Abstract
The effect of creatine phosphate (PCr) on sarcoplasmic reticulum (SR) Ca2+ regulation was studied in mechanically skinned skeletal muscle fibres from rat extensor digitorium longus (EDL). Preparations were perfused with solutions mimicking the intracellular milieu and the [Ca2+] within the muscle was monitored continuously using fura-2.
Brief application of 40 mM caffeine caused a transient increase in [Ca2+] due to SR Ca2+ release, and an associated tension response. Withdrawal of PCr resulted in (i) a slow transient release of Ca2+ from the SR (ii) a marked prolongation of the descending phase of the caffeine-induced fluorescence ratio transient and (iii) a decrease in the Ca2+ transient amplitude to 69.2 ± 2.7% (n = 16) of control responses.
Prolongation of the caffeine-induced Ca2+ transient also occurred following application of the SR Ca2+ pump inhibitor cyclopiazonic acid (CPA). This suggests that (i) the descending phase of the caffeine-induced Ca2+ transient is dependent on the rate of Ca2+ uptake by the SR and (ii) prolongation associated with PCr withdrawal may also reflect a decrease in the net Ca2+ uptake rate.
The effects of PCr withdrawal were mimicked by addition of the creatine kinase (CK) inhibitor 2,4-dinitro-1-fluorobenzene (DNFB). Hence, reducing the [PCr] may influence SR Ca2+ regulation by limiting local ATP regeneration by endogenous CK. After treatment with DNFB, PCr withdrawal had no effect on the Ca2+ transient, confirming that PCr does not have an additional direct effect on the SR.
The Ca2+ efflux associated with PCr withdrawal was insensitive to ryanodine or Ruthenium Red, but was effectively abolished by pretreatment with the SR Ca2+ pump inhibitor cyclopiazonic acid (CPA). This suggests that the Ca2+ efflux associated with PCr withdrawal is independent of the SR Ca2+ channel, but may involve reversal or inhibition of the Ca2+ ATPase.
These data suggest that Ca2+ regulation by the SR is strongly dependent on the supply of ATP via endogenous CK. Depletion of PCr may contribute to impaired SR Ca2+ regulation known to occur in intact skeletal muscle under conditions of fatigue.
Previous studies on isolated skeletal muscle fibres have established that Ca2+ uptake and release by the SR are impaired in fatigue induced by intermittent tetanic stimulation (for reviews, see Fitts, 1994; Allen et al. 1995) During the early stages of fatiguing stimulation, tetanic [Ca2+]i increases transiently. This is followed by prolongation of the [Ca2+]i transient and a gradual increase in resting [Ca2+]i. These effects are consistent with a reduction in the rate of Ca2+ accumulation by the SR. In the final stages, tetanic [Ca2+]i and force decline markedly due to failure of the SR Ca2+ release mechanism (Westerblad et al. 1998).
Recent experiments have shown that Ca2+ release can be restored in the later stages of fatigue by a rapid increase in [ATP]i induced by flash photolysis of the caged compound (Allen et al. 1997). Similar results were obtained by application of caffeine, which is known to increase the opening probability of the SR channel (Westerblad & Allen, 1991). This has led to the suggestion that release failure may result from desensitization of the SR Ca2+ channel due to a local fall in [ATP]i in the triads. However, the mechanism underlying the reduction in SR Ca2+ uptake observed earlier in the fatigue process remains uncertain. One possibility considered in previous studies is that the progressive increase in the intracellular inorganic phosphate (Pi) concentration, which occurs following the onset of fatiguing stimulation, inhibits SR Ca2+ uptake. However, recent work has shown that intracellular injection of Pi increases the rate of relaxation and decreases resting [Ca2+]i in mouse skeletal muscle fibres (Westerblad & Allen, 1996a). These effects may be explained by stimulation of the SR Ca2+ pump due to calcium phosphate (Ca-Pi) precipitation within the SR lumen. In mechanically skinned skeletal muscle fibres, Ca-Pi precipitation within the SR has also been shown to occur and the reported actions of Pi cannot explain the reduced rate of SR Ca2+ accumulation observed in fatigue (Fryer et al. 1995).
An alternative possibility is that reduced SR Ca2+ uptake may result from creatine phosphate (PCr) depletion. Studies on isolated skeletal and cardiac SR vesicles have provided evidence that the maximum capacity of the SR and the rate of Ca2+ accumulation can be influenced by local ATP regeneration via bound creatine kinase (CK; Korge et al. 1993). Recent work on cultured myotubes from CK-deficient mice also suggests that CK is of importance in maintaining the efficiency of the SR Ca2+ uptake and release mechanisms (Steeghs et al. 1997). However, such experiments are difficult to interpret, because CK deficiency is associated with increased mitochondrial density and changes in SR structure.
In the present study, we have investigated the role of PCr in SR Ca2+ regulation using mechanically skinned skeletal muscle fibres from the rat. In this preparation, the SR remains in situ and the ionic conditions closely match those in intact fibres. Caffeine was rapidly applied and the resulting release of Ca2+ from the SR detected using fura-2 fluorescence. In the presence of millimolar levels of bathing ATP, withdrawal of PCr or inhibition of CK resulted in loss of Ca2+ from the SR, and prolongation of the caffeine-induced Ca2+ transient. These results suggest that (i) Ca2+ accumulation by the SR is strongly dependent on the supply of ATP via CK and (ii) depletion of PCr may contribute to impaired SR Ca2+ uptake during skeletal muscle fatigue. The mechanisms underlying the efflux of Ca2+ observed on withdrawal of PCr and possible effects on the release process are considered.
METHODS
Preparation
Rats (Wistar, 200-350 g) were killed by stunning followed by cervical dislocation according to standard Shedule 1 procedures. The extensor digitorium longus (EDL) muscle was removed rapidly and placed in ‘relaxing’ solution approximating to the intracellular milieu (see below). Single muscle fibres, or pairs of adjacent muscle fibres were then mechanically skinned and attached between an isometric force transducer (SensoNor, Norway) and a fixed support using monofilament snares (diameter 30 μm) within stainless steel tubes (Goodfellow Metals, UK). In most experiments, two adjacent muscle fibres were mounted in parallel, as this increased the amount of light collected by the objective and improved the signal-to-noise ratio on the Ca2+ signal (see below). However, in all protocols shown, qualitatively similar results were obtained on single fibres. In these experiments, fibres were mechanically skinned in the presence of a low [Ca2+] (40 nM), which is likely to inactivate the t-tubule depolarization-induced Ca2+ release mechanism (Lamb & Stephenson, 1990). Consistent with this, substitution of K+ with Na+ failed to release Ca2+ in these preparations.
Apparatus
The apparatus for simultaneous measurement of tension and SR Ca2+ release has been described previously (Duke & Steele, 1998a). Briefly, the mounted preparation was lowered close to the bottom of a shallow bath with a glass coverslip base. A Perspex column (5 mm diameter) was lowered close to the surface of the muscle to minimize the volume of solution above the preparation. Throughout the experimental protocols, preparations were perfused at 0.8 ml min−1 via a narrow duct (200 μm diameter) passing longitudinally through the centre of the column. Waste solution was collected continuously at the column edge. The volume of solution between the coverslip and the base of the column (i.e. the effective bath volume) was approximately 6 μl. The basic perfusing solution was changed using a series of valves positioned above the column. Using this method, the solution within the bath could be exchanged within 10-15 s. The comparatively slow solution exchange reflected the mixing of solutions in the tubing between the valves and the column. Solutions containing caffeine were rapidly applied (20 ml min−1) for 2 s duration via a narrow plastic tube connected to an injection duct at the column base. The higher flow rate and the smaller dead space allowed a more rapid exchange of solutions within the bath. Previous measurements based on quench of indo-1 fluorescence by caffeine (O'Neill et al. 1990) under similar conditions have shown that the caffeine concentration within the bath typically increased to 50% of the concentration injected within 8-10 ms (Smith & Steele, 1993).
