Abstract
Using whole-cell patch clamp recording from neurones in an in vitro slice preparation, we have examined opioid- and orphanin FQ (OFQ)-mediated modulation of synaptic transmission in the rat arcuate nucleus and ventromedial hypothalamus (VMH).
Application of OFQ activated a Ba2+-sensitive and inwardly rectifying K+ conductance in ≈50% of arcuate nucleus neurones and ≈95% of VMH neurones. The OFQ-activated current was blocked by the nociceptin antagonist [Phe1Ψ(CH2NH)Gly2]-nociceptin(1-13) NH2 (NCA), a peptide that on its own exhibited only weak agonist activity at high concentrations (> 1 μm). Similar current activation was observed with the μ agonist DAMGO but not δ (DPDPE) or κ (U69593) agonists.
In arcuate nucleus neurones, DAMGO (1 μm), U69593 (1 μm) and OFQ (100 nM to 1 μm) but not DPDPE (1 μm) were found to depress the amplitude of electrically evoked glutamatergic postsynaptic currents (EPSCs) and decrease the magnitude of paired-pulse depression, indicating that opioid receptors were located presynaptically.
In VMH neurones, DAMGO strongly depressed the EPSC amplitude in all cells examined. DAMGO decreased the magnitude of paired-pulse depression, indicating that μ receptors were located presynaptically. U69593 weakly depressed the EPSC while OFQ and DPDPE had no effect.
In VMH neurones, DAMGO depressed the frequency of miniature EPSCs (-58%) in the presence of tetrodotoxin and Cd2+ (100 μm), suggesting that the actions of μ receptors could be mediated by an inhibition of the synaptic vesicle release process downstream of Ca2+ entry.
The data presented show that presynaptic modulation of excitatory neurotransmission in the arcuate nucleus occurs through μ, κ and the orphan opioid ORL-1 receptors while in the VMH presynaptic modulation only occurs through μ opioid receptors. Additionally, postsynaptic μ and ORL-1 receptors in both the arcuate nucleus and VMH modulate neuronal excitability through activation of a K+ conductance.
In addition to their effects on pain perception, opioids have been implicated in the control of food ingestion (Grandison & Guidotti, 1977; Levine & Billington, 1997), sexual behaviour (Vathy et al. 1991) and the release of hormones from the pituitary (Delitala, 1991). Opioid receptors and their endogenous ligands have been identified in many areas of the hypothalamus thought to be involved in the control of the neuroendocrine system, including the arcuate nucleus and the ventromedial hypothalamus (VMH). In the forebrain, expression of β-endorphin is confined to arcuate neurones which project to the median eminence and throughout the brain (Akil et al. 1984; Loughlin et al. 1995). Arcuate neurones have also been shown to contain dynorphin A (Akil et al. 1984; Loughlin et al. 1995), enkephalins (Sar et al. 1978; Akil et al. 1984; Loughlin et al. 1995) and the recently described μ-specific peptide endomorphin-2 (Schreff et al. 1998). Ligand binding and immunohistochemical studies have identified diffusely distributed μ and κ opioid receptors in the basal hypothalamus whereas δ receptors were less well represented (Loughlin et al. 1985; Mansour et al. 1995; Bausch et al. 1995; Mansour et al. 1996).
Hypothalamic actions of the recently described orphan opioid receptor ORL-1 (opioid receptor-like 1) and the endogenous ligand orphanin FQ or nociceptin (OFQ) have also been reported, including stimulation of food intake (Pomonis et al. 1996; Stratford et al. 1997), stimulation of prolactin and growth hormone secretion (Bryant et al. 1998), control of urinary sodium and water excretion (Kapusta et al. 1997), and facilitation of lordosis (Sinchak et al. 1997). Expression of ORL-1 (Mollereau et al. 1994; Anton et al. 1996) and OFQ (Schulz et al. 1996) was observed within the hypothalamus with highest receptor levels in the VMH. The overlapping tissue distribution of the ORL-1 and opioid receptors suggests that the endogenous opioid system has physiological regulatory functions in this region of the brain.
Activation of opioid and orphan opioid receptors has been shown to modulate neuronal excitability and synaptic communication by hyperpolarizing neurones through the activation of G protein-gated K+ channels (GIRKs) (North & Williams, 1985; Vaughan & Christie, 1996), depression of a hyperpolarization-activated cation current (IH) (Svoboda & Lupica, 1998), inhibition of voltage-gated Ca2+ channels (Rhim & Miller, 1994), and actions on the presynaptic nerve terminal leading to modulation of neurotransmitter release ‘downstream’ of Ca2+ entry (Capogna et al. 1996; Vaughan & Christie, 1997). The mechanism of opioid action in the medial hypothalamus is less clear. Previous studies of the effects of opioids in the VMH have shown that μ and δ (Charpak et al. 1988) and more recently orphan opioids (Lee et al. 1997) hyperpolarize and depress the firing rate of spontaneously active VMH neurones. μ and δ (Loose & Kelly, 1990) as well as orphan opioid receptors (Wagner et al. 1998) have also been shown to hyperpolarize neurones of the guinea-pig arcuate nucleus through the activation of a K+ conductance. Although κ opioid receptors are present in the medial hypothalamus, κ opioids have not been found to mediate hyperpolarization of either arcuate (Loose & Kelly, 1990) or VMH (Charpak et al. 1988) neurones. However, activation of κ receptors was found to weakly inhibit the spontaneous firing of VMH neurones (Zhang et al. 1996). In the present study, we have examined the modulation of excitatory neurotransmission in the arcuate nucleus and VMH by opioid and orphan opioid receptors. The data show that activation of presynaptic μ, κ and orphan opioid receptors in the arcuate nucleus and of presynaptic μ opioid receptors in the VMH inhibits excitatory synaptic transmission. In addition, we have further characterized the ionic current activated by the orphan opioid receptor in the VMH and the activity of a putative antagonist for this receptor (Guerrini et al. 1998).
METHODS
Preparation of brain slices
The methods for preparation of thin hypothalamic brain slices were described previously (Glaum et al. 1994). Experiments were conducted on Holtzman rats of either sex, of postnatal age 11-20 days. Animals were anaesthetized by ether inhalation and killed by decapitation using a guillotine. The brain was removed rapidly by dissection and placed in cold (0-4°C) extracellular bathing solution containing (mM): NaCl, 126; NaHCO3, 26; KCl, 3; NaH2PO4, 1.25; MgSO4, 1.3; CaCl2, 2.5; and D-glucose, 10 (gassed with 95% O2-5% CO2, osmolarity 315 mosmol l−1). Thin (200 μm) coronal slices of the rat brain containing the arcuate nucleus and ventromedial hypothalamus (VMH) were cut using a vibrating tissue slicer (Vibratome). Slices were maintained at 22-25°C until used.
For recording, a slice was transferred to a submersion chamber mounted on the stage of an upright microscope (Leitz Laborlux) and viewed with a Zeiss × 40 water immersion objective with Hoffman contrast optics. The slices were perfused continuously (3-5 ml min−1) with extracellular solution at room temperature (22-25°C). All recordings were made from visually identified neurones located in the arcuate nucleus or ventromedial hypothalamus.
Electrophysiology
For whole-cell patch clamp recording (Hamill et al. 1981) from visually identified arcuate nucleus and VMH neurones, pipettes were made from thin-walled borosilicate glass (outer diameter 1.5 mm, inner diameter 1.12 mm) (World Precision Instruments) using a Flaming-Brown horizontal pipette puller (Sutter Instruments). The pipette resistances were 4-8 MΩ when filled with pipette solution containing (mM): potassium gluconate, 145; MgCl2, 2; K2ATP, 5; GTP, 0.25; EGTA, 1.1; CaCl2, 0.1; and Hepes, 5; pH 7.2 (osmolarity adjusted to 280-290 mosmol l−1). After obtaining whole-cell access, transmembrane voltage and current were recorded using an Axoclamp-2A amplifier (Axon Instruments) in the discontinuous voltage clamp mode (filtered at 5 kHz and acquired at 20 kHz), and stored on computer (Quantex) and chart recorder (Gould). Data were acquired and analysed using pCLAMP 6.0.1 software (Axon Instruments). The cellular input resistance was calculated from the slope of the current-voltage relationship (-80 to -60 mV). Single excitatory postsynaptic currents (EPSCs) were evoked by local stimulation (0.05-0.1 Hz) using a monopolar tungsten stimulating electrode (0.2 mm diameter) (stimuli: 0.2 ms, 3-20 V). The stimulating electrode was placed 150-500 μm from the recording electrode either in the ventrolateral arcuate nucleus or ventral to the recording electrode in the VMH. Paired-pulse stimuli were recorded at 0.05 Hz using a 30 ms interpulse interval. For the purpose of data analysis, 3-5 min of evoked EPSCs were averaged (12-20 EPSCs) and the peak of the mean EPSC was measured. Cells that responded to drug application with a 25% or greater reduction in EPSC amplitude (a change greater than the 99% confidence limits of the control window mean) were considered to have responded positively. Miniature EPSCs (mEPSCs) were recorded from VMH neurones at a holding potential of -60 mV in the presence of 1 μm tetrodotoxin (TTX), 100 μm cadmium chloride (Cd2+), 100 μm dl-2-amino-5-phosphonovaleric acid (dl-AP5) and 10 μm bicuculline. Currents were filtered at 1-2 kHz, sampled at 8 kHz, and acquired to disk using pCLAMP software. mEPSCs were analysed using MiniAnalysis Program (Jaejin Software, Leonia, NJ, USA). The root mean square (r.m.s.) noise was ∼1 and ∼2 pA when filtered at 1 and 2 kHz, respectively. Miniature events were screened automatically using an amplitude threshold of 5 pA and an area threshold of 10 fC. Events were then visually accepted or rejected based upon rise and decay times. Analysed mEPSCs from a 3-4 min recording period were pooled for statistical analysis (Student's paired t test) using GraphPad Prism (GraphPad Software, San Diego, CA, USA) and were considered statistically significant if P < 0.05. Data are expressed as means ±s.e.m. Current-voltage relationships were obtained using either a voltage ramp from -147 to +3 mV (1 s duration) or by 300 ms voltage steps from -127 to -52 mV (in 5 mV increments). For statistical analysis, the effects of opioid agonist treatment on membrane holding currents were quantified as the relative current activation determined at the most negative voltage tested in these experiments (-127 mV). Current-voltage data were sampled at 5 kHz and filtered at 1 kHz. Membrane potentials were corrected for a junction potential of +7 mV measured according to the method described by Neher (1992).
