Abstract
Peristalsis was evoked in guinea-pig small intestine by slow fluid infusion and recorded onto video and digitized. Spatio-temporal maps of diameter and longitudinal movement were constructed and parameters of motion were calculated.
During the filling of the isolated segments of intestine, rhythmic local longitudinal movements were observed at several points along the preparation. These phasic longitudinal muscle contractions were associated with small but significant local increases in diameter and probably reflect a passive mechanical coupling by connective tissue in the gut wall. In addition, occasional synchronized longitudinal muscle contractions caused net shortening of the preparation and always preceded the onset of peristaltic emptying.
Peristaltic emptying was characterized by a contraction of the circular muscle which usually started at the oral end of the preparation, that propagated aborally, propelling the contents. However, in 19% of trials, the first circular muscle contraction occurred in the aboral half of the preparation.
The propagation of peristalsis consisted of separate sequential circular muscle contractions several centimetres long, particularly in the oral half of the preparation, giving a ‘step-like’ appearance to the spatio-temporal map. The gut was transiently distended aboral to the propagating circular muscle contraction due to the propulsion of contents.
At each point in the preparation, the longitudinal muscle remained contracted during the propulsive part of the circular muscle contraction. Only when the circular muscle contraction became lumen occlusive did lengthening of the longitudinal muscle take place.
Spatio-temporal maps are a powerful tool to visualize and analyse the complexity of gastrointestinal motility patterns.
The controlled progression of contents along the gastrointestinal tract is an essential part of digestion. Different patterns of intestinal movements are involved in the physiological progression of contents along the digestive tract and are the result of the interplay between spontaneous activity of intestinal smooth muscle and enteric neural circuits (Costa & Furness, 1982; Huizinga et al. 1998). Almost one hundred years ago, Bayliss and Starling (1899) revealed the presence of polarized reflex pathways in the intestine and suggested that they were responsible for the propulsion of contents. The analysis of intestinal propulsion was significantly advanced by Trendelenburg in 1917 who showed that reproducible propulsive motor patterns could be triggered in isolated segments of guinea-pig ileum by liquid distension. This form of intestinal peristalsis elicited in vitro is dependent on the activation of enteric circuits as many investigators have demonstrated (Kosterlitz, 1968; Tonini et al. 1981; Waterman et al. 1994b).
Slow distension of isolated segments of guinea-pig intestine by liquid infusion produces a neurally-mediated shortening of the longitudinal muscle (Kosterlitz & Robinson, 1959) and an increase in diameter coinciding with an inhibitory reflex mechanism involving nitric oxide (intestinal accommodation; Waterman et al. 1994a). This initial response to liquid distension has been named the ‘preparatory phase’ (Trendelenburg, 1917; Kosterlitz, 1968). At a threshold volume or intraluminal pressure, a contraction of the circular muscle occurs at the oral end and propagates aborally to empty the segment. This propulsive event is called the ‘emptying phase’ and involves the activation of different enteric neural pathways (Waterman & Costa, 1994; Waterman et al. 1994b).
Despite the common description of this motor behaviour as the ‘peristaltic reflex’ (Kosterlitz, 1968), it has become apparent that there is a sequential activation of neural pathways. Thus, peristalsis should not be regarded as a simple reflex, but rather as a motor pattern, similar to some co-ordinated locomotor patterns (Tonini et al. 1996).
In order to analyse the components of this motor pattern, we have developed a simple method of constructing spatio-temporal maps of changes in diameter and longitudinal muscle length from video recordings. Using this method, we have analysed quantitatively the dynamics of peristalsis in the guinea-pig small intestine and have revealed new patterns of motor activity. Some of these results have been communicated previously (Hennig et al. 1998).
METHODS
Tissue preparation
Guinea-pigs (IMVS coloured) of either sex, weighing 250-400 g were killed by cervical dislocation, followed by bleeding from the carotid arteries (protocol approved by the Animal Welfare Committee of Flinders University). A ventral midline incision was made to expose the peritoneal cavity and a segment of ileum (6-8 cm long) was removed 10 cm oral to the ileo-caecal junction. The segment was flushed clean, cannulated at the oral and aboral ends, and placed in a heated organ bath containing Krebs solution of the following composition (mM): NaCl, 118; KCl, 4.7; NaH2PO4, 1.0; NaHCO3, 25; MgSO4, 1.2; D-glucose, 11.0; and CaCl2, 2.5; pH 7.4.
Experimental set-ups
Two experimental arrangements based on Tonini et al. (1981) and Waterman et al. (1994b) were used to study peristalsis elicited by slow intraluminal infusion of Krebs solution (Fig. 1A and B). A different experimental arrangement was used to study spontaneous peristalsis when intraluminal volume was held at a constant level (Fig. 1C).
Figure 1. Experimental arrangements used to study peristalsis.

A segment of ileum (black), cannulated at each end, was placed in oxygenated warmed Krebs solution. Intraluminal pressure was monitored with a pressure transducer (P) and Krebs solution was infused via the oral cannula by a syringe pump. Movements of the preparation were recorded onto S-VHS video tape. The preparations emptied their luminal contents via the aboral cannula, through a one-way valve or into a vertical tube. In A, both ends of the preparation were fixed to prevent the overall shortening of the segment and the height of the outflow was adjusted to control outflow resistance. In B, the preparation was able to shorten during filling as the outflow cannula was mounted on a flexible tube. In C, the preparation emptied via the aboral cannula into a large diameter tube, thus maintaining intraluminal pressure constant. Fluid flowed back into the intestine after emptying.
The oral end of a segment of small intestine was cannulated to allow slow infusion of Krebs solution into the lumen. The aboral end was cannulated and fitted with a one-way valve to prevent back flow of Krebs solution. Intraluminal pressure was measured at each end using pressure transducers (Statham P23X) connected to a MacLab data acquisition system (AD Instruments, Sydney, Australia). Both the oral and aboral ends were fixed to prevent shortening of the segment in the longitudinal direction (Fig. 1A).
Krebs solution was infused via the oral cannula at a constant rate (4.25 μl s−1) until a propagated peristaltic contraction occurred (emptying phase). At the beginning of the circular muscle contraction, the infusion was stopped and the segment was allowed to empty by the propagated peristaltic contraction. Cycles of peristalsis were repeated every 5 min.
In a second variation of the above preparation, the aboral end was cannulated and fitted with a one-way valve connected to a long piece of silastic tubing that was free to move in the longitudinal direction. Surface markers (black silk knots) were tied onto the mesentery approximately every 10-15 mm to monitor the motion of the longitudinal muscle layer (Fig. 1B). This arrangement permitted the changes in the longitudinal direction along the segment to be better investigated.
In a third variation, the aboral end was connected to a vertical open tube with no valve and was free to move in the longitudinal direction. Intraluminal pressure was set by varying the height of the column of Krebs solution in the aboral cannula by slowly infusing Krebs solution to a sub-threshold volume for the triggering of peristalsis (approximately 60%). In these experiments, surface markers were fixed as described above (Fig. 1C).
Image acquisition
A video camera (Panasonic WV-CL504) was positioned above the ileum (Fig. 1) to record intestinal motor patterns on a high resolution S-VHS video recorder (Panasonic AG-7355) in PAL format at 25 frames per second (frames s−1). Frames of motion were digitized on an Apple Power Macintosh (7600/132) from video using a modified version of the image analysis software developed by the National Institute of Health (NIH Image 1.62), at 25 frames s−1.
The maximum resolution achievable using S-VHS video tape is approximately 400-410 columns by 625 rows. Depending on the final magnification (approximately × 2.5), this corresponded to a vertical resolution of 0.15-0.25 mm and horizontal resolution of 0.2-0.3 mm. To achieve a workable balance between the capture rate and the size of the digitized image, a half sized capture screen was used (384 × 256 pixels) which enabled the video stream to be captured at video rate (25 frames s−1). The temporal accuracy of capture from the S-VHS video recorder onto the computer was very stable, with time discrepancies of less than 1 s h−1. The correlation between the video and the pressure recordings was established by using visual markers synchronized to time points in the pressure recording.
