Abstract
Myocyte-dependent regulation of acetylcholine (ACh) quantal secretion from developing motoneurons was studied in day-3 Xenopus nerve-muscle co-cultures. Spontaneous synaptic currents (SSCs) were measured in manipulated synapses by using whole-cell voltage-clamped myocytes. Changes in SSC amplitude were assumed to reflect changes in the ACh content of secreted quantal packets. Compared with natural synapses, motoneurons without any contact with a myocyte (naive neurons) released ACh in smaller quantal packets.
Bipolar cultured motoneurons, which were in contact with a myocyte with one axon branch (contact-end) but remained free at another axon branch (free-end), were further used to examine quantal ACh secretion. The ACh quantal size recorded at free-end terminals was similar to that of naive neurons and was smaller than that at the contact-end, indicating that myocyte contact exerts differential regulation on quantal secretion in the same neuron.
Some of the neurons that formed a natural synapse with a myocyte continued to grow forward and ACh quantal secretion from the free growth cone was examined. The ACh quantal size recorded at free growth cones was inversely proportional to the distance to the natural synapse, implying localized regulation of quantal secretion by the myocyte.
Chronic treatment of day-1 cultures with veratridine and d-tubocurarine, respectively, increased and decreased the neurotrophic action of myocytes when assayed on day 3.
Taken together, these findings suggest that the myocyte is an important postsynaptic target in the regulation of quantal secretion and that the trophic action is spatially restricted to the neighbourhood of the neuromuscular junction.
During the course of normal development, the survival and differentiation of spinal cord motoneurons are influenced by many different environmental cues (McManaman et al. 1990; Henderson et al. 1993). The contact between presynaptic motoneurons and target cells leading to the exchange of electrical signals and chemical factors serves to co-ordinate their spatial and temporal differentiation (Hall & Sanes, 1993; Connor & Smith, 1994). Motoneurons have played an essential role in establishing the concept that target tissues have an important influence on the development of innervating neurons. The critical role of the peripheral target for developing motoneurons was first highlighted by experiments which showed that motoneuron death was increased by removal of the target and reduced by the presence of an additional limb bud during early developmental stages of chick embryos (Hamburger, 1975; Purves, 1988). These observations gave rise to the theory of target-dependent neurotrophic action and resulted in the discovery of various neurotrophic factors.
Extensive studies have elucidated the powerful effects of neurotrophic factors on neuronal survival and differentiation of motoneurons (Korsching, 1993; Isackson, 1995). We have previously shown that chronic treatment of day-1 cultures with brain-derived neurotrophic factor (BDNF), neurotrophin-4 (NT-4), ciliary neurotrophic factor (CNTF), glial cell line-derived neurotrophic factor (GDNF) and neurotrophin-3 (NT-3) for 2 days increased the acetylcholine (ACh) quantal size of naive motoneurons, indicating that many neurotrophic factors are involved in the regulation and maintenance of synaptic function in developing motoneurons (Liou & Fu, 1997; Liou et al. 1997). In the present study, we further characterized the nature of target-dependent trophic action by taking advantage of multiform encounters between growing motoneurons and myocytes in day-3 Xenopus nerve-muscle co-cultures. In addition, motoneurons also contact other cell types present in the culture system, which provides a good model for characterizing the specificity of target-derived neurotrophic activity. Here we found that myocytes, but not melanocytes or fibroblasts, increase ACh quantal packets, indicating that the regulation of quantal secretion is target dependent. Furthermore, myocyte modulation of quantal secretion is spatially localized to a restricted neighbourhood around the neuromuscular junction.
METHODS
Cell culture
All experiments were carried out in accordance with the guidelines laid down by our Institution's animal welfare committee. Xenopus nerve-muscle cultures were prepared as described by Tabti & Poo (1991). Briefly, the neural tube and the associated myotomal tissue of 1-day-old Xenopus embryos (stage 20–22) (Nieuwkoop & Faber, 1967) were dissected and dissociated in Ca2+- and Mg2+-free Ringer solution supplemented with 0.15 mM EDTA. The dissociated cells were plated on clean coverslips and were used for experiments after incubation in culture medium for 3 days at room temperature. The culture medium consisted of 50 % (v/v) Ringer solution (115 mM NaCl, 2 mM CaCl2, 2.5 mM KCl, 10 mM Hepes; pH adjusted to 7.6 with NaOH), 49 % L-15 Leibovitz medium (Sigma), 1 % fetal bovine serum (Life Technologies, Gaithersburg, MD, USA) and antibiotics (100 U ml−1 penicillin and 100 μg ml−1 streptomycin sulphate). There are at least four types of cells in these cultures. As shown in Fig. 1, they can be easily identified by their characteristic features. Myocytes of either spherical (myoballs) or spindle shape have a clear large nucleus. Spinal neurons are identified by their long axons. Fibroblasts are thinner than other cells and have many processes. Melanocytes are characterized by the black cytoplasmic melanophores.
Figure 1. Cell types in culture.
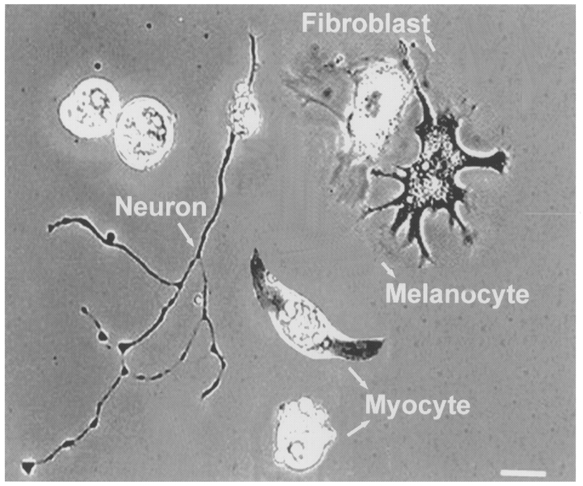
Xenopus cultured cells visualized with a phase-contrast microscope. Four types of cells were generally present in the culture, as labelled. Scale bar, 30 μm.
