Abstract
The activation of G protein-regulated inward rectifying potassium (GIRK) channels is modulated by G protein-coupled receptors (GPCRs) via the G protein βγ subunits and is accelerated by regulators of G protein signalling (RGS). In the present study we investigated the ratio dependence of receptor-mediated activation and deactivation and the influence of new members of the RGS protein family on GIRK currents by coexpressing the recombinant protein subunits in Xenopus oocytes and further analysis of the whole cell currents.
The activation of GIRK channels by the muscarinic acetylcholine receptor M2 (M2 mAChR) is strongly dependent on the ratio of receptor to channel in Xenopus oocytes. The increase and on-rate of the amplified current is affected by this ratio. An excess of receptor over channel is necessary for current amplification, while the reverse excess of channel over receptor abolishes the effect.
The speed of receptor-mediated activation of GIRK currents is accelerated for a high ratio of receptor to channel, while the time of deactivation is independent of this ratio.
Coexpression of RGS2, 5 and 8 accelerates the speed for ACh-mediated activation and deactivation of GIRK1/2 and GIRK1/4 currents. Thereby the receptor/channel/RGS ratio determines the amount of current amplification.
Bordetella pertussis toxin completely abolished ACh-mediated current amplification of GIRK channels coexpressed with or without RGS2.
Two single point mutations in the RGS2 protein (RGS2(N109S) and RGS2(L180F)) reduced the acceleration of current amplification after ACh application on GIRK1/4 channels compared with RGS2 wild-type protein.
The G protein-regulated inward rectifying potassium (GIRK) channel and G protein signal transduction pathway regulates many crucial physiological responses such as cardiac rate and synaptic transmission via neurotransmitters, hormones and sensory stimuli. The duration and intensity of the signal are dependent on the receptor activation of the heterotrimeric G protein complex. The modulation of GIRK channels is due to a membrane-delimited pathway (Sakmann et al. 1983; Soejima & Noma, 1984). Recent work supports the idea that released Gβγ subunits mediate the activation of potassium channels (Breitwieser & Szabo, 1985; Pfaffinger et al. 1985; Logothetis et al. 1987; Reuveny et al. 1994; Wickman et al. 1994; Kofuji et al. 1995; Kurachi, 1995; Nair et al. 1995). Besides the effect of Gβγ subunits on channel modulation, it has been shown that in addition PIP2 is necessary for sufficient activation of GIRK channels (Huang et al. 1998; Sui et al. 1998).
To date four GIRK subunits (GIRK1-4) have been cloned and identified in mammals. GIRK1 and 4 are found in atrial cells (Dascal et al. 1993; Kubo et al. 1993; Krapivinsky et al. 1995a), whereas GIRK1, 2, 3 and 4 (Karschin et al. 1996; Iizuka et al. 1997; Murer et al. 1997) are expressed in brain. GIRK1 and 2 subunits are likely to form heteromultimers in the mammalian nervous system, because of their overlapping distribution, co-immunoprecipitation and reduced protein levels in GIRK2 null mutant mice and the weaver mice (for review see Jan & Jan, 1997). However, expression of cloned GIRK2 subunits alone in a heterologous expression system leads to the formation of functional channels, and therefore the occurrence of homomeric GIRK2 channels cannot be excluded (Duprat et al. 1995; Kofuji et al. 1995; Krapivinsky et al. 1995a,b; Lesage et al. 1995; Hedin et al. 1996). Coexpression of muscarinic receptors and GIRK subunits in Xenopus oocytes results in a variable current amplification following M2 mAChR stimulation, ranging from 0.5- to 20-fold for amplification and 0.35 to 14 μA for current increase (Dascal et al. 1993; Kubo et al. 1993; Fidler Lim et al. 1995; Kofuji et al. 1996; Schreibmayer et al. 1996; Doupnik et al. 1997; Kim et al. 1997; Luchian et al. 1997; Saitoh et al. 1997; Slesinger et al. 1997).
Recently a superfamily of GTPase-activating proteins (GAPs) known as regulators of G protein signalling (RGS proteins) were identified (for reviews see Siderovski et al. 1996; Dohlman & Thorner, 1997; Koelle, 1997; Neer, 1997; Berman & Gilman, 1998). All RGS members share a 130 amino acid domain which is conserved among vertebrates and invertebrates and responsible for the GTPase activity. These proteins mediate the fast kinetics of GTP hydrolysis for the Gi/o and Gq family and are responsible for up to 100-fold increases in in vivo GTP hydrolysis compared with in vitro biochemical studies (Zerangue & Jan, 1998). A similar difference was observed in the kinetics of GIRK current amplification after receptor stimulation in Xenopus oocytes compared with neurons or cardiomyocytes. Doupnik et al. (1997) discovered that receptor-stimulated GIRK current kinetics in Chinese hamster ovary cells (CHO) or Xenopus oocytes coexpressing muscarinic M2 receptor and RGS1, 3 and 4 were accelerated and comparable to the native GIRK currents in atrial myocytes and hippocampal neurons. Similar studies demonstrating the modulation of GIRK channels by RGS8 have been carried out (Saitoh et al. 1997). Here we describe the modulation of GIRK activity by three RGS proteins, RGS2, 5 and 8. We demonstrate that the activation kinetics and ACh-mediated current amplification of GIRK channels are dependent on the ratio of expression between the M2 mAChR, GIRK channel and RGS proteins. Thus these results imply a critical ratio for efficient function between receptor, channel and RGS. In addition oocytes treated with pertussis toxin displayed no ACh-mediated current amplification, suggesting a Gαi/o-mediated event. To ensure that the acceleration effects were mediated by RGS proteins, we introduced two point mutations in the highly conserved GTPase domain and showed a significant decrease in the acceleration of Gαi/o-mediated GIRK channel activation and deactivation.
METHODS
RGS2, 5 and 8 cDNAs
Reverse transcription and PCR amplification were used to clone RGS2, 5 and 8 from mouse brain RNA using the following oligonucleotide primers:
for RGS2 sense, 5′-GCTCAAGCTTCGATGCAAAGTGCCATGTTCCTGGCT-3′;
antisense, 5′-GGTGGATCCTCATGTAGCATGGGGCTCCGTGGT-3′;
for RGS5 sense, 5′-AGATCTCGAGCTATGTGTAAGGGACTGGCAGCTCTG-3′;
antisense, 5′-CGCGGTACCCTACTTGATTAGCTCCTTATAAAA-3′;
for RGS8 sense, 5′-GCTCAAGCTTCGATGGCTGCCTTACTGATGCCACGC-3′;
antisense, 5′-GCAGAATTCCTAGCTGAGCCTCCTCTGGCTTTG-3′.
The entire nucleotide sequence was verified to the mouse RGS2 (GenBank accession no. U67187), mouse RGS5 (GenBank accession no. U67188) or rat RGS8 (GenBank accession no. AB006013) by cDNA sequencing.
Expression of clones and mutagenesis
cDNAs encoding GIRK subunits 1, 2 and 4 and RGS2, 5 and 8 were subcloned into pBF1, and M2 mAChR into pBSK. Plasmids were linearized at the 3′ end of the poly(A) stretch. cRNAs were synthesized in vitro with SP6 polymerase for GIRK and RGS and with T7 polymerase for M2 mAChR using the mMessage mMachine Ambion kits (Ambion, Inc.). For each set of experiments respective mRNAs were synthesized on the same day under the same condition. Concentrations were determined by comparing the RNA ladder which contains 0.5 μg (3 μl)−1 for each component (Gibco BRL; 0.24-9.5 kb RNA ladder) to the synthesized RNA on an agarose gel. mRNAs were diluted for each sample to approximately the same concentration. The stock solution with a concentration of 100 ng μl−1 was stored at -80°C and was classified as the 1:1 dilution. Further dilutions were made out of this stock. For RGS experiments a stock of a 2:1 dilution (200 ng μl−1) for M2 mAChR mRNA was used. Point mutations for RGS2 were designed by the methods of Herlitze & Koenen (1990) and Ho et al. (1989). Mutations were verified by cDNA sequencing.