Construction of spatio-temporal maps of intestinal motion
The movement of the intestinal wall was portrayed by mapping changes in diameter (diameter map or DMap) and changes in length between marked points along the intestinal segment (longitudinal map or LMap) over time.
Diameter maps (Fig. 2)
Figure 2. Construction of spatio-temporal map of intestinal diameter (DMaps).

A single frame of a video recording of the intestine (A) was subjected to the threshold procedure (see Methods) and converted into a binary image (B). The number of black pixels in each column (corresponding to diameter of the intestine at each point along its length) was converted into a grey scale pixel (bar above preparation, in B). Multiple frames were treated in this way to produce a diameter map (C), showing the diameter at each point from the oral end of the preparation to the aboral end: the preparation was 55 mm long. In this map, the minimum diameter was 2.1 mm (represented by white pixels) and the maximum was 7.5 mm (black pixels) and the map represents a time period of approximately 18 s. Time starts at the top of the diameter map. The white dotted line corresponds to the frame shown in A and B. Using the diameter map, the profile of the intestine could be reconstructed at any moment (D).
A threshold procedure was applied to video frames (Fig. 2A) to define the edges of the gut. Images were converted to binary (1bit/black or white) with black representing the intestinal wall and white the background (Fig. 2B). The diameter of the preparation at every pixel column along the length of the segment was determined by the number black pixels in each column. These diameters were represented as single grey-scale pixels with the smallest diameter coded as white and largest diameter as black (Fig. 2B). Intermediate diameters were assigned a proportional level of grey. The grey-scale coded pixels from each frame of the movie were used to construct a single row. Sequential rows were placed underneath each other in a separate image to produce a spatio-temporal map of intestinal diameters (diameter map; DMap: Fig. 2C). The vertical displacement of the preparation from the top of the video image was measured by calculating the position of the first black pixel, and was stored in a separate image (not shown). The vertical displacement information was used in conjunction with the diameter map to reconstruct gut profiles (Fig. 2D).
Longitudinal maps (Fig. 3)
Figure 3. Construction of spatio-temporal maps of longitudinal motion (LMaps).

A single frame of a video recording shows five black surface markers along the length of the preparation (A). A DMap, constructed from 20 s of recording (B) shows the movements of the surface markers as vertical, pale, wavy bands - the black dotted line represents the frame shown in A. A tracking routine was used to define the positions of the surface markers (black wavy lines, C) and the midpoint between each band was determined (grey lines, C). The distance between adjacent surface marker lines was then determined and represented as a grey scale pixel, at the calculated midpoint. Linear interpolation of grey scales was used to fill in the areas between the midpoints (D). The resulting LMap shows local shortening of the preparation, compared with resting conditions (prior to Krebs infusion) as pale grey. Local lengthening of the preparation is shown as dark grey. The right hand end of the LMap (D) represents the net shortening of the preparation in each video frame.
In preparations with surface markers attached at regular intervals along the preparation (Fig. 3A), a diameter map was constructed as described above. In these maps, the longitudinal markers appeared as vertically oriented white lines (Fig. 3B). The movement of the longitudinal markers over time was extracted from the DMap using tracking algorithms within NIH Image 1.62 (Fig. 3C; black lines). The distance between the markers was calculated and encoded as a grey-scale value between each pair of markers (Fig. 3C; grey lines). A linear interpolation routine was applied to each row (representing a single time frame), resulting in a smooth transition of grey-scale values along the intestinal segment (Fig. 3D). Whiter areas in the LMap indicate regions between surface markers that were closer together with darker areas indicating regions that were further apart. The absolute movement between each pair of markers was scaled so that white represents the minimum distance, and black the maximum distance.
Reconstruction of intestinal profiles
Single profiles of an intestinal segment could be reconstructed from any row of the diameter map. Diameter values from a row were converted into pixel columns of corresponding diameter (Fig. 2D) with the vertical displacement providing the start position for each pixel column. Movies of reconstructed profiles could be produced that were indistinguishable from the original binary movie, provided that the outline of the intestine was free from specular noise.
The changes of diameter over time at a particular point along the intestine could be plotted as a time plot by converting grey-scale coded pixels at a particular position along the segment into vertical co-ordinates (Fig. 4, CM). To ensure that the diameter values were taken from the same point along the segment, the trajectories of the surface markers (Fig. 4) were used as guides from which diameter values were extracted. This time plot is equivalent to an isotonic recording from one point along the intestinal segment. The distance between two surface markers could be converted into a time plot using a similar technique (Fig. 4, LM). Smoothing routines were applied to reduce excessive pixellation of the time plots (3 or 5 point running means).
Figure 4. Example of peristalsis evoked by slow liquid infusion at 4.25 μl s−1.
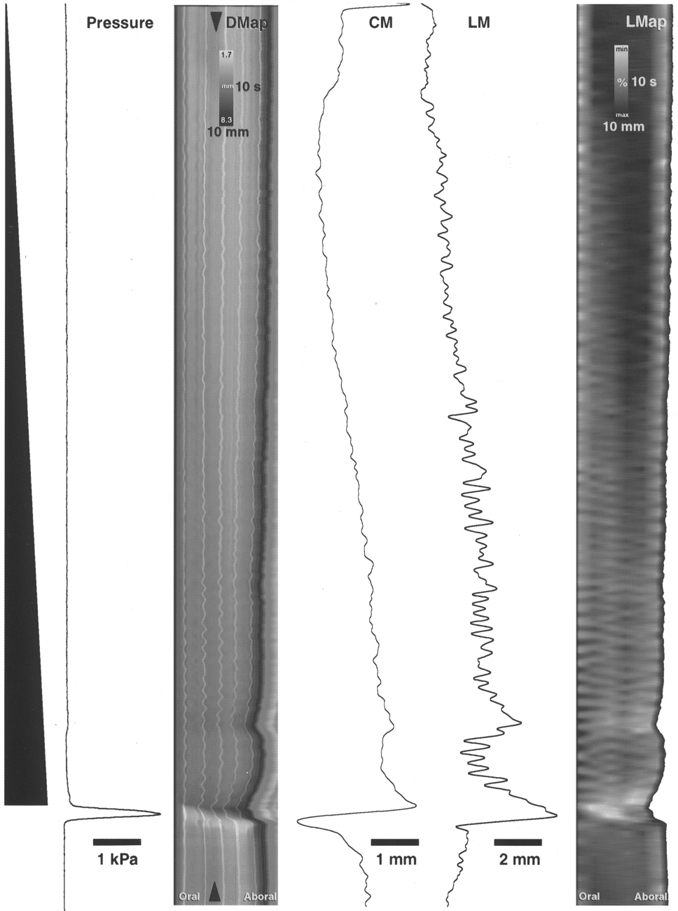
Infused fluid volume is shown schematically on the left as a black ramp. Time starts at the top of the figure. Intraluminal pressure is represented in the adjacent trace, showing a large, abrupt increase in pressure during peristaltic emptying. The diameter map (DMap) of the same period shows a gradual darkening during the course of the infusion, reflecting the gradual increase in diameter (see circular muscle diameter trace, CM). The arrowheads on the DMap show the point of the intestine at which circular muscle (CM) and longitudinal muscle (LM) traces were generated. Longitudinal muscle activity also increased during the preparatory phase (shown by the overall shortening of the preparation in the DMap and by the trace of longitudinal muscle activity, LM). The longitudinal map of the same period (right hand part of figure, LMap) shows a gradual shortening of the segment of intestine during the infusion (revealed as a gradual lightening of the map). The pattern of oblique stripes in the LMap during the preparatory phase corresponds to phasic changes in longitudinal muscle length (at approximately 37 c.p.m.) that occur with some delay along the length of the preparation, suggesting that the longitudinal muscle contractions propagate sometimes orally (sloping to the left) and at other times aborally (sloping to the right). There is a sudden net shortening of the longitudinal muscle just prior to the onset of peristalsis (visible in both the DMap and LMap). The circular muscle initially lengthens at the start of peristaltic emptying, then rapidly shortens (leftward deflection on CM trace). In contrast the longitudinal muscle, at the same point in the intestine, shortens prior to emptying (rightward deflection on LM trace).
