Abstract
The membrane properties and conduction velocities of antidromically activated medial septum-diagonal band (MS-DB) neurons were examined using whole-cell recordings in a longitudinally cut rat brain slice preparation containing the MS-DB and the dorsal fornix.
MS-DB neurons were divided into three groups according to their action potential characteristics and firing properties. Slow firing neurons displayed a broad action potential followed by a prominent after-hyperpolarization. Burst firing neurons, when depolarized from hyperpolarized holding potentials, exhibited a high-frequency burst of spikes on the crest of a slow depolarizing potential. Fast firing neurons did not fire bursts of spikes when depolarized from hyperpolarized holding potentials.
Eighteen MS-DB neurons were identified as septohippocampal by antidromic activation. Of the septohippocampal neurons, four were slow firing neurons, five were burst firing neurons and nine were fast firing neurons. The mean axon conduction velocities of these neurons fell into two significant groups, termed slow conducting and fast conducting. Slow firing septohippocampal neurons had significantly slower conduction velocities than either fast firing or burst firing neurons (P < 0.05), being 0.7 ± 0.5 ms−1 for slow firing neurons and 2.9 ± 2.0 and 2.0 ± 1.4 ms−1 for burst firing and fast firing neurons, respectively.
On the basis of previous evidence which has linked firing properties with the neurochemical identities of the neurons, we propose that the slow firing septohippocampal neurons are cholinergic whereas the burst firing and fast firing septohippocampal neurons are GABAergic.
Neurons in the medial septum-diagonal band area (MS-DB) which project to the hippocampus are believed to play an important role in the generation and maintenance of the hippocampal theta rhythm (reviewed by Stewart & Fox, 1990). The theta rhythm is a large amplitude, 4–12 Hz, periodic field potential recorded in the hippocampal formation from conscious or urethane-anaesthetized animals, and is thought to reflect the synchronized discharge of a large population of neurons (Green & Arduini, 1954; Vanderwolf, 1969; Green & Rawlins, 1979). A significant proportion of the septohippocampal neurons also display a rhythmically bursting activity (Petsche et al. 1962; Lamour et al. 1984) that is tightly coupled to the frequency of the hippocampal theta rhythm during various behavioural states (Petsche et al. 1962; Apostol & Creutzfeldt, 1974; Sweeney et al. 1992). The cells in the MS-DB have therefore been described as the pacemaker of the hippocampal theta rhythm (Green & Arduini, 1954; Petsche et al. 1962; Gogolàk et al. 1968; Apostol & Creutzfeldt 1974; Stewart & Fox, 1989b, 1990; Vinogradova, 1995) and lesions of the MS-DB or fimbria- fornix abolish hippocampal theta (Green & Arduini, 1954; Petsche et al. 1962; Ranck, 1973; Rawlins et al. 1979).
Two types of hippocampal theta rhythm are distinguished: one is associated with movements such as walking and rearing and is mostly resistant to atropine, and the other occurs spontaneously during immobility under urethane anaesthesia, and is abolished by atropine (Kramis et al. 1975). The atropine sensitivity of the hippocampal theta rhythm reflects the involvement of acetylcholine, and the major source of cholinergic innervation of the hippocampus comes from the MS-DB, via the fimbria-fornix pathway (Lewis & Shute, 1967; Amaral & Kurz, 1985; Wainer et al. 1985). It has been shown more recently that GABAergic MS-DB neurons project to the hippocampus, also via the fimbria-fornix pathway (Köhler et al. 1984; Kiss et al. 1990). The cholinergic neurons synapse onto all hippocampal cell types while the GABAergic neurons terminate on hippocampal GABAergic neurons (Frotscher & Leranth, 1985; Freund & Antal, 1988). Electrophysiological studies in vivo also indicate the presence of two types of septohippocampal neuron (identified by antidromic activation) based on the differential sensitivity of their burst firing to atropine (Stewart & Fox, 1989a). Stewart & Fox (1990) proposed that the atropine-insensitive bursting cells are GABAergic and that they act as ‘pacemaker’ cells, i.e. their intrinsic properties allow the generation of rhythmic oscillations. It is now believed that the hippocampal theta rhythm relies on the coactivation of cholinergic and GABAergic MS-DB inputs to the hippocampal formation (Stewart & Fox, 1990; Smythe et al. 1992), but it is apparent that the GABAergic neurons play a more dominant role in this, since selective depletion of the cholinergic component of the septohippocampal pathway reduces the amplitude of the theta rhythm but does not abolish it (Lee et al. 1994; Bassant et al. 1995).
A number of studies have been carried out on the basic electrophysiological properties of MS-DB neurons in vitro, in order to identify which neurons possess the intrinsic properties necessary to act as pacemaker cells in vivo (Griffith & Matthews, 1986; Segal, 1986; Griffith, 1988; Markram & Segal, 1990; Griffith et al. 1991). Three types of neurons have been identified based on active and passive membrane properties: (1) slow firing neurons with a broad action potential and a long duration postspike after-hyperpolarization (AHP), (2) fast firing neurons with short duration action potential and short duration AHP and (3) neurons that fire in a bursting mode when depolarized from a hyperpolarized holding potential. Double-labelling techniques have shown that slow firing cells possess acetylcholinesterase activity (Griffith & Matthews, 1986), and choline acetyltransferase immunoreactivity (Markram & Segal, 1990; Gorelova & Reiner, 1996), indicating that they are cholinergic. The neurochemistry of the fast firing and bursting cells is not known, but given that the majority of cells in the MS-DB are either GABAergic or cholinergic (Gritti et al. 1993), it is assumed, by exclusion, that the fast firing and bursting cells in the MS-DB are GABAergic. Peptide neurotransmitters also exist within the MS-DB but are co-localized with GABA or acetylcholine (review by Jakab & Leranth, 1995).
Understanding the role of the GABAergic and cholinergic neurons in the hippocampal theta rhythm is important, but it has been difficult to correlate the in vitro electrophysiological properties of the neurons with their behaviour in vivo. In slices containing MS-DB neurons, although rhythmic firing is occasionally observed (Griffith & Matthews, 1986; Segal, 1986; Serafin et al. 1996), it has not been possible to induce a maintained burst firing pattern in the theta range of frequencies. Some studies have adopted other measures to correlate in vivo with in vitro properties of MS-DB neurons, either by performing intracellular recording in vivo (Barrenechea et al. 1995; Brazhnik & Fox, 1997), or by correlating the extracellular waveform of the action potential in vivo (King et al. 1998) with that recorded from MS-DB neurons in vitro (Matthews & Lee, 1991). In the present study we correlate the in vitro electrophysiological properties of the neurons with the conduction velocities of their axons, a parameter that is relatively easy to measure in vivo (Dutar et al. 1985; Barrenechea et al. 1995). Previous in vitro studies have employed coronal brain slices, acutely dissociated cells or cells in primary culture, which preclude the identification of septohippocampal neurons by antidromic activation. Consequently, the aim of the present study was to determine the intrinsic membrane properties and firing characteristics of antidromically activated neurons recorded in a parasagittal slice of the MS-DB, in which the connection with the fornix is retained. Preliminary observations have been presented to The Physiological Society (Norris, 1996).
