Abstract
We examined interactions between haemodynamic and autonomic neural oscillations during passive upright tilt, to gain better insight into human autonomic regulatory mechanisms.
We recorded the electrocardiogram, finger photoplethysmographic arterial pressure, respiration and peroneal nerve muscle sympathetic activity in nine healthy young adults. Subjects breathed in time with a metronome at 12 breaths min−1 (0.2 Hz) for 5 min each, in supine, and 20, 40, 60, 70 and 80 deg head-up positions. We performed fast Fourier transform (and autoregressive) power spectral analyses and integrated low-frequency (0.05-0.15 Hz) and respiratory-frequency (0.15-0.5 Hz) spectral powers.
Integrated areas of muscle sympathetic bursts and their low- and respiratory-frequency spectral powers increased directly and significantly with the tilt angle. The centre frequency of low-frequency sympathetic oscillations was constant before and during tilt. Sympathetic bursts occurred more commonly during expiration than inspiration at low tilt angles, but occurred equally in expiration and inspiration at high tilt angles.
Systolic and diastolic pressures and their low- and respiratory-frequency spectral powers increased, and R-R intervals and their respiratory-frequency spectral power decreased progressively with the tilt angle. Low-frequency R-R interval spectral power did not change.
The cross-spectral phase angle between systolic pressures and R-R intervals remained constant and consistently negative at the low frequency, but shifted progressively from positive to negative at the respiratory frequency during tilt. The arterial baroreflex modulus, calculated from low-frequency cross-spectra, decreased at high tilt angles.
Our results document changes of baroreflex responses during upright tilt, which may reflect leftward movement of subjects on their arterial pressure sympathetic and vagal response relations. The intensity, but not the centre frequency of low-frequency cardiovascular rhythms, is modulated by the level of arterial baroreceptor input. Tilt reduces respiratory gating of sympathetic and vagal motoneurone responsiveness to stimulatory inputs for different reasons; during tilt, sympathetic stimulation increases to a level that overwhelms the respiratory gate, and vagal stimulation decreases to a level below that necessary for maximal respiratory gating to occur.
Human cardiovascular function can be characterized by steady-state measures of muscle sympathetic nerve activity, arterial pressure, R-R intervals and respiration. Additional information can be obtained from the study of the oscillations of these parameters, as they exist individually, and in relation to each other. The magnitude of oscillations can be gauged with frequency domain methods, including fast Fourier transformation and autoregressive modelling, and the coherence between these measures and their phase relations can be gauged with cross-spectral analysis. We closely examined haemodynamic and autonomic neural periodicities in a group of healthy young volunteers in order to understand better the underlying neurophysiological mechanisms. We used a physiological manoeuvre, precisely graded passive upright tilt, and asked subjects to control one important variable, breathing frequency.
Our results suggest that autonomic changes during progressive upright tilt reflect leftward movement of subjects on their arterial pressure sympathetic and vagal response relations. The changes of oscillatory behaviour we recorded indicate further that the intensity, but not the centre frequency of low-frequency human cardiovascular rhythms is modulated by the level of arterial baroreceptor input. Tilt reduces respiratory gating of sympathetic and vagal motoneurone responsiveness to stimulatory inputs. Sympathetic gating may be reduced because increases of sympathetic stimulation overwhelm respiratory influences, and vagal gating may be reduced because vagal stimulation declines to a level below that necessary for maximal respiratory gating to occur.
METHODS
Subjects
We studied nine healthy volunteers (seven men and two women; age 29.5 ± 2.8 years (mean ±s.e.m.); height 169.5 ± 5.9 cm; weight 68.6 ± 4.5 kg) in the morning, after they abstained from caffeine and exercise for 24 h, and had not eaten for at least 3 h. All subjects were non-smokers who had never fainted, and were taking no medications. This study was approved by the human research committees of the Hunter Holmes McGuire Department of Veterans Affairs Medical Centre and the Medical College of Virginia at Virginia Commonwealth University. All subjects gave their written, informed consent prior to participating.
Measurements
We recorded data on digital tape, and subsequently re-digitized them at 500 Hz with commercial hardware and software (WINDAQ, Dataq Instruments, Akron, OH, USA). We continuously measured electrocardiographic R-R intervals, respiration (uncalibrated pneumobelt), and finger photoplethysmographic arterial pressure (Finapres, Model 2300, Ohmeda, Englewood, CO, USA). The subject's left arm was placed in a sling with the hand at heart level at all tilt angles. End-tidal carbon dioxide (CO2) concentrations were measured on a breath-by-breath basis at the mouth (Infrared Analyser, Gambro, Engström, Sweden).
We recorded muscle sympathetic nerve activity directly (Nerve Traffic Analyser, Model 662C-1, University of Iowa Bioengineering, Iowa City, IA, USA), as described previously (Wallin & Eckberg, 1982). Briefly, multifibre efferent sympathetic traffic from peroneal nerve muscle fascicles was led off with tungsten microelectrodes with uninsulated tip diameters of about 2 μm. A reference electrode was inserted subcutaneously 1-2 cm from the recording electrode. Both electrodes were connected to a differential pre-amplifier, and then to an amplifier (total gain of 70 000) where the nerve signal was band-pass filtered (700-2000 Hz), and integrated (time constant, 0.1 s) to obtain mean voltage neurograms. Satisfactory recordings of muscle sympathetic nerve activity were defined by pulse-synchronous bursts that increased during end-expiratory apnoea or Valsalva straining, and did not change during tactile or auditory stimulation. All nerve recordings were made with the subject's weight-bearing foot resting on a block, such that during tilt, the foot of the recording leg did not touch the footrest of the tilt table.
Experimental protocol and data analysis
We recorded data for 5 min each, during supine and 20, 40, 60, 70 and 80 deg head-up tilt, with subjects breathing with a metronome at 12 breaths min−1 (0.2 Hz). We analysed sympathetic nerve activity with custom programs developed for use with commercial hardware and software (WINDAQ, Dataq Instruments, Akron, OH, USA). We automatically detected sympathetic bursts with signal-to-noise ratios > 3:1, and time-to-peak burst latencies from preceding R waves of about 1.3 s (Fagius et al. 1987). One observer manually over-read results of automated analyses. The integral of all sympathetic bursts occurring during the initial 5 min supine rest period was divided by the number of bursts to derive an average control burst area. The area of each sympathetic burst recorded subsequently was divided by this number to derive normalized burst areas. (For example, if the area of a burst was exactly equal to the average control burst, it would be assigned a value of 1.0.) We expressed sympathetic nerve activity as bursts per minute, average normalized burst area, and total activity (bursts per minute multiplied by average normalized burst area). We gauged respiratory influences on sympathetic motoneurone firing by measuring the number and integrated areas (advanced by 1.3 s to account for peripheral conduction delays; Fagius et al. 1987) during early-inspiration to early-expiration ‘inspiration’, and early-expiration to early-inspiration ‘expiration’.
We analysed R-R intervals, muscle sympathetic nerve activity, and arterial pressure in the frequency domain as follows. We registered each R wave and peak systolic pressure at their times of occurrence in the data stream. The non-equidistant waveforms were then spline interpolated (cubic), resampled at 4 Hz, and passed through a finite low-pass impulse response filter with a cut-off frequency of 0.5 Hz. Data sets comprising 64 s (256 samples), sliding in 10 s steps, were fast Fourier transformed. Power spectral densities were calculated with the Welch algorithm for seven overlapping sections of 256 points (64 s) staggered by 128 points. Spectral power was expressed as the integrated areas in low (0.05-0.15 Hz), respiratory (0.15-0.5 Hz), and total (0.05-0.5 Hz) frequency ranges. Muscle sympathetic nerve spectral power was characterized both as oscillations of frequency and integrated burst areas.
