Abstract
Skeletal muscle is the major producer of lactic acid in the body, but its oxidative fibres also use lactic acid as a respiratory fuel. The stereoselective transport of L-lactic acid across the plasma membrane of muscle fibres has been shown to involve a proton-linked monocarboxylate transporter (MCT) similar to that described in erythrocytes and other cells. This transporter plays an important role in the pH regulation of skeletal muscle. A family of eight MCTs has now been cloned and sequenced, and the tissue distribution of each isoform varies. Skeletal muscle contains both MCT1 (the only isoform found in erythrocytes but also present in most other cells) and MCT4. The latter is found in all fibre types, although least in more oxidative red muscles such as soleus, whereas expression of MCT1 is highest in the more oxidative muscles and very low in white muscles that are almost entirely glycolytic. The properties of MCT1 and MCT2 have been described in some detail and the latter shown to have a higher affinity for substrates. MCT4 has been less well characterized but has a lower affinity for L-lactate (i.e. a higher Km of 20 mm) than does MCT1 (Km of 5 mm). MCT1 expression is increased in response to chronic stimulation and either endurance or explosive exercise training in rats and humans, whereas denervation decreases expression of both MCT1 and MCT4. The mechanism of regulation is not established, but does not appear to be accompanied by changes in mRNA concentrations. However, in other cells MCT1 and MCT4 are intimately associated with an ancillary protein OX-47 (also known as CD147). This protein is a member of the immunoglobulin superfamily with a single transmembrane helix, whose expression is known to be increased in a range of cells when their metabolic activity is increased.
The rapid transport of lactic acid across the plasma membrane is of fundamental importance for the metabolism of almost all cells; it is also vital for intracellular pH (pHi) homeostasis (see Poole & Halestrap, 1993). Glycolysis produces two molecules of lactic acid for every glucose molecule consumed and these must be transported out of the cell if high rates of glycolysis are to be maintained. In contrast, tissues such as heart and red skeletal muscle that use lactic acid as a major respiratory fuel require lactic acid to be transported into the cell. Transport of lactate, or more accurately lactic acid, across the plasma membrane of all cells is catalysed by proton-linked monocarboxylate transporters (MCTs). MCTs are also responsible for enabling the transport of pyruvate and the ketone bodies acetoacetate, β-hydroxybutyrate and acetate. As such they are critical for metabolic communication between cells. This article will review recent progress in our understanding of lactate transport in skeletal muscle with particular emphasis on the recent cloning and sequencing of several new members of the MCT family. For further information on the role and characterization of monocarboxylate transport in other tissues and the integration of lactate metabolism between tissues the reader is referred elsewhere (Poole & Halestrap, 1993; Halestrap et al. 1997).
Lactate metabolism and transport in skeletal muscle
Although high rates of glycolysis in skeletal muscle make it the main producer of lactic acid in the body, lactic acid can also be taken up by skeletal muscle and heart and used as a respiratory fuel. The balance of the two depends on the type of muscle fibre and the energy demand. The net production of lactic acid by muscle is particularly pronounced in the transition from rest to heavy exercise when there is a rapid increase in energy demand. This is not normally due to insufficient oxygenation (see Gladden, 1996) but to two other reasons. First, the acceleration of glycolysis at the onset of muscle activity is fast when compared with that of the oxidative pathway. Second, the maximal glycolytic capacity of muscle exceeds the maximal oxidative capacity. The extent to which the ATP requirements can be met by glycolysis or oxidative phosphorylation is fibre-type dependent with white muscle relying more on the former and red muscle on the latter. In either case, lactate is an important metabolic intermediate, which can exchange rapidly between different cells within a given muscle, between different muscles and between muscle and blood. These processes all require lactic acid transport across the sarcolemma (see Juel, 1997; Bonen et al. 1997).
Direct evidence for carrier-mediated transport in skeletal muscle
More than fifty years ago it was reported that lactate did not move freely from muscle to blood. However, evidence gathered in later experiments with in situ and isolated muscle preparations implied that, for the most part, translocation of lactate across the plasma membrane was mediated by a transport system which could be inhibited by specific inhibitors of the erythrocyte lactate transporter (see Juel, 1997). Accurate transport kinetics are difficult to determine in intact skeletal muscles because of their multicellular nature and because of the possible metabolism of added lactate; these problems can be overcome by the use of sarcolemmal membrane vesicles, both small and ‘giant’, derived from them. Such vesicles enabled the kinetics of both influx and efflux of lactate to be determined (reviewed by Juel, 1997; Bonen et al. 1997). These studies confirmed that lactate and other monocarboxylates cross skeletal muscle sarcolemma by means of a saturable, stereospecific transport system which shows an obligatory 1 : 1 coupling between lactate and H+. The concentration of L-lactate for half-maximal rates of transport (the Michaelis constant, Km) was found to be between 13 and 40 mM in most studies with rat and human sarcolemmal vesicles. Transport has been reported to be inhibited reversibly by α-cyano-4-hydroxycinnamate (CHC) and irreversibly by organo-mercurials such as p-chloromercuribenzene sulphonate (pCMBS). These characteristics of skeletal muscle lactic acid transport resemble those of many other cells (see Poole & Halestrap, 1993; Juel, 1997).
The monocarboxylate transporter family
Identification, cloning and sequencing of MCT1
Lactate transport has been studied most extensively in erythrocytes since large quantities of these cells can be obtained readily. Identification of the protein responsible for transport was achieved by specific covalent labelling with 4,4′-diisothiocyanatostilbene-2,2′-disulphonate (DIDS) followed by detergent solubilization and subsequent purification (Poole & Halestrap, 1992, 1994). N-terminal protein sequencing showed that erythrocyte MCT is identical to a membrane protein previously cloned and sequenced by Kim et al. (1992) and predicted to be a transporter. These authors stumbled across the transporter because a single point mutation in it greatly enhanced the uptake of mevalonate into Chinese hamster ovary (CHO) cells. Mevalonate is a precursor of cholesterol biosynthesis which they were investigating at the time. They subsequently expressed the wild-type protein in a breast carcinoma cell line and demonstrated that it catalysed inhibitor-sensitive monocarboxylate transport (Garcia et al. 1994a). The transporter was named MCT1, and subsequently MCT1 from human, rat and mouse were cloned (Garcia et al. 1994b; Jackson et al. 1995; Carpenter et al. 1996). They share about 95 % identity with the CHO MCT1. Human MCT1 has been mapped to chromosome band 1p13.2-p12 (Garcia et al. 1994b; Jackson et al. 1995; Carpenter et al. 1996).