Analysis of motion from maps
The maximum and minimum diameter and rate of contraction during peristalsis were averaged over a 5 mm region at oral and aboral ends, at least 5 mm away from the edge of the cannulae. The velocity of propagation was calculated by fitting a least squares regression line to the mid-diameter level during the peristaltic contraction. To compare preparations in which the aboral end moved freely with those in which the aboral end was fixed, the DMaps that were free to move, were artificially ‘stretched’ by using nearest neighbour interpolation at each time point along the preparation, to maintain a fixed resting length. In differentiated maps, the rate of change of diameter was calculated using 0.2 s intervals.
Shortening of the longitudinal muscle was expressed as a percentage of the resting length.
Statistics
Statistical comparisons were made using factorial ANOVA, with Fisher's PLSD post hoc tests. A probability of less than 0.05 was considered significant.
RESULTS
Peristalsis elicited by slow liquid distension
Slow infusion of Krebs solution into isolated segments of guinea-pig small intestine evoked peristalsis in a similar manner to that described in numerous previous studies (Trendelenburg, 1917; Tonini et al. 1981; Waterman & Costa, 1994). Notably, there was a graded longitudinal shortening of the preparation during the preparatory phase of peristalsis (Trendelenburg, 1917; Kosterlitz & Lees, 1964), then at a threshold volume, there was an abrupt contraction of the circular muscle at the oral end which propagated aborally, expelling intraluminal contents and correlated with a large transient increase in intraluminal pressure (Fig. 4, Pressure). These changes in the dimensions of preparation were apparent in spatio-temporal maps (Fig. 4, DMap). During the preparatory phase, there was an overall darkening of the DMap over time, reflecting the increase in diameter evoked by distension. This increase in diameter was also apparent in the diameter trace (Fig. 4; CM, dilatation indicated by a rightward deflection) extracted from between the arrowheads in the DMap. In parallel with this, the preparation shortened as demonstrated by the graded lightening of the LMap in Fig. 4 and in the longitudinal muscle trace (Fig. 4; LM, shortening indicated by a rightward deflection).
The LMap also revealed patterns of longitudinal motor activity along the length of the intestine during the preparatory phase. These patterns consist of oblique stripes and are due to delays of rhythmic longitudinal muscle oscillations along the preparation. The slope of the stripes indicate the direction and speed of propagation of longitudinal muscle contractions (see legend of Fig. 4). At each measured point along the intestine, the longitudinal muscle oscillated rhythmically at about 37 cycles min−1. This longitudinal muscle motor activity had little effect on the overall length of the preparation. Close to the threshold for peristaltic emptying, the preparation shortened substantially (Fig. 4, indentations on right edge of the LMap).
At the beginning of the emptying phase, there was a contraction of the circular muscle at the oral end, which propagated aborally, invading the entire segment. This appears as the diagonal white streak in the DMap (Fig. 4, DMap). After emptying, the intestine often flattened and twisted significantly, causing artefacts in DMaps. For this reason, no analysis was performed in this period.
Circular muscle during the preparatory phase
The DMaps show a gradual darkening during the infusion of fluid, reflecting an overall increase in diameter of the preparation as it accommodated the increasing volume. The aboral end of preparations was distended more rapidly than the oral end. The mean rate of diameter change was determined throughout the preparatory phase for the oral, middle and aboral ends of preparations which were free to shorten (Fig. 5). The diameter at the aboral end increased more quickly than at the oral end during slow filling, suggesting that the tissue aborally was more compliant.
Figure 5. Rate of change of diameter varied along the intestine during slow filling.

Measurements of circular muscle diameter at the oral, middle and aboral ends of 5 preparations were used to calculate the mean rate of increase of diameter during slow filling. The oral end distended significantly more slowly during infusion than the aboral end (P < 0.05), indicating that the oral end is less distensible. This probably reflects greater inhibitory neuronal input to the circular muscle at the aboral end during filling.
Relationship between longitudinal and circular muscle layers during the preparatory phase
Before the threshold volume for peristalsis had been reached, DMaps often showed small changes in diameter (11/19 animals) that resembled the patterns of activity in the longitudinal muscle (Fig. 6A and B). These oblique dark ‘bands’ became more evident with greater distension volumes and appeared to propagate orally. Small changes in diameter also occurred when preparations were held at a constant volume (approximately 60% of the threshold volume) although the direction of propagation of the small diameter changes was more variable. In order to establish whether these small diameter changes were correlated with longitudinal muscle activity, DMaps and LMaps were compared (see Figs 6 and 9). As the longitudinal muscle shortened locally, there was a significantly correlated increase in diameter (darkening) in the corresponding DMap (correlation coefficient r2= 0.95 at LM oscillations between 4-5 mm) measured in the middle of the preparation. For every 5 mm of longitudinal muscle shortening between two surface markers (12-14 mm apart) there was approximately 1 mm increase in diameter. This indicates that mechanical interactions occur between the muscle layers in isolated tubular preparations and that these interactions can be restricted to small localized regions.
Figure 6. Relationship between longitudinal and circular muscle during the preparatory phase.

During slow filling of the intestine, the longitudinal muscle showed characteristic phasic contractions that appeared to propagate orally (LMap in B). These correlated with small phasic increases in overall diameter of the preparation revealed in DMaps (see dark bands in A). This suggests that localized longitudinal muscle shortening may cause a localized small increase in diameter.
Figure 9. Relationship between circular and longitudinal shortening during the emptying phase.

Local changes in diameter and length (calculated from surface markers) were plotted for the oral, middle and aboral regions of a preparation during the preparatory and emptying phases of peristalsis. Values were calculated at intervals of 40 ms (each represented by a single dot). During filling, there were phasic contractions of the longitudinal muscle which correlated with phasic increases in diameter (between points a and b). At the onset of peristaltic emptying (point b), there was a sudden decrease in diameter, initially without lengthening, indicating that the longitudinal muscle did not relax during the initiation of peristalsis. Lengthening only commenced during the later stages of the circular muscle contraction (point c). Similarly, in the middle and aboral regions, there was no significant localized lengthening during the circular muscle contraction. However, particularly at the aboral end of the preparation there was a significant increase in diameter just prior to the circular muscle contraction due to fluid displaced from the contraction further orally.
If the aboral end of the preparation was free to move, there was always a shortening of the preparation prior to initiation of the emptying phase by 10.8 ± 0.8 mm, corresponding to a change in length of approximately 19% (n = 4, 17 samples). A progressive shortening of the longitudinal muscle occurred during the entire period of filling at 0.1 ± 0.002 mm s−1. Just prior to the emptying phase, the rate of longitudinal shortening increased to 1.63 ± 0.17 mm s−1. This shortening is visible in Fig. 7C as a horizontal shift of the aboral end and in Fig. 4 as a whitening in the LMap.
Figure 7. Comparison of peristaltic emptying in preparations that were either fixed at both ends or free to move at the aboral end.
