Abstract
The fluorescent dye 5-N-hexadecanoyl-aminofluorescein (HAF) was used to study the mechanisms involved in maintaining a relatively constant luminal surface pH (pHs) in the distal colon of the guinea-pig. The fatty acyl chain of the HAF molecule inserts into the apical membrane of epithelial cells. This allows a continuous measurement of the surface pH for several hours.
The localization of HAF was confirmed by confocal laser-scanning microscopy and by using monoclonal antibodies against fluorescein. The insertion of HAF into the apical membrane of the colonocytes did not change the transepithelial conductance or the short-circuit current of the epithelium.
With the HAF method a pH microclimate was confirmed at the colonic surface. Although the pH of the bulk luminal solution was decreased in bicarbonate-containing solution from 7.4 to 6.4 the pHs changed only in the range 7.54-6.98.
In the absence of bicarbonate pHs almost followed changes of bulk luminal pH. In the presence of bicarbonate there was a decrease in pHs after removal of chloride from the luminal side and an increase in pHs after addition of butyrate to the luminal solution. This suggests the involvement of a bicarbonate-anion exchange in bicarbonate secretion: a Cl−-HCO3− as well as a short-chain fatty acid−-HCO3− exchange.
The apical K+-H+-ATPase in the distal colon of guinea-pig has little influence on pHs in the presence of physiological buffer concentrations.
Our findings indicate that bicarbonate plays a major role in maintaining the pH microclimate at the colonic surface.
The existence of a constant pH microclimate at the luminal surface of the large intestinal mucosa had been shown by various research groups in recent years. These measurements hitherto had been performed almost exclusively by means of pH-sensitive microelectrodes. In studies with the rat, guinea-pig and human colon (McNeil & Ling, 1984; Rechkemmer et al. 1986) it was demonstrated that this surface pH was relatively independent of changes in proton concentration of the bulk luminal fluid. Neither removal of sodium from the perfusion solution, nor the inhibition of Na+-H+ exchange by mucosal amiloride affected the pH microclimate in the colon of guinea-pig (Rechkemmer et al. 1986).
Such a region of relatively constant pH has a decisive influence on the absorption of weak electrolytes such as short-chain fatty acids (SCFAs) (Rechkemmer, 1991). A constant luminal pH microclimate is efficient, for instance, in preventing the rapid entry of protonated, lipid-soluble SCFA molecules into the enterocytes, when the pH in the luminal bulk phase is decreased. Lowering the pH of the luminal solution from 7.2 to 5.5 in the human colon (McNeil et al. 1978), from 7.4 to 5.4 in the rat caecum (Flemming et al. 1991), from 8.6 to 5.0 or from 7.4 to 5.6 in the guinea-pig colon (Rechkemmer & Engelhardt, 1988; Oltmer & Engelhardt, 1994) had little or no effect on SCFA absorption rates. This independence of SCFA absorption from pH can be attributed to a microclimate of constant pH at the luminal surface of the large intestine.
The reasons for the constancy of the pH microclimate at the surface of the large intestine are not known. A limiting factor for studies in this area has been the lack of an appropriate method for surface pH estimations. Measurement using microelectrodes provides several possible sources of error, including the lack of a defined position for the electrode tip and, over the course of time, the uncertainty of the location of the tip due to movement of the mucosa, especially when a change of the perfusion solution is required. Therefore, estimations of surface pH over a longer period of time are generally not possible with microelectrodes. Shiau et al. (1985) estimated the surface pH in rat jejunum with the pH indicators Kodak 5325 and Bromocresol Purple. For measuring the luminal pH in colonic crypts of mice Chu & Montrose (1995, 1996) used the pH-sensitive fluorescent dye SNARF-1.
It was the aim of our study to use a pH-sensitive dye that attaches to the luminal surface of enterocytes. With such a probe factors affecting and regulating the pH at the luminal surface of colonocytes could be studied. We used the pH-sensitive fluorescent dye 5-N-hexadecanoyl-aminofluorescein (HAF). Due to its lipophilic character the alkyl tail of this probe inserts into the membrane of the colonocytes. Hitherto, HAF had been used for investigations of lateral diffusion of lipids in biomembranes (Dragsten et al. 1981; Dupou et al. 1987; Tournier et al. 1989) and for measurements of resonance energy transfer in lipid membranes (Remmers & Neubig, 1993).
The localization of the dye molecule at the epithelial surface was demonstrated by means of a confocal laser-scanning microscope and by using IgG antibodies. With the pH-sensitive dye attached to the apical membrane the influence of bicarbonate, chloride and SCFAs on the pH microclimate were investigated. Our findings underline the importance of bicarbonate in establishing and maintaining the pH microclimate at the surface of the colonic mucosa of guinea-pig.
METHODS
Animals and tissue preparation
Male guinea-pigs (body weight of 550-650 g) were fed on a pelleted standard diet (Altromin No. 3122, Altromin, Lage, Germany). Water and food were available ad libitum. The guinea-pigs were maintained on a 12 h light: 12 h dark photoperiod with two or three animals being held together in one cage. For experiments the guinea-pigs were killed by decapitation. The distal colon was cut in approximately 3 cm-long sections, and these sections were immediately put into ice-cold, carbogen (95% O2, 5% CO2)-gassed Krebs-Henseleit solution (KH; pH 7.4) after the luminal contents had been flushed off. The sections were stored in this solution until the beginning of the preparations. A section of the distal colon was cut open longitudinally at the mesenterial side and was fixed on a PVC plate by needles with the mucosal side uppermost. The mucosa was stripped off the submucosal tissue with glass slides, and was mounted, slightly stretched with its serosal side on a supporting silicone ring, with Histoacryl (Braun-Melsungen, Melsungen, Germany). The experiments were carried out according to the guidelines of our local Animal Care Committee.
Fluorescent probe for the estimation of the luminal surface pH
The pH-sensitive fluorescent dye 5-N-hexadecanoylaminofluorescein (HAF) was purchased from Molecular Probes (Eugene, OR, USA). The HAF molecule consists of two domains, the fluorescein molecule and a palmitoyl chain connected to this fluorescing moiety. Dragsten et al. (1981) have shown that this palmitoyl chain inserts into the apical membrane of cells due to its lipophilic character. The sensitivity of HAF to pH changes relies on the presence of dissociable groups in the fluorescing moiety.
A stock solution of HAF in a concentration of 30 mmol l−1 was prepared with ethanol (98%; Merck, Darmstadt, Germany) and stored at -20°C for no longer than 1 week. A final HAF concentration of 15 μmol l−1 was used to superfuse the mucosal side of the epithelium of the distal colon for 20 min. An excitation spectrum of HAF was determined with the help of a Kontron SFM 23 spectrofluorometer (Kontron, Zürich, Switzerland).
Intracellular pH
The intracellular pH (pHi) was measured using the fluorescent dye 2′,7′-biscarboxyethyl-5,6-carboxyfluorescein acetoxymethylester (BCECF AM) as described for an HT29 cell line (Busche et al. 1993).
Chemicals
Freeze-dried monoclonal IgG antibody (750 μg) against free and bound fluorescein (Boehringer Mannheim GmbH, Germany) was dissolved in 50 μl of Hepes buffer. Three drops of this stock solution were used to quench the fluorescence intensity of the epithelia labelled with HAF. Concentrations of 10 mmol l−1 of 1,4-dithio-L-threitol (DTT; Sigma, Deisenhofen, Germany) were used before and during dye loading to diminish the amount of mucus at the epithelial surface. During the following perfusion DTT was not present in the solutions. Nigericin was purchased from Sigma, carbonyl cyanide 4-trifluoromethoxyphenyl-hydrazone (FCCP) from Fluka (Buchs, Switzerland) and BCECF AM from Serva (Heidelberg, Germany). For the inhibition of the apical K+-H+-ATPase ouabain (4β,20[22]-cardenolide-1β,3b,5α,14,19-hexol-3-[6′deoxy-α-L-mannopyranosyl]-G-strophanthin (Sigma)) was added to the mucosal superfusate.
Perfusion solutions
For measuring the resting surface pH, the epithelium was superfused with Krebs-Henseleit (KH) solution containing (in mmol l−1): 140 Na+, 124 Cl−, 21 HCO3−, 5.4 K+, 2.4 HPO42−, 0.6 H2PO4−, 1.2 Mg2+, 1.2 Ca2+, 10 glucose; pH 7.4. In bicarbonate-free solutions bicarbonate was replaced by 21 mmol l−1 of N-[2-hydroxyethyl]piperazine-N′-[2-ethane sulfonic acid] (Hepes) buffer. In chloride-free solutions, chloride was replaced by gluconate. In butyrate-containing solutions 21 or 113.6 mmol l−1 butyrate was added as the sodium salt. The sodium concentration was kept constant by reducing the amount of sodium chloride as appropriate. Therefore, in the solution containing 113.6 mmol l−1 butyrate the concentration of chloride was only 10.4 mmol l−1. To adjust the pH of the bicarbonate-free Hepes solutions between 6.5 and 8 the solutions were titrated with NaOH; to obtain the required sodium concentration an appropriate amount of sodium gluconate was added. To adjust the lower pH in the bicarbonate-containing solution less bicarbonate was added (for pH 6.4, 1 mmol l−1; for pH 6.9, 4.5 mmol l−1; for 7.2, 10 mmol l−1), and the solutions were gassed with carbogen. The osmolarity of all solutions was adjusted to 300 mosmol l−1 with mannitol. All of the chemicals required for the solutions were purchased from Merck (Darmstadt, Germany). During the experimental procedure the solutions were kept in stock bottles in a water-bath at 37°C. All solutions containing bicarbonate were gassed with carbogen and bicarbonate-free solutions were gassed with O2. To ensure that the solutions had a constant pH they were heated to 37°C and gassed for at least 30 min before the experiments were started.
