Abstract
The pharmacological profile of receptors activated by vasopressin (AVP) in freshly dissociated supraoptic magnocellular neurones was investigated using specific V1a- and V2-type AVP receptor agonists and antagonists.
In 97 % of AVP-responding neurones (1–3000 nm) V1a or V2 receptor type agonists (F-180 and dDAVP, respectively) elicited dose-dependent [Ca2+]i transients that were suppressed by removal of external Ca2+.
The [Ca2+]i response induced by 1 μm F-180 or dDAVP was selectively blocked by 10 nm of V1a and V2 antagonists (SR 49059 and SR 121463A, respectively). The response to V1a agonist was maintained in the presence of the V2 antagonist, and the V2 agonist-induced response persisted in the presence of the V1a antagonist.
The [Ca2+]i response induced by 1 μm AVP was partially (61 %) blocked by 10 nm SR 121463A. This blockade was increased by a further 31 % with the addition of 10 nm SR 49059. Similarly, the AVP-induced response was partially (47 %) decreased by SR 49059, and a further inhibition of 33 % was achieved in the presence of SR 121463A.
We demonstrate that AVP acts on the magnocellular neurones via two distinct types of AVP receptors that exhibit the pharmacological profiles of V1a and V2 types. However, since V2 receptor mRNA is not expressed in the supraoptic nucleus (SON), and since V1b receptor transcripts are observed in the SON, we propose that the V2 receptor agonist and antagonist act on a ‘V2-like’ receptor or a new type of AVP receptor that remains to be elucidated. The possibility that V2 ligands act on the V1b receptor cannot be excluded.
Vasopressin (AVP) magnocellular neurones in the hypothalamus exhibit phasic electrical activity that depends on intrinsic membrane properties and is controlled by extrinsic factors such as plasma osmolarity, blood volume and pressure (see review by Armstrong, 1995), and also by AVP itself. Indeed, a somato-dendritic release of AVP in the extracellular space of hypothalamic nuclei has been demonstrated by several studies (Leng & Mason, 1982; Di Scala-Guenot et al. 1987; Pow & Morris, 1989; Landgraf & Ludwig, 1991). Recently, we showed that AVP favours the expression of a specific phasic activity known to optimize the systemic release of AVP (Gouzènes et al. 1998a). This autocontrol was complex and depended on the initial state of activation of AVP neurones: AVP being excitatory for quasi-silent neurones or inhibitory for highly active neurones. These opposite effects may suggest the involvement of different types of AVP receptors in the control of the phasic pattern. Indeed, results from extracellular recordings performed in vivo or from rat hypothalamic slice preparation suggests that AVP-induced changes in firing rate of AVP neurones are mediated through the V1 receptor (Inenaga & Yamashita, 1986; Dayanithi et al. 1995; Ludwig & Leng, 1997; Gouzènes et al. 1998b). On the other hand, in guinea-pig hypothalamic slices, AVP applied to neurones from the supraoptic nucleus (SON) inhibited spike generation by membrane depolarization (Abe et al. 1983). This inhibitory effect of AVP was mimicked by cAMP and enhanced by phosphodiesterase inhibitors (Abe et al. 1983), which suggests the involvement of V2 receptors. Microspectrofluorimetric studies on dissociated supraoptic magnocellular neurones have shown that AVP induces an increase in [Ca2+]i (Dayanithi et al. 1996) that requires an influx of external Ca2+ via voltage-dependent channels (Sabatier et al. 1997). The [Ca2+]i response results from the activation of multiple intracellular transduction mechanisms involving the phospholipase C and adenylyl cyclase pathways (Sabatier et al. 1998), i.e. the classical second messenger systems activated by V1- and V2-type receptors, respectively (Orloff & Handler, 1967; Stephens & Logan, 1986). The mediation of AVP-induced [Ca2+]i response by V1a receptors has been previously suggested by the use of a specific V1a receptor antagonist, SR 49059 (Dayanithi et al. 1995, 1996). In addition, the expression of V1a and V1b but not V2 mRNAs in magnocellular neurones has recently been reported (Hurbin et al. 1998).
In the present study, we have investigated the physiological significance of AVP receptors on freshly dissociated neurones from SON by evaluating the [Ca2+]i responses evoked by specific V1a and V2 receptor agonists, F-180 and dDAVP, respectively. A preliminary report of this work has appeared in abstract form (Gouzènes et al. 1998c).
METHODS
Preparation of dissociated magnocellular neurones
For each experiment, two adult male Wistar rats (Iffa-Credo, France; 150–300 g) were killed by decapitation with a guillotine following the guidelines laid down by the French/European ethical committee (licence no. 005039/04-08-1992/G. Dayanithi), and supraoptic neurones were isolated as previously described (Lambert et al. 1994) with modifications (Sabatier et al. 1997). Briefly, the isolated SONs were incubated for 45 min at 25°C in oxygenated Locke buffer supplemented with deoxyribonuclease I (0.5 mg ml−1), proteases X and XIV (1 mg ml−1 each). All enzymes and other standard chemicals were purchased from Sigma (USA). The Locke buffer contained (mM): 140 NaCl, 5 KCl, 1.2 KH2PO4, 1.2 MgSO4, 1.8 CaCl2, 10 glucose and 10 Hepes; pH 7.2, and the osmolarity was 295–300 mosmol l−1. Tissue pieces were then rinsed with Locke buffer and submitted to mechanical trituration. Dissociated cells were plated onto glass coverslips and incubated for 1 h at 37°C with Locke buffer supplemented with 1.5 μm fura-2 AM (acetoxymethyl ester form of fura-2) plus 0.1 % (w/v) Pluronic F-127 (Molecular Probes Inc., Eugene, OR, USA). [Ca2+]i measurements were performed with the fast fluorescence photometer system (FFP; Zeiss, Oberkochen, Germany; for details, see Dayanithi et al. 1996). Only cells with dendritic processes and a soma diameter more than 12 μm were used, since these large neurones have been previously demonstrated to contain either AVP or OT (Oliet & Bourque, 1992; Lambert et al. 1994). Control and test solutions were applied in the vicinity of the recorded neurone (1 mm) using a gravity-driven perfusion system with a flow rate of 100 μl min−1. Complete solution change around the neurone was achieved within 2 s. The free Ca2+/EGTA buffer contained (mM): 0.1 EGTA, 140 NaCl, 5 KCl, 1.2 KH2PO4, 1.2 MgSO4, 10 glucose and 10 Hepes; pH 7.2, and the osmolarity was 295–300 mosmol l−1. The Ca2+ concentration in the Ca2+-free EGTA buffer was approximately 100 nm, which corresponds to the resting [Ca2+]i typically observed in neurones.