Cell manipulation experiment
Cultures were viewed with the phase-contrast optics of an inverted microscope (Nikon Diaphot-TMD), and cell manipulation was done with glass microelectrodes controlled by a micromanipulator (WR-88; Narishige, Tokyo, Japan). In these cultures motoneurons may either form natural synapses resulting from the random encounter of muscle cells by growing neurites or stay unconnected (naive neurons). Isolated embryonic myoballs have a more scattered distribution of ACh receptors and thus were used for manipulated contact (Evers et al. 1989). A myoball was first loosened from its attachment to the glass substratum by ‘rolling’ the cell across the substratum surface with a heat-polished tight-seal micropipette. The loosening of attachment allowed a myoball to be lifted up from the substratum and then translocated to make direct contact with the free growth cone region of the tested neuron to form a manipulated synapse. In some experiments, the postsynaptic myocyte of a natural synapse was mechanically destroyed by a micropipette and the scattered debris of the myocyte removed carefully. Another isolated myoball was then manipulated into contact with the so-called ‘vacated’ nerve terminals for measuring ACh release. Spontaneous synaptic current (SSC) recordings were made 3 min after contact of the manipulated myocyte with the free growth cone or vacated nerve terminals. These currents have been shown to be caused by spontaneous ACh secretion from the neuron, because they are abolished by bath application of d-tubocurarine (dTC) and unaffected by tetrodotoxin, which blocks action potentials in neurons (Xie & Poo, 1986). Besides myocytes, the neurites of spinal neurons may also have random encounters with melanocytes or fibroblasts. The quantal secretion of such neurons was also measured by manipulating a myoball into contact with the junctional region between neuron-melanocyte or neuron-fibroblast.
Drug administration
At 24 h incubation after culture plating (defined as day-1 culture), the culture medium was renewed and dTC, veratridine or neurotrophic factors NT-3 and CNTF (PeproTech, London, UK) were bath applied to the cultures. The cultures were used for experiments after 2 days further incubation (defined as day-3 culture). The drugs were removed by several washes with Ringer solution before the cultures were used for patch-clamp experiments.
Electrophysiology and data analysis
Gigaohm-seal whole-cell recording methods were used (Hamill et al. 1981). Patch pipettes were pulled with a 2-stage electrode puller (pp-83; Narishige) and the tips were polished immediately before the experiment using a microforge (MF-83; Narishige). SSCs from the natural or manipulated synapses were detected using a whole-cell voltage-clamped myocyte. Recordings were made at room temperature in Ringer solution and the solution inside the recording pipette contained (mM): 150 KCl, 1 MgCl2, 1 NaCl and 10 Hepes (the pH was adjusted to 7.2 with KOH). In some of the experiments, whole-cell recordings were made in the neuronal soma. The solution in the recording pipette contained (mM): 145 CsCl, 0.1 CaCl2, 3 MgCl2, 5 EGTA and 5 Hepes, pH 7.2; 1 mM amphotericin B was added to the internal solution to obtain a perforated patch. In all recordings, the membrane current or membrane potential was monitored by a patch-clamp amplifier (Axopatch 200A; Axon Instruments) and filtered at 10 kHz. The data were stored on a videotape recorder for later playback onto a storage oscilloscope or a polygraph (Gould RS 3200, Valley View, OH, USA) and also for amplitude analysis using the SCAN computer program (Dagan, Minneapolis, MN, USA). The detection threshold level was set as low as possible to capture most of the SSCs for SSC amplitude analysis. The results were expressed as means ±s.e.m. (n represents the number of cells examined) and statistical significance was evaluated by Student's t test and Dunnett's test. P < 0.05 was considered significant. The microscopic images were displayed on a video monitor (Kolin) or captured by computer with image processing software (Snappy, Play Incorporated, CA, USA) through a CCD camera mounted on the inverted microscope.
RESULTS
Localized regulation of motoneurons by myocytes
Isolated embryonic spinal neurons in Xenopus cultures established functional synaptic transmission with co-cultured myocytes soon after nerve-muscle contact to form natural synapses (Xie & Poo, 1986; Evers et al. 1989), although complete morphological maturation of the synapse requires many days (Takahashi et al. 1987). SSCs can be readily detected from the innervated muscle cell by whole-cell voltage-clamp recordings. The amplitude of SSCs depends on the amount of ACh contained in each secretory packet as well as the ACh receptor density in the postsynaptic membrane. In order to exclude a postsynaptic contribution to the size distribution of SSCs, the postsynaptic myocyte of natural synapses in day-3 cultures was mechanically removed by a micropipette, and another isolated myocyte was manipulated into contact with the so-called vacated nerve terminals. In our previous studies, we have shown that, compared with vacated nerve terminals, motoneurons without contact with any myocyte (naive neurons) release ACh in smaller quantal packets. The quantal size of the naive neuron is inversely proportional to the days in culture. These results suggest that muscle-derived neurotrophic factors participate in the regulation of transmitter packets at developing neurons (Liou & Fu, 1997; Liou et al. 1997). Whether the myocyte contact has universal trophic action in the regulation of quantal secretion throughout the whole neuron was further investigated in the current study. The properties of ACh quantal secretion were examined in a bipolar motoneuron which formed a natural synapse with a myocyte at one axon branch (called ‘contact-end’) and remained free at another axon branch (called ‘free-end’) in day-3 cultures (Fig. 2A, left panel). As shown in Fig. 2B, the SSC amplitude recorded from the free-end terminal of a bipolar neuron was similar to that of a naive neuron (Fig. 2A, right panel), but smaller than that from the contact-end nerve terminal (measurement in vacated nerve terminal as mentioned above). The SSC amplitudes were 81.3 ± 6.4 pA (n = 3), 55.1 ± 1.2 pA (n = 3) and 48.2 ± 1.9 pA (n = 7) for vacated synapse, free-end and naive neuron, respectively (Fig. 3). These results suggest that myocyte contact increases ACh quantal size on the contacted, but not on the free, axon branches of the same neuron.