Calibration buffers
For calibrations nigericin (10 μg l−1) was added to the calibration buffers containing (in mmol l−1: 152 Cl−, 133.4 K+, 25 Hepes, 15 Na+, 1.8 Ca2+, 0.8 Mg2+, 0.8 SO42−). The pH of these solutions was adjusted to a defined pH in the range 6.0-8.0 by different amounts of 1 n NaOH. The osmolarity of the buffers was 300 mosmol l−1. The calibration buffers were added to both the mucosal and serosal sides of the epithelium.
Experimental set-up
The stripped epithelium fixed to the supporting ring was placed in a microperfusion chamber (Busche et al. 1993) with the basolateral side uppermost. Measurements were performed by means of an inverted microscope (Axiovert 35M; Zeiss, Oberkochen, Germany). The microperfusion chamber, consisting of two silicone rubber gaskets and two in- and outflow cannulas at each side, allowed separate perfusion of the mucosal and the serosal sides of the epithelium. A Zeiss LD40 objective (NA 0.60) with a working distance of 1.5 mm (no oil immersion) was used. The volume of each side of the chamber was 20 μl. The perfusion rate was 100 μl min−1. It could be altered by varying the height of the table on which the solutions were kept in stock bottles. For the measurements the chamber was placed onto the heatable stage of the microscope. The chamber was kept at 33°C during the experiments. Two to three surface cells were focused on for measuring the fluorescence intensity. A computer-directed, motor-driven stage allowed a parallel measurement of fluorescence intensity at three different locations within the epithelium. A minimal time interval of 15 s was necessary between two measurements. The correct position of the focus could be controlled visually using a video camera connected to the microscope.
For the fluorescence measurements a 75 W xenon lamp was used and two wavelengths (436 and 485 nm) were selected by bandpass filters for the excitation of the fluorescent dye. The excitation intensity at 485 nm was reduced to 25% by a neutral density filter to allow measurements using the same gain settings of the photomultiplier at both wavelengths. To minimize photobleaching, the intensity at both wavelengths was further reduced to 10% by a neutral density filter, and the excitation time was restricted to 200 ms for each time point using a high-speed shutter. The fluorescence emission was collected by a photomultiplier in the range 515-530 nm. The ratio of the fluorescence intensities (excitation at 436 and 485 nm) was calculated continuously. After calibration the actual surface pH (pHs) was calculated by taking the ratios of at least two defined pH values and assuming a linear relation between ratio and pH within this range (Busche et al. 1993).
Experimental procedure for measurements of surface pH (pHs)
The epithelium mounted into the microperfusion chamber was superfused with KH (carbogen gassed, 37°C), and the autofluorescence of the epithelium was determined at the excitation wavelengths of 436 and 485 nm. After an equilibrium period of 15 min the mucosal KH was replaced by the dye solution of 15 μmol l−1 HAF in KH for 20 min. Thereupon the remaining non-inserted dye was washed off with standard KH. At the end of the experiments calibration with at least two calibration buffers of different pH was performed.
Confocal laser-scanning microscopy and quenching of the fluorescence intensity with antibodies
The position of HAF after the epithelium was stained with dye was investigated using confocal laser-scanning microscopes (Bio-rad, MRC 500: Hemel Hempstead, UK; and Leica TCS NT: Benzheim, Germany) using a ×20 objective (NA 0.5) and a ×40 objective (NA 1.25), respectively. The HAF-labelled epithelium was focused through in the z-direction in micrometre steps.
For quenching the fluorescence intensity of an HAF-labelled epithelium monoclonal IgG antibodies against free and bound fluorescein (Boehringer Mannheim GmbH, Germany) were used. After mounting the stripped epithelium of guinea-pig distal colon into the microperfusion chamber and staining with HAF, the fluorescence intensity of this epithelium was measured near the isosbestic point. To diminish the influence of mucus 10 mmol l−1 of DTT was added to the mucosal perfusion solution prior to dye loading. Three drops of the antibody solution were added to the mucosal side of the epithelium. After 45 min of incubation with the antibody the fluorescence intensity was measured again.
Ussing chamber experiments
The viability of the dye-labelled epithelium was controlled with mucosal sheets of the distal colon of guinea-pig mounted in Ussing chambers as described by Engelhardt et al. (1993). Both sides of the epithelium were superfused with 10 ml of Krebs-Henseleit solution at pH 7.4 and 37°C. Transepithelial conductance (gt) and short-circuit current (Isc) were determined.
Statistics
Results are presented as means and standard deviations. Differences were evaluated using Student's paired and unpaired two-sided t tests as appropriate. n corresponds to the number of epithelia that were used for the experiments.
RESULTS
Validity of the fluorometric method for estimation of the surface pH
Excitation spectrum of HAF
The relative fluorescence intensity of a HAF-labelled epithelium fixed to a plastic slide positioned in a cuvette with solutions of different pH levels (pH 4-8) was measured with the excitation wavelength varying from 400 to 510 nm and the fluorescence emission collected at 530 nm (Fig. 1). Because of the constant pH microclimate, the pH of the solutions is not necessarily the same as the actual pH at the surface of the epithelium. At about 455 nm all spectra meet at one point called the ‘isosbestic point’. At this wavelength the obtained fluorescence intensity only depends on the concentration of the dye and is independent of the solution pH. The largest pH-dependent changes are observed at an excitation wavelength of about 495 nm.
Figure 1. Excitation spectrum of HAF bound to the apical membrane of the colonocytes of the distal colon of guinea-pig at different pH levels.
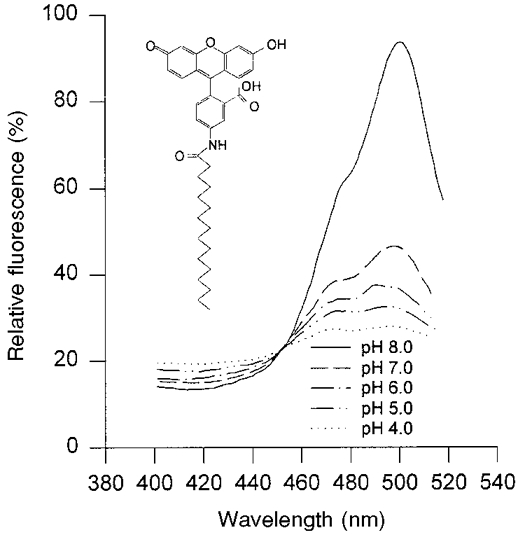
The pH of the solution in the cuvette is indicated in the figure. The fluorescence intensity was measured at 530 nm with excitation wavelength varying from 400 to 510 nm. The molecular structure of HAF is shown in the inset.
Loading of the epithelium with dye
A dye-loading experiment is shown in Fig. 2. The epithelium mounted in the perfusion chamber was superfused with a dye-containing Krebs-Henseleit solution on the mucosal side. The rapid increase of the fluorescence intensity at the beginning is caused by the dye in the perfusion solution. The slow increase of fluorescence during the superfusion represents the attachment of the dye to the epithelium. This increase was not saturated during the time of incubation. At the end of the incubation time the dye was washed out of the chamber, which led to a rapid decrease of fluorescence. The difference between the fluorescence intensities before and after dye loading represents the dye bound to the epithelium. The increase of fluorescence due to dye binding was linearly dependent on the dye concentration in the range 10-30 μmol l−1 (data not shown). A concentration of 15 μmol l−1, which allowed the stable detection of the fluorescence of the dye for at least 2 h, was chosen for all further experiments. This resulted in an increase of fluorescence by a factor of 2-4 above background. Interestingly, the solvent used for the stock solution of the dye is of importance for the dye loading. Using ethanol, we could achieve a two times higher amount of dye attached to the epithelium than if DMSO was used as a solvent.
Figure 2. Time course of the fluorescence during loading of the epithelium of the distal colon of guinea-pig.
