Abstract
Proteases regulate cells by cleaving proteinase-activated receptors (PARs). Thrombin and trypsin cleave PAR-1 and PAR-2 on neurons and astrocytes of the brain to regulate morphology, growth and survival. We hypothesized that thrombin and mast cell tryptase, which are generated and released during trauma and inflammation, regulate enteric neurons by cleaving PAR-1 and PAR-2.
We detected immunoreactive PAR-1 and PAR-2 in > 60 % of neurons from the myenteric plexus of guinea-pig small intestine in primary culture. A large proportion of neurons that expressed substance P, vasoactive intestinal peptide or nitric oxide synthase also expressed PAR-1 and PAR-2. We confirmed expression of PAR-1 and PAR-2 in the myenteric plexus by RT-PCR using primers based on sequences of cloned guinea-pig receptors.
Thrombin, trypsin, tryptase, a filtrate from degranulated mast cells, and peptides corresponding to the tethered ligand domains of PAR-1 and PAR-2 increased [Ca2+]i in > 50 % of cultured myenteric neurons. Approximately 60 % of neurons that responded to PAR-1 agonists responded to PAR-2 agonists, and > 90 % of PAR-1 and PAR-2 responsive neurons responded to ATP.
These results indicate that a large proportion of myenteric neurons that express excitatory and inhibitory neurotransmitters and purinoceptors also express PAR-1 and PAR-2. Thrombin and tryptase may excite myenteric neurons during trauma and inflammation when prothrombin is activated and mast cells degranulate. This novel action of serine proteases probably contributes to abnormal neurotransmission and motility in the inflamed intestine.
Certain proteases specifically regulate cells by cleaving members of a growing family of proteinase-activated receptors (PARs) which couple to heterotrimeric G-proteins (reviewed by Déry et al. 1998). Thrombin cleaves PAR-1, and trypsin and mast cell tryptase cleave PAR-2, exposing tethered ligand domains that bind to and activate the cleaved receptors (Vu et al. 1991; Nystedt et al. 1994; Corvera et al. 1997; Molino et al. 1997). Proteases and synthetic peptides corresponding to the tethered ligands trigger PAR-1 and PAR-2 in multiple cell types to activate signalling cascades that are directed towards inflammation and repair (Déry et al. 1998). Thrombin, which is generated after injury, causes vasodilatation and plasma extravasation, induces neutrophil adhesion and infiltration, and stimulates proliferation of fibroblasts, endothelial cells and smooth muscle cells (Déry et al. 1998). Tryptase, which is released from degranulated mast cells, is mitogenic for fibroblasts, smooth muscle cells and epithelial cells, and stimulates intercellular adhesion molecule-1 (ICAM-1) expression by epithelial cells (Brown et al. 1991; Ruoss et al. 1991; Cairns & Walls, 1995). PAR-2 may mediate these effects since PAR-2 agonists stimulate proliferation of endothelial and smooth muscle cells (Mirza et al. 1996; Bono et al. 1997), and the mitogenic effects of tryptase depend on PAR-2 cleavage (Mirza et al. 1997).
Proteases regulate neurons and glia in the central nervous system by cleaving PARs. Prothrombin and PAR-1 are widely expressed by neurons and glia in the brain, and in certain regions neuronal thrombin may cleave PAR-1 (Weinstein et al. 1995). Circulating thrombin may also regulate neurons and glia expressing PAR-1 when the blood-brain barrier is disrupted by trauma. PAR-1 agonists act on neurons and astrocytes to regulate morphology (Suidan et al. 1992; Beecher et al. 1994), proliferation (Perraud et al. 1987), release of growth factors (Ehrenreich et al. 1993; Neveu et al. 1993), and expression of receptors (Miller et al. 1996). PAR-1 agonists are neurotoxic but also protect neurons and astrocytes from death induced by environmental stresses (Smith-Swintosky et al. 1995; Vaughan et al. 1995). Thrombin also stimulates infiltration of inflammatory cells and proliferation of astrocytes in vivo (Nishino et al. 1993). Protease nexin-1, a specific thrombin inhibitor that is expressed in the brain, modulates these effects of thrombin (Cavanaugh et al. 1990; Smith-Swintosky et al. 1995). Less is known about the function of tryptic proteases and PAR-2 in the central nervous system (CNS). However, PAR-2 is expressed by cultured hippocampal neurons and PAR-2 agonists are neurotoxic (Smith-Swintosky et al. 1997).
The expression and functions of PAR-1 and PAR-2 have not been examined in the enteric nervous system (ENS), which regulates gastrointestinal motility, secretion and absorption. We hypothesized that thrombin and tryptase, which are generated and released during trauma and inflammation, regulate enteric neurons by cleaving PAR-1 and PAR-2. This activation may contribute to functional disturbances of the inflamed intestine. Our aims were to: (a) examine PAR-1 and PAR-2 mRNA expression in the myenteric plexus; (b) localize PAR-1 and PAR-2 in neurons by immunohistochemistry; (c) determine whether thrombin and tryptase excite neurons by cleaving PAR-1 and PAR-2; and (d) identify receptors that are co-expressed with PAR-1 and PAR-2 on neurons.
METHODS
Reagents
Thrombin was obtained from Boehringer-Mannheim. Trypsin was purchased from Worthington Biochemical Co. Tryptase was extracted and purified from human lungs as described previously for purification of tryptase from human mast cells (Corvera et al. 1997). Human lungs were obtained from autopsies using procedures that were approved by the Human Subject Committee at the University of California, San Francisco. The tryptase inhibitor bis(5-amidino-2-benzimidazolyl)methane (BABIM) was from Dr R. Tidwell, University of North Carolina. Synthetic peptides corresponding to the tethered ligand sequences of human PAR-1 (SFLLRN-NH2), rat/mouse PAR-2 (SLIGRL-NH2) and human PAR-2 (SLIGKV-NH2) were synthesized by solid phase methods and purified by reverse-phase high pressure liquid chromatography. Analogues of these peptides that are highly selective for PAR-1 (TFLLR-NH2, AF(pF)RChaCitY-NH2) and PAR-2 (tc-LIGRLO-NH2) have been characterized (Hollenberg et al. 1997; Vergnolle et al. 1998). The reverse sequence of the mouse PAR-2 peptide (LRGILS-NH2) was used as a control. Fura-2 AM and Pluronic were from Molecular Probes. Bradykinin was from Bachem Bioscience Inc. (King of Prussia, PA, USA). A bradykinin B2 receptor antagonist HOE 140 was from Dr K. Wirth (Hoechst, Frankfurt, Germany). A neurokinin receptor-1 selective antagonist SR 140333 was a gift from Dr X. Emonds-Alt (Sanofi Recherche, France). Other reagents were from Sigma Chemical Co.
Antibodies
The sources of antibodies to PAR-1, PAR-2, substance P (SP), vasoactive intestinal peptide (VIP), protein gene product 9.5 (PGP 9.5) and nitric oxide synthase (NOS) are shown in Table 1. PAR-2 antibody 9717 was raised in rabbits to a peptide corresponding to the C-terminal six residues of mouse PAR C-S394VKTSY399 (C for conjugation) conjugated to keyhole limpet haemocyanin (KLH). KLH (25 mg) was dissolved in 1 ml 0.1 M phosphate buffer, pH 7.5, containing 0.1 M EDTA. Maleimido-benzoyl-N-hydroxysuccinimide ester (10 mg, Pierce, Rockford, IL, USA) was dissolved in 1 ml dimethylformamide. This solution was added drop-wise to the KLH solution, mixed for 30 min at room temperature, and passed through a Bio-Spin column (Bio-Rad, Hercules, CA, USA). Peptide (10 mg) was dissolved in 1 ml of the column eluent (pH 7.5) and mixed for 3 h at room temperature and overnight at 4°C. The efficiency of conjugation, assessed by including peptide labelled with 125I using chloramine T, was ∼33 %. Two New Zealand rabbits (females, 8 weeks) were immunized at 6–8 week intervals with the 100 μg conjugate, and the generation of antibodies was determined by ELISA. This antibody strongly stained Kirsten murine sarcoma virus transformed rat kidney epithelial (KNRK) cells transfected with human PAR-2 (not shown). Staining was absent from non-transfected cells and was abolished by preabsorption of the antibody with 10 μm of the peptide used for immunization. Affinity-purified goat anti-rabbit or anti-mouse IgG conjugated to fluorescein isothiocyanate (FITC) or Texas Red were from Cappel Research Products (Durham, NC, USA) and Jackson Laboratories (West Grove, PA, USA).
Table 1.
Sources and dilutions of the primary antibodies used for immunofluorescence
| Antigen | Host | Dilution | Source |
|---|---|---|---|
| Human PAR-129–68 (61–1) | Mouse | 18.3 μg ml−1 | Dr B. Coller, Cor Therapeutics Inc., South San Francisco, CA, USA (Norton et al. 1993) |
| Rat PAR-230–46(B5) | Rabbit | 1 : 500 | (Kong et al. 1997) |
| Mouse PAR-2394–399 (9717) | Rabbit | 1 : 250–500 | see Methods |
| PGP 9.5 | Mouse | 1 : 100 | Accurate Chemical Co. (Westbury, NY, USA) |
| PGP 9.5 | Rabbit | 1 : 10 000 | Biogenesis (Sandown, NH, USA) |
| SP | Rat | 1 : 500 | Chemicon Inc. (Temecula, CA, USA) |
| SP | Rabbit | 1 : 50 | Zymed (South San Francisco, CA, USA) |
| VIP (V55) | Mouse | 1 : 500 | Dr J. H. Walsh, UCLA |
| VIP (7913) | Rabbit | 1 : 1000 | Dr J. H. Walsh, UCLA |
| NOS | Mouse | 1 : 10 000 | Dr D. Bredt, UCSF |
SP, substance P; VIP, vasoactive intestinal peptide; NOS, nitric oxide synthase.