METHODS
Preparation of brain slices
All experiments were carried out under a UK Home Office licence and in accordance with the regulations of the UK Animals (Scientific Procedures) Act 1986. Parasagittal slices (500 μm thick), cut near the midline, and containing the MS-DB and dorsal fornix (Fig. 1A) were prepared from the brains of 21-day-old Wistar rats deeply anaesthetized with sodium pentobarbitone (80 mg kg−1i.p.) prior to decapitation. The slices were cut in an ice-cold solution that had the following composition (mM): 252 sucrose, 3 KCl, 2 MgSO4, 24 NaHCO3, 1.25 NaH2PO4, 1.5 CaCl2, 10 glucose, with a pH of 7.4, and which was saturated with 95 % O2-5 % CO2. Single slices were then transferred to an interface or submerged bath and perfused with the same solution at 25°C and at a rate of 2–4 ml min−1 for 30 min. Following this, the perfusate was changed to one in which the sucrose was replaced with 126 mM NaCl, the CaCl2 concentration increased to 2.0 mM, and the MgSO4 concentration reduced to 1.5 mM. The slice was perfused with this solution for a further hour before recording commenced.
Figure 1. Slice preparation used for antidromic activation of medial septal-diagonal band neurons.
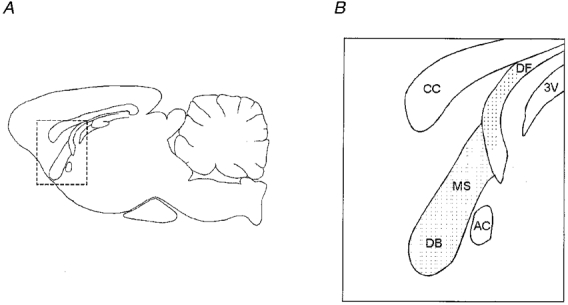
A and B, diagrams of parasagittal sections through the rat brain at the level of the medial septum-diagonal band complex (MS-DB). The diagram in B is an enlargement of the area in A bounded by dashed lines. It shows the appearance of the MS-DB, in which the recording electrode was placed, and the dorsal fornix (DF), in which the stimulating electrode was placed, in a parasagittal slice taken from near the midline of the brain. The anterior commissure (AC) is caudal to the MS-DB, and the corpus callosum (CC) is dorsal to the MS-DB. 3V, third ventricle.
Whole-cell recordings
Prior to obtaining a whole-cell recording, a concentric bipolar stimulating electrode (Clark Electromedical) was positioned in the dorsal fornix (Fig. 1B). Whole-cell patch-clamp recordings were made from neurons located throughout the MS-DB. Recording pipettes (4–6 MΩ) were filled with a solution containing (mM): 140 potassium methane sulphonate, 10 Hepes, 2 MgCl2, 0.6 EGTA, 2 Na2-ATP, 0.3 Na-GTP, and with a pH of 7.4. The osmolality of the solution was adjusted to 275–285 mosmol kg−1. Voltage recordings were made using an Axopatch-1D amplifier (Axon Instruments) in current-clamp mode. Data were digitized at 33 kHz and stored on digital audio tape (Sony, Japan) for off-line analysis. Data acquisition and analysis were performed using a CED 1401-plus interface and ‘SIGAVG’ and ‘Spike2’ software (both from Cambridge Electronic Design, UK).
Classification of MS-DB neurons
The resting membrane potential (Vm) of each neuron was noted once a stable recording was obtained. The protocol was only continued provided the resting membrane potential was more negative than −45 mV, spikes overshot 0 mV and the series resistance was less than 30 MΩ. Input resistance and time constants of the cell were then measured from the voltage response to small hyperpolarizing current pulses (20 pA, 200 ms). A protocol based on that employed by Jahnsen & Llinás (1984) was then used to characterize the firing properties of MS-DB neurons and determine if they were fast, burst, or slow firing in nature. The membrane potential of each cell was first held at around −75 mV and a series of depolarizing pulses (0–300 pA, 700 ms) was applied. The membrane was then held at −60 mV, and the same series of depolarizing pulses was applied. Initial and steady firing frequencies were determined for each neuron at membrane potentials close to −60 mV, using the same current pulse required to depolarize the cell to threshold from −75 mV. Differences between initial and steady firing frequency were taken to indicate the degree of spike frequency adaptation, a phenomenon where the rate of firing slows during the length of the pulse. Instantaneous firing frequency was measured from the inter-spike interval between the first two to three spikes and steady firing frequency was taken from interspike intervals from the final four to five spikes. Action potential properties were determined for each neuron by taking an average from at least three single spikes evoked by threshold pulses (700 ms) from −60 mV. Measurements were made of the amplitude of the spike, and the width of the spike at 50 % amplitude (half-width). Spikes were generally followed by a complex series of after-polarizations. Using the initial rise of the spike as a baseline, the amplitude and duration of these potentials were measured. On the whole, these were a fast (2–5 ms) AHP followed by a much slower (200–600 ms) AHP. These AHPs were separated by what could be an after-depolarization but this rarely overshot the baseline and therefore no meaningful measurements could be made. Long duration (700–800 ms) hyperpolarizing current steps were applied from holding potentials of −55 to −60 mV, to potentials around −100 to −120 mV, to test for voltage-dependant inward rectification (depolarizing sag), and time-independent inward rectification (Jahnsen & Llinás, 1984).
Antidromic activation of MS-DB neurons
Once the neuronal firing characteristics had been obtained, the neuronal membrane potential was held at around −60 mV. Presumed septohippocampal neurons were identified by their antidromic response to electrical stimulation of the fornix (rectangular pulses of 0.2 ms duration, 10–90 V amplitude, and applied at intervals of 3 or 4 s). The criteria for antidromic activation of the neuron were all-or-nothing action potentials which overshot 0 mV, constant latency of the response and the consistent occurrence of spikes after two or more stimuli applied at 100–200 Hz (‘high frequency following’). The distance between the stimulating and recording electrodes was measured with a scale bar placed in the recording chamber, and the conduction velocity of each antidromically activated neuron was calculated. Although this method assumes the axon travels directly to the fornix, this assumption is not unreasonable in that anatomical data from filled cells clearly show axons taking a linear course to the fornix (Z. Henderson, unpublished observations). In addition, if the axon did follow a more tortuous route then it is unlikely that it would remain in the plane of a 400–500 μm-thick slice, in which case the parent cell would not respond to fornical stimulation. If no antidromic response was seen the stimulating electrode was moved down through the brain slice in 10 μm steps. Lateral movement of the stimulating electrode could not be achieved without disturbing the recording, hence the low yield. Other methods of cutting the brain slice were employed to achieve a greater yield but had no significant benefit. Statistical comparisons were made using either a one-way ANOVA (Student-Newman-Keuls test) or an ANOVA on ranks (Kruskal-Wallis test). Measures were considered statistically significant if P < 0.05.