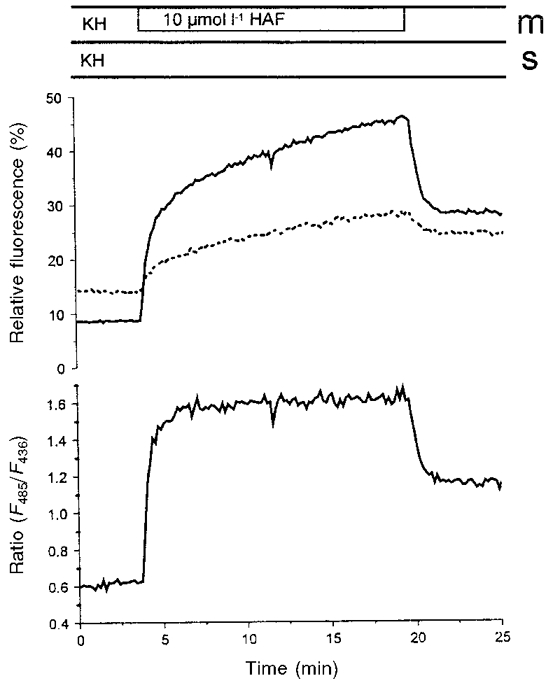
HAF (10 μmol l−1) in KH was added to the mucosal side of the mounted epithelium. The upper traces show the relative fluorescence intensities at excitation wavelengths of 485 nm (continuous line) and of 435 nm (dotted line). The lower trace shows the ratio of the two fluorescence intensities. Mucosal (m) and serosal (s) buffer solutions, respectively, are indicated above the figure. In this figure background fluorescence was not subtracted. In all other experiments the background was subtracted before the calculation of the ratio.
Localization of the dye molecule: confocal laser-scanning microscopy
To ensure that HAF remained in the apical membrane of the epithelial cells and did not move to the basolateral side of the epithelium by passing the tight junctions or by ‘flip-flop’, the localization of the dye was checked with a confocal laser-scanning microscope. Figure 3 shows four images of the fluorescing layer at the apical membrane of the distal colon of guinea-pig collected in the confocal microscope. The right panel shows transmission images, and the left panel shows recordings of the fluorescence at the corresponding depths. Since the surface of the epithelium is not in a plane, fluorescence is seen at different depths. In image A the focal plane is mostly above the surface of the apical membranes, and only traces of fluorescence are seen. In B the image is taken 10 μm deeper; in most areas fluorescence exists. In C, a further 10 μm deeper, fluorescence is seen in a lower patch in the upper right area. Five micrometres deeper (image D) only very minor fluorescence is recognizable in the region of the deeper patch. In deeper focal planes of the enterocytes and also in the lumen of the crypts there was no, or only a trace of, fluorescence visible. In the corresponding transmission images cell borders were clearly seen as soon as the focal plane was within the epithelial layer. There were no signs of fluorescence intensity in the intercellular space or at the basolateral membrane. Changes in the surface structure of the fluorescence layer represent the position of several crypts. Rarely could we see HAF in the crypt lumen. This absence may be due to the narrow opening of the unstimulated crypts of the distal colon.
Figure 3. Localization of the fluorescence intensity in HAF-labelled colonic epithelium.
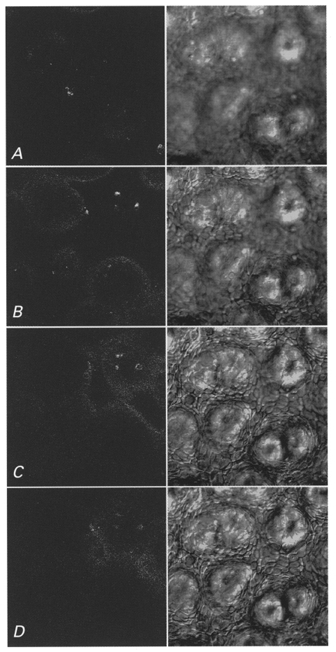
A series of confocal images (Leica TCS NT) of the dye-labelled epithelium of the distal colon of guinea-pig was taken at several depths. In the left panel fluorescence images and in the right panel the corresponding transmission images are shown. As soon as the focal plane reaches the epithelial surface cell borders come in focus. The dark areas are the openings of the crypts. In A the focal plane is mostly above the surface, only traces of fluorescence are seen. In B the focal plane is 10 μm, in C 20 μm, and in D 25 μm deeper than in A. In B patches in the left upper and in the lower right areas are labelled. In C the surface of the patch in the upper right is labelled with HAF. In D no fluorescence is visible, except traces in the upper right. Each image represents 250 μm× 250 μm.
Quenching of the fluorescence of HAF-labelled epithelium with antibodies
A monoclonal antibody was used for quenching the fluorescence emission of the dye-labelled epithelium. The antibody added to the mucosal side of the epithelium was not able to pass the apical membrane of the cells or the tight junctions. Therefore, only the fluorescence intensity of those HAF molecules bound to the apical membrane with the dye moiety projecting to the luminal side of the cells could have been quenched (Fig. 4). HAF was excited at 436 nm. The resulting fluorescence intensity was set to 100%. The fluorescence intensity decreased by about 90% after the addition of the antibody. This indicates that most of the pH-sensitive fluorescing moieties at the epithelial surface must have been outwardly orientated.
Figure 4. Quenching of the fluorescence intensity of a dye-labelled epithelium by the addition of a monoclonal antibody against free and bound fluorescein.
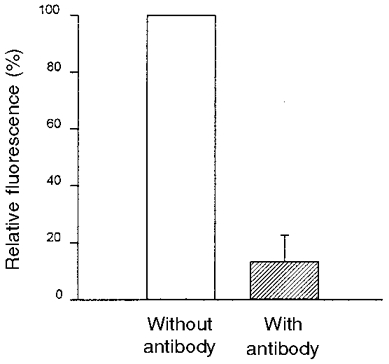
The fluorescence intensity (λEx 436 nm) was measured directly after staining the epithelium with HAF and 45 min after addition of the monoclonal IgG antibodies against fluorescein (shaded bar) (n = 3). Results are given as percentage of the dye-loaded epithelium.
Viability of the dye-labelled epithelium
Transepithelial conductance (gt) and short-circuit current (Isc) of the epithelium of the distal colon were measured in Ussing chamber experiments before, during and after superfusion with HAF solution (Fig. 5). No significant changes of Isc or gt were observed during or after the incorporation of the fluorescent probe. We therefore conclude that the integration of HAF with the epithelium at the concentrations used in our experiments does not affect membrane functions.
Figure 5. Short-circuit current (Isc) and transepithelial conductance (gt) of the distal colonic epithelium before, during and after the epithelium was stained with HAF.
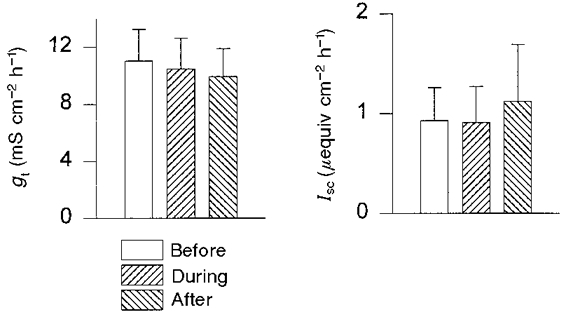
The experiments were performed in Ussing chambers; the concentration of the dye solution was 20 μmol l−1. The epithelium was superfused with the dye solution for a period of 20 min. Subsequently, the HAF solution was replaced by standard KH (n = 6).
Calibration
Differences in calibration were observed between calibrations with HAF in solution and with HAF fixed to the apical membrane of intestinal epithelia (data not shown). It was therefore not sufficient to use the calibration curve obtained with HAF in solution for the calculation of pH at the surface of intestinal epithelia. Consequently, a calibration with at least two calibration buffers of different pH was performed with the HAF-labelled epithelia after each experiment. The deviation of the calibration curves obtained with HAF in solution compared with those obtained with HAF-labelled epithelia can be explained by a pKa shift of the dye after the insertion of the palmitoyl chain into the cell membrane. The lipophilic milieu and the net charge of the epithelium may have caused a change of the molecular structure of the dye, and thereby the apparent dissociation constant of the dye's dissociable groups can change (Fromherz & Masters, 1974). According to the shape of the calibration curve the optimal range for measuring the surface pH with HAF is between pH 6.5 and 8 (Fig. 7).
Figure 7. Calibration curve for HAF inserted into the epithelium of the distal colon of guinea-pig.
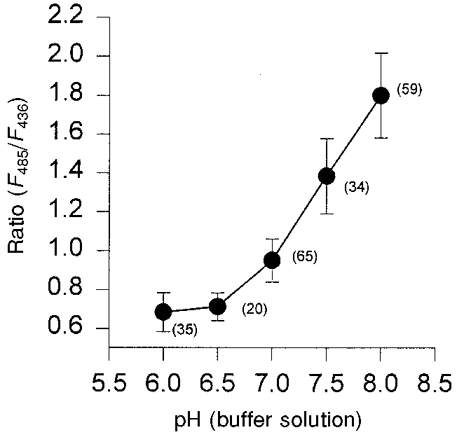
The ratio of the fluorescence intensities at the excitation wavelengths of 485 nm and 436 nm is plotted against the pH of the calibration buffers. The number of experiments is indicated in parentheses in the figure.