The bath was placed on the stage of a S200 Nikon Diaphot inverted microscope. The muscle was viewed via a × 40 objective lens (Fluor, oil immersion, Nikon) and the length increased to approximately 20% above slack length. In control experiments, we have found that length does not have a direct effect on SR Ca2+ release. The preparation was alternately illuminated with light of wavelengths 340 and 380 nm at 40 Hz frequency using a spinning wheel spectrophotometer (Cairn Research, Faversham, Kent, UK). The average [Ca2+] within the visual field containing the preparation, was indicated by the ratio of light intensities emitted at > 500 nm. Light emitted from areas of the field not occupied by the muscle image was reduced using a variable rectangular diaphragm on the side port of the microscope.
Solution composition
All chemicals were purchased from Sigma unless otherwise stated. In all experiments, the ionic composition of the solution was adjusted to maintain the [Ca2+], [Mg2+], [Na+], [K+] and pH constant. In brief, for most experiments a basic solution was prepared containing: KCl, 100 mM; Hepes, 25 mM; EGTA, 0.2 mM; phosphocreatine, 10 mM; ATP, 5 mM; fura-2, 1.5 μm. MgCl2 and CaCl2 were added (from 1 M BDH stock) to produce free concentrations of 1.2 mM and 100 nM, respectively. In most experiments where the [PCr] was reduced from 10 to 0 mM, 20 mM NaCl was added to maintain the [Na+] at 30 mM. This means that the [Cl−] increased by 20 mM, from 111.6 to 131.2 mM. The MgCl2 was reduced by approximately 0.2 mM in PCr-free solution to maintain the free Mg2+ at a constant level. However, we found that the effect of PCr withdrawal was similar, whether or not 20 mM NaCl was added to the solution lacking PCr. This suggests that (i) compensation for the change in [Na+] that accompanied PCr withdrawal had little effect and (ii) a 20 mM increase in [Cl−] had no apparent influence on the phenomena. Furthermore, increasing the [Cl−] from 112 to 132 mM at a constant [PCr], also had no significent effect on the caffeine-induced Ca2+ transients. In control experiments, we studied the effects PCr withdrawal when potassium propionate was used in place of KCl. However, the effects of PCr withdrawal reported in this study were apparently the same when propionate replaced Cl− as the principal anion. Corrections for ionic strength, details of pH measurement and the principles of the calculations are described else where (Fabiato & Fabiato, 1979; Smith & Miller, 1985). The total concentrations of Na+ and K+ were 30 mM and 130 mM, respectively. The pH was adjusted to 7.0 by addition of KOH. In most experiments, azide (5 mM) was added to inhibit possible mitochondrial activity. However, 5 mM azide had no apparent effect on the results obtained. All experiments were done at room temperature (22-24°C).
Data recording and analysis
In all experiments, the ratio and individual wavelength intensities and the isometric tension signals were low passed filtered (-3 dB at 30 Hz) and digitized for later analysis using an IBM compatible 80486 computer with a Data Translation 2801A card.
RESULTS
Effect of PCr withdrawal in mechanically skinned fibres
Figure 1A shows simultaneous recordings of the 340 nm:380 nm fluorescence ratio (upper panel) and isometric tension (lower panel) from two mechanically skinned muscle fibres. In this experiment the preparation was perfused constantly with solutions containing 100 nM Ca2+. Caffeine (40 mM) was applied for 1.5 s at 2 min intervals. Each caffeine application induced a transient increase in the fluorescence ratio due to SR Ca2+ release (which will be referred to as a Ca2+ transient) and an associated tension response. Application of caffeine for 1.5 s was sufficient to produce responses of maximal amplitude under control conditions, i.e. a more prolonged application did not increase the amplitude of the Ca2+ or tension transients (not shown).
Figure 1. Effects of PCr withdrawal on Ca2+ regulation.
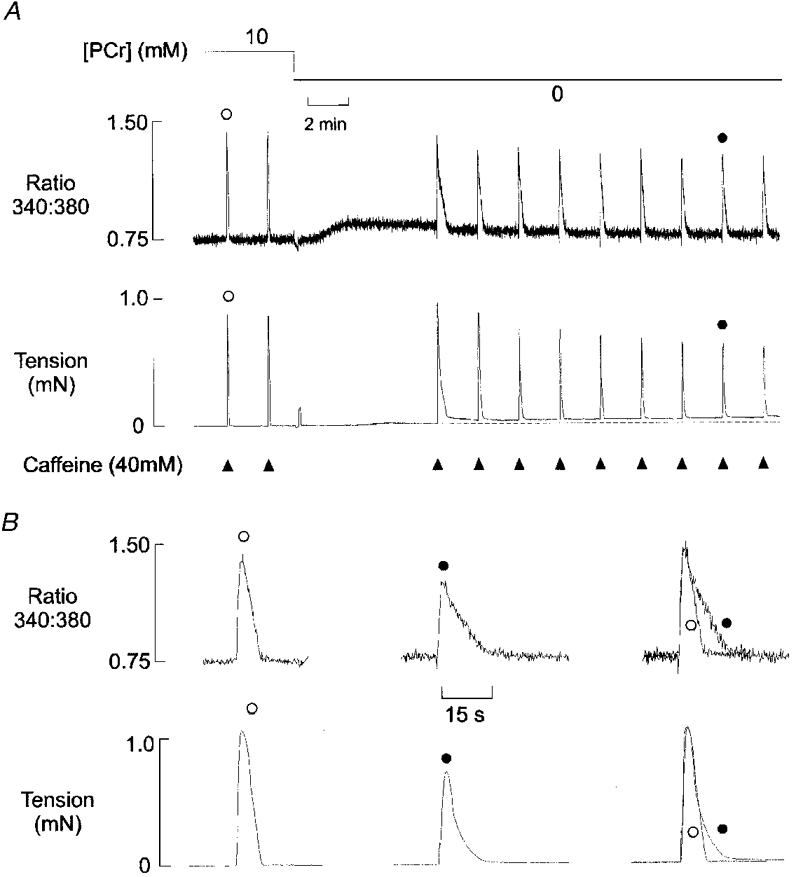
A, simultaneous records of the 340 nm:380 nm fluorescence ratio (upper panel) and isometric tension (lower panel) from a mechanically skinned EDL muscle fibre in the presence of 100 nM bathing Ca2+. Caffeine (40 mM) was briefly applied (for 1.5 s) at 2 min intervals as indicated. Each application resulted in a transient increase in the fluorescence ratio and a corresponding tension response. After two control responses, the [PCr] was reduced from 10 mM to zero, and caffeine application was stopped. PCr withdrawal resulted in a slow prolonged release of Ca2+ from the SR. When caffeine application was resumed, the amplitude of the Ca2+ and tension transients decreased to a new steady-state level over 6-8 min. B, selected Ca2+ (upper panel) and tension (lower panel) transients (shown as •and ○in A) in the presence and absence of PCr. In each panel, the superimposed responses (right) are normalized.