Dispersion and culture of guinea-pig myenteric neurons
Newborn male guinea-pigs (Duncan-Hartley, Simonsen, Gilroy, CA, USA) were killed with pentobarbital sodium (200 mg kg−1i.p.). Myenteric neurons were isolated from the entire small intestine and cultured exactly as described previously (McConalogue et al. 1998). Neurons were plated on collagen-coated glass coverslips and cultured for 7–14 days in 95 % air-5 % CO2 at 37°C.
Cloning of guinea-pig PAR-1 and PAR-2 and detection in cultured neurons by RT-PCR
RNA from the lung of an adult male guinea-pig was reverse-transcribed and cDNA was amplified using primers based on rat and mouse PAR-1 and PAR-2. The PAR-1 primers were: forward, 5′-AAGCTTCCCGCTCATTTTTTCTCAGGAA-3′ (Hin dIII site emboldened); reverse, 5′-GAATTCAATCGGTGCCGGAGAAGT-3′ (Eco RI site emboldened). The PAR-2 primers were: forward, 5′-CACCACCTGTCACGATGTGCT-3′; reverse, 5′-CCCGGGCTCAGTAGGAGGTTTTAACAC-3′ (Sma 1 site emboldened). The PCR reaction included 2 μl of the template cDNA, 2.5 units of Taq DNA polymerase (Promega, Madison, WI, USA), 1.5 mM MgCl2, 50 mM KCl, 0.1 % v/v Triton X-100 and 0.2 mM each of deoxynucleotide triphosphates in 50 μl 10 mM Tris-HCl buffer, pH 9.0. PCR conditions were: denaturation of 3 min, 35 cycles at 94°C for 1 min, 55°C for 1 min and 72°C for 1 min, and elongation at 72°C for 15 min. PCR products were subcloned into PGEM-T vector (Promega) and sequenced in the 5′ and 3′ directions by the dideoxynucleotide chain termination method.
Total RNA from cultured neurons (0.3 μg) was reverse-transcribed and amplified using primers based on the partial sequences of guinea-pig receptors. PAR-1 primers (forward, 5′-CCGCTCATTTTTTCTCAGGAATCC-3′; reverse, 5′-TAGCTGATCTTGAAGGGGAGCACG-3′) were chosen to amplify a 372 bp fragment. PAR-2 primers (forward, 5′-CATGTTCAGCTACTTCCTCTCCTT-3′; reverse, 5′-GGTTTTAACACTGGTGGAGCTTGA-3′) were chosen to amplify a 472 bp fragment. PCR conditions were: denaturation of 9 min, 35 cycles at 94°C for 1 min, 62°C for 1 min and 72°C for 1 min, and elongation at 72°C for 9 min. Cloned guinea-pig PAR-1 and PAR-2 cDNA were used as positive controls. Water was used instead of RNA as a negative control. The specificity of the PCR reaction was verified using primer pairs of PAR-1 with PAR-2 cDNA as template and vice versa. Under these conditions, no amplification was obtained. PCR products were analysed by electrophoresis on a 1 % agarose gel with ethidium bromide.
Detection of PAR-1 and PAR-2 by immunofluorescence
Cultured neurons were fixed in 4 % paraformaldehyde in 0.1 M phosphate buffered saline (PBS), pH 7.4, for 20 min at 4°C, washed in PBS and incubated with PBS containing 10 % normal goat serum, 2 % bovine serum albumin, and 0.1 % saponin for 30 min. Cultures were incubated with combinations of primary antibodies to PAR-1 or PAR-2 and PGP 9.5, SP, VIP or NOS raised in different species for 24 h at 4°C (see Table 1 for dilutions). Cultures were washed and incubated with secondary antibodies (1 : 200) conjugated to FITC, to detect PARs, and Texas Red, to detect other markers, for 2 h at room temperature. The percentage of neurons expressing PGP 9.5, SP, VIP or NOS and PAR-1 or PAR-2 was determined by counting > 100 stained cells in more than two cultures. To detect PARs in intact tissues, the ileum was opened along the mesenteric border, pinned to balsa wood, and placed in 4 % paraformaldehyde overnight at 4°C. Whole mounts of the myenteric plexus were prepared, washed in PBS, and incubated with PBS containing 20 % normal goat serum and 0.3 % Triton X-100 for 30 min. Tissue was incubated with antibodies as described. In control experiments, antibodies to PARs were incubated with 10 μm of the peptide used for immunization for 24–48 h before staining. Cultures were observed with a Zeiss Axioplan microscope and photographed using a Sony DKC5000 digital camera. Whole mounts were examined with a Zeiss Axiovert and an MRC 1000 laser scanning confocal microscope (McConalogue et al. 1998).
Measurement of [Ca2+]i in myenteric neurons
Cultured myenteric neurons were incubated in physiological salt solution (PSS of composition (mM): 137 NaCl, 4.7 KCl, 0.56 MgCl2, 2 CaCl2, 1.0 Na2HPO4, 10 Hepes, 2.0 L-glutamine and 5.5 D-glucose; pH 7.4) containing 0.1 % bovine serum albumin (BSA), 5 μm fura-2 AM and 0.2 % Pluronic for 20 min at 37°C (McConalogue et al. 1998). They were washed and mounted in a micro-incubator containing 1 ml of PSS-BSA at 37°C on the stage of a Zeiss Axiovert 100 TV microscope. To determine the role of extracellular Ca2+, cultures were incubated in Ca2+-free PSS containing 2 mM EDTA. Agonists and antagonists were directly added to the bath. Neurons were observed with a Zeiss Fluar × 40 objective (numerical aperture 1.30) and fluorescence was detected in individual neurons using an intensified charged coupled device camera (Stanford Photonics, Stanford, CA, USA) and a video microscopy acquisition program (Imaging Workbench, Axon Instruments). Fluorescence was measured at 340 and 380 nm excitation and 510 nm emission. The ratio of the fluorescence at the two excitation wavelengths, which is proportional to the [Ca2+]i, was determined for the soma of the neurons. Neurons were distinguished from non-neuronal cells in two ways (Kimball et al. 1996). (1) By morphology: neurons were compact, phase-bright cells that were clustered in ganglia. (2) By KCl depolarization: at the end of experiments, cultures were challenged with 55 mM KCl, which depolarizes neurons, increasing [Ca2+]i, whereas non-neuronal cells do not possess voltage-gated Ca2+ channels and would not respond. Only the results from cells that responded to KCl by a large increase in [Ca2+]i, and are therefore neurons, are presented. Fresh neurons were studied with each agonist concentration, and a minimum of 15 neurons were analysed in three experiments on different days.
Degranulation of mast cells
The human mast cell line HMC-1 was from Dr J. Butterfield, Mayo Clinic, Rochester, MI, USA. Cells (70 million cells ml−1) were suspended in PSS-BSA containing 25 μg ml−1 heparin. To induce degranulation, cells were incubated with 100 μm SP for 5 min, which stimulated maximal release of tryptase activity into the medium (Corvera et al. 1997). The suspension was passed through a 0.45 μm filter and the filtrate was immediately assayed for tryptase activity and for its ability to mobilize Ca2+ in neurons. Tryptase activity was measured by spectrophotometry using 0.1 mM of the substrate N-p-tosyl-Gly-L-Pro-Lys-p-nitroanilide in PSS-BSA. Human lung tryptase was used as a standard. Activity was measured using the kinetic mode at 405 nm for 2 min on a UV-visible recorder spectrophotometer (Shimadzu, Kyoto, Japan).
RESULTS
Molecular cloning of guinea-pig PAR-1 and PAR-2 and detection in cultured myenteric plexus by RT-PCR
A PAR-1 clone encoding 409 bp and 130 residues, and a PAR-2 clone encoding 527 bp and 174 residues were obtained. The partial sequence of guinea-pig PAR-1 corresponds to the 124–503 bp region of rat PAR-1, and the encoded protein is 98 % identical to rat PAR-1. The partial sequence of guinea-pig PAR-2 corresponds to the 670–1194 bp region of rat PAR-2, and the encoded protein is 83 % identical to rat PAR-2. Although we did not clone a sufficient number of bases to identify the tethered ligand domains of guinea-pig PAR-1 and PAR-2, it is probable that these are identical to other rodent tethered ligands, which are conserved.
We used primers based on the partial sequences of guinea-pig PAR-1 and PAR-2 to amplify PAR-1 and PAR-2 mRNA from cultured myenteric plexus. A PAR-1 product of the predicted size of 372 bp was amplified after 70 cycles, and a PAR-2 product of the predicted size of 472 bp was amplified after 35 cycles (Fig. 1). These products migrated identically to PCR products that were amplified from cloned guinea-pig PAR-1 and PAR-2, which served as a positive control. They were absent from the negative control. A non-specific band, probably due to primer dimerization, appeared in both the negative control and the RT-PCR reactions of PAR-1. This may explain the lower amplification of PAR-1. These results indicate that PAR-1 and PAR-2 are expressed by primary cultures of the guinea-pig myenteric plexus.
Figure 1. Detection of PAR-1 and PAR-2 in cultures of myenteric plexus by RT-PCR.