Test compounds
The V2 receptor agonist dDAVP (1-deamino-8-D-AVP), the V1a receptor agonist [Phe2, Orn8]vasotocin and the V2 receptor antagonist desGlyNH2-d(CH2)5-[D-Ile2, Ile4] AVP were generously given by Dr C. Barberis (INSERM U-469, Centre de Pharmacologie-Endocrinologie, Montpellier, France). The V1a receptor antagonist, SR 49059 ((2S)1[(2R, 3S)-(5-chloro-3-(2-chlorophenyl)-1-(3, 4-dimethoxybenzene-sulphonyl))-3-hydroxy-2, 3-dihydro-1H-indole-2-carbonyl]-pyrrolidine-2-carboxamide), and V2 receptor antagonist SR 121463A (1-[4-(N-tert-butylcarbamoyl) −2-methoxybenzene sulphonyl]-5-ethoxy-3-spiro-[4-(2-morpholino ethoxy)cyclohexane]indol-2-one, fumarate; equatorial isomer) were gifts from Sanofi Recherche (Centre de Toulouse, Toulouse, France). The V1a agonist F-180 ({underline 1}Hmp-Phe-Ile-Hgn-Asn-Cys{underline}-Pro-Dab(Abu)-Gly-NH2) was kindly supplied by Dr J. L. Junien (Ferring France, Gentilly, France). AVP and OT were purchased from Boehringer Mannheim (Meylan, France). Concentrated stock solutions of substances were prepared in DMSO (SR 49059 and SR 121463A) or distilled water (dDAVP) and stored at −20°C.
Data analysis
In the figures, the [Ca2+]i traces represent the ratio values between two fluorescence wavelengths (A: 340 nm/B: 380 nm). The dose- response relationship (Fig. 3) was constructed with peak amplitude values all normalized to that of the response induced by 100 nm agonist. The dDAVP dose-response curve was fitted with a single Hill equation:
Figure 3. Dose-dependent effect of V1a and V2 agonists.
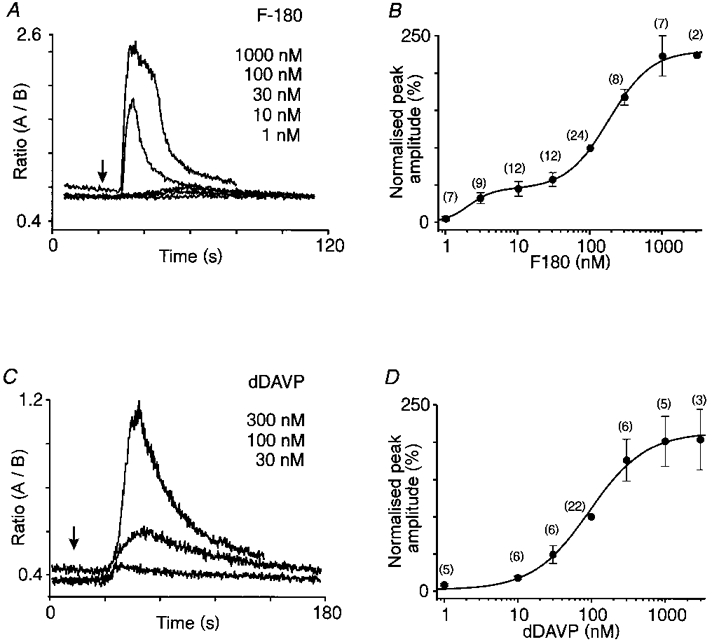
The [Ca2+]i traces in A and B illustrate the responses to increasing concentrations of F-180 and dDAVP, respectively. The graphs in C and D show the dose-response relationships of the mean [Ca2+]i transients induced by F-180 (n = 24) and dDAVP (n = 22), respectively. For each cell tested, values of peak amplitude responses were expressed as a percentage of that induced by 100 nm agonist (normalized peak amplitude, %).
The dose-response curve for F-180 was fitted with a double Hill equation:
where Y is the normalized peak amplitude (%), Ymax is the maximum normalized peak amplitude (%), EC50 is the concentration required to obtained the half-maximum peak amplitude (nm), X is the concentration of the agonist (nm) and nH is the Hill coefficient.
Fits were made using Origin software (Microcal, Northampton, USA). All values are expressed as means ±s.e.m., and the results were analysed using Student's paired t test.
RESULTS
This study was performed on a total of 206 AVP-responding neurones (from 40 preparations) that displayed stable resting [Ca2+]i levels. Those neurones initially displaying spontaneous [Ca2+]i oscillations were discarded. Consistent with our previous study (Dayanithi et al. 1996), we also observed that neurones responding to OT (n = 13) were insensitive to AVP (1 μm), F-180 (V1a receptor agonist, 1 μm) and dDAVP (V2 receptor agonist, 1 μm).
Characteristics of the agonist-induced [Ca2+]i increase
The [Ca2+]i responses to F-180 and dDAVP (1 μm) were compared with those induced by AVP (1 μm) in 58 neurones (Fig. 1). Most of these AVP-responding neurones (72 %) exhibited [Ca2+]i responses to both F-180 and dDAVP, 16 % responded to F-180 only, 9 % responded to dDAVP only, and 3 % responded neither to F-180 nor to dDAVP. Responses to F-180 and AVP displayed a short latency (8.4 ± 0.4 s and 7.4 ± 0.5 s, n = 51), a comparable amplitude (peak [Ca2+]i rise evoked by F-180 represented 119 ± 17 % of that induced by AVP; n = 51), and similar profiles, although individual variations were noted. Conversely, the response to dDAVP markedly differed from that observed in response to AVP (see Fig. 1), with a significantly longer latency (29.3 ± 7.3 vs. 7.3 ± 0.5 s, P < 0.005, n = 47), a smaller peak amplitude (48 ± 6 % of the AVP response; n = 47) and more varied profiles (see examples in Fig. 4).
Figure 1. The traces represent the [Ca2+]i responses induced by F-180 (V1a agonist) and dDAVP (V2 agonist) in a selected neurone responding to AVP.