Figure 2. Differential regulation of ACh quantal secretion by myocyte contact.
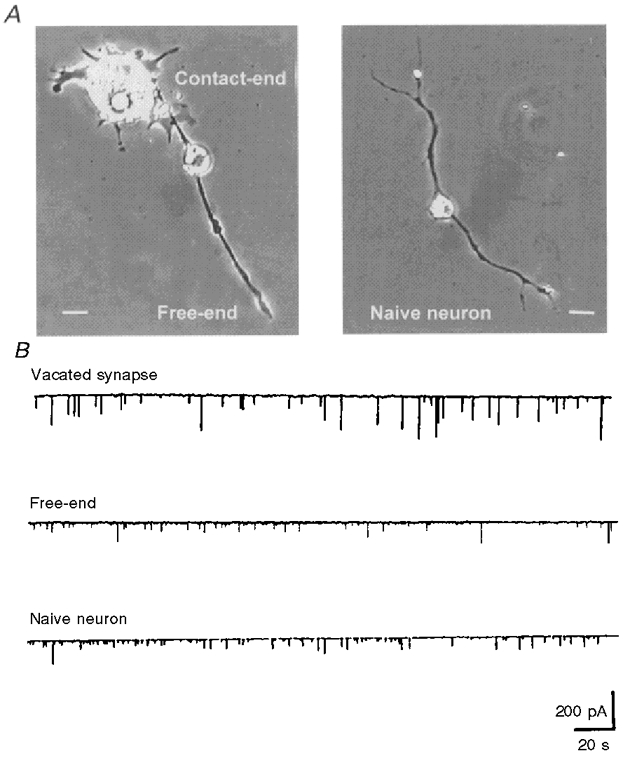
A, phase-contrast photograph showing a naive neuron (right) and a bipolar neuron (left) which formed a natural synapse with a myocyte at one axon branch (Contact-end) and remained free at another axon branch (Free-end). Scale bars, 10 μm. B, continuous traces showing the SSCs of a manipulated synapse at the contact-end or free-end of a bipolar neuron or at a growth cone of the naive neuron. The postsynaptic myocyte of the contact-end was mechanically destroyed by a micropipette and the scattered debris of the myocyte was removed carefully. Another isolated myoball was then manipulated into contact with the vacated nerve terminals for measuring ACh release (top trace). The manipulated myoball was whole-cell voltage clamped at −70 mV.
Figure 3. Summary of SSC amplitudes at manipulated synapses.
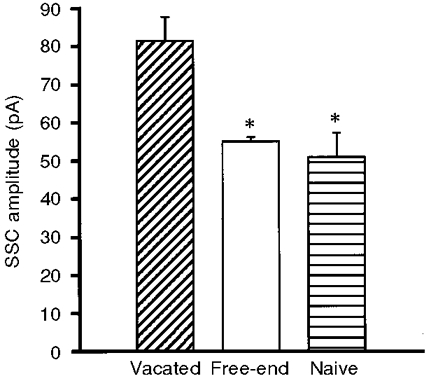
Spontaneous ACh release was recorded by moving an isolated myoball into contact with vacated-end terminals or free-end terminals of a bipolar neuron or terminals of a naive neuron (3–7 terminals). Note that the SSC amplitude at the free-ends was similar to that of naive neurons and smaller than that of vacated synapses. *P < 0.05, compared with vacated synapses (Dunnett's test).
To further characterize the localized neurotrophic effect of myocytes, the following experiments were performed. The growth cone continued to grow forward in some of the neurons that formed natural synapses with myocytes (Fig. 4A; as shown by the arrow). The ACh secretion of the free growth cone region was recorded using an isolated myoball as a detector and the distance between the free growth cone and the nearest myocyte of the natural synapse was measured along the neuritic trajectory (as shown by the dashed line in Fig. 4A). It was found that the SSC amplitude was inversely proportional to the distance (Fig. 4B), indicating the restrictive trophic action of the myocyte in the regulation of ACh quantal size. The SSC amplitude showed no significant difference from that recorded from a naive neuron (dashed line; Fig. 4B) when the distance between the free growth cone and natural synapse was larger than 70 μm. These results suggest that myocyte regulation of neurotransmitter secretion is strictly localized to the neighbouring area of the neuromuscular junction. The trophic effect on the nerve terminal is greater when the free growth cone is closer to the innervated myocyte.
Figure 4. Local action of myocyte on ACh quantal secretion.
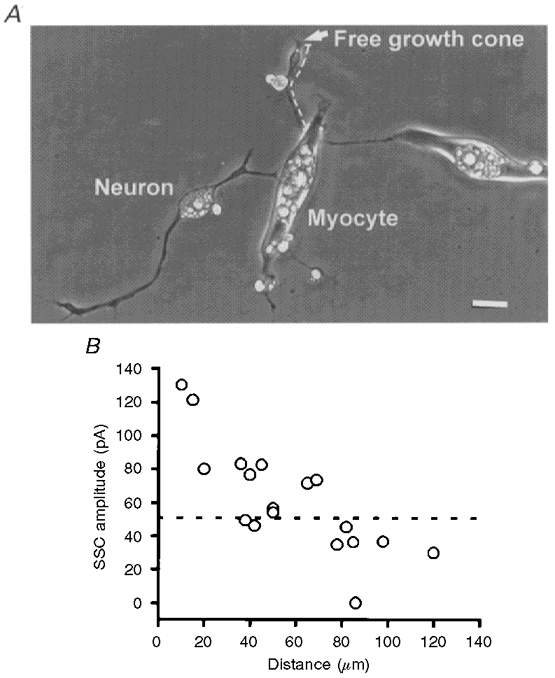
A, phase-contrast photograph showing a natural synapse and a neurite continuing to grow forward. The arrow indicates the free growth cone upon which the manipulated synapse was formed using an isolated myoball (not shown). The distance between the free growth cone and the natural synapse on the myocyte was measured along the neuritic trajectory, as shown by the dashed line. Scale bar, 10 μm. B, SSCs from the free growth cone were recorded using an isolated myoball as a detector and the distance between the growth cone and natural synapse was measured and plotted on the X-axis. Each data point represents the mean SSC amplitude recorded from a growth cone. For comparison, the mean SSC amplitude recorded from naive neurons is shown as the horizontal dashed line.