Electrophysiology
Xenopus laevis frogs were anaesthetized by immersion in water containing 0.1% aminobenzoic acid ethyl ester (Sigma) until they were unresponsive to touch. Oocytes were removed under sterile conditions, and the abdomen was closed with sutures. Following surgery, the frogs were washed with water and returned to the aquarium.
Experiments carried out were approved by the Institution's animal welfare committee.
Xenopus laevis oocytes were injected with a 50 nl solution containing the respective cRNAs dissolved in water and incubated at 19°C in OR2 solution (5 mM Hepes pH 7.3, 82.5 mM NaCl, 2.5 mM KCl, 1 mM Na2HPO4, 0.5 g l−1 polyvinylpyrolidone, 1 mM MgCl2, 1 mM CaCl2, 0.1% penicillin/streptomycin). The follicle layers of the oocytes were removed 24 h after injection by incubation in OR2 solution containing collagenase Type 1A (0.5 mg ml−1; Sigma). Then 72-96 h after injection of cRNA, currents were measured using a standard two-microelectrode voltage clamp in 90 mM K+ modified frog Ringer solution (10 mM Hepes pH 7.3, 27.5 mM NaCl, 90 mM KCl, 1.8 mM CaCl2). Oocytes were placed in a continous flow chamber perfused with modified frog Ringer solution at room temperature. Oocytes were clamped at 0 mV and voltage ramps from -100 to +50 mV were applied every second. The current and voltage electrodes were filled with 3 M KCl and had a resistance of 0.1-0.6 MΩ. Currents were recorded online. Time constants for activation and deactivation were fitted with a single exponential.
Pertussis toxin (PTX; Sigma; 50 nl of 50 ng μl−1 dissolved in water) was injected into Xenopus oocytes coexpressing the GIRK1/4 (1:10) channel combination, M2 mAChR (2:1) and when indicated with RGS2 (1:5) 30 min prior to recording.
RESULTS
Ratio-dependent activation of GIRK channels
The activation of GIRK channels is most probably mediated by Gβγ subunits released from heterotrimeric G proteins after receptor stimulation. However, coexpression of M2 mAChR and GIRK subunits in Xenopus oocytes resulted in a variable current amplification following M2 mAChR stimulation (Dascal et al. 1993; Kubo et al. 1993; Fidler Lim et al. 1995; Kofuji et al. 1996; Schreibmayer et al. 1996; Kim et al. 1997; Luchian et al. 1997; Slesinger et al. 1997). This amplification of current is given by the ratio between the control current and the current after transmitter application at a given potential. Another way to quantify the Gβγ subunit effect is to take the difference between the current before and after transmitter application. This value defines the total current increase for a given experiment. To analyse receptor-dependent G protein activation of GIRK channels in Xenopus oocytes, we coexpressed GIRK1/4 and M2 mAChR at various mRNA concentration ratios in Xenopus oocytes and measured the amplification of current as the current increase after a 10 μm ACh application by two-microelectrode voltage clamp. Mean values for control current and current increase are plotted in Fig. 1B and C for each recording at -65 mV. Muscarinic AChR M2 was diluted 1:1, 1:2, 1:5 and 1:10 and injected in an equivalent volume with a 1:5 dilution of GIRK1/4 mRNA. Saturated ACh concentrations (10 μm) increased currents by 2861 ± 309 nA (n = 23) for the highest receptor concentration (1:1). The current amplification was strongly dependent on the receptor concentration, since a 1:5 and 1:10 dilution decreased the elicited current significantly by approximately 80%. No amplification was detected in the absence of M2 mAChR. The receptor concentration had no effect on the expression of the GIRK channels, since the control currents measured before transmitter application remained the same (Fig. 1B).
Figure 1. Ratio-dependent expression and current increase of GIRK1/4 channels activated by M2 mAChR.
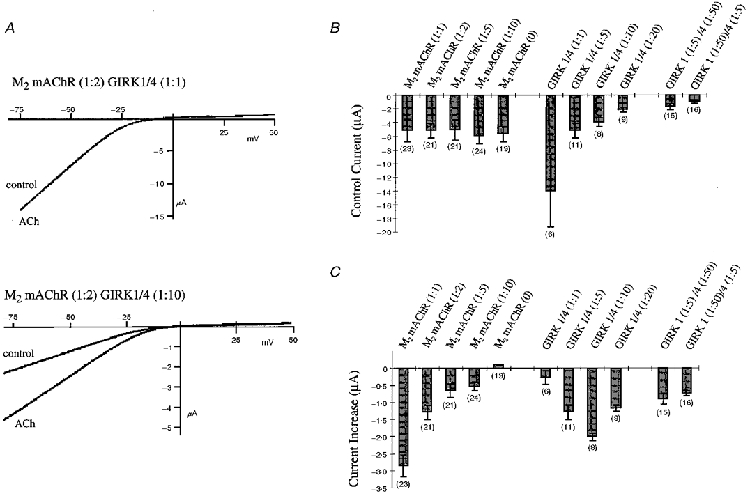
A, comparison of voltage ramps of GIRK1/4 (1:1) (upper panel) and GIRK1/4 (1:10) (lower panel) coexpressed with M2 mAChR (1:2) from -80 to +50 mV before (control) and 30 s after application of 10 μm ACh. Potassium channel currents were measured in a 90 mM K+ solution. B, control currents were measured in the absence of ACh at -65 mV. Values were determined for the dilutions of M2 mAChR (1:1; 1:2; 1:5; 1:10 and 0 M2 mAChR) coexpressed with a 1:5 dilution of GIRK1/4; for dilutions of GIRK1/4 (1:1; 1:5; 1:10 and 1:20) coexpressed with a 1:2 dilution of M2 mAChR; and for the dilutions of GIRK1 (1:5 and 1:50) in combination with GIRK4 (1:50 and 1:5, respectively) coexpressed with a 1:2 dilution of M2 mAChR. Numbers in parentheses indicate the number of oocytes tested. C, current increases were determined by subtracting the current after 30 s of ACh application at -65 mV from the control current at -65 mV. Values are given for the dilutions of M2 mAChR (1:1; 1:2; 1:5; 1:10 and 0 M2 mAChR) coexpressed with a 1:5 dilution of GIRK1/4; for dilutions of GIRK1/4 (1:1; 1:5; 1:10 and 1:20) coexpressed with a 1:2 dilution of M2 mAChR; and for the dilutions of GIRK1 (1:5 and 1:50) in combination with GIRK4 (1:50 and 1:5, respectively) coexpressed with a 1:2 dilution of M2 mAChR. Numbers in parentheses indicate the number of oocytes tested.
To confirm that the receptor/channel ratio determines the K+ current increase, we varied the concentration of GIRK1/4 channels at a constant concentration (1:2) of M2 mAChR (Fig. 1B and C). As expected, the control currents decreased accordingly with increasing dilutions of the GIRK1/4. Surprisingly, we observed no significant amplification with the highest channel concentration (1:1), despite the presence of the receptor. This result suggests that overexpressed GIRK channels can sequester endogenous Gβγ subunits and may prevent the formation of heterotrimeric G proteins which are necessary for interaction with the receptor. Successive dilutions of the channel increased currents to a maximal level of about -2 μA at the 1:10 dilution of channel. A higher dilution of GIRK channels (1:20) produced a significant decrease of the ACh-elicited current suggesting that the amount of activatable channels becomes rate limiting. Collectively, these results indicate that there is an optimal ratio between the M2 mAChR and the GIRK channel to obtain maximal channel activation.