For pH calibration nigericin, in combination with high extracellular potassium concentrations, was added to the mucosal and serosal calibration buffers (Thomas et al. 1979; Busche et al. 1993). Nigericin removes proton gradients between intracellular and extracellular space. High concentrations of potassium were used to prevent a re-establishment of transmembrane potential differences. Surface pH and intracellular pH were therefore expected to be the same as the pH of the calibration buffers. With identical buffer pH levels on the mucosal and serosal sides of the epithelium the fluorescence intensity was measured until the signal remained constant. Subsequently, the calibration buffers were simultaneously changed on both sides to a buffer of a lower pH. A representative calibration experiment is shown in Fig. 6. The ratios obtained for different pH values in several experiments are compared in Fig. 7.
Figure 6. A calibration experiment showing the ratios of the fluorescence intensities of HAF-labelled epithelium.
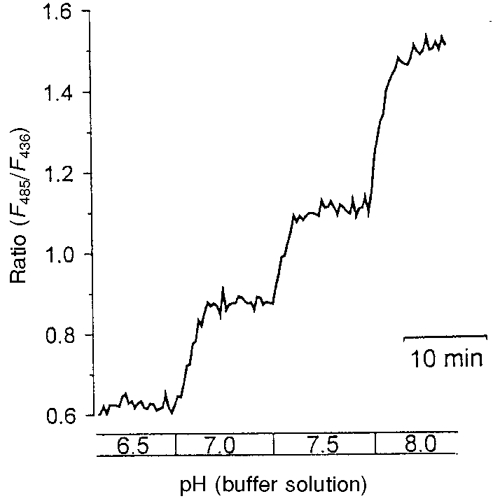
The epithelium was superfused with calibration buffers containing 10 μg l−1 nigericin. Solutions were adjusted to the pH indicated below the figure.
In order to control whether nigericin abolished existing proton gradients reliably another ionophore, FCCP, was used for calibrations. In combination with the high potassium concentrations used to clamp the membrane potential this protonophore should also abolish transmembrane proton gradients. Compared with nigericin, FCCP also inhibits oxidative energy metabolism of the cells. No differences were seen between calibration curves obtained with nigericin and those obtained with FCCP. A direct comparison in bicarbonate-free calibration buffers of pH 8 (n = 3) is shown in Fig. 8. In all further experiments nigericin was used for calibration.
Figure 8. Comparison of the calibration of the surface pH using nigericin or FCCP.
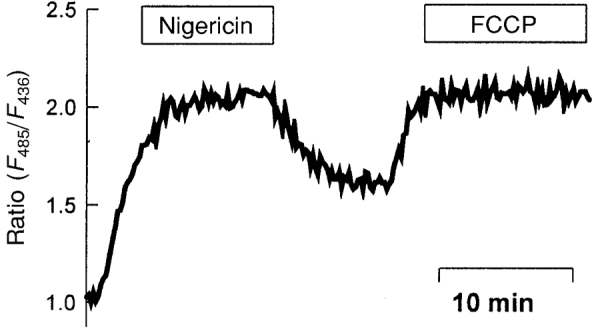
Both drugs were solubilized to a concentration of 10 μmol l−1 in the bicarbonate-free calibration buffer of pH 8. The mucosal and the serosal sides were perfused with the buffer.
Constancy of the membrane-bound dye
For the exact and continuous measurement of the surface pH it is important that the fluorescent probe remains in the apical membrane without being washed off, dipping into the membrane or decaying. According to the excitation spectrum of HAF (Fig. 1), the concentration-dependent part of the fluorescence intensity can be controlled by measuring the fluorescence intensity at the isosbestic point. The concentrations of the membrane-bound HAF did not change significantly within 2-3 h (decrease within 2 h: 2 ± 9%, n = 33). Furthermore, there was no measurable decrease in the membrane-bound dye concentration as a response to solution changes.
Microclimate pH at the surface of the distal colon of guinea-pig
Resting surface pH of the distal colon in the presence of bicarbonate
For measuring the resting surface pH isolated epithelia of the distal colon of guinea-pig labelled with HAF were superfused with standard KH containing 21 mmol l−1 bicarbonate at pH 7.4 at both sides of the epithelia. The resting surface pH under these experimental conditions was 7.54 ± 0.14 (n = 82). Surface pH measured in the microperfusion chamber by means of HAF remained constant for at least 2.5-3 h.
Surface microclimate pH when pH of the bulk luminal solution was changed
The dependence of the surface pH on the luminal pH was examined by superfusing the mucosal side of the epithelium with solutions of varying pH. The pH of the serosal solution remained constant at pH 7.4. In the presence of bicarbonate the surface pH in the distal colon of guinea-pig remained more alkaline when the pH of the luminal superfusate was below 7.5. When the pH of the luminal solution was 6.4 the surface pH at the apical membrane was 6.98 ± 0.10. Below a luminal solution pH of 7.0, pHs was significantly higher than luminal pH. In the absence of bicarbonate on the mucosal and the serosal sides of the epithelium the pH of the mucosal solution was changed from 6.5 to 8.0 in steps of 0.5 pH units. The pH of the serosal bicarbonate-free solution remained at 7.4. In the absence of bicarbonate the surface pH almost linearly followed changes of bulk luminal pH (Fig. 9).
Figure 9. Correlation between surface pH and the pH of the bulk luminal solution in the presence and the absence of bicarbonate.
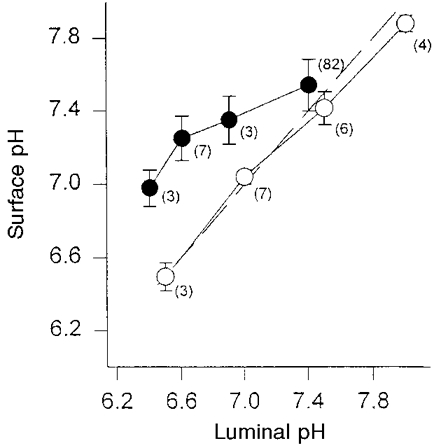
KH solutions with bicarbonate (•) or bicarbonate-free solutions (○) were added to both sides of the epithelium of the distal colon of guinea-pig. The pH of the luminal bicarbonate-containing and the bicarbonate-free perfusion solutions was changed in steps of 0.5 or less pH units. The pH of the serosal superfusate remained at pH 7.4. The dashed line is the line of identity. Numbers of experiments are indicated by the values in brackets.
Titration curves showed that the buffering powers of KH and bicarbonate-free 21 mmol l−1 Hepes solution were similar between pH 7.0 and 7.5. Above pH 7.5 the buffering power of KH was slightly lower, and between pH 6.4 and 7.0 it was higher than the respective Hepes solutions. Although the bicarbonate concentration at the low pH was low, the buffering power was high due to the gassing with CO2 and thereby buffering in the open system. Although KH at pH 6.4 has a higher buffering power than the Hepes solution, at that pH a larger deviation from the luminal pH was seen. That indicates that a lower buffering capacity cannot be an explanation for the higher pHs at the low pH of the luminal bicarbonate-containing solution. We interpret the higher pHs as being due to HCO3− secretion, enhanced by the HCO3− gradient across the epithelium at the lower pH.
Role of bicarbonate
When the bicarbonate-containing KH (pH 7.4) on the mucosal side was replaced by a bicarbonate-free Hepes-buffered solution (pH 7.4) the surface pH became acidified by 0.14 ± 0.06 pH units (n = 15, P < 0.05). As shown in Fig. 10, this acidification was reversible and reproducible. Removal of bicarbonate from the serosal side of the epithelium, on the other hand, increased the mucosal surface pH by 0.11 ± 0.04 pH units (n = 10, P < 0.02). Removal of bicarbonate from both sides of the epithelium caused a decrease of surface pH by 0.13 ± 0.04 pH units (n = 4; P < 0.05).
Figure 10. The mucosal surface pH (pHs) before and after removal of bicarbonate from the mucosal or serosal perfusion solutions.
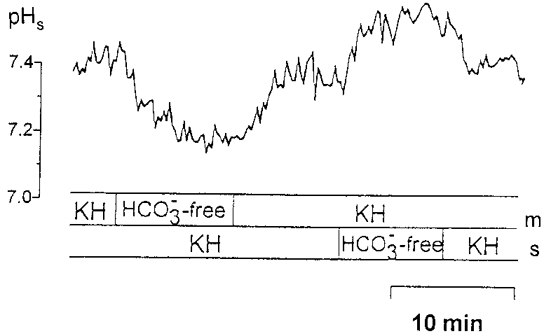
The mucosal (m) and the serosal (s) sides of the epithelium of the distal colon of guinea-pig were superfused with bicarbonate-containing KH (pH 7.4). After a pre-incubation period the KH solution was replaced either at the mucosal or the serosal side by a bicarbonate-free solution buffered with 21 mmol l−1 of Hepes. The pH of all solutions was 7.4.
To investigate the participation of the apical Cl−-HCO3− exchanger on the maintenance of the pH microclimate, chloride was removed from the mucosal or serosal perfusion solution alternately (Fig. 11A). Removal of chloride from the mucosal solution (pH 7.4) in the presence of bicarbonate on both sides of the epithelium caused a significant decrease of the surface pH by 0.12 ± 0.04 pH units (n = 7, P < 0.02). With bicarbonate-free media on both sides of the epithelium, a supplementary removal of chloride did not change the surface pH significantly (0.03 ± 0.02; n = 4, P < 0.05; Fig. 11B). Interestingly, the presence of 21 mmol l−1 butyrate on both sides of the epithelium caused an increase in pHs of 0.06 ± 0.01 (n = 3, P < 0.05; Fig. 11B). When chloride was removed from the mucosal solution in the presence of butyrate, pHs acidified back to the level of the chloride-free and butyrate-free solution. This may indicate that butyrate can substitute for bicarbonate.