Effect of synaptic activity on the local trophic action of myocytes
Synaptic activity is known to play a critical role in regulating the pattern of synaptic connections. Our previous study has shown that the retrograde effect of muscle cells on secretion from presynaptic nerve terminals depends on synaptic activity (Liou & Fu, 1997). Muscle-derived neurotrophic factors participate in the maturation of normal transmitter packets in developing neurons. Chronic treatment of the culture with dTC for 2 days resulted in a marked reduction of quantal size, when assayed after extensive washing of the culture with Ringer solution. On the other hand, co-treatment with the sodium channel activator veratridine effectively reversed the effect of dTC on the quantal size. We thus further examined whether the relationship between SSC amplitude and distance was affected by treating cultures with dTC or veratridine. Either dTC (50 μm) or veratridine (1 μm) was bath applied to cultures at day 1 and the SSC amplitude of the day-3 culture was measured. Neurons which formed a natural synapse with a myocyte and continued to grow forward for different distances were chosen for this kind of experiment. The ACh-releasing properties of the free growth cone were examined by manipulating a myoball into contact as a detector. As shown in Fig. 5, veratridine (1 μm) shifted the SSC curves to the right and dTC (50 μm) markedly inhibited the spatial neurotrophic effect of myocytes. The neuromuscular blocking action of dTC can be reversed following removal of dTC by extensive washing of cultures with fresh Ringer solution (Liou & Fu, 1997). Therefore, the reduced SSC amplitude was not due to an incomplete clearance of dTC from the muscle cells. In order to confirm that veratridine increased the synaptic activity, we examined the acute synaptic effect of veratridine by local perfusion of veratridine with another glass pipette (6 μm opening) in day-1 cultures. It was found that veratridine (1 μm) depolarized the neuronal soma (from −62.0 ± 2.1 to −52.5 ± 2.2 mV, n = 5) and myocyte (from −71.3 ± 0.25 to −64.1 ± 1.0 mV, n = 5) within 5 min, when the cells were whole-cell current clamped. Veratridine (1 μm) increased the maximum SSC frequency by 57 ± 40-fold in natural synapses in five out of nine experiments while it had no significant effect on SSC frequency in the other four experiments. Increasing the concentration of veratridine to 2 μm enhanced maximum SSC frequency 173 ± 49-fold (n = 4). These results suggest that the enhancement or reduction of synaptic activity increased or decreased the muscular neurotrophic action, respectively. Therefore, synaptic activity plays an important role in the regulation of ACh quanta of presynaptic nerve terminals. The myocyte may release more diffusible trophic factors when synaptic activity is enhanced and the larger neighbouring area receives trophic support.
Figure 5. Effect of dTC and veratridine on the local trophic action of myocytes.
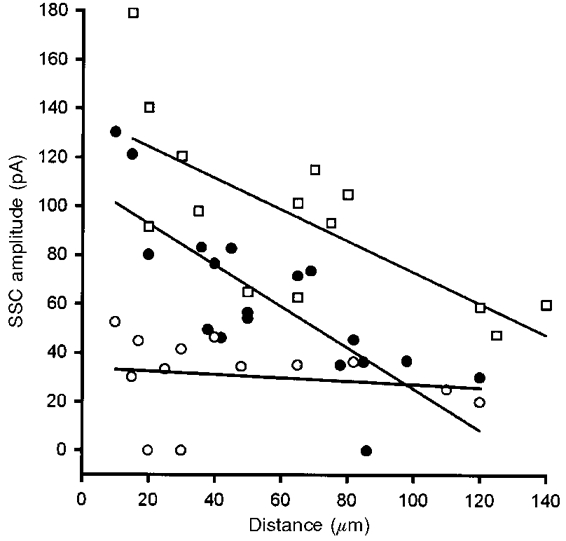
Day-1 cultures were treated with either dTC (50 μm) or veratridine (1 μm) and the SSCs of manipulated synapses at a free growth cone on the same axon branch as the natural synapse were recorded with whole-cell voltage-clamped myocytes (holding potential (Vh) =−70 mV) at day 3, after washout of the drugs. The distance between the free growth cone and the natural synapse was measured (as indicated in Fig. 4) and plotted on the X-axis. Each symbol represents data collected from one synapse and the continuous lines represent the best linear fits of the data points. ○, dTC; □, veratridine; •, control.