To investigate whether the ratio between the GIRK subunits 1 and 4 influences the amplified current, a 1:5 dilution of one subunit was combined with a 1:50 dilution of the other subunit and coinjected with a 1:2 dilution of receptor (Fig. 1B and C). There were no significant differences in the control current or current increase between these two ratios. However, the control current and current increase were both lower than the 1:20 dilution of both subunits due to the limited amount of one subunit available to assemble active channels (Fig. 1B and C).
Ratio-dependent effects on activation and deactivation of GIRK channels
Past studies have demonstrated that the density of receptors influences the GIRK channel activation following receptor stimulation (Bünemann et al. 1997; Chuang et al. 1998). Based on our findings that high levels of GIRK expression drastically reduce G protein-mediated activation, we propose that GIRK channels sequester Gβγ proteins within Xenopus oocytes. Thus a high channel concentration should decrease the G protein concentration available to be activated by the receptor. A reduction in the number of available G proteins for activation of GIRK channels and the change in the ratio between receptor and channel should alter the activation time at various concentrations of GIRK channels and a constant level of receptor. In agreement with this hypothesis, we found that ACh-stimulated currents increased faster for low channel concentrations (1:50; τon= 3.23 ± 0.47 s (n = 7)) compared with high concentrations (1:1; τon= 6.45 ± 0.33 s (n = 3)) at a constant receptor level (Fig. 2). The speed of current deactivation was independent of the receptor to channel ratio (Fig. 2C). Our data show that the time course of current activation is critically influenced by the receptor/channel ratio.
Figure 2. Ratio dependence of time constants, τon and τoff, of GIRK1/4 channel amplification by M2 mAChR.
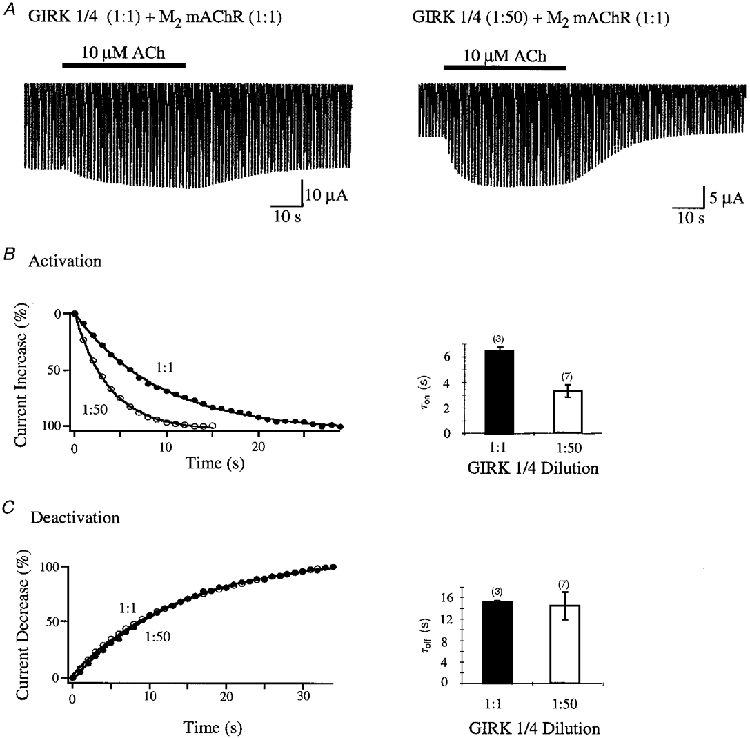
A, comparison of voltage ramps of GIRK1/4 (1:1) (left trace) and GIRK1/4 (1:50) (right trace) coexpressed with M2 mAChR (1:1) from -100 to +50 mV before and after application of 10 μm ACh. Voltage ramps were applied every second. After 5 ramps 10 μm ACh was applied for 35 s and then washed out. Recordings were done in a 90 mM K+ solution. B, time constants of current increase after ACh application for different dilutions of GIRK1/4 channels. The left panel shows the time-dependent current increase as a percentage (%) after 10 μm ACh application. Values are given at -65 mV and were related to the highest current reached after ACh application. Curves for GIRK1/4 (1:1) (•) and GIRK1/4 (1:50) (○) were fitted with a single exponential. The right panel displays the time constants (τon) of current increase for GIRK channel dilutions (1:1 (filled bars) and 1:50 (open bars)) coexpressed with M2 mAChR (1:1). Numbers in parentheses indicate the number of oocytes tested. C, time constants of current decrease after removal of ACh for different dilutions of GIRK1/4 channels. The left panel shows the time-dependent current decrease as a percentage (%) after 10 μm ACh application. Values are given at -65 mV and were related to the lowest current reached after removal of ACh. Curves for GIRK1/4 (1:1) (•) and GIRK1/4 (1:50) (○) were fitted with a single exponential. The right panel depicts the time constants (τoff) of current decrease for GIRK channel dilutions (1:1 (filled bars) and 1:50 (open bars)) coexpressed with M2 mAChR (1:1). Numbers in parentheses indicate the number of oocytes tested.
Effects of RGS2, 5 and 8 on GIRK1/4 and GIRK1/2 channel modulation
The family of GIRK channels is composed of four subunits: 1, 2, 3 and 4 (Jan & Jan, 1997). The GIRK1/4 combination is found in heart and mediates ACh-dependent effects in the cardiac muscle. Although all four subunits are expressed in brain the functional roles and possible assembly between the members are not very well defined. However, colocalization and immunoprecipitation studies suggest that GIRK1 and 2 are most probably coassembled in the central nervous system and act as a functional unit. Discrepancies between native and recombinant GIRK channel kinetics by receptor-mediated activation and deactivation could be explained by studies from Doupnik et al. (1997) and Saitoh et al. (1997). They demonstrated an acceleration of activation and deactivation by RGS1, 3, 4 and 8 of GIRK1/2 and GIRK1/4 currents. However, no effects were reported for RGS2 (Doupnik et al. 1997) and RGS5.
In our studies we analysed the regulation of GIRK channels by RGS2, 5 and 8. To determine the effects of individual RGS members on the kinetics of channel activation and deactivation, we coexpressed the RGS proteins in a 1:1 dilution (unless indicated otherwise) with GIRK1/4 and GIRK1/2 (each as a 1:10 dilution) and a high concentration (2:1) of M2 mAChR to ensure sufficient GIRK channel activation (see Fig. 1). Surprisingly all RGS proteins, even RGS2, influenced the kinetics of GIRK1/4 and GIRK1/2 channel amplification (Fig. 3A, B and C and Fig. 3D and E, respectively). The kinetic effects are more pronounced for channel deactivation than for activation. Deactivation time constants for GIRK1/4 were reduced from approximately 16.5 to 5.5 s (Fig. 3C) and from 20 to 8 s for GIRK1/2 channels coexpressed with RGS (Fig. 3E). The activation time constants were accelerated from approximately 5.5 to 2.5 s for GIRK1/4 (Fig. 3B) and from 3.4 to 2 s for GIRK1/2 (Fig. 3D). The acceleration effects of RGS were reduced by dilution of RGS cRNA (Fig. 3F and G). As shown for the 1:50 dilution of RGS2 τon and τoff increased significantly from 1.7 to 2.3 s for the activation and from 9 to 16 s for the deactivation. These results indicate that G protein-mediated activation and deactivation of GIRK1/4 and GIRK1/2 current can be accelerated by RGS2, 5 and 8 in a concentration-dependent manner.