Cloning and sequencing of other MCT isoforms
Extensive studies on the kinetics and substrate and inhibitor specificity of monocarboxylate transport into a range of tissues, and most especially the heart, suggested the existence of a family of MCTs (reviewed by Poole & Halestrap, 1993; Halestrap et al. 1997). Confirmation of this was first provided by the cloning, sequencing and functional expression of a second isoform of MCT (MCT2) with 60 % identity with MCT1 (Garcia et al. 1995). MCT2 has since been cloned and sequenced from rat, mouse and human, in which it has been mapped to chromosome band 12q13 (Jackson et al. 1997; Gerhart et al. 1998; Koehler-Stec et al. 1998; Lin et al. 1998). The next MCT to be cloned and sequenced was from a chicken retinal pigment epithelium cDNA library and was named MCT3 since it appeared to be different from both MCT1 and MCT2 with which it shares 43 and 45 % identity, respectively. The genomic structure of MCT3 has been elucidated and the protein expressed in a mammalian cell line and confirmed to transport lactate (Philp et al. 1995; Yoon et al. 1997; Yoon & Philp, 1998). However, detailed characterization of its properties has not been reported. Most recently, by searching the database of expression sequence tags (dbEST) for fragments of MCT-like sequences, four new members of the MCT family have been identified, cloned and sequenced. These exhibit 30–60 % identity with MCT1 and were initially named MCT3-MCT6, since one of the new isoforms appeared to be the mammalian equivalent of chicken MCT3 (Price et al. 1998). However, following identification of the true mouse MCT3 (Philp et al. 1998), they have since been renamed MCT4-MCT7 (Wilson et al. 1998). MCT4 has recently been expressed in Xenopus oocytes and confirmed to be a proton-linked monocarboxylate transporter (S. Bröer, personal communication). However, this has not yet been achieved for MCT5-MCT7. Another MCT-related sequence has been identified to be encoded within the human X-chromosome genomic sequence (Lafreniere et al. 1994). The translated protein sequence possesses a long N-terminal extension containing the PEST sequence motif indicative of rapid protein degradation. Thus it was originally named XPCT for X-linked PEST-containing transporter but has since been renamed MCT8 (Price et al. 1998; Wilson et al. 1998). A mouse homologue of this protein has recently been identified (Debrand et al. 1998).
The existence of a new transporter family with at least eight members in humans is now established. Homologues of MCTs have been identified in both Caenorhabditis elegans (4 isoforms) and Saccharomyces cerevisiae (4 isoforms) and a distant relative was found in the archebacterium Sulpholobus sulfataricus, suggesting that the MCT family has an early evolutionary origin (Price et al. 1998). Full sequence alignments of the different isoforms and their relatedness as judged by percentage identity and similarity are reported elsewhere (Price et al. 1998). Conservation of sequence between the isoforms is greatest for MCT1-MCT4 (> 50 %) but is significantly less (< 30 %) for the other isoforms, which may reflect a quite distinct substrate specificity.
Common features of the MCT family
Topology predictions suggest that all MCT family members have 12 transmembrane-spanning (TM) helices with intracellular C- and N-termini and a large intracellular loop between TM segments 6 and 7. This topology, which is illustrated in Fig. 1, has been confirmed for MCT1 by proteolytic cleavage and labelling studies (Poole et al. 1996; Poole & Halestrap, 1997). The sequence conservation between different family members is greatest in the TM segments and least in the intervening hydrophilic regions of the sequences (the N-terminal residues preceding TM1, the loop region between TM segments 6 and 7, and the C-terminal residues succeeding TM12). Such divergent hydrophilic sequences are a common feature of 12-TM-helix transporter family members (see Saier, 1994), and it is unlikely that these regions are directly involved in transport. Rather they may be critical for other aspects of function such as substrate specificity or regulation of transport activity. The C-terminal half of the molecule (TM helices 7–12) shows a slightly lower level of conservation than the N-terminal half (TM helices 1–6) and this is the same pattern as is observed in most transporter families (Saier, 1994). Indeed, it has been proposed that the two halves of the molecule (TM helices 1–6 and 7–12) have different functional roles. The N-terminal domains may be more important for energy coupling (e.g. via H+ or Na+ cotransport), membrane insertion, and/or correct structure maintenance, whereas the C-terminal domains may be more important for the determination of substrate specificity. Indeed, there is direct evidence for the latter since conversion of Phe360 to Cys in TM segment 10 of CHO MCT1 changes MCT1 from a lactate/pyruvate transporter to a mevalonate transporter (Kim et al. 1992; Garcia et al. 1994a). Furthermore, the binding site of MCT1 for DIDS, which binds at or near the substrate binding site, is in the C-terminal half of the transporter (Poole et al. 1996; Poole & Halestrap, 1997).
Figure 1. Proposed topology of MCT1.
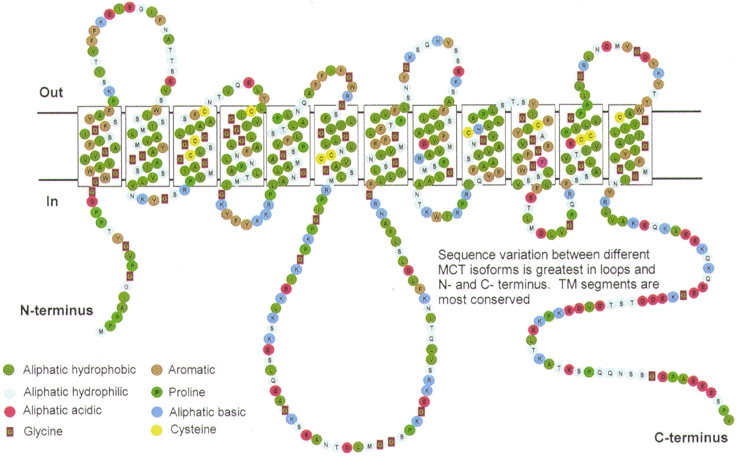
The model shown is that predicted from the primary sequence using hydropathy plots and subsequently confirmed by proteolytic cleavage and labelling experiments as described by Poole et al. (1996) and Poole & Halestrap (1997).
Tissue distribution and properties of the mammalian MCT isoforms
Expression of all the different MCT isoforms has been studied at the mRNA level by Northern blot analysis in a wide range of human tissues (Price et al. 1998; Lin et al. 1998). In addition, for MCT1 and MCT2, rat, mouse and hamster tissues have also been investigated (Kim et al. 1992; Jackson et al. 1997; Koehler-Stec et al. 1998; Pellerin et al. 1998), whereas for MCT3, chicken tissues have been studied (Philp et al. 1995). Expression of MCT1 and MCT2 at the protein level has been determined in many different rat and hamster tissues by both immunofluorescence microscopy and Western blotting (Garcia et al. 1994, 1995; Jackson et al. 1997; Gerhart et al. 1997, 1998; Wilson et al. 1998) whereas less extensive studies have been reported for MCT3 and MCT4 (Philp et al. 1998; Wilson et al. 1998). There are currently no published data on expression of MCT5-MCT8 at the protein level and these members of the MCT family will not be considered further.