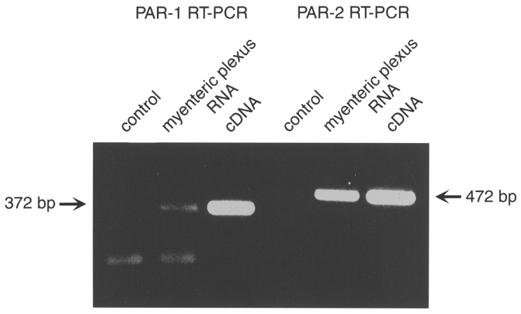
Cloned PAR-1 and PAR-2 cDNA were used as templates for the positive control. Water was used in place of RNA for the negative control.
Detection of immunoreactive PAR-1 and PAR-2 in cultured neurons and whole mounts of myenteric plexus
To determine whether PAR-1 and PAR-2 are expressed by neurons, we simultaneously localized the receptors with the neuronal marker PGP 9.5 in cultures (Fig. 2). Immunoreactive PAR-1 was detected in 76 % of neurons that were positive for PGP 9.5, where it was mainly detected in intracellular locations in the soma and in neurites. Immunoreactive PAR-2 was detected in the soma and neurites of most PGP 9.5 positive neurons using 9717 and B5 antibodies (9717, 62 %; B5, 68 %). To immunochemically characterize neurons, we simultaneously localized PAR-1 or PAR-2 with SP, VIP or NOS. Of neurons expressing SP, 89 % expressed PAR-1, and 51 and 52 % were stained with 9717 and B5, respectively, and thus express PAR-2. Of neurons expressing VIP, 44 % expressed PAR-1, 88 % were stained with 9717, and 92 % were stained with B5. Of neurons expressing NOS, 73 % expressed PAR-1. Thus, PAR-1 and PAR-2 are expressed by a large population of myenteric neurons in culture that contain excitatory and inhibitory transmitters. We stained whole mounts of the myenteric plexus to confirm expression of PARs in intact tissues. In whole mounts of the myenteric plexus, PAR-1 and PAR-2 were detected in a large proportion of neurons where they were principally localized intracellularly in the soma (Fig. 2). The intensity of staining for both receptors varied from neuron to neuron, suggesting heterogeneous levels of expression. Neuronal processes were also stained within the ganglia. There was similar staining with PAR-2 antibodies 9717 and B5. Staining of cultures and whole mounts was markedly diminished by preabsorption of the PAR-1 and PAR-2 antibodies with the receptor fragments that were used for immunization, and is thus specific (Fig. 2).
Figure 2. Simultaneous localization of immunoreactive PAR-1 and PAR-2 with PGP 9.5, SP, NOS and VIP in myenteric neurons in culture.
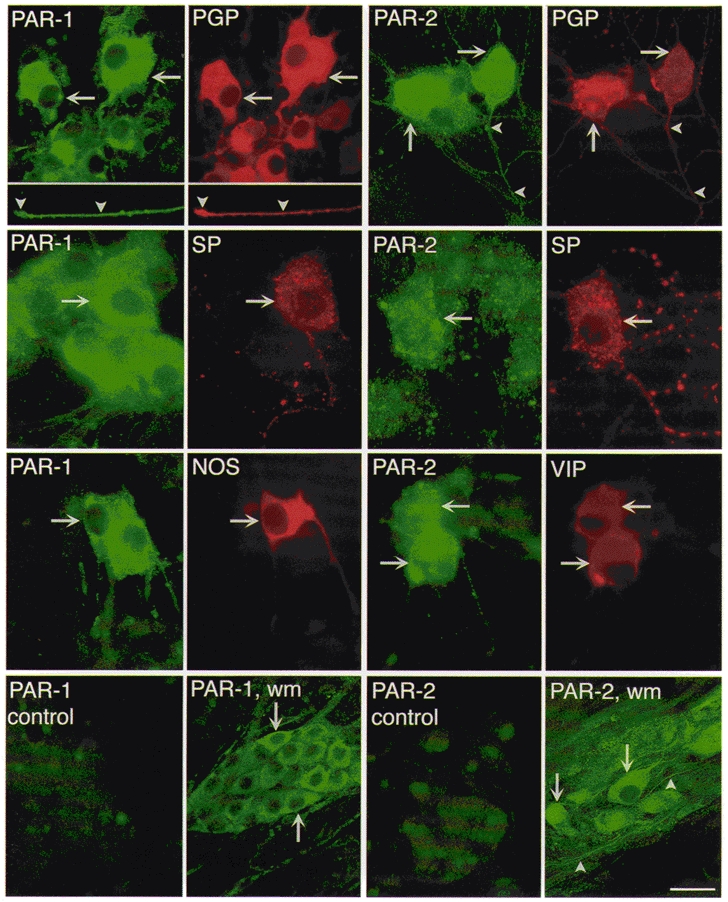
To localize PAR-1, cultures were incubated with mouse PAR-1 61-1 and rabbit PGP 9.5, or rat SP, or rabbit NOS antibodies. To localize PAR-2, cultures were incubated with rabbit PAR-2 B5 and mouse PGP 9.5 antibodies, or rabbit PAR-2 9717 and rat SP, or mouse VIP antibodies. The arrows indicate colocalization in neurons of PAR-1 and PAR-2 with PGP 9.5, SP, VIP or NOS. The arrowheads indicate stained neurites. In the control experiments, antibodies to 61-1 or B5 were preincubated with 10 μm of the peptides used for immunization. PAR-1 wm (whole mount) and PAR-2 wm show localization in whole mounts of the myenteric plexus using 61-1 and 9717, respectively. Scale bar, 20 μm for top row and controls, 28 μm for middle two rows, and 50 μm for whole mounts.
PAR-1- and PAR-2-mediated Ca2+ mobilization in cultured neurons
Both PAR-1 and PAR-2 couple to phospholipase Cβ and hence mobilize intracellular Ca2+. We examined the effects of PAR agonists on [Ca2+]i to provide functional evidence for expression of PARs in myenteric neurons. Thrombin stimulated a rapid increase in [Ca2+]i in a large proportion of neurons (72/153 neurons for 10 nm thrombin, 47.6 ± 5.5 %, mean ±s.e.m., n = 3 cultures) with a threshold of ∼0.1 nm and an EC50 of ∼1 nm (Fig. 3 and Fig. 4A). A peptide corresponding to the tethered ligand of human PAR-1 (SFLLRN-NH2) similarly increased [Ca2+]i in many neurons (100 μm SFLLRN-NH2: 116/192 neurons, 74.7 ± 9.3 %) with a comparable efficacy but reduced potency to thrombin (EC50, ∼50 nm). Since SFLLRN-NH2 also activates PAR-2 (Blackhart et al. 1996), we also assayed PAR-1 selective analogues. AF(pF)RChaCitY-NH2 and TFLLRN-NH2 increased [Ca2+]i in many neurons (100 nm AF(pF)RChaCitY-NH2, 183/271 neurons, 63.6 ± 9.5 %; 1 μm TFLLR-NH2, 121/189 neurons, 66.5 ± 4.2 %). TFLLRN-NH2had a similar potency to SFLLRN-NH2 but AF(pF)RChaCitY-NH2 was surprisingly potent (EC50, ∼10 nm).
Figure 3. Effects of PAR-1 agonists on [Ca2+]i in myenteric neurons.
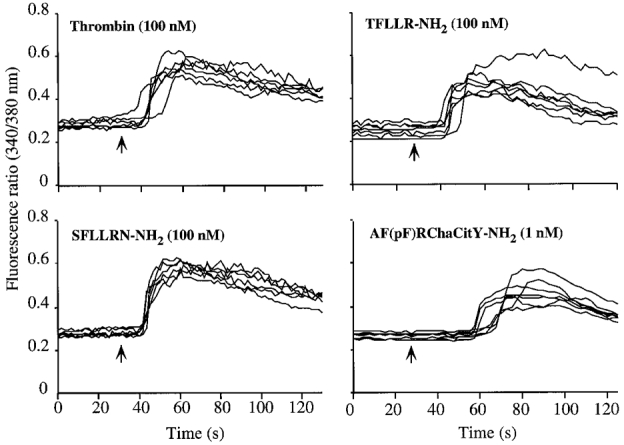
Neurons were exposed to agonists at the indicated concentrations (arrows) and the fluorescence ratio (340/380 nm) was measured in the soma of individual neurons. Each line is a trace from a single cell. Experiments were repeated on three different neuronal cultures, with > 20 neurons recorded per culture.
Figure 4. Effects of graded concentrations of PAR-1 agonists (A) or PAR-2 agonists (B) on [Ca2+]i in myenteric neurons.
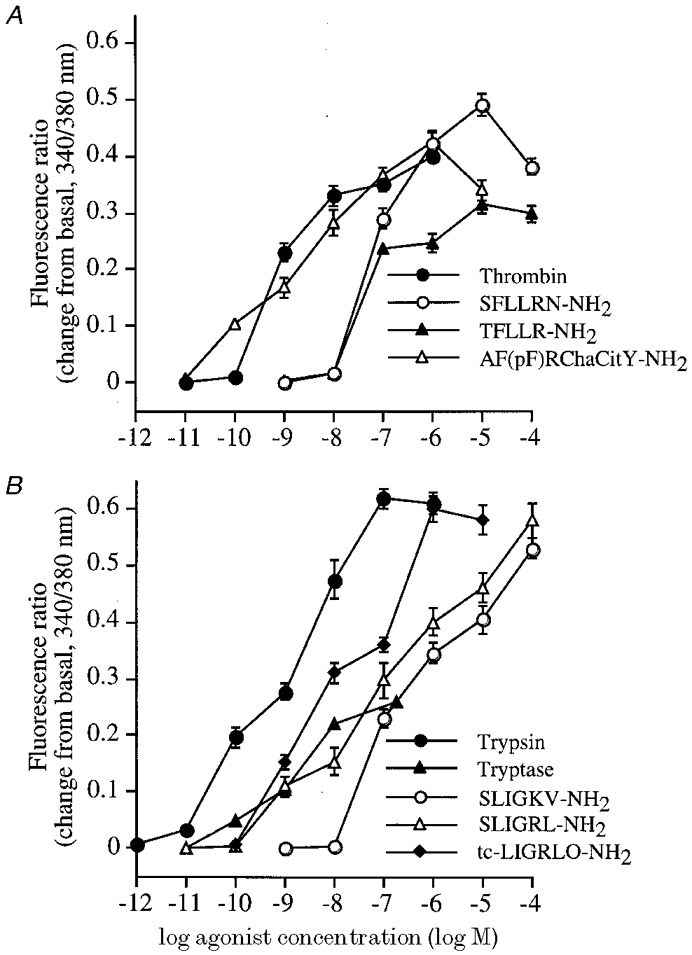
Neurons were exposed to a single concentration of agonist. Results are expressed as the change from basal level of the fluorescence ratio (340/380 nm). Each point represents the mean ±s.e.m. of observations from > 20 neurons.