RESULTS
Recordings were made from 94 neurons, throughout the MS-DB. Neurons were divided into three groups, slow firing, fast firing and burst firing (Griffith & Matthews, 1986; Griffith, 1988; Markram & Segal, 1990; Gorelova & Reiner, 1996), on the basis of their response to injection of long duration current pulses (600–800 ms) at membrane potentials of −75 and −60 mV (Fig. 2A, D and G). Briefly, slow firing cells fire regularly in response to depolarizing current pulses applied from −60 or −75 mV, and they fire at a significantly lower steady frequency than burst and fast firing cells (Table 1). Fast firing neurons display tonic repetitive discharge in response to depolarizing current pulses from −60 or −75 mV, whereas burst firing neurons show a voltage-dependent burst response from −75 mV but not from - 60 mV, with two or more spikes riding on a slow depolarizing wave (Fig. 2D and D1).
Figure 2. Identification of slow firing, burst firing and fast firing neurons in the medial septum-diagonal band.
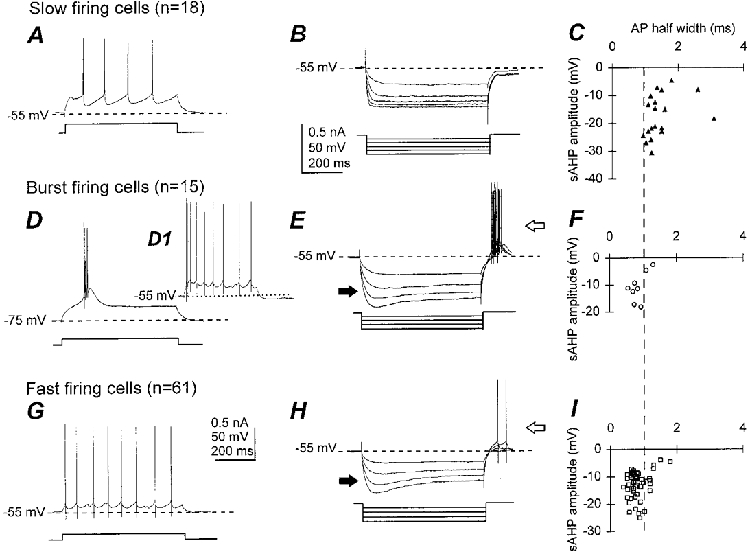
A, D and G, responses to injection of depolarizing current pulses, as indicated by the square pulses beneath the traces. B, E and H, overlay of responses to a series of hyperpolarizing current pulses, indicated beneath the traces. C, F and I, scatter plots for all cells showing relationship of action potential (AP) half-width with slow after-hyperpolarization (sAHP) amplitude. Slow firing neurons fire slow repetitive action potentials followed by pronounced, long-lasting after-hyperpolarizations (A) and lack time-dependent inward rectification, or depolarizing sag (B). They can be clearly distinguished from fast firing and burst firing neurons on the basis of their action potential waveform (C, F and I). Burst firing neurons show a burst of action potentials overriding a slow depolarization in response to a threshold depolarizing current pulse from −75 mV and repetitive firing when depolarized from - 55 mV (D and D1), and have depolarizing sag (filled arrow in E) and rebound firing (open arrow in E). Fast firing neurons possess higher firing rates and smaller after-hyperpolarizations (G) than slow firing neurons, and show depolarizing sag (filled arrow in H) and rebound firing (open arrow in H).
Table 1. Membrane properties and firing characteristics of MS–DB neurons.
| Slow firing | Burst firing | Fast firing | ||||
|---|---|---|---|---|---|---|
| Number of neurons | 18 | (4) | 15 | (5) | 61 | (9) |
| Resting membrane potential (mV) | −56.3 ± 6.3† | (−51.9 ± 1.9) | −53.9 ± 6.5 | (−56.8 ± 3.8) | −51.7 ± 5.5 | (−53.8 ± 3.8) |
| Input resistance (MΩ) | 370 ± 210 | (241 ± 221) | 273 ± 210 | (253 ± 191) | 320 ± 230 | (265 ± 131) |
| Time constant (ms) | 17.5 ± 7.5 | (14.7 ± 11.6) | 12.2 ± 5.6 | (11.5 ± 4.5) | 14.4 ± 7.2 | (14.4 ± 8.3) |
| Instantaneous firing frequency (Hz) | 19.4 ± 15.4* | (23.8 ± 11.4) | 123.9 ± 54‡ | (124.4 ± 5.4) | 45.1 ± 29.9 | (56.1 ± 29.9) |
| Steady firing frequency (Hz) | 9.4 ± 3.1* | (12.5 ± 4.2) | 18.2 ± 7.7 | (16.0 ± 4.2) | 20.5 ± 9.8 | (23.0 ± 10.9) |
| Action potential amplitude (mV) | 72.3 ± 12.1* | (67.2 ± 9.8) | 85.8 ± 15.8 | (97.5 ± 7.3) | 88.7 ± 16.3 | (84.6 ± 16.9) |
| Action potential half-width (ms) | 1.5 ± 0.5* | (1.5 ± 0.1) | 0.9 ± 0.2 | (0.7 ± 0.1) | 0.8 ± 0.3 | (1.0 ± 0.4) |
| Fast AHP amplitude (mV) | – | – | 20.3 ± 10.9 | (21.2 ± 10.4) | 20.3 ± 9.4 | (19.9 ± 8.8) |
| Fast AHP duration (ms) | – | – | 2.5 ± 1.7 | (3.5 ± 2.6) | 2.4 ± 2.1 | (4.6 ± 4.0) |
| Slow AHP amplitude (mV) | 17.4 ± 7.7* | (13.0 ± 6.8) | 10.9 ± 5.5 | (10.4 ± 1.5) | 12.2 ± 6.3 | (10.4 ± 4.5) |
| Slow AHP duration (ms) | 267.1 ± 94.5† | (187 ± 61.4) | 275.4 ± 89.2 | (287 ± 89.1) | 218.6 ± 65.8 | (196.7 ± 49.4) |
Input resistances and time constants were calculated from small hyperpolarizing current pulses (−20 pA). Instantaneous and steady firing frequencies were determined using a depolarizing current pulse from −60 mV. The size of the current pulse was that required to bring the membrane to threshold from −75 mV. Action potential measurements were taken using a long duration (700 ms) depolarization that brought the membrane to threshold from −60 mV. Statistical tests were performed on all cells. Significant differences (P < 0.05) are indicated:
slow firing versus burst firing and fast firing neurons.
slow firing versus fast firing neurons
burst firing versus slow firing and fast firing neurons. The numbers in parentheses represent values from antidromic cells.
The passive and active membrane properties of these three cell types are summarized in Table 1. Significant differences in resting membrane potentials, firing characteristics and action potential properties were seen between the three groups. A wide range of values for input resistance and time constants were observed within each group, with no statistically significant differences between the three groups (Table 1). Hyperpolarizing current steps applied from holding potentials of −55 to - 60 mV to potentials around −100 to −120 mV evoked a rapid membrane hyperpolarization, followed in some neurons by a depolarizing sag to plateau level 200–400 ms after the start of the current pulse (filled arrows, Fig. 2E and H). On termination of the hyperpolarizing pulse the membrane became transiently depolarized, and in many neurons this rebound depolarization reached threshold and initiated one or more action potentials (open arrows, Fig. 2E and H). This rebound firing was only seen in those cells that possessed the time-dependent inward rectification. Time-independent rectification was observed in a proportion of the neurons, but was not linked to any particular cell type, or to the occurrence of time-dependent inward rectification (depolarizing sag).