After two control responses, the perfusing solution was changed to one lacking PCr. Withdrawal of PCr was associated with a net release of Ca2+ from the SR. This release was slow in onset and lasted several minutes. In this example, caffeine application was resumed approximately 6 min after PCr withdrawal, and before the fluorescence ratio had completely returned to control levels. The first caffeine-induced transient following PCr withdrawal was reduced in amplitude by about 20% and markedly prolonged. Over subsequent Ca2+ load and release cycles, the amplitude declined further until a new steady-state was achieved after 6-8 min. It is apparent that the first Ca2+ transient following PCr withdrawal is more prolonged than the steady-state responses obtained after the baseline fluorescence returned to control levels. However, as shown in Fig. 1B, the steady-state Ca2+ and tension responses were still markedly reduced and prolonged relative to the preceding control responses obtained in the presence of PCr. These effects were fully reversible and the Ca2+ and tension responses returned to control levels when PCr was re-introduced (not shown)
The tension responses generally reflected the changes in the Ca2+ transient, with an increase in duration and a decrease in amplitude. However, comparison of the Ca2+ and tension transients on an expanded time scale (Fig. 1B) shows that the base of the tension transient was prolonged, while there was little effect on the initial rate of decline of tension. The descending phase of the Ca2+ transient was also unaffected until the fluorescence ratio had declined to approximately 2/3 of the peak value. This suggests that the initial decrease in the Ca2+ transient to about 2/3 of the peak value results in a decline of more than 50% in the associated tension response. This may reflect the differing relationship between [Ca2+] and the fura-2 fluorescence ratio or tension production, but this has not been investigated in detail. PCr withdrawal also resulted in a small maintained increase in resting force in most fibres. This probably results from a local decrease in the ATP:ADP.Pi ratio in the vicinity of the myofilaments. Similar results were obtained using this protocol in eight other experiments.
Effects of 2,4-dinitro-1-fluorobenzene (DNFB) on the caffeine-induced Ca2+ and tension responses
The effects of PCr withdrawal were compared with inhibition of the CK enzyme with DNFB (Fig. 2A). After two control responses to caffeine, 20 μm DNFB was introduced into the perfusing solution. This resulted in a slow maintained rise in the fluorescence ratio and a gradual decline in the caffeine-induced Ca2+ transient over 6-8 min. The superimposed responses (Fig. 2B) show that, as with PCr withdrawal, the time course of the descending phase of the Ca2+ transient was markedly prolonged. However, one clear difference is that the increase in fluorescence ratio is maintained following addition of DNFB, while a transient net Ca2+ release occurs after PCr withdrawal. This is because the 340 and 380 nm fluorescence signals were slightly reduced in the presence of DNFB (not shown). The decrease in the 380 nm signal was proportionally smaller, resulting in a maintained rise in the fluorescence ratio, which may have obscured any transient component due to SR Ca2+ release. Similar results were obtained in four other preparations. In control experiments, it was found that withdrawal of PCr had no apparent influence on the Ca2+ transients after DNFB treatment (n = 3). Similarly, addition of DNFB had no apparent effect after complete withdrawal of PCr, other than a small maintained increase in the fluorescence ratio due to the Ca2+-independent effects on fluorescence described above (n = 3). In all experiments, the effects of DNFB were found to be irreversible, as the amplitude and time course of the Ca2+ transients failed to return to control levels after removal of the drug.
Figure 2. Effects of DNFB on the caffeine-induced Ca2+ transients.
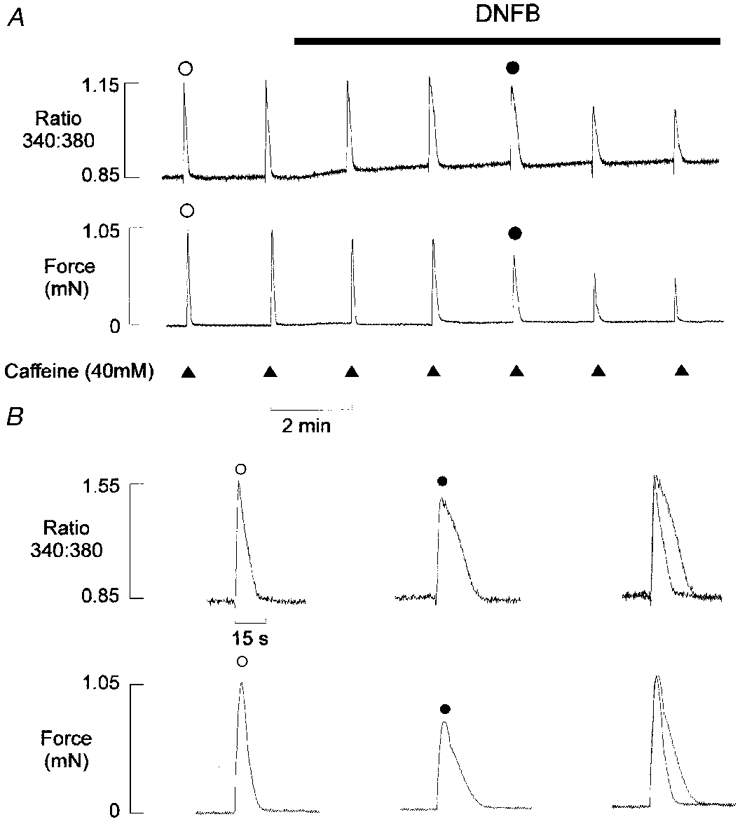
A, simultaneous records of the fluorescence ratio (upper panel) and isometric tension (lower panel) from a mechanically skinned EDL muscle fibre in the presence of 100 nM bathing Ca2+. Caffeine (40 mM) was briefly applied (for 1.5 s) at 2 min intervals. After two control responses, introduction of DNFB (20 μm) resulted in a progressive decrease in the amplitude and an increase in duration of the caffeine-induced Ca2+ and tension transients. The effects of DNFB were not reversible within 30 min (not shown). B, selected Ca2+ (upper panel) and tension (lower panel) transients are shown individually and normalized (right). 10 mM PCr was present throughout the protocol.
Effects of PCr withdrawal on Ca2+ and tension transients: accumulated data
Accumulated data showing the characteristic changes in the Ca2+ and tension transients following PCr withdrawal is given in Fig. 3. In these experiments, the preparation was initially perfused with solutions containing 10 mM PCr and caffeine was briefly applied at 2 min intervals (Fig. 3A). After two control responses, PCr was rapidly withdrawn, resulting in a slow increase in the fluorescence ratio due to net SR Ca2+ release. However, unlike the protocol shown in Fig. 1, brief caffeine application was continued at 2 min intervals throughout the period following PCr withdrawal. Under these conditions, the resting [Ca2+] typically returned to control levels within two to three Ca2+ load and release cycles. The first Ca2+ transient following withdrawal of PCr was markedly prolonged and the Ca2+ and tension transients declined to a new steady state within 4-6 min. The amplitude and time course of the 6th Ca2+ transient was not significantly different from subsequent responses (not shown). The sixth response in the series can, therefore, be considered as being representative of the steady state.
Figure 3. Time course of changes in Ca2+ and tension transients following PCr withdrawal.