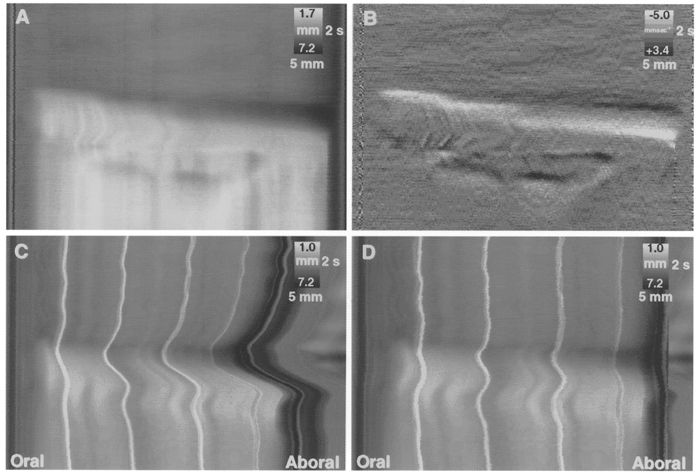
A DMap of a preparation in which both oral and aboral ends were attached to fixed cannulae (A) shows an abrupt start to the emptying phase. The same map differentiated, to show the dynamic changes in circular muscle length (B), reveals that the emptying occurred as a sequence of three contractions at various points along its length, giving rise to ‘step-like’ propagation. In C, the aboral end of the preparation was attached to a freely moving cannula (see Fig. 1B) and surface markers were used to map the local shortening of the preparation. A pronounced shortening of the preparation occurred prior to the initial contraction of the circular muscle and the preparation lengthened during the ensuing emptying phase. The DMap was subsequently processed to maintain a constant overall length (D), making it possible to compare directly the emptying phase of fixed preparations (A) and preparations that were free to shorten (D), revealing that the rate of propagation of circular muscle contraction was not affected by shortening of the longitudinal muscle.
Circular muscle movements during the emptying phase of peristalsis
The contraction of the circular muscle usually started at the oral end and propagated aborally. The circular muscle at the oral end remained contracted for the entire emptying phase which lasted 1.83 ± 0.09 s. It should be noted that the liquid infusion was switched off at the beginning of the emptying phase to prevent fluid from refilling the preparation. Aboral to the advancing circular muscle contraction, the intestine rapidly increased in diameter, as revealed by the marked darkening in the DMap (Fig. 7A).
A number of parameters of motions of the circular muscle during the emptying phase were calculated and are shown in Table 1.
Table 1.
Parameters of motion
| Fixed Means ± s.e.m. | Free Means ± s.e.m. | Significance P | |
|---|---|---|---|
| Threshold diameter oral (mm) | 4.9 ± 0.05 5 | 6 ± 0.09 | * < 0.001 |
| Maximum diameter aboral (mm) | 5.9 ± 0.1 | 6.5 ± 0.07 | * < 0.001 |
| Empty diameter oral (mm) | 2.7 ± 0.06 | 3.2 ± 0.09 | * < 0.01 |
| Empty diameter aboral (mm) | 2.5 ± 0.6 | 3.2 ± 0.08 | * < 0.001 |
| Rate of CM contraction oral (mm s−1) | 2.2 ± 0.6 | 2.2 ± 0.6 | > 0.05 |
| Rate of CM contraction aboral (mm s−1) | 3.3 ± 0.09 | 2.5 ± 0.06 | * < 0.001 |
| Propagation velocity of CM contraction (mm s−1) | 30.2 ± 0.9 | 29.6 ± 1.5 | > 0.05 |
Parameters of motion calculated during the emptying phase of peristalysis in preparations that were fixed at each end (Fixed, n = 21; 4 animals) or free to move at the aboreal end (Free, n = 31; 5 animals). CM, circular motion
To visualize and analyse the dynamics of the propagated circular muscle contraction more clearly, DMaps were differentiated with respect to time. In such maps, high rates of circular muscle shortening are shown in white, while high rates of dilatation are shown in black (Fig. 7B). DMaps and their differentiated versions, revealed a number of features not previously recognized. The propagated peristaltic contractions often had a ‘step-like’ appearance, rather than appearing as a smooth wave of contraction. In these cases, peristalsis was equally effective in emptying the segment and consisted of a series of sequential, more separate localized contractions (Fig. 8B top and bottom; 8C top and 8D bottom). These contractions, in some cases, occurred almost simultaneously over a significant length of the segment (Fig. 8C and D both middle) and thus no measurement of the speed of propagation of the separate contractions was attempted. In all cases the mean speed of propagation of the peristaltic contraction was calculated as described in Methods, by fitting a least squares regression line to the mid-diameter level during the peristaltic contraction (Table 1). In 3 of 12 preparations a localized contraction of the circular muscle occurred at the aboral end, preceding the oral initiation of peristalsis contraction (Fig. 8B middle; 19% of all trials from 12 preparations). This was a consistent feature in 1 of 12 preparations.
Figure 8. Dynamics of peristaltic emptying in preparations maintained at a fixed length.

Three differentiated diameter maps from each of four animals (A, B, C and D) show features of propagation of the circular muscle contraction during the emptying phase. Most examples show a ‘step-like’ activation of the circular muscle contraction as it propagated aborally (B top and bottom, C top and D bottom). The initial circular muscle contraction usually occupied a small region at the oral end of the segment; however, occasionally the contraction occurred in a large region simultaneously (C and D middle). Occasionally, a circular muscle contraction occurred aborally before the peristaltic contraction was initiated at the oral end (B middle). The dark bands after the peristaltic contraction (B) are artefacts due to excessive bending caused by longitudinal muscle movement in a fixed preparation.
The differentiated DMaps also revealed that the maximum rate of contraction of the circular muscle was greater towards the aboral end of the segment during peristalsis (Table 1), perhaps because the starting diameter was greater, due to fluid displaced aborally by the propagating circular muscle contraction. The parameters of peristalsis analysed in preparations in which the aboral end was free to move (permitting net shortening of the intestinal segment) were compared with preparations in which the aboral end was fixed. DMaps from freely contracting preparations were artificially stretched as described in Methods, to permit direct comparison with fixed preparations (Fig. 7C and D). The threshold diameter at which peristalsis was evoked at the oral end was significantly greater in preparations that were free to shorten (Table 1). Similarly, the maximum diameter reached during peristalsis was greater in preparations that were free to shorten. The minimum diameter after emptying was greater in preparations that were free to shorten, at both oral and aboral ends. The mean rate of circular muscle contraction in preparations of fixed length was greater aborally than orally. The mean rate of propagation of the circular muscle contraction did not differ significantly after correcting for the overall change in length of freely shortening preparations (Table 1).
Relationship between longitudinal and circular muscle during the emptying phase of peristalsis
As described above, the contraction of the circular muscle usually occurred first at the oral end and subsequently propagated aborally. In the middle and aboral ends the contraction was preceded by a distension due to the displacement of fluid from further orally. The relationship between longitudinal and circular shortening at the oral, middle and aboral ends of the preparation were determined by plotting local changes in length and diameter versus each other (Fig. 9). During the preparatory phase (Fig. 9, from points a to b) there were phasic contractions of the longitudinal muscle which correlated closely with small increases in diameter. After the threshold for peristalsis (point b), there was a rapid decrease in diameter but the length remained constant (between points b and c). This indicates that the longitudinal muscle remained active during the circular muscle contraction. The longitudinal muscle at the oral end of the segment remained active for 0.89 ± 0.04 s (n = 5; 31 samples) during the circular muscle contraction before noticable elongation occurred. This corresponds to 48% of the duration of the circular muscle contraction to its peak. The corresponding figures at the anal end were 1.00 ± 0.03 s (47% of duration circular muscle contraction). Lengthening of the longitudinal muscle occurred when the diameter approached a minimum (point c) and is probably due to passive elongation due to the ongoing circular muscle shortening.
