Abstract
Western diets high in saturated fat are associated with an increased incidence of cardiovascular diseases. In this study we have evaluated vascular endothelial function and oxidative stress in virgin rats fed a normal (VC) or high in saturated fat diet (VHF) (20% lard and corn oil w/w) from weaning until adulthood, and throughout subsequent pregnancy (PC and PHF, respectively).
The saturated fat diet was associated with enhanced noradrenaline sensitivity in small mesenteric arteries from VHF rats (VHF vs. VC, P < 0.05) and blunted endothelium-dependent relaxation in VHF and PHF rats (VHF vs. VC, P < 0.001; PHF vs. PC, P < 0.05). Endothelial dysfunction was attributable to a reduced nitric oxide component of relaxation in VHF rats, and blunted prostacyclin and endothelium-derived hyperpolarizing factor components in PHF rats.
Other than plasma cholesterol, which was reduced in VHF and PHF rats, plasma lipids were normal. Fasting plasma insulin and glucose concentrations were raised in VHF rats (P < 0.05) and the plasma marker of oxidative stress, 8-iso PGF2α, was increased in PHF animals (P < 0.01).
These findings suggest that endothelial dysfunction induced by a saturated fat diet is cholesterol independent and likely to be of different mechanistic origin in virgin and pregnant rats.
A high saturated fat intake has been implicated in the development of cardiovascular disease. Epidemiological studies have shown an increased incidence amongst populations consuming a typical Western diet high in saturated fat (Keys, 1997) and a low incidence of disease when consumption of polyunsaturated fats is high (Kushi et al. 1995). Vascular endothelial disorders are now considered to be an important underlying pathology which predisposes to atherosclerosis, thrombosis and hypertension (De Meyer & Herman, 1997). Whilst largely attributed to hypercholesterolaemia (Goode et al. 1995), other factors have recently been implicated in endothelial dysfunction, particularly the metabolic sequelae of insulin resistance (Chowienczyk & Watts, 1997). This disorder is associated with obesity (Walker, 1995) and high saturated fat intake (Storlien et al. 1991; Fryer & Kruszynska, 1993). Affected patients (Steinberg et al. 1996), including those with non-insulin dependent diabetes (McVeigh et al. 1992), demonstrate blunted endothelium-dependent vasodilatation. Free radical synthesis is also evoked by high saturated fat consumption (Erhardt et al. 1997) and may contribute to vascular disorders as reactive oxygen species have been implicated in endothelial cell damage (Busse & Fleming, 1996).
Interest in the relationship between saturated fat intake and cardiovascular disease has focused on the general population, but dietary saturated fat intake during pregnancy also warrants consideration. Obese women are more likely to suffer from hypertensive disorders of pregnancy and pre-eclampsia (Calandra et al. 1981) as well as impaired glucose metabolism and gestational diabetes (Ekblad & Grenman, 1992). Diminished reproductive performance is also reported amongst obese women (Rogers & Mitchell, 1952) and rats on a high saturated fat intake (Shaw et al. 1997). Additionally, and because recent studies have suggested that adulthood cardiovascular function can be programmed in utero (Barker, 1994), it is possible that oxidative stress and metabolic disturbance resulting from saturated fat intake in pregnant women could compromise the fetus with long-lasting consequences.
The aim of the present investigation was to investigate endothelium-dependent vasodilatation in virgin and pregnant rats fed a diet high in saturated fat and to determine whether oxidative stress and/or insulin resistance may be important determinants of the disorders observed. Unlike the human, the rat paradoxically responds to a diet high in saturated fat by the reduction of plasma cholesterol (Salter et al. 1991), and so provides an ideal model by which to study cholesterol-independent mechanisms of vascular dysfunction. Vascular constrictor responses and endothelium-dependent and -independent vasodilatation were assessed in isolated small mesenteric arteries using a small vessel myograph. Plasma lipids, insulin and glucose were measured and, for the first time in saturated fat-fed animals, plasma concentrations of the stable marker of oxidative stress, 8-iso PGF2α, were evaluated.
METHODS
The entire protocol was reviewed and approved by the local Ethical Committee for Animal Procedures (K.U. Leuven, Belgium).
Dietary protocol
Virgin female Wistar rats were fed either normal rat chow (4% fat (corn oil), 21% protein and 51% carbohydrate; VC group) or a semi-synthetic diet containing 20% saturated fat (Mucaron High Fat 821910; VHF group) from weaning until adulthood (100-120 days of age) (Special Diet Services, Witham, Essex, UK). The diet consisted of 16% lard (lard fatty acid constituents: 2.7% palmitoleic, 32.8% oleic, 8.1% linoleic, 0.4% linolenic, 1.55% myristic, 21.2% palmitic and 9.6% stearic) and 4% corn oil (corn oil fatty acid constituents: 23.4% oleic, 42.9% linoleic, 0.8% linolenic, 9.8% palmitic and 2% stearic) supplemented with essential micronutrients and vitamins to ensure the same final content (w/w) as in normal chow. Rats were weighed weekly from 7-98 days of age. At 90-100 days of age, subgroups of VC and VHF rats (termed PC and PHF, respectively) were mated and then remained on the same diet until 19-22 days gestation when vascular function was assessed. The number of fetuses and fetal weights were determined after dams (19-22 days gestation) were killed by inhalation of a rising concentration of CO2.
Plasma measurement of glucose, insulin, cholesterol, triglycerides and non-esterified fatty acids
After an overnight fast, blood was taken from virgin female rats (100 days old) from an incision made at the tip of the tail for determination of plasma glucose, insulin, cholesterol, triglycerides and non-esterified fatty acids. In some cases the sample obtained was insufficient for complete analysis. In pregnant rats, fasting plasma samples were taken the day before the experiments, but only for estimation of plasma cholesterol. Plasma glucose and insulin varies significantly from day to day in late pregnancy and the data from the previous day would not be relevant to the vascular function assessed on the following day. An overnight fast immediately prior to the experiments might have influenced vascular function. Non-fasting samples were taken immediately prior to assessment of vascular function for estimation of lipids in both pregnant and non-pregnant animals. Plasma glucose was determined with the glucose oxidase method (glucose analyser 2300STAT; Yellow Springs Instruments, Yellow Springs, OH, USA). Plasma insulin was assessed by radioimmunoassay using rat insulin as a standard (Holemans et al. 1997). Plasma triglycerides (Triglycerides: GPO-PAP), cholesterol (Cholesterol: CHOL-PAP) and non-esterified fatty acids (free fatty acids, half-micro test) were evaluated using commercially available kits.
