Abstract
The involvement of P2 purinoceptors in chemosensory function in the ventrolateral regions of the medulla oblongata was investigated in the anaesthetized rat. We have investigated the effect of antagonizing, or desensitizing, P2 receptors in the retrofacial area of the ventrolateral medulla on factors modifying respiratory activity.
Bilateral microinjection of suramin (50 nl, 0.02 M), a P2 purinoceptor antagonist, into the retrofacial area in the artificially ventilated rat reduced resting phrenic nerve discharge. It also markedly affected the response of the phrenic nerve to increases in arterial CO2. Under conditions of hyperoxic, hypocapnic apnoea, the mean threshold for inducing phrenic nerve activity was raised significantly (from an end-tidal CO2 of 2.5 % to 4.5 %, n = 9).
In addition, the slope of the respiratory response curve to increases in CO2 was reduced after suramin. A similar effect was observed after desensitization of certain P2X receptors with αβ-methyleneATP. As arterial levels of O2 were greater than 100 mmHg, and an equivalent pattern of response was observed in sino-aortically denervated and vagotomized animals, we believe any contribution of the peripheral chemoreceptors to be minimal.
Our data suggest that respiratory neurones within the retrofacial area (Bötzinger complex) represent part of the central site of action of CO2 on respiration. Moreover, our observations lead us to suggest that CO2-evoked changes in respiration are mediated at least in part by P2X purinoceptors.
Central chemoreceptive areas responsible for sensitivity to increases in carbon dioxide (CO2) have been shown to be located in several areas of the brainstem including the nucleus tractus solitarii, the locus coeruleus, medullary raphe and the ventrolateral medulla (VLM) (Loeshcke, 1982; Nattie, 1995; Oyamada et al. 1998; Wang et al. 1998). Whilst CO2 provides the major stimulus for sustaining rhythmic respiration, the molecular mechanisms of central chemoreception remain unknown. Changes in the arterial partial pressure of CO2 (Pa,CO2) evoke rapid alterations in respiration. It has been suggested that the changes in extracellular pH that follow changes in Pa,CO2 may mediate the mechanism for central chemoreception (Cherniack, 1993), although recent evidence also indicates a role for changes in intracellular pH in chemoreception (Ritucci et al. 1998).
The VLM contains an extensive network of respiratory neurones, known collectively as the ventral respiratory group (VRG), involving the nucleus ambiguus (NA), and Bötzinger and pre-Bötzinger complexes (Richter et al. 1997). Data obtained using in vitro techniques have provided strong evidence that significant numbers of neurones, including respiratory neurones, contained within the VLM can be activated directly by changes in CO2 and pH (Richerson, 1995; Kawai et al. 1996).
Recent studies in our laboratory showed that exogenously applied ATP, and its stable analogue αβ-methyleneATP (αβ-meATP), can evoke profound changes in cardiovascular and respiratory activity when microinjected into the region of the VLM that includes the VRG (Ralevic et al. 1998). We observed that they caused an increase in arterial pressure and heart rate, but more interestingly a marked inhibition of respiratory activity resulting in an expiratory apnoea. Since the animals were ventilated artificially and the respiratory effects were often accompanied by small cardiovascular changes, the inhibition of respiration could not be attributed to reflex inputs to the central nervous system. In fact, recent electrophysiological studies in our laboratory indicate that ATP and αβ-meATP applied ionophoretically can increase the activity of inspiratory, expiratory and pre-inspiratory neurones recorded from the VLM (V. Ralevic, T. Thomas & K. M. Spyer, unpublished observations). These actions of αβ-meATP, together with the fact that ATP effects were blocked by suramin, suggested the involvement of P2X purinoceptors.
Certain purinoceptors within the P2X subfamily are highly sensitive to pH. For example, extracellular acidification to pH 7.1 enhances the inward currents generated by P2X2 receptors expressed in oocytes approximately 5-fold (King et al. 1996). Similar observations have been made in HEK 293 cells (Stoop et al. 1997). However, the sensitivity of P2X1, P2X3 and P2X4 receptors expressed in HEK 293 cells is decreased by lowering pH (Stoop et al. 1997). This has led to the present study, in which we have tested the hypothesis that P2 receptors localized within the VLM may provide a target through which increases in inspired CO2 alter respiratory activity. To this end we have performed bilateral microinjections of suramin and αβ-meATP into the VLM, and observed the effects on the respiratory response to increases in inspired CO2. We have compared these effects with those obtained after similar bilateral microinjections into the inferior olives, a non-respiratory region of the brainstem, known to be CO2 insensitive (see Ritucci et al. 1998).