Spontaneous peristalsis in partially distended preparations
In order to study patterns of activity during the filling phase, four preparations that were free to shorten were maintained at a constant volume distension, corresponding to approximately 60% of the threshold volume for peristaltic emptying. In all these preparations there were patterned oscillations of longitudinal muscle shortening (mean frequency, 32 cycles min−1) and periodic larger shortenings of the entire preparation by 11.7 ± 2.2% occurring at 3.5-4 cycles min. Interestingly, after considerable delays, peristaltic emptying occurred without further fluid infusion, at mean intervals of 8.0 ± 0.7 min (29 bouts of emptying). This indicates that there is considerable variation in the threshold for peristalsis when the gut is partially distended for long periods. Each bout of peristaltic emptying was preceded by a larger-than-average shortening of the preparation by 23.1 ± 2.9% of resting length (n = 17; from 4 animals). Measurement of parameters of peristaltic emptying revealed no differences from preparations distended to threshold which were free to shorten, with one exception. The rate of propagation of the circular muscle contraction was significantly slower than in preparations distended using a ramp distension (22.0 ± 2.3 mm s−1; P < 0.01, n = 19; from 4 animals). This suggests that the volume of intraluminal contents may play a significant role in the rate of propagation of peristalsis along the intestine.
DISCUSSION
In this paper, a simple method was developed to generate spatio-temporal maps of patterns of movement in the isolated guinea-pig ileum similar to that used by (Benard et al. 1997). These maps were used to quantify changes in the length and diameter of the intestine during peristalsis evoked by slow fluid infusion, with high spatial and temporal resolution. From such detailed analysis, organized patterns of longitudinal muscle contractility became apparent during the preparatory or filling phase. It was also clear that the longitudinal muscle contracts in synchrony with the circular muscle during the propagating wave of circular muscle contraction, as suggested by some authors (Bayliss & Starling, 1899; Yokoyama & Ozaki, 1990; Smith & Robertson, 1998) rather than relaxing as others have suggested (Kottegoda, 1969). In addition, the emptying phase of peristalsis was shown to consist, in many preparations, of a ‘step-wise’ sequential activation of separate circular muscle contractions at different points along the intestine, rather than as a smooth propagating wave of contraction.
The longitudinal muscle in the preparatory phase of peristalsis
The gradual shortening of the intestine during the preparatory phase, together with superimposed longitudinal oscillations has been previously described (Trendelenburg, 1917; Hukuhara et al. 1969; Tsuchiya, 1972; Yokoyama & North, 1983). Oscillations of the longitudinal muscle, without circular muscle activity have been reported in isolated preparations of intestine from other laboratory animals (Cannon, 1912; Gonella, 1971; Cheung & Daniel, 1980). The small longitudinal muscle oscillations, which occurred at frequencies of 32-37 cycles min−1, may well be caused by slow waves with superimposed smooth muscle action potentials. Slow waves accompanied by bursts of action potentials have been recorded from longitudinal muscle in different species, including the guinea-pig (Yokoyama & North, 1983; Szurszewski, 1987; Bolton, 1989). With this method, combined with electrophysiological recordings from smooth muscle cells, it will be possible to investigate the spatio-temporal patterns of these longitudinal muscle oscillations, and their relation with slow waves.
The rhythmic longitudinal oscillations probably correspond to the pendular movements of the intestine described last century. Krishnan (1932) observed pendular movements in cats and concluded that they were solely due to longitudinal muscle activity and produced a to and fro movement of the contents, with an associated small passive dilatation of the circular muscle.
Our results support this interpretation. We calculated that for every 5 mm of longitudinal shortening between two surface markers there was an associated increase in diameter of 1 mm. This increase in diameter is likely to be due to passive mechanical interactions between the longitudinal and circular dimensions of the intestine that have been described previously (Gregory & Bentley, 1968; Wood & Perkins, 1970). Indeed, Hukuhara & Fukuda (1965) demonstrated that fluid distension of tubes made of inert material can cause significant shortening. It has been suggested that longitudinal muscle movements are likely to result in effective mixing of the intestinal contents (Melville et al. 1975).
The progressive shortening of the preparation during the filling in the preparatory phase is generally considered as a reflex contraction of the longitudinal muscle and has been reported to be sensitive to both hexamethonium and atropine (Baur, 1928; Kosterlitz & Robinson, 1959; Kosterlitz, 1968; Tsuchiya, 1972) although it has also been suggested that passive mechanisms may contribute (Hukuhara & Fukuda, 1965).
The rapid oscillations in local longitudinal muscle correlated with small changes of length of the entire preparation. However, in all preparations in which one end was free to move, considerably larger phasic shortenings occurred occasionally (up to 4 min−1) at higher levels of distension and the onset of the emptying phase was always preceded by a large contraction. The LMaps showed that this was due to synchronous shortening of the longitudinal muscle at many points along the length of the preparation, which overrode the rhythmicity of the local contractions. Since longitudinal muscle layer of the guinea-pig ileum is innervated by motor neurons with short projections, 2-3 mm long (Brookes et al. 1992), it seems likely that a burst of firing in interneuronal pathways must synchronize activity in longitudinal muscle motor neurons along the preparation to cause these large contractions. At least three descending interneuronal pathways and one orally projecting pathway are known to exist in the guinea-pig ileum (Costa et al. 1996). Which of these neuronal pathways leads to the shortening of the entire segment remains to be established.
The circular muscle in the preparatory phase of peristalsis
In contrast to the longitudinal muscle, which showed considerable contractile activity during the preparatory phase, the circular muscle showed very little activity. The diameter of the gut increased gradually with distension, revealed as a graded darkening of DMaps, but essentially no motor activity was detected in the circular muscle until the threshold for peristalsis was reached. However, any change in diameter of less than 0.25 mm would not be detected with the present system (see Methods). There are a number of possible reasons for the differences in activation of the external muscle layers. It is likely that the circular muscle is relatively less excitable (or more inhibited) and requires considerable excitatory motor input to reach the threshold for contraction, whereas the longitudinal muscle layer is normally very close to threshold, judged by the presence of spontaneous contractions. This difference in state could be due to a preferential inhibition of circular muscle, but not longitudinal muscle, by prostaglandins released from damaged tissue (Bennett et al. 1976; Maggi et al. 1994). Another possibility is that the circular muscle, but not the longitudinal muscle, is actively inhibited by enteric inhibitory motor neurones. There is a substantial innervation of the circular muscle by inhibitory motor neurones (Brookes et al. 1991) whereas the longitudinal muscle is innervated nearly exclusively by excitatory motor neurons in the guinea-pig ileum (Brookes et al. 1992). There is ample evidence for ongoing inhibitory neural activity in the circular muscle of isolated segments of intestine (Wood, 1972; Biber & Fara, 1973; Tonini et al. 1974), including the guinea-pig ileum circular muscle (Smith, 1989). This inhibitory activity results in maximal muscle relaxation during the accommodatory reflex in the guinea-pig small intestine during slow distension (Waterman et al. 1994b).
The initiation of peristaltic emptying
Based on visual monitoring, the start of the emptying phase of peristalsis has long been considered to be due to an abrupt, large amplitude contraction of the circular muscle at the oral end of the preparation. This is confirmed by the DMaps where the initiation of emptying is shown as a sudden whitening at the oral end of the map. However, in 19% of trials, contractions of the circular muscle occurred first in the aboral half of the preparation (e.g. Fig. 8B), revealing some variability in the patterning of circular muscle contraction. The occurrence of an abrupt start to the contraction of the circular muscle is not because the circular muscle is incapable of generating graded responses; it is well established that the ascending excitatory reflex, evoked by rapid distension (usually with an intraluminal balloon), is graded with stimulus intensity (Bayliss & Starling, 1899; Costa & Furness, 1976; Smith et al. 1990; Tonini et al. 1996). Additional mechanisms may be involved that are responsible for its abrupt start (Tonini et al. 1996). The larger shortening of the preparation that always precedes the peristaltic contraction may contribute to the initiation of peristalsis as suggested by Kottegoda (1969). As discussed above, the shortening of the intestine results in an increase in diameter due to the mechanical interaction between the two muscle layers. This increase in diameter would further activate the excitatory reflex activity of the circular muscle motor neurons. A burst of activity of these excitatory motor neurons releasing acetylcholine onto the circular muscle would initiate the peristaltic contraction. However, the overall shortening of the segment is not a prerequisite for the initiation of the emptying phase, as anchoring both ends of the preparation did not prevent peristaltic emptying (Kosterlitz et al. 1956).