Figure 11. The chloride dependence of the surface pH (pHs) in the distal colon of guinea-pig.
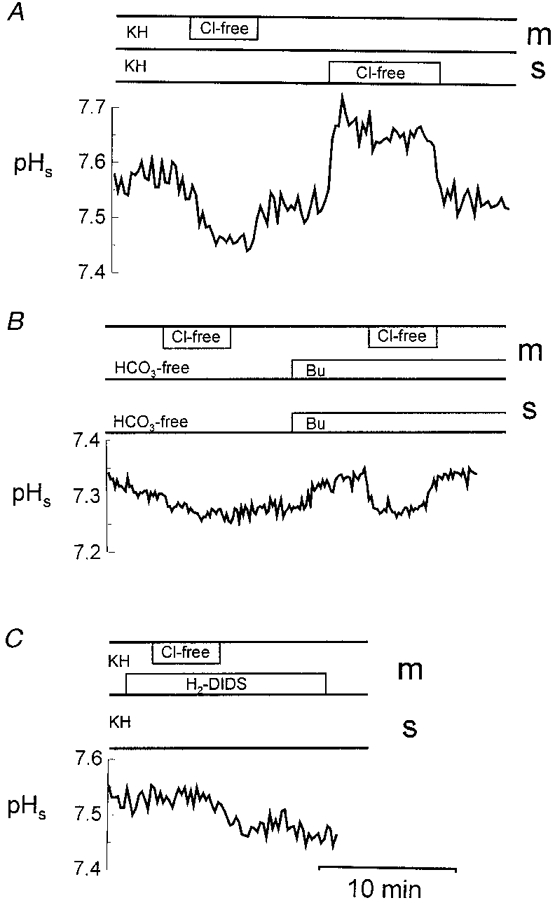
A, in the presence of bicarbonate (KH) chloride was removed (Cl-free) from the mucosal (m) or the serosal (s) side. B, in bicarbonate-free 21 mmol l−1 Hepes buffer solution (HCO3-free), chloride was removed from the mucosal solution in the absence or in the presence of 21 mmol l−1 butyrate (Bu) on both sides of the epithelium. C, addition of 0.2 mmol l−1 H2-DIDS to the mucosal KH in the presence or absence of chloride. All solutions had been titrated to pH 7.4.
The addition of 0.2 mmol l−1 H2-DIDS, an inhibitor of Cl−-HCO3− exchange, to the mucosal perfusion solution reduced the steady-state pHs by 0.08 ± 0.03 (n = 8, P < 0.05). Additionally, the decrease of pHs due to chloride removal from the mucosal side in the presence of bicarbonate, as seen in Fig. 11A, was not observed in the presence of 0.2 mmol l−1 H2-DIDS at the mucosal side (Fig. 11C). The addition of DIDS resulted, however, in a slight decrease of pHs over time.
An apical SCFA−-HCO3− exchanger?
Harig et al. (1991, 1996), Mascolo et al. (1991) and Engelhardt et al. (1994, 1997) have provided evidence for a SCFA−-HCO3− exchanger in the apical membrane of enterocytes in the large intestine. Such a SCFA−-HCO3− exchanger is assumed to be involved in the absorption of SCFA anions. In addition, findings from unidirectional flux studies (Engelhardt et al. 1993; Sellin & De Soignie, 1998; Charney et al. 1998), in situ perfusion experiments (Oltmer & Engelhardt, 1994), changes of the pH in colonic crypts (Chu & Montrose, 1996) and the intracellular pH of colonocytes (Sellin & De Soignie, 1998) clearly indicate that besides carrier-mediated transport non-ionic diffusion of SCFA also plays a major role in the transepithelial transport of SCFA. The transport of SCFA via non-ionic diffusion or by anion exchange may have different effects on pHs. Recently such effects have been discussed in relation to changes of pH in the lumen of mouse colonic crypts (Chu & Montrose, 1996).
In the presence of bicarbonate-containing solutions (pH 7.4) on both sides of the epithelium, 113.6 mmol l−1 butyrate was added to the mucosal superfusate (Fig. 12, recordings on the left). This addition of butyrate caused an increase in pHs of 0.13 ± 0.04 pH units (n = 20, P < 0.01). At the same time the intracellular pH (pHi) decreased slightly by 0.07 ± 0.03 pH units (n = 3, P = 0.05). These changes of pHs and of pHi could be explained by non-ionic diffusion into the enterocytes as well as by SCFA−-HCO3− exchange. When butyrate was added to the mucosal side in the absence of bicarbonate on both sides of the epithelium (buffered with 21 mmol l−1 Hepes), no significant changes of pHs (0.1 ± 0.06, n = 6) or of pHi were observed. This result supports the existence of a SCFA−-HCO3− exchanger. It is interesting to note that after lowering the concentration of the Hepes buffer in the mucosal bicarbonate-free solution to 5 mmol l−1, butyrate added to the mucosal superfusate also caused a significant alkalization of pHs by 0.27 ± 0.01 pH units (n = 3, P < 0.01). This indicates that besides the SCFA−-HCO3− exchanger, SCFAs are also transported by non-ionic diffusion across the distal colon of guinea-pig. At a higher buffering power this net proton flux due to non-ionic SCFA uptake from the lumen into the cell could, however, not be detected by changes in pHs.
Figure 12. The effect of butyrate on the microclimate pH (pHs) and on the intracellular pH (pHi).
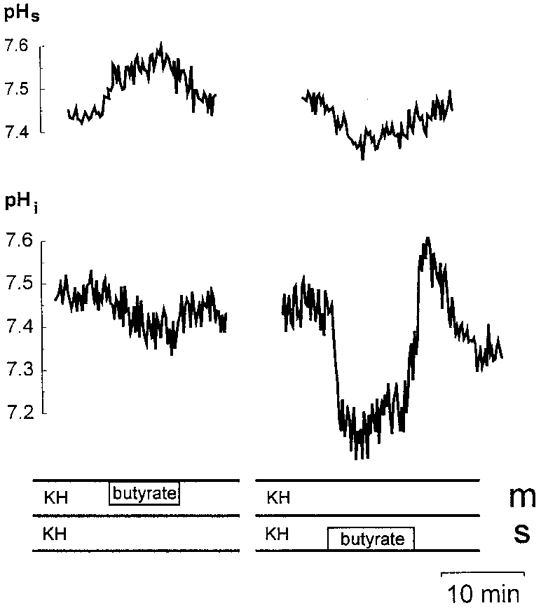
The mucosal (m) and serosal (s) sides were superfused with bicarbonate-containing KH. Butyrate (113.6 mmol l−1) was added to the mucosal (recordings on the left) or serosal (recordings on the right) side.
To determine whether the increase of pHs is chloride dependent in a further experiment butyrate was added to the mucosal solution after mucosal perfusion with gluconate- (i.e. Cl− free) and bicarbonate-containing solution (serosal-side KH). The increase in pHs (0.11 ± 0.01; n = 4) was similar to that after preperfusion with chloride-containing solution. This indicates that the butyrate effect seen in Fig. 12 does not depend on the removal of chloride. In this context it may be important that the chloride concentration in normal colonic lumen is only 6-12 mmol l−1 (authors' unpublished observations). This corresponds to the chloride concentration (10.4 mmol l−1) in our solution with 113.6 mmol l−1 butyrate; therefore this butyrate-containing solution at the luminal side was similar to that of the physiological colonic contents.
Butyrate added to the serosal side of the epithelium in the presence of bicarbonate caused a marked decrease of pHi by 0.35 ± 0.04 pH units (n = 4, P < 0.05) (Fig. 12, recordings on the right); pHs was acidified by 0.10 ± 0.02 pH units (n = 4, P < 0.01). A similar decrease of pHs was seen when butyrate was added to the serosal solution in the absence of bicarbonate on both sides of the epithelium (0.11 ± 0.03 pH units; n = 6, P < 0.02). This decrease in pHs could not be inhibited by the addition of 0.1 mmol l−1 ouabain to the mucosal solution.
Importance of luminal buffering capacity and influence of the apical K+-H+-ATPase
When the buffer concentration of the mucosal and serosal bicarbonate-free, Hepes solutions was lowered from 21 mmol l−1 to 5 mmol l−1 of Hepes, a significant decrease in pHs of 0.15 ± 0.04 pH units (n = 3, P < 0.05) (Fig. 13) was seen. This supports the presence of proton secretion across the apical membrane. The apical K+-H+-ATPase in the distal colon of guinea-pig (Suzuki & Kaneka, 1989; Watanabe & Suzuki, 1990) supplies protons, and it is assumed that these protons allow protonation of SCFA anions and subsequent permeation by non-ionic diffusion across the cell membranes (Engelhardt et al. 1993). To check whether the apical K+-H+-ATPase of the distal colon is involved in the acidification of the surface pH seen in the presence of low buffering capacity, 0.1 mmol l−1 ouabain was added to the mucosal bicarbonate-free superfusate. As a result, an alkalization of the surface pH by 0.12 ± 0.03 pH units (n = 3, P < 0.05) was observed (Fig. 13). In a similar experiment performed with bicarbonate-free 21 mmol l−1 Hepes-buffered solutions no such changes of pHs were seen when ouabain was added.