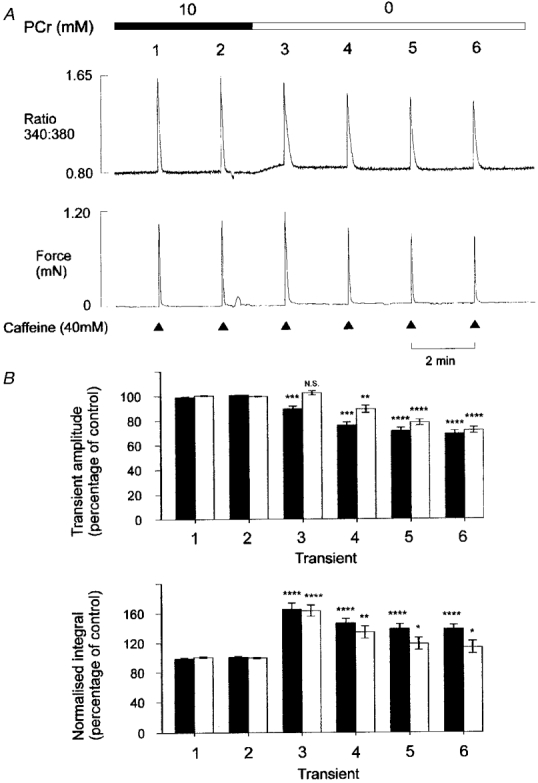
A, simultaneous records of the fluorescence ratio (upper panel) and tension (lower panel) in a mechanically skinned EDL muscle fibre. Protocol as Fig. 1, except that caffeine application was continued at 2 min intervals following PCr withdrawal. B, cumulative data showing the effects of PCr withdrawal on the caffeine-induced Ca2+ and tension transients using the protocol shown in A. It should be noted that 4-5 control responses were generally obtained prior to withdrawal of PCr, although only 2 are shown. The responses were expressed relative to the mean of all 4-5 control responses obtained in each preparation. The upper panel indicates the mean (±s.e.m.) amplitude of the caffeine-induced Ca2+ (▪) and tension (□) transients (n = 16). The lower panel shows the corresponding integrals of the normalized Ca2+ (▪) and tension (□) transients. The response number is indicated below each pair of bars, such that 1 and 2 are the control responses, and 3-6 are the transients obtained following PCr withdrawal. *P < 0.05; **P < 0.01; ***P < 0.001; **** P < 0.0001.
Figure 3B shows the changes in the amplitude and time course of the Ca2+ transients from several preparations using the protocol shown in Fig. 3A. The upper panel indicates the mean (±s.e.m.) amplitude of the Ca2+ and tension transients before and after PCr withdrawal. The lower panel shows the mean (±s.e.m.) integral of the normalized caffeine-induced Ca2+ and tension transients. As the rising phases of the Ca2+ or tension transients were apparently unaffected by PCr withdrawal (Fig. 1B), an increase in the integral of the normalized response reflects prolongation of the Ca2+ transient. The mean data show that the amplitudes of the fluorescence ratio and tension transients decrease progressively to 69.2 ± 2.7% (n = 16) and 71.8 ± 2.5% (n = 16), respectively, 6 min after PCr withdrawal. The integral of the normalized fluorescence ratio and tension transients increased markedly to 165.9 ± 7.9% and 163.4 ± 7.6% (n = 16), respectively, during the first response following PCr withdrawal and then declined slightly to a new steady state over two to three Ca2+ load and release cycles.
Concentration dependence of PCr effects on Ca2+ and tension transients
Figure 4A shows Ca2+and tension transients obtained from the same preparation in the presence of various [PCr]. Each response is representative of the steady-state transient obtained following equilibration with a solution containing the [PCr] indicated. The amplitudes of the Ca2+ and tension transients decreased progressively as the [PCr] was reduced below 5 mM. However, the Ca2+ transient was significantly prolonged as [PCr] was decreased from 10 to 5 mM. Prolongation of the Ca2+ transient was more pronounced as the [PCr] was further reduced. The accumulated data in Fig. 4B also show that the average Ca2+ and tension transient amplitudes were not significantly affected by a reduction in the [PCr] from 10 to 5 mM. However, the time course of the fluorescence ratio transient increased significantly to 109.9 ± 4.9% (n = 5) as indicated by the increase in the normalized transient integrals. As the [PCr] was decreased further to 2, 1 and 0 mM, the amplitude of the Ca2+ and tension transients decreased progressively and the normalized integrals increased, indicating further prolongation of the transients.
Figure 4. Effects of PCr withdrawal: concentration dependence.
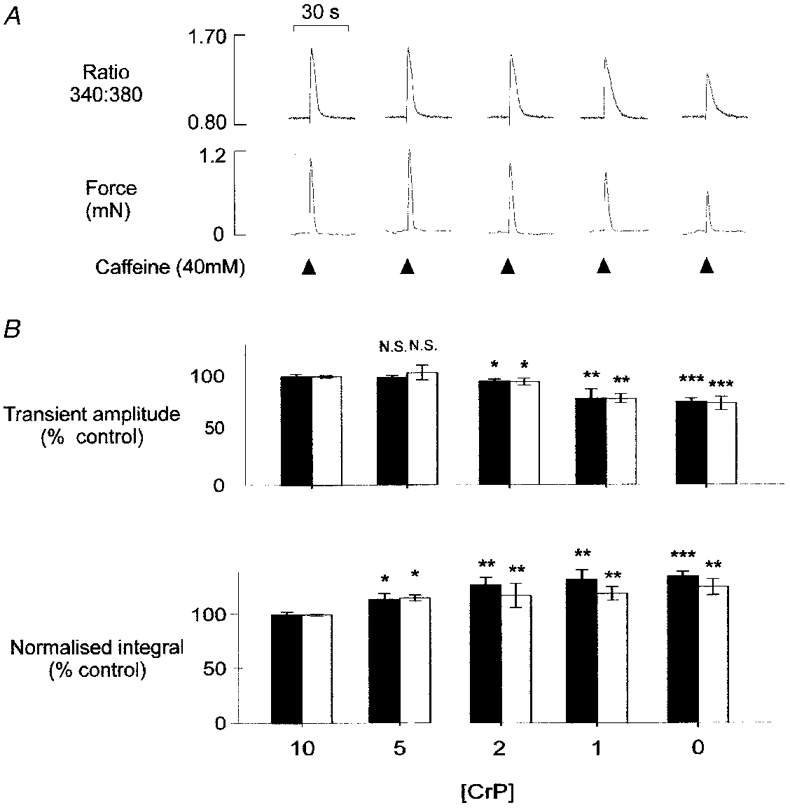
A, steady-state Ca2+(upper panel) and tension (lower panel) transients in the presence of 10, 5, 2, 1 and 0 mM PCr. All responses are from the same mechanically skinned preparation, after a minimum of 8 min equilibration at each [PCr]. B, accumulated data showing the mean (±s.e.m.) amplitude (upper panel) and integral of the normalized Ca2+ (▪) and tension (□) transients (lower panel) in the presence of various [PCr]. In each preparation, steady-state transients at 5, 2, 1 and 0 mM PCr were expressed relative to the mean of 4-5 control responses obtained in the presence of 10 mM PCr. *P < 0.05, **P < 0.01, ***P < 0.0001 compared with control response at 10 mM PCr. N.S., not significant. With 5 mM, 2 mM and 1 mM PCr, n = 6. With 0 mM PCr, n = 16.