METHODS
Experiments were carried out on nineteen Sprague-Dawley rats (280–320 g), in which anaesthesia was induced by pentobarbitone sodium (Sagatal, May & Baker; 60 mg kg −1i.p.) and maintained with supplemental doses as required (10 mg kg −1i.v.). Depth of anaesthesia was monitored by the absence of a withdrawal response to a pinch of the paw, and stable records of cardiovascular and respiratory variables.
Arterial blood pressure (ABP) was recorded via a catheter placed in the femoral artery. The femoral vein was cannulated for administration of anaesthetic. Heart rate (HR) was derived from the electrocardiogram recorded by transcutaneous electrodes. Body temperature was maintained between 37 and 37.5°C with a servo-controlled heating pad. The trachea was cannulated for artificial ventilation with positive pressure (Harvard rodent ventilator, model 683) with a mixture of 21 % oxygen (O2) and 79 % nitrogen unless otherwise stated in the protocol (see below). Levels of end-tidal CO2 were monitored on-line using a fast-response CO2 analyser (Analytical Development, Herts, UK). Arterial partial pressures of oxygen (Pa,O2), carbon dioxide (Pa,CO2) and pH were monitored regularly using a Corning pH/blood gas analyser (model 238) to ensure they remained within the physiological range under resting conditions. In two animals, the vagi and aortic nerves were sectioned, and the carotid sinus nerves sectioned at the junction with the glossopharyngeal trunk.
In all cases, the animal was then placed in a stereotaxic frame. Phrenic nerve activity was recorded as an indication of central respiratory activity. The activity was amplified (× 10 000), filtered (500–1500 Hz) and rectified and smoothed (τ= 100 ms). An occipital craniotomy was performed and the cerebellum aspirated to expose the dorsal surface of the medulla oblongata. Multibarrelled micropipettes (tip diameter, 40–60 μm) were placed bilaterally using stereotaxic co-ordinates into either the ventrolateral medulla (VLM), within the retrofacial area (2.2–2.5 mm rostral to obex, 2 mm lateral to mid-line, 2.5–3 mm ventral) or within the region of the inferior olives (1.5–2 mm rostral to obex, 0.5–0.8 mm lateral to mid-line, 2.8–3 mm ventral) (Paxinos & Watson, 1998). Micropipettes were filled with L-glutamate (0.2 M, pH 7.4; Sigma) and suramin (0.02 M, pH 7.4, Sigma), saline (0.9 %, pH 7.4), or αβ-meATP (12 mM, pH 7.4, Sigma) and Pontamine Sky Blue (2 % in 0.2 M sodium acetate). All drugs (except for Pontamine Sky Blue) were dissolved in saline.
Protocol
The positions of the micropipettes were adjusted in the VLM until a brisk cardiovascular and respiratory response was evoked by pressure injection of L-glutamate (50 nl; NeuroPhore BH-2 system, Medical Systems Corp., Greenvale, NY, USA). This indicated that the observed effects were a result of excitation of perikarya but not fibres of passage (Fries & Zieglgansberger, 1974). At least 30 min was allowed between successive injections of L-glutamate. Microinjections of glutamate were also made into the inferior olives, although responses obtained there were highly variable. The procedure of microinjection was monitored using a binocular microscope with a calibrated micrometre disk.
Respiratory apnoea (cessation of phrenic nerve activity) was induced by mechanically hyperventilating the animal, adding supplemental oxygen if necessary. Arterial levels of CO2 were increased by titrating CO2 into the respiratory mixture in steps of 1–2 %, up to a level of 8–10 % end-tidal CO2. Each step was maintained until phrenic nerve activity had stabilized (∼1 min). At low levels of CO2 each step was maintained until phrenic nerve activity had stablized, but at high levels (> 4 % CO2) this was not the case and phrenic discharge was often still increasing. The animal was then ventilated normally to re-establish physiological levels of arterial gases and pH, and to resume normal phrenic nerve activity.
