Abstract
Neurotrophin-3 (NT-3) supports the survival and differentiation of neurones in the central and peripheral nervous systems through a number of mechanisms that occur in a matter of hours or days. NT-3 may also have a more rapid mode of action that influences synaptic activity in mature neurones. In the present study, the effect of NT-3 on developing GABAergic synapses was investigated in 3- to 7-day-old cultures of rat hypothalamic neurones with whole-cell patch-clamp recording.
NT-3 induced a substantial dose-dependent potentiation of the frequency of spontaneous postsynaptic currents (sPSCs; 160 %) in developing neurones during a period when GABA evoked inward (depolarizing) current, as determined with gramicidin-perforated patch recordings. The NT-3 effect was long lasting; continued enhancement was found > 30 min after NT-3 wash-out. NT-3 evoked a substantial 202 % increase in total GABA-mediated inward current, measured as the time-current integral. Action potential frequency was also increased by NT-3 (to 220 %).
The frequency of GABA-mediated miniature postsynaptic currents in developing neurones in the presence of tetrodotoxin was potentiated (to 140 %) by NT-3 with no change in the mean amplitude, suggesting a presynaptic locus of the effect.
In striking contrast to immature neurones, when more mature neurones were studied, NT-3 did not enhance the frequency of GABA-mediated spontaneous postsynaptic currents (sPSCs), but instead evoked a slight (16 %) decrease. The frequency of miniature post-synaptic currents was also slightly decreased (16 %) by the NT-3, with no change in amplitude. These results were recorded during a later period of neuronal maturity when GABA would evoke outward (hyperpolarizing) currents. NT-3 had no effect on the mean amplitude of GABA-evoked postsynaptic currents in either developing or mature neurones.
Intracellular application of K252a, a non-selective tyrosine kinase inhibitor, did not block the NT-3 effect postsynaptically. In contrast, bath application of K252a prevented the enhancement of sPSCs by NT-3, consistent with NT-3 acting through presynaptic induction of tyrosine kinase. Decreasing extracellular calcium with BAPTA or inhibiting calcium channels with Cd2+ blocked the augmentation of sPSC frequency by NT-3, suggesting that an increase of calcium entry may be required for the facilitation of NT-3.
Together, our results suggest NT-3 enhances GABA release during the developmental period when GABA is depolarizing and calcium elevating, but not later when GABA is inhibitory, suggesting that one mechanism through which NT-3 may influence neuronal development is via presynaptic potentiation of GABA excitation.
Neurotrophic factors regulate proliferation, differentiation, process outgrowth and survival of specific neuronal populations, and thus play a vital role in vertebrate neuronal development. Neurotrophin-3 (NT-3), a member of the nerve growth factor gene family, supports the survival and differentiation of various peripheral sensory neurones (Ernfors et al. 1990; Hohn et al. 1990; Ernfors et al. 1994; Farinas et al. 1994). NT-3 enhances the survival and differentiation of spinal cord neurones (Henderson et al. 1993), cultured Purkinje cells (Lindholm et al. 1993), auditory neurones (Avila et al. 1993) and hippocampal neurones (Collazo et al. 1992; Ip et al. 1993). NT-3 induces neuronal differentiation of cortical precursor cells (Ghosh & Greenberg, 1995), and enhances sprouting of the corticospinal tract (Schnell et al. 1994). Hypothalamic neurones express TrkC, the primary receptor for NT-3 (Escandón et al. 1994; Berg-von der Emde et al. 1995), suggesting that NT-3 may influence hypothalamic neurones. Despite extensive evidence demonstrating important roles for NT-3 in neuronal development, there is little physiological work indicating how the neurotrophins act on developing central neurones and synapses. Much of our understanding of NT-3 is based on data obtained from the peripheral nervous system (Lohof et al. 1993; Liou et al. 1997).
GABAergic synaptic transmission appears earlier than glutamatergic transmission in the development of the CNS (Reynolds & Brien, 1992; Chen et al. 1995, 1996; Ben-Ari et al. 1997). During early development of hypothalamic and other CNS neurones, due to a relatively positive Cl− reversal potential, GABA is excitatory, depolarizing the membrane potential, evoking action potentials and raising cytosolic calcium (Obrietan & van den Pol, 1995; Chen et al. 1996; Gao et al. 1998). The excitatory synaptic transmission mediated by GABAA receptors may comprise the majority of the excitatory driving force in the developing hypothalamus and other regions of the CNS (Ben-Ari et al. 1989; LoTurco et al. 1995; Obrietan & van den Pol, 1995; Chen et al. 1996; Owens et al. 1996; Leinekugel et al. 1997; Gao et al. 1998). Growing evidence indicates that NT-3 potentiates neuronal activity in mature cortical neurones (Kim et al. 1994) and induces long-lasting enhancement of synaptic transmission in mature hippocampal slices (Kang & Schuman, 1995). NT-3 was reported to inhibit GABAergic synaptic transmission in cortical neurones, which may be the mechanism responsible for the enhanced firing of action potentials in mature neurones (Kim et al. 1994).
Given that hypothalamic neurones show a strong expression of the NT-3 receptor, TrkC, in development (Lamballe et al. 1994), in the present paper we studied the actions of NT-3 on GABAergic synaptic transmission in cultured hypothalamic neurones. We used cultures to allow rapid onset of response and complete wash-out of reagents, and to compare our work with work on neurotrophic factor actions on cultures of more mature neurones described previously (Berninger et al. 1993; Kim et al. 1994; Jarvis et al. 1997; Sakai et al. 1997). Cultures in defined media also allowed us to control the external cellular milieu to avoid complications due to uncharacterized trophic factors in serum. In striking contrast to previous work that focused on more mature neurones and found that NT-3 depressed GABA synaptic transmission (Kim et al. 1994), we found that in developing neurones NT-3 enhanced GABA transmission, probably by a Trk calcium-dependent mechanism at a presynaptic site that increased GABA release.
METHODS
Cell culture
Hypothalamic neurones were cultured from rat embryos as described previously (Gao et al. 1998). Serum-free culture medium was used to prevent the masking effect of animal sera that may contain unknown amounts of different neurotrophic factors. The experiments were carried out in accordance with the guidelines of the Yale University animal welfare committee. Pregnant Sprague-Dawley rats were heavily anaesthetized with a lethal dose of Nembutal (80 mg kg−1) and the E15-E18 embryos were removed. These embryos were decapitated under hypothermic anaesthesia and the dorsomedial hypothalami were dissected out of the brains. After being cut into small pieces (smaller than 1 mm3), the tissue was incubated at 37°C for 15-20 min in an enzyme solution containing 20 units ml−1 papain, 0.5 mM EDTA, 1.5 mM CaCl2 and 0.2 mg ml−1 L-cysteine, and then washed with culture medium containing 10 % fetal calf serum and mechanically triturated to obtain dissociated cells. The cells were plated into 35 mm culture dishes at a high density of 200 000-300 000 cells per dish, and maintained in an incubator at 37°C with 5 % CO2. Serum-containing medium was replaced with serum-free medium 1-2 h after plating. The serum-free culture medium contained neurobasal medium (Gibco), 5 % B27 supplement (Gibco), 0.5 mM L-glutamine, 100 units ml−1 penicillin-streptomycin and 6 % glucose. Neurones were fed twice a week.