Propagation of the circular muscle contraction during peristaltic emptying
The spatio-temporal maps clearly show the emptying phase of peristalsis as an oblique white streak preceded by a darker dilated area. Detailed analysis of differentiated DMaps revealed that the circular muscle often contracted simultaneously over several centimetres of intestine so that the propagation actually consisted of a series of separate ‘step-like’ contractions along the segment. Interestingly, in most cases, these ‘steps’ were clearest in the oral half of the preparation. At the beginning of the emptying phase, relatively little fluid was displaced by the circular muscle contraction which was restricted to the oral end. As emptying proceeded, the propelled contents accumulated, causing a wave of dilatation that preceded the contraction (Alvarez & Zimmermann, 1927). This dilatation was most marked in the aboral half of the preparation and may explain the smoother propagation of the peristaltic contraction in this region. From the apparent smoothness of the pressure wave recorded at the aboral end of the preparation, the mechanical consequence of the ‘step-like’ progression of the circular muscle contraction may be minimal. The average speed of propagation of the peristalsic contraction, calculated by ignoring the ‘step-like’ progression of the contractions, was approximately 30 mm s−1. This speed was not different in preparations which were allowed to shorten during emptying, indicating that longitudinal muscle activity does not influence the speed of propagation of the circular muscle contraction. Interestingly, when preparations were held at a constant volume (60% of threshold) for extended periods of time, the average speed of propagation of circular muscle contraction was reduced to approximately 20 mm s−1. This suggests that there is some flexibility in the propulsive peristaltic contraction and reinforces our suggestion that peristalsis should be regarded as a motor pattern rather than as a simple reflex.
Relation between longitudinal and circular muscle layers during the emptying phase
There has long been uncertainty about the exact timing of longitudinal and circular muscle activities during peristaltic emptying although evidence that they can contract together has been provided (Bayliss & Starling, 1899; Yokoyama & Ozaki, 1990; Smith & Robertson, 1998). Our method has provided an accurate description of these events at multiple points along a preparation. By plotting length against diameter for restricted regions at the oral, middle and aboral ends of the preparation, it was possible to correlate the activity of the circular and longitudinal muscle layers during peristaltic emptying (Fig. 6).
As the emptying phase commenced, there was an abrupt shortening of the circular muscle, suggesting that there is a switching from a prevailing effect of inhibitory motor neurons, responsible for the accommodation in the preparatory phase (Waterman et al. 1994a), to a sudden recruitment of circular muscle excitatory motor neurons. The plots in Fig. 9 (between points b and c) clearly show that the longitudinal muscle does not relax during this period. This would indicate that both longitudinal and circular muscle excitatory motor neurons are active during the first half of the circular muscle contraction. It also indicates that excitatory motor neurons to both muscle layers are active over half the duration of the propagation of the peristaltic contraction. These observations support the conclusion that the longitudinal muscle contraction persisted during the circular muscle contraction (Tsuchiya, 1972).
In the later part of the circular muscle contraction, there is a considerable elongation of the longitudinal muscle (Fig. 9, after point C). This elongation appears to occur when the particular segment of intestine has emptied its contents. This increase in length may simply reflect mechanical interaction of the two layers as suggested by Wood & Perkins (1970). It is unlikely that it is due to a reciprocal inhibition of the longitudinal muscle as proposed by Kottegoda (1969), since the longitudinal muscle layer receives a very sparse innervation by inhibitory motor neurons (Brookes et al. 1992). At some point during the emptying phase the drive to longitudinal muscle motor neurons must be switched off since the preparation returns to its resting length after emptying is completed.
Physiological recordings of peristalsis
The movements of the gut involve simultaneous contractions and relaxations of both circular and longitudinal smooth muscle layers at every point along its length. The resulting visual and numerical complexity has been a major hindrance to the analysis of the physiological mechanisms that underlie gut motility. Initially, recording methods aimed at simplifying this complexity by recording accurately from just one or a few sites. Mechanical recordings of physiological events can be traced back to the kymographic method invented by Carl Ludwig in 1847 (quoted in Holmes & Olesko, 1995). This was developed further by Bayliss & Starling (1899) with the development of the ‘enterograph’ which allowed them to record, via a system of levers, movements of the gut wall at several sites simultaneously. Mechanical recordings at several sites along segments of gut have subsequently been made, both in vivo and in vitro, by many workers, allowing some analysis of propagating contractions (Costa & Furness, 1976; Schemann & Ehrlein, 1986; Huizinga et al. 1998).
An alternative strategy to document gut motility has been to use image analysis of whole segments of gut. This was first developed by Cannon (1902) who recorded the motions of radio-opaque material in the lumen of the intestine of cats. Since then, many workers have analysed radiographic images in vivo and cinematographic images in situ or in isolated preparations (Fleisch & Wyss, 1923; Alvarez & Zimmermann, 1927; Baur, 1928; Hukuhara, 1931; Schulze-Delrieu et al. 1991; Waterman et al. 1994b). The manual analysis of such images is very labour intensive. These observations have led to the recognition of some patterns of motility including peristalsis, but description of more complex patterns and activity over protracted periods has not been forthcoming.
The method that we developed here, combines these two methodological approaches and is based on the production of spatio-temporal maps of intestinal activity in which either the diameter (DMap) or the longitudinal length (LMap) of every segment of gut is portrayed as a grey scale, as described by Benard et al. (1997). This allows a complete record of many minutes of activity to be compressed into one or two small maps, giving a visual immediacy that allows the detection of subtle patterns that are not obvious in frame-by-frame analysis. Since the maps are made up of quantified diameter measurements, movements at any particular site can be readily extracted, providing traces which are the equivalent of isotonic recordings at any point in the preparation. Thus, to some extent, spatio-temporal maps can bridge the gap between image analysis of entire preparations and physiological recording at a restricted number of sites.
Limitations of spatio-temporal maps
The spatial resolution of spatio-temporal maps is determined only by pixel density and the magnification used: in this study one pixel corresponded to approximately 0.2-0.3 mm. The images were thus equivalent to approximately 300 isotonic transducers attached along a 7 cm segment of intestine, without the interference that would be caused by physical attachment. The spatial resolution could theoretically be increased by using higher magnification of smaller pieces of tissue, or by combining images from multiple video cameras. The temporal resolution of the system was determined by the 25 frames s−1 sampling rate of the PAL recording format. The information stored in the spatio-temporal maps could be used to reconstruct the silhouette of the segment of intestine, and thus represents an efficient way to store images of motility. Indeed, we have calculated that with appropriate compression routines, 95 h of intestinal motion could be stored as spatio-temporal maps on a single CD-ROM (21 h uncompressed).
