NK-3 receptor antagonist SR 142801 (1 μm) significantly decreased the amplitude and duration of the sEPSPs; the NK-1 receptor antagonist CP-99,994 (1 μm) was ineffective. Atropine (0.5 μm) increased the duration but not the amplitude of the sEPSPs.
Microejection of 100 mM sodium butyrate onto the neurones induced in 90% of the DiI-labelled neurones a transient depolarization associated with an increased excitability. In neurones with SHPs sodium butyrate evoked, additionally, a late onset hyperpolarization. Perfusion of 0.1-10 mM sodium butyrate induced a dose-dependent increase in neuronal excitability. Sodium butyrate was ineffective when applied directly onto the mucosa.
Mucosally projecting myenteric neurones of the colon are multipolar AH neurones with NK-3-mediated slow EPSPs and somal butyrate sensitivity.
Neural regulation of a variety of gastrointestinal functions is primarily achieved by the enteric nervous system which is embedded within the gastrointestinal wall. It is usually formed from two major plexus: the myenteric plexus located between the circular and the longitudinal muscle layer and the submucosal plexus situated between the submucosa and the mucosa (for review, see Wood, 1994).
In the early part of the 1970s, the first attempt to classify enteric neurones was made using extracellular recording techniques (Wood, 1970). Nishi & North (1973) and Hirst et al. (1974) then used intracellular microelectrode techniques to determine the electrophysiological characteristics of myenteric neurones in the guinea-pig small intestine. Although their classification schemes did not recover exactly the same neuronal populations, two major groups of neurones were revealed. Following the classification of Hirst et al. (1974), S neurones (for synaptically driven) and AH neurones (named after the characteristic long-lasting membrane potential hyperpolarization following the action potential) made up the two populations. Further studies by Wood and his collaborators revealed the presence of different classes of myenteric neurones depending on the region of the gut studied (Wood, 1994). Additionally, enteric neurones may be subdivided into neurochemically distinct populations (Costa et al. 1996), whose number exceeds the broadly defined electrophysiological classes.
The use of intracellular injection of biocytin or neurobiotin allowed the morphology of electrophysiologically classified neurones to be determined. AH neurones were extensively studied in the ileum (for review, see Furness et al. 1998) where they were shown to have morphological characteristics of multipolar Dogiel type II neurones with most processes projecting in the circumferential direction (Iyer et al. 1988; Bornstein et al. 1991). Neuronal tracing methods showed that at least one process of myenteric Dogiel type II neurones in the ileum or colon projected to the mucosa (Song et al. 1991; Neunlist & Schemann, 1997). Immunohistochemical studies revealed that a majority of myenteric Dogiel type II neurones were immunoreactive for choline acetyltransferase (ChAT) (Costa et al. 1996; Neunlist & Schemann, 1997), for calbindin (Calb) (Song et al. 1994; Costa et al. 1996; Neunlist & Schemann, 1997) and for substance P (SP) (Song et al. 1991; Costa et al. 1996).
Myenteric Dogiel type II AH neurones have been of particular interest to researchers over the last few years, primarily because it has been suggested that they represent the intrinsic primary afferent neurones, at least in the guinea-pig ileum. In the ileum, AH neurones have been shown to respond to mucosal stimuli such as hydrochloric acid, acetate and also to muscle stretch (Kunze et al. 1995, 1998; Bertrand et al. 1997). The responses of these neurones to mucosal application of acid could be blocked by removing the mucosa and then putting it back in its original position (Kunze et al. 1995), suggesting that myenteric neurones responding to mucosal stimuli project to the mucosa. These studies have used short chain fatty acids (SCFAs) or hydrochloric acid as a stimulus in the ileum (Kunze et al. 1995; Bertrand et al. 1997) where high concentrations of these substances occur only under pathological conditions. Activation of some intrinsic primary afferent neurones in the ileum by acetate very probably involves release of intermediary substances (Bertrand et al. 1997). Support for this comes from reports that enterochromaffin cells might act as ‘taste buds’ to sense mechanical stimulation of the mucosa and as a consequence release serotonin which then activates primary afferent neurones in the submucosal plexus (Kirchgessner et al. 1992). So far the sensitivity of mucosal terminals of enteric neurones, as well as of non-neuronal cells in the mucosa, has been demonstrated but somal sensitivity of enteric neurones to chemicals like SCFAs has not been investigated. The colon, which is the site of bacterial production of SCFAs would be the appropriate organ to study the effects of SCFAs, in particular those of butyrate. In the colon, SCFAs appear to have concentration-dependent effects, i.e. low concentrations (< 100 μm) increased and high concentrations decreased motility (Yajima, 1985; Squires et al. 1992). It has been shown that enteric neurones are involved in the response of the gut to SCFAs (Yajima, 1985; Diener et al. 1996).
AH neurones occur in different regions of the gut, namely in the antrum (Tack & Wood, 1992), the duodenum and ileum (Nishi & North, 1973; Hirst et al. 1974), in the proximal colon (Messenger et al. 1994), the distal colon (Wade & Wood, 1988) and the rectum of the guinea-pig (Tamura & Wood, 1989). However, their putative functions in these regions are unknown. The population of AH neurones may be subdivided into subclasses with different properties, which led to the suggestion that they might fulfil more than one specific function (see Wood, 1994). In the guinea-pig ileum numerous studies have investigated the synaptic properties of myenteric AH neurones, considering the kind of input AH neurones receive as well as the input they generate in other enteric neurones; fast and slow excitatory and inhibitory inputs to AH neurones have been described (see Wood, 1994; Furness et al. 1998). It remains unknown whether differences in electrophysiological behaviour are related to functional specialization and it is therefore important to investigate closely the electrophysiology, neurochemistry and neuropharmacology of enteric neurones with identified projections.
In the present study, we have used a 1,1′-didodecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI)-based tracing method to identify the myenteric neurones projecting to the mucosa. Such a method has already allowed us to show that myenteric neurones with Dogiel type II morphology project to the mucosa (Neunlist & Schemann, 1997). Once identified, we have investigated their electrophysiological and synaptic characteristics as well as their sensitivity to butyrate.
METHODS
Organotypic culture of proximal colon segments
Guinea-pigs (200-350 g) were killed by cervical dislocation followed by exsanguination. The experiments conformed to the German national guidelines and were approved by the Animal Welfare Department of the School of Veterinary Medicine in Hannover. Specimens of proximal colon (5 cm distal to the caeco-colic junction; 5-6 cm in length) were removed and placed in Krebs solution of the following composition (mM): NaCl, 117; KCl, 4.7; MgCl2, 1.2; NaH2PO4, 1.2; NaHCO3, 25; CaCl2, 2.5; glucose, 11.5; and 1-3 μm nifedipine; pH = 7.4. The organotypic culture method has previously been described in detail (Neunlist & Schemann, 1997). After removal from the animal and several washes, a 7 cm × 2 cm segment of proximal colon was pinned out flat in a Sylgard (Dow Corning)-covered Petri dish and the mucosa was carefully removed except for a small patch of about 1 cm × 1 cm. A DiI (1,1′-didodecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; Molecular Probes)-coated glass bead (diameter 50-100 μm) was slightly pressed onto the mucosa with forceps. Care was taken not to push the bead too deep into the mucosa.
Following the bead application, 20 ml of culture medium with 1 μm nifedipine was added to the culture dish. The culture medium (Dulbecco's modified Eagle's medium/F-12 Ham; Sigma, Deisenhofen, Germany) was supplemented with 10% heat inactivated fetal calf serum (CC Pro, Karlsruhe, Germany), 100 IU ml−1 penicillin, 100 μg ml−1 streptomycin, 2.75 μg ml−1 amphotericin B and 50 μg ml−1 gentamicin (all from Sigma) and adjusted to pH 7.4. The tissue was maintained in a humidified incubator at 37°C and equilibrated with 5% CO2 in air for a period of 48-72 h. The dishes were placed on a rocking tray shaking at a frequency of about 0.5 Hz. The culture medium was changed once daily.
Electrophysiology
After 48-72 h in organotypic culture the tissue was prepared for electrophysiological recordings. A piece 4 cm × 2 cm with the DiI application site in the centre was used for electrophysiological studies. A peninsula (about 1 cm × 1 cm) was created at the anal and oral border of the mucosa patch. In this area, the circular muscle layer was carefully removed in order to expose the myenteric plexus. The mucosa was left on the preparation in order to preserve some of the processes projecting to the mucosa. The tissue was then pinned in the Sylgard-coated recording chamber (5.5 cm × 4 cm; 10-12 ml) and continuously superfused at a flow rate of 13.5 ml min−1 with oxygenated Krebs solution at 35-37°C containing 1-3 μm of nifedipine to prevent muscle activity. The preparation was placed on the stage of an IMT2 Olympus inverted microscope (Olympus, Japan) fitted with a rhodamine filter and a 100 W halogen light source (Osram, Germany).