We calculated the squared coherence between each of the pairs of measurements by dividing the cross-spectral densities by the product of the individual power spectral densities, and the phase angle by multiplying the tangent by the quotient of the coincident and the quadrature spectral density functions. The square root of the ratio between the R-R interval and systolic pressure variabilities was calculated for those subjects whose squared coherence was > 0.5 (de Boer et al. 1985), and was used as a measure of baroreflex gain (α, at low frequencies, within the range 0.05-0.15 Hz). We also used autoregressive modelling to determine the centre frequencies of oscillations, with automatic selection of the model to minimize Akaike's final prediction of merit (Akaike, 1969). Finally, we performed cross-spectral analysis on R-R interval and systolic pressure time series without smoothing or resampling to determine what influence, if any, our data treatment had on phase relations.
Statistical analysis
We performed statistical tests with commercial (SAS Institute, Cary, NC, USA) and in-house software (bend point analysis). Dependent variables were evaluated for effects due to tilt angle with a one-way repeated measures analysis of variance (ANOVA; general linear model). The influence of respiratory phase on muscle sympathetic nerve activity was evaluated with a two-way repeated measures ANOVA (breath phase by tilt angle). Significant global effects were probed further with the Duncan multiple range test (α level adjustments were made to account for numerous pair-wise comparisons). Significant interactions were evaluated using analysis of simple main effects. The point of pre-syncope was defined with a statistical program to calculate a ‘bend point’, based on iterative least-squares linear regression analysis as described previously (Eckberg, 1979). We used linear regression analysis to gauge relations between variables and the sine of the tilt angle (which reflects the body axis component of gravity; Iwase et al. 1987). Differences between means were considered significant when P < 0.05.
RESULTS
Three of the nine subjects experienced pre-syncope during tilt. Pre-syncope occurred 175 and 225 s into the 80 deg tilt stage for subjects 1 and 7, and 200 s into the 60 deg tilt stage for subject 3. Our analysis of responses to tilt for these subjects includes only data obtained prior to pre-syncope (defined by a significant reduction of arterial pressure as calculated by the bend point analysis; Eckberg, 1979). The remaining subjects tolerated all stages of tilt; however, tilt dislodged the recording electrode at 70 deg in subject 2, and at 80 deg in subject 6. Because of this, data from all nine subjects are included from the supine position to 60 deg tilt, and data from seven and six subjects are included for 70 and 80 deg tilt. All data are given as means ±s.e.m., unless specified otherwise.
Table 1 summarizes all measurements. Subjects controlled their respiratory rates well in supine and tilted positions (P = 0.21). Progressive head-up tilt reduced R-R intervals (P = 0.0001), and increased systolic and diastolic pressures (both P = 0.0001). Average end-tidal CO2 concentrations decreased moderately (from 5.0 to 3.9%), and significantly (P = 0.0001) with tilt.
Table 1.
Cardiovascular and respiratory variables during head-up tilt
| Degrees of tilt | Supine | 20 deg | 40 deg | 60 deg | 70 deg | 80 deg |
|---|---|---|---|---|---|---|
| Number of subjects | 9 | 9 | 9 | 9 | 7 | 6 |
| R–R intervals (s) | 1.06 ± 0.18 | 0.98 ± 0.17 | 0.88 ± 0.15 | 0.79 ± 0.12 | 0.81 ± 0.12 | 0.75 ± 0.14 |
| A | AB | BC | CD | CD | D | |
| Systolic pressure (mmHg) | 115.9 ± 13.6 | 122.7 ± 12.6 | 129.3 ± 14.8 | 137.5 ± 12.6 | 138.4 ± 13.8 | 140.6 ± 16.2 |
| A | B | B | C | C | C | |
| Diastolic pressure (mmHg) | 61.6 ± 6.9 | 68.9 ± 7.8 | 77.7 ± 7.9 | 84.6 ± 8.5 | 87.6 ± 9.8 | 89.9 ± 10.3 |
| A | B | C | D | DE | E | |
| Mean pressure (mmHg) | 79.7 ± 7.9 | 86.8 ± 8.4 | 94.9 ± 8.9 | 102.3 ± 8.7 | 104.5 ± 9.3 | 106.8 ± 10.1 |
| A | B | C | D | DE | E | |
| CO2 (%) | 5.1 ± 0.79 | 4.7 ± 0.94 | 4.6 ± 0.99 | 4.4 ± 0.99 | 4.1 ± 1.04 | 3.9 ± 1.20 |
| A | AB | B | B | C | C | |
| Breath number (breaths min−1) | 12.1 ± 0.67 | 12.2 ± 0.86 | 12.3 ± 0.76 | 12.1 ± 0.58 | 12.2 ± 0.68 | 11.8 ± 0.74 |
| A | A | A | A | A | A |
Values are means ± s.d.; means with the same letter are not significantly different across tilt angles.
Muscle sympathetic nerve activity
Figure 1 depicts original arterial pressure and muscle sympathetic nerve recordings (without latency corrections) from one subject after a 1 min stabilization period, at 0 and 60 deg tilt. In this and other subjects, head-up tilt increased arterial pressure, arterial pressure oscillations, muscle sympathetic nerve activity and muscle sympathetic nerve activity oscillations.
Figure 1. Experimental record from one subject.

Representative arterial pressure and muscle sympathetic nerve tracings from one subject during supine and 60 deg passive upright tilt.
Figure 2 depicts average changes of muscle sympathetic nerve activity at all tilt angles. Upright tilt significantly increased the number of sympathetic bursts per minute, average normalized integrated sympathetic burst areas, and total muscle sympathetic nerve activity. Increases of all three measures correlated significantly with the sine of the tilt angle. The absolute number of sympathetic bursts increased significantly at 40, 60, 70 and 80 deg (ANOVA, P = 0.0001). Normalized burst areas and total muscle sympathetic nerve activity increased significantly at 60, 70 and 80 deg (ANOVA, P = 0.0009), compared with supine measurements. The time to the peak of bursts from the preceding R-waves (not shown) decreased significantly, from 1.52 ± 0.12 s in the supine position, to 1.10 ± 0.03 s at 80 deg (ANOVA, P = 0.0002), and correlated significantly with the sine of the tilt angle (r = -0.56, P = 0.0001).
Figure 2. Average muscle sympathetic nerve activity for all subjects.
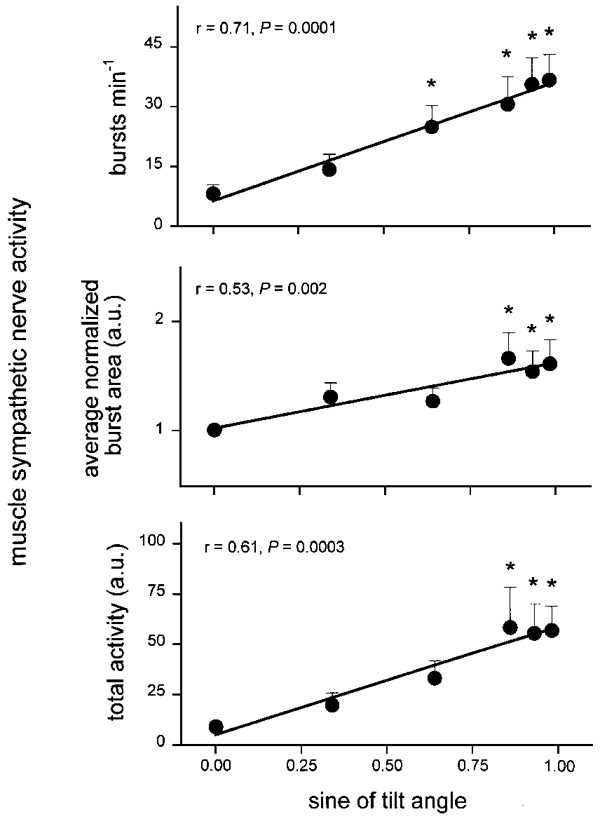
Mean ±s.e.m. changes of muscle sympathetic nerve activity as a function of the sine of the tilt angle. * Significantly different from supine.