Assessment of vascular function
Rats were killed by inhalation of a rising concentration of CO2 at 100-120 days of age and at the same time of day for each experiment. Small mesenteric resistance arteries (internal diameter, mean ±s.e.m.: VC, 296 ± 11 μm (n = 17) vs. VHF, 300 ± 8 μm (n = 18), not significant; PC, 319 ± 12 μm (n = 17) vs. PHF, 330 ± 15 μm (n = 16), not significant) were mounted on a small vessel wire myograph as previously described (Mulvany & Halpern, 1977). Briefly, third-order branches of the mesenteric tree were dissected free of connective tissue and mounted on fine tungsten wires in pairs as ring preparations for the measurement of isometric tension, and bathed in physiological salt solution (PSS, constituents (mM): NaCl, 119; KCl, 4.7; CaCl2, 2.5; MgSO4, 1.17; NaHCO3, 25; KH2PO4, 1.16; EDTA, 0.026; and glucose, 6.0; pH 7.4 at 37°C) gassed with 5% CO2 in O2. The passive tension-internal circumference characteristics of the arteries were determined by stretching to achieve an internal circumference equivalent to 90% of that which would be attained when relaxed in situ under a transmural pressure of 100 mmHg. To confirm viability of the arteries, four contractions (4 min duration) were performed to 5 μm noradrenaline (NA), 125 mM KCl in PSS, or a combination of both. Arteries failing to produce active tension equivalent to 100 mmHg were rejected. Concentration-response curves, at increments of 2 min duration, were then constructed to NA (0.1-10 μm) and following repeated washing and recovery, endothelium-dependent relaxation to acetylcholine (ACh; 1 nM-10 μm) and endothelium-independent relaxation to sodium nitroprusside (SNP; 1 nM-10 μm) were assessed in arteries submaximally preconstricted with 5 μm NA. To deduce the relative contributions of prostaglandins and/or nitric oxide to the ACh-induced relaxation, two further concentration responses to ACh were repeated after, firstly, incubation (20 min) with, and in the presence of, indomethacin (10 μm) and, secondly, with indomethacin (10 μm), Nω-nitro-L-arginine methyl ester (L-NAME, 100 μm) and the soluble guanylate cyclase inhibitor, oxadiazole quinoxalin (ODQ, 1 μm). Lastly, to investigate the potential role of a hyperpolarizing factor, ACh responses were performed with the prostaglandin and nitric oxide inhibitors in partially depolarizing PSS (25 mM KCl). For this protocol arteries were preconstricted with 2-4 μm NA, the concentration being adjusted in order to evoke similar preconstrictor tone to that observed in normal PSS (5 mM KCl) without inhibitors.
Determination of F2-isoprostanes
Blood samples were taken for F2-isoprostane (8-iso PGF2α) and PGF2α analysis by cardiac puncture in virgin rats at 100-120 days of age and pregnant dams between 19 and 22 days gestation (when vascular function was assessed). Samples were collected into 3.8% trisodium citrate (blood: anticoagulant ratio, 9:1), 15 μm indomethacin (in phosphate buffer adjusted to pH 7.4) and 20 μm butylated hydroxytoluene (in ethanol), final concentration. The blood was then centrifuged after standing at 4°C for 30 min, (2500 g, 15 min, 4°C) and 1 ml aliquots of the plasma were stored in butylated hydroxytoluene and frozen at -70°C until analysis. Total (sum of free and esterified) F2-isoprostanes were assessed in the plasma of all groups as previously described (Nourooz-Zadeh et al. 1996). Briefly, after KOH hydrolysis of esterified F2-isoprostanes, PGF2α-d4 (1 ng (ml plasma)−1) was added as an internal standard and the samples were subjected to solid-phase extraction and derivatization. Derivatized samples and the appropriate internal standard were analysed subsequently by gas chromatography-mass spectrometry (GC-MS). This was carried out with a Hewlett-Packard 5890 gas chromatograph (Bracknell, UK) linked to a VG70SEQ mass spectrometer (Fisons Instruments, Manchester, UK) using ammonia as the reagent gas. Quantitative analysis was performed using selected ion monitoring (SIM) of the carboxylate ion [M-181]− at m/z 569 for the F2-isoprostanes and m/z 573 for PGF2α-d4.
Analysis of total body fat tissue mass by dual energy X-ray absorptiometry
In separate groups of VHF (n = 9) and VC (n = 9) rats, the animals were killed (100-120 days of age) by inhalation of a rising concentration of CO2 and, after exsanguination by cardiac puncture, the carcasses were stored at -20°C for subsequent analysis of body composition. This was performed by dual energy X-ray absorptiometry (DEXA) using a Hologic QDR-1000/W absorptiometer (line spacing, 0.1511 cm; point resolution, 0.076 cm). The total lean and fat tissue mass and bone mineral content were recorded for each rat; the total mass was calculated as the sum of these three.
Materials
Noradrenaline was obtained from Winthrop (Guildford, UK); acetylcholine, indomethacin, sodium nitroprusside and L-NAME were from Sigma; ODQ was from Alexis Corporation (Nottingham, UK). PGF2α, PGF2α-d4 and 8-iso PGF2α were purchased from Cayman Chemicals (Ann Arbor, MI, USA). Sep-Pak C-18 and NH2 cartridges for solid-phase extraction were obtained from Waters Chromatography (Watford, UK). Commercially available kits were obtained from Boehringer-Mannheim. Rat insulin as standard for radioimmunoassay was from Novo Industry. All other chemicals were of AnalaR grade from Merck Ltd.
Data analysis
Data are given as means ±s.e.m. For vascular protocols noradrenaline was expressed as a percentage of K+ (125 mM KCl)-induced tension and sensitivity calculated by the EC50 (pEC50 (-logEC50)). Relaxation to ACh was assessed by the EC50 and maximum relaxation (% NA-induced tone) using the curve fitting program Graphpad (Graphpad Software, San Diego, CA, USA). Two arteries were used from each animal and mean values calculated. When it was not possible to fit accurate sigmoidal curves, comparisons were made between maximal responses. PGF2α and total 8-iso PGF2α were calculated by equating the ratio of the peak areas of either PGF2α or 8-iso PGF2α (R and S enantiomer) to the internal standard PGF2α-d4 calculated from the original mass spectrometry traces for each individual sample. From this ratio, quantification of PGF2α or 8-iso PGF2α was obtained from calibration curves previously constructed from purchased standards extracted in PSS as described above (but to known concentrations). Comparisons were made between groups using Student's paired and unpaired t tests (InStat Graphpad, Graphpad Software). Significance was assumed if P < 0.05.