Figure 3. Effects of RGS2, 5 and 8 on GIRK1/4 and GIRK1/2 channel modulation.
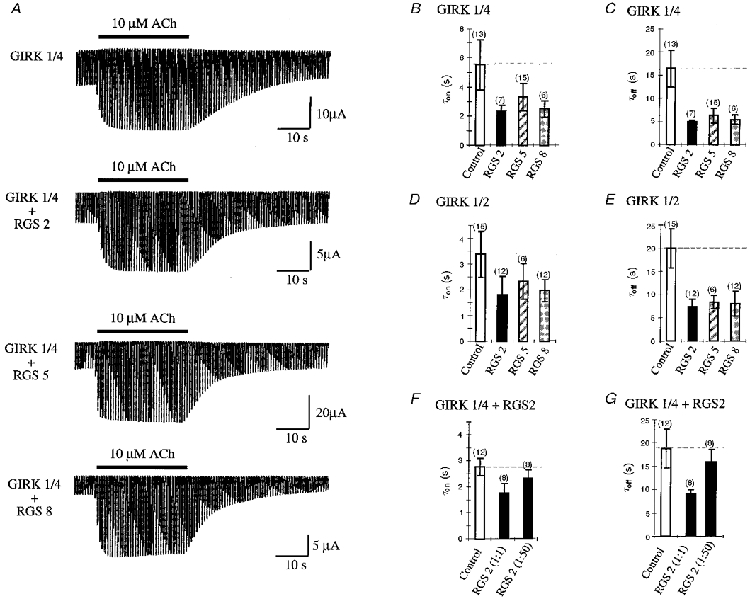
A, comparison of GIRK1/4 currents elicited by voltage ramps from -100 to +50 mV before and after application of 10 μm ACh. GIRK1/4 (1:10) was coexpressed with M2 mAChR (1:1) in the presence or absence of different RGS proteins (1:1). Voltage ramps were applied every second. Five ramps of 10 μm ACh were applied for 35 s and then washed out. Recordings were done in a 90 mM K+ solution. B, time constants of current increase after ACh application for GIRK1/4 channel (1:1) coexpressed with M2 mAChR (2:1) and RGS2, 5 and 8 (1:1). In B-G, controls indicate GIRK1/4 or GIRK1/2 coexpressed with M2 mAChR in the absence of RGS (open bars); numbers in parentheses indicate the number of oocytes tested. C, time constants of current decrease after ACh removal for GIRK1/4 channel (1:1) coexpressed with M2 mAChR (2:1) and RGS2, 5 and 8 (1:1). D, time constants of current increase after ACh application for GIRK1/2 channel (1:1) coexpressed with M2 mAChR (2:1) and RGS2, 5 and 8 (1:1). E, time constants of current decrease after ACh removal for GIRK1/2 channel (1:1) coexpressed with M2 mAChR (2:1) and RGS2, 5 and 8 (1:1). F, time constants of current increase after ACh application for GIRK1/4 channel (1:10) coexpressed with M2 mAChR (2:1) and RGS2 in a 1:1 or 1:50 dilution. G, time constants of current decrease after ACh removal for GIRK1/4 channel (1:10) coexpressed with M2 mAChR (2:1) and RGS2 in a 1:1 or 1:50 dilution. Time constants for B-G were determined for current increase or decrease at -65 mV. Curves were fitted with a single exponential.
Effects of RGS2, 5 and 8 on receptor-mediated current increase
Previous studies report conflicting results for RGS effects on the control current and current amplification (Doupnik et al. 1997; Saitoh et al. 1997; Chuang et al. 1998; Bünemann & Hosey, 1998). To investigate whether the RGS to channel ratio affects the control current and current increase, we coexpressed the RGS proteins in a 1:1 dilution with a high (1:1 dilution) and a low (1:50 dilution) concentration of GIRK1/4 and 1/2 channels together with the M2 mAChR (2:1 dilution). At high concentrations of GIRK channels, RGS had no effects on ACh-elicited current increase (Fig. 4B and D). However, the current increase was significantly reduced in the presence of RGS and low concentrations of channel (Fig. 4B and D). This effect cannot be due to a change in expression of GIRK channels, since the control currents are comparable (Fig. 4A and C). Collectively, an excess of RGS to channel reduced ACh-elicited currents, while equivalent RGS and channel concentrations demonstrated no effect on the current amplification.
Figure 4. Effects of RGS2, 5 and 8 on GIRK1/4 and GIRK1/2 on control current and ACh-mediated current increase.
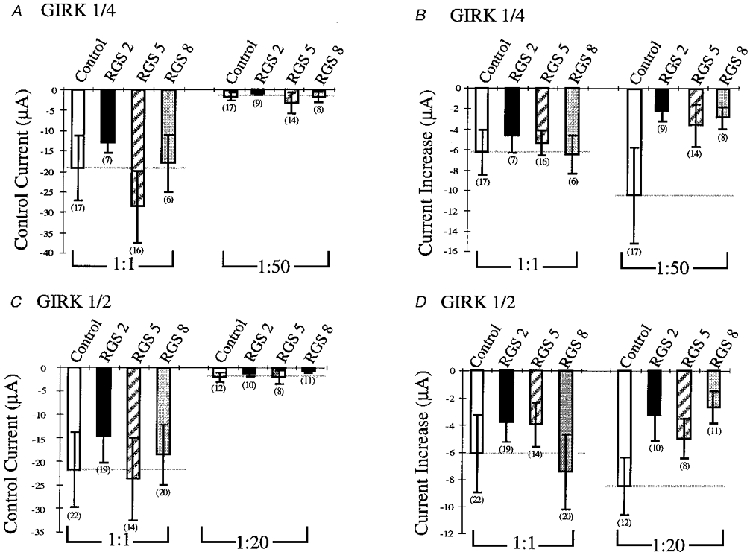
A, control current for different dilutions of GIRK1/4 channels coexpressed with a 2:1 dilution of M2 mAChR and a 1:1 dilution of RGS2, 5 and 8. Control indicates the GIRK1/4 dilution expressed in the absence of RGS (open bars) for A and B. Control currents were determined without ACh application at -65 mV. GIRK1/4 was diluted 1:1 and 1:50 as indicated. Numbers in parentheses indicate the number of oocytes tested for all panels. B, current increase for different dilutions of GIRK1/4 channels coexpressed with a 2:1 dilution of M2 mAChR and a 1:1 dilution of RGS2, 5 and 8. Current increases were determined by subtracting the current after 30 s of ACh application at -65 mV from the control current at -65 mV. GIRK1/4 was diluted 1:1 and 1:50 as indicated. C, control current for different dilutions of GIRK1/2 channels coexpressed with a 2:1 dilution of M2 mAChR and a 1:1 dilution of RGS2, 5 and 8. Control indicates the GIRK1/2 dilution expressed in the absence of RGS (open bars) for C and D. Control currents were determined without ACh application at -65 mV. GIRK1/2 was diluted 1:1 and 1:20 as indicated. D, current increase for different dilutions of GIRK1/2 channels coexpressed with a 2:1 dilution of M2 mAChR and a 1:1 dilution of RGS2, 5 and 8. Current increases were determined by subtracting the current after 30 s of ACh application at -65 mV from the control current at -65 mV. GIRK1/2 was diluted 1:1 and 1:20 as indicated.