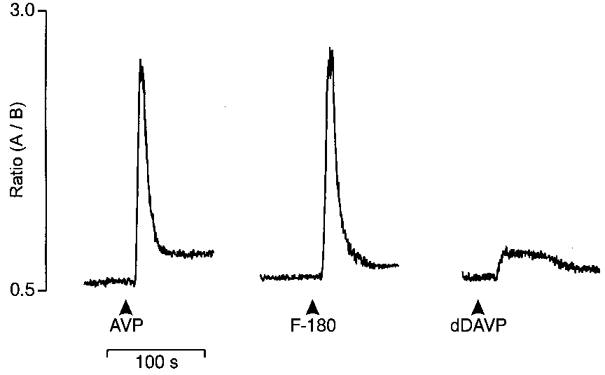
The three substances were tested at 1 μm. Traces are expressed as the ratio between two fluorescence wavelengths: 340 nm (A)/380 nm (B).
Figure 4. Specificity of the responses to V1a and V2 receptor agonists.
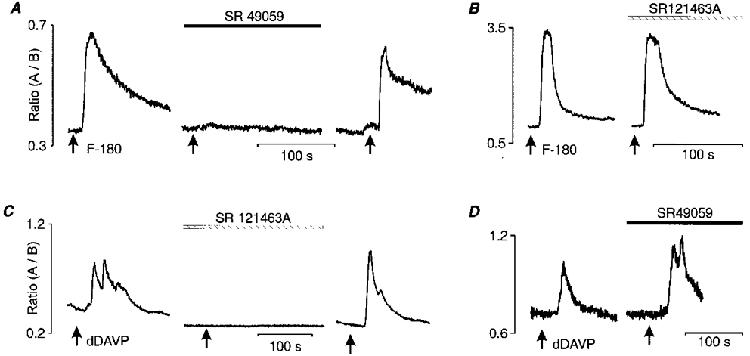
The [Ca2+]i transient elicited by F-180 (1 μm) was totally blocked by 10 nm of the specific V1a receptor antagonist (SR 49059, A), but was maintained in the presence of 10 nm V2 receptor antagonist (SR 121463A, B). Similarly, response to dDAVP (1 μm) was abolished after pre-incubation with 10 nm SR 121463A (C) but persisted in the presence of 10 nm SR 49059 (D). A and B show the reversibility of the blockade induced by each antagonist.
Twelve AVP-responsive neurones were subjected to four successive applications of F-180 (1 μm given every 2–3 min; Fig. 2A). In all neurones tested, F-180 induced reproducible [Ca2+]i responses. Similarly, the [Ca2+]i responses to successive applications of AVP (1 μm given every 2–3 min) were generally stable in each of the five neurones tested (Fig. 2B). In contrast, the responses to successive applications of dDAVP (1 μm, given every 2–3 min) showed a strong tachyphylaxis, as the third application of dDAVP induced a response with a peak amplitude reaching only 21 ± 9 % of that to the first test (n = 10; Fig. 2C). However, tachyphylaxis could be prevented by increasing the delay between applications to 10 min (n = 6; see Fig. 2D). Consequently, all subsequent experiments with dDAVP were performed with one application every 10 min.
Figure 2. The [Ca2+]i responses to repeated applications of 1 μm F-180 (A), AVP (B) and dDAVP (C and D).
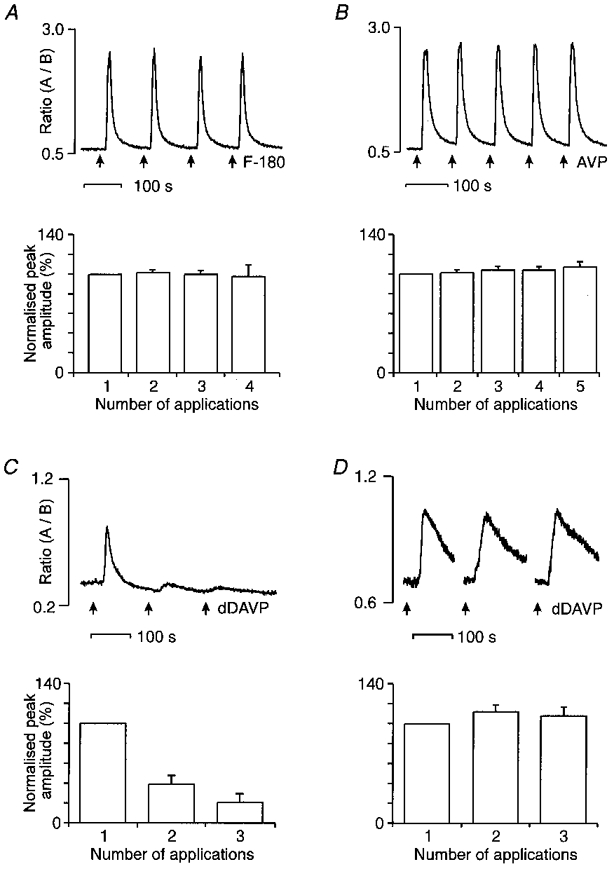
In each case, the upper Ca2+ traces show individual examples of responses. The lower histograms correspond to the mean values of the responses obtained in several neurones. The peak amplitude of the successive responses were expressed as a percentage of the first peak amplitude (% normalized peak amplitude). A and B, reproducibility of the responses to successive application of F-180 (n = 15 neurones) and AVP (n = 5 neurones). C, tachyphylaxis obtained with dDAVP applications every 2 min (n = 10 neurones). D, a longer interval (8–10 min) between successive applications of dDAVP prevented tachyphylaxis (n = 6 and 3 for the second and the third applications, respectively).
Dose dependence of the responses
To evaluate the dose-response relationships for the two agonists (F-180 and dDAVP), the neurones were systematically tested with each compound at 100 nm. Two or three other concentrations (from 1 nm to 3 μm) were applied to the same neurone in a random order. Threshold concentration was 3 nm for F-180 and 30 nm for dDAVP. The dose-response curve obtained with F-180 (Fig. 3B) was best fitted with the sum of two Hill equations (EC50= 2.1 ± 0.5 and 184 ± 18 nm, nH= 2.64 ± 1.24 and 1.49 ± 0.21, respectively), whereas the dose-response curve with dDAVP (Fig. 3D) presented a single slope only (EC50= 95 ± 13 nm and nH= 1.15 ± 0.15).
