Abstract
We have used fluorescence confocal laser scanning microscopy to attain the three-dimensional (3-D) microstructure of perimysial collagen fibres over the range of sarcomere lengths (1.9–2.3 μm) in which passive force of cardiac muscle increases steeply.
A uniaxial muscle preparation (right ventricular trabecula of rat) was used so that the 3-D collagen configuration could be readily related to sarcomere length. Transmission electron microscopy showed that these preparations were structurally homologous to ventricular wall muscle.
Trabeculae were mounted on the stage of an inverted microscope and fixed at various sarcomere lengths. After a trabecula was stained with the fluorophore Sirius Red F3BA and embedded in resin, sequential optical sectioning enabled 3-D reconstruction of its perimysial collagen fibres. The area fraction of these fibres, determined from the cross-sections of seven trabeculae, was 10.5 ± 3.9 % (means ± s.d.).
The reconstructed 3-D images show that perimysial collagen fibres are wavy (as distinct from coiled) cords which straighten considerably as the sarcomere length is increased from 1.85 ± 0.06 μm (near-resting length) to 2.3 ± 0.04 μm (means ± s.d., n = 4). These observations are consistent with the notion that the straightening of these fibres is responsible for limiting extension of the cardiac sarcomere to a length of ≈2.3 μm.
When cardiac muscle is stretched from its resting sarcomere length of ∼1.9 μm, passive force increases steeply such that extension beyond a sarcomere length of ∼2.3 μm is prevented (Pollack & Huntsman, 1974; ter Keurs et al. 1980; Kentish et al. 1986; de Tombe & ter Keurs, 1992). This elastic behaviour of passive cardiac muscle restricts the myocytes to operating over a sarcomere length range in which Ca2+-activated force increases (ter Keurs et al. 1980; Kentish et al. 1986). The relative contributions of intra- and extracellular structures to this elastic property have been deduced by comparing the passive force-sarcomere length relations of rat ventricular muscle preparations which are collagen free (myocytes) and collagen intact (trabeculae) (Granzier & Irving, 1995). That work showed that, at sarcomere lengths less than 2.1 μm, the sarcomeric protein titin (also called connectin) is the major contributor to elasticity whereas, at longer lengths, fibrillar collagen becomes the predominant source. Hence, titin is the principal structure dictating the mechanical properties of passive cardiac muscle over most of its physiological working range.
Titin is an impressively large protein (∼3 MDa) which extends from the Z-line to the M-line of the sarcomere (for review, see Labeit et al. 1997). The molecular mechanism by which titin exhibits elasticity upon sarcomere extension involves the unfolding and straightening of domains in its I-band region (Granzier et al. 1996; Kellermayer et al. 1997; Tskhovrebova et al. 1997). In addition to acting as a molecular spring, titin may also contribute to the stiffness of the sarcomeres by interacting with the thin filament (Linke et al. 1997). Furthermore, Stuyvers et al. (1997) have recently proposed that the inverse relation between diastolic intracellular [Ca2+] ([Ca2+] < 200 nm) and sarcomere stiffness may be due to a Ca2+-dependent interaction of titin with the thin filament.
The mechanism by which fibrillar collagen contributes to the elastic properties of passive cardiac muscle is not as well understood as it is for titin. In an elaborate study of rat papillary muscle, Robinson et al. (1988) have shown that large perimysial collagen fibres, up to 10 μm diameter, are arranged parallel to the sarcomeres in relative abundance (1 fibre: several myocytes). On the basis of scanning electron micrographs, these perimysial fibres appeared to be configured as helical coils. It follows that stretching of cardiac muscle in the direction of the myocytes would be expected to produce straightening of these putatively coiled perimysial fibres. This would explain the observation of Gay & Johnson (1967) that perimysial collagen fibres, distinguishable in living and thin ventricular trabeculae of rabbit using light microscopy, appeared wavy at near slack length and could be reversibly stretched to a straight configuration.
The object of the present study was to determine whether perimysial collagen fibres are transformed from a coiled (or other) configuration to a straightened structure over the sarcomere length range in which passive force of cardiac muscle increases steeply. We used fluorescence confocal microscopy to reconstruct the three-dimensional configuration of perimysial collagen fibres (stained with Sirius Red F3BA) in rat cardiac trabeculae fixed at near-resting and extended sarcomere lengths.