DiI-labelled myenteric neurones were first visualized using a × 20 objective lens and impaled with a microelectrode advanced near vertically. Borosilicate glass microelectrodes (World Precision Instruments) were filled with 0.5% neurobiotin in 0.5 mM KCl and 0.1% acetate and backfilled with 0.5 mM KCl and had resistances between 150 and 250 MΩ. DiI-labelled cells were filled with neurobiotin for 3 min using depolarizing pulses (300 ms duration, 0.3 nA amplitude, 0.5 Hz frequency). Synaptic inputs to the cells were tested by electrical stimulation of the interganglionic strands with a 25 μm Teflon-coated platinum unipolar electrode connected to a constant voltage isolation unit. Electrical pulses had durations of 300 μs and amplitudes varying between 1 and 15 V.
Electrical signals were amplified with a microelectrode amplifier (MEZ 8300, Nihon Kohden, Japan), displayed on an oscilloscope (Tektronik 2230, Japan) and a chart recorder (Astro Med, Dash IV, Germany). This system was also used to inject intracellular constant current pulses. Stimulus current pulse and membrane potential were acquired with a pulse code modulator and stored on videotape (AR Vetter, Rebersburg, PA, USA). Data were analysed and displayed off-line using a Macintosh computer and a MacLab system, using a sampling frequency of either 2 or 4 kHz (MacLab 4 s/e with Chart software 3.5.1, ADInstruments, Castle Hill, Australia).
Immunohistochemistry
After the electrophysiological studies, the tissue was fixed overnight in 2% paraformaldehyde and 0.2% picric acid in 0.1 M phosphate buffer at 4°C (pH = 7.44). The fixed tissue was then repeatedly washed with phosphate buffer. The DiI application site was marked by making a hole with a fine needle. Further dissection was done to obtain a longitudinal muscle-myenteric plexus preparation with or without the mucosa patch still present.
Immunohistochemical detection of neuronal antigens was achieved using a procedure described in detail by Neunlist & Schemann (1997). After the membrane permeabilization procedure, the tissue was exposed at room temperature with the following primary antibodies: rabbit anti-choline acetyltransferase (1:2000; Schemann et al. 1993) or goat anti-ChAT (1:100; Chemicon International, Temecula, CA, USA), mouse anti-calbindin (1:200; SWANT, Bellinzona, Switzerland) and rabbit anti-vasoactive intestinal peptide (1:1000; Peninsula, Heidelberg, Germany). The following affinity-purified secondary antibodies were used: goat anti-rabbit IgG conjugated to indodicarbocyanin (Cy5), goat anti-mouse IgG conjugated to either dichloro-triazinyl aminofluorescin (DTAF) 1:100 or 7-amino-4-methylcoumarin-3-acetate (AMCA) 1:50 (all secondary antibodies from Dianova, Hamburg, Germany). Neurobiotin-filled cells were labelled with streptavidin DTAF or AMCA (1:50-100). An Olympus IX70 microscope fitted with the following Olympus filter cubes was used for immunohistochemical studies: UM41007 (Beam splitter: 565 DCLP, Ex.: BP 530-560, Em.: BP 575-645) for DiI, U-MNIBA (Beam splitter DM 505, Ex.: BP 470-490, Em.: D520) for DTAF, U-MWU (Beam splitter DM400, Ex.: BP 330-385, Em.: BP 460-490) for AMCA and U-M41008 (Beam splitter Q660 LP, Ex.: BP 540-650, Em.: BP 667-735) for Cy5. No cross-detection between the four fluorophore-filter-cube combinations was observed. The preparations were further analysed using an image analysis system. Pictures were acquired with a black and white video camera (Mod. 4910, Cohu Inc., San Diego, CA, USA) connected to a Macintosh Computer and controlled by the IPLab Spectrum 3.0 software (Signal Analytics, Vienna, VI, USA). Frame integration and contrast enhancement were used for picture processing. Digitized photomicrographs of each individual neurone were stored on the computer for later analysis.
Cell counting
The position of the DiI-labelled neurone with respect to the application site was measured using a computerized stage mapping system (Märzhäuser and Kassen, Wetzlar, Germany) with a precision of 1 μm. The DiI-labelled neurone was located according to its X and Y co-ordinates. The application site had the co-ordinate (0,0). The distance (d) from the centre of the DiI-labelled neurone to the centre of the dye application site was calculated as d =√(X2+ Y2). DiI-labelled neurones were defined as descending or ascending if their cell body was located orally or anally from the DiI application site, respectively, and if their co-ordinates were |X| > |Y|. DiI-labelled cell bodies whose co-ordinates had |X| < |Y| were defined as circumferentially projecting neurones.
Pharmacological study
Solutions
Solutions were applied to the cells either via superfusion in the bath or via a glass pipette filled with the drug-containing Krebs solution and connected to a constant pressure application system (35 p.s.i.). Fast Green (0.5 mM) was added to check the application and to verify that the solution did not leak out from the pipette. For the microejection application, solutions were delivered at a constant volume rate of 15.4 ± 11.6 nl s−1 (4 pipettes) leading to a total ejection volume per application in the range of 1-50 nl applied from a distance of about 100 μm from the cell soma.
Solutions of sodium butyrate or choline butyrate (Sigma) were applied to the cell soma of the DiI-filled neurones by pressure microejection. Sodium butyrate (stock 1 M; pH = 7.4) was used in concentrations ranging between 10 and 100 mM and dissolved in Krebs solution with a complementary concentration of NaCl to compensate for the increase in osmolarity.
Drugs
Most drugs were dissolved in Krebs solution. The following drugs were purchased from Sigma: nifedipine, atropine, mecamylamine, tetrodotoxin (TTX). Guinea-pig vasoactive intestinal peptide (VIP) was purchased from Peninsula (Belmont, CA, USA). The neurokinin-1 (NK-1) agonist [(SAR9, Met(O2)11]-SP and the NK-3 agonist (MePhe7)-NKB were purchased from Novabiochem (Bad Soden, Germany). The specific NK-1 receptor antagonist CP-99,994 was donated by Pfizer (Groton, CT, USA). The specific NK-3 antagonist SR 142801 was from Sanofi (Montpellier, France) and was dissolved in 100% DMSO at a concentration of 10 mM.
Statistics
Unless otherwise specified all values are given as means ±s.d.; n, number of cells. The χ2 test was used to test for significant differences in proportions. Means were compared with Student's paired or unpaired t test or the Spearman Rank test for data not normally distributed. One-way analysis of variance (Student- Newman-Keuls test) was performed for multiple comparisons. Differences were considered as significant for P < 0.05.
RESULTS
The spatial distribution pattern of DiI-labelled myenteric neurones projecting to the mucosa was identical to that previously reported (Neunlist & Schemann, 1997).
Neurochemical and morphological characteristics
The DiI-labelled myenteric neurones were stained for choline acetyltransferase (ChAT) and 28 kDa calbindin (Calb) protein. Calbindin has been suggested to label the primary intrinsic afferent neurones in the guinea-pig ileum and colon (Furness et al. 1990; Song et al. 1994; Neunlist & Schemann, 1997). Our study revealed that 61% of the DiI-labelled neurones were ChAT/Calb immunoreactive (40/66 cells) (Fig. 1A, C and D), and 23% (15/66) of the DiI-labelled neurones were ChAT immunoreactive but Calb negative. Of the remaining 11 cells, five were ChAT negative but Calb positive, and six cells were neither ChAT nor Calb positive. Similar results were obtained in a previous study (Neunlist & Schemann, 1997). The projection direction of the neurones was not related to a specific neurochemical code.
Figure 1. Immunohistochemical, morphological and electrophysiological properties of DiI-labelled myenteric AH neurones projecting to the mucosa in the proximal colon.
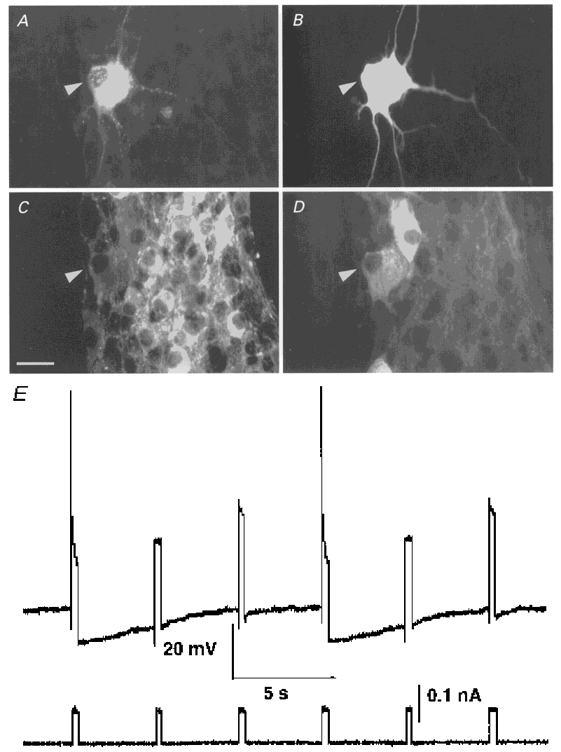
A-D, micrographs of an electrophysiologically characterized DiI-labelled myenteric neurone projecting to the mucosa (DiI-labelling in A) which was filled with neurobiotin to reveal a dendritic Dogiel type II morphology (shown in B). This neurone (marked by an arrowhead) was immunoreactive for choline acetyltransferase (C) and calbindin (D). Bar represents 25 μm. E, a DiI-labelled myenteric neurone behaved like an AH neurone: membrane response shown above and current trace shown below. Intracellular depolarizing pulses induced action potentials followed by a long-lasting late after-hyperpolarization during which the excitability of the cell was decreased.