Figure 13. Mucosal surface pH (pHs) in the absence of bicarbonate at low buffer concentration and during inhibition of the apical K+-H+-ATPase.
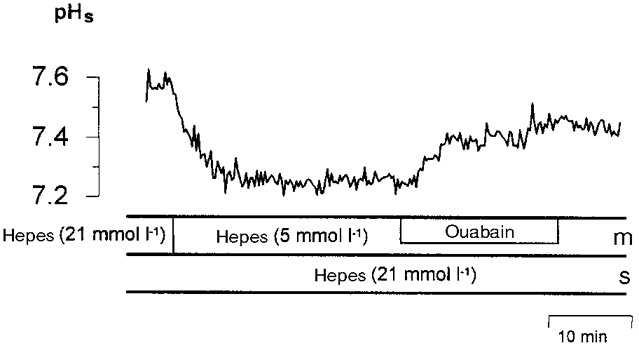
pHs in the presence of bicarbonate-free Hepes buffer of low (5 mmol l−1) buffer concentration on the mucosal (m) side and of high (21 mmol l−1) buffer concentration on the serosal (s) side of the epithelium of the distal colon of guinea-pig. The apical K+-H+-ATPase was inhibited by 0.1 mmol l−1 ouabain added to the mucosal superfusate.
DISCUSSION
Estimation of microclimate pH at the apical surface of colonocytes with HAF
The pH-sensitive fluorescent dye 5-N-hexadecanoylaminofluorescein (HAF) is a well-suited probe for measuring the microclimate pH at the intestinal surface of the distal colon of guinea-pig. As observed earlier by Dragsten et al. (1981) for epithelial cell lines derived from the kidney, and as confirmed in our experiments, the lipophilic palmitoyl chain of HAF inserts into the apical membrane of the colonocytes, and the pH-sensitive fluorescing moiety is thus located at the epithelial surface. By means of a confocal laser-scanning microscope it was shown that fluorescence intensity was not seen in the intracellular space or at the basolateral membrane. This implies that the dye molecule is exclusively bound to the apical membrane of the cells (Fig. 3). This corresponds to the observations by Dragsten et al. (1981). These authors reported that the length of the fatty acyl chain was responsible for the behaviour of such membrane-bound probes. With an acyl chain consisting of 16 carbon atoms (like HAF) the fluorescein moiety remains at the outer leaflet of the membrane, whereas a chain length of 12 carbon atoms causes the fluorescein moiety to dive into the lipid membrane (Dragsten et al. 1981). The localization of fluorescein at the luminal surface of the apical membrane of the colonocytes was confirmed by a 90% quenching of the fluorescence intensity after adding monoclonal IgG antibodies against fluorescein (Fig. 4). According to Ahlers et al. (1992) the antibody-fluorescein complex does not emit fluorescence radiation. In their experiments the fluorescence emission of the fluorescein molecules was subsequently quenched by addition of the antibody.
The calibration technique used in our experiments was adopted from measurements of the intracellular pH of different cell types (Thomas et al. 1979; Busche et al. 1993). To confirm that the high K+-nigericin technique is suitable for calibrating the microclimate pH measurements with HAF, another ionophore, FCCP, was used. Calibration curves obtained in our experiments using either nigericin or FCCP did not differ.
Resting microclimate pH at the luminal surface of the distal colon of guinea-pig
The microclimate pH measured with HAF at the luminal surface of the distal colonic epithelium of guinea-pig was 7.54 ± 0.14 when the pH of the luminal solution was 7.4. This pH is more alkaline than the mean microclimate pH obtained with a pH-sensitive microelectrode in the proximal colon (pH 7.1) and in the distal colon (pH 6.9) of guinea-pig (Rechkemmer et al. 1986). McNeil et al. (1987) had estimated even lower values for the rat and human distal colon. One explanation for the differing microclimate pH levels obtained with HAF and microelectrodes is probably the difference between the methods. The position of the tips of the glass electrodes cannot be defined exactly at any time of the measurement, and the position can easily change as a result of minimal movements of the epithelium. Furthermore, the surface of the epithelium of the colon is not smooth because of crescentic folds and crypts. It should also be noted that the tip might occasionally injure the apical membrane of the enterocytes. In contrast, the distance between the fluorescing moiety of HAF molecules and the epithelial surface is considerably smaller than the distance between electrode tip and epithelial surface, and this distance does not change with epithelial movements. Therefore, by using HAF, the pH at the luminal side of the apical membrane can be estimated directly at the surface.
Chu & Montrose (1995) used a soluble pH-sensitive fluorescent dye (carboxy-SNARF-1) for measuring the crypt luminal pH in the murine distal colon. They also obtained a slightly alkaline pH (about pH 7.3), and findings are similar to those we had measured with HAF. Thus using two different pH-sensitive fluorescent dyes, similar results have been observed.
Bulk luminal pH and microclimate pH
Rechkemmer et al. (1986) observed with microelectrodes a constant independent pH microclimate at the surface of the proximal and distal colon of guinea-pig that was independent of changes of pH in the bulk luminal solution. In in situ as well as in in vitro studies the surface pH remained constant when the luminal pH was changed between pH 5 and pH 8.6. A similar constant pH microclimate was reported by Shiau et al. (1985) for the rat small intestine. In our studies the pH at the surface of the colonocytes in the distal colon of guinea-pig changed by 0.56 pH units (from pH 7.54 to 6.98) when the pH in the bulk luminal solution was decreased by 1.0 pH unit (from pH 7.4 to 6.4; Fig. 9). Thus, in our studies a slight decrease in surface pH was seen when the pH of the bulk luminal solution was decreased step by step, whereas in in vitro experiments with microelectrodes (Shiau et al. 1985; Rechkemmer et al. 1986) no major changes of surface pH were observed. In in vivo experiments in rat proximal jejunum (Lucas, 1983) the surface pH changed, as we also found in the colon, when the pH in the bulk luminal solution was altered. With pH microelectrodes it seems likely that the mean pH value had been measured in the unstirred layer at the surface of the colonic epithelium. These mean values may explain the reported lower values and the missing changes when bulk luminal pH was altered.
Bicarbonate: the most important factor in maintaining a microclimate surface pH
A continuous secretion of bicarbonate at the luminal surface of the colonic epithelium (Feldman et al. 1990) might be the cause of the alkaline surface pH measured with HAF at the colonic surface (Fig. 9) and also with SNARF-1 in the colonic crypt lumen (Chu & Montrose, 1995). A similar explanation for the neutral surface pH was given by Rechkemmer (1991). Removal of bicarbonate and CO2 from the luminal side creates a bicarbonate and CO2 gradient across the cell membrane. The luminal CO2 pool would be ‘refilled’ by diffusion of CO2 from the serosal to the luminal side of the epithelium. This promotes the reaction of CO2 and H2O towards HCO3− and H+ at the luminal side, which is additionally accelerated by the high carbonic anhydrase activity in the mucin attached to the epithelial surface (Kleinke et al. 1998). As a consequence more protons would be available at the surface of the epithelium, and pHs would decrease. Similarly, Chu & Montrose (1996) have shown a decrease in pH in the lumen of mouse crypts by 0.12 units with CO2-HCO3− at the serosal side and HCO3−-free solution at the mucosal side. Moreover, due to a bicarbonate gradient HCO3− anions would be transported out of the cell by the apical Cl−-HCO3− exchanger. Bicarbonate anions would neutralize the free protons at the luminal surface of the epithelium, and thus pHs would increase. However, due to a rapid CO2 diffusion-mediated equilibrium reaction the effect of slower bicarbonate transport becomes less apparent (Fig. 10).
When bicarbonate-free solutions were added to the serosal side of the epithelium CO2 diffusion equilibrium reactions and bicarbonate secretion were directed towards the serosal side. The flux of CO2 from the mucosal to the serosal side would provoke an alkalization of the luminal surface as seen in our experiments and also in the colonic mouse crypt (Chu & Montrose, 1996). After removal of bicarbonate from the mucosal as well as the serosal side only the very small amounts of CO2 and H2O produced by intracellular metabolism are present, and thus very little bicarbonate would be available for the apical Cl−-HCO3− exchanger. This explains the lower pHs, which is similar to that when only the luminal solution was bicarbonate free. Under these bicarbonate-free conditions an alkaline microclimate pHs cannot be maintained, and when the bulk luminal pH is decreased the pHs decreases correspondingly (Fig. 9).