METHODS
The methods (approved by the Animal Ethics Committee of the University of Auckland) for anaesthetizing rats and isolating trabeculae have been previously described (Hanley & Loiselle, 1998). In brief, Wistar rats (weight, 200–300 g) were anaesthetized by intraperitoneal injection of 3.5–4.5 ml kg−1 of a warmed (37–38°C) mixture containing (mg ml−1): 0.079 fentanyl citrate, 2.5 fluanisone and 1.25 midazolam hydrochloride. Anaesthesia-induced respiratory depression was countered by providing intermittent positive pressure ventilation with 100 % oxygen. In addition, an intravenous load of 2–3 ml of warmed plasma volume expander (Haemaccel, Behringwerke AG, Marburg, Germany) was slowly infused via a tail vein cannula. After the thorax was opened, the heart was carefully excised and immersed in chilled (4°C) dissection solution to induce arrest. Within 30 s of excising the heart, the aorta was perfused with physiological salt (modified Krebs-Henseleit) solution containing (mM): 118 NaCl, 13.82 KCl, 0.25 CaCl2, 1.18 MgSO4, 1.18 KH2PO4, 24.8 NaHCO3, 20 2,3-butanedione monoxime (BDM) and 10 D-glucose. After equilibration with 95 % O2-5 % CO2, the pH of this solution was 7.4.
Thin trabeculae (width, < 200 μm) were dissected from the right ventricle and transferred to a miniature bath (volume, 450 μl) located on the stage of an inverted microscope (Nikon Diaphot 300). The preparation was then mounted between a force transducer (AE801, SensoNor, Horten, Norway) and a micromanipulator. After mounting, the preparation was superfused at a rate of 8–9 ml min−1 with a BDM-free solution which contained 5 mM K+.
Passive force-sarcomere length determination
The passive force-sarcomere length relation was determined in five trabeculae. Sarcomere length (SL) was readily measurable after a central portion of the trabecula was magnified × 400 (using a × 40 Nikon objective), viewed by a charge-coupled device (CCD) camera and displayed on a video monitor. To ensure that no cross-bridges were activated during this series of experiments, the preparation was superfused with Ca2+-free solution, containing 1 mM EGTA.
Staining and resin embedding
The sarcomere length of trabeculae was increased by ∼0.1 μm before fixing, dehydration and resin embedding in order to account for the ∼5 % shrinkage associated with this process. We obtained four resin-embedded trabeculae at SL 1.85 ± 0.06 μm and four at SL 2.3 ± 0.04 μm (means ±s.d.). Trabeculae were fixed by rapidly exchanging physiological salt solution for Bouin's (fixative) solution. This fixative was used since it provides the acidic environment required for the staining of collagen with Sirius Red F3BA (Young et al. 1998). After 30 min, the ends of the fixed trabecula were carefully detached from the force transducer assembly and fixed support and the preparation stored in Bouin's solution. The preparation was stained with the collagen-specific fluorophore Sirius Red F3BA by immersing it in Picrosirius Red (0.1 % w/v Sirius Red F3BA in saturated picric acid) for at least 2 h. It was dehydrated by sequential immersions in increasing concentrations of ethanol, followed by 100 % propylene oxide. Subsequently, the preparation was impregnated with a freshly prepared monomer (Agar 100 resin) which was polymerized by incubation at 60°C for 48 h. A Reichert-Jung ultramicrotome was used to trim the resin block to within 10 μm of the specimen. The resin block holder could be readily interchanged between the ultramicrotome and a custom-made Perspex receptacle located on the stage of the confocal microscope. The Perspex receptacle was specially designed so that the specimen was positioned coplanar with the microscope stage.
Confocal microscopy
Resin-embedded trabeculae were imaged using a confocal (argon-krypton) laser scanning microscope (Leica TCS 4D, Heidelberg, Germany). Confocal images of a trabecula were obtained via a × 63 (1.4 NA) or × 100 (1.3 NA) Leica oil immersion objective lens when the preparation was excited with the 568 nm emission line of the laser and fluorescence detected at > 590 nm. The refractive indices of the polymerized resin and immersion oil have previously been measured and found to be well matched (Young et al. 1998). The pinhole size was optimized for the particular objective lens used and eight line averages were performed.
When using the × 63 objective lens, a 512 × 512 pixel image matrix was obtained for a 158.74 μm × 158.74 μm field of view (giving 0.31 μm per pixel) and optical sections were obtained at 0.31 μm intervals. This imaging protocol provided isometric voxels (with 0.31 μm sides), which optimized the execution of image processing by the volume-rendering software employed (VoxelView, Vital Images, Fairfield, IA, USA). In selected experiments, a × 100 objective lens was used to obtain images of single collagen fibres.