Specificity of the [Ca2+]i responses
The specificity of the two agonists was studied using specific V1a and V2 receptor antagonists (Fig. 4). The [Ca2+]i response to F-180 was inhibited by 97 ± 2 % by 10 nm SR 49059, a specific V1a receptor antagonist (n = 6; Fig. 4A). SR 49059 did not affect the resting [Ca2+]i level and inhibition of the response to F-180 recovered after wash-out of the antagonist. Similarly, the responses to 100 nm[Phe2, Orn8]vasotocin, another V1a receptor agonist, were suppressed by 10 nm SR 49059 (by 97 ± 1 %; n = 6; figure not shown). In contrast, the mean amplitude of the response induced by F-180 was unaffected by the presence of 10 nm SR 121463A, a specific V2 receptor antagonist, (105 ± 5 % of respective control response; n = 10; Fig. 4B; P = 0.59). The same concentration of SR 121463A, reversibly inhibited (by 91 ± 5 %) the response to dDAVP (n = 9; Fig. 4C; P < 0.001). The mean amplitude of the [Ca2+]i rise induced by dDAVP was unchanged in the presence of the V1a antagonist, SR 49059 (127 ± 15 % of the respective control response, n = 5; Fig. 4D; P = 0.09).
The effects of the V1a and V2 receptor antagonists were also tested on the [Ca2+]i rise induced by 1 μm AVP (Fig. 5). The V2 receptor antagonist SR 121463A (10 nm) and desGlyNH2-d(CH2)5-[D-Ile2, Ile4] AVP (100 nm) reduced the response to AVP by 61 ± 6 % (n = 9; see example in Fig. 5A; P < 0.001) and by 53 ± 15 % (n = 4; figure not shown), respectively. A near complete inhibition (92 ± 3 %; n = 9; P < 0.001) of the AVP response was obtained by combined application of SR 49059 and SR 121463A (see example in Fig. 5A). Similarly, the response to AVP was reduced by 46 ± 7 % (n = 5) with SR 49059 given alone, and further decreased up to 79 ± 4 % (n = 5) by subsequent addition of SR 121463A (see example in Fig. 5B).
Figure 5. Simultaneous activation of V1a- and V2-type receptors by AVP.
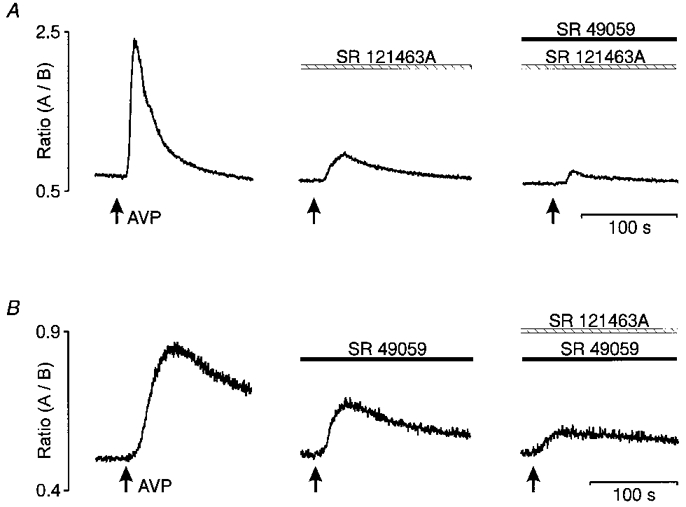
Pre-incubation with 10 nm SR 49059 or SR 121463A (middle panels in A and B) partially inhibited the [Ca2+]i response normally induced by AVP (left panels in A and B). At the same dose, each antagonist was shown to prevent the [Ca2+]i transients elicited by the respective agonist (see Fig. 4). Blockade of AVP response was nearly complete when the two antagonists were pre-incubated simultaneously (right panels in A and B).
Origin of the Ca2+ rise induced by the agonists
The responses induced by V1a or V2 receptor agonists were also tested in Ca2+-free EGTA buffer to investigate the dependence upon external Ca2+ (Fig. 6A and B). The [Ca2+]i responses induced by 1 μm F-180 or dDAVP were completely abolished in a Ca2+-free EGTA buffer (n = 10 and 4, respectively), suggesting that extracellular Ca2+ is necessary for the action of either agonist.
Figure 6. Block of [Ca2+]i responses to V1a and V2 receptor agonists by suppression of extracellular Ca2+.
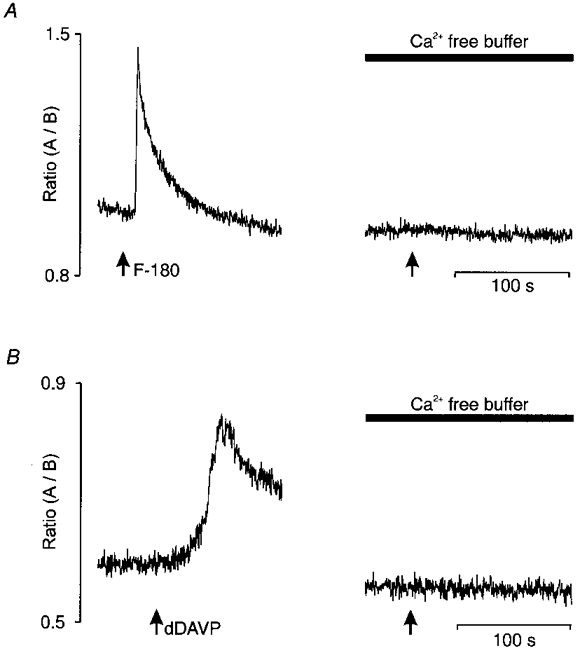
In A and B, the left panel represents the [Ca2+]i response induced by 1 μm F-180 and dDAVP, respectively, in the presence of normal Locke buffer containing 1.8 mM external Ca2+. Pre-incubation in a Ca2+-free EGTA buffer (see Methods) for 10 min abolished the response to each agonist (right panels).