Using these different techniques MCT1 was found to be present in almost all tissues, in many cases with specific locations within each tissue. In contrast, MCT2 is expressed in fewer tissues and is absent or barely detectable in skeletal muscle. It is found together with MCT1 in several tissues such as liver, kidney, testis and brain but its exact location within each tissue differs from that of MCT1 suggesting a distinct functional role. For example, in spermatozoa, MCT1 is found exclusively in the head region and MCT2 in the tail (Garcia et al. 1995). MCT2 is unusual in that there appears to be substantial species differences both in sequence and tissue distribution (Jackson et al. 1997). Furthermore, several sizes of mRNA transcript are detected in different cell types suggesting that alternatively spliced forms of MCT2 mRNA may exist (Jackson et al. 1997; Lin et al. 1998). MCT3 appears to be exclusively located in the basal membrane of the retinal pigment epithelium in both chicken and rat. Northern blot analysis has shown MCT4 (previously MCT3) to have a fairly broad tissue distribution and these studies together with Western blotting and confocal microscopy have shown that this isoform is expressed particularly strongly in skeletal muscle (Price et al. 1998; Wilson et al. 1998).
MCT1 and MCT4 are the major isoforms expressed in skeletal muscle
MCT1 expression in individual muscles correlates with their mitochondrial content; muscles such as soleus, which predominantly contain slow oxidative fibres, express large amounts of MCT1 whereas muscles with a high proportion of fast-twitch glycolytic fibres such as semimembranosus and semitendinosus contain almost none (McCullagh et al. 1996b). These data suggest that MCT1 expression in muscle fibres may reflect the extent to which transport of lactic acid into the cell is required for its oxidation as a respiratory fuel. The presence of high levels of MCT1 in heart muscle is consistent with this view (Halestrap et al. 1997). In contrast, MCT4 is present in all muscles, but at a lower concentration in predominantly oxidative muscle such as soleus (Fig. 2; and Wilson et al. 1998). These data suggest that this isoform is most important for lactic acid efflux from muscles that rely more on glycolytic metabolism. This is also reflected in the metabolic requirements of other cells that express large amounts of MCT4. For example, MCT4 is the only MCT expressed in human white blood cells, which are also highly glycolytic and net lactic acid exporters (Wilson et al. 1998). In human skeletal muscle a more detailed analysis of the distribution of MCT1 and MCT4 between different fibre types has been performed, using myosin heavy chain isoforms as fibre-type markers. There is a positive relationship between MCT1 density and occurrence of type I fibres, whereas MCT4 density was independent of fibre type and the inter-individual variation was large (Pilegaard et al. 1999b).
Figure 2. Distribution of MCT1 and MCT4 in different rat skeletal muscles.
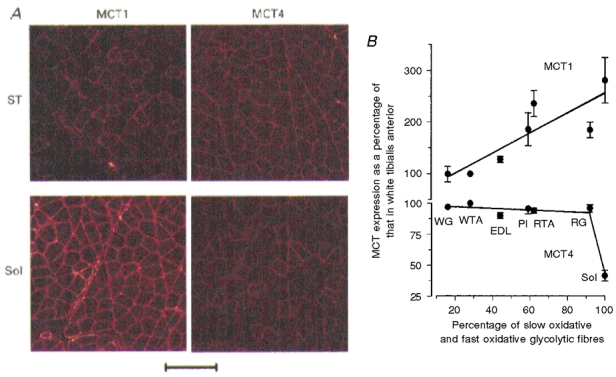
A, the presence of MCT1 and MCT4 in semitendinosus (ST) and soleus (Sol) muscles was revealed by immunofluorescence confocal microscopy. In ST muscle the smaller MCT1 staining cells were shown to be those with the greatest succinate dehydrogenase activity. Scale bar, 20 μm. B, quantification of the amount of MCT1 and MCT4 in homogenates from a range of muscles was performed using Western blotting. Muscles used were white gastrocnemius (WG), white tibialis anterior (WTA), extensor digitorum longus (EDL), plantaris (Pl), red tibialis anterior (RTA), red gastrocnemius (RG) and soleus (Sol). Data are adapted from Wilson et al. (1998).
Unique properties of the different MCT isoforms
MCT1
The kinetics of MCT1 have been thoroughly characterized in erythrocytes and tumour cells (Poole & Halestrap, 1993; Carpenter & Halestrap, 1994), and similar data have been obtained following expression of the protein in Xenopus oocytes (Bröer et al. 1998). The mechanism of transport involves an ordered sequential mechanism whereby a proton first binds to the transporter and then the lactate anion. This is followed by the translocation of lactate and proton across the membrane and their sequential release from the transporter on the other side of the membrane (see Fig. 3). The process is freely reversible with equilibrium being reached when:
Figure 3. Proposed kinetic scheme for MCT1.
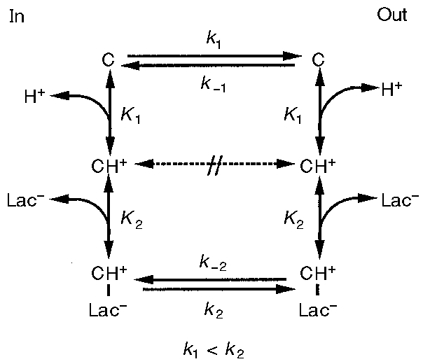
See text for details. Rate constants (k1, k-1, etc.) are given for the forward and backward translocation of the different species of carrier across the membrane. Equilibrium constants (K1 and K2) are given for proton and lactate binding, respectively.
although it is unlikely that this situation is ever reached in muscle. The rate-limiting step for net lactic acid flux appears to be the return of the free carrier across the membrane, which is required to complete the translocation cycle, and this is reflected in rates of monocarboxylate exchange being substantially faster than those of net transport. Between pH values of about 6 and 8, a lowering of pH on the same side as lactate is added stimulates transport primarily through decreasing the Km for lactate. Transport can also be stimulated by raising the pH on the opposite side of the membrane, which may act to increase the Vmax of transport by increasing the rate at which the unloaded carrier reorientates in the membrane.
The substrate and inhibitor specificity have also been extensively studied for MCT1 and are summarized in Table 1. A wide range of short chain monocarboxylates are transported with Km values decreasing as the chain length increases from 2 to 4. Monocarboxylates with longer branched aliphatic or aromatic side chains also bind to the transporter but are not readily released following translocation and act as inhibitors. One of the most potent of these is the classical inhibitor of lactate transport, CHC. Substitution of the side chain is tolerated with C2 being preferred over C3, and this allows the carrier to transport a range of naturally occurring monocarboxylates such as pyruvate, lactate, acetoacetate and β-hydroxybutyrate, whose Km values are given in Table 2. Indeed substitution on C2 may actually enhance binding, especially in the case of a carbonyl group, with pyruvate and 2-oxobutyrate having the lowest Km values of the natural substrates. For lactate, MCT1 is stereospecific, with the Km for the L-isomer (5–10 mM) being an order of magnitude lower than that for the D-isomer. However, this is not true for other monocarboxylates such as 2-chloropropionate or β-hydroxybutyrate.