Bilateral microinjections of either suramin (n = 9), αβ-meATP (n = 3) or saline (n = 3) were then made into the VLM. Repeated doses of αβ-meATP were given 5 min apart until the evoked cardiorespiratory response disappeared (see Ralevic et al. 1998), indicating desensitization of the receptors. In four other animals, bilateral microinjections of suramin were made into the inferior olives. Five to ten minutes were allowed before the animal was again hyperventilated with oxygen-enriched air to provoke apnoea. The respiratory responses to increasing levels of CO2 were then retested. Blood gas analysis confirmed that levels of Pa,CO2 and pH reached at the highest level of end-tidal CO2 were similar before, and after, the drugs (55 ± 9 vs. 55 ± 7 mmHg, and 7.23 ± 0.03 vs. 7.21 ± 0.04, respectively, P > 0.05, Student's paired t test). After a suitable recovery period, bilateral microinjection of L-glutamate was repeated to assess whether suramin had non-specific effects at glutamate receptors (see Nakazawa et al. 1995). The dose of suramin was chosen on the basis that it antagonizes the action of ATP in the VLM (see Ralevic et al. 1998), but does not block glutamate-evoked responses (see Results).
Sites of microinjection were marked by microinjection of Pontamine Sky Blue. The animal was killed by an overdose of anaesthetic. The brain was removed, fixed in 10 % formal saline, frozen, sectioned serially (40 μm) and counterstained with Neutral Red. The sites were then examined by light microscopy and mapped using a stereotaxic atlas (Paxinos & Watson, 1998).
Data analysis
Records of ABP, HR and phrenic discharge were stored on videotape via a digital interface (Instrutech VR-100B). Data were analysed off-line on a computer accessed by an analog-to-digital interface (1401 plus, Cambridge Electronic Design (CED), Cambridge, UK) using Spike 2 software (CED).
Values are given as means ±s.e.m. Levels of ABP and HR were taken immediately before, and during the highest level of end-tidal CO2 attained during hyper capnia. Absolute levels of ABP and HR, and changes in ABP and HR elicited during increasing levels of inspired CO2 were compared before, and after, the relevant drugs by Student's paired t test.
The threshold level of end-tidal CO2 for inducing phrenic discharge from apnoea was noted, and compared before, and after, microinjection of each drug by Student's paired t test. Once phrenic discharge had resumed, respiratory activity was quantified (in arbitrary units) by determining the sum of the area under the curve of phrenic nerve activity over a 10 s period. Measurements were taken at the end of each step-wise increase in end-tidal CO2. Respiratory activity was plotted against end-tidal CO2. A line of best fit was applied and linear regression was performed. The slope of the curve was used as an indication of the gain of the CO2 respiratory response. This was compared before, and after, microinjection of suramin, αβ-meATP or saline by Student's paired t test. In all cases, P < 0.05 was considered significant.
RESULTS
Bilateral microinjection of L-glutamate (0.2 M) into the VLM evoked a short-lasting increase in ABP, variable changes in HR and a reduction, or cessation, of phrenic nerve activity. Similar microinjections into the inferior olive produced very variable changes in ABP, HR and phrenic nerve activity.
The threshold level of end-tidal CO2 for inducing phrenic nerve activity from hypocapnic apnoea before microinjection of suramin ranged from 1.4 to 3.2 % (mean 2.3 ± 0.3 %; see Fig. 1). As expected, step-wise increases in levels of inspired CO2 resulted in step-wise increases in end-tidal CO2 and an increase in phrenic nerve activity (Fig. 1). Hypercapnia also elicited a bradycardia (−17 ± 4 beats min−1, P < 0.01) and slight increase in ABP (8 ± 6 mmHg) (Fig. 1).
Figure 1. Raw data from an anaesthetized and ventilated rat showing effects of bilateral microinjection of suramin into the VLM on the phrenic nerve and cardiovascular responses to increases in inspired CO2.