Muscle sympathetic nerve frequency spectral power (the variability in the frequency of muscle sympathetic bursts) did not change significantly with tilt in either low-frequency or respiratory-frequency ranges (not shown, ANOVA, P = 0.06 and 0.08). Furthermore, the spectral power of sympathetic burst frequency did not correlate with the sine of the tilt angle in either low- or respiratory-frequency ranges (r = -0.21 and -0.04, P = 0.14 and 0.75). That is, although the frequency of sympathetic bursts increased with the tilt angle (Fig. 2), fluctuations of the frequency of sympathetic bursts at low and respiratory frequencies did not change significantly.
Figure 3 shows spectral power of the integrated areas of sympathetic bursts (which reflect the number firings of sympathetic motoneurones within bursts; Macefield et al. 1994). In Figs 3, 5, 6, 7 and 8, significant regressions are shown in the lower graphs as thick lines, and non-significant relations are shown as thin lines. Muscle sympathetic nerve area spectral power increased significantly at 60, 70 and 80 deg compared with supine levels, in both low- and respiratory-frequency ranges (ANOVA, P = 0.007), and correlated significantly with the sine of the tilt angle.
Figure 3. Muscle sympathetic nerve area spectral power.
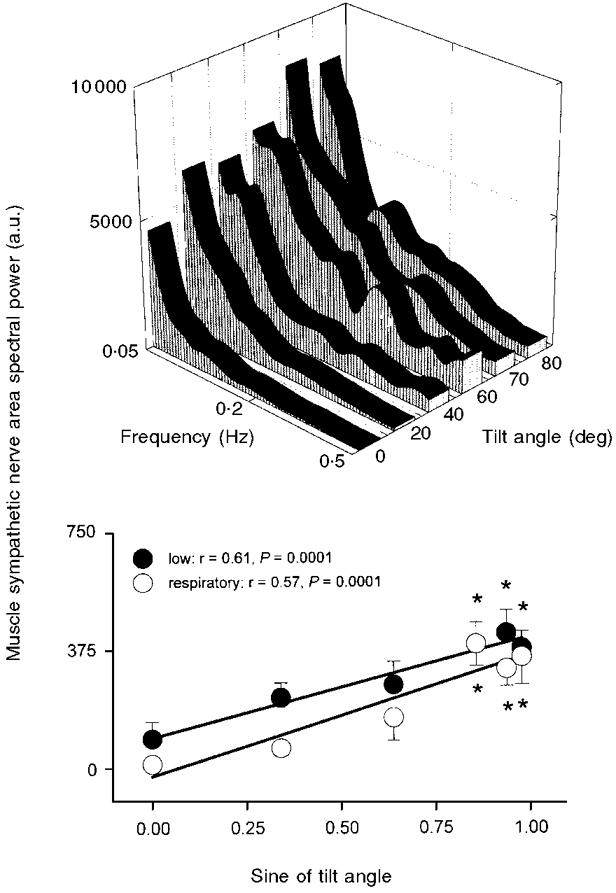
Average muscle sympathetic nerve area spectral power at each tilt angle (top panel), and integrated spectral power in low- and respiratory-frequency bands, plotted as functions of the sine of tilt angle (bottom panel). a.u., arbitrary units. Bottom panel: thick lines denote significant regressions. r, correlation coefficient derived from least-squares linear regression. * Significantly different from supine.
Figure 5. Systolic pressure spectral power.
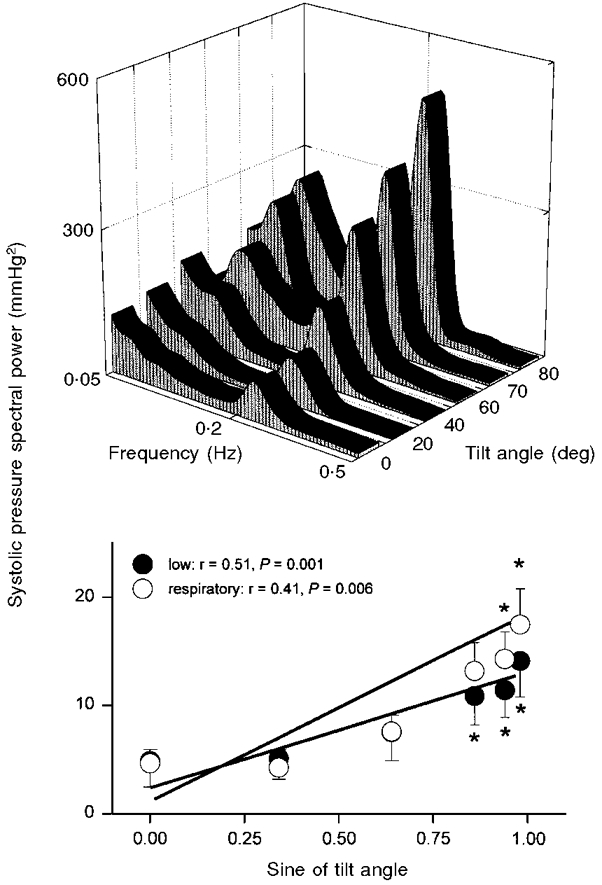
Average systolic pressure spectral power at each tilt angle (top panel), and at low- and respiratory-frequency bands, plotted as functions of the sine of tilt angle (bottom panel). Bottom panel: thick lines denote significant regression. * Significantly different from supine.
Figure 6. Diastolic pressure spectral power.
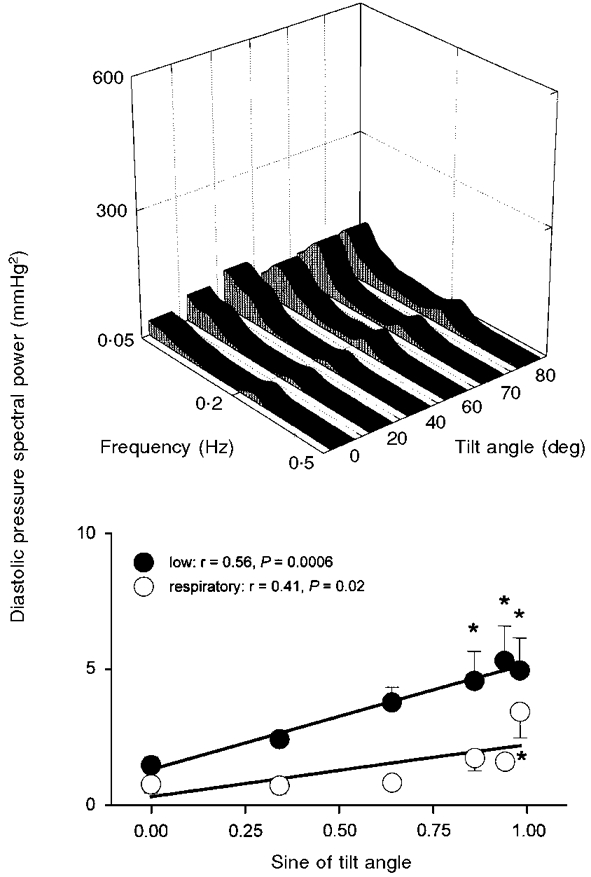
Average diastolic pressure spectral power at each tilt angle (top panel), and at low- and respiratory-frequency bands, plotted as functions of the sine of tilt angle (bottom panel). Bottom panel: thick lines denote significant regression. * Significantly different from supine.{
Figure 7. R-R interval spectral power.