RESULTS
Plasma glucose, insulin, triglycerides, non-esterified fatty acids and cholesterol
Fasting glucose and insulin levels were significantly higher in VHF compared with VC rats at 100 days of age (Table 1). Plasma cholesterol concentration was significantly lower in 100-day-old VHF rats (Table 1) and also at the time vascular function was measured (data not shown; P < 0.01). Plasma triglyceride concentrations were no different between 100-day-old VC and VHF rats (Table 1). Fasting non-esterified fatty acids were not significantly different between the two groups at 100 days and at the time of vascular function measurement (Table 1). Fasting plasma cholesterol was higher in PC than PHF rats (2.16 ± 0.07 mM (n = 11) vs. 1.65 ± 0.10 mM (n = 6); P < 0.001). Non-fasting triglycerides were similar in VHF and VC groups (1.62 ± 0.68 mM (n = 15) vs. 1.06 ± 0.15 mM (n = 7)) and in PHF and PC groups (5.90 ± 0.75 mM (n = 11) vs. 4.43 ± 0.87 mM (n = 11)).
Table 1.
Levels of plasma glucose, insulin, triglycerides, non-esterified fatty acids and cholesterol from virgin rats (100 days old) fed either normal or saturated fat diets
| Fasting glucose (mm) | Fasting insulin (pm) | Triglycerides (mm) | Fasting non-esterified fatty acids (mm) | Cholesterol (mm) | |
|---|---|---|---|---|---|
| Virgin control group(VC) | 5.01 ± 0.27 (10) | 40.73 ± 3.35 (10) | 0.74 ± 0.06 (10) | 0.53 ± 0.06 (8) | 1.94 ± 0.07 (10) |
| Virgin high fat group(VHF) | 5.62 ± 0.27 (10)* | 122.68 ± 33.54 (10)* | 0.63 ± 0.05 (10) | 0.65 ± 0.08 (7) | 1.42 ± 0.07 (10)† |
Data are means ± s.e.m.
P < 0.05 for VHF vs. VC
P < 0.001 for VHF vs. VC. n values are given in parentheses.
Effects of high saturated fat diet in non-pregnant rats
The high saturated fat intake led to a significantly greater increase in weight in the virgin rats (VHF) compared with the control rats (VC), but only from 98 to 120 days of age (day 98: mean VHF group wt, 218.33 ± 4.31 g (n = 23) vs. mean VC group wt, 202.37 ± 2.38 g (n = 21); P < 0.05). Growth curve profiles from weaning until 98 days of age were similar between the two groups (Fig. 1). VHF rats consumed significantly less chow than VC rats at 100 days of age (VHF, 12.85 ± 0.40 g day−1 (n = 10) vs. VC, 15.16 ± 0.37 g day−1 (n = 10); P < 0.001). Despite the reduction in food intake, daily calorific intake was similar in the two groups (VHF, 0.247 ± 0.007 kJ day−1 (n = 10) vs. VC, 0.228 ± 0.005 kJ day−1 (n = 10)).
Figure 1. Growth curves.
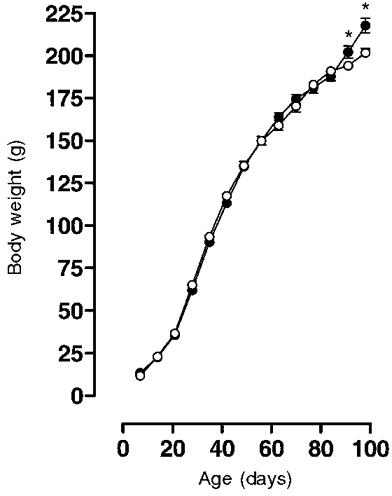
Growth curves for female virgin rats fed a control (○; n = 21) or saturated fat diet (•; n = 23) from 7 until 98 days of age. Values are given as means ±s.e.m.*P < 0.05.
Total body fat content (DEXA)
Table 2 shows the total body analyses performed in the VC and VHF rats. Despite lower total body food intake in VHF rats, these animals demonstrated a disproportionate increase in total body fat content when body fat was expressed as a percentage of total mass.
Table 2.
Body composition measured by dual energy X-ray absorptiometry in control rats fed normal chow (VC) and rats fed a saturated fat diet (VHF) at 100–120 days of age
| Group | n | BMC (g) | Lean tissue mass (g) | Fat tissue mass (g) | Fat tissue (% of total mass) | Total mass (g) |
|---|---|---|---|---|---|---|
| VC | 9 | 7.24 ± 0.15 | 186.14 ± 4.31 | 15.68 ± 1.23 | 7.57 ± 0.69 | 209.06 ± 3.68 |
| VHF | 9 | 8.38 ± 0.16† | 208.71 ± 2.75† | 25.92 ± 2.56* | 10.54 ± 0.85* | 243.02 ± 4.97‡ |
Data are represented as means ± s.e.m. Total mass represents the sum of lean tissue mass, fat tissue mass and BMC (bone mineral content).
P < 0.01
P < 0.001
P < 0.0001 vs. VC.
Vascular function
Arteries from VHF rats showed a significantly enhanced sensitivity to NA when compared with those from VC rats but similar maximum constrictor tension (Table 3 and Fig. 2A). Preconstriction to NA was no different between VC and VHF arteries, or in the presence of any inhibitor. There was a significant shift in sensitivity, but not maximum relaxation to endothelium-dependent ACh-induced relaxation in the saturated fat-fed group (Table 3 and Fig. 3A). Indomethacin caused a significant decrease in sensitivity in both groups but the defect in sensitivity remained in VHF rats in the presence of indomethacin (Table 3 and Fig. 3B). In the presence of both nitric oxide and cyclo-oxygenase blockade the difference between the VC and VHF groups was no longer evident (Table 3 and Fig. 3C). Relaxation was no different and was completely inhibited in both groups in the presence of indomethacin, L-NAME, ODQ and partially depolarizing PSS (25 mM KCl) (Table 3 and Fig. 3C). Relaxation to the endothelium-independent vasodilator sodium nitroprusside (SNP) was no different between VHF and VC animals (Table 3).