Effect of NT-3 and CNTF
From the results depicted above, the free growth cone with a natural synapse in the proximal region receives insufficient trophic support from the myocyte when the distance between the free growth cone and natural synapse is larger than 70 μm. We have previously demonstrated that the neurotrophic factors CNTF and NT-3 regulate transmitter packets at developing motoneurons (Liou et al. 1997; Liou & Fu, 1997). We thus evaluated the neurotrophic activity of these two factors on the ACh quantal size of the free growth cone at a distance greater than 70 μm from a natural synapse of the same axon branch. Either NT-3 (50 ng ml−1) or CNTF (100 ng ml−1) was bath applied to the culture at day 1 and the SSC amplitude of the day-3 growth cones with a distance of between 70 and 120 μm from the natural synapse in the same axon branch was recorded by manipulating an untreated myoball as detector. As shown in Fig. 6, the SSC amplitudes recorded from NT-3- and CNTF- treated growth cones were 90.2 ± 16.2 pA (n = 6) and 103.6 ± 23.0 pA (n = 5), respectively, which were significantly larger than that of untreated growth cones (36.7 ± 2.5 pA, n = 5). These results suggest that the smaller SSC amplitude of free growth cones partly results from insufficient support by neurotrophic factors derived from the myocyte. Indeed, if neurotrophic factors are endogenously produced by the myocyte and their actions are local at the synapse, it is expected that endogenous effects should occlude the effect of exogenous neurotrophic factors in natural synapses. We found that the mean SSC amplitude at NT-3-treated natural synapses (184.2 ± 33.1 pA, n = 22) was not significantly different from that at synapses in control day-3 cultures (155.3 ± 19.1 pA, n = 27). However, treatment with CNTF in day-1 cultures increased the SSC amplitude of natural synapses in day-3 cultures (240.4 ± 28.5 pA, n = 20), indicating that CNTF may be derived from other neighbouring cells besides myocytes in vivo.
Figure 6. Regulation of ACh quantal secretion by exogenous NT-3 and CNTF.
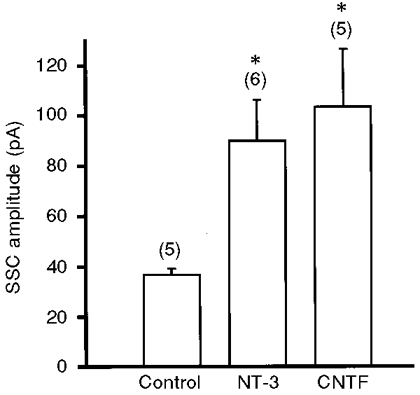
NT-3 (50 ng ml−1) or CNTF (100 ng ml−1) was bath applied to day-1 cultures and the SSCs of manipulated synapses were recorded at day 3. Growth cone free-ends at a distance of between 70 and 120 μm from the natural synapse were chosen for these experiments. A myoball was manipulated into contact with the growth cone and whole-cell voltage clamped at −70 mV to record SSCs. The numbers in parentheses indicate the total number of free growth cones examined. *P < 0.05, compared with control (Dunnett's test).
Postsynaptic target determines the trophic action on motoneurons
There are at least four cell types in the Xenopus cultures: spinal neuron, muscle cell, fibroblast and melanocyte (Fig. 1). We further clarified the target specificity by taking advantage of contacts between motoneurons and other cell types. The ACh-releasing property of fibroblast- or melanocyte-contacting neurons was measured by manipulating a myoball into contact with the junctional region between fibroblast-neuron or melanocyte-neuron. As shown in Fig. 7, the SSC amplitude of neurons which made contact with a fibroblast or melanocyte was similar to that of naive neurons and was smaller than that of vacated synapses. The SSC amplitudes of fibroblast- and melanocyte-contacting neurons were 52.1 ± 6.7 pA (n = 5) and 51.1 ± 7.8 pA (n = 4), respectively. The lower SSC amplitude recorded from neurons in contact with a fibroblast or melanocyte might result from the absence of regulation of ACh quantal secretion by these two cell types or from the distance between the release sites on the motor terminal and the detector myoball surface being greater.
Figure 7. Target-dependent regulation of ACh quantal secretion.
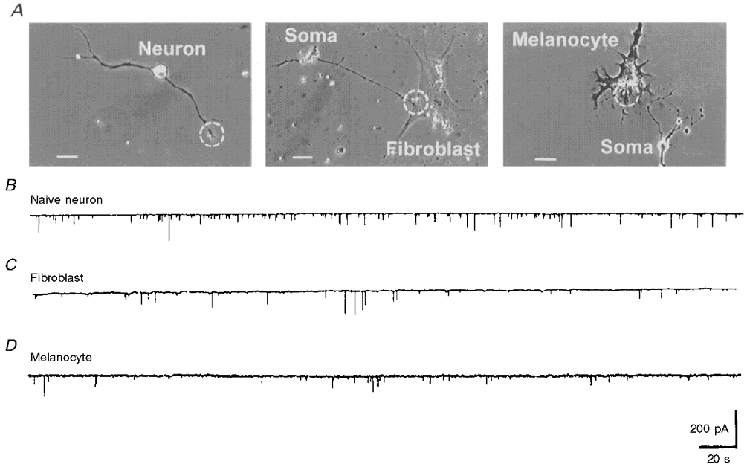
A, phase-contrast photographs showing a naive neuron (left), a fibroblast-contacting neuron (middle) and a melanocyte-contacting neuron (right). Dashed circles depict the contact area for the manipulated myoball. Scale bars, 20 μm. B-D, the downward continuous traces represent the SSCs recorded from manipulated synapses built using an isolated myoball in contact with the naive neuron (B), the fibroblast-contacting neuron (C) or the melanocyte-contacting neuron (D).