Area fraction of collagen
Seven trabeculae were transversely sectioned so that the fractional area of collagen fibres in the preparation could be determined. Confocal images of the cross-sections were captured by the microscope host computer and image analysis software (NIH Image, version 1.61) was used to measure selected pixel areas. Specifically, the area fraction of collagen was determined by dividing the total pixel area of collagen by the pixel area of the cross-section. It should be noted that the capillaries were collapsed in the (immersion-fixed) trabeculae used to measure area fraction attributable to collagen. For comparison, the mean area fraction of collagen was similarly determined from the mid-wall region of a 2.43 mm3 volume of rat left ventricular free wall. This volume was reconstructed in 3-D (with a resolution of 0.31–1.56 μm per side of a cubic voxel) using extended confocal microscopy (Young et al. 1998). For calculation of mean area fraction, eleven optical slices with a resolution of 0.31 μm pixel−1 were taken transverse to the myocyte direction at 1.55 μm intervals. The area occupied by blood vessels was excluded.
Sarcomere length determination
Sarcomere striations were readily visible in trabeculae stained with Sirius Red F3BA and resin embedded. A Nikon Fluor × 40 (1.3 NA) or Leica × 100 (1.25 NA) oil immersion objective lens was used to image a central portion of the trabecula. A Sony CCD/RGB camera (Tokyo, Japan) and Power Macintosh computer with a video frame grabber were used to digitize the image and mean sarcomere length was then determined using image analysis software (NIH Image, version 1.61). A calibration slide with 10 μm spaced stripes was used to calibrate the measurement system, the resolution of which was 4.88 pixels μm−1 using the × 40 objective lens.
Transmission electron microscopy
The cellular composition of right ventricular trabeculae, dissected from glutaraldehyde-fixed rat hearts, was examined using transmission electron microscopy. Glutaraldehyde was used as the fixative since it provides better preservation of cellular ultrastructure than Bouin's solution. Eight hearts were attached to a vertically mounted cannula via the aorta and perfused with 2.5 % glutaraldehyde (electron microscopy grade) in phosphate buffer. After fixation, hearts were transferred to a dissection dish and a suitable trabecula, if present, was excised from the right ventricle. Four suitable trabeculae were obtained. After overnight storage in fixative, trabeculae were post-fixed in 1 % osmium tetroxide and then dehydrated in increasing concentrations of ethanol before being embedded in Agar 100 resin.
Ultrathin (∼90 nm) cross-sections of a central portion of the resin-embedded trabecula (see Fig. 1A) were cut using a diamond knife. Before being viewed in an electron microscope, the sections were stained with tannic acid to enhance visualization of collagen. Thicker sections (∼10 μm) were cut for light microscopic examination. The latter sections were mounted on a slide, stained with Toluidine Blue and viewed on a video monitor via a Nikon Fluor × 40 (1.3 NA) oil immersion objective lens and Sony CCD/RGB camera. Video frames of these images were digitized for later analysis. An image of a calibration slide was used to scale these images.
Figure 1. Ultrastructure of rat cardiac trabeculae.
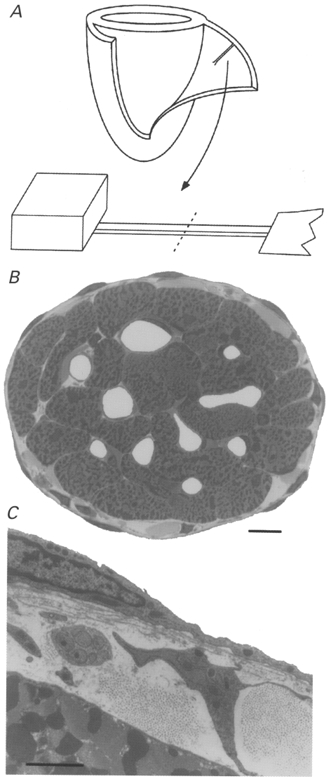
A, schematic diagram showing a suitable trabecula before and after its isolation from the right ventricular free wall of a glutaraldehyde-fixed heart (only ventricles depicted). The dashed line indicates where the trabecula was transversely sectioned after resin embedding. B, low-power image, obtained by light microscopy, of the cross-section of a trabecula. Scale bar, 10 μm. C, electron micrograph of the periphery of the above trabecula. Note the presence of a fibroblast, a bundle of nerve axons and fibrillar collagen in the space between the endothelium and a myocyte. Scale bar, 2 μm.