Materials
Bicuculline methobromide, (5α, 7α, 8β)-(+)-N-methyl-N-(7-(1-pyrrolidinyl)-1-oxaspiro[4.5]dec-8-yl)-benzeneacetamide (U69593), (d-Pen2, D-Pen5)-enkephalin (DPDPE), Tyr-D-Ala-Phe-Glu-Val-Val-Gly-NH2 (deltorphin II), naloxone hydrochloride (NLX) and Tyr-D-Ala-Gly-MePhe-Gly-ol (DAMGO) were obtained from Sigma. (±)-2-Amino-5-phosphonovaleric acid (dl-AP5), nor-binaltorphimine dihydrochloride (nor-BNI) and [Phe1Ψ(CH2NH)Gly2]-nociceptin (1-13)NH2 (NCA) were obtained from Tocris Cookson (Ballwin, MO, USA). Orphanin FQ/nociceptin (OFQ) was obtained from Bachem (King of Prussia, PA, USA). TTX was obtained from Alamone Laboratories (Jerusalem, Israel). All drugs were dissolved in distilled water or DMSO (< 0.1% final concentration in perfusate) and applied by bath perfusion.
RESULTS
Whole-cell recordings were obtained from 127 arcuate nucleus neurones and 132 VMH neurones in 160 preparations of 200 μm thick coronal slices of the rat hypothalamus. The mean input resistance of arcuate neurones was 1.87 ± 0.09 GΩ (n = 84) while that for VMH neurones was 804 ± 37 MΩ (n = 81). All experiments were performed in the discontinuous voltage clamp mode at a holding potential of -60 to -70 mV unless otherwise noted.
Postsynaptic modulation of hypothalamic neurones
Application of OFQ (100-1000 nM) activated an outward current (36.8 ± 2.9 pA) in 95% (65 of 68) of VMH neurones held at a membrane potential of -67 mV (Fig. 1Aa). Brief (1-2 min) applications of OFQ (100 nM) activated an outward current which returned to control after washing for 15-20 min. Following activation of the current by OFQ, application of BaCl2 (300 μm) in the continued presence of OFQ was found to completely reverse the activated current (n = 10, data not shown). The sensitivity of the OFQ-activated current to block by external BaCl2 suggests this conductance is mediated by potassium ions. Current-voltage relationships were determined before and during OFQ application (Fig. 1Aa and C). The current activated by OFQ exhibited inward rectification (Fig. 1C, inset). In normal saline (3 mM KCl), slope conductances measured from -50 to -70 mV and -100 to -120 mV in the absence of OFQ were 1.49 ± 0.27 and 1.85 ± 0.27 nS, respectively (n = 11, P > 0.05, paired t test) (Fig. 1C). Following the addition of OFQ the conductances increased to 2.55 ± 0.40 nS (P < 0.01, paired t test) and 5.75 ± 0.91 nS (P < 0.01, paired t test), respectively (Fig. 1C). The OFQ-activated current reversed potential at -89.2 ± 0.7 mV (n = 28). The hyperpolarizing effect of this outward current on a VMH cell is shown in the example cell in Fig. 1C. The reversal potential of the whole-cell current (equivalent to the resting membrane potential in current clamp) was decreased by 24 mV (from -54 to -78 mV) in the presence of OFQ. The reversal potential and magnitude of the OFQ-activated current were dependent upon [K+]o. Increasing [K+]o from 3 to 16 mM resulted in a linear change (slope = 53.1 ± 1.5 mV (logM)−1) in the reversal potential (Fig. 1D) indicating the activation of an inwardly rectifying K+ current by OFQ.
Figure 1. Activation of an outward current by OFQ and DAMGO in arcuate and VMH neurones.
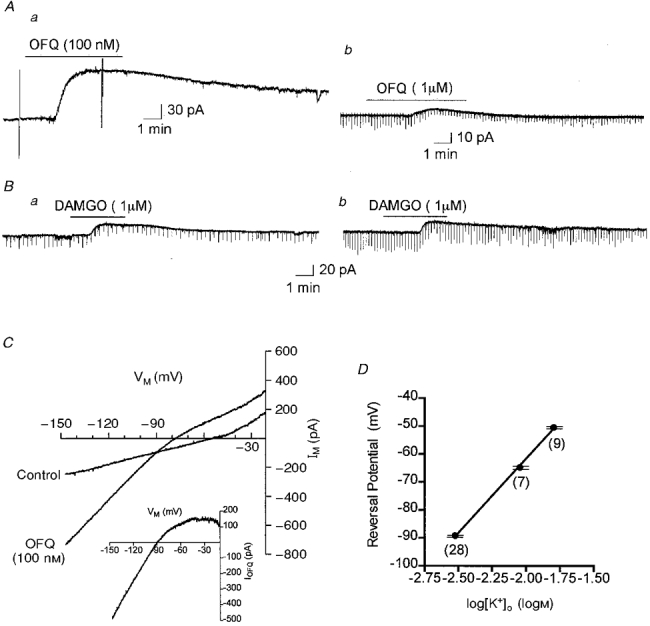
Application of OFQ (100 nM or 1 μm) (A) and DAMGO (B) to VMH (a) and arcuate (b) neurones activated an outward current from a holding potential of -67 mV. OFQ application in this VMH neurone was done in the presence of TTX (1 μm) and the deflections from baseline indicate the times at which current-voltage ramps were acquired (C). Traces are scanned images from a chart recording of the holding current. The downward deflections in Ab and B represent synaptically evoked currents. C, current-voltage relationship from the points indicated in the VMH cell shown in Aa. The current was measured during a voltage ramp from -147 to -27 mV, 1 s duration, from a holding potential of -67 mV. Inset, the OFQ-activated current obtained by subtraction of these traces. D, dependence of the reversal potential of the OFQ-activated current on [K+]o in the VMH. Reversal potentials for the current were -89.2 ± 0.7 mV in 3 mM K+, -64.7 ± 1.4 mV in 9 mM K+ and -50.4 ± 1.3 in 16 mM K+. Symbols are means ±s.e.m. (error bars) for the number of observations indicated below each point. The continuous line indicates the linear regression (slope = 53.1 ± 1.5 mV (logM)−1 determined for the data.
The concentration-response relationship for the OFQ-activated current in the VMH was determined by cumulatively applying increasing concentrations (1-1000 nM) of the peptide to slices while recording the transmembrane current-voltage relationship (Fig. 2). Current activation could be detected at concentrations as low as 1 nM (Fig. 2). Maximum current activation was achieved at a concentration of ∼100 nM with an EC50 of 7.8 nM (Fig. 2).
Figure 2. Concentration dependence of the OFQ-activated current in VMH neurones.
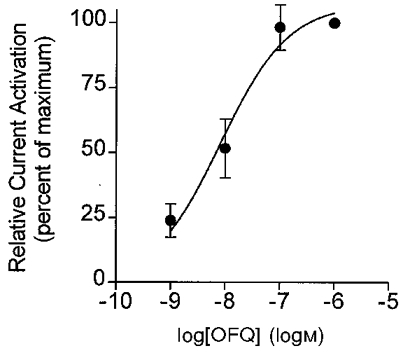
Increasing concentrations of OFQ were cumulatively applied to VMH neurones voltage clamped at -67 mV. Current-voltage relationships at the indicated concentrations of OFQ were determined by 300 ms voltage steps to membrane potentials between -127 and -52 mV, in 5 mV increments. The relative current activation was determined by normalizing to the maximum inward current (VH -127 mV) obtained with OFQ (1 μm). Data are means ±s.e.m. Data from the concentration-response curves were pooled and fitted to a sigmoidal function, logEC50 = -8.11 ± 0.15, EC50 = 7.8 nM (n = 5).
The novel OFQ analogue [Phe1Ψ(CH2-NH)Gly2]-nociceptin(1-13)NH2 (NCA) was recently described as the first antagonist of the ORL-1 receptor in the guinea-pig ileum and mouse vas deferens (Guerrini et al. 1998). However, in another study which utilized a cell line expressing the human ORL-1 homologue, NCA was shown to be a full agonist exhibiting a maximal inhibition of adenylyl cyclase at ∼100 nM (Butour et al. 1998). We further studied the actions of NCA on the K+ current activated by OFQ (100 nM) in rat VMH neurones. Initially, NCA was applied alone to determine whether the peptide activated an outward current in VMH neurones. Application of the peptide weakly activated an outward current in VMH neurones but only at high concentrations (100 nM, 0 of 3; 1 μm, 12.7 ± 2.8 pA, 3 of 17; 3 μm, 13.0 ± 1.5 pA, 4 of 5; data not shown) illustrating some weak agonist activity for the peptide. Subsequently, the ability of NCA to occlude OFQ-activated currents was tested. Application of the peptide (1 μm) for 5 min prior to the application of OFQ (100 nM) was found to reduce the number of cells responding to OFQ and the magnitude of current activation (20 pA, 2 of 6 cells; data not shown). Furthermore, application of NCA (3 μm) following activation of a K+ current by OFQ (100 nM) was found to reverse the activated current (Fig. 3A), suppressing the maximum inward current (holding potential (VH) -127 mV) by 79.5 ± 7.9% (n = 6) (Fig. 3B). These experiments suggested that NCA acted as a weak partial agonist at the orphan opioid receptor.