In order to reveal the morphology of the DiI-labelled myenteric neurones, they were intracellularly labelled with neurobiotin. Except for one unipolar neurone exhibiting multiple short processes and electrophysiological S neurone characteristics (see below), all neurones had large cell bodies with ovoid or round shapes. All DiI-labelled myenteric neurones were multipolar with short and long processes and had Dogiel type II morphology (Fig. 1B). The size of the neurobiotin-filled soma, not including short and long processes, was on average 1039 ± 334 μm2 (major axis, 51 ± 7 μm; minor axis, 28 ± 5 μm; n = 63). The majority of the neurones (70%; 26/37) had a combination of short dendritic-like processes and long processes, which is typical for dendritic Dogiel type II neurones (Stach, 1989). The number of long processes was on average 3.5 ± 1.1 (n = 41; range, 2-6) and 81 ± 20% of them (n = 41) projected in the circumferential direction. A circumferential projection was defined when the circumferential projection was greater than the longitudinal projection (whenever the endings of the processes had co-ordinates with |X| < |Y| relative to the location of the DiI-filled cell body). In addition, the majority of the circumferentially projecting processes made numerous varicose-like swellings in the ganglia. One of the processes also projected towards the DiI application site. In the majority of the cases, we could not follow the process up to the application site as it was cut as soon as it entered the circular muscle layer that was removed during the preparation. In those cases where proximal process potentials (for description, see below) were elicited by electrical stimulation of the mucosa at the DiI application site, the process could be followed close to the DiI application site.
Electrophysiological characteristics
Intracellular recordings were made from a total of 85 DiI-labelled myenteric neurones with projections to the mucosa (46 preparations). Impalements were made from 60 ascending neurones, 10 descending neurones and 15 circumferentially projecting neurones. This proportion reflected the conscious choice of the experimenter. The DiI-labelled neurones impaled were located at a distance of 2.7 ± 0.9 mm for ascending neurones, 2.0 ± 0.7 mm for descending neurones and 1.9 ± 0.8 mm for circumferentially projecting neurones from the DiI application site.
All DiI-labelled neurones, except one, had electrophysiological characteristics of AH neurones in response to intracellular electrical stimulation (Fig. 1E). The non-AH cell was an S neurone with a resting membrane potential of -52 mV and responded to interganglionic fibre tract stimulation with fast excitatory postsynaptic potentials (fEPSPs). The AH neurones responded to depolarizing current pulses of sufficient amplitude with one to two action potentials. The half-maximal duration of the action potential was 2.3 ± 0.3 ms (n = 8). The maximal amplitude of the late after-hyperpolarization was on average 6.8 ± 1.8 mV (n = 7) and had a duration of 10 ± 3 s (n = 7). The above values were analysed after discharge of a single action potential. The mean resting potential of the DiI-labelled AH neurones was -65.0 ± 6.0 mV (n = 84). Descending, ascending and circumferentially projecting neurones had similar resting potentials of -64.4 ± 6.0, -64.9 ± 6.4 and -65.0 ± 4.2 mV (P = 0.97), respectively.
In order to test if the characteristics of the AH neurones could have been modified by the 2-3 days of organ culture, we recorded from AH neurones from fresh preparations of myenteric neurones in the proximal colon. In fresh preparations, AH neurones (n = 6) had a resting membrane potential of -60.8 ± 7.65 mV, a half-maximal duration of the action potential of 2.4 ± 0.5 ms and an after-hyperpolarization amplitude of 7.9 ± 3.1 mV; all parameters were not significantly different from those from neurones impaled in cultured tissue.
Some DiI-labelled AH myenteric neurones (28/84; 33%) exhibited spontaneously occurring hyperpolarizing potentials (SHPs) (Fig. 2). SHPs occurred in 40% of the descending, 32% of the ascending and 40% of the circumferentially projecting DiI-labelled neurones. Neurones with or without SHPs had a similar resting membrane potential which was measured during the depolarized phase (-65.2 ± 5.8 vs. -64.3 ± 6.0 mV; P = 0.53). Furthermore, there was no significant difference in the projection distance between neurones with or without SHPs (2.67 ± 0.66 vs. 2.32 ± 1.02 mm; P = 0.12). SHPs started to occur a few minutes after the impalement and appeared to be elicited after discharge of the first action potential or after activating the neurones by stimulation of interganglionic fibre tracts. SHPs could be recorded on average for a period of 28 ± 15 min (range 11-41 min; n = 7) (Fig. 2A). The SHPs occurred periodically on average every 59.4 ± 24.4 s (range 27-115 s; n = 12). The duration of the SHPs measured at the half-maximal amplitude was 10.8 ± 1.8 s (n = 11). The average amplitude of the SHPs was 9.1 ± 1.8 mV (n = 8). The amplitude of the SHPs increased in five out of seven cells when the membrane potential was clamped to more positive values. The input resistance decreased during the SHPs (Fig. 2A). Compared with the resting phase the excitability was decreased during the SHPs (Fig. 2B). SHPs persisted in the presence of 0.5 μm atropine (n = 8) or 1 μm tetrodotoxin (TTX) (n = 4). During perfusion of TTX, neurones with SHPs stayed quiescent for an average of 3.3 ± 1.3 min before resuming their SHPs. Furthermore, TTX induced in all cells (with or without SHPs) tested a slow membrane depolarization of 3.9 ± 1.4 mV (n = 7). A small membrane depolarization of 2.3 ± 1.6 mV was also observed after perfusion of 0.5 μm atropine (n = 6) that was not significantly different from that induced by TTX (P = 0.09). There was no relation between the presence or absence of SHPs and the neurochemical coding.
Figure 2. DiI-labelled mucosally projecting myenteric AH neurones exhibited spontaneously occurring hyperpolarizing potentials (SHPs).
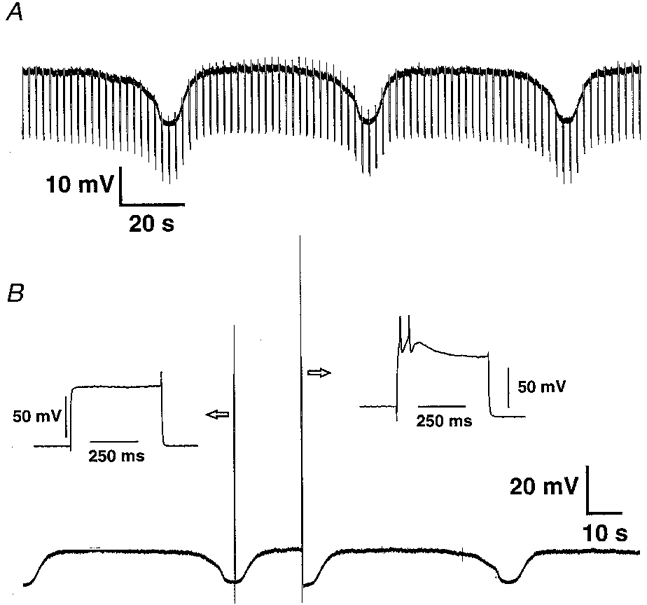
A, the SHPs were associated with a decrease in membrane resistance (shown by the decrease in the membrane response to hyperpolarizing pulses of 0.03 nA; current trace not shown). B, the excitability of the cell changed during the hyperpolarizing phase where only a passive change in membrane potential was observed (see left inset). The same pulse intensity was able to induce action potentials during the depolarized phase (see right inset).
During the experiments, illumination of the tissue to identify the DiI-labelled neurones was kept to a minimum (5 s maximum) in order to avoid potential photodynamic damage. Intentional illumination of the DiI-filled cells induced in the majority of the cells after about 10 s of illumination a slow reversible hyperpolarization associated with a decrease in cell excitability and membrane resistance.
Synaptic characteristics
The synaptic inputs to DiI-labelled myenteric neurones projecting to the mucosa were studied by focal electrical stimulation of the interganglionic fibre strands. We were unable to detect, except in the S neurone, a fEPSP in the AH neurones projecting to the mucosa (Fig. 3A). For technical reasons, descending and ascending neurones were stimulated by electrodes placed on fibre tracts located circumferentially or orally and circumferentially or anally, respectively. Stimulation of one interganglionic strand induced an action potential (probably antidromic) in 19 out of 27 neurones; this number reached 4 out of 5 by stimulating two different strands. The non-synaptic nature of this action potential was verified since hyperpolarizing the cell never resulted in an increased amplitude of the electrically induced response (n = 6), which would be typical for a fEPSP (see also Brookes et al. 1995).