Comparative effects of Ca2+ pump inhibition and increased SR Ca2+ permeability
We have shown previously in skinned frog skeletal muscle fibres that inhibition of the SR Ca2+ pump with CPA markedly prolongs the descending phase of the caffeine-induced Ca2+ transient (Duke & Steele, 1998a). If this also occurs in rat EDL fibres, then the prolongation associated with PCr withdrawal may also reflect a reduced SR Ca2+ uptake rate. However, an alternative explanation is that prolongation of the Ca2+ transient reflects an increased Ca2+‘leak’ or efflux from the SR. If net Ca2+ accumulation reflects a balance between uptake (via the SR Ca2+ pump) and efflux (via a Ca2+ leak pathway), an increase in efflux may similarly prolong the SR Ca2+ transient.
To investigate these possibilities, the effects of CPA were compared with increased SR Ca2+ permeability induced by exposure to ionomycin (Fig. 5A). Introduction of 4 μm CPA resulted in (i) a slow net release of Ca2+ from the SR, (ii) potentiation of the following caffeine-induced Ca2+ transient, followed by (iii) a progressive and marked decline in the amplitude of the Ca2+ transient. The normalized responses (right) show that CPA also slowed the descending phase of the Ca2+ transient in rat EDL. For comparison, the effects of 100 nM ionomycin on the caffeine-induced Ca2+ transient are shown in Fig. 5B. This level of ionomycin produced an efflux of Ca2+ from the SR, which was similar in time course and magnitude to that induced by 4 μm CPA, and a progressive decline in the Ca2+ transient. Nevertheless, it was found consistently that ionomycin had little or no effect on the descending phase of the Ca2+ transient (right). Furthermore, the first Ca2+ transient after introduction of ionomycin was always reduced in amplitude. Similar data were obtained in four other preparations. Qualitatively similar results were also obtained, when the SR Ca2+ permeability was increased by reducing the [Mg2+] or inclusion of low levels of caffeine in the perfusing solution (not shown). These data suggest that pump inhibition tends to prolong the Ca2+ transient, while an increase in SR Ca2+ permeability has little effect on the time course under these conditions (see Discussion).
Figure 5. Comparative effects of CPA and ionomycin.
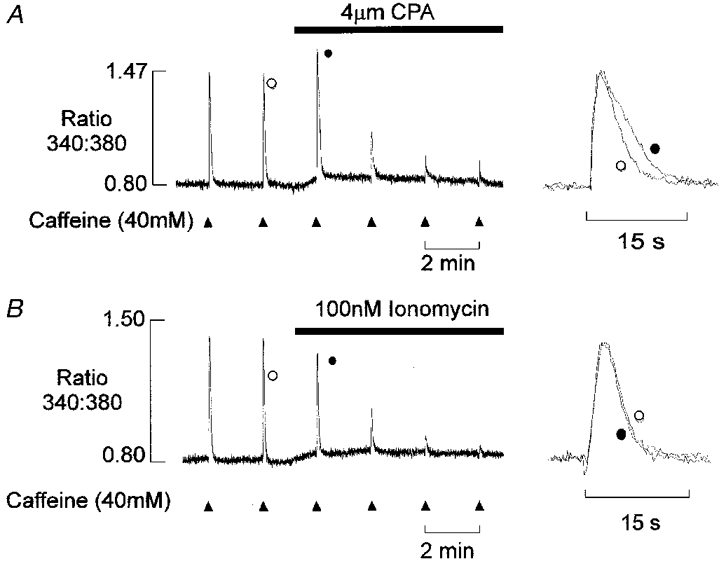
A, after two control responses to caffeine, 4 μm CPA was introduced into the perfusing solution. This resulted in a transient increase in the amplitude of the caffeine-induced Ca2+ transient followed by a marked decline over 4-6 min. The superimposed responses on an expanded time scale (right) show that the descending phase of the caffeine-induced Ca2+ transient is prolonged. B, after two control responses the Ca2+ permeability of the SR was increased by addition of 100 nM ionomycin. This resulted in a progressive decline in the amplitude of the Ca2+ transient, with little effect on the time course (right). Introduction of both CPA and ionomycin caused a slow transient release of Ca2+ from the SR.
Mechanism underlying the Ca2+ efflux associated with PCr withdrawal
The mechanism underlying the net Ca2+ efflux associated with PCr withdrawal was further investigated by exposing the preparations to the SR Ca2+ channel inhibitors ryanodine and Ruthenium Red. In Fig. 6A, the preparation was perfused with a solution containing 100 nM Ca2+ and caffeine was briefly applied at 2 min intervals. After three control responses, PCr was withdrawn from the solution, resulting in a net release of Ca2+ from the SR. After 6 min, the [PCr] was increased again to 10 mM (not shown) and the caffeine-induced Ca2+ transients returned to near-control levels as shown (Fig. 6B). Subsequent exposure of the muscle to 20 μm ryanodine effectively abolished the caffeine-induced Ca2+ transient (Fig. 6C). However, in the continued presence of ryanodine, withdrawal of PCr induced a net efflux of Ca2+ from the SR, which was similar in amplitude and time course to that obtained under control conditions (Fig. 6D). The net efflux of Ca2+ obtained on withdrawal of PCr was also unaffected by further exposure to the SR Ca2+ channel blocker Ruthenium Red (50 μm) at a level, which completely blocks caffeine-induced Ca2+ release (Fig. 6E). The lack of effect of SR Ca2+ channel inhibitors suggests that the net Ca2+ efflux observed on PCr withdrawal does not occur via the SR Ca2+ channel. Similar results were obtained in five other preparations.
Figure 6. Ca2+ efflux associated with PCr withdrawal is insensitive to ryanodine or Ruthenium Red.
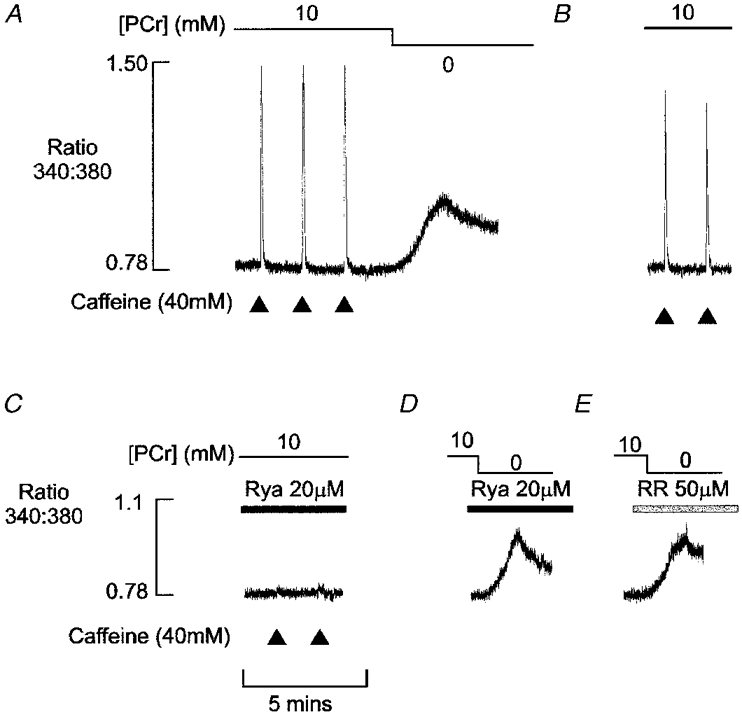
A, after three caffeine-induced Ca2+transients, PCr was withdrawn, resulting in a slow prolonged release of Ca2+ from the SR. B, after 6 min, the [PCr] was increased again to 10 mM (not shown) and the steady-state caffeine-induced Ca2+ transients returned to near-control level. C, after treatment with 20 μm ryanodine for 10 min, the caffeine-induced Ca2+ transients were almost completely abolished. The Ca2+ release associated with PCr was essentially unaffected by ryanodine (D) or by further treatment with 50 μm Ruthenium Red (E). All responses were obtained in the same preparation.