Statistical analyses
An analysis of variance (ANOVA) for repeated measures was used to determine statistical significance at the 0.95 level of confidence.
RESULTS
Ultrastructure of right ventricular trabeculae
The ultrastructure of four trabeculae, dissected from glutaraldehyde-fixed rat hearts, was examined. Figure 1B shows a low-power image, obtained by light microscopy, of a transverse section of a typical trabecula. It can be seen that the cross-sectional area of the preparation is chiefly occupied by cardiac myocytes and, in this particular case, approximately five cells span its 100 μm diameter. Note for comparison that a single cell of mammalian skeletal muscle is ∼50-100 μm in diameter. The capillaries (seen as white holes) are open since the heart from which this trabecula was dissected was fixed at 10 kPa perfusion pressure. An electron micrograph of the periphery of this trabecula (Fig. 1C) illustrates the multicellular nature of the preparation and the diffusion barrier imposed between the outer rim of cardiac cells and endocardial environment. Note the presence of a fibroblast, fibrillar collagen, a bundle of nerve axons and endothelium.
Passive force-sarcomere length relation
In agreement with previous work, we found that the passive force-sarcomere length relation of trabeculae increased steeply when the resting sarcomere length was increased from 1.94 ± 0.06 μm to 2.3 μm (n = 5). The striking feature of the relation shown in Fig. 2 is the steepness with which passive force increases as the muscle is stretched to a sarcomere length of 2.3 μm. We used confocal microscopy to test whether this steep increase in passive force was associated with the straightening of coiled (or wavy) perimysial collagen fibres.
Figure 2. Relation between passive force and sarcomere length of ventricular trabeculae.
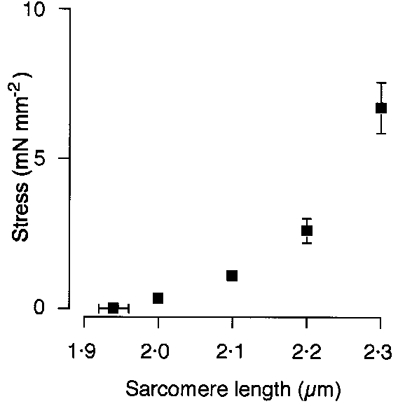
Preparations were progressively stretched so that sarcomere length increased from its resting value (1.94 ± 0.02 μm, mean ±s.e.m., n = 5) to 2.0, 2.1, 2.2 and 2.3 μm.
Confocal microscopy
The area fraction of collagen fibres was determined for seven transversely sectioned trabeculae using fluorescence confocal microscopy. The fibres were generally round or ellipsoid in cross-section and ranged in diameter from less than 1 μm to 11 μm. Although we recognize that fluorescence confocal microscopy tends to exaggerate the dimensions of collagen (by around 20 % when compared with transmission electron microscopy (Young et al. 1998)), the area fraction was nevertheless surprisingly high (10.5 ± 3.9 %, mean ±s.d.; range, 6.1–16.4 %; n = 7 trabeculae). In the mid-wall of the left ventricle of the whole heart, the estimate was only slightly lower: 7.8 ± 0.9 % (mean ±s.d.; n = 11 optical slices). Whereas these values exceed the 3–6 % range reported by Weber (1989) and MacKenna et al. (1994, 1996), they are substantially less than the mean value of 18 % reported by Whittaker et al. (1994) for the same tissues.
Serial optical sectioning and volume rendering software was used to obtain three-dimensional images of perimysial collagen fibres in trabeculae fixed at either near-resting or extended sarcomere lengths. The 3-D structure of perimysial collagen fibres at near-resting sarcomere length was examined in four trabeculae (SL, 1.85 ± 0.06 μm, n = 4). Figure 3 shows a 3-D image of a perimysial collagen fibre in a trabecula fixed at a sarcomere length of 1.81 μm. When the path of the centre of this fibre was followed in 3-D, it appeared to be wavy in a planar fashion, rather than coiled as proposed by Robinson et al. (1988). In order to test this further, we obtained global co-ordinates (X, Y and Z) of the centre of this fibre at 0.78 μm intervals along its length in order to display orthogonal views of its path. These are shown on the right side of Fig. 3 where the Z and Y co-ordinates of the centreline of the fibre are separately plotted as a function of X. It can be clearly seen that the fibre does not follow a helical path.