DISCUSSION
In this study, the use of specific agonists and antagonists allowed us to define the pharmacological properties of AVP receptors expressed by AVP-responding neurones of the SON (i.e. vasopressinergic neurones, see Dayanithi et al. 1996). In most of these neurones, both the V1a and V2 receptor agonists, F-180 and dDAVP, induced dose-dependent rises in [Ca2+]i that were totally abolished in a Ca2+-free EGTA buffer and inhibited by their respective selective antagonist. The response to AVP was decreased by both V1a and V2 receptor antagonists, suggesting the simultaneous activation of V1a- and V2-type receptors by AVP. Because AVP was previously shown to induce an increase in [Ca2+]i in AVP-sensitive neurones (Dayanithi et al. 1996), and because F-180 and dDAVP elicited responses only in AVP-sensitive neurones, these agonists are most likely to act on AVP neurones.
Characteristics of the responses to V1a and V2 receptor agonists
The [Ca2+]i responses to the V1a receptor agonist, F-180, and to AVP were similar, i.e. the responses occurred with a short latency and did not desensitize. The reproducibility of the AVP responses could be due to the use of a rapid and short duration of application of the peptide rather than static incubation over long periods, which might have caused desensitization as previously reported (Dayanithi et al. 1996). The dose-response relationship to F-180 was best described by a double Hill function, which suggests the activation of two receptor types with different affinities. Such a hypothesis is plausible since the existence of low- and high-affinity states for the V1a receptor has been demonstrated in smooth muscle cells (Stassen et al. 1987; Gopalakrishnan et al. 1991) and in the liver (Gopalakrishnan et al. 1988). The biphasic dose-response to F-180 could result from complex interactions between different second messenger pathways (see Sabatier et al. 1998), which could modulate the activation of Ca2+ channels. Indeed, it has been shown that the rise in [Ca2+]i activates the neurospecific type I calmodulin-sensitive adenylyl cyclase, which in turn exerts a positive feedback regulation of Ca2+ channels by cAMP-dependent protein kinase (Choi et al. 1993). In the present work, [Ca2+]i responses induced by F-180 were also abolished in a free Ca2+ buffer, suggesting the involvement of a Ca2+ influx but not excluding an additional participation of Ca2+ from internal stores, as has been previously demonstrated for AVP (Dayanithi et al. 1996; Sabatier et al. 1997). In cultured hippocampal (Brinton et al. 1994) and cortical (Son & Brinton, 1998) neurones, the absence of Ca2+ in the extracellular medium has been shown to abolish the rise in [Ca2+]i induced by a V1 vasopressin receptor agonist, but the inositol-1-phosphate formation persisted. These studies indicate that the absence of extracellular Ca2+ does not affect the binding characteristics of the V1 vasopressin receptor. They further showed that the activation of phospholipase C, and thus the production of the phosphatidylinositols, was involved in the response to the V1 receptor agonist, but was not sufficient to increase the [Ca2+]i.
Conversely, [Ca2+]i responses induced by the V2 receptor agonist, dDAVP, were smaller and delayed compared with those elicited by either AVP or F-180, and the dose-response curve showed a single slope. Figure 3 clearly illustrates the fact that the latency of the responses to dDAVP and F-180 does not depend on the concentrations that induced a significant response (i.e. over 30 nm). Indeed, the latency to dDAVP response is similar from 30 nm (i.e. below the half-maximal dose) to 300 or 1000 nm (illustration not shown), and is longer than the latency to F-180 responses from 100 to 1000 nm. The threshold of the dDAVP response was 30 nm, i.e. much higher than the concentration (0.7 nm) required to elicit [Ca2+]i changes in rat medullary collecting tubules (Champigneulle et al. 1993). The tachyphylaxis obtained with a short interval between applications of dDAVP seems to be directly linked to the agonist characteristics rather than the receptor itself, since AVP showed reproducible responses. In the present study, dDAVP triggered a rise in [Ca2+]i that was abolished in a Ca2+-free buffer, as has been demonstrated in rat inner medullary collecting tubes (Ishikawa & Saito, 1990; Naruse, 1992). This result supports the activation of Ca2+ influx by V2 receptors, although the possibility of an additional mobilization of Ca2+ from internal stores is not excluded, as is the case for AVP (Dayanithi et al. 1996). Indeed, mobilization of Ca2+ from intracellular stores by V2-like receptors has been observed in a subpopulation of neurones from circumventricular organs (Jursak et al. 1995). Whatever the origin of the Ca2+, the transduction pathways responsible for the [Ca2+]i rise induced by dDAVP in the present work are still unknown. The involvement of the cAMP pathway is an interesting issue, since typically the V2 receptor has been reported to be linked to activation of adenylyl cyclase (see Zingg, 1996).
Which AVP receptors subtypes are present on magnocellular neurones?
Based on their pharmacological profile and transduction signals, three types of vasopressin receptors have been described in the periphery: V1a- and V1b-types, both coupled to the phospholipase C, and V2-type, coupled to the adenylate cyclase (see Zingg, 1996). The V1a receptor agonist, F-180, is a selective vasoconstrictor and appears to be the most specific ligand of the V1a receptor available to date (Aurell et al. 1991; Bernadich et al. 1998). Similarly, SR 49059 is a potent, non-peptidic antagonist of rat and human vasopressin V1a receptors (Serradeil-Le-Gal et al. 1993; Guillon et al. 1995). The dDAVP and SR 121463A are respectively considered as a specific agonist (Manning et al. 1976) and a selective antagonist of the V2 receptor (Serradeil-Le-Gal et al. 1996).
The affinities of AVP for peripheral vasopressin receptors in the rat are: Kd= 1.7 nm for the V1a receptor and Kd= 0.4 nm for the V2 receptor. The affinities of the V2 agonist dDAVP are: Kd= 250 nm for the V1a receptor and Kd= 0.3 nm for the V2 receptor. Concerning the V1a agonist, F-180, the relative affinities studied in COS cells are: Kd= 5.8 nm for the V1a receptor and Kd > 10 000 nm for the V2 receptor. Therefore, the V1a agonist presents a very good selectivity between V1a and V2 receptors (see Barberis & Tribollet, 1996).
The antagonists we used are very potent and selective. The affinities of the V1a antagonist for peripheral receptors in the rat are: Kd= 2 nm for the V1a receptor and Kd= 275 nm for the V2 receptor. The affinities of V2 antagonist SR 121463A are: Kd= 10 600 nm and Kd= 1.42 nm for V1a and V2 receptors, respectively (see Serradeil-Le-Gal et al. 1996). At the concentrations we used, the V2 agonist (1 μm) is able to bind to V1a receptors, but in our experiments the response to V2 agonist is not affected by the V1a antagonist (10 nm), while it is totally blocked by the V2 antagonist (10 nm). However, we also have to consider that the relative affinities and even the nature of vasopressin receptors expressed in the central nervous system are not well known and remain to be clearly elucidated.