Slow firing neurons
In our preparation, 18 slow firing neurons were identified. The instantaneous (19.4 ± 15.4 Hz) and steady-state firing frequencies (9.4 ± 3.1 Hz) of these cells were significantly lower than that of the burst and fast firing neurons, and little or no spike frequency spike adaptation occurred throughout the duration of the pulse (Table 1). Action potentials of slow firing cells were significantly broader (half-width 1.5 ± 0.5 ms) than those of the other cell types (Table 1) and were followed by a deep and slow after-hyperpolarization (amplitude 17.4 ± 7.7 mV, duration 267.1 ± 94.5 ms). This broad spike and slow AHP appears to be specific to the slow firing neuron type (Fig. 2C, F and I) which has previously been shown to be cholinergic in nature (Griffiths & Matthews, 1986; Markram & Segal, 1988; Gorelova & Reiner, 1996). The average amplitude of the spikes (72.3 ± 12.1 mV) of the slow firing neurons was significantly smaller than in other cell types and the resting membrane potential (−56.6 ± 6.3 mV) was significantly more hyperpolarized than that of the fast firing neurons (Table 1). Application of hyperpolarizing current pulses revealed that the majority of cells (n = 13/18) did not exhibit depolarizing sag (Fig. 2B).
Burst firing neurons
Fifteen neurons were classified as burst firing. Steady firing frequency was 18.2 ± 7.7 Hz, and the instantaneous firing frequency (123.9 ± 54 Hz) was significantly higher than for other cell types (Table 1). Action potentials were narrow (half-width 0.9 ± 0.2 ms) and followed by a fast AHP (amplitude 20.3 ± 10.9 mV, duration 2.5 ± 1.7 ms) and a slow AHP (amplitude 10.9 ± 5.5, duration 275.4 ± 89.2 ms). The majority of these cells displayed depolarizing sag (n = 11/14; Fig. 2E).
Fast firing neurons
On entry into the cell, most fast firing neurons were spontaneously active whereas burst firing and slow firing neurons tended to be quiescent (Fig. 3A and D). This compared well with the observation that the resting membrane potential for fast firing cells was more depolarized than that of the other cells (Table 1). Fast firing neurons (n = 61) fired repetitively upon depolarization from −60 or −75 mV, with an average instantaneous firing frequency of 45.1 ± 29.9 Hz and an average steady firing frequency of 20.5 ± 9.8 Hz (Table 1). Marked spike frequency adaptation was seen in a subset of these cells (n = 14/61). Action potentials were narrow (half-width 0.8 ± 0.3 ms) and followed by a fast AHP similar to burst firing neurons (2.4 ± 2.1 ms) and a slow AHP that was less pronounced (amplitude 12.2 ± 6.3 mV) and shorter (duration 218.6 ± 65.8 ms) than the slow AHP of slow firing cells. Depolarizing sag was present in the majority of cells (n = 48/59; Fig. 2H).
Figure 3. Lack of clustering of action potentials in burst firing and fast firing neurons in the medial septum-diagonal band of the rat.
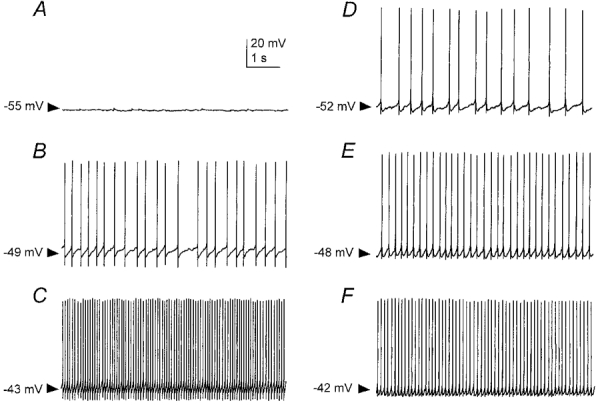
A, discharge of a burst firing neuron at rest; membrane potential (Vm) −55 mV. B and C, increased firing frequency of the burst firing neuron in the presence of 0.1 and 0.2 nA injected direct current, respectively. D, discharge of a fast firing neuron at rest; Vm−52 mV (the majority of these neurons are spontaneously active). E and F, increased firing frequency of the fast firing neuron in the presence of 0.1 and 0.2 nA injected direct current, respectively. Clusters of action potentials interspersed with subthreshold membrane oscillations are not apparent in this preparation, as has been reported in the guinea-pig MS-DB (Serafin et al. 1996).
In summary, the three types of cells within the MS-DB slow firing, fast firing and burst firing possess significant differences in their firing characteristics and action potential properties. Slow firing cells in the MS-DB have wide action potentials, a deep and slow AHP, and these cells do not display depolarizing sag or spike frequency adaptation. Burst firing cells fire a burst of action potentials when depolarized to threshold from −75 mV, have narrow action potentials, display depolarizing sag and possess both fast and slow AHPs. Fast firing cells have narrow action potentials, exhibit depolarizing sag, and possess both fast and slow AHPs.
Rhythmic firing, in the form of action potential clusters interspersed with subthreshold membrane oscillations, and observed in non-cholinergic neurons in the guinea-pig MS-DB slice preparation (Serafin et al. 1996), was not seen in either burst firing or fast firing neurons of the rat MS-DB (Fig. 3).
Antidromic activation of MS-DB neurons
Stimulation of the fornix evoked antidromic responses in 18 out of the 94 cells with an mean threshold intensity of 56.4 ± 29.4 V. Of these cells, four were slow firing, five burst firing and nine fast firing, and the latency of the antidromic spike depended upon cell type (Fig. 4A, C and E). The neurons identified as antidromic showed typical all-or-nothing responses, and could follow stimuli at 109.7 ± 77.7 Hz (Fig. 4B, D and F). High frequency following was not possible in one burst firing neuron because it fired an all-or-nothing burst of three action potentials. The passive and active membrane properties of these neurons were comparable to their respective larger populations (Table 1). Due to the blind nature of the recordings it is possible that some recordings were made from dendrites as opposed to the soma. This is unlikely for the majority of the neurons tested, since the mean action potential amplitude was 101.9 ± 35.2 mV, and attenuation of the amplitude of the last spike in the train at the maximum stimulation frequency was not less than 83.7 ± 22.1 % of the first (Fig. 4B, D and F).
Figure 4. Antidromic activation of slow firing, burst firing and fast firing neurons in the medial septum-diagonal band after stimulation of the dorsal fornix.