Figure 3. Current activation by OFQ was partly reversed by [Phe1Ψ(CH2NH)Gly2]-nociceptin(1-13)NH2 (NCA) in VMH neurones.
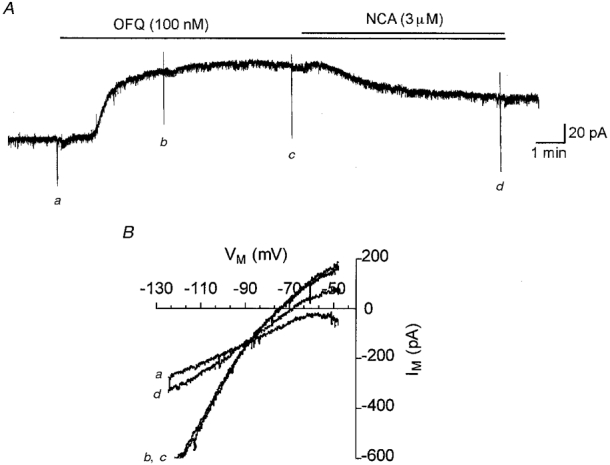
A, application of OFQ (100 nM) activated an outward current in this VMH neurone from a holding potential of -67 mV. OFQ was applied to the VMH neurone for ≈9 min during which time no desensitization of the outward current was observed. NCA (3 μm) application in the continued presence of OFQ caused the current to partially relax to baseline. B, current-voltage relationship showing the reversal of the OFQ-activated current by NCA. Shown are the current-voltage relationships determined by a continuous voltage ramp from -127 to -47 mV, 1 s duration, determined at the time points indicated in A prior to the addition of OFQ (a), during OFQ application (b and c) and following partial reversal of the current by NCA (d).
Comparison of the effects of OFQ in the VMH with those of other opioid receptor agonists showed significant outward current activation by the μ opioid DAMGO. Application of DAMGO (1 μm) was found to activate an outward current in 58% of VMH cells (19.7 ± 2.8 pA, 23 of 40 cells) (Fig. 1Ba). Perfusion of the slice with BaCl2 (300 μm) in the continued presence of DAMGO reversed the activated current (n = 2). Prior application of a low concentration of the opioid antagonist naloxone (100 nM) blocked activation of this current (12 pA, 1 of 7 cells; data not shown). The δ-specific agonists deltorphin II (1 μm) (11 pA, 1 of 5 cells) and DPDPE (1 μm) (16 pA, 1 of 10) were found to weakly activate an outward current in the same cell that also responded to both OFQ and DAMGO. The κ opioid agonist U69593 (1 μm) was not found to activate an outward current in any of the VMH cells tested (n = 10).
OFQ has recently been shown to activate a K+ conductance in guinea-pig arcuate neurones (Wagner et al. 1998), an effect similar to that reported earlier for DAMGO in arcuate neurones of the guinea-pig and rat (Loose & Kelly, 1990; Loose et al. 1991). In the rat arcuate nucleus, application of OFQ (100-1000 nM) was found to activate an outward current (13.0 ± 1.5 pA, 43 of 78 cells) (Fig. 1Ab). As with the current activated in VMH neurones, this current was inwardly rectifying with a reversal potential of -92.8 ± 2.2 mV (n = 3) (data not shown). Application of BaCl2 (300 μm, n = 6) in the continued presence of OFQ reversed the activated current (data not shown) indicating activation of an inwardly rectifying K+ conductance by OFQ in rat arcuate neurones. DAMGO was also found to activate an outward current of similar magnitude to OFQ, although in relatively few cells (9.6 ± 1.9 pA, 10 of 45 cells) (Fig. 1Bb). Arcuate neurones were found to respond to both OFQ and DAMGO (3 of 32 cells), OFQ alone (13 of 32), or neither peptide (16 of 32). Application of U69593 (1 μm) (n = 53) and DPDPE (1 μm) (n = 15) was not found to activate an outward current in any arcuate cells examined.
Opioid receptor-mediated synaptic depression
In addition to the observed postsynaptic modulation of K+ conductances, G protein-coupled receptors may also act presynaptically to inhibit the release of neurotransmitters (presynaptic inhibition) (Miller, 1998). To examine the potential modulation of synaptic transmission by opioid receptors, local stimulation (15-20 s interval) was used to evoke glutamatergic EPSCs while recording from arcuate nucleus or VMH neurones in the discontinuous voltage clamp mode (VH -60 to -70 mV). Excitatory inputs into the recording cell were pharmacologically isolated by inclusion of bicuculline (10 μm) and dl-AP5 (100 μm) in the bathing solution. Under these conditions evoked EPSCs were completely blocked by the AMPA/kainate (KA) receptor antagonist CNQX (10 μm) (data not shown).
In the arcuate nucleus, application of OFQ (100 nM) was found to depress the amplitude of the evoked EPSC (-43.5 ± 2.2%, 23 of 38 cells) (Figs 4, 5B and 6). Following removal of the peptide, the EPSC returned to baseline within 15-20 min (Fig. 4). This concentration of OFQ produced a maximal effect since application of a 10-fold higher concentration of OFQ (1 μm) was found to depress the EPSC to a similar extent (-53.7 ± 5.8%, 7 of 10 cells) (Fig. 6). Many of the cells that responded to OFQ (100 nM to 1 μm) with a depression of the EPSC also exhibited activation of an outward current (14 of 30 cells, see Fig. 1Ab). Application of NCA (1 μm) was not found to depress the EPSC amplitude in any cells tested (+0.46 ± 3.6%, n = 12) (Figs 5B and 6). In the presence of NCA, OFQ (100 nM) no longer depressed the EPSC amplitude (-12.3 ± 4.3%, n = 12) (Figs 5B and 6) nor activated any outward currents. However, in one of these cells following 60 min of wash, OFQ application was found to both depress the EPSC amplitude (Fig. 5B) and activate an outward current (+18 pA), further supporting the antagonist actions of NCA. Application of DAMGO (1 μm) produced a rapidly reversible (∼10 min) depression of the EPSC in arcuate neurones (-52.6 ± 3.6%, 24 of 32 cells) (Figs 4A and 6). DAMGO depression of the EPSC was reversed to control by naloxone (100 nM) (-3.4 ± 8.3%, n = 5) (Figs 5B and 6). In contrast to OFQ, DAMGO infrequently modulated both the EPSC and the membrane holding current of the recording cell (1 of 24). Although U69593 (1 μm) was not found to activate an outward current in any arcuate nucleus neurones, it was found to depress the EPSC amplitude (-50.9 ± 3.0%, 26 of 44 cells) (Figs 4B, 5A and 6). Following removal of U69593, the inhibitory effect required substantially longer washing periods than other opioids tested (> 45 min) to return to control (Fig. 4B). The depression caused by U69593 was rapidly reversed to control by the addition of a low concentration of the κ antagonist nor-BNI (100 nM) (-12.8 ± 4.4%, n = 8) (Figs 5A and 6). DPDPE (1 μm) had little effect on the EPSC amplitude (-14.8 ± 2.8%, n = 9) (Fig. 6). Depression of the EPSC by multiple opioid agonists was often observed within the same arcuate neurone, including OFQ and U69593 (9 of 20 cells) (Fig. 4B), OFQ and DAMGO (Fig. 4A and 5B) (10 of 21 cells), DAMGO and U69593 (10 of 24 cells), or all three agonists (4 of 17 cells).
Figure 4. Opioid agonists cause a depression of AMPA receptor-mediated excitatory synaptic transmission in the arcuate nucleus.
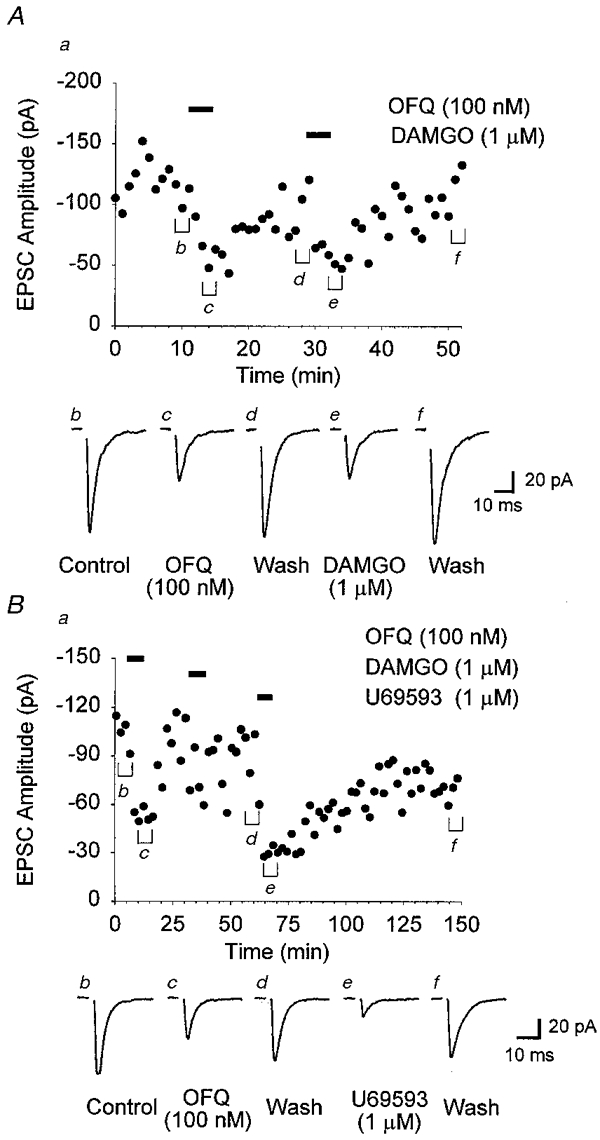
Electrically evoked EPSCs were recorded in the presence of dl-AP5 (100 μm) and bicuculline (10 μm). EPSCs were evoked every 15 s. A, data from an arcuate neurone illustrating a reversible depression of the evoked EPSC following application of OFQ (100 nM) and DAMGO (1 μm). B, data from a different arcuate neurone showing a reversible depression of the EPSC following application of OFQ (100 nM) and U69593 (1 μm). Application of DAMGO (1 μm) did not elicit a response in this cell. For both A and B, a represents the time course of the effects of opioid agonists on the mean EPSC amplitude (each point represents the mean amplitude of 4 responses), whereas b-f are averaged EPSCs (3 min) taken from the time points indicated in a. Neurones were voltage clamped at a holding potential of -60 mV.