The PAR-2 agonist trypsin stimulated a rapid increase in [Ca2+]i in a large proportion of neurons (1 nm trypsin, 104/223 neurons, 46.6 ± 3.7 %) with a threshold of ∼0.01 nm and an EC50 of ∼5 nm (Fig. 4B and Fig. 5). There was no response to 1 nm trypsin that was preincubated with 10 mg ml−1 soybean trypsin inhibitor (SBTI) for 60 min at 37°C before assay, indicating the requirement for enzymatic activity. Peptides corresponding to the tethered ligand of rat PAR-2 (SLIGRL-NH2) and human PAR-2 (SLIGKV-NH2) similarly increased [Ca2+]i (10 μm SLIGRL-NH2, 73/120 neurons, 60.8 ± 3.0 %; 10 μm SLIGKV-NH2, 86/146 neurons, 58.9 ± 11.9 %) with a comparable efficacy but reduced potency relative to trypsin (EC50 values, ∼500 nm). The rat peptide was marginally more potent than the human peptide. An analogue of these peptides, tc-LIGRLO-NH2, increased [Ca2+]i in neurons (10 nm, 130/180 neurons, 72.4 ± 6.9 %) although it was more potent than SLIGRL-NH2 and SLIGKV-NH2 (EC50, ∼10 nm). The reverse sequence of the tethered ligand peptide LRGILS-NH2 had no effect on [Ca2+]i, confirming specificity. These results provide functional evidence that PAR-1 and PAR-2 are expressed by many myenteric neurons of the guinea-pig small intestine.
Figure 5. Effects of protease agonists (A) and peptide agonists (B) of PAR-2 on [Ca2+]i in myenteric neurons.
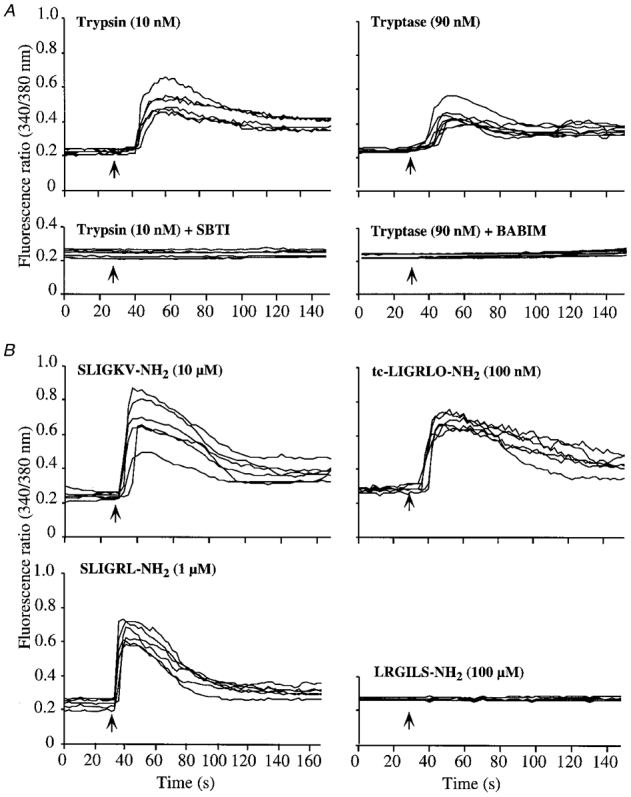
Neurons were exposed to agonists at the indicated concentrations (arrows) and the fluorescence ratio (340/380 nm) was measured in the soma of individual neurons. Each line is a trace from a single cell. Experiments were repeated on three different neuronal cultures, with > 20 neurons recorded per culture. Trypsin and tryptase were incubated with SBTI and BABIM, respectively, before the assay.
Tryptase, a major secretory granule protease of human mast cells (Metcalfe et al. 1997) that activates PAR-2 in several cell types (Corvera et al. 1997; Mirza et al. 1997; Molino et al. 1997), stimulated a rapid increase in [Ca2+]i in neurons (90 nm, 123/214 neurons, 57.6 ± 7.9 %) with a threshold-detectable response to ∼1 nm (Fig. 4B and Fig. 5). Due to limited availability, we could not determine the potency or efficacy of the effect of tryptase. The Ca2+ response to 90 nm tryptase was abolished by pre-incubation with 1 μm BABIM for 5 min at room temperature, and is thus dependent on its enzymatic activity.
To determine if mast cells release factors activate neurons, we degranulated HMC-1 cells with SP and assayed the filtrate to ascertain its effect on [Ca2+]i in neurons. Enzymatic assay indicated that 10 μl HMC-1 filtrate contained activity equivalent to ∼2-4 μm tryptase (equivalent to ∼20-40 nm in assay) that was completely inhibited by preincubation with 1 μm BABIM and 1 μm leupeptin for 5 min at room temperature. HMC-1 filtrate (10 μl) stimulated a large increase in [Ca2+]i (Fig. 6). Preincubation with 1 μm BABIM or 1 μm leupeptin inhibited the magnitude of the response by 42.0 ± 8.1 and 48.3 ± 6.9 %, respectively, suggesting that Ca2+ mobilization depends to a large extent on tryptase. To verify that the Ca2+response to HMC-1 filtrate was not due to activation of the neurokinin 1 receptor by SP, we pretreated neurons with the neurokinin 1 receptor antagonist SR 140333 (1 μm). This antagonist abolished the Ca2+ response of myenteric neurons to 1 μm SP (McConalogue et al. 1998), but had no effect on the response to the HMC-1 filtrate. Thus, the Ca2+ response to the HMC-1 filtrate is not due to exogenous SP.
Figure 6. Effects of a filtrate of degranulated HMC-1 cells on [Ca2+]i in myenteric neurons.
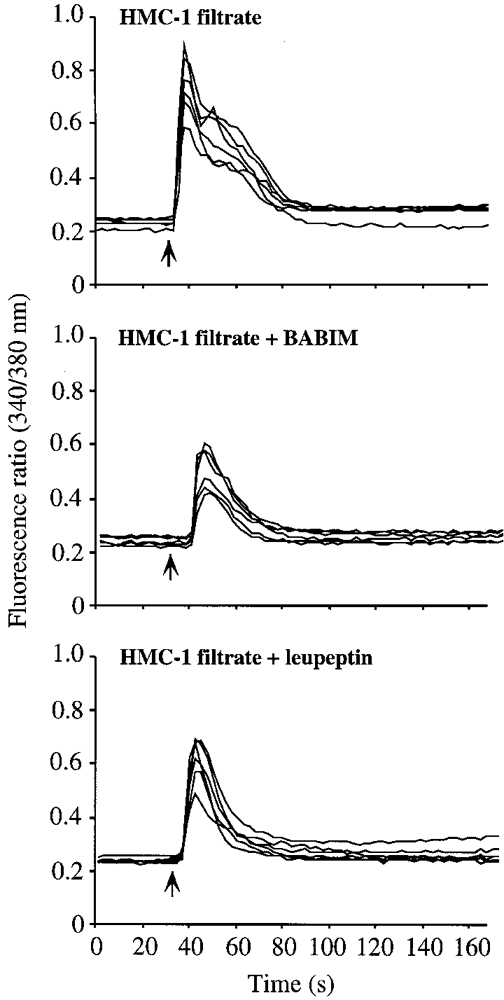
Neurons were exposed to 10 μl of the mast cell filtrate which was obtained as described in Methods. The filtrate was preincubated with BABIM or leupeptin immediately before the assay.
We measured [Ca2+]i in the absence of extracellular Ca2+, to determine the relative contributions of intracellular and extracellular Ca2+. In the presence of extracellular Ca2+, agonists of PAR-1 (10 nm AF(pF)RChaCitY-NH2) and PAR-2 (10 nm tc-LIGRLO-NH2) caused a prompt increase in [Ca2+]i that declined to basal levels within 100 s even in the continued presence of agonist (Fig. 7A and B). In the absence of extracellular Ca2+, a similar profile was observed, indicating that the response is mainly due to mobilization of intracellular Ca2+. However, when Ca2+ was added to the extracellular fluid in the continued presence of agonist, a small increase in [Ca2+]i was detected. This response is thus dependent on a minor influx of Ca2+ from the extracellular fluid.
Figure 7. Effects of extracellular Ca2+ on changes in [Ca2+]i to agonists of PAR-1 (A) and PAR-2 (B) in myenteric neurons.