Table 1.
Substrates and inhibitors of MCT1
| Substrates | Short chain fatty acids, e.g. acetate, propionate, butyrate Note: formate, aromatic and branched chain monocarboxylates are poor substrates |
| Monocarboxylates substituted with halogens, hydroxyl, cyano or azido groups in 2 or 3 position, e.g. monochloroacetate, dichloroacetate, l-lactate, l-2-chloropropionate, β-hydroxybutyrate and 3-chloro-propionate | |
| 2 or 3 oxo-acids, e.g. pyruvate, 2-oxobutyrate, acetoacetate Note: oxamate and glycollate are not substrates. Aromatic and branched chain monocarboxylates substituted in the 2 position are poor substrates but good inhibitors | |
| Inhibitors | Substituted aromatic monocarboxylates, e. g. α-cyano-4-hydroxycinnamate (CHC), phenyl-pyruvate, α-fluorocinnamate |
| Anion transport inhibitors, e.g. stilbene disulphonates (4,4′-diisothiocyanatostilbene-2,2′-disulphonate (DIDS), 4,4′-dibenzamidostilbene-2,2′-disulphonate (DBDS) etc.), niflumic acid, 5-nitro-2-(3-phenylpropylamino)-benzoate (NPPB) | |
| Lysine and arginine reagents, e.g. 2,4-dinitrofluorobenzene, pyridoxal phosphate and phenylglyoxal | |
| Sulfhydryl reagents, e.g. p-chloromercuribenzene sulphonate but not iodoacetate or N-ethylmaleimide | |
| Miscellaneous, e.g. phloretin, quercetin, isobutylcarbonyl-lactyl anhydride (IBCLA), 3-isobutyl-1-methylxanthine |
Information is taken from Poole & Halestrap (1993).
Table 2.
Comparison of Km and Ki values for various substrates and inhibitors of different MCT isoforms
| Tumour cell | MCT1 oocyte | MCT2 oocyte | Muscle vesicles | |
|---|---|---|---|---|
| Substrate Km(mm) | ||||
| l-Lactate | 4.5 | 3.5 | 0.5 | 13–40 |
| d, l-β-Hydroxybutyrate | 12.5 | 12.5 | 1.2 | High |
| Pyruvate | 0.72 | 1.0 | 0.08 | > 50 |
| α-Ketoisovalerate | – | 1.3 | 0.3 | > 50 |
| α-Ketoisocaproate | – | 0.7 | 0.1 | – |
| Acetoacetate | 5.5 | 5.5 | 0.8 | High |
| Inhibitor Ki(μm) | ||||
| CHC | 166 | 425 | 24 | 4000 |
| Phloretin | 5.1 | 28 | 14 | > 1500 |
| DIDS | 434 | – | – | > 1000 |
| p-CMBS (% inhibition at 0.1 mm) | 50 | 90 | 0 | 25 |
Data for tumour cells and muscle vesicles are taken from Carpenter & Halestrap (1994) and Juel (1997), respectively. Data for MCT1 expressed in Xenopus oocytes are from Bröer et al. (1998) and for MCT2 from Bröer et al. (1999).
Inhibitors of MCT1 fall into five broad categories that are summarized in Table 1. The most frequently used of these are CHC and the stilbene disulphonates such as DIDS, but none are specific for inhibition of MCT1 and their use in studies on the role of MCT needs to take this into account. Often overlooked is that CHC is two orders of magnitude more potent at inhibiting pyruvate transport into mitochondria than it is at inhibiting lactate transport across the plasma membrane (Halestrap, 1975). Thus, when used with cells in which some oxidation of glucose to CO2 and water is occurring, CHC causes a huge increase in lactic acid production and intracellular acidification independent of its effect on MCT (Halestrap & Denton, 1975).
Other MCT isoforms
In comparison with MCT1, the properties of MCT2 are poorly understood. Recently, Lin et al. (1998) expressed human MCT2 in Xenopus oocytes and showed it to have a very high affinity for pyruvate (Km, 25 μm). These observations have recently been confirmed and extended for rat MCT2 (Bröer et al. 1999). Measured Km and Ki values for most substrates and inhibitors are one-sixth to one-tenth of those of MCT1 with Km values for pyruvate, L-lactate, acetoacetate and D, L-β-hydroxybutyrate being 0.08, 0.5, 0.8 and 1.2 mM, respectively (Table 2). Unlike MCT1, MCT2 is insensitive to inhibition by pCMBS. No detailed analyses of the properties of either MCT3 or MCT4 have been described. In a monkey kidney epithelial cell line (COS) and bovine kidney epithelial cell line (NBL1), which both express large amounts of MCT4, transport kinetics are similar to those for MCT1 (Wilson et al. 1998). Furthermore, in frog sartorius muscle, which as a white muscle might be expected to predominantly express MCT4, microelectrode studies gave a Km for L-lactate of about 10 mM (Mason & Thomas, 1988). However, when expressed in Xenopus oocytes MCT4 gave a Km for L-lactate of 22 mM (S. Bröer, personal communication). This value is higher than that determined for MCT1, but is similar to Km values obtained for lactate transport into giant sarcolemmal vesicles from glycolytic muscle fibres (Juel, 1997). In such vesicles, the derived Ki values for CHC and stilbene disulphonates were also significantly higher than those for MCT1, consistent with MCT4 having a lower affinity for both substrates and inhibitors. However, another study using giant sarcolemmal vesicles derived from either red or white fibres has demonstrated that Km values for L-lactate are the same (15 mM), despite their different content of MCT1 and MCT4 (Juel & Pilegaard, 1998).
Regulation of MCT activity in muscle
Lactate transport activity increases in response to increased physical activity of muscle
Both endurance and high-intensity training have been demonstrated to increase the maximal rate of lactate transport into sarcolemmal vesicles by 30–100 % (McDermott & Bonen, 1993; Pilegaard et al. 1993); chronic electrical stimulation of the hindlimb of rats has a similar effect (McCullagh et al. 1996a). It is unclear whether changes in Km can occur under these conditions but the availability of specific antibodies against MCT1 has revealed that expression of this isoform, which is present predominantly in the red fibres, is increased (McCullagh et al. 1996a, b; Baker et al. 1998). No changes in MCT4 expression were observed after chronic stimulation of the hindlimb, despite a 2- to 3-fold increase in MCT1 expression (Wilson et al. 1998). However, in other studies, also using sarcolemmal vesicles, it was found that the majority of the increase in lactate transport capacity in response to training took place in the white fibres (Juel & Pilegaard, 1998). This would imply that an increase in MCT4 expression or activity does occur. Irrespective of which MCT isoforms are involved, it seems clear that an increase in physical activity can improve lactate-H+ transport capacity of skeletal muscle. This conclusion is reinforced by the finding that extremely low muscle activity, as in spinal cord injured patients (Pilegaard et al. 1998) or in denervated rat muscle (Pilegaard & Juel, 1995; McCullagh & Bonen, 1995), is accompanied by a reduction in lactate transport capacity. In the latter case this was shown to be associated with a significant decrease in the expression of both MCT1 and MCT4 (Wilson et al. 1998).