Electrophysiological recording
All experiments were performed in neurones cultured for 3-7 days in vitro (DIV) at room temperature. The recording chamber was continuously perfused at a rate of 1.5-2 ml min−1 with a bath solution containing (mM): 150 NaCl, 2.5 KCl, 2 CaCl2, 10 Hepes and 10 glucose; pH 7.3 with NaOH. Whole-cell voltage clamp was used to observe spontaneous and miniature postsynaptic currents at -60 mV with an L/M EPC-7 amplifier. The patch pipette was made of borosilicate glass (World Precision Instruments) with a Narashige puller (PP-83). The tip resistance of the recording pipettes was 4-6 MΩ after filling with a pipette solution containing (mM): 145 KCl, 1 MgCl2, 10 Hepes, 1.1 EGTA, 2 Mg-ATP and 0.5 Na2-GTP; pH 7.3 with KOH. In some experiments with perforated patch recording, gramicidin (50-100 μg ml−1) was added to the pipette solution to verify inward currents and depolarizing actions of GABA transmission. The preparation of gramicidin was as described previously (Gao et al. 1998). After a gigohm seal was formed, the series resistance was between 20 and 40 MΩ and partially compensated for by the amplifier. Both input resistance and series resistance were monitored throughout experiments. Only those recordings with input resistance higher than 0.8 GΩ and stable series resistance were accepted. In some experiments, GABA (50 μM) was ejected by pressure (4 p.s.i.) through micropipettes (with 2-3 μm tip diameter, 4 μm from the soma) with a picospritzer (General Valve, Fairfield, NJ, USA) to study GABA-evoked postsynaptic currents. The duration of GABA application was 10 ms, except in some cells where GABA at this duration evoked an action potential, in which case a duration of 5 ms was used. A constant duration was used for any single cell. All data were sampled at 3-10 kHz and filtered at 1-3 kHZ with an Apple Macintosh computer using AxoData 1.2.2 software (Axon Instruments). Data were analysed with Axograph 3.5 (Axon Instruments) and plotted with Igor Pro software (WaveMetrics, Lake Oswego, OR, USA). Both miniature and spontaneous postsynaptic currents were detected and measured with an algorithm in Axograph 3.5 (Clements & Bekkers, 1997). Briefly, a simulated template with the width and time course of a typical synaptic event, is moved along the recorded data trace one point at a time. At each position, this template is optimally scaled and offset to fit the data so that an event can be detected. The template function used to approximate the time course of miniature and spontaneous synaptic events has a flat baseline region followed by an exponential rise and decay typical of a synaptic event:
where RISE is the time constant of the rising phase, DECAY is the time constant of the falling phase of the template, and t is the time from onset of idealized synaptic event.
The same criteria were used during control periods and after drug application. To eliminate electronic noise, we only used signals > 4 pA. All data were reported as means ±s.e.m. Student's t test and analysis of variance were used to compare groups.
Recombinant human neurotrophin-3 (NT-3) was obtained from PeproTech (NJ, USA). The lyophilized protein was reconstituted in water to a concentration of 100 μg ml−1 and stored at -20°C, as instructed by the supplier. The stock solution was diluted to the working concentration of 100 ng ml−1 just prior to use. 2-Amino-5-phosphono-pentanoic acid (AP5), 6-cyano-7-nitroquinoxaline-2, 3-dione (CNQX) and the tyrosine kinase inhibitor K252a were obtained from Research Biochemicals International (RBI, USA).
RESULTS
Spontaneous GABAergic synaptic activity was recorded in cultures less than a week old in the presence of the glutamate receptor antagonists CNQX (10 μM) and AP5 (50 μM). The spontaneous postsynaptic currents (sPSCs) were inward in all five cells cultured in serum-free culture when recorded with gramicidin-perforated whole-cell recordings as shown in Fig. 1, and thus they were excitatory. The frequency range of sPSCs was 0.28 to 4.5 Hz. The amplitude of the sPSCs was 4.0 pA to more than 200 pA. Bicuculline (30 μM) completely blocked the sPSCs. In the experiments described below, the effect of NT-3 on developing GABAergic synapses was investigated with hypothalamic cultures of the same age, a period when GABA exerted excitatory actions. To reduce the high series resistance of perforated patch recordings, the experiments described below with NT-3 were done on hypothalamic neurones of the same age with conventional whole-cell access.
Figure 1. Excitatory GABAergic synaptic transmission in 3- to 7-day-old hypothalamic cultures.
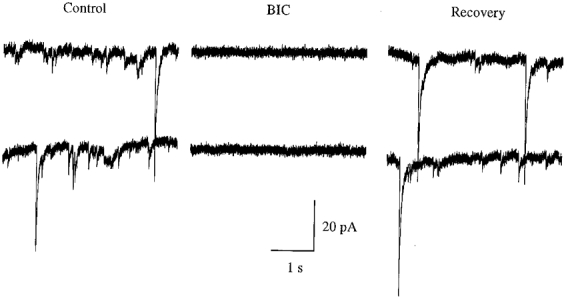
The traces are spontaneous postsynaptic currents from gramicidin-perforated whole-cell recording under voltage clamp (-60 mV) in the presence of the glutamate receptor antagonists CNQX and AP5. Bicuculline (BIC, 30 μM) blocked the recorded postsynaptic currents, indicating a GABAergic source for this activity.
NT-3 increases the frequency of postsynaptic currents in developing neurones
Immature neurones
Despite the potential importance of NT-3 in developing CNS neurones, previous electrophysiological studies have focused on more mature neurones (Kim et al. 1994; Kang & Schuman, 1995), and found that NT-3 reduced GABAergic activity. As NT-3 may participate in mechanisms related to synaptic development, we focused on the effect of NT-3 on GABA transmission at a developmental time period when GABA actions are depolarizing. Ten to fifteen minutes after whole-cell access was achieved and a stable baseline recorded, 100 ng ml−1 human recombinant NT-3 was applied to the hypothalamic culture in the bath solution. Within 8 min of the application of NT-3, an enhancement of sPSC frequency was observed in 13 of 16 cells. Three cells showed no response.
The enhancement of GABAergic sPSC frequency reached its peak within 2-4 min after the initiation of NT-3 application, and remained at a steady state during NT-3 exposure (Fig. 2B). Figure 2A depicts the traces recorded in a typical experiment. In Fig. 2C, data from all 13 neurones were analysed and plotted. The extent of this enhancement was from 129.6 % of control to 318.4 %, with a mean increase to 160.2 ± 18.6 % of control (P < 0.004, t test, n = 13). The frequency of spontaneous GABAergic activity was 156.1 ± 19.1 % of control (P < 0.01, t test, n = 13) 10 min after the withdrawal of NT-3. The amplitude of sPSCs was also increased by the NT-3 treatment. This was determined by finding the maximal current in each 2-min interval of an experiment, and determining the cell mean (for maximal synaptic current) before and during NT-3 application for each cell (n = 13). The maximal synaptic current was increased by NT-3 from 151.2 ± 35.4 to 205.8 ± 51.3 pA, a statistically significant 40 % increase (P < 0.05).
Figure 2. Synaptic potentiation induced by neurotrophin-3 (NT-3) at GABAergic synapses.
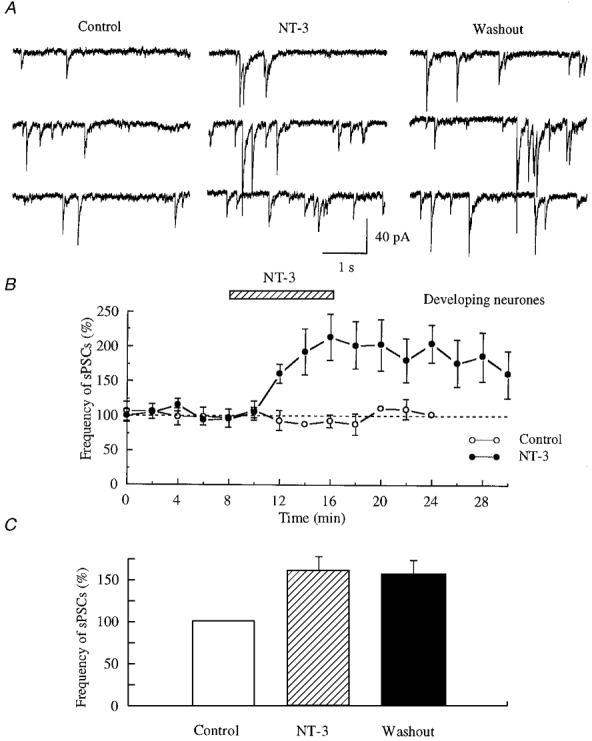
A, traces were recorded in the presence of CNQX and AP5 to block ionotropic glutamate receptors. NT-3 (100 ng ml−1) dramatically increased the frequency of GABAergic spontaneous postsynaptic currents (sPSCs). This potentiation still existed 10 min after withdrawal of NT-3. B, normalized data from 5 cells are plotted. The frequency of GABAergic sPSCs was normalized to the mean of control sPSC frequency. During application of NT-3 (100 ng ml−1), a potentiation of GABAergic synaptic transmission developed. The maximum potentiation was 202.3 ± 36.5 % of control. The potentiation declined to 159.9 ± 33.9 % of control, 14 min after wash-out of NT-3. Also plotted is the mean of two typical control cells that received no NT-3, and showed a stable frequency of sPSCs. C, all data from 13 cells were analysed and are plotted in this bar graph.