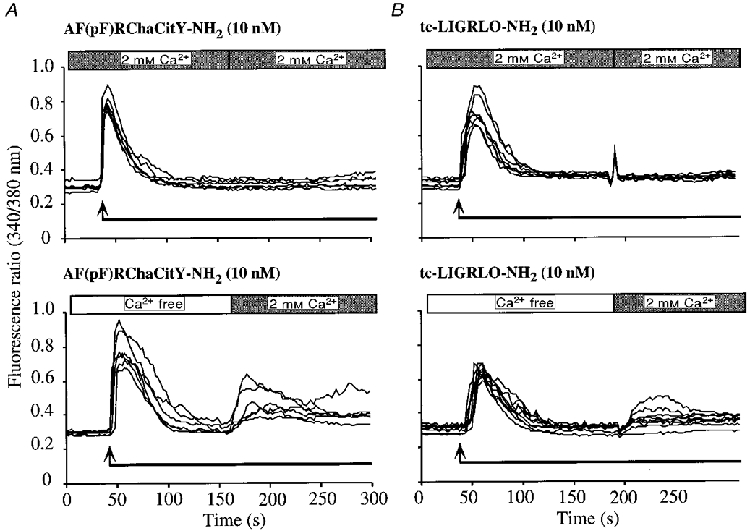
Agonists were added at the arrows and remained in the bath for the entire experimental period. Cultures were bathed in PSS containing 2 mM Ca2+ (upper panels) or in Ca2+-free PSS (lower panels). After [Ca2+]i returned to baseline, the medium was replaced with PSS containing 2 mM Ca2+. The fluorescence ratio (340/380 nm) was measured in the soma of individual neurons. Each line is a trace from a single cell. Experiments were repeated on three different neuronal cultures, with > 20 neurons recorded per culture.
Desensitization of PAR-1 and PAR-2
To provide evidence that proteases and peptide agonists activate the same receptors, we examined desensitization of Ca2+ responses to repetitive exposure of neurons to PAR-1 or PAR-2 agonists. Thrombin (10 nm) strongly desensitized the Ca2+ response to a second challenge with 10 nm thrombin or 100 nm AF(pF)RChaCitY-NH2 applied 3 min later (without an intervening wash) (Fig. 8, Table 2). AF(pF)RChaCitY-NH2 (100 nm) strongly desensitized the response to a second challenge with 100 nm AF(pF)RChaCitY-NH2, or 10 nm thrombin. These results suggest that thrombin and AF(pF)RChaCitY-NH2 activate the same receptor (PAR-1). Trypsin (10 nm) strongly desensitized the Ca2+ response to a second challenge with 10 nm trypsin, 10 μm SLIGKV-NH2, or 90 nm tryptase applied 2 min later (without an intervening wash) (Fig. 8, Table 2). SLIGKV-NH2 (10 μm) also desensitized the response to a second challenge with 10 μm SLIGKV-NH2, 10 nm thrombin, or 90 nm tryptase. These results suggest that thrombin, tryptase and SLIGKV-NH2 activate the same receptor (PAR-2).
Figure 8. Desensitization of [Ca2+]i in myenteric neurons to repeated application of agonists.
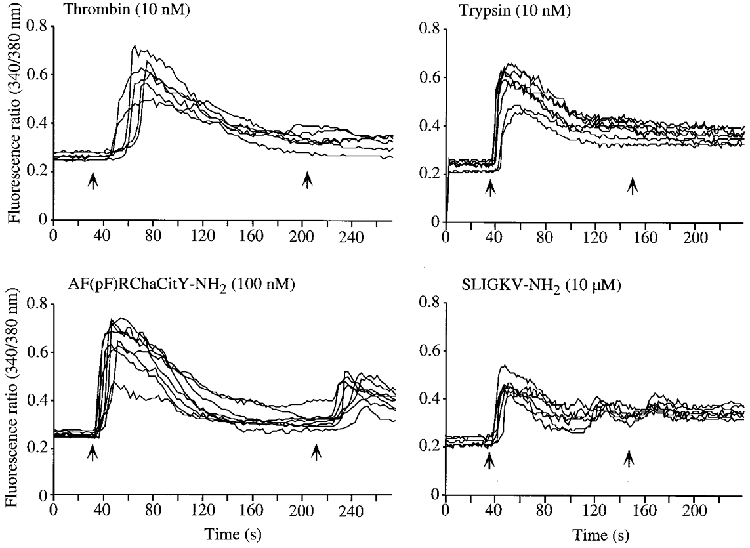
Neurons were exposed sequentially to agonists at the indicated concentrations (arrows) without an intervening wash, and the fluorescence ratio (340/380 nm) was measured in the soma of individual neurons. Each line is a trace from a single cell. Experiments were repeated on three different neuronal cultures, with > 20 neurons recorded per culture.
Table 2.
Desensitization of [Ca2+]i in myenteric neurons in response to repeated application of agonists
| First agonist | Response | Second agonist | Response | Desensitization (%) |
|---|---|---|---|---|
| Thrombin (10 nm) | 0.31 ± 0.05 | Thrombin (10 nm) | 0.018 ± 0.005 | 99.9* |
| AF(pF)RChaCitY-NH2 (100 nm) | 0.002 ± 0.001 | 99.4* | ||
| AF(pF)RChaCitY-NH2 (100 nm) | 0.40 ± 0.09 | AF(pF)RChaCitY-NH2 (100 nm) | 0.114 ± 0.06 | 71.5* |
| Thrombin (10 nm) | 0.09 ± 0.03 | 77.5* | ||
| Trypsin (10 nm) | 0.41 ± 0.05 | Trypsin (10 nm) | 0.002 ± 0.001 | 99.5* |
| SLIGKV-NH2(10 μm) | 0.14 ± 0.013 | 65.9* | ||
| Tryptase (90 nm) | Undetectable | 100* | ||
| SLIGKV-NH2(10 μm) | 0.40 ± 0.03 | SLIGKV-NH2(10 μm) | 0.09 ± 0.014 | 77.5* |
| Trypsin (10 nm) | 0.02 ± 0.004 | 95.0* | ||
| Tryptase (90 nm) | * Undetectable | 100* |
Neurons were exposed sequentially to agonists at a 2 (PAR-2 agonists) or 3 min (PAR-1 agonists) interval at the indicated concentrations without an intervening wash, and the fluorescence ratio (340/380 nm) was measured in the soma of individual neurons. Experiments were repeated on three different neuronal cultures, with > 20 neurons recorded per culture.
P < 0.05 compared with response to the same first agonist (ANOVA and Student–Newman–Keuls test).
Neurons that were exposed to 10 nm thrombin or 10 nm trypsin, and showed strong desensitization of PAR-1 and PAR-2, respectively, still responded to 100 nm ATP and 55 mM KCl (Fig. 9 and Fig. 10). These finding suggest that the diminished Ca2+ response to a second thrombin or trypsin challenge is not due to depletion of intracellular Ca2+ stores but rather to homologous desensitization of PAR-1 or PAR-2.
Figure 9. Effects of a PAR-1 agonist, ATP and KCl on [Ca2+]i in myenteric neurons.
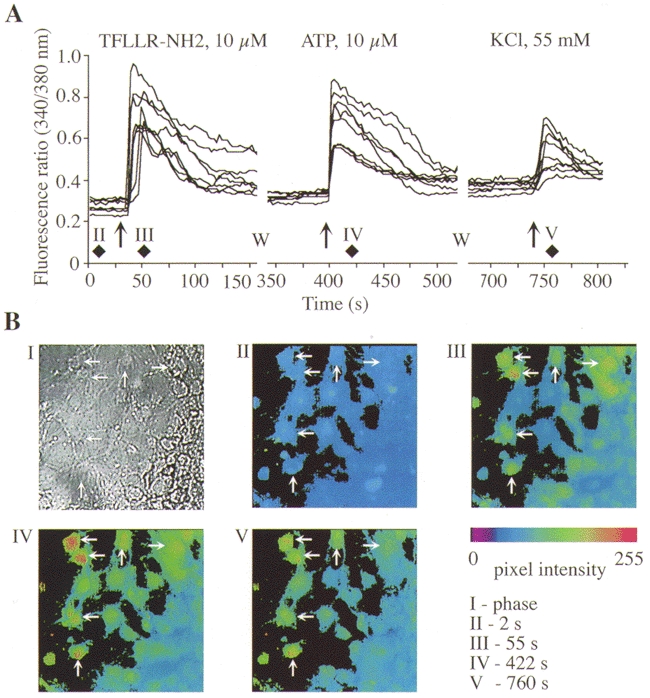
Neurons were exposed to the PAR-1 agonist, washed (W), and exposed to ATP and then KCl. A, each trace shows the 340/380 nm fluorescence ratio for a single neuron. B, I, phase contrast image of neurons. II-V, pseudo-colour images of the 340/380 fluorescence ratio for these neurons at the indicated times. Diamonds in A indicate the times at which these images were obtained.
Figure 10. Effects of a PAR-2 agonist, ATP and KCl on [Ca2+]i in myenteric neurons.