The large (up to 100 %) training-induced increase in lactate-H+ cotransport found in rat muscle must be evaluated against the extremely sedentary (untrained) state of control rats. Thus it is important to establish whether the lactate transport capacity in normal active human subjects can also be improved by training. Numerous studies have demonstrated that training can change the kinetics of lactate turnover in the human body, but a large fraction of such changes can be ascribed to metabolic changes and not to increased membrane transport. Nevertheless, an increase in MCT expression at the sarcolemma would seem an appropriate response to meet the increased release of H+ and lactate from intensely active human muscle. In a cross-sectional study of human subjects of widely differing states of fitness, it was shown that vesicles prepared from their muscle have widely ranging differences in lactate transport capacity; some extremely well-trained subjects seem to have a very high capacity (Pilegaard et al. 1994). Does this mean that training per se can increase the membrane transport capacity in humans? If so, does this increased transport have any functional significance? Two studies have addressed this question. In one case it was reported that 7 days of intense bicycle training increased the MCT1 concentration of vastus lateralis muscle by 18 % and the femoral venous lactate concentration during activity increased in proportion to the rise in exercise-induced muscle lactate (Bonen et al. 1998). In the second study with high intensity knee-extensor exercise it was found that the content of the two isoforms MCT1 and MCT4 was increased by 76 and 32 %, respectively. These increases in MCT expression were associated with a 12 % increase in sarcolemmal lactate transport measured in vesicles formed from needle biopsies. Furthermore, in the trained subjects the release of lactate and H+ was higher at a given cellular to interstitial concentration gradient than that before training (Pilegaard et al. 1999a). It can therefore be concluded that the lactate-H+ transport capacity in man may be improved by training, and that the increased density of membrane transporter proteins is of functional importance for the translocation of H+ and lactate from muscle to blood.
In contrast to these long-term regulations associated with training, there is currently no evidence for any medium or short-term hormonal regulation of the lactate-H+ cotransport.
Mechanisms involved in the regulation of lactate transport in skeletal muscle
Quantitative Northern blot analysis did not detect a significant increase in MCT1 mRNA in rat hindlimb following chronic stimulation, despite MCT1 protein expression increasing severalfold. This suggests that regulation of expression may be translational (i.e. an increase in the rate at which ribosomes translate mRNA into protein), a phenomenon whose importance is becoming increasingly recognized (Sachs et al. 1997; Proud & Denton, 1997). Such regulation usually involves specific sequences and secondary structure in the 5′ untranslated region with which initiation factors and regulatory factors interact to enhance or repress translation. However, it is now clear that the 3′ untranslated region may also play a role (Miyamoto et al. 1996). This may either be by looping back and interacting with the 5′ untranslated region or by binding to regulatory factors/binding proteins which make the mRNA unavailable for translation. In this context it may be significant that the 3′ untranslated region of MCT1 is very long (some 1.2 kb longer than that of either MCT2 or MCT4) (Jackson et al. 1995, 1997).
An additional means by which MCT activity might be regulated is through its interaction with an ancillary protein. MCT1 is not itself glycosylated (Carpenter et al. 1996), but in erythrocytes it is closely associated with a 70 kDa membrane-spanning glycoprotein of the immunoglobulin superfamily known as embigin or GP70, to which it can be specifically cross-linked with DIDS (Poole & Halestrap, 1997). Embigin is mainly expressed in embryonic tissues (Ozawa et al. 1988) but antibodies directed against a closely related protein, OX-47, coimmunoprecipitate both MCT1 and MCT4, suggesting that these proteins also associate specifically in the membrane (Kirk et al. 1998). OX-47 is also known as CD147, basigin, CE9, neurothelin, M6 or EMMPRIN in other species (Fossum et al. 1991; Seulberger et al. 1992; Schuster et al. 1996) and is found in most adult tissues although with considerable variation in glycosylation state. Expression of OX-47 is upregulated by stimuli that enhance the metabolic activity of cells and lead to stimulation of glycolysis and increased expression of glucose transporters (Nehme et al. 1995). Thus an attractive possibility is that OX-47 may be involved in a parallel stimulation or modulation of monocarboxylate transport under such conditions. This effect could be exerted either through a direct effect on the catalytic activity of the transporter or through regulating its translocation to the membrane. There are precedents for these suggestions. An ancillary protein is involved in the stimulation of neutral amino acid transport into cultured cells by system A in response to hypertonic shock (Ruiz-Montasell et al. 1994), whereas glycophorin associates with the anion exchanger, AE1, and acts as a chaperon to increase its translocation from the endoplasmic reticulum to the Golgi and plasma membrane (Groves & Tanner, 1992, 1994).
Role of lactate-H+ transport in pH regulation
In general, cellular pH homeostasis in muscle is a balance between H+ accumulation (H+ influx and metabolic production of acid) and H+ removal mediated by transporters located in the sarcolemma (Juel, 1998). Whether lactate crosses the sarcolemmal membrane via the lactate-H+ cotransporter or via diffusion of undissociated lactic acid, its movement is always coupled to the movement of H+ in a 1 : 1 ratio. Since it is lactic acid that is both the product of glycolysis and the substrate for respiration, this enables transport and metabolism to be matched without an imbalance of protons. It would therefore be expected that lactate transport is of importance for muscle pH regulation. This was confirmed in rat model systems where the lactate-H+ cotransporter possesses a higher capacity for mediating H+ efflux than either the Na+-H+ exchanger or the bicarbonate-dependent transport systems (Juel, 1995). In human subjects, it has been possible to evaluate the importance of the cotransporter for pH regulation during exercise by comparing the lactate release with total H+ release from muscle to blood during intense knee-extensor exercise. These data confirm the prominent role of the lactate-H+ cotransporter in mediating H+ removal from muscle during intense exercise and in the first part of subsequent recovery. Lactate release is approximately two-thirds of the total H+ release under these conditions (Juel et al. 1990) but is less during submaximal exercise (Bangsbo et al. 1997).