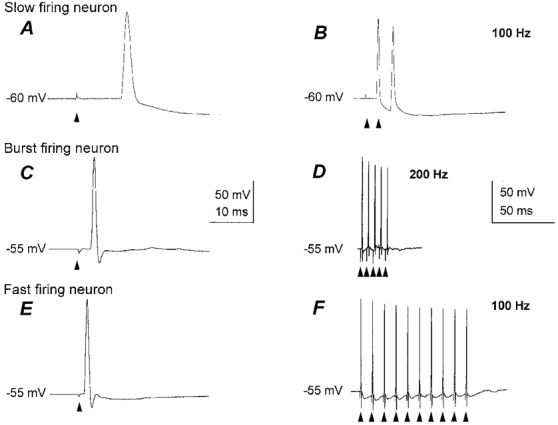
A, C and E, all-or-nothing antidromic spikes recorded in a slow firing neuron (A), burst firing neuron (C) and fast firing neuron (E), showing different latencies and action potential widths after threshold stimuli (arrowheads) for the three neuron types. The conduction velocities were 0.7 ± 0.5, 2.9 ± 2.0 and 2.0 ± 1.4 ms−1, respectively. B, D and F, the antidromically activated spikes in the slow firing (B), burst firing (D) and fast firing neurons (F) faithfully followed trains of two or more stimuli presented at 100 Hz or faster.
Analysis of the conduction velocities of the antidromically activated neurons revealed two populations, fast conducting and slow conducting. Slow firing neurons had conduction velocities of 0.7 ± 0.5 ms−1, at 25°C, whereas burst firing neurons and fast firing neurons conducted faster, at 2.9 ± 2.0 and 2.0 ± 1.4 ms−1, respectively, at 25°C. The difference between the conduction velocities of slow firing neurons and the burst firing and/or fast firing neurons was statistically significant (P < 0.05).
Location of MS-DB neuron types
Recordings were made from all parts of the MS-DB in the longitudinally oriented slice cut near the midline, in which the MS-DB appears as a pear-shaped structure (Figs 1 and 5; the MS-DB in the living slice is identified by its greater level of myelination than the other nearby structures). Slow firing neurons were predominantly located at the margins of the MS-DB (Fig. 5A), where numerous choline acetyltransferase-positive neurons are also distributed (data not shown). Burst firing neurons were clustered in the central region of the MS-DB (Fig. 5B), and fast firing neurons were scattered throughout the MS-DB (Fig. 5C). Antidromically activated cells of each class were distributed in regions comparable to their larger respective populations (Fig. 5A-C).
Figure 5. Location of antidromically activated slow firing, burst firing and fast firing neurons in the medial septum (MS) and diagonal band (DB) slice.
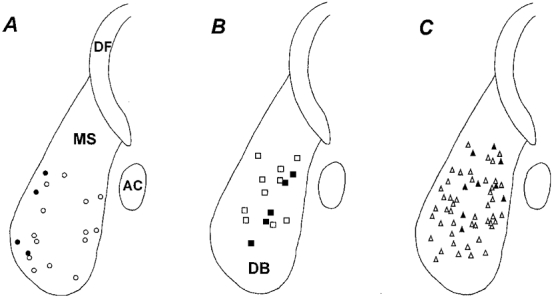
A, slow firing neurons (•). B, burst firing neurons (▪). C, fast firing neurons (▴). The open symbols in each panel show the positions of the same respective cell types that could not be antidromically activated upon stimulation of the dorsal fornix (DF). The anterior commissure (AC) marks the caudal border of the slice, and the dorsal fornix (DF) is dorsal to the MS-DB.
DISCUSSION
By recording from presumed septohippocampal neurons in vitro, we have shown that slow firing neurons have a slow mean axon conduction velocity of 0.7 ms−1 (25°C) and burst firing and fast firing neurons with fast mean axon conduction velocities of 2.0 and 2.9 ms−1 (25°C), respectively. Although the neurochemistry of these projection neurons was not determined directly, in the light of previous evidence of cell types in the MS-DB, it is most likely that the slow firing neurons are cholinergic and the burst firing and fast firing neurons are GABAergic.
Technical considerations
The electrophysiological properties of the neurons recorded here, and their classifications, are similar to those recorded by other laboratories (Griffith & Matthews, 1986; Segal, 1986; Griffith, 1988; Markram & Segal, 1990; Gorelova & Reiner, 1996). The neurons were divided into three types, slow firing, burst firing and fast firing, in response to depolarizing and hyperpolarizing stimuli. Furthermore, the slow firing cells had other specific attributes such as lack of adaptation and depolarizing sag, broad action potentials and large amplitude AHPs, all which have previously been observed in this category of cell which has also been shown to be cholinergic (Griffith & Matthews, 1986; Griffith, 1988; Markram & Segal, 1990; Gorelova & Reiner, 1996). However, we were not able to show that these slow firing cells have a substantially longer AHP than the other neurons, as has been done before (Griffith & Matthews, 1986; Griffith, 1988). This may have been due to our using the whole-cell patch configuration for recording rather than sharp electrodes. In another study employing whole-cell patch electrodes, it was found that a class of long AHP cells was not cholinergic (Gorelova & Reiner, 1996). Although subtypes within the burst firing and fast firing neurons have been observed with sharp electrode recording (Morris et al. 1999), we did not attempt to subdivide the three neuron types obtained any further, due to the relatively low number of antidromically activated cells obtained.
The neurons that were antidromically activated in our slice preparation are assumed to be septohippocampal neurons, since the stimulating electrode was placed in the fornix rather than in the upper part of the MS-DB preparation, so it is most likely that these cells were projecting to the hippocampus, rather than sending widely distributed axons in the MS-DB, although this possibility cannot be precluded for a minority of cells.
Of the total number of cells from which recordings were made, only 18/94 cells could be antidromically activated. Although the remainder may be interneuronal, it is more likely that most if not all are septohippocampal, since the types of non-antidromically activated cells corresponded closely to the three classes of neuron that could be antidromically activated. There are many reasons for not obtaining a higher proportion of antidromically activated cell. In a slice preparation of 500 μm, even one cut at optimal orientation, it is impossible to retain the axons of all of the soma in their entirety all the way to the fornix. Also, some septohippocampal neurons take other routes, i.e. via the supracallosal stria or the ventral forebrain (Peterson, 1994). The other routes may be particularly applicable to the slow firing neurons, since the only slow firing neurons that could be activated antidromically were located at the rostral border of the MS-DB in the longitudinally cut slice.
Analyses of the conduction velocities of the neurons showed that the slow firing neurons conduct more slowly than the fast firing and burst firing neurons. Given the assumption that slow firing neurons are cholinergic and the others are GABAergic, this would provide for a distinction between these cell types in vivo. The burst firing neurons in the preparation had a tendency for a faster conduction velocity than the fast firing neurons, but this was not statistically significant. It is possible that this non-significant gap between conduction velocities would be widened by other conditions of recording, since our recordings were made at 25°C and from 3-week-old rats, conditions standard for whole-cell patch recording experiments in vitro. Conduction velocity, however, is dependent on temperature and on the level of myelination. Although myelination was visible in our preparations, it is possible that the process of myelination was not yet completed or fully matured. Thus it is most likely that conduction velocities of the respective neurons would be greater recorded in vivo at 37°C and in an older rat. All these disadvantages of recording conduction velocities in vitro are nevertheless counteracted by the fact that measurement of the conduction path is probably subject to less error than that in vivo, where it is not possible to measure directly the distance of the pathway between the recording and stimulating electrodes.