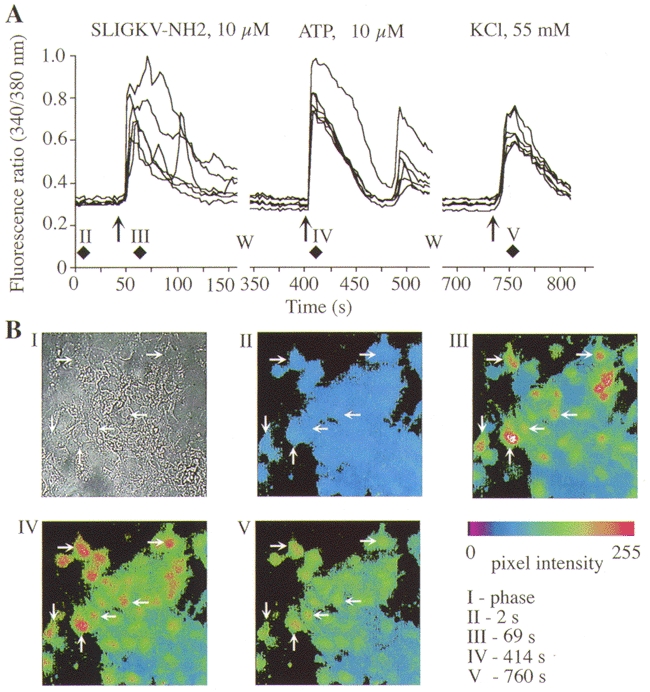
Neurons were exposed to the PAR-2 agonist, washed (W), and exposed to ATP and then KCl. A, each trace shows the 340/380 nm fluorescence ratio for a single neuron. B, I, phase contrast image of neurons. II-V, pseudo-colour images of the 340/380 fluorescence ratio for these neurons at the indicated times. Diamonds in A indicate the times at which these images were obtained.
Pharmacological characterization of neurons expressing PAR-1 and PAR-2
To determine which other receptors are coexpressed with PAR-1 and PAR-2 on myenteric neurons, we sequentially challenged neurons with selective PAR-1 or PAR-2 agonists followed by agonists of other functionally important receptors. Of neurons that responded to 10 μm TFLLR-NH2 (PAR-1 agonist), 66.4 ± 7.2 % (51/93 neurons, n = 3 cultures) also responded to 10 μm SLIGKV-NH2 (PAR-2 agonist). Similarly, of neurons that responded to 10 μm SLIGKV-NH2, 56.8 ± 13.6 % (68/103) also responded 10 μm TFLLR-NH2. Thus, PAR-1 and PAR-2 are coexpressed by > 57 % of myenteric neurons. Notably, of neurons that responded to 10 μm TFLLR-NH2 or SLIGKV-NH2, 95.4 ± 2.3 % (79/83) and 98.8 ± 5.7 % (151/154) also responded to 10 μm ATP (Fig. 9 and Fig. 10). In contrast, fewer than 20 % of TFLLR-NH2- or SLIGKV-NH2-responsive neurons also responded to 100 μm ACh. Therefore, most neurons expressing functional PAR-1 or PAR-2 appear to coexpress purinergic receptors.
The stimulatory effects of PAR-1 and PAR-2 agonists on [Ca2+]i are probably due to a direct effect of agonists on PAR-expressing neurons, since many cultured neurons express immunoreactive PAR-1 and PAR-2. However, PAR agonists may indirectly stimulate Ca2+ mobilization by inducing the release of neurotransmitters from adjacent neurons. In an attempt to exclude any indirect effects, we examined the actions of antagonists of other receptors on the proportion of neurons responding to PAR-1 and PAR-2 agonists. We exposed neurons to 10 μm atropine (muscarinic receptor antagonist), 10 μm thioperamide maleate (histamine H3 receptor antagonist), 1 μm SR140333 (neurokinin 1 receptor) or 1 μm HOE-140 (bradykinin B2 receptor antagonist). These antagonists abolished the Ca2+ responses of neurons to 1 μm ACh, 10 μm histamine, 1 μm SP and 1 μm bradykinin, respectively, indicating their effectiveness (data not shown). However, in the presence of these antagonists, > 60 % of neurons still responded to the same extent to 1 μm AF(pF)RChaCitY-NH2 or 10 μm SLIGKV-NH2. Therefore, the proportion of neurons responding to PAR-1 and PAR-2 agonists, and the magnitude of the responses were unaffected by antagonists of other G-protein-coupled receptors (GPCRs) that induce Ca2+ mobilization. Similarly, responses to 1 μm AF(pF)RChaCitY-NH2 or 10 μm SLIGKV-NH2 were unaffected by the 100 μm indomethacin (cyclo-oxygenase inhibitor) or by 1 μm tetrodotoxin (inhibits neuronal Na+ channels to block axonal conduction in some enteric neurons). These results suggest that agonists of PAR-1 and PAR-2 stimulate Ca2+ mobilization in myenteric neurons by a direct action rather than by the release of neurotransmitters from other neurons.
DISCUSSION
We used molecular, immunochemical and functional approaches to show that > 50 % of neurons in the myenteric plexus of the guinea-pig small intestine express PAR-1 and PAR-2. This is the first demonstration that enteric neurons express these receptors. Thrombin and tryptase, which are generated and released during trauma and inflammation, are likely to regulate myenteric neurons by cleaving PAR-1 and PAR-2, and may thereby influence multiple functions of the intestine.
Myenteric neurons express PAR-1 and PAR-2 and respond to thrombin and tryptase
Several observations suggest that myenteric neurons express PAR-1. Firstly, PAR-1 mRNA was detected in cultured myenteric plexus by RT-PCR. However, the cultures contain neurons, glial cells and muscle cells, which may also express PARs. Therefore, this result does not prove that neurons express PAR-1. Secondly, immunoreactive PAR-1 was detected in > 70 % of PGP 9.5-positive neurons in culture and was also localized to neurons in whole mounts of the myenteric plexus. We cannot exclude completely the possibility that the PAR-1 antibody cross-reacts with another member of this receptor family or with a different protein, but our results with this well-characterized monoclonal antibody strongly suggest that immunoreactive PAR-1 is expressed by myenteric neurons. Thirdly, thrombin, SFLLRN-NH2 and the PAR-1 selective agonists AF(pF)RChaCitY-NH2 and TFLLRN-NH2, stimulated Ca2+ mobilization in 50–75 % of neurons. Thrombin and peptide agonists strongly desensitized responses to a second challenge, suggesting that they activate the same receptor. Proteases produced stronger desensitization than peptides, even though agonist concentrations were chosen to induce a comparable Ca2+ response. This more complete desensitization by a protease may be due to physical cleavage of receptors, which would generate a receptor that is unable to respond to proteolysis. It is possible that thrombin and the peptides activate PARs other than PAR-1. Thrombin also cleaves PAR-3 and PAR-4, which are expressed in the intestine (Ishihara et al. 1997; Xu et al. 1998). SFLLRN-NH2 also activates PAR-2 (Blackhart et al. 1996), but AF(pF)RChaCitY-NH2 and TFLLRN-NH2 do not activate PAR-2 (Hollenberg et al. 1997; Vergnolle et al. 1998), and PAR-3 is not activated by peptides corresponding to its tethered ligand domain (Ishihara et al. 1997). In addition, PAR-4 does not respond to peptides corresponding to the tethered ligands of PAR-1 (Xu et al. 1998). Therefore, the most straightforward explanation of our results is that thrombin and peptide analogues of the tethered ligand excite myenteric neurons through PAR-1. In support of our results, PAR-1 is expressed by discrete populations of neurons and glia in the CNS (Weinstein et al. 1995).
Similar observations indicate that myenteric neurons also express PAR-2. We detected PAR-2 mRNA in cultured myenteric plexus by RT-PCR, and localized immunoreactive PAR-2 to ∼65 % of PGP 9.5-positive neurons in cultures and to myenteric neurons in whole mounts with two different antibodies. Trypsin and peptides corresponding to the tethered ligand domains of PAR-2 stimulated Ca2+ mobilization in 50–70 % of neurons. Trypsin or peptide agonists strongly desensitized responses to a second challenge, which suggests that they activate the same receptor. Although trypsin also activates PAR-4, which is expressed in the intestine (Xu et al. 1998), and peptide agonists of PAR-2 may also activate other PARs, our results strongly suggest that these agonists excite myenteric neurons through PAR-2. In support of our results, PAR-2 is expressed by hypothalamic neurons (Smith-Swintosky et al. 1997).
Purified tryptase stimulated Ca2+ mobilization in myenteric neurons. Activation of PAR-2 with trypsin or peptide agonists strongly desensitized the response to tryptase, suggesting that tryptase excites neurons through PAR-2. In support of our results, tryptase and trypsin cleave human PAR-2 at the same site (SKGR * SLIGK) to expose a tethered ligand (SLIGK) (Mirza et al. 1997; Molino et al. 1997). Tryptase also activates endothelial cells, epithelial cells, myocytes and transfected cell lines by cleaving PAR-2 (Corvera et al. 1997; Mirza et al. 1997; Molino et al. 1997). We used human tryptase to trigger guinea-pig PAR-2. However, human and guinea-pig tryptase have similar specificity (McEuen et al. 1996), and the cleavage site of PAR-2 is conserved between species. Our observation that a tryptase inhibitor (BABIM) strongly suppresses Ca2+ mobilization in response to a filtrate of degranulated mast cells indicates that tryptase is a major mast cell component that has the capacity to regulate myenteric neurons through PAR-2. The BABIM-insensitive component was not identified, although it is unlikely to be a protease since leupeptin, a broad inhibitor of serine and cysteine proteases of tryptic specificity, was no more effective than BABIM.
We consistently observed that a higher proportion of neurons responded to peptide agonists of PAR-1 and PAR-2 than to proteases. Approximately 48 % of neurons responded to thrombin whereas 75 % responded to SFLLRN-NH2, possibly because this agonist also excites PAR-2 as well as PAR-1 (Blackhart et al. 1996). Approximately 45–55 % of neurons responded to trypsin and tryptase, whereas ∼50-70 % responded to the peptide agonists of PAR-2. One explanation for the higher proportion of neurons responding to the peptides compared with proteases may be that the neurons or glia of the ENS produce protease inhibitors. Indeed, the thrombin inhibitor protease nexin-1 is expressed in the CNS (Cavanaugh et al. 1990; Smith-Swintosky et al. 1995). Another explanation could be that the peptides are more diffusable than proteases and thus activate more cells. In support of this possibility, tryptase binds to extracellular matrix and is not highly diffusable (Schwartz et al. 1989). We also cannot exclude the possibility that peptides interact with PARs that are not triggered by thrombin or trypsin. However, despite the variability in the number of neurons responding to various PAR agonists by increased [Ca2+]i, the proportion of responsive neurons is similar to the percentages of PGP 9.5-positive neurons expressing immunoreactive PARs. Notably, proteases and peptides stimulated Ca2+ mobilization in neurons with a particularly high potency. The high potency of agonists may reflect the increased sensitivity of the single cell assay system compared with assays that measure [Ca2+]i in populations of cells (author's unpublished observations).