Figure 3. DiI-labelled mucosally projecting myenteric AH neurones responded to electrical stimulation of an interganglionic fibre tract with slow excitatory post synaptic potentials (sEPSPs) but not with fast EPSPs.
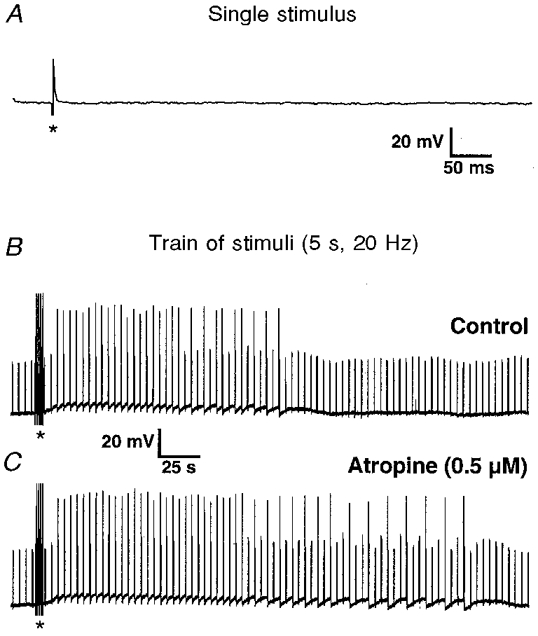
A, a single pulse failed to evoke a fast EPSP. The deviation in the membrane potential trace is the stimulus artefact (marked by an asterisk). B, a train of extracellular pulses (5 s; 20 Hz; 300 μs; marked by asterisks in B and C) induced a sEPSP. Increased excitability is illustrated by the discharge of action potentials during previously subthreshold intracellular current pulses of 0.09 nA (current trace not shown). C, the sEPSP was potentiated in atropine (0.5 μm) as indicated by the prolonged duration of the sEPSP and the associated increased discharge of action potentials. Calibration bars in B also apply to C.
Trains of 50 or 100 pulses delivered at 20 Hz induced in 94% of the cells tested (34 out of 36) a slow EPSP (sEPSP) associated with an increase in cellular excitability (Fig. 3B). The average amplitudes of the sEPSP were 7.4 ± 4.8 mV (n = 23) and 6.2 ± 3.4 mV (n = 31) for trains of 50 and 100 pulses, respectively.
Results from myenteric neurones in the ileum indicated that AH neurones communicate with other AH neurones via sEPSPs (Kunze et al. 1993). Since the vast majority of myenteric neurones with mucosal projections were ChAT immunoreactive and a proportion were also SP immunoreactive (Song et al. 1991; Neunlist & Schemann, 1997), the role of both acetylcholine and SP as putative mediators of sEPSPs was investigated. During atropine superfusion (0.5 μm; n = 6) the duration of sEPSPs measured at half-maximal amplitude increased by 51% (100 ± 35 vs. 151 ± 80 s; n = 6; P = 0.04) but the maximum amplitude remained unchanged (7.0 ± 6.0 vs. 7.3 ± 5.9 mV; n = 6; P = 0.36; paired t test) (Fig. 3C).
The putative involvement of SP in the sEPSPs was investigated by studying the effect of the NK-1 antagonist CP-99,994 (1 μm) and the NK-3 antagonist SR 142801 (1 μm). Perfusion of CP-99,994 for at least 10 min did not significantly modify the sEPSP (n = 3). In agreement, DiI-labelled AH neurones did not respond to pressure microejection of 20 μm of the NK-1 agonist [(SAR9, Met(O2)11]-SP (n = 4). However, pressure microejection of the NK-3 agonist (MePhe7)-NKB (20 μm) induced in all neurones tested (n = 8) a sEPSP-like response consisting of membrane potential depolarization associated with increased action potential discharge and a decrease in the duration of the late after-hyperpolarization. Bath perfusion of the NK-3 antagonist SR 142801 (up to 60 min) at a concentration of 1 μm significantly reduced the amplitude and the duration (measured at the half-maximal amplitude) of the sEPSP by 50% (6.1 ± 4.4 vs. 3.1 ± 3.5 mV; n = 7; P = 0.002) and by 53% (180 ± 59 vs. 85 ± 57 s; n = 6; P = 0.009; paired t test), respectively (Fig. 4). In one neurone we were able to fully block the sEPSP after a 10 min superfusion of SR 142801 (Fig. 4).
Figure 4. sEPSPs in mucosally projecting myenteric AH neurones were mediated via activation of NK-3 receptors.
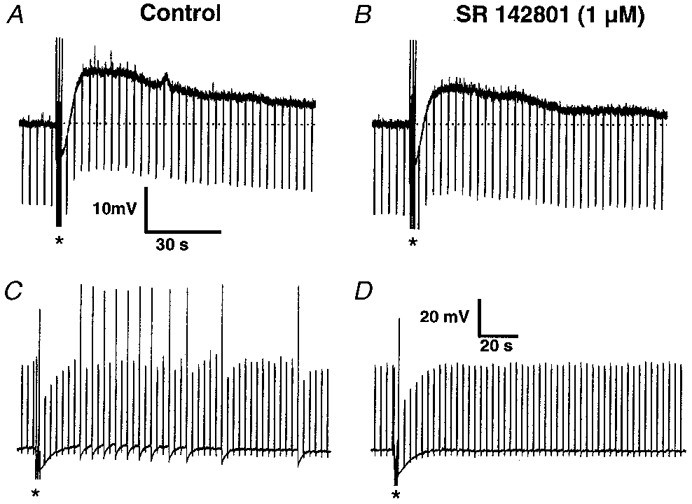
A, a train of extracellular pulses (2.5 s; 20 Hz; 300 μs; indicated by the asterisks) induced a sEPSP. During the depolarization the membrane resistance was increased as indicated by the increased amplitude of the membrane response to hyperpolarizing current pulses of 0.03 nA (current trace not shown). B, the amplitude and duration of the sEPSP was reduced following 15 min perfusion of the NK-3 receptor antagonist SR 142801 (1 μm). Dotted lines indicate the resting membrane potential which was -72 mV in this neurone. Calibration bars in A also apply to B. C, a train of extracellular pulses (5 s; 20 Hz; 300 μs; indicated by the asterisks) induced a sEPSP with a small amplitude of 4 mV. In this neurone depolarizing pulses of 0.08 nA were applied to indicate the increased excitability during the sEPSP (current trace not shown). D, the depolarization and the action potential discharge during the sEPSP was totally abolished following 10 min perfusion of the NK-3 receptor antagonist SR 142801 (1 μm). Calibration bars in D also apply to C.
Although all DiI-labelled neurones were VIP negative, VIP-positive terminals were abundant in the myenteric ganglia. In agreement with this observation, pressure microejection of VIP (20 μm) did elicit a slow membrane depolarization associated with an increase in membrane resistance and cellular excitability (n = 7).
Responses to electrical stimulation of the mucosa
Electrical stimulation with an unipolar electrode placed directly onto the mucosal DiI application site evoked in 8 out of 13 cells potentials with two distinct patterns (Fig. 5A and C). In the cells not responding to electrical stimulation we observed, following the neurobiotin labelling, that the axons projecting to the DiI application site were cut as they entered the circular muscle layer due to the dissection. The apparent conduction velocity was calculated to be 0.32 ± 0.13 m s−1 (n = 8). Current clamping the cells to more negative potentials reduced the amplitude in 3 out of 4 cells tested; in the remaining cell there was no change in amplitude (Fig. 5A and B). The potentials had a rise time of 1.2 ± 0.1 ms (n = 6; measured between 10 and 90% amplitude) and a half-maximal duration of 4.7 ± 0.9 ms (n = 6). Taken together, these properties indicated that proximal process potentials have been evoked, i.e. potentials arising from a process belonging to the impaled neurone. In six of the neurones responding to electrical stimulation of the mucosa we also observed spontaneously occurring proximal process potentials (Fig. 5D). Their rise time and duration did not differ from those of the proximal process potentials induced by electrical stimulation of the mucosa. Like the electrically induced response, the spontaneously occurring proximal process potentials decreased in amplitude (n = 2) or remained constant (n = 1) with increasing hyperpolarization.
Figure 5. Proximal process potentials in two DiI-labelled mucosally projecting myenteric AH neurones.
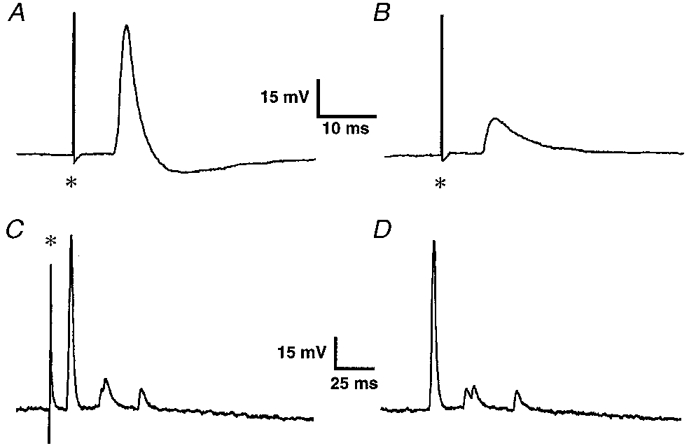
A, a potential was induced following a single focal electrical stimulation of the mucosa at the DiI application site; resting membrane potential was -68 mV. B, with hyperpolarization to -80 mV the amplitude of the potential decreased, suggesting its non-synaptic origin. C, in another neurone, a single electrical stimulation of the mucosa evoked a burst of potentials. D, an almost identical pattern occurred spontaneously in the same neurone. Asterisks indicate the stimulus artefact.