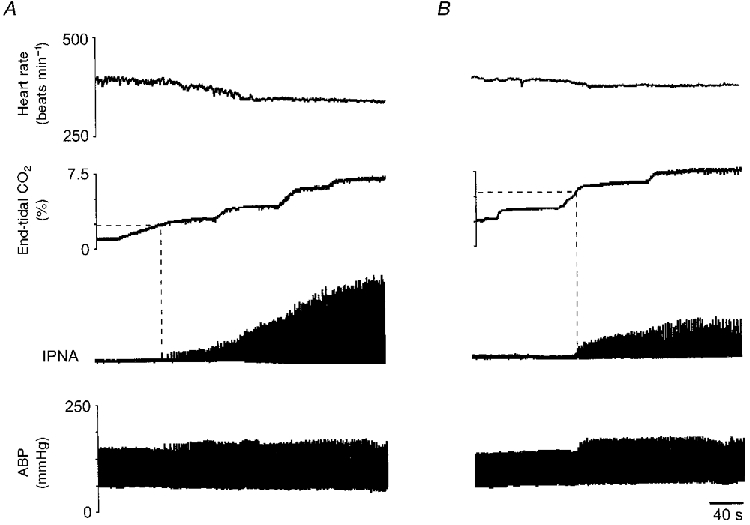
A, response to increasing levels of CO2 before suramin. Note that the threshold level of end-tidal CO2 required to induce phrenic nerve activity from apnoea (shown by interrupted line) is 2.4 %. B, response to increasing CO2 levels after suramin. Apnoeic threshold was 5.8 % end-tidal CO2. Note a reduction in the amplitude of phrenic bursts in response to hypercapnia after suramin. IPNA, integrated phrenic nerve activity; HR, heart rate; ABP, arterial blood pressure.
After bilateral microinjections of suramin (0.02 M) into the VLM, there was a small, but significant, increase in HR (380 ± 7 vs. 357 ± 7 beats min−1, P < 0.01). Baseline levels of ABP were unaffected by suramin (103 ± 9 vs. 94 ± 9 mmHg, P > 0.05). In three animals suramin caused a reduction in resting phrenic nerve activity (−23 ± 13 %, P > 0.05) and complete cessation of phrenic nerve activity in three other animals. Suramin had no effect on phrenic activity in one case. Microinjection of αβ-meATP, or saline, had no effect on baseline levels of HR, ABP and phrenic nerve activity.
The magnitudes of the bradycardia and hypertension elicited during raised levels of CO2 were unaffected by suramin (−22 ± 3 beats min−1 and 9 ± 5 mmHg, respectively, P > 0.05 compared with control; see Fig. 1). After suramin, increasing levels of inspired CO2 again caused an increase in the amplitude and frequency of phrenic nerve bursts (n = 6). However, after suramin, the mean threshold level of end-tidal CO2 required to induce phrenic nerve activity from hypocapnic apnoea was raised significantly to 4.5 ± 0.7 % (P < 0.01, see Fig. 1; range 1.7–6.1 %). In one further animal, increasing levels of inspired CO2 failed to induce phrenic nerve activity after suramin. However, reducing levels of Pa,O2, by ventilating with a gas mixture in which O2 was reduced to 10 %, induced phrenic discharge in this animal.
In five of six cases, the increase in the rate and amplitude of phrenic nerve bursts in response to increasing CO2 levels was reduced after suramin (see Fig. 1). Consequently, the slope of the CO2 respiratory response curve was reduced in these animals by a mean of −56 ± 19 % (P < 0.05; Fig. 3A). In the remaining animal the slope of the respiratory response curve to CO2 was increased slightly. Suramin had similar effects on the apnoeic threshold and on the slope of the respiratory response curve to increasing levels of inspired CO2 in the two denervated animals. In all cases, the effects of suramin were not reversible within the time limits of our experiments (1–2 h after microinjection).
Figure 3. Effect of bilateral microinjection of suramin into the VLM and inferior olives, and of αβ-meATP and saline into the VLM, on respiratory response curve to increasing levels of inspired CO2.

Examples of respiratory response curves to increasing end-tidal CO2 are shown for five different animals. Respiratory activity is plotted against end-tidal CO2. ○, control response; •, response after microinjection of suramin into the VLM (responses of two different animals shown) (A) and the inferior olives (B). C, ▴ indicate the response after microinjection of αβ-meATP into the VLM; D, ▪ indicate the response after microinjection of saline into the VLM. Numbers in parentheses give the slope in arbitrary units.