Figure 5. Antagonism of opioid-induced depression of arcuate nucleus AMPA receptor-mediated EPSCs.
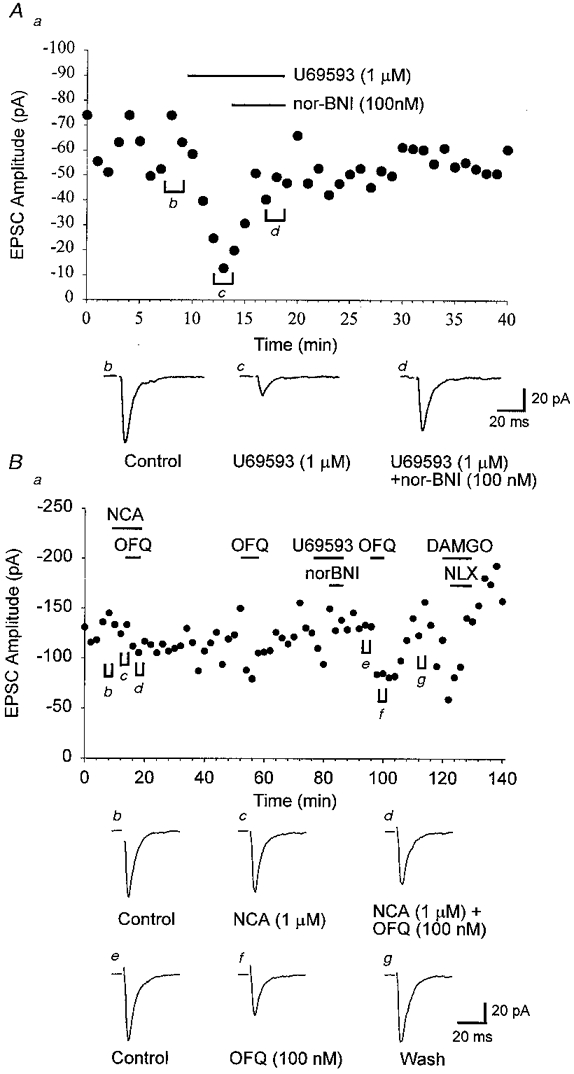
Electrically evoked EPSCs were recorded in the presence of dl-AP5 (100 μm) and bicuculline (10 μm). EPSCs were evoked every 15 s. A, data from an arcuate neurone illustrating a depression of the evoked EPSC following application of U69593 (1 μm) and subsequent reversal following inclusion of the κ opioid antagonist nor-BNI (100 nM). B, in another arcuate neurone application of NCA (1 μm) or the combination of NCA (1 μm) and OFQ (100 nM) did not reduce the EPSC amplitude. However, ≈60 min after the removal of peptides, OFQ (100 nM) alone was found to reversibly depress the EPSC amplitude. Application of DAMGO (1 μm) also produced a strong depression of the EPSC which was reversed by the addition of naloxone (NLX, 100 nM). Application of U69593 (1 μm) alone or in combination with nor-BNI (100 nM) had no effect on the EPSC amplitude in this neurone. For both A and B, a represents the time course of the effects of opioid agonists on the mean EPSC amplitude (each point represents the mean amplitude of 4 (A) or 8 (B) responses), whereas b-f are averaged EPSCs (3 min) taken from the time points indicated in a. Neurones were voltage clamped at a holding potential of -60 mV.
Figure 6. Summary of data for opioid depression of EPSCs in the arcuate nucleus.
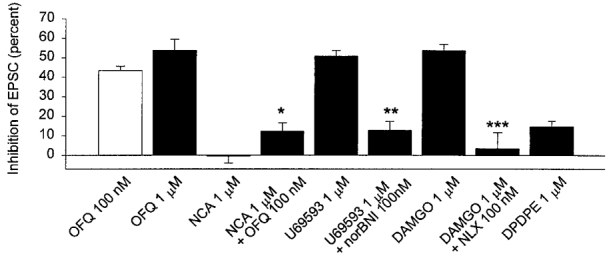
Columns represent the degree of inhibition of the EPSC produced by the indicated opioid. The number of observations for each peptide was (number of positive trials per total number of trials): 100 nM OFQ, 23 of 38; 1 μm OFQ, 7 of 10; 1 μm NCA, 12 of 12; 1 μm NCA plus 100 nM OFQ, 12 of 12; 1 μm U69593, 26 of 44; 1 μm U69593 plus 100 nM nor-BNI, 8 of 8 (data from 8 cells responding to U69593), 1 μm DAMGO, 24 of 32; 1 μm DAMGO plus 100 nM naloxone, 5 of 5 (data from 5 cells responding to DAMGO); 1 μm DPDPE, 9 of 9. * Significantly different from the depression observed with OFQ (100 nM) (P < 0.01, unpaired t test). ** Significantly different from U69593 (1 μm), and *** significantly different from DAMGO (1 μm) (P < 0.01, paired t test).
To determine the location of opioid receptors modulating excitatory synaptic transmission in the arcuate nucleus, the effect of opioid agonists on paired-pulse depression (Baskys & Malenka, 1991) (0.05 Hz, 30 ms interpulse interval) was studied in a population of the cells described above. In cells for which application of DAMGO (1 μm) resulted in a depression of the first evoked EPSC (EPSC1, -57.1 ± 4.2; n = 9), an increase in the paired-pulse ratio (EPSC2/EPSC1) from 0.88 ± 0.08 in control to 1.65 ± 0.21 (P < 0.01, paired t test) in the presence of DAMGO was observed (Fig. 7). U69593 depression of the EPSC (EPSC1, -47 ± 6.1; n = 9) increased the paired-pulse ratio from 0.88 ± 0.07 to 1.24 ± 0.07 (P < 0.01, paired t test), and OFQ depression of the EPSC (EPSC1, -44.4 ± 3.3; n = 11) increased the paired-pulse ratio from 0.73 ± 0.09 to 1.06 ± 0.08 (P < 0.01, paired t test) (Fig. 7). In cells that did not respond to these agonists, no significant change in the paired-pulse ratio was found (data not shown). These data suggest that μ, κ and ORL-1 agonists modulate neurotransmitter release in the arcuate nucleus through receptors located presynaptically.
Figure 7. Application of opioids reduced paired-pulse depression in the arcuate nucleus.
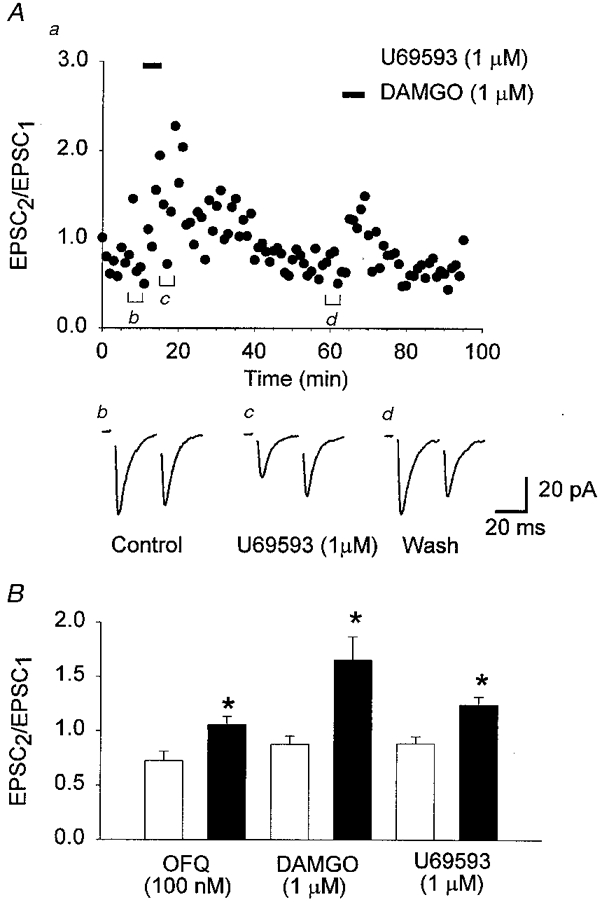
Evoked EPSCs were recorded in the presence of dl-AP5 (100 μm) and bicuculline (10 μm). Paired EPSCs (interpulse interval 30 ms) were evoked every 20 s. A, application of U69593 (1 μm) and DAMGO (1 μm) caused a reversible depression of the initial ESPC (EPSC1), and an increase in the paired-pulse ratio (EPSC2/EPSC1). For A, a represents the time course of the effects of U69593 and DAMGO on the ratio of EPSC2/EPSC1, and b-d show the mean EPSCs (3 min) from the time points indicated in a. B, histogram of the mean paired-pulse ratio illustrating the decrease in paired-pulse depression following application of U69593 (n = 9), DAMGO (n = 9) and OFQ (n = 11). □, the mean control paired-pulse ratio; ▪, the mean paired-pulse ratio following application of the indicated opioid. The pooled data show a significant increase in the paired-pulse ratio for all opioids tested (*P < 0.01, paired t test). Neurones were voltage clamped at a holding potential of -60 mV.