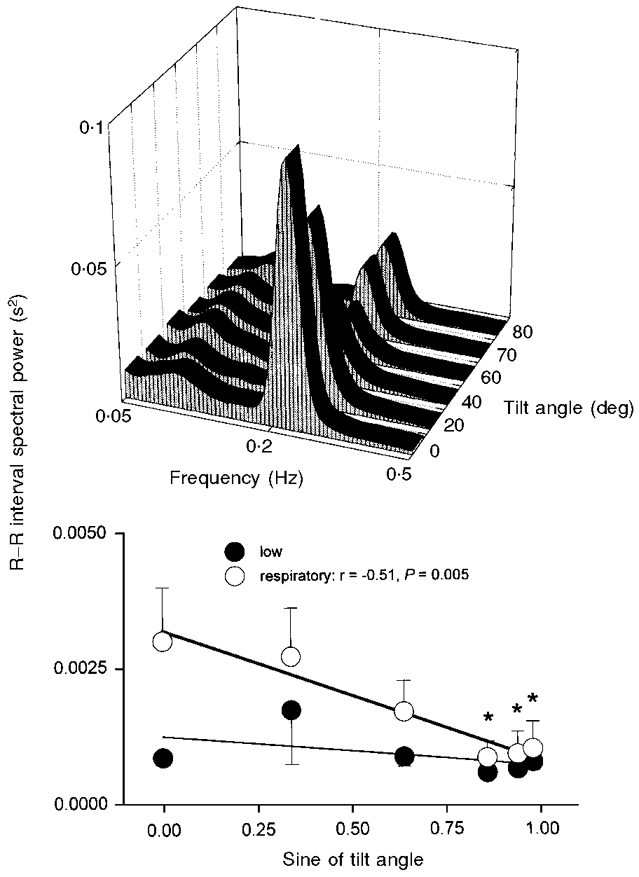
Average R-R interval spectral power at each tilt angle (top panel), and at low- and respiratory-frequency bands, plotted as functions of the sine of tilt angle (bottom panel). Bottom panel: thick line, significant regression; thin line, insignificant regression. * Significantly different from supine.
Figure 8. Systolic pressure and R-R interval phase angles.
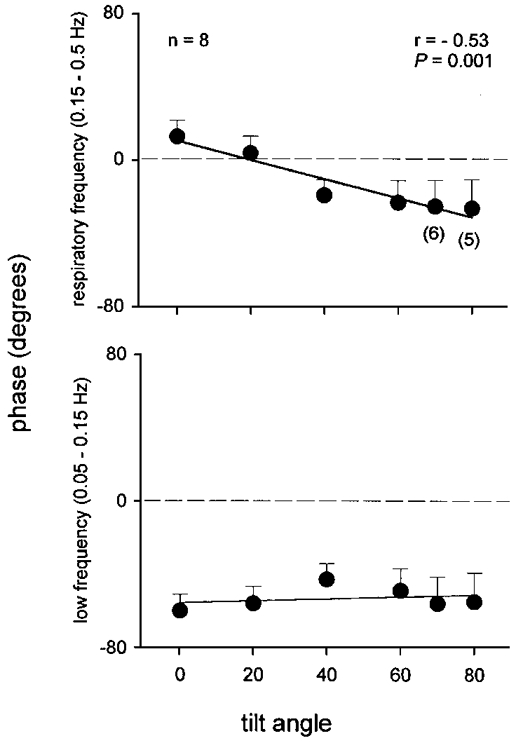
Average phase angles between systolic pressures and R-R intervals, derived from cross-spectral analysis in subjects whose squared coherence was > = 0.50. Numbers in parentheses indicate the number of subjects at higher tilt angles. Thick line, significant regression; thin line, insignificant regression.
Figure 4 shows the distribution of sympathetic burst frequency (upper panel), and the percentage of all bursts occurring at each tilt angle (lower panel) during inspiration and expiration (see Methods). Sympathetic burst frequency (bursts min−1) increased in both inspiration and expiration during tilt (ANOVA, P = 0.0001). Sympathetic burst frequency was significantly greater during expiration than inspiration at 0, 20 and 40 deg (Fig. 4, upper panel; ANOVA, P = 0.04). Similarly, the percentage of sympathetic bursts was greater during expiration than inspiration at 0, 20 and 40 deg (Fig. 4, lower panel; ANOVA, P = 0.05). Burst frequency and the percentage of sympathetic bursts was distributed evenly between expiration and inspiration at higher tilt angles.
Figure 4. Inspiratory/expiratory distribution of muscle sympathetic nerve activity.
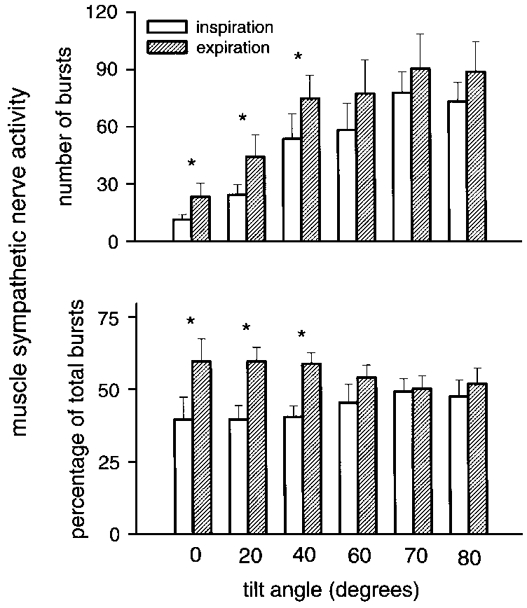
Respiratory gating of muscle sympathetic nerve activity during inspiration and expiration for all subjects. * Significant difference between breath phases.
As with burst frequency, sympathetic burst area (not shown) was significantly greater in both inspiration and expiration during tilt (ANOVA, P = 0.0001). Sympathetic burst area was significantly greater during expiration than inspiration at 0, 20 and 40 deg (ANOVA, P = 0.05). Similarly, total sympathetic burst area was significantly greater in expiration than inspiration at 0, 20 and 40 deg (ANOVA, P = 0.04). Burst area was distributed evenly between expiration and inspiration at higher tilt angles.
Arterial pressure and R-R intervals
Figures 5 and 6 show that both low- and respiratory-frequency systolic and diastolic pressure spectral powers correlated directly and significantly with the sine of the tilt angle. Systolic pressure spectral power (Fig. 5) increased at the respiratory frequency at 70 and 80 deg (ANOVA, P = 0.03), and at low frequencies at 60, 70 and 80 deg (ANOVA, P = 0.002), compared with supine measurements. Diastolic pressure spectral power (Fig. 6) - which was substantially less than systolic pressure spectral power - increased at low frequencies at 60, 70 and 80 deg (ANOVA, P = 0.004) and at the respiratory frequency at 80 deg (ANOVA, P = 0.05), compared with supine measurements. Average arterial pressure centre frequencies were 0.09 ± 0.005 Hz for low frequencies, and 0.22 ± 0.002 Hz for respiratory frequencies, and were not significantly affected by tilt (P = 0.62 and 0.31).