Effects of PTX on control current and receptor-mediated current increase in the presence of RGS2
Neuronal and biochemical studies show that RGS2 stimulates the GTPase activity of Gαq and Gαi/o (Heximer et al. 1997; Ingi et al. 1998). In order to demonstrate that current amplification in the absence or presence of RGS2 is mediated by the Gαi/o pathway, we injected 2.5 ng of PTX 30 min prior to recording Xenopus oocytes expressing receptor and channel either with or without RGS2. ACh-elicited currents were blocked completely by PTX, while the control currents were not significantly affected (Fig. 5A and B). These results indicate that ACh-mediated current amplification of GIRK channels in the presence of RGS is mediated by PTX-sensitive G proteins.
Figure 5. Effects of PTX on control current and ACh-mediated current increase of GIRK1/4 expressed in the presence or absence of RGS2.
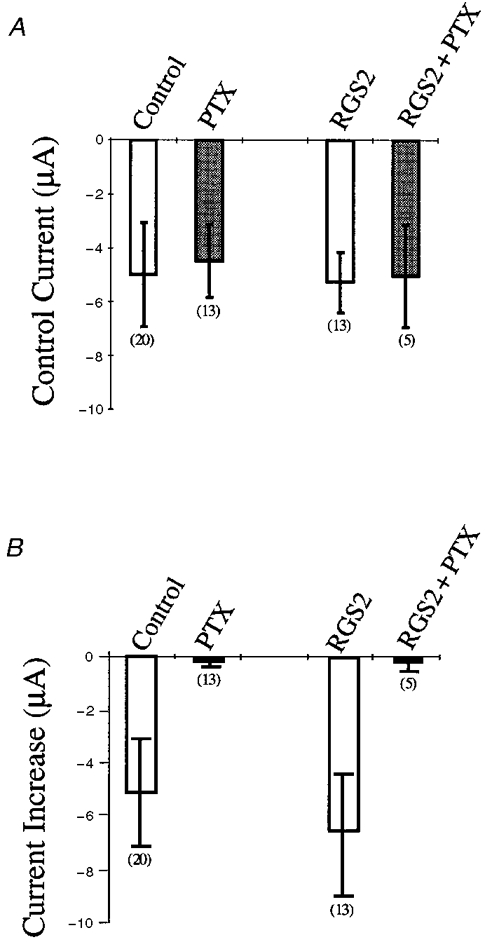
A, control current for GIRK1/4 channels coexpressed with a 2:1 dilution of M2 mAChR and with or without a 1:1 dilution of RGS2. PTX or RGS2 + PTX indicate oocytes expressing channel and receptor or channel, receptor and RGS injected with 50 nl of 50 ng μl−1 PTX solution 30 min prior to recording. Control indicates the GIRK1/4 dilution expressed with or without RGS in the absence of PTX (open bars). Control currents were determined without ACh application at -65 mV. B, current increase for GIRK1/4 channels coexpressed with a 2:1 dilution of M2 mAChR and with or without a 1:1 dilution of RGS2. PTX or RGS2 + PTX indicate oocytes expressing channel and receptor or channel, receptor and RGS injected with 50 nl of 50 ng μl−1 PTX solution 30 min prior to recording. Control indicates the GIRK1/4 dilution expressed with or without RGS in the absence of PTX (open bars). Current increases were determined by subtracting the current after 30 s of ACh application at -65 mV from the control current at -65 mV. Numbers in parentheses indicate the number of oocytes tested.
Effect of RGS2 point mutations on GIRK1/4 channel modulation
The crystal structure of RGS4 complexed with Gαi1-GDP- AlF4− identified several interacting amino acids between these two proteins (Tesmer et al. 1997). Based on this study Druey & Kehrl (1997) mutated the contacting amino acids N88S and L159F in RGS4 and abolished both RGS4 binding to Gαi1 and GTPase activity in biochemical assays. Alignment of RGS domains between the different RGS proteins revealed that these two amino acids are conserved among the RGS family members (Fig. 6A). In order to verify the modulation of GIRK channels by RGS, we introduced the two corresponding point mutations into RGS2, RGS2(N109S) and RGS2(L180F), and analysed their effects on the kinetics of amplification for GIRK1/4. Both point mutations, RGS2(N109S) and RGS2(L180F), considerably abolished acceleration effects on the deactivation (Fig. 6C). Effects on the activation were not as pronounced. The acceleration of current increase was significantly reduced for L180F but not for N109S, where the variability of the results did not allow an interpretation of this mutation. However, both point mutations within the RGS2 diminished the kinetic effects of wild-type RGS on G protein-mediated current amplification of GIRK channels indicating a clear role of RGS in GIRK channel regulation.
Figure 6. Effects of point mutations within RGS2 on GIRK1/4 channel modulation.
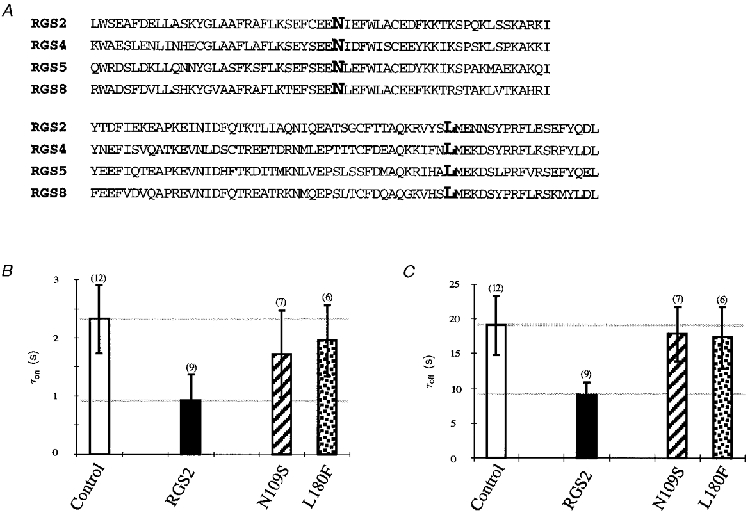
A, comparison of the amino acid sequences between the core domain of RGS2, 4, 5 and 8. Amino acids N109 and L180 for RGS2 and corresponding amino acids in the other RGS proteins are shown emboldened. B, time constants of current increase after ACh application for RGS2 (1:5) and mutated RGS2 (1:5) coexpressed with GIRK1/4 (1:10) and M2 mAChR (2:1). Control indicates GIRK1/4 (1:10) expressed in the absence of RGS (open bars). Values were determined for current increase at -65 mV. Curves were fitted with a single exponential. C, time constants of current decrease after removal of ACh for RGS2 (1:5) and mutated RGS2 (1:5) coexpressed with GIRK1/4 (1:10) and M2 mAChR (1:1). Control indicates GIRK1/4 (1:10) expressed in the absence of RGS (open bars). Values were determined for current decrease at -65 mV. Curves were fitted with a single exponential. Numbers in parentheses indicate the number of oocytes tested.
DISCUSSION
The ratio between receptor, channel and RGS determines the amount of current amplification
The stoichiometry of interaction between G protein-coupled receptors, GIRK channels, G proteins and RGS in neurons and myocardiocytes is unknown. However, we show in this study that the expression of certain amounts of GIRK channels in combination with various receptor concentrations gives rise to different current increases in Xenopus oocytes. More G proteins can be activated at high receptor concentrations, thus more current is elicited. Presumably, dissociated Gβγ subunits will bind to the channel and lead to current increase probably by favouring a closed state from which opening of GIRK channels is easier to achieve (Soejima & Noma, 1984; Breitwieser, 1996; Hosoya et al. 1996). On the other hand, the lower the receptor concentration the fewer G proteins can be activated. Therefore the elicited current is small. Suprisingly, high channel to receptor ratios abolish current increase. A likely explanation is that at a very high channel concentration G proteins could be depleted within the oocytes, since the channels sequester all G proteins. The amount of available G proteins is diminished for coupling to the receptor, and therefore G proteins cannot be activated. Competition for G protein binding is likely, since GIRK channels bind to the Gβγ subunit (Huang et al. 1995).