Although the lactate-H+ cotransporter can function as a pH-regulating system, it must be noted that its activity is mainly driven by the lactate gradient and is only slightly sensitive to internal pH. In this respect it differs from the classical pH-regulatory mechanisms such as Na+-H+ exchange, which are strongly activated if internal pH is reduced. The latter systems are better suited for fine adjustments of pH and for pH regulation at rest, where the lactate gradient is small or absent. In contrast, during intense exercise, where lactate production is large, the lactate-H+ cotransporter will be responsible for most of the H+ removal from the cell. It might be argued that it would be advantageous for the muscle to have very high lactate-H+ transport activity so as to allow rapid loss of glycolytic lactic acid without appreciable accumulation of lactate and H+ and a consequent decrease in pHi. Yet this seems not to be the case. The lactate-H+ cotransporter only increases the capacity for transmembrane fluxes of lactate and H+ by 3- to 4-fold, and, as a consequence, both lactate and H+ do accumulate during exercise. Not only is MCT expression in muscles that are primarily glycolytic low relative to other more oxidative tissues such as heart and ‘red’ muscle, but also the lactate Km for the predominant MCT isoform (MCT4) is relatively high (10–20 mM). It seems probable that by adapting to restrict the lactate-H+ transport capacity of skeletal muscle, the body is protected from acidosis during intense exercise. This is because intracellular accumulation of lactic acid and drop in muscle pHi will inhibit glycolysis and induce fatigue before the lactic acid lost to the blood overwhelms the pH regulatory mechanisms of the body. Such an explanation may also explain why the greatest expression of MCT activity is found in oxidative muscle cells, which produce less lactic acid (McCullagh et al. 1996b; Wilson et al. 1998). MCT1 is the predominant MCT isoform in these cells and has a lower Km for lactate than does MCT4. At physiological blood lactate concentrations this makes MCT1 better suited for the rapid uptake of lactic acid for oxidation as a respiratory fuel.
Acknowledgments
This work was supported by grants to A. P. H. by The Wellcome Trust, British Heart Foundation and Medical Research Council and to C. J. by The Danish National Research Foundation.
References
- Baker SK, McCullagh KJA, Bonen A. Training intensity-dependent and tissue-specific increases in lactate uptake and MCT-1 in heart and muscle. Journal of Applied Physiology. 1998;84:987–994. doi: 10.1152/jappl.1998.84.3.987. 10.1063/1.368165. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Juel C, Hellsten Y, Saltin B. Dissociation between lactate and proton exchange in muscle during intense exercise in man. The Journal of Physiology. 1997;504:489–499. doi: 10.1111/j.1469-7793.1997.489be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonen A, Baker SK, Hatta H. Lactate transport and lactate transporters in skeletal muscle. Canadian Journal of Applied Physiology. 1997;22:531–552. doi: 10.1139/h97-034. [DOI] [PubMed] [Google Scholar]
- Bonen A, McCullagh KJA, Putman CT, Hultman E, Jones NL, Heigenhauser GJF. Short-term training increases human muscle MCT1 and femoral venous lactate in relation to muscle lactate. American Journal of Physiology. 1998;274:E102–107. doi: 10.1152/ajpendo.1998.274.1.E102. [DOI] [PubMed] [Google Scholar]
- Bröer S, Bröer A, Schneider H-P, Stegen C, Halestrap AP, Deitmer JW. Characterisation of the high-affinity monocarboxylate transporter MCT2 in Xenopus laevis oocytes. Biochemical Journal. 1999. (in the Press) [DOI] [PMC free article] [PubMed]
- Bröer S, Schneider HP, Bröer A, Rahman B, Hamprecht B, Deitmer JW. Characterization of the monocarboxylate transporter 1 expressed in Xenopus laevis oocytes by changes in cytosolic pH. Biochemical Journal. 1998;333:167–174. doi: 10.1042/bj3330167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter L, Halestrap AP. The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells studied with the intracellular pH indicator BCECF. Biochemical Journal. 1994;304:751–760. doi: 10.1042/bj3040751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter L, Poole RC, Halestrap AP. Cloning and sequencing of the monocarboxylate transporter from mouse Ehrlich Lettre tumour cell confirms its identity as MCT1 and demonstrates that glycosylation is not required for MCT1 function. Biochimica et Biophysica Acta. 1996;1279:157–163. doi: 10.1016/0005-2736(95)00254-5. [DOI] [PubMed] [Google Scholar]
- Debrand E, Heard E, Avner P. Cloning and localization of the murine Xpct gene: evidence for complex rearrangements during the evolution of the region around the Xist gene. Genomics. 1998;48:296–303. doi: 10.1006/geno.1997.5173. 10.1006/geno.1997.5173. [DOI] [PubMed] [Google Scholar]
- Fossum S, Mallett S, Barclay AN. The MRC OX-47 antigen is a member of the immunoglobulin superfamily with an unusual transmembrane sequence. European Journal of Immunology. 1991;21:671–679. doi: 10.1002/eji.1830210320. [DOI] [PubMed] [Google Scholar]
- Garcia CK, Brown MS, Pathak RK, Goldstein JL. cDNA cloning of MCT2, a second monocarboxylate transporter expressed in different cells than MCT1. Journal of Biological Chemistry. 1995;270:1843–1849. doi: 10.1074/jbc.270.4.1843. [DOI] [PubMed] [Google Scholar]
- Garcia CK, Goldstein JL, Pathak RK, Anderson RGW, Brown MS. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell. 1994a;76:865–873. doi: 10.1016/0092-8674(94)90361-1. [DOI] [PubMed] [Google Scholar]
- Garcia CK, Li X, Luna J, Francke U. cDNA cloning of the human monocarboxylate transporter 1 and chromosomal localization of the SLC16A1 locus to 1p13.2-p12. Genomics. 1994b;23:500–503. doi: 10.1006/geno.1994.1532. 10.1006/geno.1994.1532. [DOI] [PubMed] [Google Scholar]
- Gerhart DZ, Enerson BE, Zhdankina OY, Leino RL, Drewes LR. Expression of monocarboxylate transporter MCT1 by brain endothelium and glia in adult and suckling rats. American Journal of Physiology. 1997;273:E207–213. doi: 10.1152/ajpendo.1997.273.1.E207. [DOI] [PubMed] [Google Scholar]
- Gerhart DZ, Enerson BE, Zhdankina OY, Leino RL, Drewes LR. Expression of the monocarboxylate transporter MCT2 by rat brain glia. Glia. 1998;22:272–281. 10.1002/(SICI)1098-1136(199803)22:3<272::AID-GLIA6>3.3.CO;2-T. [PubMed] [Google Scholar]