DISCUSSION
Localized action of myocyte-derived neurotrophic factors
The present results using a cell manipulation technique demonstrate that myocytes provide trophic support for developing motoneurons by regulating the ACh content of quantal packets. Upon reaching their targets, axons extending from motoneurons undergo structural and molecular differentiation during which the growth cone is reconfigured into a specialized axon terminal. Several lines of evidence suggest that this unique period of intimate contact between nerve and muscle cell triggers functional changes within the nerve terminals. Manipulated contact between a nerve growth cone and muscle cell induces elevation of the presynaptic calcium concentration followed by a rapid and persistent increase in ACh release, as measured by the appearance of synaptic currents in the muscle cell (Xie & Poo, 1986; Evers et al. 1989; Dai & Peng, 1993). The increased secretion of ACh, as well as many other modulatory substances, including calcitonin gene-related peptide, agrin and ATP (Mason et al. 1984; McMahan & Wallace, 1989; Reist et al. 1992; Fu, 1995) trigger specializations of postsynaptic muscle cells (Kidokoro & Saito, 1988; Jennings & Burden, 1993; Hall & Sanes, 1993). However, the muscle cell is not merely a passive recipient of inductive signals from the nerve terminals. Following initial contact and the early phase of neurotransmitter secretion, activity-dependent release of diffusible retrograde factors from myocytes stimulates neuronal development and maturation (Hall & Sanes, 1993; Connor & Smith, 1994; Liou & Fu, 1997; Liou et al. 1997). The successful co-ordination of nerve-myocyte is thus achieved through interactions between the neuron and its target.
The various forms of contact between motoneurons and myocytes in Xenopus cell cultures offer a good model for further understanding myocyte-derived trophic actions in the regulation of presynaptic nerve terminals. The ACh quantal size recorded at the free-end terminal of a bipolar neuron was similar to that of naive neurons, and was smaller than that at the contact-end of a bipolar neuron, indicating that myocyte contact exerts localized regulation of ACh quantal size in the same neuron. Moreover, the ACh quantal size recorded from the free growth cone was inversely proportional to the distance to the natural synapse in the same axon branch, implying localized regulation by the myocyte. Campenot, (1982, 1987, 1994) demonstrated that nerve growth factor (NGF) supplied to distal neurites in one compartment did not promote neurite growth from the same cultured rat sympathetic neuron in a different compartment. These experiments suggest that local application of NGF not only supports neuronal survival but also produces local and acute changes in growth cone morphology. Other evidence for spatially restricted influences in neuronal differentiation comes from a recent study in Xenopus cell cultures (Wang et al. 1998). These authors demonstrated that presynaptic potentiation is restricted to synapses on myocytes overexpressing NT-4, with no effect on nearby synapses formed by the same neuron on control myocytes. This presynaptic potentiation was spatially restricted to < 60 μm from the site of NT-4 secretion. The current study also indicates that the quantal size-promoting action of myocyte contact involves a mechanism localized to the neighbourhood of the neuromuscular junction. Even though the two axon branches of the same neuron were formed simultaneously, the regulation of ACh quantal secretion was quite different and depended on myocyte contact. A simple explanation is that the immediate mechanisms linking the binding of a neurotrophic factor to its receptor on the cell surface to quantal size regulation are localized near the site of neurotrophic factor binding and do not involve a retrograde intracellular signal to the cell body or an anterograde signal to more distant free growth cones, whose distance is greater than 70 μm from the natural synapse. This distance-SSC amplitude relationship is very similar to that reported by Wang et al. (1998). Shigemoto et al. (1996) demonstrated that the concentration of presynaptic metabotropic glutamate autoreceptors (mGluR7) depends on the identity of the postsynaptic neuron. That distinct levels of mGluR7 are found at different synapses made by individual pyramidal axons or even single boutons further indicates that the postsynaptic target can regulate the properties of individual synapses.
Role of synaptic activity
The activity of neuromuscular transmission at developing synapses is crucial in synaptic maturation and competition as well as in the differentiation of postsynaptic properties (Balice-Gordon & Lichtman, 1993; Bear & Malenka, 1994; Dan & Poo, 1994). The release of neurotrophin may also be activity dependent, as demonstrated in hippocampal neurons (Zafra et al. 1992) and in neuromuscular junctions (Liou & Fu, 1997). Here we show that prolonged blockade of neuromuscular transmission by bath application of dTC markedly inhibited the trophic effect of a neighbouring myocyte, whereas chronic treatment with veratridine to depolarize neuronal and muscle membrane potentials or increase synaptic transmission shifted the relationship between distance and quantal size to the right. These results suggest that synaptic activity plays an important role in the functional regulation of quantal size. The inhibitory action on the size of ACh quanta by chronic treatment with dTC suggests that postsynaptic activity is required for the retrograde effect on presynaptic nerve terminals. If the retrograde factor were a neurotrophic factor, exogenously supplied neurotrophic factor should obliterate the dTC effect. We have previously found that chronic treatment of cultures with exogenous neurotrophic factors NT-3, NT-4, BDNF, CNTF and GDNF could increase the ACh quantal size of naive motoneurons (Liou et al. 1997). In the current study, we used two neurotrophic factors, NT-3 and CNTF, to show a regulatory action on ACh quanta in free growth cones at a distance greater than 70 μm from the natural synapse in the same axon branch. Application of neurotrophic factors increased the SSC amplitude of manipulated synapses in free growth cones, indicating that a free growth cone receiving insufficient trophic support from myocytes can respond to exogenous application of neurotrophic factors. On the other hand, endogenously released NT-3 from myocytes occludes the effect of exogenous NT-3 at natural synapses. CNTF, which appears in extracellular compartments after injury, lacks a signal peptide typical of released proteins and normally remains confined within Schwann cells in vivo (Sendtner et al. 1992). Therefore, chronic treatment with exogenous CNTF still increased the SSC amplitude of natural synapses in cell cultures. Taken together, these results suggest that activity-dependent release of neurotrophic factors from myocytes may mediate spatially restricted regulation of ACh quantal secretion.