We compared the current-time integral for six cells randomly sampled. The current-time integral represents the total current flow into neurones during a period of time. The integral was substantially greater (by 202.2 ± 34.8 %) in the presence of NT-3 than in the control period before NT-3 application, a significant increase (P < 0.05, n = 6). In current clamp, the action potential frequency was strongly increased to 220 ± 29 % (mean ±s.e.m.) of control (range, 183-306 % of control; P < 0.05, n = 4) (Fig. 3). These action potentials were dependent on GABA activity, and were not seen in the presence of bicuculline (30 μM). NT-3 evoked a long-lasting effect on GABA activity; even after NT-3 wash-out, synaptic activity was elevated for at least 30 min. Longer recordings were not made. In control neurones where no NT-3 was added, the frequency of sPSCs remained stable (Fig. 2B).
Figure 3. The frequency of action potentials was increased by NT-3 in developing neurones.
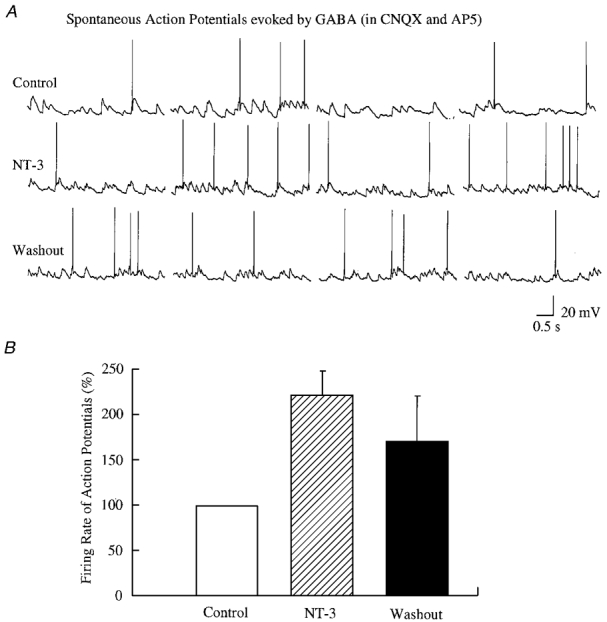
A, representative trace from a neurone recorded under current clamp in the presence of CNQX and AP5. NT-3 dramatically increased the firing rate of action potentials. Resting membrane potential, -55 mV. B, normalized data from all four neurones are plotted. The firing rate of action potentials was normalized to the mean of the control.
The enhancement of GABAergic sPSCs by NT-3 led to a second question. Is the effect of NT-3 presynaptic or postsynaptic? The investigation of miniature postsynaptic currents (mPSCs) has been extensively used to determine the site of action of a neuroactive substances on the synapse (Chen & van den Pol, 1997). In our experiments, the effect of NT-3 on GABAergic synaptic transmission was studied in the presence of 0.5-1 μM TTX. In immature 3- to 7-day-old hypothalamic cultures, the frequency of GABAergic mPSCs was from 0.52 to 1.78 Hz, and the amplitude of mPSCs was from 4.1 to 130 pA. As shown in Fig. 4, the frequency of GABAergic mPSCs was enhanced several minutes after the application of NT-3. All six neurones tested showed an NT-3-mediated increase in mPSCs; the frequency increased from 120.6 to 161.8 % of control, with a mean of 139.6 ± 6.0 % of control (P < 0.002, t test, n = 6). The frequency of mPSCs remained elevated (155.6 ± 15.6 % of control) 10 min after the wash-out of NT-3. The potentiation of mPSC frequency continued after wash-out of NT-3 for up to 30 min, the duration of the longest experiments. The effect of NT-3 on the amplitude of mPSCs was also examined. Two cells showed a decrease, two an increase, and two showed no change in amplitude. A Kolmogorov-Smirnov statistical test on the group (n = 6) revealed that NT-3 exerted no effect on the mean amplitude of mPSCs, as shown in Fig. 4C.
Figure 4. NT-3 increased the frequency of GABAergic miniature postsynaptic currents (mPSCs).
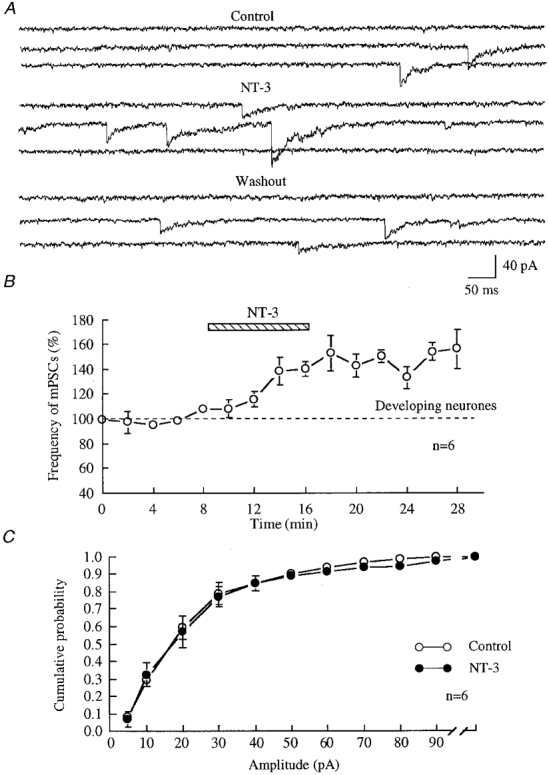
A, traces show mPSCs recorded from 4-day-old hypothalamic cultures under voltage clamp at -60 mV in the presence of 10 μM CNQX, 50 μM AP5 and 1 μM TTX. The enhancement of the frequency of mPSCs was observed in the presence of NT-3 (100 ng ml−1) and 10 min after wash-out of NT-3. B, summary of results from all six neurones. Data were normalized before analysis and plotting. The frequency of mPSCs was 139.6 ± 6.0 % of control (P = 0.0012, t test, n = 6) after 8 min of treatment with NT-3, and 155.6 ± 15.6 % of control 10 min after wash-out of NT-3. C, the cumulative probability of mPSCs from all six cells is plotted. The amplitude of mPSCs was not changed after NT-3 treatment. ○, mPSCs before application of NT-3; •, mPSCs during application of NT-3.
The effect of NT-3 on GABA-evoked postsynaptic currents was also observed by pressure application of 50 μM GABA to the soma of postsynaptic neurones. In six developing neurones tested, the mean amplitude of GABA-evoked currents was not altered by NT-3 (101.4 ± 10.6 % of control after application of NT-3, range -28 to +26 % of control). This small difference was not statistically significant (n = 6, P > 0.05) (Fig. 7C).
Figure 7. NT-3 did not significantly change the frequency or amplitude of mPSCs in mature hypothalamic neurones.