Table 3.
Responses to constrictor and dilator agonists in the presence and absence of 10 μm indomethacin (Indo), 100 μm l-NAME and 1 μm oxadiazole quinoxalin (ODQ) in rat small mesenteric arteries from virgin and pregnant Wistar rats on either normal or saturated fat diets
| Agonist | VC (n = 10) | VHF (n = 10) | PC (n = 9) | PHF (n = 9) | |
|---|---|---|---|---|---|
| NA | pEC50 (μm) | 5.78 ± 0.06 | 5.99 ± 0.06* | 6.01 ± 0.08‡ | 5.96 ± 0.11 |
| Max. constriction | 107.32 ± 3.20 | 109.87 ± 4.23 | 106.50 ± 2.91 | 108.18 ± 3.03 | |
| Ach | pEC50 (μm) | 7.22 ± 0.09 | 6.61 ± 0.12† | 7.18 ± 0.11 | 7.03 ± 0.14§ |
| Max. relaxation | 89.49 ± 3.15 | 84.56 ± 4.81 | 98.94 ± 0.62‡ | 89.57 ± 3.30* | |
| ACh + Indo | pEC50 (μm) | 6.89 ± 0.08 | 6.26 ± 0.12† | 6.62 ± 0.25‡ | 6.56 ± 0.15 |
| Max. relaxation | 89.52 ± 3.63 | 90.33 ± 2.94 | 93.58 ± 2.37 | 88.02 ± 3.99 | |
| ACh + Indo + l-NAME + ODQ | Max. relaxation | 70.12 ± 6.39 | 51.32 ± 9.70 | 73.72 ± 9.15 | 41.02 ± 11.70* |
| ACh + Indo + l-NAME + ODQ + 25 mm KCl | Max. relaxation | 3.64 ± 2.21 | 0.38 ± 5.06 | 1.62 ± 3.69 | 2.64 ± 3.15 |
| SNP | pEC50 (μm) | 7.01 ± 0.17 | 6.99 ± 0.15 | 6.91 ± 0.17 | 6.72 ± 0.22 |
| Max. relaxation | 68.68 ± 3.03 | 68.33 ± 3.69 | 66.19 ± 7.19 | 69.04 ± 7.41 |
VC, virgin control group; VHF, virgin high fat group; PC, pregnant control group; PHF, pregnant high fat group; NA, 0.1–10 μm noradrenaline; ACh, 1 nm–10 μm acetylcholine; SNP, 1 nm–10 μm sodium nitroprusside; Max. constriction, maximum constriction calculated as the percentage of K+-induced constriction; Max. relaxation, maximum relaxation calculated as the percentage of NA-induced constriction. Data are means ± s.e.m.
P < 0.05, VHF vs. VC and PHF vs. PC
P < 0.001, VHF vs. VC
P < 0.05, PC vs. VC
P < 0.05, PHF vs. VHF.
Figure 2. Concentration-response curves to noradrenaline in mesenteric small arteries.
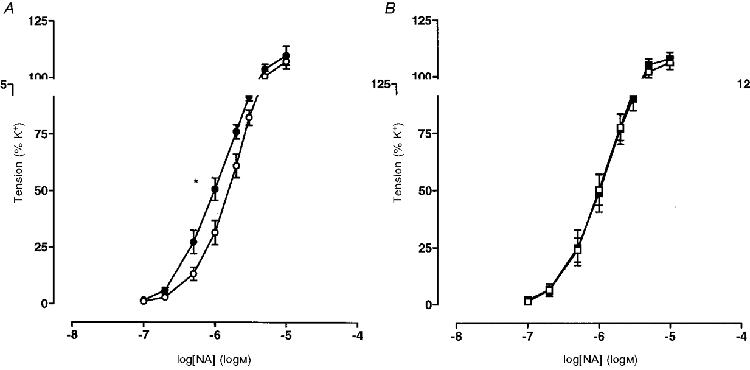
A, virgin rats fed a control (○; n = 10) or saturated fat diet (•; n = 10); B, pregnant rats fed a control (□; n = 9) or saturated fat diet (▪; n = 9). Values are given as means ±s.e.m. pEC50: *P < 0.05.
Figure 3. Concentration-response curves to acetylcholine in mesenteric small arteries from virgin rats fed a control (○; n = 10) or saturated fat diet (•; n = 10).

A, without inhibitors. B, in the presence of 10 μm indomethacin. C, in the presence of 100 μm L-NAME, 10 μm indomethacin and 1 μm ODQ and also with the same inhibitors but in the presence of depolarizing 25 mM KCl to obviate the effects of endothelium-derived hyperpolarizing factor (EDHF) (▵, control diet; ▴, saturated fat diet). Values are given as means ±s.e.m. pEC50: ***P < 0.001.
8-iso PGF2α and PGF2α
Total (free and esterified) plasma levels of the F2-isoprostane 8-iso PGF2α were similar in VHF and VC rats (8-iso PGF2α: VHF, 281.57 ± 43.02 pg (ml plasma)−1 (n = 20) vs. VC, 322.93 ± 32.01 pg (ml plasma)−1 (n = 18); Fig. 4A). Plasma levels of the cyclo-oxygenase-derived prostaglandin PGF2α were no different between VHF and VC (PGF2α: VHF, 514.99 ± 53.08 pg (ml plasma)−1 (n = 20) vs. VC, 696.34 ± 73.73 pg (ml plasma)−1 (n = 18); Fig. 4B).
Figure 4. Plasma concentrations of 8-iso PGF2α and PGF2α.

Plasma concentrations (pg (ml plasma)−1) of 8-iso PGF2α (A) and PGF2α (B) from virgin (VC; n = 18) and pregnant (PC; n = 10) rats fed the control diet and virgin (VHF; n = 20) and pregnant (PHF; n = 10) rats fed the saturated fat diet. Values are given as means ±s.e.m.A:**P < 0.01, PHF vs. PC and ***P < 0.001, VHF vs. PHF; B:**P < 0.01, PC vs. VC and ***P < 0.001, PHF vs. VHF.