Figure 3. 3-D reconstruction of a typical perimysial collagen fibre in a ventricular trabecula fixed at near-resting sarcomere length.
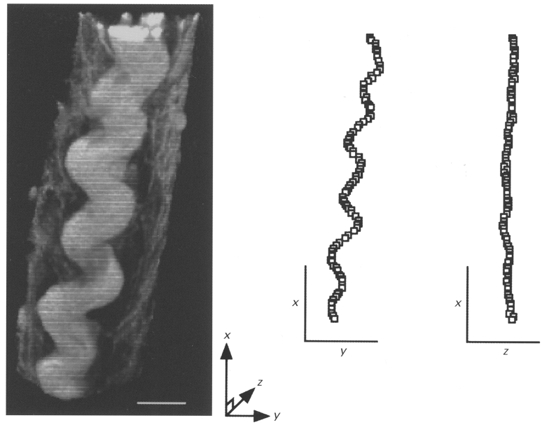
The trabecula was fixed at a sarcomere length of 1.81 μm and stained with a collagen-specific fluorophore (Sirius Red F3BA). Confocal laser scanning microscopy (via a × 100 objective lens) was used to obtain the 30 serial optical images (taken at 0.39 μm intervals) used in this reconstruction. 3-D analysis of this fibre showed that it was wavy in a planar fashion rather than coiled. White scale bar, 10 μm. On the right, the centre of the fibre has been plotted in orthogonal planes. Note that the fibre is wavy in the X-Y plane (viewed from above) whereas it is not in the orthogonal X-Z (or profile) view. The axes serve as scale bars and are each 20 μm.
We examined the three-dimensional morphology of at least three perimysial collagen fibres in each of the four trabeculae fixed at near-resting sarcomere length. As has previously been reported for rat ventricular muscle (Robinson et al. 1988; MacKenna et al. 1996; Young et al. 1998), the collagen fibres we observed were typically branched (for example, this was the case for the upper part of the fibre shown in Fig. 3) and were parallel to the long axis of the myocytes. In further accord with the observations of Robinson et al. (1988), there was considerable variation in the morphology along the length of a fibre and amongst fibres. However, on no occasion did we see any coiled collagen fibres. Absence of coiling obtained whether the fibre observed was on the surface of the trabecula or within it.
We tested whether perimysial collagen fibres were straightened in stretched trabeculae. Indeed, in trabeculae fixed at long sarcomere lengths (2.3 ± 0.04 μm; n = 4) all perimysial collagen fibres were considerably straightened in comparison with those fixed at short sarcomere lengths. This is clearly illustrated in Fig. 4, which shows a 3-D image of perimysial collagen fibres in a trabecula fixed at a sarcomere length of 2.27 μm. When these fibres were followed in 3-D, no waviness was observed in either the X-Z or X-Y planes. Note that small kinks (not breaks) are evident in Fig. 4. These kinks were observed in most of the straightened fibres we examined and could reflect damage to the fibres as a consequence of overstretching the preparation prior to fixation. Whereas similar kinks may be expected from the straightening of a structure configured as a helical coil (Robinson et al. 1988), we think it more likely that these kinks reflect tethering of the perimysial collagen fibres to other components of the extracellular connective tissue matrix (see Robinson et al. 1983, 1988).
Figure 4. 3-D reconstruction of perimysial collagen fibres in a ventricular trabecula fixed at an extended sarcomere length.
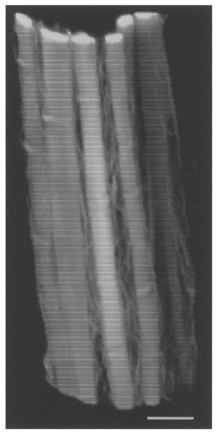
The preparation was fixed at a sarcomere length of 2.27 μm. Confocal laser scanning microscopy (via a × 63 objective lens) was used to obtain the fifty optical sections (taken at 0.31 μm intervals) used in this reconstruction. Note that some of the fibres show small kinks. Scale bar, 10 μm.
DISCUSSION
The object of our present study was to determine how the three-dimensional microstructure of perimysial collagen fibres changed over the range of sarcomere lengths in which cardiac muscle exhibits a steep increase in passive force. As a first step, we examined cross-sections of trabeculae using light- and transmission electron microscopy in order to characterize the cellular composition of these commonly used preparations.