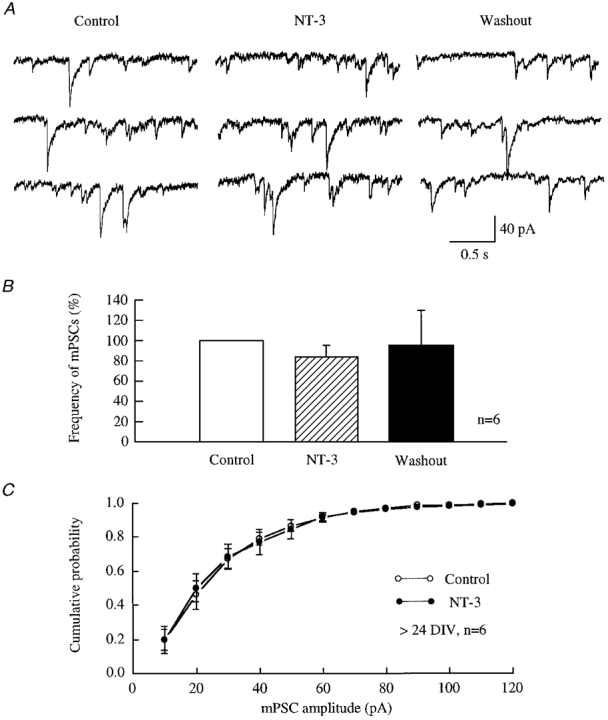
A, raw data recorded from hypothalamic neurones after 3.5 weeks in culture in the presence of 10 μM CNQX, 50 μM AP5 and 1 μM TTX under voltage clamp (-60 mV). B, all data from six neurones are plotted. A slight decrease in the frequency of mPSCs was induced by NT-3. The frequency of mPSCs was reduced by 16.3 % during application of NT-3 (100 ng ml−1) and by 5 % of control after wash-out of NT-3. C, the cumulative probability of mPSCs from all six cells is plotted. No change in the amplitude of mPSCs was detected with a Kolmogorov-Smirnov test.
To characterize the potentiating action of NT-3 further, we observed the effect of different concentrations of NT-3. Three concentrations were used. As shown in Fig. 5, the frequency of sPSCs in the presence of different concentrations of NT-3 was normalized as a percentage of control. During treatment with 10, 50 and 100 ng ml−1 NT-3, the frequency of sPSCs was 106.0 ± 10.8 (n = 8), 131.8 ± 15.1 (n = 9) and 160.2 ± 18.6 % (n = 13) of control, respectively, indicating a concentration-dependent action of NT-3.
Figure 5. Dose dependence of NT-3 effect.
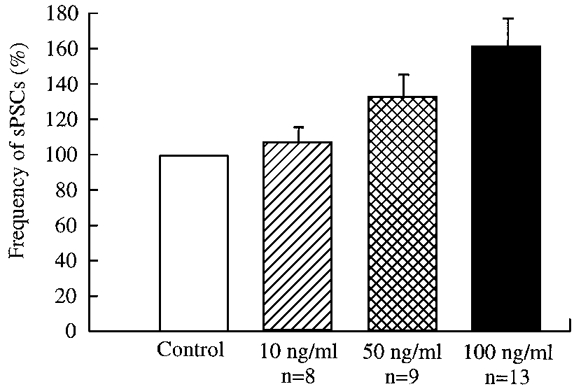
The frequency of spontaneous postsynaptic currents in the presence of NT-3 at concentrations of 10, 50 and 100 ng ml−1 is plotted. A greater concentration of NT-3 generated a larger response.
Thus our results suggest that NT-3 can potentiate the frequency of both spontaneous and miniature postsynaptic currents mediated by GABA in developing neurones. Our results also suggest a presynaptic locus for the effect of NT-3 on the enhancement of GABAergic transmission.
Mature neurones
NT-3 has been reported to potentiate excitatory synaptic transmission by inhibiting GABAergic synaptic transmission in adult cortical neurones (Kim et al. 1994). In contrast, our results suggested a potentiating effect of NT-3 on the developing synapses of hypothalamic neurones. This raised the question whether NT-3 had the same effect on more mature synapses in the hypothalamus as reported in cortical neurones. In our experiments, hypothalamic neurones cultured for 3-4 weeks were used. In these older neurones, 3.2-16.3 Hz spontaneous postsynaptic currents were recorded. The frequency of sPSCs was 84.1 ± 8.6 % of control (n = 8) during application of NT-3 in our experiments on mature neurones. The frequency of sPSCs was 96.1 ± 18.7 % of control (n = 8) 10 min after removing NT-3 (Fig. 6). Thus in contrast to our findings with developing neurones where NT-3 increased GABA activity in thirteen of sixteen neurones, in mature neurones we were unable to detect any increase, and in fact found a slight decrease (P = 0.1, t test, n = 8).
Figure 6. NT-3 did not potentiate the frequency of sPSCs in mature hypothalamic cultures.
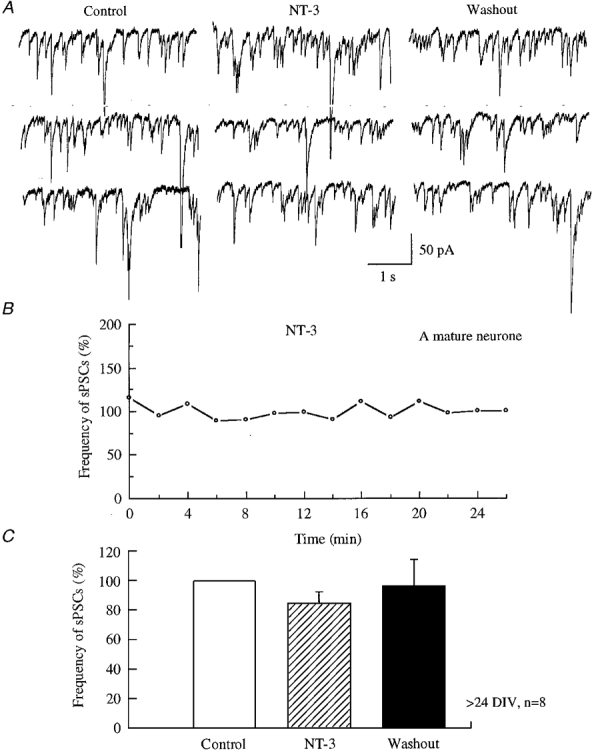
A, raw data recorded from 3.5-week-old hypothalamic neurones in the presence of 10 μM CNQX and 50 μM AP5 under voltage clamp (-60 mV). The frequency of sPSCs was not changed either during application of NT-3 (100 ng ml−1) or after application of NT-3. B, an example of a typical neurone is presented to show that the frequency of sPSCs did not change throughout the entire experiment. C, all data from eight neurones are plotted.
We compared the NT-3-mediated actions in immature and mature neurones and found a statistically significant difference in NT-3 actions (P < 0.01, t test). The same result was obtained using analysis of variance to compare mature and immature neurones during or before NT-3 application (P < 0.001). Because a KCl-containing pipette solution was used in the experiment above, the sPSCs recorded at -60 mV under voltage clamp were inward. However, our previous work with gramicidin perforated recordings that do not disturb the normal intracellular Cl− level has shown that GABA consistently evoked outward currents at this stage of development due to a more negative Cl− reversal potential (Chen et al. 1996; van den Pol et al. 1996; Gao et al. 1998).
The effect of NT-3 on mPSCs in older neurones was also studied. In the presence of TTX (0.5-1 μM), the frequency of mPSCs was reduced to 83.7 ± 11.3 % of control (n = 6) during the application of NT-3, and 95.0 ± 34.5 % of control (n = 6) 10 min after withdrawal of NT-3 (Fig. 7). The amplitude of mPSCs was not changed by the application of NT-3 in older neurones, as demonstrated by a Kolmogorov-Smirnov statistical test.