- Gladden LB. Lactate transport and exchange during exercise. In: Rowell L, Shepherd J, editors. Handbook of Physiology, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 614–648. section 12, chap. 14. [Google Scholar]
- Groves JD, Tanner MJA. Glycophorin-A facilitates the expression of human band-3-mediated anion transport in Xenopus oocytes. Journal of Biological Chemistry. 1992;267:22163–22170. [PubMed] [Google Scholar]
- Groves JD, Tanner MJA. The effects of glycophorin A on the expression of the human red cell anion transporter (band 3) in Xenopus oocytes. Journal of Membrane Biology. 1994;140:81–88. doi: 10.1007/BF00234488. [DOI] [PubMed] [Google Scholar]
- Halestrap AP. The mitochondrial pyruvate carrier: Kinetics and specificity for substrates and inhibitors. Biochemical Journal. 1975;148:85–96. doi: 10.1042/bj1480085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP, Denton RM. The specificity and metabolic implications of the inhibition of pyruvate transport in isolated mitochondria and intact preparations by α-cyano-4-hydroxycinnamate and related compounds. Biochemical Journal. 1975;148:97–106. doi: 10.1042/bj1480097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP, Wang XM, Poole RC, Jackson VN, Price NT. Lactate transport in heart in relation to myocardial ischemia. American Journal of Cardiology. 1997;80:A17–25. doi: 10.1016/s0002-9149(97)00454-2. 10.1016/S0002-9149(97)00454-2. [DOI] [PubMed] [Google Scholar]
- Jackson VN, Price NT, Carpenter L, Halestrap AP. Cloning of the monocarboxylate transporter isoform MCT2 from rat testis provides evidence that expression in tissues is species-specific and may involve post-transcriptional regulation. Biochemical Journal. 1997;324:447–453. doi: 10.1042/bj3240447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson VN, Price NT, Halestrap AP. cDNA cloning of MCT1, a monocarboxylate transporter from rat skeletal muscle. Biochimica et Biophysica Acta. 1995;1238:193–196. doi: 10.1016/0005-2736(95)00160-5. [DOI] [PubMed] [Google Scholar]
- Juel C. Regulation of cellular pH in skeletal muscle fiber types, studied with sarcolemmal giant vesicles obtained from rat muscles. Biochimica et Biophysica Acta. 1995;1265:127–132. doi: 10.1016/0167-4889(94)00209-w. 10.1016/0167-4889(94)00209-W. [DOI] [PubMed] [Google Scholar]
- Juel C. Lactate-proton co-transport in skeletal muscle. Physiological Reviews. 1997;77:321–358. doi: 10.1152/physrev.1997.77.2.321. [DOI] [PubMed] [Google Scholar]
- Juel C. Muscle pH regulation: role of training. Acta Physiologica Scandinavica. 1998;162:359–366. doi: 10.1046/j.1365-201X.1998.0305f.x. 10.1046/j.1365-201X.1998.0305f.x. [DOI] [PubMed] [Google Scholar]
- Juel C, Bangsbo J, Graham T, Saltin B. Lactate and potassium fluxes from skeletal muscle during intense dynamic knee-extensor exercise in man. Acta Physiologica Scandinavica. 1990;140:147–159. doi: 10.1111/j.1748-1716.1990.tb08986.x. [DOI] [PubMed] [Google Scholar]
- Juel C, Pilegaard H. Lactate/H+ transport kinetics in rat skeletal muscle related to fibre type and changes in transport capacity. Pflügers Archiv. 1998;436:560–564. doi: 10.1007/s004240050672. [DOI] [PubMed] [Google Scholar]
- Kim CM, Goldstein JL, Brown MS. cDNA cloning of MEV, a mutant protein that facilitates cellular uptake of mevalonate, and identification of a point mutation responsible for its gain in function. Journal of Biological Chemistry. 1992;267:23113–23121. [PubMed] [Google Scholar]
- Kirk P, Wilson MC, Halestrap AP, Barclay AN, Brown MH. The immunoglobulin superfamily member CD147 interacts with a lactate transporter. Immunology. 1998;95(suppl. 1):9.8. [Google Scholar]
- Koehler-Stec EM, Simpson IA, Vannucci SJ, Landschulz KT, Landschulz WH. Monocarboxylate transporter expression in mouse brain. American Journal of Physiology. 1998;275:E516–524. doi: 10.1152/ajpendo.1998.275.3.E516. [DOI] [PubMed] [Google Scholar]
- Lafreniere RG, Carrel L, Willard HF. A novel transmembrane transporter encoded by the XPCT gene in Xq13.2. Human Molecular Genetics. 1994;3:1133–1139. doi: 10.1093/hmg/3.7.1133. [DOI] [PubMed] [Google Scholar]
- Lin RY, Vera JC, Chaganti RSK, Golde DW. Human monocarboxylate transporter 2 (MCT2) is a high affinity pyruvate transporter. Journal of Biological Chemistry. 1998;273:28959–28965. doi: 10.1074/jbc.273.44.28959. [DOI] [PubMed] [Google Scholar]
- McCullagh KJA, Bonen A. Reduced lactate transport in denervated rat skeletal muscle. American Journal of Physiology. 1995;268:R884–888. doi: 10.1152/ajpregu.1995.268.4.R884. [DOI] [PubMed] [Google Scholar]
- McCullagh KJA, Juel C, O'Brien M, Bonen A. Chronic muscle stimulation increases lactate transport in rat skeletal muscle. Molecular and Cellular Biochemistry. 1996a;156:51–57. doi: 10.1007/BF00239319. [DOI] [PubMed] [Google Scholar]
- McCullagh KJA, Poole RC, Halestrap AP, O'Brien M, Bonen A. Role of the lactate transporter (MCT1) in skeletal muscles. American Journal of Physiology. 1996b;271:E143–150. doi: 10.1152/ajpendo.1996.271.1.E143. [DOI] [PubMed] [Google Scholar]
- McDermott JC, Bonen A. Endurance training increases skeletal muscle lactate transport. Acta Physiologica Scandinavica. 1993;147:323–327. doi: 10.1111/j.1748-1716.1993.tb09505.x. [DOI] [PubMed] [Google Scholar]
- Mason MJ, Thomas RC. A microelectrode study of the mechanisms of L-lactate entry into and release from frog sartorius muscle. The Journal of Physiology. 1988;400:459–479. doi: 10.1113/jphysiol.1988.sp017132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, Chiorini JA, Urcelay E, Safer B. Regulation of gene expression for translation initiation factor elF-2 alpha: Importance of the 3′ untranslated region. Biochemical Journal. 1996;315:791–798. doi: 10.1042/bj3150791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehme CL, Fayos BE, Bartles JR. Distribution of the integral plasma membrane glycoprotein CE9 (MRC OX-47) among rat tissues and its induction by diverse stimuli of metabolic activation. Biochemical Journal. 1995;310:693–698. doi: 10.1042/bj3100693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M, Huang RP, Furukawa T, Muramatsu T. A teratocarcinoma glycoprotein carrying a developmentally regulated carbohydrate marker is a member of the immunoglobulin gene superfamily. Journal of Biological Chemistry. 1988;263:3059–3062. [PubMed] [Google Scholar]