Immunological and pharmacological characterization of myenteric neurons expressing PAR-1 and PAR-2
We simultaneously localized immunoreactive PAR-1 and PAR-2 with markers of a distinct population of myenteric neurons. A large proportion of cultured neurons expressing SP, a primary excitatory transmitter to muscle, also expressed PAR-1 (89 %) and PAR-2 (∼50 %). Similarly, a large percentage of neurons expressing VIP, an inhibitory motor transmitter, co-expressed PAR-1 (44 %) and PAR-2 (∼90 %). Of neurons expressing NOS, a marker of inhibitory motor neurons, 73 % expressed PAR-1. In future studies it will be important to define in detail the neurochemical content of neurons expressing PAR-1 and PAR-2 in intact tissues, for such analysis will provide insight into the potential role of these receptors in the ENS.
We characterized PAR-expressing neurons by a challenge with agonists of other receptors. More than 50 % of neurons expressing PAR-1 also expressed PAR-2. Both receptors are also co-expressed by other cell types, such as endothelial cells where they may co-operate to regulate growth and release of vasodilators (Mirza et al. 1996). Over 90 % of PAR-expressing neurons responded to ATP and presumably express purinergic receptors. Due to the lack of highly selective agonists and antagonists, we did not further characterize the receptors responsible for ATP-induced Ca2+ mobilization. Purinoceptors of the P2Y subtype are G-protein-coupled receptors that appear to mediate ATP-induced Ca2+ mobilization in a large proportion of neurons in the myenteric plexus of the guinea-pig taenia coli (Kimball et al. 1996). P2X receptors are ligand-gated ion channels that are expressed by many myenteric neurons involved in descending inhibitory pathways in the small intestine of the guinea-pig (LePard et al. 1997). Although it is likely that ATP increased [Ca2+]i mobilization via P2Y receptors in our experiments, we cannot exclude the possibility that activation of P2X receptors also contributed to this response. Thus, neurons expressing PAR-1 and PAR-2 may express P2Y and P2X receptors.
Potential functions of PARs in the ENS
Thrombin and tryptase are probably the natural agonists of PAR-1 and PAR-2 in the ENS. Thrombin is generated in the circulation during injury and could have access to enteric neurons when there is plasma extravasation at sites of inflammation. Prothrombin may be expressed by enteric neurons because brain neurons express prothrombin (Weinstein et al. 1995). Tryptase-containing mast cells are resident in the human intestine and infiltrate inflamed tissues (Metcalfe et al. 1997). Tryptase is found in almost all human mast cells and is the major secretory protease of mast cells in the intestine (Metcalfe et al. 1997). We used guinea-pigs since in this species, as in humans, all mast cells that stain with Alcian Blue contain tryptase, and guinea-pigs may be a model species for examining the pathophysiological role of tryptase (McEuen et al. 1996). Mast cells from mice and rats are more heterogeneous with respect to their protease content, although they too express proteases with tryptic specificity. Thus, tryptase may have access to enteric neurons when mast cells degranulate during inflammation. Anatomical and functional experiments indicate that there is a close association between mast cells and intestinal nerves. Mast cells are in close proximity to SP-containing nerves in the normal and inflamed intestine (Stead et al. 1987). Stimulation of parasympathetic nerves induces mast cell degranulation and histamine release in the ileum (Bani-Sacchi et al. 1986), and mast cell activation alters electrical activity of enteric neurons in part through release of histamine (Frieling et al. 1994). Our observation that tryptase excites neurons through PAR-2 is a novel mechanism by which mast cells regulate neuronal activity.
Further experimentation is required to determine the effects of thrombin and tryptase on the function of the ENS and on regulation of the intestine. In the CNS, PAR-1 agonists induce neurite retraction (Suidan et al. 1992), are neurotoxic, and protect neurons from death induced by hypoglycaemia and oxidative stress (Smith-Swintosky et al. 1995; Vaughan et al. 1995). PAR-1 agonists act on brain astrocytes to induce characteristic shape changes (Beecher et al. 1994), stimulate proliferation (Perraud et al. 1987), release endothelin-1 and nerve growth factor (Ehrenreich et al. 1993; Neveu et al. 1993), inhibit expression of the metabotropic glutamate receptor (Miller et al. 1996), and protect from environmental stresses (Vaughan et al. 1995). PAR-2 agonists are toxic to hippocampal neurons (Smith-Swintosky et al. 1997). Similar effects of PAR-1 and PAR-2 agonists on myenteric neurons may have marked functional consequences in view of the important role of the ENS in the reflex regulation of intestinal motility, secretion and transport, and may contribute to the marked disturbances of intestinal function that are observed during trauma and inflammation.
We detected immunoreactive PAR-1 and PAR-2 on a large proportion of neurons expressing excitatory (SP) and inhibitory (VIP and NOS) motor transmitters. We also found that most myenteric neurons responding to PAR-1 or PAR-2 agonists also responded to ATP, an agonist of the P2Y and P2X purinoceptors, which may also participate in the regulation of intestinal motility (LePard et al. 1997). Excitation or damage of these neurons by thrombin or tryptase would dramatically impair intestinal motor function, and may partly explain the effects of PAR-1 and PAR-2 agonists on gastrointestinal motility (Saifeddine et al. 1996; Corvera et al. 1997). Proteolytic activation of submucosal neurons may account for altered secretion of the inflamed intestine.
In summary, we have demonstrated that PAR-1 and PAR-2 are expressed by a large proportion of neurons of the myenteric plexus of the guinea-pig intestine. Thrombin and mast cell tryptase regulate enteric neurons by triggering PAR-1 and PAR-2, which may contribute to motility disturbances during intestinal trauma and inflammation.
Acknowledgments
This work was supported by NIH grants DK39957, DK43207, NS21710 (N. W. B.), HL24136 (G. H. C.) and The Medical Research Council of Canada (M. D. H.). K. M. was supported by a C. J. Martin Fellowship of the National Health and Medical Research Council of Australia. We thank Dr J. Butterfield (Mayo Clinic) for HMC-1 cells, Dr R. Tidwell (University of North Carolina) for BABIM, Dr B. Coller (COR Therapeutics, South San Francisco) for the PAR-1 antibody, Dr J. H. Walsh (UCLA) for VIP antibodies (generated by the CURE/Gastroenteric Biology Center, Antibody/RIA Core, NIH grant DK41301) and Dr D. Bredt (UCSF) for the NOS antibody.