Figure 7 shows average R-R interval spectral power at all tilt angles. R-R interval spectral power at the low frequency was unaffected by tilt (ANOVA, P = 0.42). R-R interval spectral power at the respiratory frequency decreased significantly at 60, 70 and 80 deg tilt (ANOVA, P = 0.003). R-R interval centre frequencies were 0.09 ± 0.004 Hz for low frequencies, and 0.22 ± 0.002 Hz for respiratory frequencies, and did not change significantly during tilt (P = 0.62 and 0.31).
Figure 8 shows average phase angles derived from systolic pressure and R-R interval cross spectral analysis (n = 8 from supine to 60 deg, n = 6 at 70 deg, and n = 5 at 80 deg). The systolic pressure and R-R interval phase angle at the low frequency averaged -53 ± 11 deg, was not affected by tilt (P = 0.70), and did not correlate with the tilt angle (r = 0.20). The systolic pressure and R-R interval phase angle at the respiratory frequency shifted significantly (P = 0.001) from positive to negative with tilt, and correlated with the tilt angle (P = 0.001). The average phase angle at both low and respiratory frequencies was similar (P = 0.63 and 0.48) without smoothing, windowing, and resampling. We found significant coherence between muscle sympathetic nerve activity and diastolic pressure in only three subjects; in these, coherence was not significant at all tilt angles.
Figure 9 shows baroreflex gain, as reflected by the square root of the ratios of R-R interval and systolic pressure spectral powers in regions where squared coherence was ≥0.50 (see Methods). Baroreflex gain decreased significantly at 60, 70 and 80 deg (P = 0.0001) compared with supine, and 20 and 40 deg tilt levels.
Figure 9. Cross-spectral baroreflex modulus.

Average spontaneous baroreflex gain derived from cross-spectral analysis in subjects with significant squared coherence. * Significantly different from 0, 20 and 40 deg.
DISCUSSION
We measured autonomic neural and haemodynamic responses of healthy young men and women to graded passive head-up tilt during controlled frequency breathing. Our data indicate that tilt progressively increases average muscle sympathetic nerve activity and systolic and diastolic pressures, and their spectral powers at low and respiratory frequencies. Conversely, tilt progressively decreases vagal baroreflex gain and vagal cardiac nerve activity, as reflected by R-R intervals and respiratory-frequency R-R interval spectral power, but does not alter low-frequency R-R interval spectral power. Our observations support a variety of inferences.
Human responses to upright posture
Assumption of upright posture leads to displacement of blood towards the lower body, and sets in motion a cascade of haemodynamic and autonomic adjustments. Right and left ventricular stroke volumes decline, heart rate increases secondary to vagal withdrawal and sympathetic stimulation; and muscle sympathetic nerve activity and forearm vascular resistance increase.
In agreement with Iwase et al. (1987), we documented increases of muscle sympathetic nerve activity in proportion to the tilt angle (Fig. 2). We showed further that as the degree of tilt increases, the areas and amplitudes of bursts increase (Figs 1 and 2), and the latencies of bursts from prior R waves decrease. Wallin and his co-workers (1994) also documented an inverse relation between muscle sympathetic burst amplitude and R wave - burst latency, and suggested two possible mechanisms: reductions of central synaptic delays and recruitment of sympathetic motoneurones with faster conduction velocities. Our study shows that progressive increases of burst areas provoked physiologically by graded tilt also are associated with progressive, major (by 28%) reductions of sympathetic burst latencies.
Our subjects, like others reported earlier (Asmussen, 1943; Morillo et al. 1997), increased their arterial pressures during passive upright tilt (Table 1). This response raises the question: what receptors mediate autonomic responses to upright posture? It is highly likely that arterial baroreceptors figure prominently, and probably prepotently, in these adjustments. During mild lower body negative pressure, intracarotid artery pressure, diameter (Lacolley et al. 1992), and presumably carotid baroreceptor firing decline, according to the reduction of the hydrostatic gradient, by about 20-25 cmH2O. Moreover, arterial baroreceptor denervation leads to orthostatic hypotension in animals (Cowley et al. 1973) and humans (Holton & Wood, 1965) (but cardiopulmonary denervation does not; Banner et al. 1990). Therefore, absence of arterial pressure decreases, and even arterial pressure increases, do not exclude major contributions from arterial baroreceptors to the autonomic adjustments that occur during upright tilt.
Low-frequency rhythms
In 1877, Mayer published a study conducted in spontaneously breathing rabbits, which documented arterial pressure waves at frequencies slower than respiration. Subsequent reports confirmed the observation that Mayer waves are slower than respiration (Cherniack et al. 1969), and are associated with waxing and waning of vascular resistance and sympathetic and vagal nerve activities (Koizumi et al. 1984). Mayer waves may not be present in animals, unless they are caused by laboratory manoeuvres, including haemorrhage, carotid artery occlusion, metabolic acidosis, or elevation of subarachnoid pressure. Therefore, humans, who usually have such low-frequency cardiovascular rhythms at rest (Eckberg et al. 1985), may be more appropriate for study of low-frequency rhythms than animals.
Two theories have been put forward to explain low-frequency cardiovascular rhythms. The resonance theory, propounded by Guyton & Harris (as the ‘oscillator hypothesis’ (1951)) and de Boer and co-workers (1987), is based primarily on the time constants of effector responses to neuronally released noradrenaline. Sinoatrial node and arteriolar responses to brief sympathetic stimulation develop and dissipate slowly, with a total period of about 10 s (Wallin & Nerhed, 1982). Therefore, a single sympathetic burst or a brief volley of sympathetic bursts can initiate a cycle of rising and falling arterial pressures, and decreasing and increasing sympathetic nerve activities, at about 0.1 Hz.
The second theory is that low-frequency cardiovascular rhythms are driven by a pacemaker. Low-frequency sympathetic rhythms persist in anaesthetized dogs when arterial pressure fluctuations are abolished (Preiss & Polosa, 1974), and therefore cannot represent exclusively reactions to arterial pressure changes. It is unclear where a low-frequency pacemaker might be located. Since low-frequency sympathetic rhythms are present in animals after acute cervical spinal cord transection (Fernandez de Molina & Perl, 1965), and since low-frequency arterial pressure and R-R interval rhythms are present in patients with chronic complete cervical spinal cord lesions (Guzzetti et al. 1994; Koh et al. 1994), the pacemaker responsible for low-frequency rhythms may be in the spinal cord. Evidence from humans regarding this possibility is inconclusive. Although muscle sympathetic nerve activity has been recorded in patients with chronic cervical spinal cord injuries (Stjernberg et al. 1986), its frequency has not been determined. It is not known if muscle sympathetic nerve activity in tetraplegic patients is organized with a periodicity of about 0.1 Hz.
Our results provide some new insights into low-frequency human autonomic rhythms. We found that the magnitude, but not the centre frequency of low-frequency muscle sympathetic nerve and arterial pressure spectral powers increases directly with the tilt angle (Figs 3, 5 and 6). Although these results do not permit us to say where a putative low-frequency pacemaker might be, they indicate that its firing frequency is independent of the level of baroreceptor input.