Target dependence in the regulation of ACh quantal secretion
During the course of normal development, spinal cord motoneurons are subjected to many different environmental cues which influence their survival and differentiation (McManaman et al. 1990). In addition to myocyte-derived neurotrophic factors, neurons might receive other trophic support from afferent neurons, axon-ensheathing glial cells, or other supportive cells (Henderson et al. 1993, 1994). Our analysis and comparison of neuronal function with different cell pairings (neuron-myocyte, neuron-fibroblast, neuron- melanocyte) suggest that myocytes are the main physiologically relevant target for regulating quantal secretion from developing motoneurons. The possibility that the lower SSC amplitude recorded from neurons in contact with a fibroblast or melanocyte results from a greater distance between the release sites on the motor terminal and the detector myoball surface should not be excluded. Although fibroblast contact does not increase ACh quantal secretion, the number of surviving neurons increases following contact with fibroblasts (J.-C. Liou & W.-M. Fu, unpublished observations). This result is consistent with our previous findings that bFGF (basic fibroblast growth factor) and IGF-1 (insulin-like growth factor) had no effect on ACh quantal secretion but are reported to increase the survival of motoneurons (Caroni, 1993; Ikeda et al. 1996). Thus, fibroblast contact may release some neurotrophic factors that support neuronal survival but have no effect on ACh quantal secretion.
The maintenance and/or potentiation of ACh quantal secretion by myocyte contact is crucial in synaptic maturation. At the developing neuromuscular junction, spontaneous synaptic activity appears immediately after nerve-muscle contact in cell cultures (Xie & Poo, 1986). Synaptic activity has a well-documented role in controlling the transcription and expression of several muscle fibre proteins (Goldman et al. 1988; Eftimie et al. 1991) and the release of neurotrophic factors from myocytes is activity dependent (Wang et al. 1995; Liou & Fu, 1997). Moreover, SSC frequency can be enhanced by the acute application of neurotrophic factors and the ACh content of quanta can be enhanced by chronic treatment with neurotrophic factors (Lohof et al. 1993; Stoop & Poo, 1996; Liou et al. 1997). The positive feedback regulation between nerve and muscle is initiated at contact and plays an important role in synaptogenesis. In conclusion, the present results suggest that there is a target selectivity in the regulation of neuronal quantal secretion and that the neurotrophic action of myocytes on the ACh content of quanta is localized to the neighbourhood of the neuromuscular junction.
Acknowledgments
This work was supported by a grant from the National Science Council (NSC 87-2314-B-002-304).
References
- Balice-Gordon RJ, Lichtman JW. In vivo observations of pre- and postsynaptic changes during the transition from multiple to single innervation at developing neuromuscular junctions. Journal of Neuroscience. 1993;13:834–855. doi: 10.1523/JNEUROSCI.13-02-00834.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Current Opinion in Neurobiology. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Campenot RB. Development of sympathetic neurons in compartmentalized cultures. I. Local control of neurite growth by nerve growth factor. Developmental Biology. 1982;93:1–12. doi: 10.1016/0012-1606(82)90232-9. [DOI] [PubMed] [Google Scholar]
- Campenot RB. Local promotion of neurite sprouting in cultured sympathetic neurons by nerve growth factor. Developmental Brain Research. 1987;37:293–301. doi: 10.1016/0165-3806(87)90250-1. [DOI] [PubMed] [Google Scholar]
- Campenot RB. NGF and the local control of nerve terminal growth. Journal of Neurobiology. 1994;25:599–611. doi: 10.1002/neu.480250603. [DOI] [PubMed] [Google Scholar]
- Caroni P. Activity-sensitive signaling by muscle-derived insulin-like growth factors in the developing and regenerating neuromuscular system. Annals of the New York Academy of Sciences. 1993;692:209–222. doi: 10.1111/j.1749-6632.1993.tb26219.x. [DOI] [PubMed] [Google Scholar]
- Connor EA, Smith MA. Retrograde signaling in the formation and maturation of the neuromuscular junction. Journal of Neurobiology. 1994;25:722–739. doi: 10.1002/neu.480250611. [DOI] [PubMed] [Google Scholar]
- Dai Z, Peng BP. Elevation in presynaptic Ca2+ level accompanying initial nerve-muscle contact in tissue culture. Neuron. 1993;10:827–837. doi: 10.1016/0896-6273(93)90199-2. 10.1016/0896-6273(93)90199-2. [DOI] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Retrograde interactions during formation and elimination of neuromuscular synapses. Current Opinion in Neurobiology. 1994;4:95–100. doi: 10.1016/0959-4388(94)90037-x. 10.1016/0959-4388(94)90037-X. [DOI] [PubMed] [Google Scholar]
- Eftimie R, Brenner HR, Buonanno A. Myogenin and MyoD join a family of skeletal muscle genes regulated by electrical activity. Proceedings of the National Academy of Sciences of the USA. 1991;88:1349–1353. doi: 10.1073/pnas.88.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers J, Laser M, Sun YA, Xie ZP, Poo MM. Studies of nerve-muscle interactions in Xenopus cell culture: analysis of early synaptic currents. Journal of Neuroscience. 1989;9:1523–1539. doi: 10.1523/JNEUROSCI.09-05-01523.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu WM. Regulatory role of ATP at developing neuromuscular junctions. Progress in Neurobiology. 1995;47:31–44. doi: 10.1016/0301-0082(95)00019-r. 10.1016/0301-0082(95)00019-R. [DOI] [PubMed] [Google Scholar]
- Goldman D, Brenner HR, Heinemann S. Acetylcholine receptor α-, β-, γ-, and δ-subunit mRNA levels are regulated by muscle activity. Neuron. 1988;1:329–333. doi: 10.1016/0896-6273(88)90081-5. 10.1016/0896-6273(88)90081-5. [DOI] [PubMed] [Google Scholar]
- Hall ZW, Sanes JR. Synaptic structure and development: the neuromuscular junction. Cell. 1993;72:99–121. doi: 10.1016/s0092-8674(05)80031-5. suppl. [DOI] [PubMed] [Google Scholar]