Comparison of relative conduction velocities of MS-DB neurons recorded in vitro with those recorded in vivo and correlation with anatomical studies
Previous studies have identified septohippocampal neurons in vivo by their antidromic response to stimulation of the hippocampus, dorsal fornix or fimbria. Actual conduction velocities have rarely been reported, since the main aim in these studies was simply to identify neurons that project to the hippocampus, and the most that have been given are latencies of conduction. Studies in urethane-anaesthetized rats by Lamour and colleagues have suggested that there are at least two types of septohippocampal neurons with respect to conduction velocity. A proportion of the septohippocampal neurons (41–45 %) discharged in rhythmic bursts and were driven antidromically at short latencies (0.5–5 ms) from the fimbria (Lamour et al. 1984; Dutar et al. 1986). The remaining septohippocampal neurons fired repetitively or irregularly and were driven antidromically at longer latencies (0.5–18 ms). Under similar experimental conditions, Stewart & Fox (1989b) also reported the existence of at least two types of septohippocampal neurons, one that fired rhythmically in bursts and was driven antidromically with a latency of 0.7–1.1 ms, and another which fired irregularly and was driven antidromically with an average latency of 6.8 ms. These results suggest that as in vitro, there are neurons recorded in vivo that fall into two groups with respect to conduction velocities.
Neuroanatomical and axonal tract tracing studies also support the observation that there are two populations of septohippocampal neurons with different axon conduction velocities. Fibres in the fimbria-fornix fall into two categories, thin unmyelinated, and thick myelinated (Nyakas et al. 1987), and it is the cholinergic septohippocampal axons that are unmyelinated or thinly myelinated (Bialowas & Frotscher, 1987; Freund, 1989) whereas it is the GABAergic septohippocampal axons that are thickly myelinated (Freund, 1989).
We can only speculate on the chemical identity of the burst firing and fast firing neurons observed in vitro, but since septohippocampal neurons are either cholinergic or GABAergic (Gritti et al. 1993), those septohippocampal neurons which do not display the cholinergic electrophysiological ‘signature’ are almost certainly GABAergic in nature. Moreover, it has been shown that the majority of GABAergic septohippocampal neurons are large and contain parvalbumin, a calcium binding protein that is often used as a GABAergic marker (Freund, 1989). This credits the opinion that these cells are GABAergic because it has recently been shown that some burst firing and fast firing cells within the medial septum contain parvalbumin (Morris et al. 1997, 1999).
Comparison of active and passive membrane properties of MS-DB neurons with studies conducted in vivo
The main aim of in vitro studies on the MS-DB has been to investigate the factors that underlie the burst firing properties of MS-DB neurons in vivo and their selective sensitivity to atropine. The in vivo studies described above, which have employed extracellular recording, have indicated that a proportion of fast conducting septohippocampal neurons (which may be GABAergic) discharge in rhythmic bursts whereas slow conducting septohippocampal neurons (which may be cholinergic) more often fire repetitively or irregularly (Lamour et al. 1984; Dutar et al. 1986; Stewart & Fox, 1989b). Intracellular recording studies, or those in which the extracellular wave form was analysed, suggest that neurons with broad spikes (presumed cholinergic cells) fire rhythmic bursts, but with fewer spikes per burst than the narrow spike neurons (Barrenechea et al. 1995; Brazhnik & Fox, 1997; King et al. 1998). Indeed, it has been shown that, during extracellular recordings from anaesthetized rats 15–16 % of rhythmically firing MS-DB neurons switch to irregular firing (Lamour et al. 1984; Dutar et al. 1986), suggesting that irregular and slow firing rhythmic bursters are part of the same group and that they are cholinergic and slow conducting.
According to Stewart & Fox (1989a), cells that fire bursts of action potentials in phase with the hippocampal theta rhythm also fall into two groups depending on whether or not intra-septal atropine disrupts their spontaneous rhythmic discharge (Stewart & Fox, 1989b). It has been recently shown that rhythmic bursters which possess narrow action potentials (presumed GABAergic) and fire many spikes per theta cycle (4–20) are insensitive to atropine, and that neurons which have broad action potentials (presumed cholinergic) and follow the theta rhythm with few spikes per cycle (1–3) or fire irregularly are sensitive to muscarinic antagonism (Brazhnik & Fox, 1997). That these cells are cholinergic is further indicated by the observation that after a selective cholinergic lesion of the fimbria-fornix, the rhythmic bursters remaining have the firing properties of the atropine-insensitive neurons (Apartis et al. 1998). Furthermore, rhythmically bursting cells, identified as septohippocampal by stimulation of the fimbria-fornix, had a much shorter antidromic latency following the cholinergic lesion (Apartis et al. 1998). Thus, non-cholinergic neurons in vivo have a fast conduction velocity similar to our own in vitro observations. It has not been possible to identify atropine-sensitive and -insensitive neurons in vitro because neurons in slices do not show the rhythmic activity seen in vivo, nor do they display selective responses to muscarinic agonists (Sim & Griffith, 1991).
In conclusion, we suggest that slow firing neurons as identified in vitro, are cholinergic, slow conducting and possess unmyelinated axons. The burst firing and fast firing cells, as identified in vitro, may be GABAergic, possess fast conduction velocities and are myelinated. This view is consistent with studies in vitro and with lesion studies that have attempted to examine the individual roles of the cholinergic and GABAergic neurons in the generation of the hippocampal theta rhythm. In a recent in vitro study, Tóth et al. (1997) found that rhythmic stimulation of septohippocampal fibres evoked rhythmic discharge in hippocampal pyramidal neurons, interpreted as being caused by local disinhibition as a result of stimulation of GABAergic septohippocampal neurons. Furthermore, whilst lesion of the fimbria-fornix abolishes the theta rhythm in urethane-anaesthetized animals (Green & Arduini, 1954; Petsche et al. 1962; Ranck, 1973; Rawlins et al. 1979), selective lesion of the cholinergic component of the septohippocampal pathway does not abolish the hippocampal theta rhythm but merely reduces its amplitude (Lee et al. 1994; Bassant et al. 1995; Apartis et al. 1998). Thus it appears that GABAergic neurons within the MS-DB play a major role in pacing the rhythmic activity in the hippocampus, whereas the cholinergic component serves to modulate it. As shown in this study, axons of putative GABAergic neurons have a faster conduction velocity than those of cholinergic neurons. The functional significance of the differences in conduction velocity between the cell types is not clear at present, except that it may impart small phase differences between the rhythmic firing of the slow and fast conducting cells when the action potentials reach the hippocampus. This may have important implications on firing patterns in the hippocampus, especially if the two varieties of neurons have different types of terminations in the hippocampus, but this would require further investigation in vivo.
Acknowledgments
We would like to thank Julie Higgins for her expert technical assistance. This research was funded by the MRC.