In most subjects, the phase angle between low-frequency systolic pressure and R-R interval spectral powers was significant (squared coherence ≥0.50), and remarkably constant, at about -53 deg (Fig. 8, lower panel). The mean phase angle of -53 deg at 0.09 Hz (period: 11.1 s), represents a phase lag of 1.6 s. This phase relation is almost identical to the lag between an abrupt increase of carotid distending pressure and maximum R-R interval prolongation (Baskerville et al. 1979). Thus the nearly fixed phase relation identified by cross-spectral analysis of low-frequency systolic pressures and R-R intervals is compatible with a simple baroreflex mechanism. Although cross-spectral analysis provides no information regarding which signal precedes which, it is highly unlikely that at low frequencies, R-R interval changes precede (and therefore cause) arterial pressure changes (with a latency of about 11.1 minus 1.6, or 9.5 s). Moreover, fixed rate atrial pacing does not alter low-frequency arterial pressure rhythms in healthy humans (Taylor et al. 1998).
Not surprisingly, baroreflex gain, as estimated by the modulus of steadily increasing systolic pressure, and constant R-R interval spectral powers (Figs 5 and 7), declined in proportion to the tilt angle (Fig. 9). Steptoe & Vögele (1990) also documented a reduction of baroreflex gain when subjects change from lying to standing positions. Our data do not indicate why vagal baroreflex gain declines with tilt. One possibility is that the subjects we studied moved from the linear to the threshold regions of their systolic pressure and R-R interval response relations (Eckberg, 1980).
In agreement with Mukai & Hayano (1995), we found no change of low-frequency R-R interval spectral power during upright tilt (Fig. 7). Since tilt clearly increases low-frequency fluctuations of muscle sympathetic nerve activity (Fig. 3), as well as their absolute levels (Fig. 2), our data provide a strong argument against use of low-frequency R-R interval spectral power as a non-invasive index of sympathetic nerve activity (Pagani et al. 1984).
We cannot be certain why low-frequency R-R interval spectral power does not increase in response to steadily increasing fluctuations of muscle sympathetic nerve activity. Transfer function analysis before and after β-adrenergic blockade (Saul et al. 1991) indicates that sympathetically mediated R-R interval changes can occur in response to arterial fluctuations as rapid as 0.1 Hz. Constant levels of low-frequency R-R interval spectral power at all tilt angles (Fig. 7) may reflect the prepotence of efferent vagal cardiac nerve activity. Although small changes of baroreceptor input trigger reciprocal changes of sympathetic and vagal cardiac nerve activities (Rea & Eckberg, 1987), the net effect of such neural changes on sinoatrial function may not be their algebraic sum. The presence of any vagal cardiac nerve traffic may nullify sympathetic influences on the sinoatrial node (Samaan, 1935).
Respiratory-frequency rhythms
In our study, respiratory fluctuations of muscle sympathetic nerve activity (Figs 3 and 4) and systolic and diastolic pressures (Figs 5 and 6) increased, and respiratory fluctuations of R-R intervals decreased (Fig. 7), in proportion to the tilt angle. Our study sheds light on all of these changes.
Respiration gates responsiveness (Lopes & Palmer, 1976) of vagal (Eckberg et al. 1980; Gilbey et al. 1984) and sympathetic (Eckberg et al. 1985) motoneurones, such that firing of both groups in response to stimulatory inputs is greater in expiration than inspiration. Our study documents inspiratory-expiratory differences in sympathetic firing in the supine position and at low tilt angles (Fig. 4), and disappearance of inspiratory-expiratory differences at high tilt angles (Fig. 4). These observations suggest that the ability of respiration to gate sympathetic motoneurone responsiveness to stimulation is finite, and can be overcome. An earlier study in humans (Eckberg & Orshan, 1977) showed that respiratory gating of vagal motoneurone responsiveness also disappears when baroreceptor stimulation is intense.
We showed (Figs 5 and 6), as did Dornhorst et al. (1952), that upright posture increases respiratory fluctuations of arterial pressure. At least two mechanisms may contribute to greater fluctuations of arterial pressure in the upright position than in the supine position. First, it is likely that downward displacement of the diaphragm augments reductions of intrathoracic pressure during inspiration, and thereby augments inspiratory reductions of left ventricular stroke volumes (Guz et al. 1987). Second, since in supine healthy humans respiratory frequency R-R interval fluctuations damp arterial pressure fluctuations (Taylor & Eckberg, 1996), progressive decreases of R-R interval fluctuations (Fig. 7) may contribute to progressive increases of arterial pressure fluctuations at respiratory frequencies.
De Boer and co-workers (1985, 1987) reported systolic pressure and R-R interval phase angles of about 0 deg in supine subjects, and proposed that respiratory frequency R-R interval fluctuations reflect simple baroreflex physiology - arterial pressure changes provoke R-R interval changes. These authors argued that a baroreflex mechanism at respiratory frequencies is plausible, given the extraordinarily short latency between arterial baroreceptor stimuli and vagally mediated R-R interval lengthening (as short as 0.24 s; Eckberg, 1976). Although our data are very similar, we interpret them differently. First, we found that in the supine position, R-R intervals lead arterial pressure changes, by an average of 12 ± 10.3 deg, or 0.15 s at a breathing frequency of 0.22 Hz (Fig. 8, upper panel). Vagal baroreflex responses require some time to develop, and no alacrity of responsiveness would enable vagal motoneurones to respond to arterial pressure changes before they occur. To be certain, arterial pressure might lead R-R intervals by the latency we measured, plus an entire breathing cycle, in our study, 4.55 s (or a total latency of 4.7 s). This possibility is unlikely; after impulse baroreceptor stimuli, R-R intervals decline to baseline levels by about 3.0 s (Baskerville et al. 1979).
Second, the negative phase angles registered at higher degrees of tilt are small, and therefore also unlikely to reflect baroreflex physiology. At the highest tilt angle, the latency between systolic pressure and R-R interval changes is only -27 deg (or 0.34 s). This latency is probably too short for any serious vagal baroreflex response to be mounted. Seidel and colleagues (1997) closely analysed their own data and data published earlier, and concluded that the minimum human baroreceptor cardiac reflex latency is about 0.4 s. Moreover, the phase angles we measured are between systolic pressures and R-R intervals. We have not measured phase angles between systolic pressures and P waves, which are the critical values. If we had corrected latencies by subtracting the P-R interval (which we did not measure), the calculated latencies would be even smaller. For example, if our subjects had P-R intervals of 0.15 s, the actual average phase lead in the supine position would be 0.3 s, instead of 0.15 s, and the average phase lag for all positions would be -0.01 s, instead of -0.19 s.
Third, the variability of phase angles at different positions (Fig. 8, upper panel) argues against a unitary baroreflex explanation for respiratory frequency R-R interval changes. These variable phase angles stand in sharp contrast with the nearly constant phase angle at low frequencies. Moreover, it is unclear why the baroreceptor cardiac reflex should have a latency of 1.6 s at low frequencies, and a latency averaging 0.18 s at respiratory frequencies. Fourth, fixed rate atrial pacing (Taylor & Eckberg, 1996) and large doses of atropine (Saul et al. 1991; Toska & Eriksen, 1993) significantly increase respiratory frequency systolic pressure fluctuations. This suggests that R-R interval fluctuations modulate systolic pressure fluctuations, rather than the reverse. Finally, Cerutti and her co-workers (1994) studied conscious rats and reported that sinoaortic denervation abolishes the coherence between mean arterial pressure and heart rate at frequencies believed to correspond with low frequencies in humans, but does not affect coherence at respiratory frequencies.