- Pellerin L, Pellegri G, Martin JL, Magistretti PJ. Expression of monocarboxylate transporter mRNAs in mouse brain: Support for a distinct role of lactate as an energy substrate for the neonatal vs. adult brain. Proceedings of the National Academy of Sciences of the USA. 1998;95:3990–3995. doi: 10.1073/pnas.95.7.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philp N, Chu P, Pan T-C, Zhang RZ, Chu M-L, Stark K, Boettiger D, Yoon H, Kieber-Emmons T. Developmental expression and molecular cloning of REMP, a novel retinal epithelial membrane protein. Experimental Cell Research. 1995;219:64–73. doi: 10.1006/excr.1995.1205. [DOI] [PubMed] [Google Scholar]
- Philp NJ, Yoon H, Grollman EF. Monocarboxylate transporter MCT1 is located in the apical membrane and MCT3 in the basal membrane of rat RPE. American Journal of Physiology. 1998;274:R1824–1828. doi: 10.1152/ajpregu.1998.274.6.R1824. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Bangsbo J, Richter EA, Juel C. Lactate transport studied in sarcolemmal giant vesicles from human muscle biopsies: Relation to training status. Journal of Applied Physiology. 1994;77:1858–1862. doi: 10.1152/jappl.1994.77.4.1858. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Domino K, Noland T, Juel C, Hellsten Y, Halestrap AP, Bangsbo J. Effect of high-intensity exercise training on lactate/H+ transport capacity in human skeletal muscle. American Journal of Physiology. 1999a;276:E255–261. doi: 10.1152/ajpendo.1999.276.2.E255. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Juel C. Lactate transport studied in sarcolemmal giant vesicles from rat skeletal muscles: Effect of denervation. American Journal of Physiology. 1995;269:E679–682. doi: 10.1152/ajpendo.1995.269.4.E679. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Juel C, Wibrand F. Lactate transport studied in sarcolemmal giant vesicles from rats: effect of training. American Journal of Physiology. 1993;264:E156–160. doi: 10.1152/ajpendo.1993.264.2.E156. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Mohr T, Kjær M, Juel C. Lactate/H+ transport in skeletal muscle from spinal cord injured patients. Scandinavian Journal of Medicine and Science in Sports. 1998;8:98–101. doi: 10.1111/j.1600-0838.1998.tb00175.x. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Terzis G, Halestrap A, Juel C. Distribution of the lactate/H+ transporter isoforms MCT1 and MCT4 in human skeletal muscle. American Journal of Physiology. 1999b. (in the Press) [DOI] [PubMed]
- Poole RC, Halestrap AP. Identification and partial purification of the erythrocyte lactate transporter. Biochemical Journal. 1992;283:855–862. doi: 10.1042/bj2830855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole RC, Halestrap AP. Transport of lactate and other monocarboxylates across mammalian plasma membranes. American Journal of Physiology. 1993;264:C761–782. doi: 10.1152/ajpcell.1993.264.4.C761. [DOI] [PubMed] [Google Scholar]
- Poole RC, Halestrap AP. N-Terminal protein sequence analysis of the rabbit erythrocyte lactate transporter suggests identity with the cloned monocarboxylate transport protein MCT1. Biochemical Journal. 1994;303:755–759. doi: 10.1042/bj3030755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole RC, Halestrap AP. Interaction of the erythrocyte lactate transporter (monocarboxylate transporter 1) with an integral 70-kDa membrane glycoprotein of the immunoglobulin superfamily. Journal of Biological Chemistry. 1997;272:14624–14628. doi: 10.1074/jbc.272.23.14624. [DOI] [PubMed] [Google Scholar]
- Poole RC, Sansom CE, Halestrap AP. Studies of the membrane topology of the rat erythrocyte H+/lactate cotransporter (MCT1) Biochemical Journal. 1996;320:817–824. doi: 10.1042/bj3200817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price NT, Jackson VN, Halestrap AP. Cloning and sequencing of four new mammalian monocarboxylate transporter (MCT) homologues confirms the existence of a transporter family with an ancient past. Biochemical Journal. 1998;329:321–328. doi: 10.1042/bj3290321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proud CG, Denton RM. Molecular mechanisms for the control of translation by insulin. Biochemical Journal. 1997;328:329–341. doi: 10.1042/bj3280329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Montasell B, Gomez-Angelats M, Casado FJ, Felipe A, McGivan JD, Pastor-Anglada M. Evidence for a regulatory protein involved in the increased activity of system A for neutral amino acid transport in osmotically stressed mammalian cells. Proceedings of the National Academy of Sciences of the USA. 1994;91:9569–9573. doi: 10.1073/pnas.91.20.9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs AB, Sarnow P, Hentze MW. Starting at the beginning, middle and end: translation initiation in eukaryotes. Cell. 1997;89:831–838. doi: 10.1016/s0092-8674(00)80268-8. [DOI] [PubMed] [Google Scholar]
- Saier MH. Computer-aided analyses of transport protein sequences - gleaning evidence concerning function, structure, biogenesis, and evolution. Microbiological Reviews. 1994;58:71–93. doi: 10.1128/mr.58.1.71-93.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster VL, Lu R, Kanai N, Bao Y, Rosenberg S, Prie D, Ronco P, Jennings ML. Cloning of the rabbit homologue of mouse ‘basigin’ and rat ‘OX-47’: kidney cell type-specific expression, and regulation in collecting duct cells. Biochimica et Biophysica Acta. 1996;1311:13–19. doi: 10.1016/0167-4889(95)00186-7. [DOI] [PubMed] [Google Scholar]
- Seulberger H, Unger CM, Risau W. HT7, Neurothelin, Basigin, gp42 and OX-47 - many names for one developmentally regulated immuno-globulin-like surface glycoprotein on blood-brain barrier endothelium, epithelial tissue barriers and neurons. Neuroscience Letters. 1992;140:93–97. doi: 10.1016/0304-3940(92)90690-9. [DOI] [PubMed] [Google Scholar]
- Wilson MC, Jackson VN, Heddle C, Price NT, Pilegaard H, Juel C, Bonen A, Montgomery I, Hutter OF, Halestrap AP. Lactic acid efflux from white skeletal muscle is catalyzed by the monocarboxylate transporter isoform MCT3. Journal of Biological Chemistry. 1998;273:15920–15926. doi: 10.1074/jbc.273.26.15920. [DOI] [PubMed] [Google Scholar]
- Yoon H, Fanelli A, Grollman EF, Philp NJ. Identification of a unique monocarboxylate transporter (MCT3) in retinal pigment epithelium. Biochemical and Biophysical Research Communications. 1997;234:90–94. doi: 10.1006/bbrc.1997.6588. [DOI] [PubMed] [Google Scholar]
- Yoon H, Philp NJ. Genomic structure and developmental expression of the chicken monocarboxylate transporter MCT3. Experimental Eye Research. 1998;67:417–424. doi: 10.1006/exer.1998.0533. [DOI] [PubMed] [Google Scholar]