In the present work, 10 nm SR 49059 abolished the [Ca2+]i response to F-180, but not that to dDAVP. Similarly, 10 nm SR 121463A completely suppressed the [Ca2+]i response to dDAVP but not that to F-180. These results argue for the presence of two distinct types of AVP receptors on the cell body of the large majority of AVP-responding neurones. These two AVP receptor types appear to be simultaneously activated by AVP, since the selective V1a and V2 receptor antagonists show additivity in the blockade of the AVP-induced response. In agreement with these results, we recently demonstrated that the [Ca2+]i rise induced by AVP in AVP magnocellular neurones involved both the phospholipase C and adenylyl cyclase intracellular pathways (Sabatier et al. 1998), the established transduction pathways of V1a and V2 receptors, respectively. Furthermore, AVP has been reported to induce a rise in cAMP levels in neurones from the SON, and cAMP to mimic the electrophysiological effect of AVP on magnocellular neurones (Abe et al. 1983).
Taken together, these results clearly suggest a possible expression of V1a and V2 receptors on AVP magnocellular neurones. However, a different picture arose from a recent study from our laboratory using RT-PCR, which showed the presence of V1a and V1b receptor mRNAs, but not the presence of the V2 transcript, in the SON (Hurbin et al. 1998). By in situ hybridization, these authors further visualized V1a and V1b mRNAs in AVP magnocellular neurones. It is of interest to note that a similar observation was made by Vaccari and collaborators (Vaccari et al. 1998) that the supraoptic nucleus expresses V1b transcripts. With regard to the V1a receptor, these results fit with the present study. For the V1b receptor, the lack of specific ligands limits its pharmacological characterization (Barberis & Tribollet, 1996). Nevertheless, since a residual AVP response persisted in the presence of both V1a and V2 receptor antagonists, we suggest the presence of another receptor type on AVP magnocellular neurones, which could be the V1b receptor type. With regard to V2 receptors, the results obtained with RT-nested PCR (Hurbin et al. 1998) are in apparent contradiction with the present pharmacological data. Similarly, the V2-type receptor has not been identified in the brain by either autoradiography or immunocytochemistry (see Burbach et al. 1995), whereas several pharmacological studies attest for its central involvement in different functions such as the proconvulsive effect of AVP (Croiset & De Wied, 1997), analgesia (You et al. 1995), cardiovascular regulation (Lowes et al. 1993) and Ca2+ responses in circumventricular organs (Jursak et al. 1995). Thus the nature of the AVP receptor mimicking the V2-type pharmacology or activating the cAMP pathway remains to be elucidated.
In conclusion, our results demonstrate that AVP-responding neurones express at least two distinct AVP receptors that have the pharmacological profile of V1a and V2 receptors. The existence of functional V1a-type receptors is corroborated by the presence of V1a receptor mRNA in AVP neurones, but this is not the case for the receptor exhibiting the pharmacological profile of the V2 type. The nature of this V2-like receptor has yet to be defined. There is no direct solution offered in this paper for the discrepancies between the data (absence of V2 receptor mRNA vs. V2 agonist-induced [Ca2+]i response) obtained within our research group. However, we can clearly hypothesize that the V2-agonist could act on a different type (V2-like?) or a new type of receptor that may have a similar pharmacology to ‘classical’ V2-type receptors. Nevertheless, it appears that AVP magnocellular neurones express multiple AVP receptor types that could account for the complex effects of AVP on the electrical activity of AVP neurones observed in vivo (Gouzènes et al. 1998a). The inhibitory effect of AVP seems to be mediated via the V1a receptor, as revealed by the excitatory effect of the V1a receptor antagonist on the phasic pattern of AVP neurones (Dayanithi et al. 1995; Ludwig & Leng, 1997; Gouzènes et al. 1998b). In contrast, Inenaga & Yamashita (1986) have suggested that the activation of V1 receptors results mostly in neuronal excitation. However, our preliminary results suggest that the excitatory effect could be mediated by V2-type receptors (Gouzènes et al. 1998b). A pharmacological characterization of all receptors activated by AVP expressed on AVP magnocellular neurones, as well as intracellular pathways activated by the different agonists, is under investigation. This will help in defining the exact nature of the receptor types activated by AVP on AVP magnocellular neurones.
Acknowledgments
We are sincerely grateful to Dr C. Barberis (INSERM U-469, CCIPE, Montpellier, France) for the generous gift of dDAVP, dVDAVP and [Phe2, Orn8]vasotocin, to Dr C. Serradeil-Le Gal (Sanofi Recherche, Centre de Toulouse, France) for SR 49059 and SR 121463A, and to Dr J. L. Junien (Ferring France, Gentilly, France) for F-180. We thank Dr C. Barberis, Dr G. Guillon, Dr B. Mouillac (INSERM U-401, CCIPE, Montpellier, France) for helpful discussions and Dr M. G. Desarménien, Dr N. Hussy, Dr A. Rabié (CNRS UPR-9055, CCIPE, Montpellier, France) and Colin Brown (Edinburgh, UK) for critical reading of the manuscript.