- Hamburger V. Cell death in the development of the lateral motor column of the chick embryo. Journal of Comparative Neurology. 1975;160:535–546. doi: 10.1002/cne.901600408. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Camu W, Mettling C, Gouin A, Poulsen K, Karihaloo M, Rullamas J, Evans T, McMahon SB, Armanini MP, Berkemeier L, Phillips HS, Rosenthal A. Neurotrophins promote motor neuron survival and are present in embryonic limb bud. Nature. 1993;363:266–270. doi: 10.1038/363266a0. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simmons L, Moffet B, Vandlen RA. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266:1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Kinoshita M, Iwasaki Y. Basic fibroblast growth factor has neuroprotective effects on axotomy-induced spinal motoneuron death and wobbler mouse motoneuron disease. Muscle and Nerve. 1996;19:794–795. [PubMed] [Google Scholar]
- Isackson PJ. Trophic factor response to neuronal stimuli or injury. Current Opinion in Neurobiology. 1995;5:350–357. doi: 10.1016/0959-4388(95)80048-4. [DOI] [PubMed] [Google Scholar]
- Jennings CG, Burden SJ. Development of the neuromuscular synapse. Current Opinion in Neurobiology. 1993;3:75–81. doi: 10.1016/0959-4388(93)90038-z. [DOI] [PubMed] [Google Scholar]
- Kidokoro Y, Saito M. Early cross-striation formation in twitching Xenopus myocytes in culture. Proceedings of the National Academy of Sciences of the USA. 1988;85:1978–1982. doi: 10.1073/pnas.85.6.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsching S. The neurotrophic factor concept: a reexamination. Journal of Neuroscience. 1993;13:2739–2748. doi: 10.1523/JNEUROSCI.13-07-02739.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou JC, Fu WM. Regulation of quantal secretion from developing motoneurons by postsynaptic activity-dependent release of NT-3. Journal of Neuroscience. 1997;17:2459–2468. doi: 10.1523/JNEUROSCI.17-07-02459.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J-C, Yang R-S, Fu W-M. Regulation of quantal secretion by neurotrophic factors at developing motoneurons in Xenopus cell cultures. The Journal of Physiology. 1997;503:129–139. doi: 10.1111/j.1469-7793.1997.129bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohof AM, Ip NY, Poo MM. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- McMahan UJ, Wallace BG. Molecules in basal lamina that direct the formation of synaptic specializations at neuromuscular junctions. Developmental Neuroscience. 1989;11:227–247. doi: 10.1159/000111903. [DOI] [PubMed] [Google Scholar]
- McManaman JL, Oppenheim RW, Prevette D, Marchette D. Rescue of motor neurons from cell death by a purified skeletal muscle polypeptide: Effects of the ChAT development factor, CDF. Neuron. 1990;4:891–898. doi: 10.1016/0896-6273(90)90142-3. [DOI] [PubMed] [Google Scholar]
- Mason RT, Peterfreund RA, Sawchenko PE, Corrigan AZ, Rivier JE, Vale WW. Release of the predicted calcitonin gene-related peptide from cultured rat trigeminal ganglion cells. Nature. 1984;308:653–655. doi: 10.1038/308653a0. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin) 2. Amsterdam: North-Holland; 1967. [Google Scholar]
- Purves D. Body and Brain. A Trophic Theory of Neuronal Connections. Cambridge, MA, USA: Harvard University Press; 1988. [DOI] [PubMed] [Google Scholar]
- Reist NE, Werle MJ, McMahan UJ. Agrin released by motor neurons induces the aggregation of acetylcholine receptors at neuromuscular junctions. Neuron. 1992;8:865–868. doi: 10.1016/0896-6273(92)90200-w. [DOI] [PubMed] [Google Scholar]
- Sendtner M, Stockli KA, Thoenen H. Synthesis and localization of ciliary neurotrophic factor in the sciatic nerve of the adult rat after lesion and during regeneration. Journal of Cellular Biology. 1992;118:139–148. doi: 10.1083/jcb.118.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto R, Kulik A, Roberts JD, Ohishi H, Nusser Z, Kaneko T, Somogyi P. Target-cell-specific concentration of a metabotropic glutamate receptor in the presynaptic active zone. Nature. 1996;381:523–525. doi: 10.1038/381523a0. [DOI] [PubMed] [Google Scholar]
- Stoop R, Poo MM. Synaptic modulation by neurotrophic factors: Differential and synergistic effects of brain-derived neurotrophic factor and ciliary neurotrophic factor. Journal of Neuroscience. 1996;16:3256–3264. doi: 10.1523/JNEUROSCI.16-10-03256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabti N, Poo MM. Culturing spinal cord neurons and muscle cells from Xenopus embryos. In: Banker G, Goslin K, editors. Culturing Nerve Cells. Cambridge, MA, USA: MIT Press; 1991. pp. 137–154. [Google Scholar]
- Takahashi T, Nakajima Y, Hirosawa K, Nakajima S, Onodera K. Structure and physiology of developing neuromuscular synapses in culture. Journal of Neuroscience. 1987;7:473–481. doi: 10.1523/JNEUROSCI.07-02-00473.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Xie K, Lu B. Neurotrophins promote maturation of developing neuromuscular synapses. Journal of Neuroscience. 1995;15:4796–4805. doi: 10.1523/JNEUROSCI.15-07-04796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XH, Berninger B, Poo MM. Localized synaptic actions of neurotrophin-4. Journal of Neuroscience. 1998;18:4985–4992. doi: 10.1523/JNEUROSCI.18-13-04985.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie ZP, Poo MM. Initial events in the formation of neuromuscular synapse: rapid induction of acetylcholine release from embryonic neuron. Proceedings of the National Academy of Sciences of the USA. 1986;83:7069–7073. doi: 10.1073/pnas.83.18.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafra F, Lindholm D, Castren E, Hartikka J, Thoenen H. Regulation of brain-derived neurotrophic factor and nerve growth factor mRNA in primary cultures of hippocampal neurons and astrocytes. Journal of Neuroscience. 1992;12:4793–4799. doi: 10.1523/JNEUROSCI.12-12-04793.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