While the net Ca2+ efflux associated with PCr withdrawal was insensitive to Ruthenium Red or ryanodine, the efflux was markedly reduced by prior exposure to cyclopiazonic acid (Fig. 7). After four control responses to 40 mM caffeine, 1 μm CPA was added to the perfusing solution. This level of CPA typically induced a small transient rise in the fluorescence ratio due to loss of Ca2+ from the SR. Subsequent withdrawal of PCr consistently failed to induce a further release of Ca2+ from the SR. However, application of caffeine induced a Ca2+ transient of similar amplitude to that obtained prior to CPA treatment. The large response to caffeine suggests that failure to observe Ca2+ release on PCr withdrawal did not reflect depletion of SR Ca2+. The first caffeine-induced Ca2+ transient after PCr withdrawal was markedly prolonged, due to the combined effects of Ca2+ pump inhibition with CPA and the prolongation associated with PCr removal. Indeed, the second caffeine-induced after PCr withdrawal Ca2+ transient to about 25% of control levels, suggesting that the SR only partially refilled during the preceding 2 min period. Similar results were obtained in four other preparations.
Figure 7. Effects of CPA on PCr withdrawal efflux.
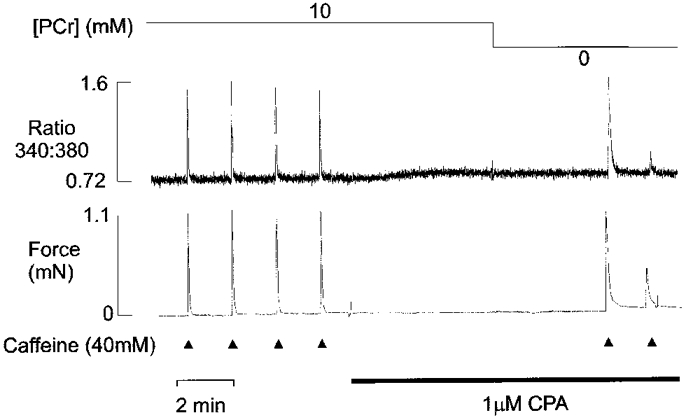
Simultaneous records of isometric tension (lower panel) and the fura-2 fluorescence ratio (upper panel). After four caffeine-induced Ca2+ transients obtained under control conditions, 1 μm CPA was introduced into the perfusing solution. As previously shown, introduction of CPA was associated with a slow prolonged release of Ca2+ from the SR, which resulted in a small transient rise in the fluorescence ratio. In the continued presence of CPA, withdrawal of PCr failed to induce Ca2+ release from the SR, while subsequent application of caffeine caused a large Ca2+ and tension transient.
DISCUSSION
In this study, we have shown that PCr withdrawal results in loss of Ca2+ from the SR, prolongation of the caffeine-induced Ca2+ transient and a decrease in the Ca2+ transient amplitude. The descending phase of the caffeine-induced Ca2+ transient was prolonged slightly by a reduction in the [PCr] from 10 to 5 mM, with little affect on the amplitude (Fig. 4). However, the amplitude of the Ca2+ transient decreased progressively as the [PCr] was reduced below 5 mM and the prolongation became increasingly marked. Prolongation of the Ca2+ transient was greatest during the net Ca2+ efflux which followed PCr withdrawal. However, prolongation of the response was still apparent under steady-state conditions, after maintained exposure to each selected [PCr] below 5 mM.
The characteristic effects of PCr withdrawal were mimicked by application of the CK inhibitor DNFB (Fig. 2). This suggests that reducing the [PCr] influences SR Ca2+ regulation by limiting the local regeneration of ATP at sites, where endogenous CK is bound within the muscle. After treatment with DNFB, PCr withdrawal had no apparent influence on the Ca2+ transient (not shown), suggesting that PCr does not have additional direct effects on the SR.
Prolongation of Ca2+ transient: increased SR Ca2+ permeability or pump inhibition?
We have shown previously that addition of CPA markedly prolongs the Ca2+ transient resulting from brief application of caffeine in frog skeletal muscle fibres (Duke & Steele, 1998a). As shown in Fig. 5, addition of CPA also prolonged the descending phase of the caffeine-induced Ca2+ transient in the rat EDL fibres used in the present study. Hence, the descending phase of the Ca2+ transient is partly dependent upon re-accumulation of Ca2+ by the SR under these conditions. This introduces the possibility that prolongation of the Ca2+ transient following withdrawal of PCr may result from a reduced rate of Ca2+ uptake by the SR.
An alternative explanation is that PCr withdrawal results in an increased Ca2+ leak or efflux from the SR. If net Ca2+ accumulation reflects a balance between uptake (via the SR Ca2+ pump) and efflux (via a Ca2+ leak pathway), an increase in efflux might also be expected to prolong the SR Ca2+ transient. However, increasing the Ca2+ permeability of the SR with ionomycin had little apparent effect on the time-course of the response, despite the fact that the Ca2+ transient was markedly reduced in amplitude (Fig. 5).
The lack of effect of increased SR Ca2+permeability on the time course of the response may reflect changes in the relationship between Ca2+ uptake and efflux during the Ca2+ transient. At the peak of the caffeine-induced Ca2+ transient, the [Ca2+] at surface of the SR is high and uptake via the Ca2+ pump may be able to compensate for a moderate rise in Ca2+ permeability. At this point, the Ca2+ leak may also be relatively small as the SR Ca2+ content is at a minimum level. However, when the cytosolic [Ca2+] approaches resting levels, any Ca2+ leak would be expected to have a greater relative influence as (i) the Ca2+ pump progressively deactivates and (ii) efflux via the Ca2+ leak pathway rises in proportion to the [Ca2+] gradient across the SR membrane. In these circumstances, it is possible that an increase in Ca2+ leak may reduce the maximum SR Ca2+ content with little effect on the time course of the caffeine-induced Ca2+ transient (Fig. 5).
Qualitatively similar effects have been reported in intact preparations following inhibition of the Ca2+ pump or when Ca2+ efflux is potentiated by maintained application of caffeine (Westerblad & Allen, 1996b). Introduction of a specific SR Ca2+ pump inhibitor during a tetanic contraction increased maximum [Ca2+]i and markedly prolonged the descending phase of the intracellular Ca2+ transient during relaxation. In mechanically skinned toad fibres, depolarization-induced Ca2+ release was also potentiated and prolonged by inhibition of the SR Ca2+pump (Owen et al. 1997) In contrast, introduction of caffeine at a concentration that increased tetanic [Ca2+]i to a similar level had little effect on the initial rapid decline in [Ca2+]i that occurs on cessation of stimulation, and only slightly prolonged the slower ‘Ca2+ tail’ (Westerblad & Allen, 1996b).