References
- Amaral DG, Kurz J. An analysis of the origins of the cholinergic and non-cholinergic septal projections to the hippocampal formation of the rat. Journal of Comparative Neurology. 1985;240:37–59. doi: 10.1002/cne.902400104. [DOI] [PubMed] [Google Scholar]
- Apartis E, Poindessous-Jazat FR, Lamour YA, Bassant MH. Loss of rhythmically bursting neurons in rat medial septum following selective lesion of septohippocampal cholinergic system. Journal of Neurophysiology. 1998;79:1633–1642. doi: 10.1152/jn.1998.79.4.1633. [DOI] [PubMed] [Google Scholar]
- Apostol G, Creutzfeldt OD. Crosscorrelation between the activity of septal units and hippocampal EEG during arousal. Brain Research. 1974;67:65–75. doi: 10.1016/0006-8993(74)90298-4. 10.1016/0006-8993(74)90298-4. [DOI] [PubMed] [Google Scholar]
- Barrenechea C, Pedemonte M, Nuñez A, García-Austt E. In vivo intracellular recordings of medial septal and diagonal band of Broca neurons: relationships with theta rhythm. Experimental Brain Research. 1995;103:31–40. doi: 10.1007/BF00241962. [DOI] [PubMed] [Google Scholar]
- Bassant MH, Apartis E, Jazat-Poindessous FR, Wiley RG, Lamour YA. Selective immunolesion of the basal forebrain cholinergic neurons: effects on hippocampal activity during sleep and wakefulness in the rat. Neurodegeneration. 1995;4:61–70. doi: 10.1006/neur.1995.0007. [DOI] [PubMed] [Google Scholar]
- Bialowas J, Frotscher M. Choline acetyltransferase-immunoreactive neurons and terminals in the rat septal complex: a combined light and electron microscope study. Journal of Comparative Neurology. 1987;259:298–307. doi: 10.1002/cne.902590209. [DOI] [PubMed] [Google Scholar]
- Brazhnik ES, Fox SE. Intracellular recordings from medial septal neurons during hippocampal theta rhythm. Experimental Brain Research. 1997;114:442–453. doi: 10.1007/pl00005653. [DOI] [PubMed] [Google Scholar]
- Dutar P, Lamour Y, Jobert A. Septohippocampal neurons in the rat: an in vivo intracellular study. Brain Research. 1985;340:135–142. doi: 10.1016/0006-8993(85)90782-6. 10.1016/0006-8993(85)90782-6. [DOI] [PubMed] [Google Scholar]
- Dutar P, Lamour Y, Rascol O, Jobert A. Septo-hippocampal neurons in the rat: further study of their physiological and pharmacological properties. Brain Research. 1986;365:325–334. doi: 10.1016/0006-8993(86)91644-6. 10.1016/0006-8993(86)91644-6. [DOI] [PubMed] [Google Scholar]
- Freund TF. GABAergic septohippocampal neurons contain parvalbumin. Brain Research. 1989;478:375–381. doi: 10.1016/0006-8993(89)91520-5. 10.1016/0006-8993(89)91520-5. [DOI] [PubMed] [Google Scholar]
- Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988;336:170–173. doi: 10.1038/336170a0. 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Leranth C. Cholinergic innervation of the rat hippocampus as revealed by choline acetyltransferase immunocytochemistry: a combined light and electron microscopic study. Journal of Comparative Neurology. 1985;239:237–246. doi: 10.1002/cne.902390210. [DOI] [PubMed] [Google Scholar]
- Gogolàk G, Petsche H, Sterc J, Stumpf C. Septum cell activity in the rabbit under reticular stimulation. Brain Research. 1968;4:508–510. doi: 10.1016/0006-8993(67)90022-4. [DOI] [PubMed] [Google Scholar]
- Gorelova N, Reiner PB. Role of the afterhyperpolarization in control of discharge properties of septal cholinergic neurons in vitro. Journal of Neurophysiology. 1996;75:695–706. doi: 10.1152/jn.1996.75.2.695. [DOI] [PubMed] [Google Scholar]
- Green JD, Arduini AA. Hippocampal electrical activity in arousal. Journal of Neurophysiology. 1954;17:533–554. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- Green KP, Rawlins JNP. Hippocampal theta in rats under urethane: generators and phase relations. Electroencephalography and Clinical Neurophysiology. 1979;47:420–429. doi: 10.1016/0013-4694(79)90158-5. 10.1016/0013-4694(79)90158-5. [DOI] [PubMed] [Google Scholar]
- Griffith WH. Membrane properties of cell types within guinea pig basal forebrain nuclei in vitro. Journal of Neurophysiology. 1988;59:1590–1612. doi: 10.1152/jn.1988.59.5.1590. [DOI] [PubMed] [Google Scholar]
- Griffith WH, Matthews RT. Electrophysiology of AChE-positive neurons in basal forebrain slices. Neuroscience Letters. 1986;71:169–174. doi: 10.1016/0304-3940(86)90553-7. 10.1016/0304-3940(86)90553-7. [DOI] [PubMed] [Google Scholar]
- Griffith WH, Sim JA, Matthews RT. Electrophysiological characteristics of basal forebrain neurons in vitro. In: Napier TC, Kalivas P, Hanin I, editors. The Basal Forebrain: Anatomy to Function. New York: Plenum Press; 1991. pp. 143–155. [Google Scholar]
- Gritti I, Mainville L, Jones B. Codistribution of GABA- with acetylcholine-synthesising neurons in the basal forebrain of the rat. Journal of Comparative Neurology. 1993;329:438–457. doi: 10.1002/cne.903290403. [DOI] [PubMed] [Google Scholar]
- Jahnsen H, Llinás R. Electrophysiological properties of guinea-pig thalamic neurons: an in vitro study. The Journal of Physiology. 1984;349:205–226. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab RL, Leranth C. Septum. In: Paxinos G, editor. The Rat Nervous System. London: Academic Press; 1995. pp. 405–441. [Google Scholar]
- King C, Recce M, O'Keefe J. The rhythmicity of cells of the medial septum/diagonal band of Broca in the awake, freely moving rat: relationships with behaviour and hippocampal theta. European Journal of Neuroscience. 1998;10:464–477. doi: 10.1046/j.1460-9568.1998.00026.x. 10.1046/j.1460-9568.1998.00026.x. [DOI] [PubMed] [Google Scholar]
- Kiss J, Patel AJ, Freund TF. Distribution of septohippocampal neurons containing parvalbumin or choline acetyltransferase in the rat brain. Journal of Comparative Neurology. 1990;298:362–372. [Google Scholar]
- Köhler C, Chan-Palay V, Wu JY. Septal neurons containing glutamic acid decarboxylase immunoreactivity project to the hippocampal region in the rat brain. Anatomy and Embryology. 1984;169:41–44. doi: 10.1007/BF00300585. [DOI] [PubMed] [Google Scholar]
- Kramis R, Vanderwoolf CH, Bland BH. Two types of hippocampal rhythmic slow activity in both the rabbit and the rat: relations to behaviour and effects of atropine, diethyl ether, urethane and pentobarbital. Experimental Neurology. 1975;49:58–85. doi: 10.1016/0014-4886(75)90195-8. 10.1016/0014-4886(75)90195-8. [DOI] [PubMed] [Google Scholar]