The most striking benefit of this type of image analysis is that extended periods of spatially complex movements can be displayed in a single plot, allowing subtle patterns to be readily recognized. In frame-by-frame analysis of video or cinematographic images, the constant updating of the images makes detailed pattern recognition nearly impossible, even when viewed in slow motion. The ability to extract quantitative data from spatio-temporal maps has been used by Benard et al. (1997) who recently published a method based on similar principles for constructing diameter maps to investigate motor behaviour of isolated rat intestine. However, their method did not include analysis of longitudinal muscle contractions and their system captured images at a lower rate (6 frames s−1versus 25). This type of methodology will be applicable in many areas where high contrast images can be obtained. We expect that the use of radio-opaque markers may allow similar analysis of motility in vivo and perhaps allow analysis of the propulsion of contents. The potential value of combining this approach with other recording methods is also apparent. For example it will be possible to monitor the activity of significant lengths of intestine while at the same time recording spontaneous or physiologically evoked muscle electrical activity (Gonella, 1971; Tsuchiya, 1972; Yokoyama & North, 1983; Lammers et al. 1993). It should also be possible to combine spatio-temporal maps with monitoring of intracellular Ca2+, intracellular recordings from neurons, multiple luminal manometry or strain gauges (Summers & Dusdieker, 1981; Bercik et al. 1994; Huizinga et al. 1998) and intraluminal flow (Bertuzzi et al. 1978; Macagno & Christensen, 1980) to improve the understanding of the biomechanical basis of intestinal motor function.
The major limitations of the technique relate to the need for high contrast images with well defined edges, which may limit its use in vivo. Movements of the wall can be caused either by localized muscle activity acting on the contents or by forces produced some distance away and coupled by passive physical means, via connective tissue or by the intraluminal contents. This means that analysis of the mechanisms that cause intestinal motility has to be approached with caution.
Despite its limitations, we are confident that combining focal mechanical or electrical recordings with spatio-temporal mapping will help unravel the mechanisms that underlie the apparent complexity of intestinal motility.
Acknowledgments
This work has been funded by the National Health and Medical Research Council of Australia and the Flinders Medical Centre Foundation. S. J. H. B. was supported, for part of this study, by a Senior Research Fellowship in Digestive Science of the Gastroenterological Society of Australia. G. W. H. was supported for part of this study by a Biomedical Research Scholarship from the Gastroenterological Society of Australia.
References
- Alvarez WC, Zimmermann A. The absence of inhibition ahead of peristaltic rushes. American Journal of Physiology. 1927;83:52–59. [Google Scholar]
- Baur M. Die Peristaltik des isolierten Meerschweinchendunndarms im Filmversuch. Archives of Experimental Pathology and Pharmacology. 1928;133:69–83. [Google Scholar]
- Bayliss WM, Starling EH. The movements and innervation of the small intestine. The Journal of Physiology. 1899;24:99–143. doi: 10.1113/jphysiol.1899.sp000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benard T, Bouchoucha M, Dupres M, Cugnenc PH. In vitro analysis of rat intestinal wall movements at rest and during propagated contraction - a new method. American Journal of Physiology. 1997;36:776–784. doi: 10.1152/ajpgi.1997.273.4.G776. [DOI] [PubMed] [Google Scholar]
- Bennett A, Eley KG, Stockley HL. Inhibition of peristalsis in guinea-pig isolated ileum and colon by drugs that block prostaglandin synthesis. British Journal of Pharmacology. 1976;57:335–340. [PMC free article] [PubMed] [Google Scholar]
- Bercik P, Armstrong D, Fraser R, Dutoit P, Emde C, Primi MP, Blum AL, Kucera P. Origins of motility patterns in isolated arterially perfused rat intestine. Gastroenterology. 1994;106:649–657. doi: 10.1016/0016-5085(94)90698-x. [DOI] [PubMed] [Google Scholar]
- Bertuzzi A, Mancinelli R, Ronzoni G, Salinari S. A mathematical model of intestinal motor activity. Journal of Biomechanics. 1978;11:41–47. doi: 10.1016/0021-9290(78)90042-8. [DOI] [PubMed] [Google Scholar]
- Biber B, Fara J. Intestinal motility increased by tetrodotoxin, lidocaine and procaine. Experientia. 1973;29:551–552. doi: 10.1007/BF01926658. [DOI] [PubMed] [Google Scholar]
- Bolton TB. Electrophysiology of the intestinal musculature. In: Schultz SG, Wood JD, Rauner BB, editors. Handbook of Physiology, The Gastrointestinal System. I. Bethesda, MD, USA: American Physiological Society; 1989. pp. 217–250. section 1. [Google Scholar]
- Brookes SJH, Song ZM, Steele PA, Costa M. Identification of motor neurons to the longitudinal muscle of the guinea pig ileum. Gastroenterology. 1992;103:961–973. doi: 10.1016/0016-5085(92)90030-3. [DOI] [PubMed] [Google Scholar]
- Brookes SJH, Steele PA, Costa M. Identification and immunohistochemistry of cholinergic and non-cholinergic circular muscle motor neurons in the guinea-pig small intestine. Neuroscience. 1991;42:863–878. doi: 10.1016/0306-4522(91)90050-x. [DOI] [PubMed] [Google Scholar]
- Cannon WB. The movements of the intestines studied by means of the roentgen rays. American Journal of Physiology. 1902;6:251–277. [PMC free article] [PubMed] [Google Scholar]
- Cannon WB. Peristalsis, segmentation and the myenteric reflex. American Journal of Physiology. 1912;30:114–128. [Google Scholar]
- Cheung DW, Daniel EE. Comparative study of the smooth muscle layers of the rabbit duodenum. The Journal of Physiology. 1980;309:13–27. doi: 10.1113/jphysiol.1980.sp013490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M, Brookes SJH, Steele PA, Gibbins IL, Burcher E, Kandiah CJ. Neurochemical classification of myenteric neurons in the guinea-pig ileum. Neuroscience. 1996;75:949–967. doi: 10.1016/0306-4522(96)00275-8. [DOI] [PubMed] [Google Scholar]
- Costa M, Furness JB. The peristaltic reflex: an analysis of the nerve pathways and their pharmacology. Naunyn-Schmiedeberg' Archives of Pharmacology. 1976;294:47–60. doi: 10.1007/BF00692784. [DOI] [PubMed] [Google Scholar]
- Costa M, Furness JB. Nervous control of intestinal motility. In: Bertacchini A, editor. Mediators and Drugs in Gastrointestinal Motility I. Morphological Basis and Neurophysiological Control. 59/I. Berlin: Springer-Verlag; 1982. pp. 279–382. [Google Scholar]
- Fleisch A, Wyss WH. Zur Kenntnis der visceralen Tiefensensibilitat. Pflügers Archiv. 1923;200:291–314. [Google Scholar]
- Gonella J. Etude electromyographique des contractions segmentaires et peristaltiques du duodenum de Lapin. Pflügers Archiv. 1971;322:217–234. doi: 10.1007/BF00602071. [DOI] [PubMed] [Google Scholar]
- Gregory JE, Bentley GA. The peristaltic reflex in the isolated guinea pig ileum during drug-induced spasm of the longitudinal muscle. Australian Journal of Experimental Biology and Medical Science. 1968;46:1–16. doi: 10.1038/icb.1968.1. [DOI] [PubMed] [Google Scholar]
- Hennig GW, Costa M, Brookes SJH. Relationship between circular and longitudinal muscle motor activity in the guinea-pig small intestine. Neurogastroenterology and Motility. 1998;10:75. [Google Scholar]
- Holmes FL, Olesko KM. The images of precision. In: Wise MN, editor. The Values of Precision. Princeton, New Jersey: Princeton University Press; 1995. pp. 198–221. [Google Scholar]