On balance, therefore, prolongation of the descending phase of the Ca2+ transient following PCr withdrawal is more consistent with pump inhibition than increased SR Ca2+ permeability. Prolongation may result from (i) raised levels of ADP, which can act locally to impair SR Ca2+ uptake or (ii) reduced supply of ATP via CK which may be functionally coupled to the SR ATPase (Korge et al. 1993). However, one noticeable difference between PCr withdrawal and pump inhibition is that introduction of CPA consistently resulted in potentiation of the first caffeine-induced Ca2+ transient after introduction of the drug (Fig. 5). This contrasts with the effects of PCr withdrawal, which never potentiated the caffeine response. One possible explanation for this is that PCr withdrawal also results in desensitization of the release mechanism. We have shown previously that the amplitude of the caffeine-induced Ca2+ transient is dependent on the cytosolic [ATP] (Duke & Steele, 1998b). Hence, the release mechanism may become desensitized following PCr withdrawal, due to abolition of local ATP buffering in the vicinity of the SR Ca2+ channel, where CK is bound (Rossi et al. 1990). Further experiments are currently in progress to investigate this possibility.
Mechanism of Ca2+ efflux observed on PCr withdrawal
Caffeine-induced Ca2+ release was completely abolished by treatment with ryanodine or Ruthenium Red, while the net Ca2+ efflux associated with PCr withdrawal was apparently unaffected (Fig. 6). This suggests that the PCr withdrawal efflux does not involve activation of the SR Ca2+ channel. The PCr withdrawal efflux was abolished by prior exposure of the preparation to CPA. As previously reported, addition of CPA was itself associated with a slow efflux of Ca2+ from the SR (Duke & Steele, 1998a). However, CPA-induced depletion of SR Ca2+ cannot explain the lack of response to PCr withdrawal, because the first response to caffeine following introduction of CPA was of similar amplitude to the control responses, despite the loss of some Ca2+ from the SR (Fig. 7). This is because re-uptake of Ca2+ normally buffers the released Ca2+ under these conditions, and buffering is markedly reduced following pump inhibition by CPA (Duke & Steele, 1998a).
The ability of CPA to block efflux of Ca2+ on withdrawal of PCr could involve a number of possible mechanisms. The first possibility is that PCr withdrawal simply inhibits the SR Ca2+ pump as suggested above. Rapid withdrawal of PCr would then be expected to cause a net efflux of Ca2+ from the SR as the Ca2+ leak transiently exceeds the ability of the Ca2+ pump to re-accumulate Ca2+. In this case, PCr withdrawal would have essentially the same effect as adding a pump inhibitor, such as CPA. Furthermore, when the SR Ca2+ pump is inhibited in the presence of high levels of CPA, subsequent removal of PCr would not be expected to have any further effect, as shown in Fig. 7. However, this explanation seems unlikely, because CPA is more effective at inhibiting the SR Ca2+ pump, but generally it induces a smaller net Ca2+ efflux than that occurring on PCr withdrawal (not shown).
An alternative explanation is that withdrawal of PCr results in loss of Ca2+ by reversal of the SR Ca2+ pump. Previous work on isolated SR vesicles has shown that increasing levels of Pi or ADP can induce Ca2+ efflux from the SR due to reversal of the Ca2+ pump (see Steele et al. 1995 for references and discussion). Pump reversal might occur in the present study because removal of PCr and the abolition of local ATP regeneration would be expected to reduce the [ATP] and increase the [ADP], both of which favour pump reversal. This possibility is supported by recent work which has shown that CPA blocks Ca2+ efflux associated with pump reversal (Du et al. 1994). Furthermore, we have found that cessation of SR Ca2+ uptake by complete withdrawal of ATP does not block the efflux of Ca2+ from the SR on PCr withdrawal (not shown). Hence, inhibition of the SR Ca2+ pump, or indeed the associated loss of some SR Ca2+ does not itself prevent the Ca2+ efflux which accompanies PCr withdrawal.
On balance, therefore, these results suggest that withdrawal of PCr may cause some Ca2+ efflux via the SR Ca2+ pump, which can be effectively blocked by CPA. It is not clear, however, whether significant Ca2+ efflux via the SR Ca2+ pump is maintained in the absence of PCr and if so, whether this contributes to prolongation of the caffeine-induced Ca2+ transient. It is possible that pump reversal may occur only transiently following withdrawal of PCr, because the Ca2+ pump cannot sustain the existing [Ca2+] gradient due to a decrease in the local [ATP]:[ADP.Pi] ratio. In this case, pump reversal may only occur until the SR Ca2+ content has fallen to a level, which can be sustained energetically.
Relationship to previous studies on intact tissue
Many previous studies on intact skeletal muscle have shown that Ca2+ uptake by the SR is impaired under conditions of fatigue. This is associated with prolongation of the descending phase of the Ca2+ transient and a progressive increase in the resting [Ca2+]. A metabolic mechanism has often been proposed, although recent work appears to have excluded a causal link between impaired SR function and acidosis (Westerblad et al. 1997) or increased levels of Pi (Westerblad & Allen, 1996a).
Experiments involving 31P NMR have shown marked depletion of PCr occurs during fatigue and that this is correlated with changes in the relaxation rate (Dawson et al. 1980). Similarly, experiments on isolated fibres from Xenopus laevis have shown that [PCr] levels can reach undetectable levels during fatiguing stimulation (Nagesser et al. 1993). The present study has shown that in skinned fibres, a reduction in the [PCr] results in a net efflux of Ca2+ from the SR and a marked prolongation of the descending phase of the caffeine-induced Ca2+ transient. Assuming this occurs in intact fibres, such effects may explain or contribute to the prolongation of the Ca2+ transient.
One additional factor which must be considered, when interpreting the present data, is that in skinned fibres, any Ca2+ which is lost from the SR can diffuse into the effectively infinite volume of solution surrounding the muscle. In these circumstances, any net efflux of Ca2+ from the SR will not result in a maintained increase in [Ca2+] within the fibre (e.g. Fig. 1). However, in cells with an intact sarcolemma, an efflux of Ca2+ from the SR would be expected to cause a maintained rise in the resting [Ca2+]. Similarly, a progressive reduction in the rate of SR Ca2+ accumulation would also tend to cause a redistribution of Ca2+ from the SR to the cytosol, as occurs in fatigue.
It is important to emphasize, however, that while reduced SR Ca2+ uptake associated with PCr depletion may explain or contribute to slowing of the Ca2+ transient in intact fibres during fatigue, it may not necessarily underlie the slowing of relaxation. Current evidence suggests that in mouse fibres, the myofilaments may limit the rate of relaxation in fatigue (Westerblad & Allen, 1993), while the SR may be more important in Xenopus laevis (Westerblad et al. 1989). Therefore, the relative importance of impaired Ca2+ regulation and altered myofilament properties may vary depending on species, muscle fibre type and the experimental conditions.
Summary
The present data show that in permeabilized rat skeletal muscle fibres, acute PCr withdrawal results in (i) loss of Ca2+ from the SR and (ii) prolongation and a decrease in the amplitude of the caffeine-induced Ca2+ transient. Prolongation of the Ca2+ transient appears most consistent with a reduced rate of SR Ca2+ uptake, while the initial Ca2+ efflux may involve reversal of the SR Ca2+ pump. These effects may explain or contribute to the characteristic changes in [Ca2+] regulation which during fatigue.