Excitatory neurotransmission was also studied in the VMH. The μ agonist DAMGO (1 μm) strongly and reversibly depressed the glutamatergic EPSC in all VMH cells tested (-54.4 ± 2.9%, 14 of 14 cells) (Fig. 8). DAMGO inhibition of the EPSC reversed to control following ∼15 min of wash (Fig. 8A). Perfusion of the brain slice with a low concentration of the opioid antagonist naloxone (100 nM) prior to DAMGO (1 μm) strongly reduced the effectiveness of the agonist (-10.2 ± 3.2%, n = 6) (Fig. 8B). In order to determine the locus of the μ opioid receptors mediating this inhibition, the effect of DAMGO on paired-pulse depression was studied. DAMGO inhibited the initial EPSC (EPSC1, -62.4 ± 7.6%; 6 of 6 cells) (Fig. 9) and increased the ratio of the EPSC amplitudes (EPSC2/EPSC1) from 0.46 ± 0.09 to 1.08 ± 0.13 (P = 0.011, paired t test) indicating that μ receptors in the VMH were located presynaptically (Fig. 9). In many cells in which DAMGO depressed the EPSC amplitude, activation of an outward current was also observed (11 of 20 cells). In VMH neurones, the κ agonist U69593 produced only a modest depression of the EPSC (-36.8 ± 3.4%, 4 of 8 cells) (Fig. 8B) while neither OFQ (100 nM) (-1.7 ± 3.9%versus control, n = 10) nor DPDPE (1 μm) (-8.05 ± 4.0 versus control, n = 6) depressed evoked glutamatergic EPSCs (Fig. 8A). Because a heterogeneous δ receptor population has been suggested to exist in the VMH (Hiller et al. 1996), the effect of a δ-2 subtype agonist, deltorphin II, was also examined. However, cells that were unresponsive to the δ-1 agonist DPDPE were also unresponsive to deltorphin II (1 μm) (-5.02 ± 7.0 versus control, n = 4) (Fig. 8A).
Figure 8. Application of DAMGO caused a selective depression of glutamatergic EPSCs in all VMH neurones.
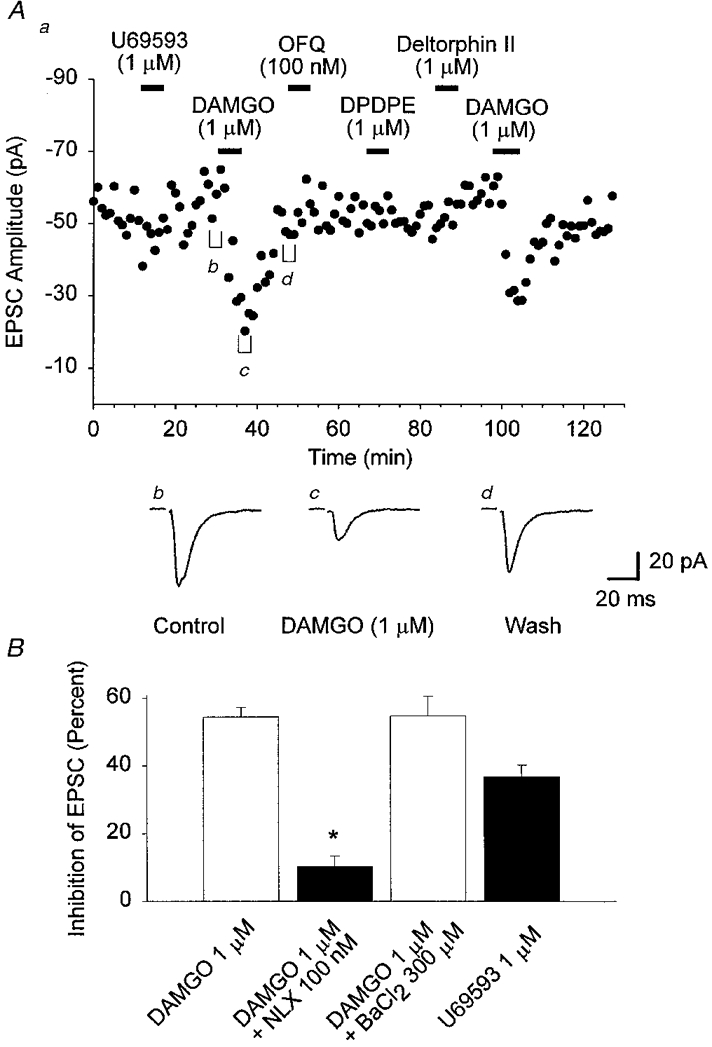
Evoked EPSCs were recorded in the presence of dl-AP5 (100 μm) and bicuculline (10 μm). EPSCs were evoked every 15 s. A, application of DAMGO (1 μm) caused a reversible depression of the EPSC (-57%) recorded from a VMH neurone. Application of U69593 (1 μm), OFQ (100 nM), DPDPE (1 μm) and deltorphin II (1 μm) did not elicit a response in this cell. A second application of DAMGO (1 μm) 60 min after the first produced a similar depression of the EPSC amplitude (-48%). For A, a-d are the same as in Fig. 4. B, histogram representing the mean inhibition of the evoked EPSC. Columns represent the degree of inhibition following application of the indicated opioids. The number of observations for each bar was (number of positive trials per total number of trials): 1 μm DAMGO, 14 of 14; 1 μm DAMGO plus 100 nM NLX, 5 of 5; 1 μm DAMGO plus 300 μm BaCl2, 5 of 5; and 1 μm U69593, 4 of 8. * Significantly different from DAMGO (1 μm) (P < 0.01, unpaired t test). Neurones were voltage clamped at a holding potential of -60 mV.
Figure 9. Application of DAMGO reduced paired-pulse depression in VMH neurones.
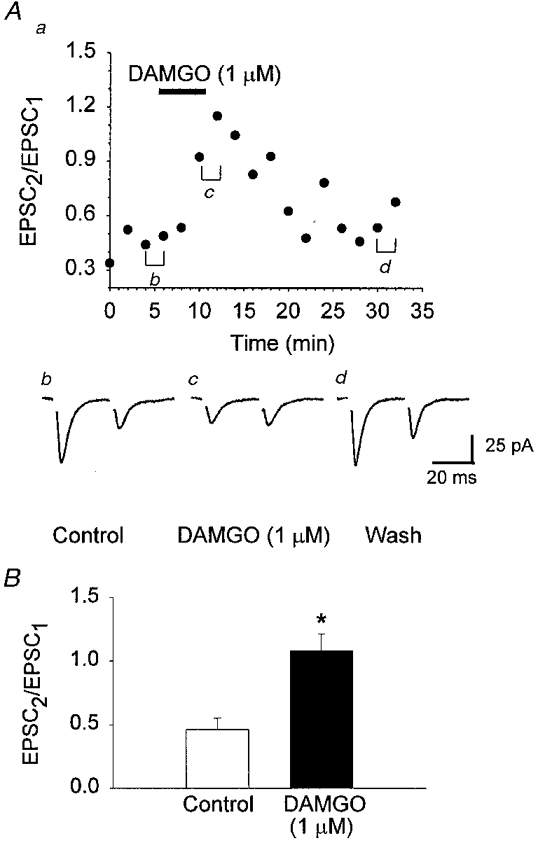
Evoked EPSCs were recorded in the presence of dl-AP5 (100 μm) and bicuculline (10 μm). Paired EPSCs (interpulse interval 30 ms) were evoked every 20 s. A, application of DAMGO (1 μm) caused a reversible depression of the initial ESPC (EPSC1), and an increase in the paired-pulse ratio (EPSC2/EPSC1). For A, a represents the time course of the effect of DAMGO on the EPSC2/EPSC1 ratio, and b-d show the mean EPSCs (3 min) from the time points indicated in a. B, histogram of the mean paired-pulse ratio illustrating the decrease in paired-pulse depression following application of DAMGO (n = 6). The pooled data show a significant increase in the paired-pulse ratio (* P < 0.001, paired t test). Neurones were voltage clamped at a holding potential of -60 mV.
Due to the robust inhibition of glutamatergic synaptic transmission by DAMGO, the mechanism of μ opioid receptor-mediated presynaptic inhibition was further examined. As described above, activation of a Ba2+-sensitive outward current by DAMGO was observed in many VMH neurones. A similar activation of this conductance in the presynaptic nerve terminal would serve to shunt the incoming action potential, reduce the influx of Ca2+ into the nerve terminal, and decrease neurotransmitter release (Miller, 1998). BaCl2 at 300 μm, a concentration that blocked the activation of outward currents by opioids, was not found to have a significant effect on the EPSC amplitude (data not shown). In the presence of BaCl2, DAMGO produced a depression of the EPSC amplitude (-54.7 ± 5.9%, n = 5) (Fig. 8B) which was not statistically different from that observed in the absence of BaCl2 (P = 0.96, unpaired t test) indicating that activation of a GIRK-like K+ conductance was not involved in the presynaptic inhibition of the EPSC. The effects of DAMGO on the amplitude and frequency distributions of mEPSCs in VMH neurones were also studied. mEPSCs were recorded in the presence of 10 μm bicuculline and 100 μm dl-AP5 to isolate AMPA/KA events, and also in the presence of Na+ (TTX, 1 μm) and Ca2+ (Cd2+, 100 μm) channel blockers to record spontaneous vesicle release independent of Ca2+ entry (Scholz & Miller, 1992). mEPSCs had a mean frequency of 3.7 ± 1.1 Hz (n = 9) and a mean amplitude of 11.9 ± 0.9 pA (n = 9). In all cells examined, application of DAMGO caused a significant reduction in the frequency of mEPSCs (-58.3 ± 5.8%, n = 9, P < 0.001, paired t test) without a significant change in the amplitude (-4.6 ± 1.7%, n = 9, P > 0.05, paired t test) (Fig. 10). Following washout of the peptide (∼20 min) the depression of mEPSC frequency was partially reversed (-35.7 ± 4.5% of control, n = 3) (Fig. 10).