Ultrastructure of trabeculae
We found trabeculae to be structurally homologous to muscle dissected from the ventricular wall of the heart (recently examined in detail by Young et al. 1998). That is, like ventricular wall muscle, the volume of these preparations is chiefly occupied by myocytes which are accompanied by parallel perimysial collagen fibres (Fig. 1B). The arrangement of myocytes is clearly much more complex in the ventricular wall where interconnected sheets of four to six cells' thickness give rise to a complex laminar structure (LeGrice et al. 1995; Young et al. 1998). Nevertheless, within a sheet, the perimysial collagen fibres are generally parallel to the direction of the myocytes and are, therefore, in a position to restrain sarcomere lengthening.
Passive force-sarcomere length relation
As expected from previous work, the passive force increased steeply as the length of sarcomeres was increased from ∼1.9 μm (slack length) to 2.3 μm. We found that when rat cardiac trabeculae are stretched to a sarcomere length of 2.3 μm, passive force increases to around 7 mN mm−2, a value comparable to that observed by ter Keurs et al. (1980). That passive force of cardiac muscle rises steeply when sarcomeres are extended to around 2.3 μm is functionally important. At this sarcomere length, both interfilament lattice spacing and the overlap between actin and myosin are optimal for force development (Fitzsimons & Moss, 1998; Solaro & Rarick, 1998).
If the sarcomeres were to lengthen beyond ∼2.3 μm then the overlap between actin and myosin would diminish, thereby reducing the number of possible cross-bridges that could be formed. Hence, it is reasonable to infer that one of the major functions of perimysial collagen fibres (and the epimysium; see Robinson et al. 1983) in the heart is to constrain the myocytes to operate on the ascending limb of the force-sarcomere length relation. It should be noted, however, that at lower sarcomere lengths (< 2.1 μm), the passive mechanical properties of cardiac muscle are dominated not by perimysial collagen fibres but, rather, by the sarcomeric protein titin (see Introduction).
In contrast to cardiac muscle, passive force in skeletal muscle is negligible until the sarcomere length is increased to ∼3 μm (Gordon et al. 1966; ter Keurs et al. 1984). Two explanations can be put forward to explain this difference. First, titin-based passive force, at any given sarcomere length, is less in skeletal muscle than in cardiac muscle because its titin isoform possesses a longer elastic (I-band) region (Granzier & Irving, 1995; Labeit & Kolmerer, 1995; Labeit et al. 1997). Second, perimysial collagen fibres of skeletal muscle generally run across (rather than parallel to) the muscle cells (Borg & Caulfield, 1980; Purslow, 1989) and, thus, further stretch is required before these fibres are both axially aligned and straightened in the direction of the myocytes.
Microstructure of perimysial collagen fibres
At near-resting sarcomere lengths (∼1.9 μm), we found that the perimysial collagen fibres in rat cardiac trabeculae are tortuous as has previously been observed in various rat heart muscle preparations (Robinson et al. 1988; MacKenna et al. 1996). In some two-dimensional views of the three-dimensional data, the perimysial fibres appeared coiled (see Fig. 3). However, when these fibres were followed axially in three dimensions, the fibres were seen to have a wavy rather than a coiled appearance. A similar configuration of collagen to that which we observed in trabeculae has been observed in muscle from the rat left ventricular wall (I. J. LeGrice & A. A. Young, unpublished observations). Furthermore, in cross-sectional views, we found that these fibres were cord-like (generally round in cross-section) rather than ribbon-like. We speculate that this cord-like structure prevents perimysial collagen fibres from buckling during sarcomere shortening.
Although we did not see the coiled perimysial fibres described by Robinson et al. (1988), perimysial collagen fibres will exhibit elastic properties whether configured as a coil or as a planar wave (sinusoid), as has been extensively discussed and modelled by MacKenna et al. (1997). Moreover, we provide two lines of evidence that these fibres are the structural correlate of the parallel elastic element in cardiac muscle that constrains sarcomere lengthening. First, these fibres are present in relative abundance. Of the total area fraction of right ventricular trabeculae and left ventricular mid-wall, perimysial collagen fibres account, respectively, for 10.5 % and 7.8 % - values which fall in the middle of the wide range (3–18 %) reported in the literature (Weber, 1989; Whittaker et al. 1994; MacKenna et al. 1996). Second, over the range of sarcomere lengths from ∼1.85 to ∼2.3 μm, perimysial collagen fibres are transformed from a wavy structure to a straightened one. In accord with our work, MacKenna et al. (1996) have shown in the whole heart that perimysial collagen fibres become less tortuous as the left ventricle is passively distended above a mean sarcomere length of ∼1.8 to ∼1.9 μm. It should be noted that once straightened, fibrillar collagen is very resistant to stretch. For example, Sasaki & Odajima (1996) have recently shown that type I collagen, the major component of fibrillar collagen in the heart, has a modulus of elasticity of ∼2.9 GPa.