Effects of high saturated fat diet in pregnant animals
There was no significant difference in maternal weights between PC and PHF rats at 20-22 days gestation (PHF, 308.05 ± 6.07 g (n = 11) vs. PC, 293.38 ± 6.05 g (n = 11)). High fat-fed dams had significantly more and heavier fetuses than pregnant controls (average number of fetuses per dam: PHF, 12.64 ± 0.49 (n = 11) vs. PC, 10.27 ± 0.83 (n = 11); P < 0.05. Fetal wt on day 20 gestation: PHF, 2.71 ± 0.07 g (n = 41) vs. PC, 1.86 ± 0.02 g (n = 56); P < 0.001).
Vascular function
Preconstriction to NA was not different between PC or PHF rat arteries, or in the presence of any inhibitor. In contrast to virgin animals, arteries from pregnant, saturated fat-fed animals had similar sensitivities and maximum responses to NA compared with pregnant controls (Table 3 and Fig. 2B). There was a significant reduction in maximum relaxation, but not sensitivity, to ACh in the pregnant saturated fat-fed group (Table 3 and Fig. 5A). Indomethacin significantly reduced the maximal response to ACh in PC rats only, thereby negating the defect in maximal response observed without indomethacin (Table 3 and Fig. 5B). Relaxation to ACh in the presence of nitric oxide and cyclo-oxygenase inhibition was significantly reduced in arteries from the PHF rats (Table 3 and Fig. 5C). However, relaxation was no different and was completely inhibited in PC and PHF rats in the presence of indomethacin, L-NAME, ODQ and partially depolarizing PSS (25 mM KCl) (Table 3 and Fig. 5C). Relaxation to the endothelium-independent vasodilator sodium nitroprusside was no different between PHF and PC (Table 3).
Figure 5. Concentration-response curves to acetylcholine in mesenteric small arteries from pregnant rats fed a control (□; n = 9) or saturated fat diet (▪; n = 9).
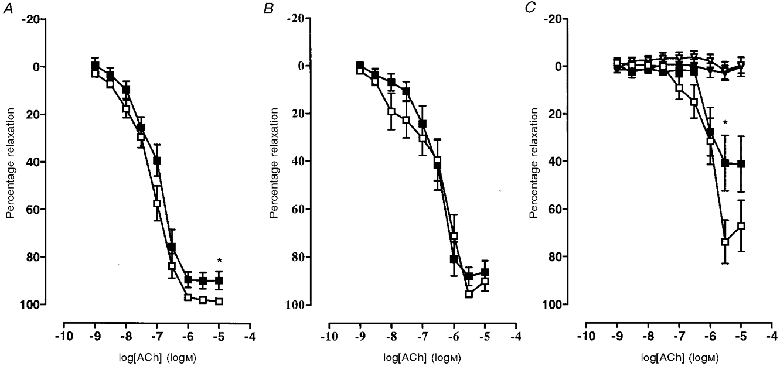
A, without inhibitors. B, in the presence of 10 μm indomethacin. C, in the presence of 100 μm L-NAME, 10 μm indomethacin and 1 μm ODQ and with the same inhibitors but in the presence of depolarizing 25 mM KCl to obviate the effects of EDHF (▿, control diet; ▾, saturated fat diet). Values are given as means ±s.e.m. Maximum relaxation: *P < 0.05.
8-iso PGF2α and PGF2α
Total (free and esterified) plasma levels of the F2-isoprostane 8-iso PGF2α were significantly higher in PHF compared with PC rats (8-iso PGF2α: PHF, 726.28 ± 112.26 pg (ml plasma)−1 (n = 10) vs. PC, 297.16 ± 14.64 pg (ml plasma)−1 (n = 10); P < 0.01; Fig. 4A). Plasma levels of the cyclo-oxygenase-derived prostaglandin PGF2α were similar in both groups (PGF2α: PHF, 1056.50 ± 96.37 pg (ml plasma)−1 (n = 10) vs. PC, 905.44 ± 37.94 pg (ml plasma)−1 (n = 10); not significant; Fig. 4B).
Comparison of non-pregnant vs. pregnant (normal and high fat diet) goups
Vascular function
Sensitivity to NA was significantly greater in PC than in VC rats, whereas sensitivity to NA in PHF and VHF rats was similar (Table 3). Preconstriction to NA, in the absence or presence of any inhibitor, was no different in arteries from pregnant or virgin rats on either dietary regime. Relaxation to ACh was significantly greater in the pregnant animals compared with virgins irrespective of diet (Table 3). In the control fed group, the enhanced relaxation to ACh in arteries from PC rats compared with VC rats was attributable to alteration in a cyclo-oxygenase-derived prostanoid and to enhanced nitric oxide synthesis or reduced degradation, whereas enhanced sensitivity to ACh in arteries from PHF compared with VHF rats was due to a cyclo-oxygenase-derived prostanoid only (Table 3). Relaxations to sodium nitroprusside were similar in virgin and pregnant animals irrespective of diet.
8-iso PGF2α and PGF2α
Plasma levels of 8-iso PGF2α were similar in PC and VC rats but were significantly raised in PHF compared with VHF rats (Fig. 4A). Plasma concentrations of PGF2α were significantly higher in the pregnant compared with virgin rats irrespective of dietary intake (Fig. 4B).
DISCUSSION
The major finding of this study was that there was impairment of endothelial vasodilator function in arteries from virgin and pregnant Wistar rats fed a 20% saturated fat diet. To our knowledge there is only one previous study demonstrating cardiovascular dysfunction in rats fed a saturated fat diet (Langley-Evans et al. 1996), in which higher systolic blood pressures in rats fed a diet supplemented with coconut oil were observed. In man it is generally considered that vascular endothelial impairment associated with cardiovascular risk factors, including a high saturated fat intake, is a consequence of increased plasma low-density lipoprotein (LDL) cholesterol (Goode et al. 1995). In the present investigation, impairment of endothelium-dependent relaxation in the rats fed saturated fat, as assessed by relaxation to ACh, did not significantly correlate with plasma cholesterol, which was reduced. A similar fall in plasma cholesterol concentration has been previously reported in rats fed a saturated fat diet (Salter et al. 1991) and is characteristic of the rodent response to a saturated fat diet.