Microinjection of αβ-meATP had no effect on the level of end-tidal CO2 that induced phrenic nerve activity from apnoea (n = 3). However, in all cases the slope of the respiratory response curve to CO2 was reduced after αβ-meATP (see Fig. 3). αβ-MeATP had no effect on the changes in ABP and HR evoked during hypercapnia.
The cardiovascular response to microinjection of L-glutamate into the VLM was not affected by suramin. In those animals in which respiratory activity was still present after suramin, L-glutamate caused a reduction in phrenic nerve activity (not shown).
Bilateral microinjection of saline into the VLM had no effect on the level of end-tidal CO2 for inducing phrenic nerve activity in apnoea (mean 3.4 ± 0.1 vs. 3.5 ± 0.2 %, P > 0.05), and also had no effect on the respiratory response curves to increasing levels of CO2 (mean change 2 ± 4 %, see Fig. 3).
Bilateral microinjection of suramin into the inferior olives had no effect on baseline levels of ABP, HR or phrenic nerve activity. Moreover, neither the apnoeic threshold (mean 5.0 ± 0.3 vs. 5.0 ± 0.6 %, P > 0.05) nor the slope of CO2 respiratory response curves to increasing level of CO2 was affected by microinjections of suramin into the inferior olives (see Figs 2 and 3, n = 4).
Figure 2. Raw data from an anaesthetized and ventilated rat showing effects of bilateral microinjection of suramin into the inferior olives on the phrenic nerve and cardiovascular responses to increases in inspired CO2.

A, response to increasing levels of CO2 before suramin. B, response to increasing CO2 levels after microinjections of suramin into the inferior olives. Suramin did not affect the apnoeic threshold or the amplitude of phrenic bursts in response to hypercapnia. Abbreviations are as in Fig. 1.
The distribution of histologically determined sites into which microinjections were made is illustrated in Fig. 4.
Figure 4. Distribution of histologically determined sites into which microinjections of suramin, αβ-meATP and saline were made into the VLM or inferior olives.

•, sites of microinjection of suramin or αβ-meATP; ▴, sites of microinjection of saline. Distances from the interaural level are indicated. NTS, nucleus tractus solitarii; Amb, ambiguus nucleus; Bo, Bötzinger complex; PrBo, pre-Bötzinger complex; Io, inferior olives.
DISCUSSION
In this study, we provide further evidence that neurones within the retrofacial area of the VLM represent a central site of action of CO2 on respiration of the rat. Specifically, our data indicate that P2 purinoceptors localized within this area provide a possible locus through which CO2-evoked changes in respiration are mediated. Although levels of tissue pH are thought not to represent the only central chemoreceptor stimulus (Nattie & Li, 1996), it has been hypothesized that decreases in both extracellular and intracellular pH represent crucial stimulating factors in central chemosensitivity (Cherniack, 1993; Ritucci et al. 1997). Thus we suggest that central CO2 sensitivity is a manifestation of the pH sensitivity of purinoceptors, possibly P2X receptors, localized on neurones in VLM. This represents the first in vivo demonstration of a direct physiological function for ATP receptors in the central nervous system.
The effect of suramin in shifting the threshold level of end-tidal CO2 for inducing phrenic nerve activity from respiratory apnoea to a higher level implies that the sensitivity of the central response to CO2 was reduced after antagonizing P2 receptors. Furthermore, as the slope of the respiratory response curve to CO2 was reduced significantly in the majority of animals, the gain of the central chemoreflex to changes in CO2 was also attenuated. A similar effect after desensitization of certain P2X receptors with αβ-meATP suggests a specific involvement of P2X receptors. Both the central and peripheral chemoreceptors monitor arterial levels of CO2. Of course, we cannot totally dismiss the possibility that some of this chemoreceptor ‘resetting’ might be due to a change in the effectiveness of peripheral chemoreceptor inputs to the medulla. However, high levels of arterial O2 were required (often > 200 mmHg), in addition to hypocapnia, to evoke respiratory apnoea in this preparation, and changes in arterial chemoreceptor drive were therefore likely to be minimal. The same result was obtained in sino-aortically denervated animals, so we suggest it is unlikely that the peripheral chemoreceptors contribute to the effects that we describe.