Figure 10. Application of DAMGO depressed mEPSC frequency in VMH neurones in the presence of Cd2+ and TTX.
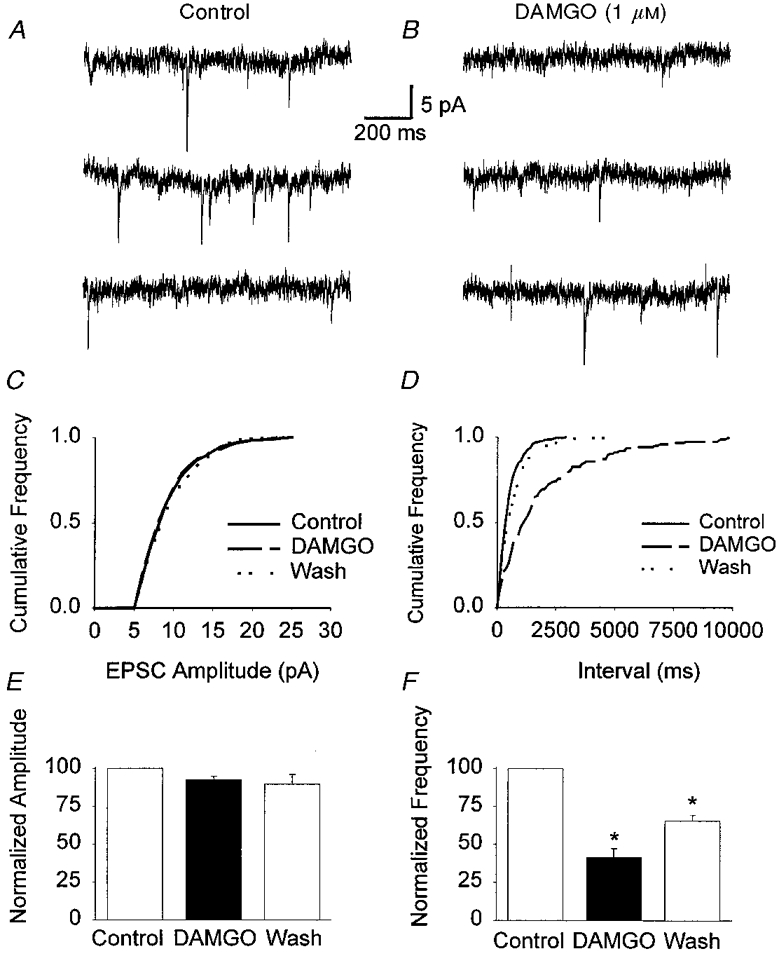
mEPSCs were recorded in the presence of TTX (1 μm), CdCl2 (100 μm), bicuculline (10 μm) and dl-AP5 (100 μm). A and B, consecutive traces before (Control) and following application of DAMGO (1 μm). C and D, cumulative probability distributions of the mEPSC amplitude and interval from 4 min periods before (494 events), during (130 events) and 25 min after (361 events) the application of DAMGO (1 μm). E and F, effect of DAMGO on the mean change in amplitude and frequency for data pooled from 9 cells and following wash from 3 cells. The pooled data showed a significant decrease in the mean frequency (* P < 0.001, paired t test), whereas no significant change in the mean amplitude was observed (P > 0.05, paired t test). Neurones were voltage clamped at a holding potential of -60 mV.
DISCUSSION
The results presented in this study demonstrate that μ and orphan opioids modulate the excitability of arcuate nucleus and VMH neurones through activation of an inwardly rectifying K+ channel. Opioids acting at μ, κ and orphan receptors were observed to act on the presynaptic nerve terminal to depress excitatory glutamatergic synaptic transmission in the arcuate nucleus. However, in the VMH only a μ opioid was found to act via a presynaptic receptor to inhibit glutamatergic transmission. Depression of neurotransmitter release in the VMH was independent of Ca2+ entry indicating that μ opioid effects under these conditions were probably mediated not by a depression of presynaptic Ca2+ channels, but rather by modulation of the release mechanism ‘downstream’ of Ca2+ entry (Scholz & Miller, 1992). However, it is possible that inhibition of terminal Ca2+ channels may also play a role.
Postsynaptic actions of opioids in the arcuate nucleus and VMH
Hypothalamic opioids are involved in the control of neuroendocrine, reproductive, thermoregulatory and orexigenic systems. Opioid receptor subtypes (μ, δ, κ and ORL-1) are present to varying degrees in areas of the hypothalamus known to control these homeostatic systems. However, the specific mechanisms by which opioids act are still not completely clear. Previous studies have focused on the effects of opioids on spontaneous firing rates and membrane hyperpolarization. These studies suggested that arcuate neurones are strongly modulated by μ (Loose & Kelly, 1990) and orphan (Wagner et al. 1998) opioids through activation of an inwardly rectifying K+ conductance, whereas weak actions were observed for δ agonists and no actions were observed for κ agonists. Localization of neurones expressing ORL-1 in rat and mouse brain indicated a strong expression within the VMH and arcuate nucleus (Mollereau et al. 1994; Anton et al. 1996). In the present study, OFQ was observed to activate a Ba2+-sensitive outward current in nearly all VMH neurones. These findings confirm those recently published by Lee et al. (1997). In the present study, the OFQ-activated current was shown to be an inwardly rectifying K+ conductance based upon the greater increase in conductance at negative potentials and the linear change in the reversal potential with increasing [K+]o. In arcuate nucleus neurones, OFQ and the μ opioid DAMGO also activated a Ba2+-sensitive outward conductance. These findings confirm the results of earlier studies in ovariectomized (OVX) adult female guinea-pigs (Loose & Kelly, 1990; Wagner et al. 1998) and normally cycling young adult female rats (Loose et al. 1991). Our findings in juvenile rats (postnatal days 11-17) do, however, differ from the previous studies in the number of arcuate cells responsive to agonist. OFQ responses were observed in ∼50% of rat arcuate neurones versus∼95% of OVX guinea-pigs and the response to DAMGO was limited to < 25% of rat arcuate neurones versus∼86% of OVX guinea-pigs and ∼68% of young adult female rats. This may indicate a dependence on age or sexual maturation in the development of opioid receptor coupling to K+ channels in the arcuate nucleus similar to that observed for δ opioid receptors in the medial vestibular nucleus (Sulaiman & Dutia, 1998). Conversely, OFQ activation of a K+ conductance in nearly all VMH neurones indicates that no such developmental maturation occurs in this area of the brain and suggests an important role for this receptor system in the control of VMH neuronal excitability.
Presynaptic actions of opioids in the arcuate nucleus and VMH
Arcuate nucleus neurones express both ionotropic (van den Pol et al. 1994) and metabotropic glutamate receptors (Ghosh et al. 1997). Excitatory neurotransmission mediated by AMPA/KA glutamate receptors has been suggested to mediate the majority of fast excitatory synaptic transmission in the medial basal hypothalamus (van den Pol et al. 1990). These glutamatergic systems have been shown to play an important role in the release of hormones from the pituitary (Brann & Mahesh, 1997). Within the arcuate nucleus, glutamatergic neurones synapse locally onto neurones whose axons then project to the median eminence, indicating their neuroendocrine function (Belousov & van den Pol, 1997). Presynaptic modulation of these glutamatergic systems may underlie the actions of neuropeptides in the hypothalamus.
In the arcuate nucleus, μ, κ and orphan opioids depressed excitatory glutamatergic postsynaptic currents. Presynaptic localization of opioid receptors controlling glutamate release in the arcuate nucleus was suggested based upon an agonist-induced decrease in paired-pulse depression (Baskys & Malenka, 1991). Although K+ current activation was observed in only a few cells in response to the μ agonist DAMGO and no cells were responsive to the κ agonist U69593, the glutamatergic inputs into 60-70% of arcuate neurones were depressed by these agonists, indicating that presynaptic modulation of glutamatergic transmission is the predominant mechanism by which these opioids modulate neurotransmission in the arcuate nucleus. OFQ was found to depress glutamatergic transmission and activate a K+ conductance in approximately the same percentage of arcuate neurones indicating both a presynaptic and a postsynaptic role for the orphan opioid receptor in the arcuate nucleus. The finding that opioid receptors function to depress glutamate release shown here, and the recent demonstration that presynaptic receptors for neuropeptide Y (Rhim et al. 1997) and galanin (Kinney et al. 1998) depress while those for hypocretin/orexin (van den Pol et al. 1998) potentiate glutamatergic neurotransmission suggests a possible mechanism by which neuropeptides may control hypothalamic systems.