Conclusion
Large (up to 11 μm diameter) perimysial collagen fibres are transformed from a wavy (as distinct from coiled) to a straightened configuration over the sarcomere length range in which cardiac muscle exhibits a steep increase in passive force. We conclude that the straightening of perimysial collagen fibres restricts cardiac sarcomere length to an upper limit of ∼2.3 μm.
Acknowledgments
This work was supported by grants from the Health Research Council of New Zealand and the National Heart Foundation of New Zealand. P. J. H. was the recipient of a Health Research Council of New Zealand Postgraduate Scholarship.
References
- Borg TK, Caulfield JB. Morphology of connective tissue in skeletal muscle. Tissue and Cell. 1980;12:197–207. doi: 10.1016/0040-8166(80)90061-0. [DOI] [PubMed] [Google Scholar]
- de Tombe PP, ter Keurs HEDJ. An internal viscous element limits unloaded velocity of sarcomere shortening in rat myocardium. The Journal of Physiology. 1992;454:619–642. doi: 10.1113/jphysiol.1992.sp019283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimons DP, Moss RL. Strong binding of myosin modulates length-dependent Ca2+ activation of rat ventricular myocytes. Circulation Research. 1998;83:602–607. doi: 10.1161/01.res.83.6.602. [DOI] [PubMed] [Google Scholar]
- Gay WA, Johnson EA. An anatomical evaluation of the myocardial length-tension diagram. Circulation Research. 1967;21:33–43. doi: 10.1161/01.res.21.1.33. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Huxley AF, Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. The Journal of Physiology. 1966;184:170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzier H, Helmes M, Trombitás K. Nonuniform elasticity of titin in cardiac myocytes: a study using immunoelectron microscopy and cellular mechanics. Biophysical Journal. 1996;70:430–442. doi: 10.1016/S0006-3495(96)79586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzier HL, Irving TC. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophysical Journal. 1995;68:1027–1044. doi: 10.1016/S0006-3495(95)80278-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley PJ, Loiselle DS. Mechanisms of force inhibition by halothane and isoflurane in intact rat cardiac muscle. The Journal of Physiology. 1998;506:231–244. doi: 10.1111/j.1469-7793.1998.231bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermayer MSZ, Smith SB, Granzier HL, Bustamante C. Folding-unfolding transitions in single titin molecules characterized with laser tweezers. Science. 1997;276:1112–1116. doi: 10.1126/science.276.5315.1112. 10.1126/science.276.5315.1112. [DOI] [PubMed] [Google Scholar]
- Kentish JC, ter Keurs HEDJ, Ricciardi L, Bucx JJJ, Noble MIM. Comparison between the sarcomere length-force relations of intact and skinned trabeculae from rat right ventricle. Circulation Research. 1986;58:755–768. doi: 10.1161/01.res.58.6.755. [DOI] [PubMed] [Google Scholar]
- Labeit S, Kolmerer B. Titins: giant proteins in charge of muscle ultrastructure and elasticity. Science. 1995;270:293–296. doi: 10.1126/science.270.5234.293. [DOI] [PubMed] [Google Scholar]
- Labeit S, Kolmerer B, Linke WA. The giant protein titin. Emerging roles in physiology and pathophysiology. Circulation Research. 1997;80:290–294. doi: 10.1161/01.res.80.2.290. [DOI] [PubMed] [Google Scholar]
- LeGrice IJ, Smaill BH, Chai LZ, Edgar SG, Gavin JB, Hunter PJ. Laminar structure of the heart: ventricular myocyte arrangement and connective tissue architecture in the dog. American Journal of Physiology. 1995;269:H571–582. doi: 10.1152/ajpheart.1995.269.2.H571. [DOI] [PubMed] [Google Scholar]
- Linke WA, Ivemeyer M, Labeit S, Hinssen H, Rüegg JC, Gautel M. Actin-titin interaction in cardiac myofibrils: probing a physiological role. Biophysical Journal. 1997;73:905–919. doi: 10.1016/S0006-3495(97)78123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenna DA, Omens JH, Covell JW. Left ventricular perimysial collagen fibers uncoil rather than stretch during diastolic filling. Basic Research in Cardiology. 1996;91:111–122. doi: 10.1007/BF00799683. [DOI] [PubMed] [Google Scholar]