Responses to somal application of butyrate
During preliminary experiments we used choline butyrate (10 and 50 mM). Application of choline butyrate induced in all S neurones tested a rapid depolarization that was blocked in the presence of 100 μm mecamylamine. Using sodium butyrate, mecamylamine-sensitive responses were never observed. These results suggest that the effect of choline butyrate was mediated via activation of nicotinic receptors.
In 24 DiI-labelled myenteric AH neurones projecting to the mucosa, the effects of microejection of different concentrations of sodium butyrate onto the cell soma were investigated. Application of 1 s puffs of sodium butyrate at concentrations lower than 20 mM in the microejection pipette induced no response in the neurones tested (n = 4). Concentrations larger than 50 mM (range 50-100 mM) induced in 90% of the cells tested (18 out of 20) a biphasic membrane potential response. An initial rapid membrane potential hyperpolarization of 1.8 ± 1.1 mV (n = 16) occurred, followed by slow membrane potential depolarization of 2.5 ± 1.9 mV (n = 16). This rapid membrane hyperpolarization was very probably an artefact caused by the transient change in the extracellular chloride concentration (see below). The slow depolarizing response had a half-maximal duration of 11.0 ± 3.7 s (n = 7) and was associated with an increased input resistance. During this slow membrane depolarization, the cellular excitability was increased (Fig. 6A). Application of sodium butyrate to AH neurones with SHPs induced in all neurones tested (n = 6), in addition to the depolarizing response, a late onset hyperpolarization of 12.5 ± 3.5 mV which occurred 29.3 ± 7.2 s after the application of sodium butyrate (Fig. 6A). Addition of 0.5 μm TTX (n = 4) did not modify the response to sodium butyrate (Fig. 6B). In addition the response induced by sodium butyrate was not affected by atropine (up to 1 μm; n = 12). The responses to sodium butyrate did not decrease in amplitude after repeated microejections. In three cells tested, clamping the membrane potential at different voltages did not affect the amplitude of the initial hyperpolarization, but the amplitudes of the depolarizing and late hyperpolarizing responses increased with more positive clamp voltages and decreased with more negative voltages and reversed around -110 mV. In addition, sodium butyrate did not modify the action potential duration but only decreased the duration of the late after-hyperpolarization (n = 3) (Fig. 7).
Figure 6. Direct application of sodium butyrate onto the cell body induced multiple responses in DiI-labelled mucosally projecting myenteric AH neurones. The neurone illustrated exhibited spontaneously occurring hyperpolarizing potentials.
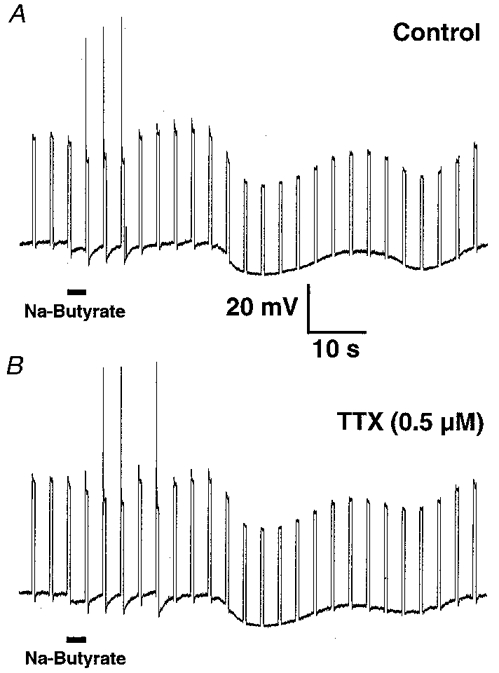
A, 3 s microejection of sodium butyrate (100 mM; duration of application marked by the bar) induced a membrane depolarization associated with action potential discharge during previously subthreshold depolarizing current pulses of 0.06 nA (current trace not shown). This was followed by a late onset membrane hyperpolarization. B, tetrodotoxin (TTX; 0.5 μm) had no detectable effect on sodium butyrate-evoked responses.
Figure 7. Superfusion of sodium butyrate increased reversibly the cellular excitability of mucosally projecting myenteric AH neurones.
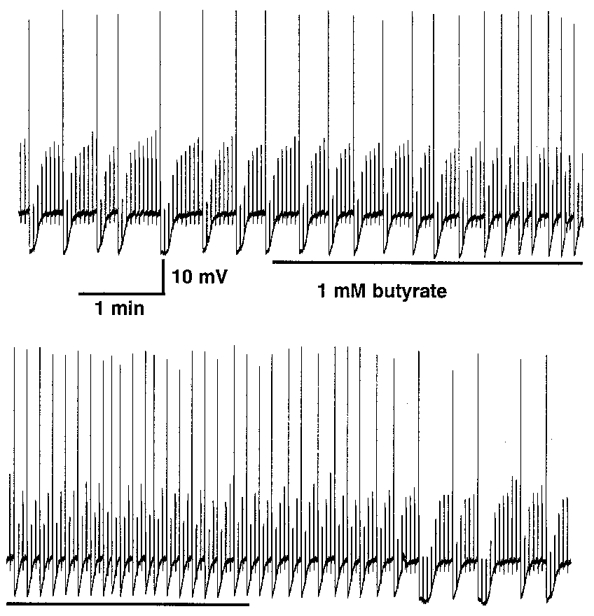
Superfusion with 1 mM sodium butyrate (time of superfusion indicated by the bar) increased the number of action potential discharges (large amplitude deflections) in response to depolarizing current pulses of 0.04 nA (current trace of depolarizing pulses of 300 ms duration given at 0.3 Hz not shown). Before perfusion of butyrate every 7th or 8th depolarizing pulse led to action potential discharge; in butyrate every 3rd or 4th depolarizing pulse led to action potential discharge. Note also the decrease in duration of the late after-hyperpolarization during butyrate superfusion. After wash-out the excitability level of the neurone returned to pre-butyrate levels.
In five cells where proximal process potentials could be evoked by electrical stimulation at the DiI application site, microejection of 100 mM sodium butyrate onto the DiI application site had no effect on the impaled neurone. In three of these cells butyrate was applied to the soma and induced the direct responses described above.
To compensate for osmolarity changes due to the high concentration of sodium butyrate in the microejection pipette the chloride concentration had to be lowered. In order to test whether the responses to butyrate were influenced by the low extracellular chloride concentration, we replaced 100 mM sodium butyrate by 100 mM sodium gluconate solution. Under these conditions, we observed in all cells tested (n = 4) the initial fast hyperpolarization but never the slow depolarization or the delayed hyperpolarization. This suggested that the initial hyperpolarization observed during sodium butyrate application could be an artefact due to the transient change in extracellular chloride concentration which, however, was not implicated in the late depolarizing or hyperpolarizing response to butyrate.
In order to obtain a dose-response curve of the butyrate effect on neuronal excitability, a total of eight DiI-labelled neurones projecting to the mucosa were superfused with different concentrations of butyrate ranging from 100 μm to 10 mM. To evaluate butyrate-induced changes in cellular excitability the number of action potentials per minute induced by intracellular current injection (300 ms, 0.02-0.1 nA, 0.3 Hz) during the butyrate perfusion was compared with the pre-infusion values (control). For all concentrations of butyrate, there was a significant increase in excitability compared with control, ranging from 38, 51 and 125% for butyrate concentrations of 100 μm (n = 6; P = 0.01), 1 mM (n = 5; n = 0.02) and 10 mM (n = 5; P = 0.004), respectively (Fig. 8).
Figure 8. Superfusion of sodium butyrate increased dose dependently the excitability of mucosally projecting myenteric AH neurones.
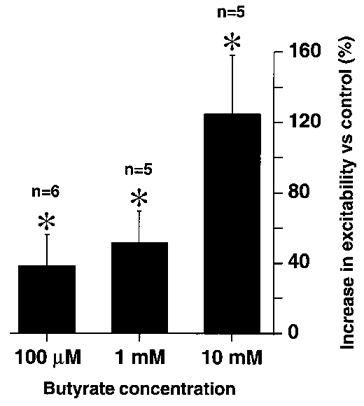
Sodium butyrate effects were expressed as percentage increase in the number of current pulses that evoked action potentials during a 1 min period in the presence of butyrate compared with 1 min pre-butyrate values. All concentrations of butyrate induced a significant increase in the number of pulses inducing action potentials. This was significantly more pronounced for 10 mM butyrate when compared with 1 or 0.1 mM. For the control period depolarizing pulses were set to suprathreshold values, had a 300 ms duration and were delivered at 0.3 Hz. The number of cells tested is indicated. * P < 0.05 compared with control.