- Huizinga JD, Ambrous K, Der-Silaphet T. Co-operation between neural and myogenic mechanisms in the control of distension-induced peristalsis in the mouse small intestine. The Journal of Physiology. 1998;506:843–856. doi: 10.1111/j.1469-7793.1998.843bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukuhara T. Die normale Dunndarmbewegung. Pflügers Archiv. 1931;226:518–542. [Google Scholar]
- Hukuhara T, Fukuda H. The motility of the isolated guinea-pig small intestine. Japanese Journal of Physiology. 1965;15:125–139. [Google Scholar]
- Hukuhara T, Neya T, Tsuchiya K. The effect of the intrinsic mucosal reflex upon the propagation of intestinal contractions. Japanese Journal of Physiology. 1969;19:824–833. doi: 10.2170/jjphysiol.19.824. [DOI] [PubMed] [Google Scholar]
- Kosterlitz HW. Intrinsic and extrinsic nervous control of motility of the stomach and the intestines. In: Code CF, editor. Handbook of Physiology. IV. Washington: American Physiological Society; 1968. pp. 2147–2171. section 6. [Google Scholar]
- Kosterlitz HW, Lees GM. Pharmacological analysis of intrinsic intestinal reflexes. Pharmacology Review. 1964;16:301–339. [PubMed] [Google Scholar]
- Kosterlitz HW, Pirie VW, Robinson JA. The mechanism of the peristaltic reflex in the isolated guinea-pig ileum. The Journal of Physiology. 1956;133:681–694. doi: 10.1113/jphysiol.1956.sp005618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosterlitz HW, Robinson JA. Reflex contractions of the longitudinal muscle coat of the isolated guinea-pig ileum. The Journal of Physiology. 1959;146:369–379. doi: 10.1113/jphysiol.1959.sp006198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottegoda SR. An analysis of possible nervous mechanisms involved in the peristaltic reflex. The Journal of Physiology. 1969;200:687–712. doi: 10.1113/jphysiol.1969.sp008717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan BT. Studies on the function of the intestinal musculature: The normal movements of the small intestine and the relations between the action of the longitudinal and circular muscle fibres in those movements. Quarterly Journal of Experimentation. 1932;22:57–63. [Google Scholar]
- Lammers WJEP, Al-Kais A, Singh S, Arafat K, El-Sharkawy TY. Multielectrode mapping of slow-wave activity in the isolated rabbit duodenum. American Journal of Physiology. 1993;74:1454–1461. doi: 10.1152/jappl.1993.74.3.1454. [DOI] [PubMed] [Google Scholar]
- Macagno EO, Christensen J. Fluid mechanics of the duodenum. Annual Review of Fluid Mechanics. 1980;12:139–158. [Google Scholar]
- Maggi CA, Patacchini R, Meini S, Giuliani S. Effect of longitudinal muscle-myenteric plexus removal and indomethacin on the response to tachykinin NK-2 and NK-3 receptor agonists in the circular muscle of the guinea-pig ileum. Journal of Autonomic Pharmacology. 1994;14:49–60. doi: 10.1111/j.1474-8673.1994.tb00589.x. [DOI] [PubMed] [Google Scholar]
- Melville J, Macagno E, Christensen J. Longitudinal contractions in the duodenum: their fluid-mechanical function. American Journal of Physiology. 1975;228:1887–1892. doi: 10.1152/ajplegacy.1975.228.6.1887. [DOI] [PubMed] [Google Scholar]
- Schemann M, Ehrlein HJ. Postprandial patterns of canine jejunal motility and transit of luminal content. Gastroenterology. 1986;90:991–1000. doi: 10.1016/0016-5085(86)90878-4. [DOI] [PubMed] [Google Scholar]
- Schulze-Delrieu K, Brown BP, Custer-Hagen T. Contraction and accommodation of guinea-pig duodenum in vitro. American Journal of Physiology. 1991;261:G364–372. doi: 10.1152/ajpgi.1991.261.2.G364. [DOI] [PubMed] [Google Scholar]
- Smith TK. Spontaneous junction potentials and slow waves in the circular muscle of isolated segments of guinea-pig ileum. Journal of the Autonomic Nervous System. 1989;27:147–154. doi: 10.1016/0165-1838(89)90096-9. [DOI] [PubMed] [Google Scholar]
- Smith TK, Bornstein JC, Furness JB. Distension-evoked ascending and descending reflexes in the circular muscle of guinea-pig ileum: an intracellular study. Journal of the Autonomic Nervous System. 1990;29:203–217. doi: 10.1016/0165-1838(90)90146-a. [DOI] [PubMed] [Google Scholar]
- Smith TK, Robertson WJ. Synchronous movements of the longitudinal and circular muscle during peristalsis in the isolated guinea-pig colon. The Journal of Physiology. 1998;506:563–577. doi: 10.1111/j.1469-7793.1998.563bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers RW, Dusdieker NS. Patterns of spike burst spread and flow in the canine small intestine. Gastroenterology. 1981;81:742–750. [PubMed] [Google Scholar]
- Szurszewski JH. Electrical basis for gastrointestinal motility. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1987. pp. 383–422. [Google Scholar]
- Tonini M, Costa M, Brookes SJH, Humphreys CMS. Dissociation of the ascending excitatory reflex from peristalsis in the guinea-pig small intestine. Neuroscience. 1996;73:287–297. doi: 10.1016/0306-4522(96)00040-1. [DOI] [PubMed] [Google Scholar]
- Tonini M, Frigo G, Lecchini S, D'Angelo L, Crema A. Hyoscine-resistant peristalsis in guinea-pig ileum. European Journal of Pharmacology. 1981;71:375–381. doi: 10.1016/0014-2999(81)90181-3. [DOI] [PubMed] [Google Scholar]
- Tonini M, Lecchini S, Frigo G, Crema A. Action of tetrodotoxin on spontaneous electrical activity of some smooth muscle preparations. European Journal of Pharmacology. 1974;29:236–240. doi: 10.1016/0014-2999(74)90021-1. [DOI] [PubMed] [Google Scholar]
- Trendelenburg P. Physiologische und pharmakologische Versuche Uber die Dunndarmperistaltik. Naunyn-Schmiedeberg's Archives of Pharmacology. 1917;81:55–129. doi: 10.1007/s00210-006-0052-7. [DOI] [PubMed] [Google Scholar]
- Tsuchiya K. Electrical and mechanical activities of the longitudinal muscle in the peristaltic wave elicited by the intraluminal pressure raising. Rendic Gastroenterology. 1972;4:115–125. [Google Scholar]
- Waterman SA, Costa M. The role of enteric inhibitory motoneurones in peristalsis in the isolated guinea-pig small intestine. The Journal of Physiology. 1994;477:459–468. doi: 10.1113/jphysiol.1994.sp020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman SA, Costa M, Tonini M. Accommodation mediated by enteric inhibitory reflexes in the isolated guinea-pig small intestine. The Journal of Physiology. 1994a;474:539–546. doi: 10.1113/jphysiol.1994.sp020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman SA, Tonini M, Costa M. The role of ascending excitatory and descending inhibitory pathways in peristalsis in the isolated guinea-pig small intestine. The Journal of Physiology. 1994b;481:223–232. doi: 10.1113/jphysiol.1994.sp020433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JD. Excitation of intestinal muscle by atropine, tetrodotoxin and xylocaine. American Journal of Physiology. 1972;222:118–125. doi: 10.1152/ajplegacy.1972.222.1.118. [DOI] [PubMed] [Google Scholar]
- Wood JD, Perkins WE. Mechanical interaction betwen longitudinal and circular axes of the small intestine. American Journal of Physiology. 1970;281:762–768. doi: 10.1152/ajplegacy.1970.218.3.762. [DOI] [PubMed] [Google Scholar]
- Yokoyama S, North RA. Electrical activity of longitudinal and circular muscle during peristalsis. American Journal of Physiology. 1983;244:G83–88. doi: 10.1152/ajpgi.1983.244.1.G83. [DOI] [PubMed] [Google Scholar]
- Yokoyama S, Ozaki T. Contractions of the longitudinal and circular muscle of the small intestine. Progress in Clinical and Biological Research. 1990;327:483–492. [PubMed] [Google Scholar]