In addition to the ratio dependence of receptor and channel, different ratios between RGS and channel also influence ACh-mediated current amplification. At low channel and high RGS concentrations current amplification is reduced compared with channels not coexpressed with RGS. This effect could be abolished by increasing the amount of channel or decreasing the amount of RGS protein (1:5 dilution) (data not shown). These results imply that the amount of RGS relative to channel can alter current amplification.
A possible explanation for a decrease of amplified currents by RGS2, 5 and 8 could be the GAP activity of the RGS itself. Fast termination of the G protein action could result in a decrease of current amplification without affecting control current. However, ACh-mediated currents are not significantly reduced at higher channel or lower RGS concentrations. Chuang and collegues (Chuang et al. 1998) showed that the coexpression of μ-opioid receptors, RGS4 and GIRK channels in Xenopus oocytes increased the population of G proteins available for receptor-mediated channel activation in the presence of RGS, and the receptor activated more G protein in a given amount of time. Therefore transmitter-amplified currents are not significantly changed in the presence of RGS. Changes in receptor-mediated GIRK current amplification in the presence of RGS could also not be observed by Doupnik et al. 1997 and Saitoh et al. 1997; both groups coexpressed RGS with GIRK channels and receptors in Xenopus oocytes. To date none of the studies have changed the relative ratio of channel and RGS in Xenopus oocytes, so the effects on GAP activity might only be obvious at certain ratios.
Since RGS proteins bind to G protein α subunits (Tesmer et al. 1997), another possibility for the reduction of ACh-mediated currents could be that RGS competes for G protein binding. High concentrations of RGS would bind up all G protein α subunits leading to a high concentration of free Gβγ subunits which should result in an increase in control current and a decrease in current amplification. These effects are described by Bünemann & Hosey (1998) who expressed M2 mAChR, GIRK channel and RGS3 and 4 in HEK 293 cells. A reduction in the number of Gα subunits can explain their results. However, in our expression system we do not observe a significant increase in control current in the presence of RGS. Therefore neither the high GAP activity of RGS nor the competition for G protein binding between GIRK channel and RGS can completely explain the observed reduction in GIRK amplification without affecting the control current. Other mechanisms, for example that GIRK binding to G protein subunits is critical for effective current amplification in the presence of RGS, cannot be excluded.
In summary, the results suggest that current amplification can be modulated by the relative expression of each G protein receptor cascade member in the Xenopus oocyte system. The physiological consequence of this result might be that in neurons or heart cells the ratio and stoichiometry of proteins involved in G protein signalling are regulated intracellularly by proteins like PSD95 (Snow et al. 1998), which cluster different components of the signal cascade to ensure an efficient stimulation pathway.
RGS2, 5 and 8 accelerate activation and deactivation of GIRK1/2 and GIRK1/4 channels
The rate of GIRK channel activation and deactivation in neurons and myocardiocytes compared with recombinant expressed channels in Xenopus oocytes revealed differences in kinetics of amplification after receptor stimulation. These differences are most probably due to the expression of RGS proteins in cells expressing fast kinetics for K+ current amplification. So far the acceleration of K+ current amplification has been shown for RGS1, 3, 4 and 8. In the present study we demonstrate that RGS2, 5 and 8 accelerate activation and deactivation of GIRK1/2 and GIRK1/4 current increase elicited by activation of the G protein-coupled receptor M2 mAChR. Two point mutations in RGS2 revealed a significant decrease in the deactivation time constant compared with the wild-type RGS, thus verifying modulation of GIRK currents by RGS proteins. Acceleration effects on GIRK current activation and deactivation mediated by RGS have been described to influence both the on-rate and the off-rate without affecting the current amplification. As shown by Chuang et al. (1998) and supported by our results RGS accelerates τon by increasing the G protein trimer concentration and τoff by increasing the GTPase activity of Gαi/o subunits. The physiological significance of this finding is important for proper regulation of heart rate and synaptic transmision. At a certain ratio of receptor/channel and RGS, the response to transmitter release is greatly accelerated without affecting the amount of current flowing through a certain amount of channels, thereby speeding up the signal transmission without diminishing its amplitude.
RGS2 effects on GIRK channel modulation involve Gαi/o pathways
This study is the first to report that RGS2 accelerates G protein modulation of GIRK channels. The acceleration effects involve PTX-sensitive G proteins, and can be drastically reduced by point mutations within the RGS2 protein. However, the observed effects with RGS2 are in contradiction with various studies. Doupnik et al. (1997) reported that RGS2 had no effect on GIRK channel modulation. Furthermore, Heximer et al. (1997) identified RGS2 as a selective inhibitor of Gαq, but not Gαi/o function using biochemical assays. No binding to Gαi/o subunits could be detected for histidine-tagged RGS2 protein. There are several explanations for these conflicting results. Initially, we overexpressed RGS2 at a high concentration (2:1 dilution). At this concentration RGS2 drastically reduced the GIRK1/4-mediated control current and amplified current by about 95%, but had no appreciable effect on the kinetics of current amplification (data not shown). At this concentration RGS might have non-specific effects, e.g. on intracellular trafficking or channel expression. However, RGS2 in a 1:1 or 1:5 dilution showed the described acceleration effect on GIRK currents (Figs 3, 4, 5 and 6). The overexpression of RGS2 might explain why Doupnik et al. (1997) did not see any effect on GIRK channel modulation.
An explanation for the lack of RGS2 binding to G proteins in the biochemical assay (Heximer et al. 1997) could be given by the fact that different NH2-terminal modifications of Gαz and Gαi1 subunits (i.e. palmitoylation, myristoylation and proteolysis) reduce the GAP activities of several RGS proteins (Tu et al. 1997). This amino-terminal region of Gα subunits is crucial for RGS protein recognition. Perhaps the modifications of the Gαi/o subunit for recognition of RGS2 were not completely preserved in the biochemical assays. Therefore RGS2 displayed no binding to Gαi/o subunits. In contrast, post-translational modifications may be more conserved in injected oocytes and this is technically a more sensitive assay to detect an effect of RGS on Gαi/o subunits. This explanation and our observed effects of RGS2 are also in agreement with the recently published result that reconstitution of RGS2 in phospholipid vesicles containing M2 mAChR inhibited Gi-dependent responses in transfected cells (Ingi et al. 1998).
There have been many discrepancies on the level of receptor-induced GIRK channel amplification in Xenopus oocytes (Dascal et al. 1993; Kubo et al. 1993; Fidler Lim et al. 1995; Kofuji et al. 1996; Schreibmayer et al. 1996; Doupnik et al. 1997; Kim et al. 1997; Luchian et al. 1997; Saitoh et al. 1997; Slesinger et al. 1997). We show that this is due to a ratio dependence for GIRK channel amplification between receptor, channel and RGS proteins, involving PTX-sensitive G proteins. Furthermore, we demonstrate the regulation of GIRK channels by RGS2 and 5. The increase in GIRK channel deactivation by RGS was abolished by two point mutations within the conserved RGS domain. Collectively, our results not only show the regulation of GIRK channels by two additonal RGS proteins, RGS2 and 5, but also demonstrate that activation of GIRK channels requires a critical balance between the members of the G protein signalling cascade.
Acknowledgments
We thank S. Waka, H. Fritzenschaft and B. Rudo for excellent technical assistance, and Drs T. Baukrowitz, S. Gründer, and J. Ludwig for reading the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft project number RU535/4-2.