This study has suggested, therefore, that cholesterol-independent pathways can evoke endothelial dysfunction in rats fed saturated fat. At 100 days of age, virgin, saturated fat-fed rats had significantly higher fasting insulin and glucose concentrations than controls fed normal chow, highly suggestive of insulin resistance. Confirmation of insulin resistance in these saturated fat-fed rats would be unequivocally proven only by euglycaemic- hyperinsulinaemic clamp (Holemans et al. 1997), but saturated fat ingestion in rats has previously been used as a model of insulin resistance (Fryer & Kruszynska, 1987) and obesity (Boozer et al. 1995). The association between insulin resistance and vascular disease, at least in man, is well recognized (McVeigh et al. 1992) and the link may well be at the level of the vascular endothelium (Steinberg et al. 1996; Chowienczyk & Watts, 1997). The defect in relaxation to ACh in the saturated fat-fed virgin rats appeared to result from reduced nitric oxide production or increased nitric oxide degradation, as the difference from control remained with cyclo-oxygenase inhibition but was no longer evident in the presence of nitric oxide synthase (NOS) and cyclo-oxygenase blockade. Abnormal relaxation to nitric oxide was likely to be of endothelial origin as smooth muscle sensitivity to exogenous nitric oxide (in the form of sodium nitroprusside) was not different. The enhanced sensitivity to NA in the virgin rats on the saturated fat diet could also potentially be explained on the same basis, as NA promotes endothelial nitric oxide synthesis (Liao & Homey, 1993). Theoretically, these abnormalities may result from insulin resistance, as endothelial dysfunction could arise from transient postprandial hyperglycaemia (Tribe & Poston, 1996) and/or dyslipidaemia associated with this disorder (Hennig et al. 1994; Vogel et al. 1997). Conversely, endothelial dysfunction could play a role in the development of insulin resistance (Petrie et al. 1996), since insulin stimulates endothelial nitric oxide production (Scherrer et al. 1994), which would aid glucose delivery to peripheral tissues by increasing local blood flow. However, this concept has recently been challenged (Scherrer et al. 1994).
The fat-fed virgin rats in this study ate a similar number of calories to the normal animals, and increased calorific intake per se can therefore be discounted as a possible cause of vascular dysfunction. The VHF rats also demonstrated evidence of fat accumulation as they had proportionately greater body fat mass than the controls and, akin to obese human subjects, this might contribute to insulin resistance. Nevertheless the diet, designed to mimic that of human subjects on a high saturated fat intake, would have resulted in a reduction in essential nutrient intake as the saturated fat-fed animals ate significantly less. Although the saturated fat diet was supplemented with minerals and vitamins, it cannot be discounted that in rats, and presumably in humans, a diet high in saturated fat may be deleterious to vascular function because of the reduced intake of other dietary components.
The reduction in endothelium-dependent relaxation in PHF rats was not as marked as in VHF rats, which may reflect the protective effect of oestrogens on endothelial function, through endothelial nitric oxide synthase (eNOS) activation (Hayashi et al. 1995). In pregnancy, saturated fat feeding revealed a reduction in two components of endothelium-dependent relaxation. Firstly, and in contrast to VHF rats, decreased dilator prostanoid activity played a role as differences between PHF and VHF disappeared upon cyclo-oxygenase inhibition. Secondly, simultaneous nitric oxide and prostaglandin inhibition unmasked a large difference in residual ACh-induced relaxation between the groups. As this was completely inhibited by partial depolarization by raised extracellular potassium levels in both groups, it suggested the reduced synthesis of a hyperpolarizing factor in the rats fed a saturated fat diet. This may be the endothelium-derived hyperpolarizing factor (EDHF) which elicits relaxation by vascular smooth muscle hyperpolarization (Garland et al. 1995).
To our knowledge, this is the first study to have evaluated the plasma concentration of the F2-isoprostane 8-iso PGF2α in response to saturated fat intake in animals. Whereas the concentration of 8-iso PGF2α was similar to that in control animals in the VHF group, it was raised in the PHF group. The normal plasma concentration of 8-iso PGF2α in the virgin animals questions the reported association between saturated fat feeding and increased free radical production. Previous studies have reported increases in oxidative stress in mice (Ibrahim et al. 1997) and man (Erhardt et al. 1997) fed a saturated fat diet, evaluated by the measurement of markers of oxidative stress now considered to be less reliable than that of the F2-isoprostane assay. One study in man has reported no change in anti-oxidant to pro-oxidant ratio with altered fat consumption and would agree with the data presented here (Velthuis-te Wierik et al. 1996). Accurate quantification of oxidative stress is problematic. Recently, the measurement of the F2-isoprostanes has been shown to be a consistently good marker of oxidative damage (Morrow & Roberts, 1996). The 8-iso PGF2α isoform is produced in plasma as a direct result of a non-enzymatic free radical attack of membrane arachidonic acid (Morrow & Roberts, 1996). The lack of involvement of enzymatic synthesis of 8-iso PGF2α in this study was confirmed by the independence of plasma 8-iso PGF2α concentrations from those of plasma PGF2α, synthesis of which is dependent upon cyclo-oxygenase. The observation that 8-iso PGF2α was raised in PHF rats but not in VHF rats suggests that pregnancy increases the likelihood of lipid peroxidation. Indeed, human pregnancy has been associated with an increase in free radical production (Wisdom et al. 1991). To our knowledge there are no reports of increased oxidative stress in rat pregnancy, but this study suggests that it is insufficient to evoke peroxidation as assessed by 8-iso PGF2α. However, the saturated fat consumption may have tipped the balance favouring a pro-oxidant state sufficient to elicit 8-iso PGF2α synthesis in pregnancy.
As was observed with the non-pregnant animals, the lack of increase in weight in the pregnant animals fed saturated fat may indicate a reduction in food intake and a relatively lower intake of nutrients compared with the control animals. This would also be anticipated in the human situation and we cannot exclude the possibility that oxidative stress and vascular dysfunction may arise in part as a consequence of this. Additionally, these animals had been fed saturated fat before conception, which may have influenced the pregnancy, as high fat-fed rats demonstrate poor reproductive performance (Shaw et al. 1997). It would be of interest therefore to determine whether fat feeding in pregnancy alone evoked similar defects in vascular function and oxidative stress.