References
- Abe H, Inoue M, Matsuo T, Ogata N. The effects of vasopressin on electrical activity in the guinea-pig supraoptic nucleus in vitro. The Journal of Physiology. 1983;337:665–685. doi: 10.1113/jphysiol.1983.sp014648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong WE. Morphological and electrophysiological classification of hypothalamic supraoptic neurones. Progress in Neurobiology. 1995;47:291–339. 10.1016/0301-0082(95)00025-9. [PubMed] [Google Scholar]
- Aurell C-J, Bengtsson B, Ekholm K, Kasprzykowska R, Nilsson A, Persson R, Trojnar J, Abbe M, Melin P. Development of vasopressor specific vasotocin analogs with prolonged effects. In: Girlt E, Andreu D, editors. Peptides - Proceedings of 21 European Peptides Symposium. Leiden, The Netherlands: ESCOMB Science Publishers; 1991. pp. 671–673. [Google Scholar]
- Barberis C, Tribollet E. Vasopressin and oxytocin receptors in the central nervous system. Critical Reviews in Neurobiology. 1996;10:119–154. doi: 10.1615/critrevneurobiol.v10.i1.60. [DOI] [PubMed] [Google Scholar]
- Bernadich C, Bandi J-C, Melin P, Bosch J. Effects of F-180, a new selective vasoconstrictor peptide, compared with terlipressin and vasopressin on systemic and splanchnic hemodynamics in a rat model of portal hypertension. Hepatology. 1998;27:351–356. doi: 10.1002/hep.510270206. [DOI] [PubMed] [Google Scholar]
- Brinton RD, Gonzales TM, Cheung WS. Vasopressin-induced calcium signaling in cultured hippocampal neurones. Brain Research. 1994;661:274–282. doi: 10.1016/0006-8993(94)91194-0. 10.1016/0006-8993(94)91204-1. [DOI] [PubMed] [Google Scholar]
- Burbach JPH, Adan RAH, Lolait SJ, van Leeuwen FW, Mezey E, Palkovits M, Barberis C. Molecular neurobiology and pharmacology of the vasopressin/oxytocin receptor family. Cellular and Molecular Neurobiology. 1995;15:573–595. doi: 10.1007/BF02071318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champigneulle A, Siga E, Vassent G, Imbert-Teboul M. V2-like vasopressin receptor mobilizes intracellular Ca2+ in rat medullary collecting tubules. American Journal of Physiology. 1993;265:F35–45. doi: 10.1152/ajprenal.1993.265.1.F35. [DOI] [PubMed] [Google Scholar]
- Choi EJ, Xia Z, Villacres EC, Storm DR. The regulatory diversity of the mammalian adenylyl cyclases. Current Opinion in Cell Biology. 1993;5:269–273. doi: 10.1016/0955-0674(93)90115-7. [DOI] [PubMed] [Google Scholar]
- Croiset G, De Wied D. Proconvulsive effect of vasopressin; mediation by a putative V2 receptor subtype in the central nervous system. Brain Research. 1997;759:18–23. doi: 10.1016/s0006-8993(97)00070-x. [DOI] [PubMed] [Google Scholar]
- Dayanithi G, Moos F, Richard Ph. Vasopressin controls magnocellular vasopressin neurones via V1-type receptors in the rat. The Journal of Physiology. 1995;489.P:184–185P. [Google Scholar]
- Dayanithi G, Widmer H, Richard Ph. Vasopressin-induced intracellular Ca2+ increase in isolated rat surpraoptic cells. The Journal of Physiology. 1996;490:713–727. doi: 10.1113/jphysiol.1996.sp021180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Scala-Guenot D, Strosser MT, Richard Ph. Electrical stimulation of perifused magnocellular nuclei in vitro elicit Ca2+-dependent, tetrodotoxin-insensitive release of oxytocin and vasopressin. Neuroscience Letters. 1987;76:209–214. doi: 10.1016/0304-3940(87)90717-8. 10.1016/0304-3940(87)90717-8. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan V, McNeill JR, Sulakhe PV, Triggle CR. Hepatic vasopressin receptor: differential effects of divalent cations, guanine nucleotides, and N-ethylmaleimide on agonist and antagonist interaction with the V1 subtype receptor. Endocrinology. 1988;123:922–931. doi: 10.1210/endo-123-2-922. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan V, Xu Y, Sulakhe PV, Triggle CR, McNeill JR. Vasopressin (V1) receptor characteristics in rat aortic smooth muscle cells. American Journal of Physiology. 1991;261:H1927–1936. doi: 10.1152/ajpheart.1991.261.6.H1927. [DOI] [PubMed] [Google Scholar]
- Gouzènes L, Desarménien M, Hussy N, Richard Ph, Moos FC. Vasopressin regularizes the phasic firing pattern of rat hypothalamic magnocellular vasopressin neurons. Journal of Neuroscience. 1998a;18:1879–1885. doi: 10.1523/JNEUROSCI.18-05-01879.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouzènes L, Sabatier N, Dayanithi G, Moos F, Richard Ph. Role for V1a- and V2-like vasopressin (AVP) receptors on rat supraoptic AVP neurones. Forum of European Neuroscience. European Journal of Neuroscience. 1998b;10:294. no. 116.03. [Google Scholar]
- Gouzènes L, Sabatier N, Moos F, Dayanithi G. V1a- and V2-vasopressin receptor agonists induced [Ca2+]i responses within vasopressin-sensitive neurones of the rat supraoptic nucleus. The Journal of Physiology. 1998c;509.P:86P. doi: 10.1111/j.1469-7793.1999.0771s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillon G, Trueba M, Joubert D, Grazzini E, Chouinard L, Côté M, Payet MD, Manzoni O, Barberis C, Robert M, Gallo-Payet N. Vasopressin stimulates steroid secretion in human adrenal glands: comparison with angiotensin-II effect. Endocrinology. 1995;136:1285–1295. doi: 10.1210/endo.136.3.7867583. 10.1210/en.136.3.1285. [DOI] [PubMed] [Google Scholar]
- Hurbin A, Boissin-Agasse L, Orcel H, Rabié A, Joux N, Desarménien MG, Richard Ph, Moos FC. The vasopressinergic magnocellular neurons of the rat hypothalamus express V1a and V1b but not V2 vasopressin receptor mRNAs. Endocrinology. 1998;139:4701–4707. doi: 10.1210/endo.139.11.6320. 10.1210/en.139.11.4701. [DOI] [PubMed] [Google Scholar]
- Inenaga K, Yamashita H. Excitation of neurones in the rat paraventricular nucleus in vitro by vasopressin and oxytocin. The Journal of Physiology. 1986;370:165–180. doi: 10.1113/jphysiol.1986.sp015928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa S, Saito T. Vasopressin-induced increases in cellular free calcium concentration measured in single cells of rat renal papillary collecting tubule. Endocrinologica Japanica. 1990;37:381–387. doi: 10.1507/endocrj1954.37.381. [DOI] [PubMed] [Google Scholar]