The findings in our experiments with chloride-free solutions support the existence of an apical Cl−-HCO3− exchange being involved in the regulation of pHs. This exchanger is obviously the main mechanism responsible for apical colonic bicarbonate secretion in the large intestine (Feldman et al. 1990; Feldman & Stephenson, 1990). The activity of the apical Cl−-HCO3− exchanger depends on the presence of luminal chloride. The transport direction of the exchanger is reversed when chloride is removed from the luminal superfusate as was shown by Chaillet et al. (1986) with the LLC-PK1 porcine renal cell line. The observed acidification of the luminal surface pH with luminal Cl−-free solution (Fig. 11A) can be explained by an inhibition of bicarbonate secretion. This results in a decrease of pHs. A chloride gradient in the opposite direction (no chloride on the serosal side) led to an increase of pHs probably due to an increased bicarbonate secretion across the apical membrane. Busche et al. (1997) have demonstrated that under these conditions (removal of chloride from the serosal side) the intracellular pH also increased. An interesting observation is that chloride has the same effect on pHs when bicarbonate is replaced by an equal amount of butyrate (Fig. 11B). This indicates that butyrate may substitute for bicarbonate in exchange with chloride in the apical membrane. This is in agreement with recent observations by Rajendran & Binder (1994), in which these authors postulate a Cl−-butyrate− exchange in apical membrane vesicles isolated from the distal colon of rats.
Proton secretion and microclimate pH
Rechkemmer et al. (1986) have speculated that the surface pH may depend on proton secretion in connection with the regulation of intracellular pH. In the distal colon of guinea-pig protons are secreted by the apical K+-H+-ATPase (Suzuki & Kaneko, 1989; Watanabe & Suzuki, 1990), not by Na+-H+ exchange. This proton secretion might affect the pH microclimate at the apical surface. However, in our experiments with epithelia from the distal colon of guinea-pig inhibition of the apical K+-H+-ATPase by addition of ouabain to the sufficiently buffered mucosal KH did not alter the basic pHs. Even after an experimental decrease of intracellular pH the pHs was not, or at least very little, affected, independently of whether or not K+-H+-ATPase was inhibited (authors' unpublished observations). Although Abrahamse et al. (1992, 1994) have postulated a contribution of the K+-H+-ATPase to intracellular pH regulation, we conclude that the K+-H+-ATPase in the distal colon of guinea-pig may not be sufficiently active under physiological conditions to cause acidification at the luminal surface. In this respect it is important to consider that mechanisms involved in intracellular pH regulation of enterocytes in the distal colon of guinea-pig as well as in the polarized layer of colonic tumour cells (Busche et al. 1993, 1997) are located primarily in the basolateral membrane, not in the apical membrane.
In bicarbonate-free low buffer concentrations (5 mmol l−1 Hepes) of luminal solution pHs was lower than in 21 mmol l−1 of Hepes, and after the inhibition of the apical K+-H+-ATPase under these conditions pHs increased slightly (Fig. 13). Obviously under physiological conditions the luminal buffer system neutralizes the protons secreted by K+-H+-ATPase. An effect of the luminal buffering power has also been observed by Chu & Montrose (1995). They obtained an acidification of the crypt luminal pH when the buffering capacity of the solution had been reduced. The buffering capacity of normal colonic contents of guinea-pig is high ([HCO3−]≈ 21 mmol l−1, [SCFA]≈ 100 mmol l−1).
Effect of SCFA transport on pHs
In the large intestine short-chain fatty acids, mainly acetate, propionate and butyrate, are produced by anaerobic bacterial fermentation. These SCFAs are absorbed in the non-ionized and also in the ionized form (Engelhardt et al. 1993, 1994). Although at the physiological pH in colonic contents 99% of these SCFAs are present as anions, in a number of recent studies SCFA absorption is believed to occur mainly by diffusion in the protonated form (Chu & Montrose, 1996; Charney et al. 1998; Sellin & De Soignie, 1998). Other investigators postulate a predominantly ionized transport of SCFAs (Mascolo et al. 1991; Harig et al. 1996; Ritzhaupt et al. 1998a). Obviously major regional and species differences exist with regard to transport in the protonated and ionized forms (Engelhardt et al. 1993, 1994, 1995, 1997).
The alkalization of pHs obtained in our experiments after butyrate was added to the mucosal side of the epithelium (Fig. 12) confirms a net proton flux from the lumen into the colonocytes. From this information alone it cannot be distinguished whether the non-ionic diffusion or a carrier-mediated transport of SCFA in exchange with bicarbonate caused the observed changes in pHs. However, this alkalization was also observed when butyrate was added in the absence of bicarbonate, although only when the mucosal buffering capacity was reduced. pHs was acidified in bicarbonate-free solutions, on the other hand, when butyrate was added to the serosal side. Both these observations suggest non-ionic diffusion of SCFA across the apical membrane. With 21 mmol l−1 of Hepes buffer in the absence of bicarbonate butyrate applied to the luminal side did not affect pHs. That is further indirect evidence for the participation of an apical SCFA−-HCO3− exchange (Mascolo et al. 1991; Engelhardt et al. 1994, 1997; Ritzhaupt et al. 1998a). It has been shown in a number of studies that bicarbonate accumulates in the lumen of the large intestine proportionally to SCFA absorption (see Engelhardt, 1995). Such a bicarbonate gain could also explain the observed increase of pHs. The stronger alkalization of pHs in our studies in the presence of bicarbonate compared with that in bicarbonate-free media substantiates the role of bicarbonate secretion. A monocarboxylate (MCT1) transporter that may be involved in SCFA transport in the apical membrane has been recently identified (Ritzhaupt et al. 1998b).
We conclude that the fluorescent dye HAF can be used for measurements of the surface pH in the colon. The integration of the fatty acyl chain of the HAF molecule into the apical membrane of the epithelial cells allows a continuous measurement of pHs for several hours. A pH microclimate does exist at the surface of the distal colon of guinea-pig only in the presence of bicarbonate. Bicarbonate plays a major role in maintaining pHs. Our findings support the presence of a SCFA−-HCO3− exchange in the apical membrane of the colon.
Acknowledgments
This project was supported by the Deutsche Forschungsgemeinschaft, SFB 280, ‘Gastrointestinale Barriere’. The skilled technical assistance of K. Hansen and G. Becker is greatly appreciated. We would like to thank Professor Forssmann, Niedersächsiches Institut für Peptidforschung, Hannover and Professor Niemann, Department of Physiological Chemistry, Medical School, Hannover, for the use of their confocal microscopes, and J. Bartels for his support in measuring the intracellular pH.