In all VMH neurones, excitatory glutamatergic postsynaptic currents were depressed by the μ opioid DAMGO while no other opioid agonists produced a significant depression. Neuronal excitation was further depressed by DAMGO in about half of VMH neurones by the simultaneous activation of a hyperpolarizing postsynaptic K+ conductance. The presynaptic localization of μ opioid receptors controlling glutamate release was suggested by a decrease in the magnitude of paired-pulse depression and also a decrease in the frequency of mEPSCs without a change in their amplitude. Although opioids have been shown to modulate the entry of Ca2+ into neurones of the nucleus tractus solitarii (Rhim & Miller, 1994) and to increase a Ba2+-sensitive K+ conductance (North & Williams, 1985), neither of these mechanisms appears to be involved in the depression of spontaneous glutamate release from presynaptic nerve terminals in the VMH. In the hippocampus (Capogna et al. 1996) as well as periaqueductal grey (Vaughan & Christie, 1997), presynaptic μ opioid receptors were shown to modulate spontaneous neurotransmitter release through a mechanism that does not involve presynaptic Ca2+ influx. In the present study, addition of Ba2+ had no effect on the observed depression of the electrically evoked EPSC by DAMGO, illustrating that presynaptic K+ channel activation was not involved. Furthermore, in the presence of Cd2+ and TTX, DAMGO still produced a depression of mEPSC frequency without an alteration of the amplitude distribution in all VMH cells tested, indicating that this depression was not dependent upon entry of Ca2+ through voltage-gated Ca2+ channels. These findings suggest that presynaptic modulation of neurotransmitter release by μ opioids in the VMH may involve a direct modulation of the vesicle release mechanism. Interestingly, the depression of mEPSC frequency (-58.3 ± 5.8%) was similar to the observed depression of the amplitude of electrically evoked EPSCs (-56.4 ± 2.7%). The similarity between the depression of electrically evoked EPSCs and mEPSCs in both the number of cells responding to DAMGO and the magnitude of this depression may suggest that modulation occurs through the same mechanism. However, these results do not exclude a possible contribution of opioid modulation of presynaptic Ca2+ influx in evoked transmission.
The activation of a K+ conductance by OFQ and inhibition of glutamatergic synaptic transmission were specific to the ORL-1 receptor since these effects were dose dependent and could be blocked by an OFQ antagonist, [Phe1Ψ(CH2NH) Gly2]-nociceptin(1-13)NH2 (NCA) (Guerrini et al. 1998). The efficacy of this peptide has generated considerable debate regarding its actions at the ORL-1 receptor. Initial studies indicated that NCA antagonized the actions of OFQ in both the guinea-pig ileum and mouse vas deferens (Guerrini et al. 1998). However, this peptide has been shown to have both agonist actions in an in vitro expression system using the human ORL-1 receptor (Butour et al. 1998) and antagonist actions on the OFQ-activated K+ conductance in rat amygdaloid neurones (Meis & Pape, 1998). In the present study, NCA was shown to weakly activate an outward current in VMH neurones and also partially reverse the response to OFQ, suggesting that NCA is a weak partial agonist. NCA was also found to weakly depress calcium currents and occlude the response to OFQ in a HEK293 cell line expressing both the mouse orphan opioid receptor and N-type calcium channels (P. J. Emmerson & R. J. Miller, unpublished observations). The observed discrepancy between our data and those obtained from a human ORL-1 expression system may be the result of differences between the efficacy of NCA at the human and rat and mouse orphan opioid receptors. The human receptor differs from the rodent receptors in several areas, most notably in the amino terminus but also at point mutations in the external loops.
Role of opioids in the arcuate nucleus and VMH
Opioid receptors clearly play an important role in the control of food intake (Levine & Billington, 1997). Injection of β-endorphin into the VMH stimulated food intake (Grandison & Guidotti, 1977) whereas injection of naloxone into the VMH or paraventricular nucleus suppressed food intake (Gosnell et al. 1986). Recent studies have also suggested that orphan opioid receptors in the VMH are involved in the control of food intake (Stratford et al. 1997). The involvement of μ opioid receptors in food intake is supported by the orexigenic action of the μ-specific endomorphin peptides (Asakawa et al. 1998). Although the actions of μ and orphan opioids in the VMH differ with respect to their actions on the presynaptic nerve terminal, both agonists result in the activation of a hyperpolarizing K+ conductance in these neurones, suggesting a possible mechanism for the observed effects on food intake. However, while OFQ activated a K+ conductance in nearly all VMH neurones, DAMGO depressed excitatory glutamatergic transmission into all VMH neurones, suggesting that an overall depression of VMH excitability may be involved in the opioid control of food intake.
Opioids acting in the hypothalamus have also been shown to result in the stimulation of the release of prolactin, growth hormone and corticosteroids, and depression of the release of luteinizing hormone, oxytocin and vasopressin (Delitala, 1991). Central administration of OFQ has been shown to stimulate prolactin and growth hormone secretion (Bryant et al. 1998) and modulate urinary excretion of water (diuresis) and sodium (antinatriuresis) in a manner similar to dynorphin A (Kapusta et al. 1997). Thus, the observed effects of opioid and ORL-1 agonists on glutamatergic neurotransmission in the arcuate nucleus and the known role of these glutamatergic neurones in hormone release (Belousov & van den Pol, 1997) suggest a possible mechanism for opioid-mediated modulation of hormone release.
Based upon the effects of opioids observed in the present study, the depression of neuronal excitation in the VMH and arcuate nucleus includes both presynaptic inhibition of glutamate release and postsynaptic membrane hyperpolarization resulting from the activation of a K+ conductance. These effects are likely to underlie the observed effects of opioids, including stimulation of food intake and modulation of hormone release. The finding that opioid receptors display only partially overlapping presynaptic and postsynaptic localization as well as differential localization of receptors between the VMH and arcuate nucleus may suggest that these opioid receptor subtypes serve distinct modulatory functions.
Acknowledgments
This work was supported by Public Health Service Grants DA02121, MH40165 and NS33502 to R. J. M. and DA07255 to P. J. E.
References
- Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM. Endogenous opioids: Biology and function. Annual Review of Neuroscience. 1984;7:223–255. doi: 10.1146/annurev.ne.07.030184.001255. 10.1146/annurev.ne.07.030184.001255. [DOI] [PubMed] [Google Scholar]
- Anton B, Fein J, To T, Li X, Silberstein L, Evans CJ. Immunohistochemical localization of ORL-1 in the central nervous system of the rat. Journal of Comparative Neurology. 1996;368:229–251. doi: 10.1002/(SICI)1096-9861(19960429)368:2<229::AID-CNE5>3.0.CO;2-5. 10.1002/(SICI)1096-9861(19960429)368:2<229::AID-CNE5>3.3.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Momose K, Ueno N, Fujino MA, Kasuga M. Endomorphins have orexigenic and anxiolytic activities in mice. NeuroReport. 1998;9:2265–2267. doi: 10.1097/00001756-199807130-00022. [DOI] [PubMed] [Google Scholar]
- Baskys A, Malenka RC. Agonists at metabotropic glutamate receptors presynaptically inhibit EPSCs in neonatal rat hippocampus. The Journal of Physiology. 1991;444:687–701. doi: 10.1113/jphysiol.1991.sp018901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bausch SB, Patterson TA, Appleyard SM, Chavkin C. Immunocytochemical localization of delta opioid receptors in mouse brain. Journal of Chemical Neuroanatomy. 1995;8:175–189. doi: 10.1016/0891-0618(94)00044-t. [DOI] [PubMed] [Google Scholar]
- Belousov AB, van den Pol AN. Local synaptic release of glutamate from neurons in the rat hypothalamic arcuate nucleus. The Journal of Physiology. 1997;499:747–761. doi: 10.1113/jphysiol.1997.sp021966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann DW, Mahesh VB. Excitatory amino acids: Evidence for a role in the control of reproduction and anterior pituitary hormone secretion. Endocrine Reviews. 1997;18:678–700. doi: 10.1210/edrv.18.5.0311. [DOI] [PubMed] [Google Scholar]
- Bryant W, Janik J, Baumann M, Callahan P. Orphanin FQ stimulates prolactin and growth hormone release in male and female rats. Brain Research. 1998;807:228–233. doi: 10.1016/s0006-8993(98)00802-6. [DOI] [PubMed] [Google Scholar]
- Butour JL, Moisand C, Mollereau C, Meunier JC. [Phe1psi(CH2-NH)Gly2] nociceptin-(1–13)-NH2 is an agonist of the nociceptin (ORL1) receptor. European Journal of Pharmacology. 1998;349:R5–6. doi: 10.1016/s0014-2999(98)00273-8. [DOI] [PubMed] [Google Scholar]
- Capogna M, Gahwiler BH, Thompson SM. Presynaptic inhibition of calcium-dependent and -independent release elicited with ionomycin, gadolinium, and alpha-latrotoxin in the hippocampus. Journal of Neurophysiology. 1996;75:2017–2028. doi: 10.1152/jn.1996.75.5.2017. [DOI] [PubMed] [Google Scholar]
- Charpak S, Dubois-Dauphin M, Raggenbass M, Dreifuss JJ. Direct inhibition by opioid peptides of neurons located in the ventromedial nucleus of the guinea pig hypothalamus. Brain Research. 1988;450:124–130. doi: 10.1016/0006-8993(88)91551-x. [DOI] [PubMed] [Google Scholar]