This study has also provided comparisons between pregnant and non-pregnant animals. In contrast to previous studies (Sladek et al. 1997), arteries from normal pregnant rats showed enhanced constrictor sensitivity to NA when compared with virgin animals. The enhanced ACh-induced relaxation in arteries from pregnant animals on either diet compared with arteries from virgin controls agrees with other studies in the same vascular bed (Pascoal et al. 1995) and may contribute to the normal cardiovascular adaptation to pregnancy (Sladek et al. 1997). The significantly greater dilator prostanoid component of ACh-induced relaxation in pregnant compared with virgin rats in both groups was paralleled by higher plasma concentrations of PGF2α, suggestive of enhanced cyclo-oxygenase activity previously reported in rat pregnancy (Sladek et al. 1997). However, the ACh increase was less pronounced in the arteries from the pregnant rats fed saturated fat and was not accompanied by any increase in the nitric oxide component of ACh-induced relaxation, as occurred in the pregnant rats fed normal chow. The saturated fat diet was therefore deleterious to the normal adaptation of the vasculature to pregnancy.
The intrauterine environment is now thought to be crucial in programming for cardiovascular diseases in adulthood, notably hypertension, insulin resistance and coronary heart disease (Barker, 1994), and we have recently shown endothelial dysfunction in the offspring of dams fed saturated fat (Koukkou et al. 1998). Langley-Evans (1996) has also shown that male offspring of rats fed saturated fat (coconut oil) are hypertensive. Maternal oxidative stress associated with saturated fat diet in this study may be amongst the factors responsible.
In summary, investigation of saturated fat intake in the rat which, in common with other rodents, is paradoxically associated with a fall in plasma cholesterol, has facilitated the investigation of cholesterol-independent pathways of vascular dysfunction. We conclude that an increase in saturated fat intake may lead to endothelial dysfunction in virgin and pregnant rats independently of plasma cholesterol, and by different mechanisms. These findings highlight the need for further detailed investigation of saturated fat intake and vascular dysfunction, particularly in pregnancy, when an increase in oxidative stress may increase the risk of cardiovascular dysfunction in both mother and fetus.
Acknowledgments
The authors would like to thank the British Heart Foundation, St Thomas' Hospital Special Trustees and Tommy's Campaign for supporting this work. We also thank Mr H. Peeters and Mr H. Borghs (Arthritis and Bone Metabolism Disease Unit, Leuven, Belgium) for performing the DEXA analysis.
References
- Barker DJP. The fetal origins of adult disease. Fetal and Maternal Medicine Reviews. 1994;6:71–80. [Google Scholar]
- Boozer CN, Schoenbach G, Atkinson RL. Dietary fat and adiposity: a dose-response relationship in adult male rats fed isocalorically. American Journal of Physiology. 1995;268:E546–550. doi: 10.1152/ajpendo.1995.268.4.E546. [DOI] [PubMed] [Google Scholar]
- Busse R, Fleming I. Endothelial dysfunction in atherosclerosis. Journal of Vascular Research. 1996;33:181–194. doi: 10.1159/000159147. [DOI] [PubMed] [Google Scholar]
- Calandra C, Abell DA, Beisher NA. Maternal obesity in pregnancy. Obstetrics and Gynecology. 1981;57:8–12. [PubMed] [Google Scholar]
- Chowienczyk PJ, Watts GF. Endothelial dysfunction, insulin resistance and non-insulin-dependent diabetes. Endocrinology and Metabolism. 1997;4:225–232. [Google Scholar]
- De Meyer GR, Herman AG. Vascular endothelial dysfunction. Progress in Cardiovascular Disease. 1997;39:325–342. doi: 10.1016/s0033-0620(97)80031-x. [DOI] [PubMed] [Google Scholar]
- Ekblad U, Grenman S. Maternal weight, weight gain during pregnancy and pregnancy outcome. International Journal of Gynecology and Obstetrics. 1992;39:277–283. doi: 10.1016/0020-7292(92)90258-k. 10.1016/0020-7292(92)90258-K. [DOI] [PubMed] [Google Scholar]
- Erhardt JG, Lim SS, Bode C. A diet rich in fat and poor in dietary fiber increases the in vitro formation of reactive oxygen species in human feces. Journal of Nutrition. 1997;127:706–709. doi: 10.1093/jn/127.5.706. [DOI] [PubMed] [Google Scholar]
- Fryer LG, Kruszynska YT. Insulin resistance in high fat fed rats. Role of glucose transporters, membrane lipids and triglyceride stores. Annals of the New York Academy of Sciences. 1993;683:91–97. doi: 10.1111/j.1749-6632.1993.tb35695.x. [DOI] [PubMed] [Google Scholar]
- Garland CJ, Plane F, Kemp BK, Cocks TM. Endothelium-dependent hyperpolarization: a role in the control of vascular tone. Trends in Pharmacological Sciences. 1995;16:23–30. doi: 10.1016/s0165-6147(00)88969-5. 10.1016/S0165-6147(00)88969-5. [DOI] [PubMed] [Google Scholar]
- Goode GK, Miller JP, Heagerty AM. Hyperlipidaemia, hypertension and coronary heart disease. Lancet. 1995;345:362–364. doi: 10.1016/s0140-6736(95)90345-3. 10.1016/S0140-6736(95)90345-3. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Yamada K, Esaki T, Kuzuya M, Satake S, Ishikawa T, Hidaka H, Iguchi A. Estrogen increases endothelial nitric oxide by a receptor-mediated system. Biochemical and Biophysical Research Communications. 1995;214:847–855. doi: 10.1006/bbrc.1995.2364. 10.1006/bbrc.1995.2364. [DOI] [PubMed] [Google Scholar]
- Hennig B, Toborek M, Cader AA. Nutrition, endothelial cell metabolism and atherosclerosis. Critical Reviews in Food Science and Nutrition. 1994;34:253–282. doi: 10.1080/10408399409527663. [DOI] [PubMed] [Google Scholar]
- Holemans K, Bree R, Verhaeghe J, Meurrens K, Van Assche FA. Maternal semistarvation and streptozotocin-diabetes in rats have different effects on the in vivo glucose uptake by peripheral tissues in their female adult offspring. Journal of Nutrition. 1997;127:1371–1376. doi: 10.1093/jn/127.7.1371. [DOI] [PubMed] [Google Scholar]