DISCUSSION
This study directly demonstrated that myenteric neurones projecting to the mucosa of the guinea-pig colon behave electrophysiologically as AH neurones. In addition, the study also revealed that postsynaptic modulation of these neurones occurred via activation of NK-3 but not NK-1 receptors. Furthermore we have demonstrated that butyrate, a short chain fatty acid synthesized in the lumen of the colon, is able to directly stimulate this class of neurones.
Electrophysiological characteristics
One of the major results of this study was that myenteric neurones projecting to the mucosa had the electrophysiological characteristics of AH neurones. Various studies in the ileum have also suggested that myenteric neurones projecting to the mucosa are AH neurones (Kunze et al. 1995; Bertrand et al. 1997). Retrograde tracing methods have shown that the vast majority of myenteric neurones projecting to the mucosa of the guinea-pig ileum have Dogiel type II morphology (Song et al. 1991, 1994). Furthermore, Dogiel type II myenteric neurones have been shown to be AH neurones in the ileum (see Furness et al. 1998). Therefore, the electrophysiological properties of myenteric neurones innervating the mucosa appear to be conserved in the small and large intestine.
The electrophysiological data were obtained in neurones which were in organotypic culture for 2-3 days. Our results showed that organ culture did not modify the electrophysiological characteristics of AH neurones. Recent studies on submucous neurones kept in tissue culture for 3-4 days have revealed that the electrophysiological characteristics of these neurones were largely preserved after the culture (Song et al. 1997). A noteworthy result was the change in electrophysiological properties of DiI-labelled neurones that occurs during intentional continuous long term illumination (> 5 s) of the preparation. Contrary to a previous study in which a depolarization was observed (Brookes et al. 1995), long term illumination of DiI-labelled neurones induced a membrane hyperpolarization in our study. The reasons for such a difference remain speculative but might result from a difference in light intensity or illumination wavelength used.
One property of the myenteric neurones projecting to the mucosa of the proximal colon is their ability to exhibit spontaneously occurring hyperpolarizing potentials (SHPs) which were observed in 33% of the neurones studied. Such SHPs were also observed by Messenger et al. (1994) in about 23% of AH myenteric neurones in the guinea-pig proximal colon. SHPs were also reported in submucosal and myenteric neurones of the small intestine (Wood et al. 1982; Surprenant, 1984). In contrast to the study by Surprenant (1984), in our study TTX did not abolish SHPs, suggesting that they were not synaptically driven but were rather an intrinsic property of the neurones. In addition, the observation that SHPs usually started to occur after action potential discharge suggests that calcium is involved in their initiation. Different indirect evidence has suggested the participation of calcium-dependent K+ (KCa) channels in the generation of the SHPs. First, the amplitude of the SHPs was similar to that of the late after-hyperpolarization of the AH neurones, which was due to the activation of charybdotoxin-sensitive KCa channels (Kunze et al. 1994). Second, the hyperpolarization during SHPs was associated with a decrease in membrane resistance, as was the case during the late after-hyperpolarization of the AH neurones. In contrast to our study, Wood et al. (1982) showed that SHPs in the ileum were associated with an increase in input resistance. No oscillatory behaviour was observed in S neurones (Messenger et al. 1994; M. Neunlist, G. Dobreva & M. Schemann, unpublished observations).
It is worth mentioning that oscillations in motor and secretory processes have been observed in the colon, although involvement of SHPs remains speculative. The frequency of the neuronal oscillation observed in our study is close to the rhythm observed in the contractile activity of the colon (Sarna, 1986). In addition, in the dog colon, minute slow neurogenic oscillations in the membrane potential of muscle cells occur near the myenteric plexus (Keef et al. 1997).
The morphological classification of the myenteric neurones projecting to the mucosa revealed that the majority of them (70%) were dendritic multipolar Dogiel type II neurones with large cell bodies. This result is consistent with previous studies in the proximal colon, which showed that the majority of myenteric Dogiel type II neurones were classified as dendritic Dogiel type II neurones (Messenger et al. 1994). In the small intestine, dendritric Dogiel type II neurones represented only 10% of the Dogiel type II neurones (Bornstein et al. 1991; Brookes et al. 1995) and were shown to have long aboral projections (Brookes et al. 1995).
Pharmacology of the sEPSP
We have shown that none of the AH neurones projecting to the mucosa exhibited fEPSPs in response to electrical stimulation of the internodal strands. Although we might have missed fast inputs because it was not possible to stimulate all the fibres connecting the ganglia which contained the impaled neurone, our observation was in agreement with previous studies showing that myenteric AH neurones in the small intestine (Bornstein et al. 1991; Kunze et al. 1993) and in the proximal colon (Messenger et al. 1994) did not receive fEPSPs. However, it has to be noted that some studies have reported fEPSPs in AH neurones in the small (see Wood, 1994) and large (Wade & Wood, 1988; Tamura & Wood, 1989) intestine. Further studies have to address the question of whether fEPSP-receiving AH neurones might fulfil functions other than primary afferent functions.
On the other hand, trains of pulses induced sEPSPs in the vast majority of the neurones tested. The ability to induce sEPSPs in this class of putative intrinsic primary afferent neurones supports the idea that these neurones are part of a self-reinforcing network (Furness et al. 1998). Such a feed-forward circuit consisting of Dogiel type II AH neurones has been proposed earlier by Wood (1994), although Wood did not consider them as primary afferent neurones at that time. Kunze et al. (1993) have shown that AH neurones receive synaptic inputs from other AH neurones via sEPSPs. The synaptic interactions between intrinsic primary afferent neurones could serve to regulate the sensitivity of these neurones, since the activation of one AH neurone could lead to the activation of numerous other AH neurones.
Since previous studies have shown that myenteric AH Dogiel type II neurones contain ChAT and probably also substance P (Song et al. 1991; Neunlist & Schemann, 1997), we have focused on the involvement of acetylcholine and substance P in the mediation of sEPSPs. It is unlikely that acetylcholine is the mediator of the sEPSPs since sEPSPs were not reduced in amplitude or duration in the presence of atropine but were rather potentiated by atropine. Such an increase in the duration of sEPSPs in the presence of a muscarinic antagonist was also observed in myenteric neurones of the guinea-pig ileum (Morita et al. 1982). Although acetylcholine usually has excitatory effects in the enteric nervous system, it has been shown to inhibit, presynaptically, its own release and that of noradrenaline (North et al. 1985) and to hyperpolarize submucosal neurones of the guinea-pig caecum (Mihara & Nishi, 1989).
Our results would indicate that the sEPSPs in mucosally projecting myenteric AH neurones were partly mediated via NK-3 receptors because the NK-3 antagonist SR 142801, but not the NK-1 antagonist CP-99,994, suppressed sEPSP amplitude and duration. Further evidence for the involvement of NK-3 receptors in the sEPSPs was the observation that in all cells tested microejection of the NK-3 agonist (MePhe7)-NKB, but not of the NK-1 receptor agonist [(SAR9, Met(O2)11]-SP, mimicked sEPSPs. In agreement with these results, it was shown in the guinea-pig ileum that NK-1 receptor immunoreactivity was primarily observed on Dogiel type I neurones (Portbury et al. 1996) and only sparsely present on ChAT/Calb positive neurones (Lomax et al. 1998), most of them very probably being Dogiel type II AH neurones. At least in the rat, NK-3 receptor immunoreactivity was shown to be colocalized, although not exclusively, on myenteric and submucosal calbindin-positive neurones (Mann et al. 1997). The role of substance P as a mediator of the sEPSP has already been shown in the guinea-pig ileum (Katayama & North, 1978) and the NK-3 receptor agonist senktide has been shown to induce sEPSP-like responses in AH neurones (Bertrand & Galligan, 1995). In addition, in myenteric neurones of the duodenum, the stomach, and the gall blader NK-3 receptor agonists were more effective in inducing sEPSP like responses than SP or NK-1 receptor agonists (Hanani et al. 1988; Schemann & Kayser, 1991; Mawe, 1995).
The inability of the NK-3 antagonist SR 142801 to fully block sEPSPs may have different causes. Longer incubation periods might have increased the effectiveness of the antagonist (Patachini et al. 1995), although we used incubation periods up to 60 min. However, a recent study revealed that the antagonistic effects on the senktide-evoked increase in cAMP levels of 15 or 60 min incubations with 1 μm SR 142801were not significantly different (Beaujouan et al. 1997). The involvement of additional neurotransmitters in the generation of sEPSPs may explain the lack of total block by the NK-3 antagonist. Candidates would be a number of amines and peptides (see Wood, 1994) including serotonin via activation of 5-HT1P (Wood & Mayer, 1978; Mawe et al. 1986), vasoactive intestinal peptide (Wood, 1994; present study) or glutamate via activation of NMDA receptors (Liu et al. 1997).