We observed a tachycardia after microinjection of suramin into the VLM. It is possible that our microinjections may have affected the neurones of the external formation of the NA, which contains vagal parasympathetic preganglionic neurones (Loewy & Spyer, 1990). Hence P2 receptors may play a role in determining vagal tone, and therefore resting levels of heart rate. The small change in arterial pressure and small fall in heart rate seen during CO2 loading are consistent with those reported in other studies in the anaesthetized rat (Walker, 1987; Rentero et al. 1997). These cardiovascular responses were unaffected by suramin or αβ-meATP, suggesting again that the changes in respiratory sensitivity that we observed cannot be explained by a change in reflex input to the medulla. Rather, we suggest that the effects of suramin and αβ-meATP on respiratory sensitivity to CO2 can be explained by blockade of pH-sensitive P2X receptors on the respiratory neurones of the Bötzinger complexes. This is supported by in vitro and in vivo data suggesting that respiratory neurones themselves may function as chemoreceptors (Marino & Lamb, 1975; Kawai et al. 1996). Recent data obtained in our laboratory have shown that ATP can modify markedly the activity of different types of individual respiratory neurones in the rostral VLM of the anaesthetized rat when applied ionophoretically (T. Thomas, V. Ralevic & K. M. Spyer, unpublished observations). The lack of effect of suramin on the respiratory response to CO2 when microinjected into the inferior olives, a non-respiratory, CO2-insensitive area of the brainstem, strengthens our argument that P2 purinoceptors are involved in chemoreception. Whether P2 receptors are involved in chemoreceptive mechanisms in other chemosensitive areas of the brainstem, such as the nucleus tractus solitarii and the medullary raphe, remains to be elucidated.
At this time we can only speculate about which P2 receptors might be responsible for the effects we have observed and the mechanisms responsible for mediating such an effect. We do not know whether the primary stimulus for increasing respiration during hypercapnia in our preparation was an decrease in extracellular or intracellular pH, although modulation of the response by a purine receptor antagonist and receptor desensitization might indicate the involvement of an extracellular locus. Data exist from other groups to suggest that either, or both, of these changes are more than likely to be important in central chemoreception (e.g. Ritucci et al. 1998). mRNA for the P2X2, P2X4 and P2X6 receptors have been demonstrated immunohistochemically in the NA (Collo et al. 1996). However, the effects we observe with suramin and αβ-meATP suggest the involvement of the P2X1, P2X2 or P2X3 receptors. Several of these subtypes show pH sensitivity (King et al. 1996; Stoop et al. 1997) and as such represent potential targets through which central CO2 sensitivity might be mediated. In fact, it is possible that several receptor proteins may form a heteromeric receptor, which could explain the pattern of pharmacological responses we have observed. The fact that we observed differential effects of suramin and αβ-meATP on the apnoeic threshold certainly suggests that more than one P2 subtype might be involved in this proposed mechanism. The P2X receptor profile in this area of the medulla has still to be identified.
Acknowledgments
This work was supported by British Heart Foundation and The Royal Society. C. A. G. is a PhD student funded by The Wellcome Trust.
References
- Cherniack NS. Physiological roles of central chemoreceptors. In: Speck DF, Dekin MS, Revelette WR, Frazier DT, editors. Respiratory Control; Central and Peripheral Mechanisms. University Press of Kentucky; 1993. pp. 138–146. [Google Scholar]
- Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning of P2X2 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. Journal of Neuroscience. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries W, Zieglgansberger W. A method to discriminate axonal from cell body activity and to analyse ‘silent cells’. Experimental Brain Research. 1974;21:441–445. doi: 10.1007/BF00237906. [DOI] [PubMed] [Google Scholar]