References
- Berman DM, Gilman AG. Mammalian RGS proteins: barbarians at the gate. Journal of Biological Chemistry. 1998;273:1269–1272. doi: 10.1074/jbc.273.3.1269. [DOI] [PubMed] [Google Scholar]
- Breitwieser GE. Mechanisms of K+ channel regulation. Journal of Membrane Biology. 1996;152:1–11. doi: 10.1007/s002329900080. [DOI] [PubMed] [Google Scholar]
- Breitwieser GE, Szabo G. Uncoupling of cardiac muscarinic and β-adrenergic receptors from ion channels by a guanine nucleotide analogue. Nature. 1985;317:538–540. doi: 10.1038/317538a0. [DOI] [PubMed] [Google Scholar]
- Bünemann M, Brandts B, Pott L. In vivo downregulation of M2 receptors revealed by measurement of muscarinic K+ current in cultured guinea-pig atrial myocytes. The Journal of Physiology. 1997;501:549–554. doi: 10.1111/j.1469-7793.1997.549bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bünemann M, Hosey MM. Regulators of G protein signaling (RGS) proteins constitutively activate Gβγ-gated potassium channels. Journal of Biological Chemistry. 1998;273:31186–31190. doi: 10.1074/jbc.273.47.31186. [DOI] [PubMed] [Google Scholar]
- Chuang H-H, Yu M, Jan YN, Jan LY. Evidence that the nucleotide exchange and hydrolysis cycle of G proteins causes acute desensitization of G-protein gated inward rectifier K+ channels. Proceedings of the National Academy of Sciences of the USA. 1998;95:11727–11732. doi: 10.1073/pnas.95.20.11727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N, Schreibmayer W, Lim NF, Wang W, Chavkin C, DiMagno L, Labarca C, Kieffer BL, Gaveriaux-Ruff C, Trollinger D, Lester HA, Davidson N. Atrial G protein-activated K+ channel: expression cloning and molecular properties. Proceedings of the National Academy of Sciences of the USA. 1993;90:10235–10239. doi: 10.1073/pnas.90.21.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohlman HG, Thorner J. RGS proteins and signaling by heterotrimeric G proteins. Journal of Biological Chemistry. 1997;272:3871–3874. doi: 10.1074/jbc.272.7.3871. 10.1074/jbc.272.7.3871. [DOI] [PubMed] [Google Scholar]
- Doupnik CA, Davidson N, Lester HA, Kofuji P. RGS proteins reconstitute the rapid gating kinetics of Gβγ-activated inwardly rectifying K+ channels. Proceedings of the National Academy of Sciences of the USA. 1997;94:10461–10466. doi: 10.1073/pnas.94.19.10461. 10.1073/pnas.94.19.10461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druey KM, Kehrl JH. Inhibition of regulator of G protein signaling function by two mutant RGS4 proteins. Proceedings of the National Academy of Sciences of the USA. 1997;94:12851–12856. doi: 10.1073/pnas.94.24.12851. 10.1073/pnas.94.24.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprat F, Lesage F, Guillemare E, Fink M, Hugnot J-P, Bigay J, Lazdunksi M, Romey G, Barhanin J. Heterologous multimeric assembly is essential for K+ channel activity of neuronal and cardiac G-protein-activated inward rectifiers. Biochemical and Biophysical Research Communications. 1995;212:657–663. doi: 10.1006/bbrc.1995.2019. 10.1006/bbrc.1995.2019. [DOI] [PubMed] [Google Scholar]
- Fidler Lim N, Dascal N, Labarca C, Davidson N, Lester HA. A G protein-gated K channel is activated via β2-adrenergic receptors and Gβγ subunits in Xenopus oocytes. Journal of General Physiology. 1995;105:421–439. doi: 10.1085/jgp.105.3.421. 10.1085/jgp.105.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedin KE, Lim NF, Clapham DE. Cloning of a Xenopus laevis inwardly recifying K+ channel subunit that permits GIRK1 expression of IKACh currents in oocytes. Neuron. 1996;16:423–429. doi: 10.1016/s0896-6273(00)80060-4. 10.1016/S0896-6273(00)80060-4. [DOI] [PubMed] [Google Scholar]
- Herlitze S, Koenen M. A general and rapid mutagenesis method using polymerase chain reaction. Gene. 1990;91:143–147. doi: 10.1016/0378-1119(90)90177-s. 10.1016/0378-1119(90)90177-S. [DOI] [PubMed] [Google Scholar]
- Heximer SP, Watson N, Linder ME, Blumer KJ, Hepler JR. RGS2/G0S8 is a selective inhibitor of Gqα function. Proceedings of the National Academy of Sciences of the USA. 1997;94:14389–14393. doi: 10.1073/pnas.94.26.14389. 10.1073/pnas.94.26.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Hosoya Y, Yamada M, Hiroyuki I, Kurachi Y. A functional model for G protein activation of the muscarinic K+ channel in guinea pig atrial myocytes. Journal of General Physiology. 1996;108:485–495. doi: 10.1085/jgp.108.6.485. 10.1085/jgp.108.6.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C-L, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature. 1998;391:803–806. doi: 10.1038/35882. 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- Huang C-L, Slesinger PA, Casey PJ, Jan YN, Jan LY. Evidence that direct binding of Gβγ to the GIRK1 G protein-gated inwardly rectifying K+ channel is important for channel activation. Neuron. 1995;15:1133–1143. doi: 10.1016/0896-6273(95)90101-9. 10.1016/0896-6273(95)90101-9. [DOI] [PubMed] [Google Scholar]
- Iizuka M, Tsunenari I, Momota Y, Akiba I, Kono T. Localization of a G-protein-coupled inwardly rectifying K+ channel, CIR, in the rat brain. Neuroscience. 1997;77:1–13. doi: 10.1016/s0306-4522(96)00460-5. 10.1016/S0306-4522(96)00460-5. [DOI] [PubMed] [Google Scholar]
- Ingi T, Krumins AM, Chidiac P, Brothers GM, Chung S, Snow BE, Barnes CA, Lanahan AA, Siderovski DP, Ross EM, Gilman AG, Worley PF. Dynamic regulation of RGS2 suggests a novel mechanism in G-protein signaling and neuronal plasticity. Journal of Neuroscience. 1998;18:7178–7188. doi: 10.1523/JNEUROSCI.18-18-07178.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Voltage-gated and inwardly rectifying potassium channels. The Journal of Physiology. 1997;505:267–282. doi: 10.1111/j.1469-7793.1997.267bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karschin C, Dissmann E, Stuhmer W, Karschin A. IRK(1–3) and GIRK(1–4) inwardly rectifying K+ channel mRNAs are differentially expressed in the adult rat brain. Journal of Neuroscience. 1996;16:3559–3570. doi: 10.1523/JNEUROSCI.16-11-03559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Watson M, Indyk V. ATP-dependent regulation of a G protein-coupled K+ channel (GIRK1/GIRK4) expressed in oocytes. American Journal of Physiology. 1997;272:H195–206. doi: 10.1152/ajpheart.1997.272.1.H195. [DOI] [PubMed] [Google Scholar]
- Koelle MR. A new family of G-protein regulators - the RGS proteins. Current Biology. 1997;9:143–147. doi: 10.1016/s0955-0674(97)80055-5. [DOI] [PubMed] [Google Scholar]