In parallel with our experiments on developing neurones that examined the effect of NT-3 on the amplitude of GABA evoked postsynaptic currents, experiments were also done on mature neurones (3-4 weeks in vitro). The mean amplitude of GABA-evoked currents after the application of NT-3 was 99.3 ± 15.7 % of control (range -42 to +57 %). Thus our data suggested no significant change (n = 7, P > 0.05) in the mean amplitude of GABA-evoked currents in mature neurones after treatment with NT-3 (Fig. 8C). We also used analysis of variance to compare GABA-evoked currents before and during NT-3 treatment in mature and immature neurones, and found no significant (P > 0.5) effect of NT-3.
Figure 8. NT-3 did not potentiate the amplitude of GABA-evoked postsynaptic currents.
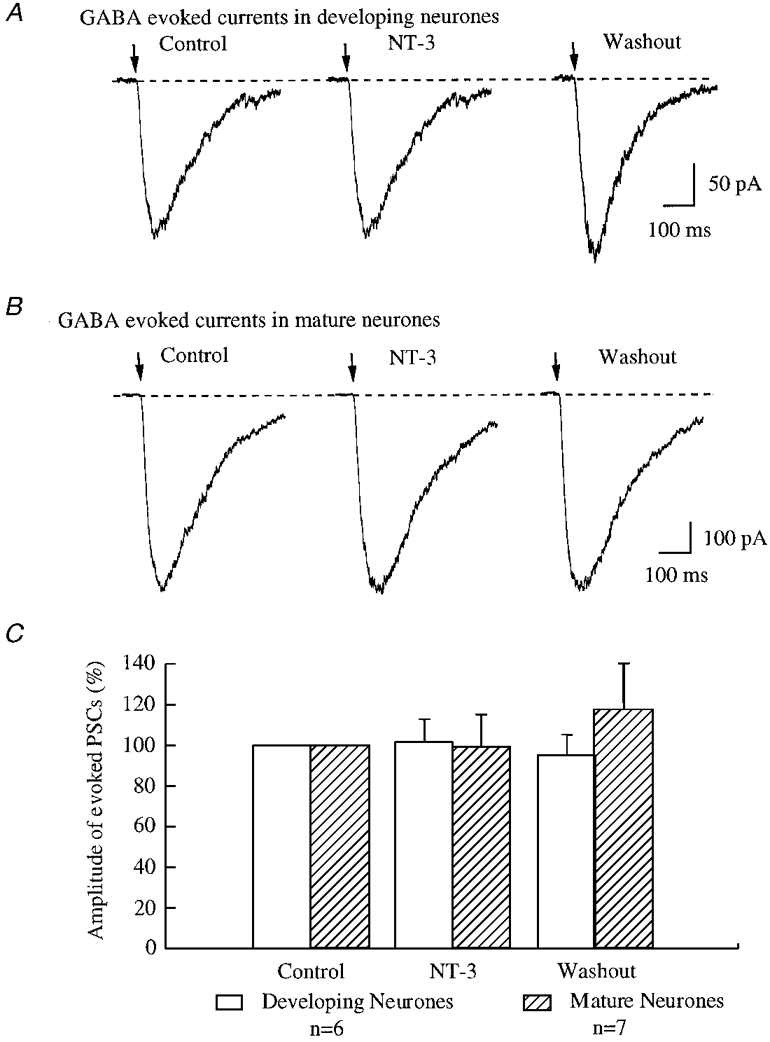
A and B, the traces are recorded from developing (A) and mature (B) neurones under voltage clamp at -60 mV. The arrows indicate the application of GABA (50 μM) to postsynaptic neurones by brief pressure pulse. C, all data from six developing neurones and seven mature neurones were analysed, and are plotted in this bar graph.
The effect of NT-3 is mediated by a tyrosine kinase cascade
NT-3 has binding sites on both the low-affinity receptor p75 and the high-affinity receptor TrkC (Chao, 1992; Rodriguez-Tebar et al. 1992). NT-3 binds to TrkC and induces biological effects such as increasing calcium concentration in hippocampal neurones (Berninger et al. 1993), inducing long-lasting enhancement of synaptic transmission in hippocampal slices (Kang & Schuman, 1995), potentiating synaptic activity in cortical neurones (Kim et al. 1994), and modulating calcium-dependent potassium channels in neurones from forebrain and cortex (Holm et al. 1997). K252a, an inhibitor of tyrosine kinase, has been demonstrated to effectively block the actions of neurotrophins (such as BDNF, NT-3 and NT-4/5, etc.) by inhibiting intracellular tyrosine kinase activity (Knüsel & Hefti, 1992).
In our experiments, in order to demonstrate the effect of NT-3 on the frequency of GABAergic sPSCs, 200 nM K252a was applied to 3- to 7-day-old cultured neurones extracellularly and intracellularly, respectively. K252a can penetrate cell membranes and inhibit intracellular tyrosine kinase activity after extracellular application. After a stable baseline of GABAergic sPSCs was recorded, K252a was added to the recording chamber by bath application for at least 10 min, and then 100 ng ml−1 NT-3 was added to the recording chamber in the presence of K252a. The application of K252a alone did not cause any change in the sPSC frequency of 0.79 ± 0.1 Hz (n = 5). As shown in Fig. 9A, in the presence of K252a, NT-3 evoked little change in the frequency of sPSCs, which was 0.8 ± 0.15 Hz (102.7 ± 9.4 %) of control. Thus there was no significant effect of NT-3 in the presence of K252a (P > 0.1, t test, n = 5). Our results suggest that extracellular application of K252a blocked the potentiation of GABAergic synaptic transmission induced by NT-3 in developing neurones.
Figure 9. Presynaptic tyrosine kinase action.
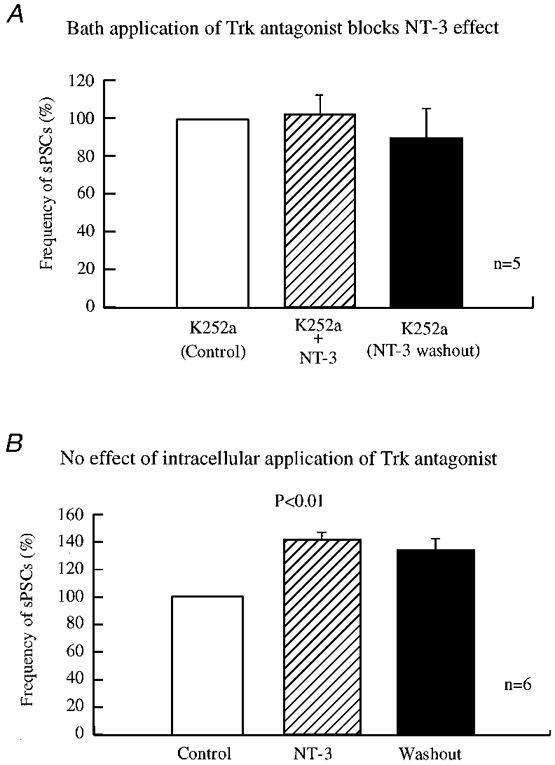
A, inhibition of bath-applied tyrosine kinase blocked NT-3 actions in developing hypothalamic cultures. K252a (200 nM), a non-selective tyrosine kinase inhibitor, was included in all buffers. K252a blocked the actions of NT-3 (100 ng ml−1). The frequency of sPSCs was 102.7 ± 9.4 % of control during application of NT-3, and 90 ± 14.7 % of control after withdrawal of NT-3. There was no increase induced by NT-3 detected in any of the five neurones. B, K252a given intracellularly to the postsynaptic neurone did not block the effect induced by NT-3 in young hypothalamic cultures. Experiments were performed in the presence of 200 nM K252a in patch pipettes. The frequency of sPSCs was 141.5 ± 5.1 % of control during application of NT-3 (100 ng ml−1) and 134.5 ± 7.3 % of control 10 min after withdrawal of NT-3. GABAergic synaptic transmission was significantly enhanced in the presence of NT-3 (P < 0.005, n = 6) and after the wash-out of NT-3 (P < 0.01, n = 6).