- Kawai A, Ballantyne D, Mückenhof K, Scheid P. Chemosensitive medullary neurones in the brainstem-spinal cord preparation of the neonatal rat. The Journal of Physiology. 1996;492:277–292. doi: 10.1113/jphysiol.1996.sp021308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BF, Ziganshina LE, Pintor J, Burnstock G. Full sensitivity of P2X2 purinoceptor to ATP revealed by changing extracellular pH. British Journal of Pharmacology. 1996;117:1371–1373. doi: 10.1111/j.1476-5381.1996.tb15293.x. (Special Report) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeschke HH. Central chemosensitivity and the reaction theory. The Journal of Physiology. 1982;332:1–24. doi: 10.1113/jphysiol.1982.sp014397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewy AD, Spyer KM. Vagal preganglionic neurons. In: Loewy AD, Spyer KM, editors. Central Regulation of Autonomic Function. New York: Oxford University Press; 1990. pp. 68–87. [Google Scholar]
- Marino PL, Lamb TW. Effects of CO2 and extracellular H+ iontophoresis on single cell activity in the cat brainstem. Journal of Applied Physiology. 1975;38:688–695. doi: 10.1152/jappl.1975.38.4.688. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Inoue K, Ityo K, Koizumi S, Inoue K. Inhibition by suramin and reactive blue 2 of GABA and glutamate receptor channels in rat hippocampal neurons. Naunyn- Schmiedeberg's Archives of Pharmacology. 1995;351:202–208. doi: 10.1007/BF00169334. [DOI] [PubMed] [Google Scholar]
- Nattie EE. Central chemoreception. In: Dempsey JA, Pack AI, editors. Regulation of Breathing. Vol. 79. New York: Dekker; 1995. pp. 473–501. [Google Scholar]
- Nattie EE, Li A. Central chemoreception in the region of the ventral respiratory group in the rat. Journal of Applied Physiology. 1996;81:1987–1995. doi: 10.1152/jappl.1996.81.5.1987. [DOI] [PubMed] [Google Scholar]
- Oyamada Y, Ballantyne D, Muckenhoff K, Scheid P. Respiration-modulated membrane potential and chemosensitivity of locus coeruleus neurones in the in vitro brainstem- spinal cord of the neonatal rat. The Journal of Physiology. 1998;513:381–398. doi: 10.1111/j.1469-7793.1998.381bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Co-ordinates. London: Academic Press; 1998. [Google Scholar]
- Ralevic V, Thomas T, Spyer KM. Effects of P2 purine receptor agonists microinjected into the rostral ventrolateral medulla on the cardiovascular and respiratory systems of the anaesthetized rat. The Journal of Physiology. 1998;509.P:127P. [Google Scholar]
- Rentero N, Bruandet N, Pequignot JM, Quintin L. Catechol changes in the rat ventrolateral medulla following changes in systemic CO2. American Journal of Physiology. 1997;273:R947–955. doi: 10.1152/ajpregu.1997.273.3.R947. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Response to CO2 of neurons in the rostral ventrolateral medulla in vitro. Journal of Neurophysiology. 1995;73:933–944. doi: 10.1152/jn.1995.73.3.933. [DOI] [PubMed] [Google Scholar]
- Richter DW, Ballanyi K, Ramirez J-M. Respiratory rhythm generation. In: Miller AD, Bianchi AL, Bishop BP, editors. Neural Control of the Respiratory Muscles. Boca Raton, FL, USA: CRC Press; 1997. pp. 119–130. [Google Scholar]
- Ritucci NA, Chambers-Kersh L, Dean JB, Putman RW. Intracellular pH regulation in neurons from chemosensitive and non-chemosensitive areas of the medulla. American Journal of Physiology. 1998;275:R1152–1163. doi: 10.1152/ajpregu.1998.275.4.R1152. [DOI] [PubMed] [Google Scholar]
- Ritucci NA, Dean JB, Putman RW. Intracellular pH response to hypercapnia in neurons from chemosensitive areas of the medulla. American Journal of Physiology. 1997;273:R433–441. doi: 10.1152/ajpregu.1997.273.1.R433. [DOI] [PubMed] [Google Scholar]
- Stoop R, Surprenant A, North RA. Different sensitivities to pH of ATP-induced currents at four cloned P2X receptors. Journal of Neurophysiology. 1997;78:1837–1840. doi: 10.1152/jn.1997.78.4.1837. [DOI] [PubMed] [Google Scholar]
- Walker BR. Cardiovascular effect of V1 vasopressinergic blockade during acute hypercapnia in conscious rats. American Journal of Physiology. 1987;252:R127–133. doi: 10.1152/ajpregu.1987.252.1.R127. [DOI] [PubMed] [Google Scholar]
- Wang W, Pizzonia JH, Richerson GB. Chemosensitivity of rat medullary raphe neurones in primary tissue culture. The Journal of Physiology. 1998;511:433–450. doi: 10.1111/j.1469-7793.1998.433bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