- Ibrahim W, Lee U, Yeh C, Szabo J, Bruckner G, Chow CK. Oxidative stress and antioxidant status in mouse liver: effects of dietary lipid, vitamin E and iron. Journal of Nutrition. 1997;127:1401–1406. doi: 10.1093/jn/127.7.1401. [DOI] [PubMed] [Google Scholar]
- Keys A. Coronary heart disease in seven countries. 1970. Nutrition. 1997;13:250–252. doi: 10.1016/s0899-9007(96)00410-8. [DOI] [PubMed] [Google Scholar]
- Koukkou E, Ghosh P, Lowy C, Poston L. Offspring of normal and diabetic rats fed saturated fats in pregnancy demonstrate vascular dysfunction. Circulation. 1998;98:2899–2904. doi: 10.1161/01.cir.98.25.2899. [DOI] [PubMed] [Google Scholar]
- Kushi LH, Lenart EB, Willett WC. Health implications of Mediterranean diets in light of contemporary knowledge. 2. Meat, wine, fats and oils. American Journal of Clinical Nutrition. 1995;61:1416–1427S. doi: 10.1093/ajcn/61.6.1407S. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC. Intrauterine programming of hypertension in the rat: nutrient interactions. Comparative Biochemistry and Physiology. 1996;A 114:327–333. doi: 10.1016/0300-9629(96)00018-7. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Clamp AG, Grimble RF, Jackson AA. Influence of dietary fats upon systolic blood pressure in the rat. International Journal of Food Science and Nutrition. 1996;47:417–425. doi: 10.3109/09637489609006955. [DOI] [PubMed] [Google Scholar]
- Liao JK, Homey CJ. The release of endothelium-derived relaxing factor via α2-adrenergic receptor activation is specifically mediated by Gi α2. Journal of Biological Chemistry. 1993;268:19528–19533. [PubMed] [Google Scholar]
- McVeigh GE, Brennan GM, Johnston GD, McDermott BJ, McGrath LT, Andrews JW, Hayes JR. Impaired endothelium dependent and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1992;35:771–776. doi: 10.1007/BF00429099. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Roberts LT. The isoprostanes. Current knowledge and directions for future research. Biochemical Pharmacology. 1996;51:1–9. doi: 10.1016/0006-2952(95)02072-1. 10.1016/0006-2952(95)02072-1. [DOI] [PubMed] [Google Scholar]
- Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circulation Research. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Nourooz-Zadeh J, Gopaul NK, Barrow S, Mallet AI, Anggard EE. Analysis of F2-isoprostanes as indicators of non-enzymatic lipid peroxidation in vivo by gas chromatography-mass spectrometry: development of a solid-phase extraction procedure. Journal of Chromatography B. 1996;667:199–208. doi: 10.1016/0378-4347(95)00035-h. [DOI] [PubMed] [Google Scholar]
- Pascoal IF, Lindheimer MD, Nalbantian-Brandt C, Umans JG. Contraction and endothelium-dependent relaxation in mesenteric microvessels from pregnant rats. American Journal of Physiology. 1995;269:H1899–1904. doi: 10.1152/ajpheart.1995.269.6.H1899. [DOI] [PubMed] [Google Scholar]
- Petrie JR, Ueda S, Webb DJ, Elliot HL, Connell JMC. Endothelial nitric oxide production and insulin insensitivity: a physiological link with implications for the pathogenesis of cardiovascular disease. Circulation. 1996;93:1331–1333. doi: 10.1161/01.cir.93.7.1331. [DOI] [PubMed] [Google Scholar]
- Rogers J, Mitchell GWJ. The relation of obesity to menstrual disturbances. New England Journal of Medicine. 1952;247:53–55. doi: 10.1056/NEJM195207102470204. [DOI] [PubMed] [Google Scholar]
- Salter AM, Hayashi R, Al-Seeni M, Brown NF, Bruce J, Sorensen O, Atkinson EA, Middleton B, Bleackley RC, Brindley DN. Effects of hypothyroidism and high fat feeding on mRNA concentrations for the low-density lipoprotein receptor and on acyl-CoA: cholesterol acetyltransferase activities in rat liver. Biochemical Journal. 1991;276:825–832. doi: 10.1042/bj2760825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer U, Randin D, Vollenweider L, Vollenweider P, Nicod P. Nitric oxide release accounts for insulin's vascular effects in humans. Journal of Clinical Investigation. 1994;94:2511–2515. doi: 10.1172/JCI117621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw MA, Rasmussen KM, Myers TR. Consumption of a high fat diet impairs reproductive performance in Sprague-Dawley rats. Journal of Nutrition. 1997;127:64–69. doi: 10.1093/jn/127.1.64. [DOI] [PubMed] [Google Scholar]
- Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. American Journal of Physiology. 1997;272:R441–463. doi: 10.1152/ajpregu.1997.272.2.R441. [DOI] [PubMed] [Google Scholar]
- Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction: implications for the syndrome of insulin resistance. Journal of Clinical Investigation. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storlien LH, Jenkins AB, Chisolm DJ, Pascoe WS, Khouri S, Kraegen EW. Influence of dietary fat consumption on development of insulin resistance in rats: relationship to muscle triglyceride and ω-3 fatty acids in muscle phospholipid. Diabetes. 1991;40:280–289. doi: 10.2337/diab.40.2.280. [DOI] [PubMed] [Google Scholar]
- Tribe RM, Poston L. Oxidative stress and lipids in diabetes: a role in endothelium vasodilator dysfunction? Vascular Medicine. 1996;1:195–206. doi: 10.1177/1358863X9600100304. [DOI] [PubMed] [Google Scholar]
- Velthuis-te Wierik EJ, Van den Berg H, Westrate JA, Van het Hof KH, De Graaf C. Consumption of reduced-fat products: effects on parameters of anti-oxidative capacity. European Journal of Clinical Nutrition. 1996;50:214–219. [PubMed] [Google Scholar]
- Vogel RA, Corretti MC, Plotnick GD. Effect of a single high-fat meal on endothelial function in healthy subjects. American Journal of Cardiology. 1997;79:350–354. doi: 10.1016/s0002-9149(96)00760-6. 10.1016/S0002-9149(96)00760-6. [DOI] [PubMed] [Google Scholar]
- Walker M. Obesity, insulin resistance, and its link to non-insulin-dependent diabetes mellitus. Metabolism. 1995;44:18–20. doi: 10.1016/0026-0495(95)90314-3. [DOI] [PubMed] [Google Scholar]
- Wisdom SJ, Wilson R, McKillop JH, Walker JJ. Antioxidant systems in normal pregnancy and in pregnancy-induced hypertension. American Journal of Obstetrics and Gynecology. 1991;165:1701–1704. doi: 10.1016/0002-9378(91)90018-m. [DOI] [PubMed] [Google Scholar]