- MacKenna DA, Omens JH, McCulloch AD, Covell JW. Contribution of collagen matrix to passive left ventricular mechanics in isolated rat hearts. American Journal of Physiology. 1994;266:H1007–1018. doi: 10.1152/ajpheart.1994.266.3.H1007. [DOI] [PubMed] [Google Scholar]
- MacKenna DA, Vaplon SM, McCulloch AD. Microstructural model of perimysial collagen fibers for resting myocardial mechanics during ventricular filling. American Journal of Physiology. 1997;273:H1576–1586. doi: 10.1152/ajpheart.1997.273.3.H1576. [DOI] [PubMed] [Google Scholar]
- Pollack GH, Huntsman LL. Sarcomere length-active force relations in living mammalian cardiac muscle. American Journal of Physiology. 1974;227:383–389. doi: 10.1152/ajplegacy.1974.227.2.383. [DOI] [PubMed] [Google Scholar]
- Purslow PP. Strain-induced reorientation of an intramuscular connective tissue network: implications for passive muscle elasticity. Journal of Biomechanics. 1989;22:21–31. doi: 10.1016/0021-9290(89)90181-4. 10.1016/0021-9290(89)90181-4. [DOI] [PubMed] [Google Scholar]
- Robinson TF, Cohen-Gould L, Factor SM. Skeletal framework of mammalian heart muscle. Arrangement of inter- and pericellular connective tissue structures. Laboratory Investigation. 1983;49:482–498. [PubMed] [Google Scholar]
- Robinson TF, Geraci MA, Sonnenblick EH, Factor SM. Coiled perimysial fibers of papillary muscle in rat heart: morphology, distribution, and changes in configuration. Circulation Research. 1988;63:577–592. doi: 10.1161/01.res.63.3.577. [DOI] [PubMed] [Google Scholar]
- Sasaki N, Odajima S. Stress-strain curve and Young's modulus of a collagen molecule as determined by the X-ray diffraction technique. Journal of Biomechanics. 1996;29:655–658. doi: 10.1016/0021-9290(95)00110-7. 10.1016/0021-9290(95)00110-7. [DOI] [PubMed] [Google Scholar]
- Solaro RJ, Rarick HM. Troponin and tropomyosin. Proteins that switch on and tune in the activity of cardiac myofilaments. Circulation Research. 1998;83:471–480. doi: 10.1161/01.res.83.5.471. [DOI] [PubMed] [Google Scholar]
- Stuyvers BDMY, Miura M, ter Keurs HEDJ. Dynamics of viscoelastic properties of rat cardiac sarcomeres during the diastolic interval: involvement of Ca2+ The Journal of Physiology. 1997;502:661–677. doi: 10.1111/j.1469-7793.1997.661bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Keurs HEDJ, Luff AR, Luff SE. Force-sarcomere-length relation and filament length in rat extensor digitorum muscle. Advances in Experimental Medicine and Biology. 1984;170:511–525. doi: 10.1007/978-1-4684-4703-3_44. [DOI] [PubMed] [Google Scholar]
- ter Keurs HEDJ, Rijnsburger WH, van Heuningen R, Nagelsmit MJ. Tension development and sarcomere length in rat cardiac trabeculae. Evidence of length-dependent activation. Circulation Research. 1980;46:703–714. doi: 10.1161/01.res.46.5.703. [DOI] [PubMed] [Google Scholar]
- Tskhovrebova L, Trinick J, Sleep JA, Simmons RM. Elasticity and unfolding of single molecules of the giant muscle protein titin. Nature. 1997;387:308–312. doi: 10.1038/387308a0. 10.1038/387308a0. [DOI] [PubMed] [Google Scholar]
- Weber KT. Cardiac interstitium in health and disease: the fibrillar collagen network. Journal of the American College of Cardiology. 1989;13:1637–1652. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- Whittaker P, Kloner RA, Boughner DR, Pickering JG. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Research in Cardiology. 1994;89:397–410. doi: 10.1007/BF00788278. [DOI] [PubMed] [Google Scholar]
- Young AA, LeGrice IJ, Young MA, Smaill BH. Extended confocal microscopy of myocardial laminae and collagen network. Journal of Microscopy. 1998;192:139–150. doi: 10.1046/j.1365-2818.1998.00414.x. 10.1046/j.1365-2818.1998.00414.x. [DOI] [PubMed] [Google Scholar]