Intracellular application of K252a is believed to block the tyrosine kinase cascade directly by diffusion of the drug from the patch pipette to the cytoplasm where it blocks tyrosine kinase only in the recorded neurone. With this protocol, the postsynaptic response to brain-derived neurotrophic factor (BDNF) is blocked by K252a in the patch pipette (Levine et al. 1995; Tanaka et al. 1997). After the formation of whole-cell access, we left the pipette on the recorded neurone for 20 min before we did any further experiments, allowing K252a to diffuse into the recorded cell. K252a given intracellularly did not block the effect of NT-3 on sPSC frequency. The frequency of sPSCs was 0.85 ± 0.05 Hz before and 1.19 ± 0.09 Hz after the NT-3 treatment. The normalized frequency of sPSCs after NT-3 treatment was 141.5 ± 12.5 % of that before NT-3 application, showing a significant increase (P < 0.005, t test, n = 6). The potentiation remained 10 min after wash-out of NT-3 (134.5 ± 7.3 % of control, P = 0.009, t test, n = 5). Thus intracellular application of K252a to the postsynaptic neurones did not abolish the NT-3 potentiation (Fig. 9B). When we used analysis of variance to examine the K252a experiments, no effect of NT-3 was found when K252a was in the bath, but the effect of NT-3 was significant if K252a was in the pipette (P < 0.01).
Our results indicate that the effect of NT-3 on sPSC frequency is mediated by a tyrosine kinase. K252a is a broad-spectrum Trk antagonist, but our results of NT-3 effects on axon terminals are consistent with previous reports that NT-3 may act via TrkC. The inhibition of postsynaptic tyrosine kinase activity did not block the potentiating effect of NT-3 on GABAergic synaptic transmission.
The effect of NT-3 is calcium dependent
Our results suggest that NT-3-induced potentiation of GABAergic synaptic activity resulted from a presynaptic action of NT-3 on tyrosine kinase. As NT-3 may increase intracellular calcium (Berninger et al. 1993), to identify the potential downstream effector of NT-3, we studied the role of calcium in the NT-3-induced potentiation of GABAergic transmission in developing neurones. First we added 5 mM BAPTA to all solutions in our experiments. The frequency of sPSCs was dramatically decreased after the normal bath solution was replaced by BAPTA-containing bath solution. The frequency of sPSCs in BAPTA solution was 43.7 ± 8.7 % (n = 8) of that before BAPTA treatment. After a stable level of synaptic activity was obtained, 100 ng ml−1 NT-3 was added to the recording chamber. As shown in Fig. 10A, the application of NT-3 did not induce potentiation of GABAergic sPSCs in the presence of BAPTA. The frequency of sPSCs was 0.8 ± 0.09 Hz before and 0.72 ± 0.11 Hz (94.3 ± 7.1 % of control, n = 6) after NT-3 treatment. Statistical analysis suggested no significant change (P > 0.4, t test, n = 6).
Figure 10. Calcium dependence.
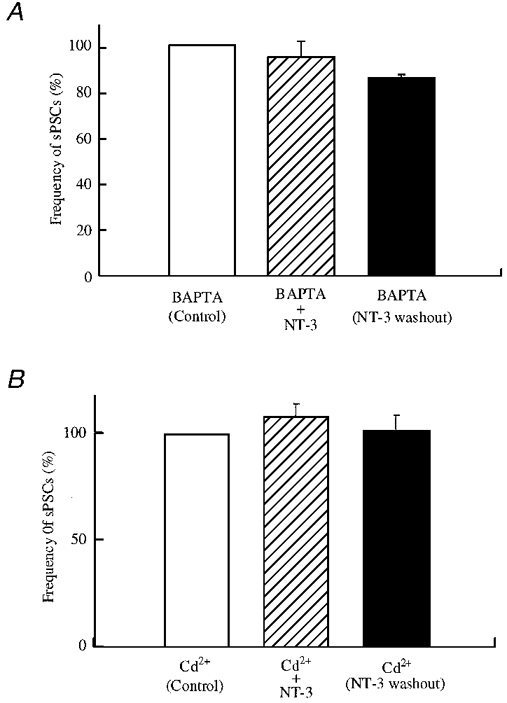
A, the effect of NT-3 on the frequency of sPSCs was blocked in low [Ca2+]o solution. BAPTA (5 mM) was applied to all solutions to decrease extracellular calcium. After application of BAPTA, the frequency of sPSCs was decreased (not shown). After synaptic activity stabilized in the presence of BAPTA, 100 ng ml−1 NT-3 was applied. The frequency during NT-3 treatment was 94.3 ± 7.1 % of control (n = 6), and 84.9 ± 1.9 % of control (n = 6) after wash-out of NT-3. No increase induced by NT-3 (100 ng ml−1) was observed in the presence of BAPTA (P = 0.46, n = 6). B, Cd2+ blocked the effect of NT-3 on the frequency of sPSCs. Cd+ (100 μM) was applied in all solutions to block calcium channels. Cd2+ at 100 μM reduced the frequency of sPSCs (not shown). After stable synaptic activity was recorded in the presence of Cd2+, 100 ng ml−1 NT-3 was applied. The frequency during NT-3 treatment was 108.0 ± 5.6 % of control (n = 3), and 101.4 ± 7.2 % of control (n = 3) after wash-out of NT-3 (no significant difference, P > 0.05), indicating that Cd2+ blocked the actions of NT-3.
Several types of calcium channels have been demonstrated to be involved in neurotransmitter release (Wheeler et al. 1994; Wu & Saggau, 1994; Mintz et al. 1995). Previous evidence suggested that NT-3 increased the concentration of intracellular calcium (Berninger et al. 1993), which may be responsible for the NT-3-induced potentiation of synaptic transmission at presynaptic sites (Lohof et al. 1993). Cd2+ is a non-selective blocker of all types of Ca2+ channels (Fox et al. 1987). In our study, 100 μM Cd2+ was added in all solutions to determine whether NT-3-induced potentiation still occurred when Ca2+ channels were blocked. After the normal bath solution was substituted by Cd2+-containing solution, the frequency of GABAergic sPSCs decreased, as Ca2+ channels were blocked in the presynaptic neurones. The frequency of sPSCs was 2.3 ± 0.24 Hz (n = 3) in the presence of Cd2+. After a stable baseline was recorded, NT-3 was applied to the recording chamber in the presence of Cd2+, as shown in Fig. 10B. The frequency of sPSCs in the presence of NT-3 was 2.4 ± 0.18 Hz. After normalization, GABAergic sPSCs frequency was 108.0 ± 5.6 % of control. There was no significant change detected (P > 0.05, t test, n = 3). With analysis of variance we found no effect of NT-3 in the presence of BAPTA or Cd2+. Our data suggest that NT-3-induced potentiation of GABAergic sPSCs is dependent on calcium influx, possibly at the presynaptic terminal.
DISCUSSION
The present study demonstrates that during development, NT-3 potentiates GABAergic synaptic transmission by increasing the frequency of spontaneous postsynaptic currents in hypothalamic neurones. This potentiating effect disappeared after neuronal maturation. Our data suggest that the site of action of NT-3 was at presynaptic axons, since the frequency of miniature postsynaptic currents was increased after NT-3 treatment. The mechanism of the NT-3 effect appears to be based on tyrosine kinase signalling and is calcium dependent in nature.