Acknowledgments
Financial support for this work was provided by The Wellcome Trust and the British Heart Foundation.
References
- Allen DG, Lännergren J, Westerblad H. The role of ATP in the regulation of intracellular Ca2+ in single fibres of mouse skeletal muscle. The Journal of Physiology. 1997;498:587–600. doi: 10.1113/jphysiol.1997.sp021885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DG, Westerblad J, Westerblad H. Muscle cell function during prolonged activity: cellular mechanisms of fatigue. Experimental Physiology. 1995;80:497–527. doi: 10.1113/expphysiol.1995.sp003864. [DOI] [PubMed] [Google Scholar]
- Dawson MJ, Gadian DG, Wilkie DR. Mechanical relaxation rate and metabolism studied in fatiguing muscle by phosphorus nuclear magnetic resonance. The Journal of Physiology. 1980;299:465–484. doi: 10.1113/jphysiol.1980.sp013137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du GG, Ashley CC, Lea TJ. Effects of thapsigargin and cyclopiazonic acid on the sarcoplasmic-reticulum Ca2+ pump of skinned fibers from frog skeletal-muscle. Pflügers Archiv. 1994;429:169–175. doi: 10.1007/BF00374309. [DOI] [PubMed] [Google Scholar]
- Duke AM, Steele DS. Effects of cyclopiazonic acid on Ca2+ regulation by the sarcoplasmic reticulum in saponin permeabilized skeletal muscle fibres. Pflügers Archiv. 1998a;436:104–111. doi: 10.1007/s004240050610. [DOI] [PubMed] [Google Scholar]
- Duke AM, Steele DS. Effects of caffeine and adenine nucleotides on Ca2+ release by the sarcoplasmic reticulum in saponin permeabilized frog skeletal muscle fibres. The Journal of Physiology. 1998b;513:43–53. doi: 10.1111/j.1469-7793.1998.043by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Calculator programs for computing the composition of solutions containing multiple metals and ligands used for experiments in skinned muscle cells. Journal de Physiologie. 1979;75:463–505. [PubMed] [Google Scholar]
- Fitts RH. Cellular mechanisms of muscle fatigue. Physiological Reviews. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- Fryer MW, Owen VJ, Lamb GD, Stephenson GD. Effects of creatine phosphate and Pi on Ca2+ movements and tension development in rat skinned skeletal muscle fibres. The Journal of Physiology. 1995;482:123–140. doi: 10.1113/jphysiol.1995.sp020504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korge P, Byrd SK, Campbell KB. Functional coupling between sarcoplasmic-reticulum-bound creatine kinase and Ca2+-ATPase. European Journal of Biochemistry. 1993;213:973–980. doi: 10.1111/j.1432-1033.1993.tb17842.x. [DOI] [PubMed] [Google Scholar]
- Lamb GD, Stephenson DG. Calcium release in skinned muscle-fibers of the toad by transverse tubule depolarization or by direct stimulation. The Journal of Physiology. 1990;423:495–517. doi: 10.1113/jphysiol.1990.sp018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagesser AS, van der Laarse WJ, Elzinga G. ATP formation and ATP hydrolysis during fatiguing, intermittent stimulation of different types of single muscle fibres from Xenopus laevis. Journal of Muscle Research and Cell Motility. 1993;14:608–618. doi: 10.1007/BF00141558. [DOI] [PubMed] [Google Scholar]
- O'Neill SC, Donoso P, Eisner DA. The role of [Ca2+]i and [Ca2+] sensitization in the caffeine contracture of rat myocytes: measurement of[Ca2+]i and [caffeine]i. The Journal of Physiology. 1990;425:55–70. doi: 10.1113/jphysiol.1990.sp018092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen VJ, Lamb GD, Stephenson DG, Fryer MW. Relationship between depolarization-induced force responses and Ca2+ content in skeletal muscle fibres of rat and toad. The Journal of Physiology. 1997;498:571–586. doi: 10.1113/jphysiol.1997.sp021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi AM, Eppenberger HM, Volpe P, Cotrufo R, Wallimann T. Muscle-type MM creatine kinase is specifically bound to sarcoplasmic reticulum and can support Ca2+ uptake and regulate local ATP/ADP ratios. Journal of Biochemistry. 1990;265:5258–5266. [PubMed] [Google Scholar]
- Smith GL, Miller DJ. Potentiometric measurements of stoichiometric and apparent affinity constants of EGTA for protons and divalent ions including calcium. Biochimica et Biophysica Acta. 1985;839:287–299. doi: 10.1016/0304-4165(85)90011-x. [DOI] [PubMed] [Google Scholar]
- Smith GL, Steele DS. Effects of 2,3 butanedione monoxime on sarcoplasmic reticulum of saponin treated rat cardiac muscle. American Journal of Physiology. 1993;265:H1493–1500. doi: 10.1152/ajpheart.1993.265.5.H1493. [DOI] [PubMed] [Google Scholar]
- Steeghs K, Benders A, Oerlemans F, Dehaan A, Heerschap A, Ruitenbeek W, Jost C, Vandeursen J, Perryman B, Pette D, Bruckwilder M, Koudijs J, Jap P, Veerkamp J, Wieringa B. Altered Ca2+ responses in muscles with combined mitochondrial and cytosolic creatine kinase deficiencies. Cell. 1997;89:93–103. doi: 10.1016/s0092-8674(00)80186-5. [DOI] [PubMed] [Google Scholar]
- Steele DS, McAinsh AM, Smith GL. Effects of creatine-phosphate and inorganic-phosphate on the sarcoplasmic-reticulum of saponin-treated rat-heart. The Journal of Physiology. 1995;483:155–166. doi: 10.1113/jphysiol.1995.sp020575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. Changes of myoplasmic calcium concentration during fatigue in single mouse muscle fibers. Journal of General Physiology. 1991;98:615–635. doi: 10.1085/jgp.98.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. The contribution of [Ca2+]i to the slowing of relaxation in fatigued single fibres from mouse skeletal muscle. The Journal of Physiology. 1993;468:729–740. doi: 10.1113/jphysiol.1993.sp019797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. The effects of intracellular injections of phosphate on intracellular calcium and force in single fibres of mouse skeletal muscle. Pflügers Archiv. 1996a;431:964–970. doi: 10.1007/s004240050092. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. Mechanisms underlying changes of tetanic [Ca2+]i and force in skeletal muscle. Acta Physiologica Scandinavica. 1996b;162:407–416. doi: 10.1046/j.1365-201X.1996.196000.x. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Allen DG, Bruton JD, Andrade FH, Lännergren J. Mechanisms underlying the reduction of isometric force in skeletal muscle fatigue. Acta Physiologica Scandinavica. 1998;162:253–260. doi: 10.1046/j.1365-201X.1998.0301f.x. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Allen DG, Lee JA. Measurements of intracellular calcium during fatiguing stimulation in single Xenopus muscle fibres. Progress in Clinical and Biological Research. 1989;315:231–232. [PubMed] [Google Scholar]
- Westerblad H, Bruton JD, Lännergren J. The effect of intracellular pH on contractile function of intact, single fibres of mouse muscle declines with increasing temperature. The Journal of Physiology. 1997;500:193–204. doi: 10.1113/jphysiol.1997.sp022009. [DOI] [PMC free article] [PubMed] [Google Scholar]