- Jursak M, Muller AR, Gerstberger R. Characterization of vasopressin receptors in cultured cells derived from the region of rat brain circumventricular organs. Neuroscience. 1995;65:1145–1159. doi: 10.1016/0306-4522(94)00539-h. 10.1016/0306-4522(94)00539-H. [DOI] [PubMed] [Google Scholar]
- Lambert RC, Dayanithi G, Moos FC, Richard Ph. A rise in the intracellular Ca2+ concentration of isolated rat supraoptic cells in response to oxytocin. The Journal of Physiology. 1994;478:275–288. doi: 10.1113/jphysiol.1994.sp020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, Ludwig M. Vasopressin release within the supraoptic and paraventricular nuclei of the rat brain: osmotic stimulation via microdialysis. Brain Research. 1991;558:191–196. doi: 10.1016/0006-8993(91)90768-q. 10.1016/0006-8993(91)90768-Q. [DOI] [PubMed] [Google Scholar]
- Leng G, Mason WT. Influence of vasopressin upon firing patterns of supraoptic neurones: a comparison of normal and Brattleboro rats. Annals of the New York Academy of Sciences. 1982;394:153–158. doi: 10.1111/j.1749-6632.1982.tb37422.x. [DOI] [PubMed] [Google Scholar]
- Lowes VL, McLean LE, Kasting NW, Ferguson AV. Cardiovascular consequences of microinjection of vasopressin and angiotensin II in the area postrema. American Journal of Physiology. 1993;265:R625–631. doi: 10.1152/ajpregu.1993.265.3.R625. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Autoinhibition of supraoptic nucleus vasopressin neurons in vivo: a combined retrodialysis/electrophysiological study in rats. European Journal of Neuroscience. 1997;9:2532–2540. doi: 10.1111/j.1460-9568.1997.tb01682.x. [DOI] [PubMed] [Google Scholar]
- Manning M, Balaspiri L, Moehring J, Haldar J, Sawyer WH. Synthesis and some pharmacological properties of deamino (4-threonine, 8-D-arginine)vasopressin and deamino (8-D-arginine)vasopressin, highly potent and specific antidiuretic peptides, and (8-D-arginine)vasopressin and deamino-arginine-vasopressin. Journal of Medical Chemistry. 1976;19:842–845. doi: 10.1021/jm00228a023. [DOI] [PubMed] [Google Scholar]
- Naruse M. Arginine vasopressin increases intracellular calcium ion concentration in isolated mouse collecting tubule cells: distinct mechanism of action through V2 receptor, but independent of adenylate cyclase activation. Nippon Jinzo Gakkai Shi. 1992;34:337–347. [PubMed] [Google Scholar]
- Oliet SH, Bourque CW. Properties of supraoptic magnocellular neurones isolated from the adult rat. The Journal of Physiology. 1992;455:291–306. doi: 10.1113/jphysiol.1992.sp019302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orloff J, Handler J. The role of adenosine 3′, 5′-phosphate in the action of antidiuretic hormone. American Journal of Medicine. 1967;42:757–768. doi: 10.1016/0002-9343(67)90093-9. 10.1016/0002-9343(67)90093-9. [DOI] [PubMed] [Google Scholar]
- Pow DV, Morris JF. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience. 1989;32:435–439. doi: 10.1016/0306-4522(89)90091-2. 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- Sabatier N, Richard Ph, Dayanithi G. L-, N- and T- but neither P- nor Q-type Ca2+ channels control vasopressin-induced Ca2+ influx in magnocellular vasopressin neurones isolated from the rat supraoptic nucleus. The Journal of Physiology. 1997;503:253–268. doi: 10.1111/j.1469-7793.1997.253bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier N, Richard Ph, Dayanithi G. Activation of multiple intracellular transduction signals by vasopressin in vasopressin-sensitive neurones of the rat supraoptic nucleus. The Journal of Physiology. 1998;513:699–710. doi: 10.1111/j.1469-7793.1998.699ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serradeil-Le-Gal C, Lacour C, Valette G, Garcia G, Foulon L, Galindo G, Bankir L, Pouzet B, Guillon G, Barberis C, Chicot D, Jard S, Vilain P, Garcia C, Marty E, Raufaste D, Brossard G, Nisato D, Maffrand JP, Le Fur G. Characterization of SR 121 463A, a highly potent and selective, oraly active vasopressin V2 receptor antagonist. Journal of Clinical Investigation. 1996;98:2729–2738. doi: 10.1172/JCI119098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serradeil-Le-Gal C, Wagnon J, Garcia C, Lacour C, Guiraudou P, Christophe B, Villanova G, Nisato D, Maffrand JP, Le Fur G, Guillon G, Cantau B, Barberis C, Trueba M, Ala Y, Jard S. Biochemical and pharmacological properties of SR 49059, a new potent, nonpeptide antagonist of rat and human vasopressin V1a receptors. Journal of Clinical Investigation. 1993;92:224–231. doi: 10.1172/JCI116554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son MC, Brinton RD. Vasopressin-induced calcium signaling in cultured cortical neurones. Brain Research. 1998;793:244–254. doi: 10.1016/s0006-8993(98)00185-1. 10.1016/S0006-8993(98)00185-1. [DOI] [PubMed] [Google Scholar]
- Stassen FL, Heckman G, Schmidt D, Aiyar N, Nambi P, Crooke ST. Identification and characterization of vascular (V1) vasopressin receptors of an established smooth muscle cell line. Molecular Pharmacology. 1987;31:259–266. [PubMed] [Google Scholar]
- Stephens LR, Logan SD. Arginine-vasopressin stimulates inositol phospholipid metabolism in rat hippocampus. Journal of Neurochemistry. 1986;46:649–651. doi: 10.1111/j.1471-4159.1986.tb13016.x. [DOI] [PubMed] [Google Scholar]
- Vaccari C, Lolait SJ, Ostrowski NL. Comparative distribution of vasopressin V1b and oxytocin receptor messenger ribonucleic acids in brain. Endocrinology. 1998;139:5015–5033. doi: 10.1210/endo.139.12.6382. 10.1210/en.139.12.5015. [DOI] [PubMed] [Google Scholar]
- You ZD, Song CY, Wang CH, Huang AJ, Lin BC. Role of locus coeruleus in analgesia caused by stimulation of supraoptic nucleus. Sheng Li Hsueh Pao. 1995;47:320–326. [PubMed] [Google Scholar]
- Zingg HH. Vasopressin and oxytocin receptors. Baillière's Clinical Endocrinology and Metabolism. 1996;10:75–96. doi: 10.1016/s0950-351x(96)80314-4. [DOI] [PubMed] [Google Scholar]