The long-term effects of NT-3 on neuronal survival and differentiation have been intensively studied. Despite the large body of data showing effects of NT-3 on neuronal survival and differentiation, there is still an absence of work on the effect of NT-3 on the synaptic activity in developing CNS networks. In our work, we found a long-term enhancement of GABA activity, far outlasting the presence of NT-3. This is similar to work in the peripheral nervous system where Lohof et al. (1993) found that NT-3 evoked a long term increase in the frequency and amplitude of activity at the cholinergic synapse of the neuromuscular junction in Xenopus cell culture. In the mature central nervous system, NT-3 was also reported to potentiate synaptic transmission. Kim et al. (1994) reported that NT-3 rapidly increased the frequency of spontaneous action potentials and synchronized excitatory synaptic activity in cortical neurones. They found that the inhibitory synaptic transmission mediated by GABAA receptors was reduced by NT-3, and suggested that the excitatory effect of NT-3 on spontaneous action potentials resulted from a reduction of GABAergic transmission. Recently, Kang & Schuman (1995) reported that the application of NT-3 to hippocampal slices induced a long-lasting potentiation of presynaptic transmission at the synapses between Schaffer collaterals and pyramidal neurones in the CA1 area.
Here we report that NT-3 enhanced synaptic transmission at developing synapses between hypothalamic neurones. Interestingly, this enhancement of synaptic transmission was not due to a reduction of GABAergic transmission as found in older cortical neurones (Kim et al. 1994), but rather an enhancement of GABAergic transmission itself. There are several differences between our study and others on CNS neurones. We think the critical difference is a developmental one. We used neurones after 3-7 days in vitro, a developmental period when GABA is depolarizing (measured by gramicidin-perforated recordings). In contrast, the other studies used older neurones in which GABA has a hyperpolarizing action. Although the other studies also used different brain regions (hippocampus and cortex), when we used mature hypothalamic cultures, we did not find an enhancement of GABA activity, but instead found a slight decrease in both spontaneous and miniature postsynaptic current frequency, consistent with the reports in non-hypothalamic tissue. In neurone cultures from cortex, the glutamatergic transmission was the dominant source of excitatory synaptic transmission, and blocking glutamate activity prevented spontaneous GABA-mediated events (Kim et al. 1994). In contrast, spontaneous GABAergic synaptic activity was readily recorded after blockade of glutamatergic transmission by CNQX and AP5 in our experiments.
In our experiments we found that NT-3 enhanced GABA release by acting at a presynaptic site of action, and by substantially increasing transmitter release in the presence of TTX. This is consistent with previous reports in the hippocampus and neuromuscular junction where presynaptic actions of NT-3 on glutamatergic and cholinergic axons were found (Lohof et al. 1993; Kang & Schuman, 1995). We do not rule out the possibility that NT-3 may also exert a postsynaptic effect on some cells. Although no difference was found in the mean GABA-mediated current response in the presence or absence of NT-3 in either immature or mature neurones, some individual cells did show an increase or decrease. Furthermore, we did find some variability in different neurones in the amplitude of the miniature GABA-mediated postsynaptic currents, although there was no change in the mean mPSC amplitude from all cells tested. Although postsynaptic actions of NT-3 have not been studied in detail, BDNF, another trophic factor, has been reported to depress the amplitude of evoked GABA currents at a postsynaptic site in mature hippocampal neurones (Tanaka et al. 1997). Further analysis of postsynaptic actions of NT-3 should clarify whether different subpopulations of identified hypothalamic cells may show some postsynaptic action.
Although tyrosine kinase was demonstrated to mediate the NT-3 effect in our data on developing neurones and in other data on more mature neurones, the downstream signalling pathway may vary at different stages of development, or in different cellular environments. There is evidence that the second messenger systems activated by neurotrophins are complex (Kaplan & Stephens, 1994). NT-3 has been demonstrated to bind and activate all three Trk receptor kinases: TrkA, TrkB and TrkC (Chao, 1992; Barbacid, 1993). Furthermore, the TrkC gene was reported to encode multiple NT-3 receptors with distinct biological properties and substrate specificities (Lamballe et al. 1993).
Our study demonstrates that calcium influx is required for the effect of NT-3. After the activation of tyrosine kinase activity by NT-3, several mechanisms may act in the presynaptic neurones in our experiments. Ion channels in the cell membrane may be modulated by protein phosphorylation induced by tyrosine kinase and/or downstream effectors, which can trigger an increase of intracellular calcium concentration and enhance the release of neurotransmitter, as suggested by our results and those of others (Berninger et al. 1993). Presynaptic calcium channels may be the primary targets that are modulated. Other ion channels may also be involved in NT-3 actions. For instance, Holm et al. (1997) reported that NT-3 could activate calcium-dependent paxilline-sensitive potassium channels (BK channels). The BK channel contributes to spike repolarization and after-hyperpolarization of neurones (Storm, 1987; Adams et al. 1992), thereby narrowing action potentials and negatively affecting action potential-evoked transmitter release. Protein phosphorylation induced by tyrosine kinase may also modulate the machinery of transmitter release and potentiate the release of neurotransmitter. For example, NGF has been demonstrated to induce the phosphorylation of synapsin I (Romano et al. 1987) via the activation of MAP kinases (Jovanovic et al. 1996), which may lead to a mobilization of synaptic vesicles and modulate synaptic efficacy (Greengard et al. 1993). The possible role of NT-3 here remains to be elucidated.
GABAergic synaptic transmission emerges earlier than glutamatergic transmission in the early development of the central nervous system (Chen et al. 1996; Ben-Ari et al. 1997), and during this early stage of neuronal development, GABA is excitatory. The excitatory GABAergic transmission may work synergistically with glutamatergic transmission to facilitate the stabilization and maturation of synapses (Gao et al. 1998). The cross-talk between potential pre- and postsynaptic neurones at this stage is crucial. Neuronal activity may induce and modulate expression of neurotrophic factors including NT-3 (Jung & Bennett, 1994; Lindholm et al. 1994; Smith et al. 1995). Potentiation of developing synapses by postsynaptic release of NT-4 has been reported (Wang & Poo, 1997). In light of the findings that in early development the excitatory actions of GABA can influence neurite growth and branching, synapse formation, division of neuronal progenitor cells, migration, and growth cone calcium levels (Meier et al. 1984; Michler, 1990; Barbin et al. 1993; LoTurco et al. 1995; Obrietan & van den Pol, 1996), our finding that NT-3 can enhance the excitatory activity of GABA suggests that NT-3 could act through GABA to influence a wide variety of developmental events.
In our previous work, we found that virtually all synaptic events in hypothalamic cultures and slices could be blocked by GABA and glutamate receptor antagonists (van den Pol & Trombley, 1993; Chen et al. 1996; Belousov & van den Pol, 1997). In the light of this, we think our experiments done in the presence of glutamate receptor blockers show an effect of NT-3 on developing GABAergic neurones. However, as hypothalamic glutamate and GABA may be colocalized with other transmitters or neuromodulators, we cannot exclude the possibility that NT-3 may modulate release or actions of an unidentified modulator, although this does not appear to be the most conservative explanation for the data.
The enhancement of excitatory GABA activity by NT-3 could hypothetically be involved in a positive feedback loop to increase NT-3 production or release from the postsynaptic cell. As activity may enhance synaptic strength and stability, the release of NT-3 from a postsynaptic neurone would enhance the excitatory actions of the presynaptic axon, which would hypothetically strengthen the developing synapse. Increased activity of a presynaptic axon may also enhance uptake or retrograde transport of NT-3 back to the cell body, where it might exert additional effects on gene expression. Thus an NT-3 enhancement of synaptic activity may be one mechanism where the excitatory nature of the early actions of GABA may lead to the stabilization of developing GABAergic synapses.
Acknowledgments
We thank Dr Peter Patrylo for helpful suggestions on the manuscript, and Dr Y. Yang for technical assistance. This work is supported by the US National Institute of Health grants NS 31573 and NS 34887, and the National Science Foundation.