Direct excitatory effect of sodium butyrate on myenteric neurones projecting to the mucosa
The lumen of the mammalian proximal colon is the site of bacterial fermentation of carbohydrates resulting in the production of a variety of short chain fatty acids. The physiological effects of short chain fatty acids (SCFAs) are wide ranging and vary from one region of the gut to the other. In the colon, SCFAs were shown to influence both motility and secretory processes. The motility effect was shown to be dose dependent, i.e. at low concentrations SCFAs induced an increase in the contraction force (Yajima, 1985), while a decrease in colonic motility was observed at higher concentrations (Squires et al. 1992). SCFAs led to an increase in chloride secretion in the distal colon and small intestine that were in part neurally mediated (Yajima, 1988; Diener et al. 1996).
Our study revealed that myenteric neurones projecting to the mucosa have a somal sensitivity for sodium butyrate and respond to doses as low as 100 μm. Although it is not known to what butyrate concentrations myenteric neurones would be normally exposed, it is worth noting that butyrate concentrations of up to 3 mM have been measured in the portal blood (Cheng et al. 1987). A recent study revealed that mucosal terminals of myenteric neurones were sensitive to acetate (Bertrand et al. 1997). However, a direct somal sensitivity was not demonstrated. Comparing the proportion of responding to non-responding mucosally projecting myenteric neurones in this study, it has to be noted that mucosal application of acetate evoked a response in about 40% of the neurones (Bertrand et al. 1997) whereas in our study direct somal application of butyrate evoked a response in 90% of the neurones. In addition, responses to somal application were readily reproducible whereas responses to mucosal application of acetate were less reliable, as indicated by a 39% intraexperimental response rate (i.e. acetate elicited neuronal responses in about one-third of the trials in the same neurone). This suggested that neuronal responses to somal application were more robust than responses to mucosal application of SCFAs. Our study revealed that application of butyrate directly onto the neurones had a biphasic effect, an initial excitatory response followed by a late inhibition; in the study by Bertrand et al. (1997) only an increase in neuronal activity after mucosal application of acetate occurred. An intermediary substance which is released by non-neuronal cells within the mucosa in response to mucosal application of chemicals may be involved in the transduction process (Bertrand et al. 1997; Furness et al. 1998). Indeed, intrinsic primary afferent neurones in the submucosal plexus were shown to be indirectly activated following mechanical stimulation of the mucosa by the release of 5-HT from enterochromaffin cells (Kirchgessner et al. 1992). In our study, the myenteric neurones did not respond to mucosal application of butyrate, although they showed proximal process potentials following electrical stimulation of the mucosa directly at the DiI application site. A similar observation was made by Bertrand et al. (1997), who showed that some AH neurones did not respond to mucosal acetate although the same neurones could be activated by electrical stimulation of the mucosa. One possible explanation for why none of the neurones in our study responded to mucosal application of butyrate might be that the release of intermediary substances, like 5-HT, would have been impaired in our preparation because the functionality of the enterochromaffin cells was very probably lost after 48-72 h of tissue culture. Another explanation could be that the amounts of sodium butyrate delivered onto the mucosa to stimulate the mucosal terminals of the AH neurone were too small, compared with the amount delivered by Bertrand et al. (1997) (1-50 nl in our study vs. 1-5 μl in their study).
In conclusion, putative intrinsic primary afferent myenteric neurones with mucosal projections form a population with uniform electrophysiological, immunohistochemical and morphological characteristics. These neurones received sEPSPs that were mediated via NK-3 receptors. In addition, they responded directly to somal application of butyrate and might be involved in the effects of short chain fatty acids.
Acknowledgments
The authors wish to thank Susanne Hoppe for her excellent technical support. This work was supported by SFB 280 to M.S., a Marie Curie Fellowship from the European Community to M.N. and a DAAD fellowship to G.D. We would like to dedicate this work to our co-author Gisela Dobreva who tragically died on August 10th, 1998.
References
- Beaujouan JC, Saffroy M, Torrens Y, Glowinski J. Potency and selectivity of the tachykinin NK3 receptor antagonist SR 142801. European Journal of Pharmacology. 1997;319:307–316. doi: 10.1016/s0014-2999(96)00848-5. [DOI] [PubMed] [Google Scholar]
- Bertrand PP, Galligan JJ. Signal-transduction pathways causing slow synaptic excitation in guinea pig myenteric AH neurons. American Journal of Physiology. 1995;269:G710–720. doi: 10.1152/ajpgi.1995.269.5.G710. [DOI] [PubMed] [Google Scholar]
- Bertrand PP, Kunze WAA, Bornstein JC, Furness JB, Smith ML. Analysis of the responses of myenteric neurons in the small intestine to chemical stimulation of the mucosa. American Journal of Physiology. 1997;273:G422–435. doi: 10.1152/ajpgi.1997.273.2.G422. [DOI] [PubMed] [Google Scholar]
- Bornstein JC, Hendriks R, Furness JB, Trussell DC. Ramifications of the axons of AH neurons injected with the intracellular marker biocytin in the myenteric plexus of the guinea pig small intestine. Journal of Comparative Neurology. 1991;314:437–451. doi: 10.1002/cne.903140303. [DOI] [PubMed] [Google Scholar]
- Brookes SJH, Song ZM, Ramsay GA, Costa M. Long aboral projections of Dogiel type II, AH neurons within the myenteric plexus of the guinea pig small intestine. Journal of Neuroscience. 1995;15:4013–4022. doi: 10.1523/JNEUROSCI.15-05-04013.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng BO, Trimble RP, Illman RJ, Stone BA, Topping DL. Comparative effects of dietary wheat bran and its morphological components (aleurone and pericarp-seed coat) on volatile fatty acid concentrations in the rat. Bristish Journal of Nutrition. 1987;57:69–76. doi: 10.1079/bjn19870010. [DOI] [PubMed] [Google Scholar]
- Costa M, Brookes SJH, Steele PA, Gibbins I, Burcher E, Kandiah CJ. Neurochemical classification of myenteric neurons in the guinea-pig ileum. Neuroscience. 1996;75:949–967. doi: 10.1016/0306-4522(96)00275-8. [DOI] [PubMed] [Google Scholar]
- Diener M, Vujicic Z, Scharrer E. Neuronally mediated anion secretion induced by short-chain fatty acids in the rat distal small intestine. Acta Physiologica Scandinavica. 1996;157:33–40. doi: 10.1046/j.1365-201X.1996.470184000.x. 10.1046/j.1365-201X.1996.470184000.x. [DOI] [PubMed] [Google Scholar]
- Furness JB, Kunze WA, Bertrand PP, Clerc N, Bornstein JC. Intrinsic primary afferent neurons of the intestine. Progress in Neurobiology. 1998;54:1–18. doi: 10.1016/s0301-0082(97)00051-8. 10.1016/S0301-0082(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Furness JB, Trussel DC, Pompolo S, Bornstein JC, Smith TK. Calbindin neurons of the guinea-pig small intestine: quantitative analysis of their numbers and projections. Cell and Tissue Research. 1990;260:261–272. doi: 10.1007/BF00318629. [DOI] [PubMed] [Google Scholar]
- Hanani M, Chorev M, Gilon C, Selinger Z. The actions of receptor-selective substance P analogs on myenteric neurons: an electrophysiological investigation. European Journal of Pharmacology. 1988;153:247–253. doi: 10.1016/0014-2999(88)90612-7. 10.1016/0014-2999(88)90612-7. [DOI] [PubMed] [Google Scholar]
- Hirst GDS, Holman ME, Spence I. Two types of neurones in the myenteric plexus of duodenum in the guinea pig. The Journal of Physiology. 1974;236:303–326. doi: 10.1113/jphysiol.1974.sp010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer V, Bornstein JC, Costa M, Furness JB, Takahashi Y, Iwanaga T. Electrophysiology of guinea-pig myenteric neurons correlated with immunoreactivity for calcium binding proteins. Journal of the Autonomic Nervous System. 1988;22:141–150. doi: 10.1016/0165-1838(88)90087-2. 10.1016/0165-1838(88)90087-2. [DOI] [PubMed] [Google Scholar]
- Katayama Y, North RA. Does substance P mediate slow synaptic excitation within the myenteric plexus? Nature. 1978;274:387–388. doi: 10.1038/274387a0. [DOI] [PubMed] [Google Scholar]