References
- Abrahamse SL, Bindels RJM, Van Os CH. The colon carcinoma cell line Caco-2 contains an H+/K+-ATPase that contributes to intracellular pH regulation. Pflügers Archiv. 1992;421:591–597. doi: 10.1007/BF00375056. [DOI] [PubMed] [Google Scholar]
- Abrahamse SL, Vis A, Bindels RJM, Van Os SH. Regulation of intracellular pH in crypt cells from rabbit distal colon. American Journal of Physiology. 1994;267:G409–415. doi: 10.1152/ajpgi.1994.267.3.G409. [DOI] [PubMed] [Google Scholar]
- Ahlers M, Grainger DW, Herron JN, Lim K, Ringsdorf H, Salesse C. Quenching of fluorescein-conjugated lipids by antibodies. Biophysical Journal. 1992;63:823–838. doi: 10.1016/S0006-3495(92)81645-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busche R, Bartels J, Genz A-K, Engelhardt Wv. Effect of SCFA on intracellular pH and intracellular pH regulation of guinea-pig caecal and colonic enterocytes and of HT29–19a monolayers. Comparative Biochemistry and Physiology. 1997;A 118:395–398. doi: 10.1016/s0300-9629(96)00327-1. [DOI] [PubMed] [Google Scholar]
- Busche R, Jeromin A, Engelhardt Wv, Rechkemmer G. Basolateral mechanisms of intracellular pH regulation in the colonic epithelial cell line HT29 clone 19A. Pflügers Archiv. 1993;425:219–224. doi: 10.1007/BF00374170. [DOI] [PubMed] [Google Scholar]
- Chaillet JR, Amsler K, Boron WF. Optical measurements of intracellular pH in single LLC-PK1 cells, demonstration of Cl−-HCO3− exchange. Proceedings of the National Academy of Sciences of the USA. 1986;83:522–526. doi: 10.1073/pnas.83.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney AN, Micic L, Egnor RW. Nonionic diffusion of short-chain fatty acids across rat colon. American Journal of Physiology. 1998;274:G518–524. doi: 10.1152/ajpgi.1998.274.3.G518. [DOI] [PubMed] [Google Scholar]
- Chu SY, Montrose MH. Extracellular pH regulation in microdomains of colonic crypts effects of short-chain fatty acids. Proceedings of the National Academy of Sciences of the USA. 1995;92:3303–3307. doi: 10.1073/pnas.92.8.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu SY, Montrose MH. Non-ionic diffusion and carrier-mediated transport drive extracellular pH regulation of mouse colonic crypts. The Journal of Physiology. 1996;494:783–793. doi: 10.1113/jphysiol.1996.sp021532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragsten PR, Blumenthal R, Handler JS. Membrane asymmetry in epithelia: is the tight junction a barrier to diffusion in the plasma membrane? Nature. 1981;294:718–722. doi: 10.1038/294718a0. [DOI] [PubMed] [Google Scholar]
- Dupou L, Gualandris L, Lopez A, Duprat AM, Tocanne JF. Alterations in lateral lipid mobility in the plasma membrane of urodelean ectodermal cells during gastrulation. Experimental Cell Research. 1987;169:502–513. doi: 10.1016/0014-4827(87)90210-2. [DOI] [PubMed] [Google Scholar]
- Engelhardt Wv. Absorption of short-chain fatty acids from the large intestine. In: Cummings JH, Rombeau JL, Sakata T, editors. Physiological and Clinical Aspects of Short Chain Fatty Acid Metabolism. Oxford: Cambridge University Press; 1995. pp. 149–170. [Google Scholar]
- Engelhardt Wv, Burmester M, Hansen K, Becker G, Rechkemmer G. Effects of amiloride and ouabain on short-chain fatty acid transport in guinea-pig large intestine. The Journal of Physiology. 1993;460:455–466. doi: 10.1113/jphysiol.1993.sp019481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt Wv, Burmester M, Hansen K, Becker G, Rechkemmer G. Effects of amiloride and ouabain on short-chain fatty acid transport across segments of the large intestine in pig, sheep, and pony compared with guinea-pig. Journal of Comparative Physiology. 1995;B 165:29–36. doi: 10.1007/BF00264683. [DOI] [PubMed] [Google Scholar]
- Engelhardt Wv, Busche R, Burmester M, Hansen K, Becker G. Transport of propionate across the distal colonic epithelium of guinea-pig in the presence and absence of bicarbonate and of chloride. Journal of Veterinary Medicine. 1997;A 44:73–78. doi: 10.1111/j.1439-0442.1997.tb01088.x. [DOI] [PubMed] [Google Scholar]
- Engelhardt Wv, Gros G, Burmester M, Hansen K, Becker G, Rechkemmer G. Functional role of bicarbonate in propionate transport across guinea-pig isolated caecum and proximal colon. The Journal of Physiology. 1994;477:365–371. doi: 10.1113/jphysiol.1994.sp020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman GM, Koethe JD, Stephenson RL. Base secretion in rat distal colon: ionic requirements. American Journal of Physiology. 1990;258:G825–832. doi: 10.1152/ajpgi.1990.258.6.G825. [DOI] [PubMed] [Google Scholar]
- Feldman GM, Stephenson RL. H+ and HCO3− flux across apical surface of rat distal colon. American Journal of Physiology. 1990;259:C35–40. doi: 10.1152/ajpcell.1990.259.1.C35. [DOI] [PubMed] [Google Scholar]
- Fleming SE, Choi SY, Fitch MD. Absorption of short-chain fatty acids from the rat cecum in vivo. Journal of Nutrition. 1991;121:1787–1797. doi: 10.1093/jn/121.11.1787. [DOI] [PubMed] [Google Scholar]
- Fromherz P, Masters B. Interfacial pH at electrically charged lipid monolayers investigated by the lipoid pH-indicator method. Biochimica et Biophysica Acta. 1974;356:270–275. doi: 10.1016/0005-2736(74)90267-3. [DOI] [PubMed] [Google Scholar]
- Harig JM, Ng EK, Dudeja PK, Brasitus TA, Ramaswamy K. Transport of n-butyrate into human colonic luminal membrane vesicles. American Journal of Physiology. 1996;250:G469–474. doi: 10.1152/ajpgi.1996.271.3.G415. [DOI] [PubMed] [Google Scholar]
- Harig JM, Soergel KH, Barry JA, Ramaswamy K. Transport of propionate by human ileal brush-border membrane vesicles. American Journal of Physiology. 1991;260:G776–782. doi: 10.1152/ajpgi.1991.260.5.G776. [DOI] [PubMed] [Google Scholar]
- Kleinke T, Wagner S, Parkkila S, Gros G. Carbonic anhydrase in the mucus of the gastrointestinal tract of guinea-pigs, rats, humans and CA-deficient mice. Pflügers Archiv. 1998;435:P36–34. suppl. [Google Scholar]
- Lucas ML. Determination of acid surface pH in vivo in rat proximal jejunum. Gut. 1983;24:734–739. doi: 10.1136/gut.24.8.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil NI, Cummings JH, James WPT. Short-chain fatty acid absorption by the human large intestine. Gut. 1978;20:819–822. doi: 10.1136/gut.19.9.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil NI, Ling KLE. Large intestinal mucosal surface pH in rat and man. In: Skadhauge E, Heintze K, editors. Intestinal Absorption and Secretion. Lancaster: MTP Press; 1984. pp. 103–109. [Google Scholar]
- McNeil NI, Ling KLE, Wager J. Mucosal surface pH of the large intestine of the rat and of normal and inflamed large intestine in man. Gut. 1987;28:707–713. doi: 10.1136/gut.28.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascolo N, Rajendran VM, Binder HJ. Mechanism of short chain fatty acid uptake by apical membrane vesicles of rat distal colon. Gastroenterology. 1991;101:311–338. doi: 10.1016/0016-5085(91)90008-9. [DOI] [PubMed] [Google Scholar]
- Oltmer S, Engelhardt Wv. Absorption of short-chain fatty acids from the in-situ-perfused caecum and colon of the guinea-pig. Scandinavian Journal of Gastroenterology. 1994;29:1009–1016. doi: 10.3109/00365529409094878. [DOI] [PubMed] [Google Scholar]
- Rajendran VM, Binder HJ. Apical membrane Cl-butyrate exchange: mechanism of short-chain fatty acid stimulation of active chloride absorption in rat distal colon. Journal of Membrane Biology. 1994;141:51–58. doi: 10.1007/BF00232873. [DOI] [PubMed] [Google Scholar]
- Rechkemmer G. Transport of weak electrolytes. In: Field M, Frizzell RA, editors. Handbook of Physiology, The Gastrointestinal System. IV. New York: Oxford University Press; 1991. pp. 371–388. [Google Scholar]
- Rechkemmer G, Engelhardt Wv. Concentration- and pH-dependence of short-chain fatty acid absorption in the proximal and distal colon of guinea-pig (Cavia porcellus) Comparative Biochemistry and Physiology. 1988;A 91:659–663. doi: 10.1016/0300-9629(88)90944-9. [DOI] [PubMed] [Google Scholar]
- Rechkemmer G, Wahl M, Kuschinsky W, Engelhardt Wv. pH-microclimate at the luminal surface of the intestinal mucosa of guinea-pig and rat. Pflügers Archiv. 1986;407:33–40. doi: 10.1007/BF00580717. [DOI] [PubMed] [Google Scholar]
- Remmers AE, Neubig RR. Resonance energy transfer between guanine nucleotide binding protein subunits and membrane lipids. Biochemistry. 1993;32:2409–2414. doi: 10.1021/bi00060a036. [DOI] [PubMed] [Google Scholar]
- Ritzhaupt A, Ellis A, Hosie KB, Shirazi-Beechey SP. The characterization of butyrate transport across pig and human colonic luminal membrane. The Journal of Physiology. 1998a;507:819–830. doi: 10.1111/j.1469-7793.1998.819bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzhaupt A, Wood IS, Ellis A, Hosie KB, Shirazi-Beechey SP. Identification and characterization of a monocarboxylate transporter (MCT1) in pig and human colon: its potential to transport L-lactate as well as butyrate. The Journal of Physiology. 1998b;513:719–732. doi: 10.1111/j.1469-7793.1998.719ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellin JH, De Soignie R. Short-chain fatty acids have polarized effects on sodium transport and intracellular pH in rabbit proximal colon. Gastroenterology. 1998;114:737–747. doi: 10.1016/s0016-5085(98)70587-6. [DOI] [PubMed] [Google Scholar]
- Shiau YF, Fernandez P, Jackson MHJ, McMonagle S. Mechanisms maintaining a low pH microclimate in the intestine. American Journal of Physiology. 1985;248:G608–617. doi: 10.1152/ajpgi.1985.248.6.G608. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Kaneko K. Ouabain-sensitive K+-H+-exchange mechanism in the apical membrane of guinea-pig colon. American Journal of Physiology. 1989;256:G979–988. doi: 10.1152/ajpgi.1989.256.6.G979. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Buchsbaum RN, Zimmiak A, Racker E. Intracellular pH measurements in Ehrlich ascites cells utilizing spectroscopic probes in situ. Biochemistry. 1979;18:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Tournier JF, Lopez A, Gas N, Tocanne JF. The lateral motion of lipid molecules in the apical plasma membrane of endothelial cells is reversibly affected by the presence of cell junctions. Experimental Cell Research. 1989;181:375–384. doi: 10.1016/0014-4827(89)90095-5. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Suzuki Y. Quabain-sensitive K+-ATPase in epithelial cells from guinea-pig distal colon. American Journal of Physiology. 1990;258:G506–511. doi: 10.1152/ajpgi.1990.258.4.G506. [DOI] [PubMed] [Google Scholar]