- Lamour Y, Dutar P, Jobert A. Septo-hippocampal and other medial septum-diagonal band neurons: electrophysiological and pharmacological properties. Brain Research. 1984;309:227–239. doi: 10.1016/0006-8993(84)90588-2. 10.1016/0006-8993(84)90588-2. [DOI] [PubMed] [Google Scholar]
- Lee MG, Chrobak JJ, Sik A, Wiley RG, Buzsaki G. Hippocampal theta activity following selective lesion of the septal cholinergic system. Neuroscience. 1994;62:1033–1047. doi: 10.1016/0306-4522(94)90341-7. 10.1016/0306-4522(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Lewis PR, Shute CCD. The cholinergic limbic system: Projection to hippocampal formation, medial cortex nuclei of ascending cholinergic reticular system and the subfornical organ and supraoptic crest. Brain. 1967;90:521–540. doi: 10.1093/brain/90.3.521. [DOI] [PubMed] [Google Scholar]
- Markram H, Segal M. Electrophysiological characterisation of cholinergic and non-cholinergic neurons in the rat medial septum/diagonal band complex. Brain Research. 1990;513:171–174. doi: 10.1016/0006-8993(90)91106-q. 10.1016/0006-8993(90)91106-Q. [DOI] [PubMed] [Google Scholar]
- Matthews RT, Lee WL. Comparison of extracellular and intracellular recordings from medial septum/diagonal band neurons in vitro. Neuroscience. 1991;42:451–462. doi: 10.1016/0306-4522(91)90388-5. 10.1016/0306-4522(91)90388-5. [DOI] [PubMed] [Google Scholar]
- Morris NP, Beeby JH, Harris SJ, Henderson Z. Characterization of burst-firing neurons in rat medial septum/diagonal band complex in vitro. Society of Neuroscience Abstracts. 1997;23:774. [Google Scholar]
- Morris NP, Harris SJ, Henderson Z. Parvalbumin-immunoreactive, fast-spiking neurons in the medial septum-diagonal band complex of the rat in vitro. Neuroscience. 1999. (in the Press) [DOI] [PubMed]
- Norris SK. Electrophysiological identification of septohippocampal neurons using whole-cell recordings from rat brain slices. The Journal of Physiology. 1996;497.P:113P. [Google Scholar]
- Nyakas C, Luiten PGM, Spencer DG, Traber J. Detailed projection patterns of septal and diagonal band efferents to the hippocampus in the rat with emphasis on innervation of CA1 and dentate gyrus. Brain Research Bulletin. 1987;18:533–545. doi: 10.1016/0361-9230(87)90117-1. 10.1016/0361-9230(87)90117-1. [DOI] [PubMed] [Google Scholar]
- Peterson GM. Differential projections to the hippocampus by neurons of the medial septum and vertical limb of the diagonal band. Brain Research. 1994;646:129–134. doi: 10.1016/0006-8993(94)90065-5. 10.1016/0006-8993(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Petsche H, Stumpf C, Gogolák G. The significance of the rabbit's septum as a relay station between the midbrain and the hippocampus. I. The control of hippocampus arousal activity by the septum cells. Electroencephalography and Clinical Neurophysiology. 1962;14:202–211. doi: 10.1016/0013-4694(62)90030-5. 10.1016/0013-4694(62)90030-5. [DOI] [PubMed] [Google Scholar]
- Ranck JB., Jr Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. I. behavioural correlates and firing repertoires. Experimental Neurology. 1973;41:461–531. doi: 10.1016/0014-4886(73)90290-2. [DOI] [PubMed] [Google Scholar]
- Rawlins JNP, Feldon J, Gray JA. Septo-hippocampal connections and the hippocampal theta-rhythm. Experimental Brain Research. 1979;37:49–63. doi: 10.1007/BF01474253. [DOI] [PubMed] [Google Scholar]
- Segal M. Properties of rat medial septal neurons recorded in vitro. The Journal of Physiology. 1986;379:309–330. doi: 10.1113/jphysiol.1986.sp016255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafin M, Williams S, Khateb A, Fort P, Mühlethaler M. Rhythmic firing of medial septum non-cholinergic neurons. Neuroscience. 1996;75:671–675. doi: 10.1016/0306-4522(96)00349-1. 10.1016/0306-4522(96)00349-1. [DOI] [PubMed] [Google Scholar]
- Sim JA, Griffith WH. Muscarinic agonists block a late-after hyperpolarization in medial septum/diagonal band neurons in vitro. Neuroscience Letters. 1991;129:63–69. doi: 10.1016/0304-3940(91)90721-5. 10.1016/0304-3940(91)90721-5. [DOI] [PubMed] [Google Scholar]
- Smythe JW, Colom LV, Bland BH. The extrinsic modulation of hippocampal theta depends on the coactivation of cholinergic and GABA-ergic medial septal inputs. Neuroscience and Behaviour Reviews. 1992;16:289–308. doi: 10.1016/s0149-7634(05)80203-9. [DOI] [PubMed] [Google Scholar]
- Stewart M, Fox SE. Two populations of rhythmically bursting neurons in rat medial septum are revealed by atropine. Journal of Neurophysiology. 1989a;61:982–993. doi: 10.1152/jn.1989.61.5.982. [DOI] [PubMed] [Google Scholar]
- Stewart M, Fox SE. Firing relations of medial septal neurons to the hippocampal theta rhythm in urethane anesthetized rats. Experimental Brain Research. 1989b;77:507–516. doi: 10.1007/BF00249604. [DOI] [PubMed] [Google Scholar]
- Stewart M, Fox SE. Do septal neurons pace the hippocampal theta rhythm? Trends in Neurosciences. 1990;13:163–168. doi: 10.1016/0166-2236(90)90040-h. 10.1016/0166-2236(90)90040-H. [DOI] [PubMed] [Google Scholar]
- Sweeney JE, Lamour Y, Bassant MH. Arousal-dependant properties of medial septal neurons in the unanesthetized rat. Neuroscience. 1992;48:353–363. doi: 10.1016/0306-4522(92)90495-n. 10.1016/0306-4522(92)90495-N. [DOI] [PubMed] [Google Scholar]
- Tóth K, Freund T, Miles R. Disinhibition of rat hippocampal pyramidal cells by GABAergic afferents from the septum. The Journal of Physiology. 1997;500:463–474. doi: 10.1113/jphysiol.1997.sp022033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalography and Clinical Neurophysiology. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- Vinogradova OS. Expression, control and probable functional significance of the neuronal theta-rhythm. Progress in Neurobiology. 1995;45:523–583. doi: 10.1016/0301-0082(94)00051-i. 10.1016/0301-0082(94)00051-I. [DOI] [PubMed] [Google Scholar]
- Wainer BH, Levey AI, Rye DB, Mesulam M-M, Mufson EJ. Cholinergic and noncholinergic septohippocampal pathways. Neuroscience Letters. 1985;54:45–52. doi: 10.1016/s0304-3940(85)80116-6. [DOI] [PubMed] [Google Scholar]