- Keef KD, Murray DC, Sanders KM, Smith TK. Basal release of nitric oxide induces an oscillatory motor pattern in the canine colon. The Journal of Physiology. 1997;499:773–786. doi: 10.1113/jphysiol.1997.sp021968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchgessner AL, Tamir H, Gershon MD. Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: activity-induced expression of Fos immunoreactivity. Journal of Neuroscience. 1992;12:235–248. doi: 10.1523/JNEUROSCI.12-01-00235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze WAA, Bornstein JC, Furness JB. Identification of sensory nerve cells in a peripheral organ (the intestine) of a mammal. Neuroscience. 1995;66:1–4. doi: 10.1016/0306-4522(95)00067-s. 10.1016/0306-4522(95)00067-S. [DOI] [PubMed] [Google Scholar]
- Kunze WAA, Bornstein JC, Furness JB, Hendriks R, Stephenson DS. Charybdotoxin and iberiotoxin but not apamin abolish the slow after-hyperpolarization in myenteric plexus neurons. Pflügers Archiv. 1994;428:300–306. doi: 10.1007/BF00724511. [DOI] [PubMed] [Google Scholar]
- Kunze WAA, Furness JB, Bertrand PP, Bornstein JC. Intracellular recording from myenteric neurons of the guinea-pig ileum that respond to stretch. The Journal of Physiology. 1998;506:827–842. doi: 10.1111/j.1469-7793.1998.827bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze WAA, Furness JB, Bornstein JC. Simultaneous intracellular recordings from enteric neurons reveal that myenteric AH neurons transmit via slow excitatory postsynaptic potentials. Neuroscience. 1993;55:685–694. doi: 10.1016/0306-4522(93)90434-h. [DOI] [PubMed] [Google Scholar]
- Liu MT, Rothstein JD, Gershon MD, Kirchgessner AL. Glutamatergic enteric neurons. Journal of Neuroscience. 1997;17:4764–4784. doi: 10.1523/JNEUROSCI.17-12-04764.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax AEG, Bertrand PP, Furness JB. Identification of the populations of enteric neurons that have NK1 tachykinin receptors in the guinea-pig small intestine. Cell and Tissue Research. 1998;294:27–33. doi: 10.1007/s004410051153. [DOI] [PubMed] [Google Scholar]
- Mann PT, Southwell BR, Ding YQ, Shigemoto R, Mizuno N, Furness JB. Localisation of neurokinin 3 (NK-3) receptor immunoreactivity in the rat gastrointestinal tract. Cell and Tissue Research. 1997;289:1–9. doi: 10.1007/s004410050846. [DOI] [PubMed] [Google Scholar]
- Mawe GM. Tachykinins as mediators of slow EPSPs in guinea-pig gall-bladder ganglia: involvement of neurokinin-3 receptors. The Journal of Physiology. 1995;485:513–524. doi: 10.1113/jphysiol.1995.sp020747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawe GM, Branchek TA, Gershon MD. Peripheral neural serotonin receptors: identification and characterization with specific antagonists and agonists. Proceedings of the National Academy of Sciences of the USA. 1986;83:9799–9803. doi: 10.1073/pnas.83.24.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenger JP, Bornstein JC, Furness JB. Electrophysiological and morphological classification of myenteric neurons in the proximal colon of the guinea-pig. Neuroscience. 1994;60:227–244. doi: 10.1016/0306-4522(94)90217-8. [DOI] [PubMed] [Google Scholar]
- Mihara S, Nishi S. Muscarinic excitation and inhibition of neurons in the submucous plexus of the guinea-pig caecum. Neuroscience. 1989;31:247–257. doi: 10.1016/0306-4522(89)90047-x. [DOI] [PubMed] [Google Scholar]
- Morita K, North RA, Tokimasa T. Muscarinic presynaptic inhibition of synaptic transmission in myenteric plexus of guinea-pig ileum. The Journal of Physiology. 1982;333:141–149. doi: 10.1113/jphysiol.1982.sp014444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunlist M, Schemann M. Projections and neurochemical coding of myenteric neurons innervating the mucosa of the guinea pig proximal colon. Cell and Tissue Research. 1997;287:119–125. doi: 10.1007/s004410050737. [DOI] [PubMed] [Google Scholar]
- Nishi S, North RA. Intracellular recording from the myenteric plexus of the guinea pig ileum. The Journal of Physiology. 1973;231:471–491. doi: 10.1113/jphysiol.1973.sp010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA, Slack BE, Surprenant A. Muscarinic M1 and M2 receptors mediate depolarization and presynaptic inhibition in guinea-pig enteric nervous system. The Journal of Physiology. 1985;368:435–452. doi: 10.1113/jphysiol.1985.sp015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patacchini R, Bartho L, Holzer P, Maggi CA. Activity of SR 142801 at peripheral tachykinin receptors. European Journal of Pharmacology. 1995;278:17–25. doi: 10.1016/0014-2999(95)00090-8. [DOI] [PubMed] [Google Scholar]
- Portbury AL, Furness JB, Young HM, Southwell BR, Vigna SR. Localisation of NK1 receptor immunoreactivity to neurons and interstitial cells of the guinea-pig gastrointestinal tract. Journal of Comparative Neurology. 1996;367:342–351. doi: 10.1002/(SICI)1096-9861(19960408)367:3<342::AID-CNE2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Sarna SK. Myoelectric correlates of colonic motor complexes and contractile activity. American Journal of Physiology. 1986;250:G213–220. doi: 10.1152/ajpgi.1986.250.2.G213. [DOI] [PubMed] [Google Scholar]
- Schemann M, Kayser H. Effects of tachykinins on myenteric neurones of the guinea-pig gastric corpus: involvement of NK-3 receptors. Pflügers Archiv. 1991;419:566–571. doi: 10.1007/BF00370296. [DOI] [PubMed] [Google Scholar]
- Schemann M, Sann H, Schaaf C, Mader M. Identification of cholinergic neurons in enteric nervous system by antibodies against choline acetyltransferase. American Journal of Physiology. 1993;265:G1005–1009. doi: 10.1152/ajpgi.1993.265.5.G1005. [DOI] [PubMed] [Google Scholar]
- Song ZM, Brookes SJH, Costa M. Identification of myenteric neurons which project to the mucosa of the guinea-pig small intestine. Neuroscience Letters. 1991;129:294–298. doi: 10.1016/0304-3940(91)90484-b. [DOI] [PubMed] [Google Scholar]
- Song ZM, Brookes SJH, Costa M. All calbindin-immunoreactive myenteric neurons project to the mucosa of the guinea-pig small intestine. Neuroscience Letters. 1994;180:219–222. doi: 10.1016/0304-3940(94)90524-x. [DOI] [PubMed] [Google Scholar]
- Song ZM, Brookes SJH, Neild TO, Costa M. Immunohistochemical and electrophysiological characterization of submucous neurons from the guinea-pig small intestine in organ culture. Journal of the Autonomic Nervous System. 1997;63:161–171. doi: 10.1016/s0165-1838(97)00005-2. [DOI] [PubMed] [Google Scholar]
- Squires PE, Rumsey RD, Edwards CA, Read NW. Effect of short-chain fatty acids on contractile activity and fluid flow in rat colon in vitro. American Journal of Physiology. 1992;262:G813–817. doi: 10.1152/ajpgi.1992.262.5.G813. [DOI] [PubMed] [Google Scholar]
- Stach W. A revised morphological classification of neurons in the enteric nervous system. In: Singer MV, Goebbel H, editors. Nerves and the Gastrointestinal Tract. Lancaster, UK.: Kluwer; 1989. pp. 29–45. [Google Scholar]
- Surprenant A. Slow excitatory synaptic potentials recorded from neurones of guinea-pig submucous plexus. The Journal of Physiology. 1984;351:343–361. doi: 10.1113/jphysiol.1984.sp015249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack JF, Wood JD. Electrical behaviour of myenteric neurones in the gastric antrum of the guinea-pig. The Journal of Physiology. 1992;447:49–66. doi: 10.1113/jphysiol.1992.sp018990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Wood JD. Electrical and synaptic properties of myenteric plexus neurones in the terminal large intestine of the guinea-pig. The Journal of Physiology. 1989;415:275–298. doi: 10.1113/jphysiol.1989.sp017722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade PR, Wood JD. Synaptic behavior of myenteric neurons in guinea pig distal colon. American Journal of Physiology. 1988;225:G184–190. doi: 10.1152/ajpgi.1988.255.2.G184. [DOI] [PubMed] [Google Scholar]
- Wood JD. Electrical activity from single neurons in Auerbach's plexus. American Journal of Physiology. 1970;219:159–169. doi: 10.1152/ajplegacy.1970.219.1.159. [DOI] [PubMed] [Google Scholar]
- Wood JD. Physiology of the enteric nervous system. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1994. pp. 423–482. [Google Scholar]
- Wood JD, Mayer CJ. Slow synaptic excitation mediated by serotonin in Auerbach's plexus. Nature. 1978;276:836–837. doi: 10.1038/276836a0. [DOI] [PubMed] [Google Scholar]
- Wood JD, Mayer CJ, Grafe P. Electrical slow waves in guinea-pig myenteric neurons. Journal of the Autonomic Nervous System. 1982;5:247–249. doi: 10.1016/0165-1838(82)90043-1. [DOI] [PubMed] [Google Scholar]
- Yajima T. Contractile effect of short-chain fatty acids on the isolated colon of the rat. The Journal of Physiology. 1985;368:667–678. doi: 10.1113/jphysiol.1985.sp015882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima T. Luminal propionate-induced secretory response in the rat distal colon in vitro. The Journal of Physiology. 1988;403:559–575. doi: 10.1113/jphysiol.1988.sp017264. [DOI] [PMC free article] [PubMed] [Google Scholar]