- Kofuji P, Davidson N, Lester HA. Evidence that neuronal G-protein-gated inwardly rectifying K+ channels are activated by G beta gamma subunits and function as heteromultimers. Proceedings of the National Academy of Sciences of the USA. 1995;92:6542–6546. doi: 10.1073/pnas.92.14.6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji P, Hofer M, Millen KJ, Millonig JH, Davidson N, Lester HA, Hatten ME. Functional analysis of the weaver mutant GIRK2 K+ channel and rescue of weaver granule cells. Neuron. 1996;16:941–952. doi: 10.1016/s0896-6273(00)80117-8. 10.1016/S0896-6273(00)80117-8. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G, Gordon EA, Wickman K, Velimirovic B, Krapivinsky L, Clapham DE. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifiying K+-channel proteins. Nature. 1995a;374:135–141. doi: 10.1038/374135a0. 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G, Krapivinsky L, Velimirovic B, Wickman K, Navarro B, Clapham DE. The cardiac inward rectifier K+ channel subunit, CIR, does not comprise the ATP-sensitive K+ channel KATP. Journal of Biological Chemistry. 1995b;270:28777–28779. doi: 10.1074/jbc.270.48.28777. 10.1074/jbc.270.48.28777. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Baldwin TJ, Jan YN, Jan LY. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993;362:127–133. doi: 10.1038/362127a0. 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- Kurachi Y. G protein regulation of cardiac muscarinic potassium channel. American Journal of Physiology. 1995;269:C821–830. doi: 10.1152/ajpcell.1995.269.4.C821. [DOI] [PubMed] [Google Scholar]
- Lesage F, Guillemare E, Fink M, Duprat F, Heureaux C, Fosset M, Romey G, Barhanin J, Lazdunski M. Molecular properties of neuronal G-protein-activated inwardly rectifying K+ channels. Journal of Biological Chemistry. 1995;270:28660–28667. doi: 10.1074/jbc.270.48.28660. 10.1074/jbc.270.48.28660. [DOI] [PubMed] [Google Scholar]
- Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. The βγ-subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987;325:321–326. doi: 10.1038/325321a0. 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- Luchian T, Dascal N, Dessauer C, Platzer D, Davidson N, Lester HA, Schreibmayer W. A C-terminal peptide of the GIRK1 subunit directly blocks the G protein-activated K+ channel (GIRK) expressed in Xenopus oocytes. The Journal of Physiology. 1997;505:13–22. doi: 10.1111/j.1469-7793.1997.013bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murer G, Adelbrecht C, Lauritzen I, Lesage F, Lazdunski M, Agid Y, Raisman-Vozari R. An immunocytochemical study on the distribution of two G-protein-gated inward rectifier potassium channels (GIRK2 and GIRK4) in the adult rat brain. Neuroscience. 1997;80:345–357. doi: 10.1016/s0306-4522(97)00001-8. 10.1016/S0306-4522(97)00001-8. [DOI] [PubMed] [Google Scholar]
- Nair LA, Inglese J, Stoffel R, Koch WJ, Lefkowitz RJ, Kwatra MM, Grant AO. Cardiac muscarinic potassium channel activity is attenuated by inhibitors of G beta gamma. Circulation Research. 1995;76:832–838. doi: 10.1161/01.res.76.5.832. [DOI] [PubMed] [Google Scholar]
- Neer EJ. Intracellular signalling: turning down G-protein signals. Current Biology. 1997;7:R31–33. doi: 10.1016/s0960-9822(06)00014-5. 10.1016/S0960-9822(06)00014-5. [DOI] [PubMed] [Google Scholar]
- Pfaffinger PJ, Martin JM, Hunter DD, Nathanson NM, Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985;317:536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- Reuveny E, Slesinger PA, Inglese J, Morales JM, Iniguez-Lluhi JA, Lefkowitz RJ, Bourne HR, Jan YN, Jan LY. Activation of the cloned muscarinic potassium channel by G protein βγ subunits. Nature. 1994;370:143–146. doi: 10.1038/370143a0. 10.1038/370143a0. [DOI] [PubMed] [Google Scholar]
- Saitoh O, Kubo Y, Miyatani Y, Asano T, Nakata H. RGS8 accelerates G-protein-mediated modulation of K+ currents. Nature. 1997;390:525–529. doi: 10.1038/37385. 10.1038/37385. [DOI] [PubMed] [Google Scholar]
- Sakmann B, Noma A, Trautwein W. Acetylcholine activation of single muscarinic K+ channels in isolated pacemaker cells of the mammalian heart. Nature. 1983;303:250–253. doi: 10.1038/303250a0. [DOI] [PubMed] [Google Scholar]
- Schreibmayer W, Dessauer CW, Vorobiov D, Gilman AG, Lester HA, Davidson N, Dascal N. Inhibition of an inwardly rectifiying K+ channel by G-protein α-subunits. Nature. 1996;380:624–627. doi: 10.1038/380624a0. 10.1038/380624a0. [DOI] [PubMed] [Google Scholar]
- Siderovski DP, Hessel A, Chung S, Mak TW, Tyers M. A new family of regulators of G-protein-coupled receptors. Current Biology. 1996;6:211–212. doi: 10.1016/s0960-9822(02)00454-2. 10.1016/S0960-9822(02)00454-2. [DOI] [PubMed] [Google Scholar]
- Slesinger PA, Stoffel M, Jan YN, Jan LY. Defective gamma-aminobutyric acid type B receptor-activated inwardly rectifying K+ currents in cerebellar granule cells isolated from weaver and Girk2 null mutant mice. Proceedings of the National Academy of Sciences of the USA. 1997;94:12210–12217. doi: 10.1073/pnas.94.22.12210. 10.1073/pnas.94.22.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow BE, Hall RA, Krumins AM, Brothers GM, Bouchard D, Brothers CA, Chung S, Mangion J, Gilman AG, Lefkowitz RJ, Siderovski DP. GTPase activating specificity of RGS12 and binding specificity of an alternatively spliced PDZ (PSD-95/Dlg/ZO-1) domain. Journal of Biological Chemistry. 1998;273:17749–17755. doi: 10.1074/jbc.273.28.17749. 10.1074/jbc.273.28.17749. [DOI] [PubMed] [Google Scholar]
- Soejima M, Noma A. Mode of regulation of the ACh-sensitive K-channel by the muscarinic receptor in rabbit atrial cells. Pflügers Archiv. 1984;400:424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]
- Sui JL, Jérôme P-J, Logothetis DE. Activation of the atrial KACh channel by the βγ subunits of G proteins or intracellular Na+ ions depends on the presence of phosphatidylinositol phosphates. Proceedings of the National Academy of Sciences of the USA. 1998;95:1307–1312. doi: 10.1073/pnas.95.3.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesmer JJG, Berman DM, Gilman AG, Sprang SR. Structure of RGS4 bound to AlF4−-activated Giα1: stabilization of the transition state for GTP hydrolysis. Cell. 1997;89:251–261. doi: 10.1016/s0092-8674(00)80204-4. [DOI] [PubMed] [Google Scholar]
- Tu Y, Wang J, Ross EM. Inhibition of brain Gz GAP and other RGS proteins by palmitoylation of G protein alpha subunits. Science. 1997;278:1132–1135. doi: 10.1126/science.278.5340.1132. [DOI] [PubMed] [Google Scholar]
- Wickman KD, Iniguez-Lluhi JA, Davenport PA, Taussing R, Krapivinsy GB, Linder ME, Gilman AG, Clapham DE. Recombinant G-protein βγ-subunits activate the muscarinic-gated atrial potassium channel. Nature. 1994;368:255–257. doi: 10.1038/368255a0. [DOI] [PubMed] [Google Scholar]
- Zerangue N, Jan LY. G-protein signaling: fine-tuning signaling kinetics. Current Biology. 1998;8:R313–316. doi: 10.1016/s0960-9822(98)70196-4. [DOI] [PubMed] [Google Scholar]