References
- Bani-Sacchi T, Barattini M, Bianchi S, Blandina P, Brunelleschi S, Fantozzi R, Mannaioni PF, Masini E. The release of histamine by parasympathetic stimulation in guinea-pig auricle and rat ileum. The Journal of Physiology. 1986;371:29–43. doi: 10.1113/jphysiol.1986.sp015960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecher KL, Andersen TT, Fenton JWN, Festoff BW. Thrombin receptor peptides induce shape change in neonatal murine astrocytes in culture. Journal of Neuroscience Research. 1994;37:108–115. doi: 10.1002/jnr.490370115. [DOI] [PubMed] [Google Scholar]
- Blackhart BD, Emilsson K, Nguyen D, Teng W, Martelli AJ, Nystedt S, Sundelin J, Scarborough RM. Ligand cross-reactivity within the protease-activated receptor family. Journal of Biological Chemistry. 1996;271:16466–16471. doi: 10.1074/jbc.271.28.16466. 10.1074/jbc.271.28.16466. [DOI] [PubMed] [Google Scholar]
- Bono F, Lamarche I, Herbert JM. Induction of vascular smooth muscle cell growth by selective activation of the proteinase activated receptor-2 (PAR-2) Biochemical and Biophysical Research Communications. 1997;241:762–764. doi: 10.1006/bbrc.1997.7847. [DOI] [PubMed] [Google Scholar]
- Brown JK, Tyler CL, Jones CA, Ruoss SJ, Hartmann T, Caughey GH. Tryptase, the dominant secretory granular protein in human mast cells, is a potent mitogen for cultured dog tracheal smooth muscle cells. Journal of Clinical Investigation. 1991;88:493–499. doi: 10.1165/ajrcmb.13.2.7626290. [DOI] [PubMed] [Google Scholar]
- Cairns JA, Walls AF. Mast cell tryptase is a mitogen for epithelial cells. Stimulation of IL-8 production and intracellular adhesion molecule-1 expression. Journal of Immunology. 1995;156:275–283. [PubMed] [Google Scholar]
- Cavanaugh KP, Gurwitz D, Cunningham DD, Bradshaw RA. Reciprocal modulation of astrocyte stellation by thrombin and protease nexin-1. Journal of Neurochemistry. 1990;54:1735–1743. doi: 10.1111/j.1471-4159.1990.tb01228.x. [DOI] [PubMed] [Google Scholar]
- Corvera CU, Dery O, Mcconalogue K, Böhm SK, Khitin LM, Caughey GH, Payan DG, Bunnett NW. Mast cell tryptase regulates rat colonic myocytes through proteinase-activated receptor 2. Journal of Clinical Investigation. 1997;100:1383–1393. doi: 10.1172/JCI119658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Déry O, Corvera CU, Steinhoff M, Bunnett NW. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. American Journal of Physiology. 1998;274:C1429–1452. doi: 10.1152/ajpcell.1998.274.6.C1429. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Costa T, Clouse KA, Pluta RM, Ogino Y, Coligan JE, Burd PR. Thrombin is a regulator of astrocytic endothelin-1. Brain Research. 1993;600:201–207. doi: 10.1016/0006-8993(93)91374-2. 10.1016/0006-8993(93)91374-2. [DOI] [PubMed] [Google Scholar]
- Frieling T, Palmer JM, Cooke HJ, Wood JD. Neuroimmune communication in the submucous plexus of guinea pig colon after infection with Trichinella spiralis. Gastroenterology. 1994;107:1602–1609. doi: 10.1016/0016-5085(94)90798-6. [DOI] [PubMed] [Google Scholar]
- Hollenberg MD, Saifeddine M, Al-Ani B, Kawabata A. Proteinase-activated receptors: structural requirements for activity, receptor cross-reactivity, and receptor selectivity of receptor-activating peptides. Canadian Journal of Physiology and Pharmacology. 1997;75:832–841. 10.1139/cjpp-75-7-832. [PubMed] [Google Scholar]
- Ishihara H, Connolly AJ, Zeng D, Kahn ML, Zheng YW, Timmons C, Tram T, Coughlin SR. Protease-activated receptor 3 is a second thrombin receptor in humans. Nature. 1997;386:502–506. doi: 10.1038/386502a0. 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- Kimball BC, Yule DI, Mulholland MW. Extracellular ATP mediates Ca2+ signaling in cultured myenteric neurons via a PLC-dependent mechanism. American Journal of Physiology. 1996;270:G587–593. doi: 10.1152/ajpgi.1996.270.4.G587. [DOI] [PubMed] [Google Scholar]
- Kong W, McConalogue K, Khitin LM, Hollenberg MD, Payan DG, Böhm SK, Bunnett NW. Luminal trypsin may regulate enterocytes through proteinase-activated receptor 2. Proceedings of the National Academy of Sciences of the USA. 1997;94:8884–8889. doi: 10.1073/pnas.94.16.8884. 10.1073/pnas.94.16.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepard KJ, Messori E, Galligan JJ. Purinergic fast excitatory postsynaptic potentials in myenteric neurons of guinea pig: distribution and pharmacology. Gastroenterology. 1997;113:1522–1534. doi: 10.1053/gast.1997.v113.pm9352854. [DOI] [PubMed] [Google Scholar]
- McConalogue K, Corvera CU, Gamp PD, Grady EF, Bunnett NW. Desensitization of the neurokinin-1 receptor (NK1-R) in neurons: effects of substance P on the distribution of NK1-R, Galphaq/11, G-protein receptor kinase-2/3, and beta-arrestin-1/2. Molecular Biology of the Cell. 1998;9:2305–2324. doi: 10.1091/mbc.9.8.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEuen AR, He S, Brander ML, Walls AF. Guinea pig lung tryptase. Localisation to mast cells and characterisation of the partially purified enzyme. Biochemical Pharmacology. 1996;52:331–340. doi: 10.1016/0006-2952(96)00211-0. 10.1016/0006-2952(96)00211-0. [DOI] [PubMed] [Google Scholar]
- Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiological Reviews. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- Miller S, Sehati N, Romano C, Cotman CW. Exposure of astrocytes to thrombin reduces levels of the metabotropic glutamate receptor mGluR5. Journal of Neurochemistry. 1996;67:1435–1447. doi: 10.1046/j.1471-4159.1996.67041435.x. [DOI] [PubMed] [Google Scholar]
- Mirza H, Schmidt VA, Derian CK, Jesty J, Bahou WF. Mitogenic responses mediated through the proteinase-activated receptor-2 are induced by expressed forms of mast cell alpha- or beta-tryptases. Blood. 1997;90:3914–3922. [PubMed] [Google Scholar]
- Mirza H, Yatsula V, Bahou WF. The proteinase activated receptor-2 (PAR-2) mediates mitogenic responses in human vascular endothelial cells. Journal of Clinical Investigation. 1996;97:1705–1714. doi: 10.1172/JCI118597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molino M, Barnathan ES, Numerof R, Clark J, Dreyer M, Cumashi A, Hoxie J, Schechter N, Woolkalis M, Brass LF. Interactions of mast cell tryptase with thrombin receptors and PAR-2. Journal of Biological Chemistry. 1997;272:4043–4049. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- Neveu I, Jehan F, Jandrot-Perrus M, Wion D, Brachet P. Enhancement of the synthesis and secretion of nerve growth factor in primary cultures of glial cells by proteases: a possible involvement of thrombin. Journal of Neurochemistry. 1993;60:858–867. doi: 10.1111/j.1471-4159.1993.tb03230.x. [DOI] [PubMed] [Google Scholar]
- Nishino A, Suzuki M, Ohtani H, Motohashi O, Umezawa K, Nagura H, Yoshimoto T. Thrombin may contribute to the pathophysiology of central nervous system injury. Journal of Neurotrauma. 1993;10:167–179. doi: 10.1089/neu.1993.10.167. [DOI] [PubMed] [Google Scholar]
- Norton KJ, Scarborough RM, Kutok JL, Escobedo MA, Nannizzi L, Coller BS. Immunologic analysis of the cloned platelet thrombin receptor activation mechanism: evidence supporting receptor cleavage, release of the N-terminal peptide, and insertion of the tethered ligand into a protected environment. Blood. 1993;82:2125–2136. [PubMed] [Google Scholar]
- Nystedt S, Emilsson K, Wahlestedt C, Sundelin J. Molecular cloning of a potential proteinase activated receptor. Proceedings of the National Academy of Sciences of the USA. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perraud F, Besnard F, Sensenbrenner M, Labourdette G. Thrombin is a potent mitogen for rat astroblasts but not for oligodendroblasts and neuroblasts in primary culture. International Journal of Developmental Neuroscience. 1987;5:181–188. doi: 10.1016/0736-5748(87)90028-1. 10.1016/0736-5748(87)90028-1. [DOI] [PubMed] [Google Scholar]
- Ruoss S, Hartmann T, Caughey G. Mast cell tryptase is a mitogen for cultured fibroblasts. Journal of Clinical Investigation. 1991;88:493–499. doi: 10.1172/JCI115330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saifeddine M, Al-Ani B, Cheng C, Wang L, Hollenberg M. Rat proteinase-activated receptor-2 (PAR-2): cDNA sequence and activity of receptor-derived peptides and in gastric and vascular tissue. British Journal of Pharmacology. 1996;118:521–530. doi: 10.1111/j.1476-5381.1996.tb15433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz L, Yunginger J, Miller J, Bokhari R, Dull D. Time course and appearance and disappearance of human mast cell tryptase in the circulation after anaphylaxis. Journal of Clinical Investigation. 1989;83:1551–1555. doi: 10.1172/JCI114051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Swintosky V, Cheo-Isaacs C, D'Andrea M, Santulli R, Darrow A, Andrade-Gordon P. Protease-activated receptor-2 (PAR-2) is present in the rat hippocampus and is associated with neurodegeneration. Journal of Neurochemistry. 1997;69:1890–1896. doi: 10.1046/j.1471-4159.1997.69051890.x. [DOI] [PubMed] [Google Scholar]
- Smith-Swintosky VL, Zimmer S, Fenton JWN, Mattson MP. Protease nexin-1 and thrombin modulate neuronal Ca2+ homeostasis and sensitivity to glucose deprivation-induced injury. Journal of Neuroscience. 1995;15:5840–5850. doi: 10.1523/JNEUROSCI.15-08-05840.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead RH, Tomioka M, Quinonez G, Simon GT, Felten SY, Bienenstock J. Intestinal mucosal mast cells in normal and nematode-infected rat intestines are in intimate contact with peptidergic nerves. Proceedings of the National Academy of Sciences of the USA. 1987;84:2975–2979. doi: 10.1073/pnas.84.9.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suidan HS, Stone SR, Hemmings BA, Monard D. Thrombin causes neurite retraction in neuronal cells through activation of cell surface receptors. Neuron. 1992;8:363–375. doi: 10.1016/0896-6273(92)90302-t. [DOI] [PubMed] [Google Scholar]
- Vaughan PJ, Pike CJ, Cotman CW, Cunningham DD. Thrombin receptor activation protects neurons and astrocytes from cell death produced by environmental insults. Journal of Neuroscience. 1995;15:5389–5401. doi: 10.1523/JNEUROSCI.15-07-05389.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnolle N, Macnaughton WK, Al-Ani B, Saifeddine M, Wallace JL, Hollenberg MD. Proteinase-activated receptor 2 (PAR2)-activating peptides: identification of a receptor distinct from PAR2 that regulates intestinal transport. Proceedings of the National Academy of Sciences of the USA. 1998;95:7766–7771. doi: 10.1073/pnas.95.13.7766. 10.1073/pnas.95.13.7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Weinstein JR, Gold SJ, Cunningham DD, Gall CM. Cellular localization of thrombin receptor mRNA in rat brain: expression by mesencephalic dopaminergic neurons and codistribution with prothrombin mRNA. Journal of Neuroscience. 1995;15:2906–2919. doi: 10.1523/JNEUROSCI.15-04-02906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Andersen H, Whitmore TE, Presnell SR, Yee DP, Ching A, Gilbert T, Davie EW, Foster DC. Cloning and characterization of human protease-activated receptor 4. Proceedings of the National Academy of Sciences of the USA. 1998;95:6642–6646. doi: 10.1073/pnas.95.12.6642. 10.1073/pnas.95.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]