Our study, and the studies cited above, suggest that the close coherence that exists between arterial pressure and R-R intervals is secondary to a third influence, respiration, acting on sympathetic and vagal motoneurone pools. This possibility was raised earlier by de Boer et al. (1987). Our interpretation does not explain why baroreflex gains, derived from separate cross-spectral analyses in low- and respiratory-frequency ranges, are so quantitatively similar (Pagani et al. 1988).
Limitations
Notwithstanding our subjects'nearly constant breathing frequencies (Figs 5-7), end-tidal CO2 levels decreased progressively during tilt. We neither measured nor controlled tidal volumes, and therefore we cannot exclude the possibility that subjects'tidal volumes increased during tilt. Increases of tidal volume, if they occurred, probably increased arterial pressure fluctuations. The influence of hypocapnia is less certain; recent studies (Cooke et al. 1998; Henry et al. 1998) suggest that the moderate degree of end-tidal CO2 reduction we observed exerted negligible effects on our results, if any. We estimated arterial pressure with non-invasive photoplethysmography. Although arterial pressure waveforms change during tilt, non-invasive photoplethysmograms correlate very well with invasive intraarterial pressure recordings during tilt (Petersen et al. 1995).
In conclusion, we measured haemodynamic and autonomic neural periodicities during precisely controlled passive upright tilt and constant breathing frequency in a group of healthy young adults. Our results document changes of baroreflex responses during upright tilt, which may reflect leftward movement of subjects on their arterial pressure sympathetic and vagal response relationships. Changes of oscillatory behaviour indicate that the intensity, but not the centre frequency, of low-frequency cardiovascular rhythms is modulated by the level of arterial baroreceptor input. Our results suggest further that tilt systematically reduces respiratory gating of sympathetic and vagal motoneurone responsiveness to stimulatory inputs. During tilt, sympathetic gating is reduced because increases of sympathetic stimulation overwhelm respiratory influences, and vagal gating is reduced because vagal stimulation declines to a level below that necessary for maximal respiratory gating to occur.
Acknowledgments
This work was supported in part by grants from the Department of Veterans Affairs, National Aeronautics and Space Administration contracts NAS9-19541 and NAG2-408, and National Heart, Lung and Blood Institute grants HL-22296 and U01HL-56417.
References
- Akaike H. Fitting autoregressive models for prediction. Annals of the Statistical Institute of Mathematics. 1969;21:243–247. [Google Scholar]
- Asmussen E. The distribution of the blood between the lower extremities and the rest of the body. Acta Physiologica Scandinavica. 1943;5:31–38. [Google Scholar]
- Banner NR, Williams M, Patel N, Chalmers J, Lightman SL, Yacoub MH. Altered cardiovascular and neurohumoral responses to head-up tilt after heart-lung transplantation. Circulation. 1990;82:863–871. doi: 10.1161/01.cir.82.3.863. [DOI] [PubMed] [Google Scholar]
- Baskerville AL, Eckberg DL, Thompson MA. Arterial pressure and pulse interval responses to repetitive carotid baroreceptor stimuli in man. The Journal of Physiology. 1979;297:61–71. doi: 10.1113/jphysiol.1979.sp013027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti C, Barres C, Paultre C. Baroreflex modulation of blood pressure variability and heart rate variabilities in rats: assessment by spectral analysis. American Journal of Physiology. 1994;266:H1993–2000. doi: 10.1152/ajpheart.1994.266.5.H1993. [DOI] [PubMed] [Google Scholar]
- Cherniack NS, Edelman NH, Fishman AP. Pattern of discharge of respiratory neurons during systemic vasomotor waves. American Journal of Physiology. 1969;217:1375–1383. doi: 10.1152/ajplegacy.1969.217.5.1375. [DOI] [PubMed] [Google Scholar]
- Cooke WH, Cox JF, Diedrich AM, Taylor JA, Beightol LA, Ames JA, Hoag JB, Seidel H, Eckberg DL. Controlled breathing protocols probe human autonomic cardiovascular rhythms. American Journal of Physiology. 1998;274:H709–718. doi: 10.1152/ajpheart.1998.274.2.h709. [DOI] [PubMed] [Google Scholar]
- Cowley AW, Jr, Liard JF, Guyton AC. Role of the baroreceptor reflex in daily control of arterial blood pressure and other variables in dogs. Circulation Research. 1973;32:564–576. doi: 10.1161/01.res.32.5.564. [DOI] [PubMed] [Google Scholar]
- de Boer RW, Karemaker JM, Strackee J. Relationships between short-term blood-pressure fluctuations and heart-rate variability in resting subjects. 1: A spectral analysis approach. Medical and Biological Engineering and Computing. 1985;23:352–358. doi: 10.1007/BF02441589. [DOI] [PubMed] [Google Scholar]
- de Boer RW, Karemaker JM, Strackee J. Hemodynamic fluctuations and baroreflex sensitivity in humans: a beat-to-beat model. American Journal of Physiology. 1987;253:H680–689. doi: 10.1152/ajpheart.1987.253.3.H680. [DOI] [PubMed] [Google Scholar]
- Dornhorst AC, Howard P, Leathart GL. Respiratory variations in blood pressure. Circulation. 1952;6:553–558. doi: 10.1161/01.cir.6.4.553. [DOI] [PubMed] [Google Scholar]
- Eckberg DL. Temporal response patterns of the human sinus node to brief carotid baroreceptor stimuli. The Journal of Physiology. 1976;258:769–782. doi: 10.1113/jphysiol.1976.sp011445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL. Carotid baroreflex function in young men with borderline blood pressure elevation. Circulation. 1979;59:632–636. doi: 10.1161/01.cir.59.4.632. [DOI] [PubMed] [Google Scholar]
- Eckberg DL. Non-linearities of the human carotid baroreceptor-cardiac reflex. Circulation Research. 1980;47:208–216. doi: 10.1161/01.res.47.2.208. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Kifle YT, Roberts VL. Phase relationship between normal human respiration and baroreflex responsiveness. The Journal of Physiology. 1980;304:489–502. doi: 10.1113/jphysiol.1980.sp013338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL, Nerhed C, Wallin BG. Respiratory modulation of muscle sympathetic and vagal cardiac outflow in man. The Journal of Physiology. 1985;365:181–196. doi: 10.1113/jphysiol.1985.sp015766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL, Orshan CR. Respiratory and baroreceptor reflex interactions in man. Journal of Clinical Investigation. 1977;59:780–785. doi: 10.1172/JCI108699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagius J, Sundlöf G, Wallin BG. Variation of sympathetic reflex latency in man. Journal of the Autonomic Nervous System. 1987;21:157–165. doi: 10.1016/0165-1838(87)90018-x. 10.1016/0165-1838(87)90018-X. [DOI] [PubMed] [Google Scholar]
- Fernandez de Molina A, Perl ER. Sympathetic activity and the systemic circulation in the spinal cat. The Journal of Physiology. 1965;181:82–102. doi: 10.1113/jphysiol.1965.sp007747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbey MP, Jordan D, Richter DW, Spyer KM. Synaptic mechanisms involved in the inspiratory modulation of vagal cardio-inhibitory neurones in the cat. The Journal of Physiology. 1984;356:65–78. doi: 10.1113/jphysiol.1984.sp015453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton AC, Harris JW. Pressoreceptor-autonomic oscillation: a probable cause of vasomotor waves. American Journal of Physiology. 1951;165:158–166. doi: 10.1152/ajplegacy.1951.165.1.158. [DOI] [PubMed] [Google Scholar]