- Delitala G. Opioid peptides and pituitary function: basic and clinical aspects. In: Motta M, editor. Brain Endocrinology. 2. New York: Raven Press; 1991. pp. 217–244. [Google Scholar]
- Ghosh PK, Baskaran N, van den Pol AN. Developmentally regulated gene expression of all eight metabotropic glutamate receptors in hypothalamic suprachiasmatic and arcuate nuclei - a PCR analysis. Brain Research Developmental Brain Research. 1997;102:1–12. doi: 10.1016/s0165-3806(97)00066-7. [DOI] [PubMed] [Google Scholar]
- Glaum SR, Alford S, Rossi DJ, Collingridge GL, Slater NT. Whole cell patch recording with simultaneous measurement of intracellular calcium concentration in mammalian brain slices in vitro. Methods in Neuroscience. 1994;19:340–358. [Google Scholar]
- Gosnell BA, Morley JE, Levine AS. Opioid-induced feeding: Localization of sensitive brain sites. Brain Research. 1986;369:177–184. doi: 10.1016/0006-8993(86)90526-3. 10.1016/0006-8993(86)90526-3. [DOI] [PubMed] [Google Scholar]
- Grandison L, Guidotti A. Stimulation of food intake by muscimol and beta-endorphin. Neuropharmacology. 1977;16:533–536. doi: 10.1016/0028-3908(77)90019-3. 10.1016/0028-3908(77)90019-3. [DOI] [PubMed] [Google Scholar]
- Guerrini R, Calo G, Rizzi A, Bigoni R, Bianchi C, Salvadori S, Regoli D. A new selective antagonist of the nociceptin receptor. British Journal of Pharmacology. 1998;123:163–165. doi: 10.1038/sj.bjp.0701640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hiller JM, Fan LQ, Simon EJ. Autoradiographic comparison of [3H]DPDPE and [3H]DSLET binding: evidence for distinct delta-1 and delta-2 opioid receptor populations in rat brain. Brain Research. 1996;719:85–95. doi: 10.1016/0006-8993(96)00090-x. [DOI] [PubMed] [Google Scholar]
- Kapusta DR, Sezen SF, Chang JK, Lippton H, Kenigs VA. Diuretic and antinatriuretic responses produced by the endogenous opioid-like peptide, nociceptin (orphanin FQ) Life Sciences. 1997;60:PL15–21. doi: 10.1016/s0024-3205(96)00593-0. [DOI] [PubMed] [Google Scholar]
- Kinney GA, Emmerson PJ, Miller RJ. Galanin receptor-mediated inhibition of glutamate release in the arcuate nucleus of the hypothalamus. Journal of Neuroscience. 1998;18:3489–3500. doi: 10.1523/JNEUROSCI.18-10-03489.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Nicholson JR, McKnight AT. Nociceptin hyperpolarises neurones in the rat ventromedial hypothalamus. Neuroscience Letters. 1997;239:37–40. doi: 10.1016/s0304-3940(97)00875-6. [DOI] [PubMed] [Google Scholar]
- Levine AS, Billington CJ. Why do we eat? A neural systems approach. Annual Review of Nutrition. 1997;17:597–619. doi: 10.1146/annurev.nutr.17.1.597. [DOI] [PubMed] [Google Scholar]
- Loose DL, Kelly MJ. Opioids act at μ-receptors to hyperpolarize arcuate neurons via an inwardly rectifying potassium conductance. Brain Research. 1990;513:15–23. doi: 10.1016/0006-8993(90)91084-t. [DOI] [PubMed] [Google Scholar]
- Loose MD, Ronnekleiv OK, Kelly MJ. Neurons in the rat arcuate nucleus are hyperpolarized by GABAB and mu-opioid receptor agonists: evidence for convergence at a ligand-gated potassium conductance. Neuroendocrinology. 1991;54:537–544. doi: 10.1159/000125979. [DOI] [PubMed] [Google Scholar]
- Loughlin SE, Leslie FM, Fallon JH. Endogenous opioid systems. In: Paxinos G, editor. The Rat Nervous System. 2. San Diego, CA, USA.: Academic Press; 1995. pp. 975–1001. [Google Scholar]
- Mansour A, Burke S, Pavlic RJ, Akil H, Watson SJ. Immunohistochemical localization of the cloned κ1 receptor in the rat CNS and pituitary. Neuroscience. 1996;71:671–690. doi: 10.1016/0306-4522(95)00464-5. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Akil H, Watson SJ. Immunohistochemical localization of the cloned μ opioid receptor in the rat CNS. Journal of Chemical Neuroanatomy. 1995;8:283–305. doi: 10.1016/0891-0618(95)00055-c. [DOI] [PubMed] [Google Scholar]
- Meis S, Pape HC. Postsynaptic mechanisms underlying responsiveness of amygdaloid neurons to Nociceptin/Orphanin FQ. Journal of Neuroscience. 1998;18:8133–8144. doi: 10.1523/JNEUROSCI.18-20-08133.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RJ. Presynaptic receptors. Annual Review of Pharmacology and Toxicology. 1998;38:201–227. doi: 10.1146/annurev.pharmtox.38.1.201. [DOI] [PubMed] [Google Scholar]
- Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, Caput D, Vassart G, Meunier JC. ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Letters. 1994;341:33–38. doi: 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods in Enzymology. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- North RA, Williams JT. On the potassium conductance increased by opioids in rat locus coeruleus neurones. The Journal of Physiology. 1985;364:265–280. doi: 10.1113/jphysiol.1985.sp015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomonis JD, Billington CJ, Levine AS. Orphanin FQ, agonist of orphan opioid receptor ORL1, stimulates feeding in rats. NeuroReport. 1996;8:369–371. doi: 10.1097/00001756-199612200-00072. [DOI] [PubMed] [Google Scholar]
- Rhim H, Kinney GA, Emmerson PJ, Miller RJ. Regulation of neurotransmission in the arcuate nucleus of the rat by different neuropeptide Y receptors. Journal of Neuroscience. 1997;17:2980–2989. doi: 10.1523/JNEUROSCI.17-09-02980.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim H, Miller RJ. Opioid receptors modulate diverse types of calcium channels in the nucleus tractus solitarius of the rat. Journal of Neuroscience. 1994;14:7608–7615. doi: 10.1523/JNEUROSCI.14-12-07608.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sar M, Stumpf WE, Miller RJ, Chang KJ, Cuatrecasas P. Immunohistochemical localization of enkephalin in rat brain and spinal cord. Journal of Comparative Neurology. 1978;182:17–37. doi: 10.1002/cne.901820103. [DOI] [PubMed] [Google Scholar]
- Scholz KP, Miller RJ. Inhibition of quantal neurotransmitter release in the absence of calcium influx by a G protein-linked adenosine receptor at hippocampal synapses. Neuron. 1992;8:1139–1150. doi: 10.1016/0896-6273(92)90134-y. [DOI] [PubMed] [Google Scholar]
- Schreff M, Schulz S, Wiborny D, Hollt V. Immunofluorescent identification of endomorphin-2-containing nerve terminals in the rat brain and spinal cord. NeuroReport. 1998;9:1031–1034. doi: 10.1097/00001756-199804200-00014. [DOI] [PubMed] [Google Scholar]
- Schulz S, Schreff M, Nuss D, Gramsch C, Hollt V. Nociceptin/orphanin FQ and opioid peptides show overlapping distribution but not colocalization in pain-modulatory brain regions. NeuroReport. 1996;7:3021–3025. doi: 10.1097/00001756-199611250-00045. [DOI] [PubMed] [Google Scholar]
- Sinchak K, Hendricks DG, Baroudi R, Micevych PE. Orphanin FQ/nociceptin in the ventromedial nucleus facilitates lordosis in female rats. NeuroReport. 1997;8:3857–3860. doi: 10.1097/00001756-199712220-00004. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Holahan MR, Kelley AE. Injections of nociceptin into nucleus accumbens shell or ventromedial hypothalamic nucleus increase food intake. NeuroReport. 1997;8:423–426. doi: 10.1097/00001756-199701200-00009. [DOI] [PubMed] [Google Scholar]
- Sulaiman RM, Dutia MB. Opioid inhibition of rat medial vestibular nucleus neurones in vitro and its dependence on age. Experimental Brain Research. 1998;122:196–202. doi: 10.1007/s002210050507. [DOI] [PubMed] [Google Scholar]
- Svoboda KR, Lupica CR. Opioid inhibition of hippocampal interneurons via modulation of potassium and hyperpolarization-activated cation (Ih) currents. Journal of Neuroscience. 1998;18:7084–7098. doi: 10.1523/JNEUROSCI.18-18-07084.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Gao XB, Obrietan K, Kilduff TS, Belousov AB. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. Journal of Neuroscience. 1998;18:7962–7971. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol A, Hermans-Borgmeyer I, Hofer M, Ghosh P, Heinemann S. Ionotropic glutamate-receptor gene expression in hypothalamus: localization of AMPA, kainate, and NMDA receptor RNA with in situ hybridization. Journal of Comparative Neurology. 1994;343:428–444. doi: 10.1002/cne.903430307. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Wuarin JP, Dudek FE. Glutamate, the dominant excitatory transmitter in neuroendocrine regulation. Science. 1990;250:1276–1278. doi: 10.1126/science.1978759. [DOI] [PubMed] [Google Scholar]
- Vathy I, van der Plas J, Vincent PA, Etgen AM. Intracranial dialysis and microinfusion studies suggest that morphine may act in the ventromedial hypothalamus to inhibit female rat sexual behavior. Hormones and Behavior. 1991;25:354–366. doi: 10.1016/0018-506x(91)90007-5. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Christie MJ. Increase by the ORL1 receptor (opioid receptor-like1) ligand, nociceptin, of inwardly rectifying K conductance in dorsal raphe nucleus neurones. British Journal of Pharmacology. 1996;117:1609–1611. doi: 10.1111/j.1476-5381.1996.tb15329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CW, Christie MJ. Presynaptic inhibitory action of opioids on synaptic transmission in the rat periaqueductal grey in vitro. The Journal of Physiology. 1997;498:463–472. doi: 10.1113/jphysiol.1997.sp021872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EJ, Ronnekleiv OK, Grandy DK, Kelly MJ. The peptide orphanin FQ inhibits β-endorphin neurons and neurosecretory cells in the hypothalamic arcuate nucleus by activating an inwardly-rectifying K+ conductance. Neuroendocrinology. 1998;67:73–82. doi: 10.1159/000054301. [DOI] [PubMed] [Google Scholar]
- Zhang C, Pfaff DW, Kow L-M. Functional analysis of opioid receptor subtypes in the ventromedial hypothalamic nucleus of the rat. European Journal of Pharmacology. 1996;308:153–159. doi: 10.1016/0014-2999(96)00293-2. [DOI] [PubMed] [Google Scholar]