- Guz A, Innes JA, Murphy K. Respiratory modulation of left ventricular stroke volume in man measured using pulsed doppler ultrasound. The Journal of Physiology. 1987;393:499–512. doi: 10.1113/jphysiol.1987.sp016836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzetti S, Cogliati C, Broggi C, Carozzi C, Caldiroli D, Lombardi F, Malliani A. Influences of neural mechanisms on heart period and arterial pressure variabilities in quadriplegic patients. American Journal of Physiology. 1994;266:H1112–1120. doi: 10.1152/ajpheart.1994.266.3.H1112. [DOI] [PubMed] [Google Scholar]
- Henry RA, Lu I-L, Beightol LA, Eckberg DL. Interactions between human CO2 chemoreflexes and arterial baroreflexes. American Journal of Physiology. 1998;274:H2177–2187. doi: 10.1152/ajpheart.1998.274.6.h2177. [DOI] [PubMed] [Google Scholar]
- Holton P, Wood JB. The effects of bilateral removal of the carotid bodies and denervation of the carotid sinuses in two human subjects. The Journal of Physiology. 1965;181:365–378. doi: 10.1113/jphysiol.1965.sp007767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase S, Mano T, Saito M. Effects of graded head-up tilting on muscle sympathetic activities in man. The Physiologist. 1987;30:S62–65. suppl. [PubMed] [Google Scholar]
- Koh J, Brown TE, Beightol LA, Ha CY, Eckberg DL. Human autonomic rhythms: vagal cardiac mechanisms in tetraplegic subjects. The Journal of Physiology. 1994;474:483–495. doi: 10.1113/jphysiol.1994.sp020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi K, Terui N, Kollai M. Relationships between vagal and sympathetic activities in rhythmic fluctuations. In: Miyakawa K, Koepchen HP, Polosa C, editors. Mechanisms of Blood Pressure Waves. Berlin: Springer-Verlag; 1984. pp. 43–56. [Google Scholar]
- Lacolley PJ, Pannier BM, Slama MA, Cuche JL, Hoeks AP G, Laurent S, London GM, Safar ME. Carotid arterial haemodynamics after mild degrees of lower-body negative pressure in man. Clinical Science. 1992;83:535–540. doi: 10.1042/cs0830535. [DOI] [PubMed] [Google Scholar]
- Lopes OU, Palmer JF. Proposed respiratory ‘gating’ mechanism for cardiac slowing. Nature. 1976;264:454–456. doi: 10.1038/264454a0. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Wallin BG, Vallbo Å B. The discharge behaviour of single vasoconstrictor motoneurones in human muscle nerves. The Journal of Physiology. 1994;481:799–809. doi: 10.1113/jphysiol.1994.sp020482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer S. Studien zur Physiologie des Herzens und der Blutgefasse. V. Über spontane Blutdruckschwankungen. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften. Mathematisch-Naturwissenschaftliche Classe. 1877;74:281–307. [Google Scholar]
- Morillo CA, Eckberg DL, Ellenbogen KA, Beightol LA, Hoag JB, Tahvanainen KU O, Kuusela TA, Diedrich AM. Vagal and sympathetic mechanisms in patients with orthostatic vasovagal syncope. Circulation. 1997;96:2509–2513. doi: 10.1161/01.cir.96.8.2509. [DOI] [PubMed] [Google Scholar]
- Mukai S, Hayano J. Heart rate and blood pressure variabilities during graded head-up tilt. Journal of Applied Physiology. 1995;78:212–216. doi: 10.1152/jappl.1995.78.1.212. 10.1063/1.360654. [DOI] [PubMed] [Google Scholar]
- Pagani M, Lombardi F, Guzzetti S, Sandrone G, Rimoldi O, Malfatto G, Cerutti S, Malliani A. Power spectral density of heart rate variability as an index of sympatho-vagal interaction in normal and hypertensive subjects. Journal of Hypertension. 1984;2(suppl. 3):383–385. [PubMed] [Google Scholar]
- Pagani M, Somers V, Furlan R, Dell'Orto S, Conway J, Baselli G, Cerutti S, Sleight P, Malliani A. Changes in autonomic regulation induced by physical training in mild hypertension. Hypertension. 1988;12:600–610. doi: 10.1161/01.hyp.12.6.600. [DOI] [PubMed] [Google Scholar]
- Petersen ME V, Williams TR, Sutton R. A comparison of non-invasive continuous finger blood pressure measurement (Finapres) with intra-arterial pressure during prolonged head-up tilt. European Heart Journal. 1995;16:1647–1654. [PubMed] [Google Scholar]
- Preiss G, Polosa C. Patterns of sympathetic neuron activity associated with Mayer waves. American Journal of Physiology. 1974;226:724–730. doi: 10.1152/ajplegacy.1974.226.3.724. [DOI] [PubMed] [Google Scholar]
- Rea RF, Eckberg DL. Carotid baroreceptor-muscle sympathetic relation in humans. American Journal of Physiology. 1987;253:R929–934. doi: 10.1152/ajpregu.1987.253.6.R929. [DOI] [PubMed] [Google Scholar]
- Samaan A. The antagonistic cardiac nerves and heart rate. The Journal of Physiology. 1935;83:332–340. doi: 10.1113/jphysiol.1935.sp003232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul JP, Berger RD, Albrecht P, Stein SP, Chen MH, Cohen RJ. Transfer function analysis of the circulation: unique insights into cardiovascular regulation. American Journal of Physiology. 1991;261:H1231–1245. doi: 10.1152/ajpheart.1991.261.4.H1231. [DOI] [PubMed] [Google Scholar]
- Seidel H, Herzel H, Eckberg DL. Phase dependencies of the human baroreceptor reflex. American Journal of Physiology. 1997;272:H2040–2053. doi: 10.1152/ajpheart.1997.272.4.H2040. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Vögele C. Cardiac baroreflex function during postural change assessed using non-invasive spontaneous sequence analysis in young men. Cardiovascular Research. 1990;24:627–632. doi: 10.1093/cvr/24.8.627. [DOI] [PubMed] [Google Scholar]
- Stjernberg L, Blumberg H, Wallin BG. Sympathetic activity in man after spinal cord injury. Outflow to muscle below the lesion. Brain. 1986;109:695–715. doi: 10.1093/brain/109.4.695. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Carr DL, Myers CW, Eckberg DL. Mechanisms underlying very low frequency R-R interval oscillations in humans. Circulation. 1998;98:547–555. doi: 10.1161/01.cir.98.6.547. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Eckberg DL. Fundamental relations between short-term R-R interval and arterial pressure oscillations in humans. Circulation. 1996;93:1527–1532. doi: 10.1161/01.cir.93.8.1527. [DOI] [PubMed] [Google Scholar]
- Toska K, Eriksen M. Respiration-synchronous fluctuations in stroke volume, heart rate and arterial pressure in humans. The Journal of Physiology. 1993;472:501–512. doi: 10.1113/jphysiol.1993.sp019958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin BG, Burke D, Gandevia S. Coupling between variations in strength and baroreflex latency of sympathetic discharges in human muscle nerves. The Journal of Physiology. 1994;474:331–338. doi: 10.1113/jphysiol.1994.sp020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin BG, Eckberg DL. Sympathetic transients caused by abrupt alterations of carotid baroreceptor activity in humans. American Journal of Physiology. 1982;242:H185–190. doi: 10.1152/ajpheart.1982.242.2.H185. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Nerhed C. Relationship between spontaneous variations of muscle sympathetic activity and succeeding changes of blood pressure in man. Journal of the Autonomic Nervous System. 1982;6:293–302. doi: 10.1016/0165-1838(82)90002-9. 10.1016/0165-1838(82)90002-9. [DOI] [PubMed] [Google Scholar]