References
- Adams PR, Constanti A, Brown DA, Clark RB. Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature. 1982;296:746–749. doi: 10.1038/296746a0. [DOI] [PubMed] [Google Scholar]
- Avila MA, Varela-Nieto I, Romero G, Mato JM, Giraldez F, Van De Water TR, Represa J. Brain-derived neurotrophic factor and neurotrophin-3 support the survival and neuritogenesis response of developing cochleovestibular ganglion neurons. Developmental Biology. 1993;159:266–275. doi: 10.1006/dbio.1993.1239. 10.1006/dbio.1993.1239. [DOI] [PubMed] [Google Scholar]
- Barbacid M. Nerve growth factor: a tale of two receptors. Oncogene. 1993;8:2033–2042. [PubMed] [Google Scholar]
- Barbin G, Pollard H, Gaiarsa J, Ben-Ari Y. Involvement of GABAA receptors in the outgrowth of cultured hippocampal neurons. Neuroscience Letters. 1993;152:150–152. doi: 10.1016/0304-3940(93)90505-f. [DOI] [PubMed] [Google Scholar]
- Belousov A, van den Pol AN. Local synaptic release of glutamate from neurons of the rat hypothalamic arcuate nucleus. The Journal of Physiology. 1997;499:747–761. doi: 10.1113/jphysiol.1997.sp021966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurons. The Journal of Physiology. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL. GABAA, NMDA and AMPA receptors: a developmentally regulated ‘ménage à trois. Trends in Neurosciences. 1997;20:523–529. doi: 10.1016/s0166-2236(97)01147-8. [DOI] [PubMed] [Google Scholar]
- Berg-von der Emde K, Dees WL, Hiney JK, Hill DF, Dissen GA, Costa ME, Moholt-Siebert M, Ojeda SR. Neurotrophins and the neuroendocrine brain: different neurotrophins sustain anatomically and functionally segregated subsets of hypothalamic dopaminergic neurons. Journal of Neuroscience. 1995;15:4223–4237. doi: 10.1523/JNEUROSCI.15-06-04223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berninger B, Garcia DE, Inagaki N, Hahnel C, Lindholm D. BDNF and NT-3 induce intracellular Ca elevation in hippocampal neurons. NeuroReport. 1993;4:1303–1306. doi: 10.1097/00001756-199309150-00004. [DOI] [PubMed] [Google Scholar]
- Chao MV. Neurotrophin receptors: a window into neuronal differentiation. Neuron. 1992;9:583–593. doi: 10.1016/0896-6273(92)90023-7. [DOI] [PubMed] [Google Scholar]
- Chen G, Trombley PQ, van den Pol AN. GABA receptors precede glutamate receptors in hypothalamic development; differential regulation by astrocytes. Journal of Neurophysiology. 1995;74:1473–1484. doi: 10.1152/jn.1995.74.4.1473. [DOI] [PubMed] [Google Scholar]
- Chen G, Trombley PQ, van den Pol AN. Excitatory actions of GABA in developing rat hypothalamic neurones. The Journal of Physiology. 1996;494:451–464. doi: 10.1113/jphysiol.1996.sp021505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, van den Pol AN. Adenosine modulation of calcium currents and presynaptic inhibition of GABA release in suprachiasmatic and arcuate nucleus neurons. Journal of Neurophysiology. 1997;77:3035–3047. doi: 10.1152/jn.1997.77.6.3035. [DOI] [PubMed] [Google Scholar]
- Clements JD, Bekkers JM. Detection of spontaneous synaptic events with an optimally scaled template. Biophysical Journal. 1997;73:220–229. doi: 10.1016/S0006-3495(97)78062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collazo D, Takahashi H, McKay RDG. Cellular targets and trophic functions of neurotrophin-3 in the developing rat hippocampus. Neuron. 1992;9:643–656. doi: 10.1016/0896-6273(92)90028-c. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Ibanez CF, Ebendal T, Olson L, Persson H. Molecular cloning and neurotrophic activities of a protein with structural similarities to nerve growth factor: developmental and topographical expression in the brain. Proceedings of the National Academy of Sciences of the USA. 1990;87:5454–5458. doi: 10.1073/pnas.87.14.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Lee K-F, Kucera J, Jaenisch R. Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell. 1994;7:503–512. doi: 10.1016/0092-8674(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Escandón E, Soppet D, Rosenthal A, Mendoza-Ramírez J-L, Szönyi É, Burton LE, Henderson CE, Parada LF, Nikolics K. Regulation of neurotrophin receptor expression during embryonic and postnatal development. Journal of Neuroscience. 1994;14:2054–2068. doi: 10.1523/JNEUROSCI.14-04-02054.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Backus C, Wang X-Y, Reichardt LF. Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature. 1994;369:658–661. doi: 10.1038/369658a0. 10.1038/369658a0. [DOI] [PubMed] [Google Scholar]
- Fox AP, Nowycky MC, Tsien RW. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurons. The Journal of Physiology. 1987;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X-B, Chen G, van den Pol AN. GABA-dependent firing of glutamate-evoked action potentials at AMPA/kainate receptors in developing hypothalamic neurons. Journal of Neurophysiology. 1998;79:716–726. doi: 10.1152/jn.1998.79.2.716. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Greenberg ME. Distinct roles for bFGF and NT-3 in the regulation of cortical neurogenesis. Neuron. 1995;15:89–103. doi: 10.1016/0896-6273(95)90067-5. 10.1016/0896-6273(95)90067-5. [DOI] [PubMed] [Google Scholar]
- Greengard P, Valtorta F, Czernik AJ, Benfenati F. Synaptic vesicle phosphorylation and regulation of synaptic function. Science. 1993;259:780–785. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Camu W, Mettling C, Gouin A, Poulson K, Karihaloo M, Rullamas J, Evans T, McMahon SB, Armanini MP, Berkemeier LR, Phillips HS, Rosenthal A. Neurotrophins promote motor neuron survival and are present in embryonic limb bud. Nature. 1993;363:266–270. doi: 10.1038/363266a0. 10.1038/363266a0. [DOI] [PubMed] [Google Scholar]
- Hohn A, Leibrock J, Bailey K, Barde Y-A. Identification and characterization of a novel member of the nerve growth factor/brain-derived neurotrophic factor family. Nature. 1990;344:339–341. doi: 10.1038/344339a0. 10.1038/344339a0. [DOI] [PubMed] [Google Scholar]
- Holm NR, Christophersen P, Olesen SP, Gammeltoft S. Activation of calcium-dependent potassium channels in rat brain neurons by neurotrophin-3 and nerve growth factor. Proceedings of the National Academy of Sciences of the USA. 1997;94:1002–1006. doi: 10.1073/pnas.94.3.1002. 10.1073/pnas.94.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip NY, Li Y, Yancopoulos GD, Lindsay RM. Cultured hippocampal neurons show response to BDNF, NT-3, and NT-4, but not NGF. Journal of Neuroscience. 1993;13:3394–3405. doi: 10.1523/JNEUROSCI.13-08-03394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis CR, Xiong ZG, Plant JR, Churchill D, Lu WY, MacVicar BA, MacDonald JF. Neurotrophin modulation of NMDA receptors in cultured murine and isolated rat neurons. Journal of Neurophysiology. 1997;78:2363–2371. doi: 10.1152/jn.1997.78.5.2363. [DOI] [PubMed] [Google Scholar]
- Jovanovic JN, Benfenati F, Siow YL, Sihra TS, Sanghera JS, Pelech SL, Greengard P, Czernik AJ. Neurotrophins stimulate phosphorylation of synapsin I by MAP kinase and regulate synapsin I-actin interactions. Proceedings of the National Academy of Sciences of the USA. 1996;93:3679–3683. doi: 10.1073/pnas.93.8.3679. 10.1073/pnas.93.8.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung AB, Bennett JP., Jr Development of striatal dopaminergic function. III: Pre- and postnatal development of striatal and cortical mRNAs for the neurotrophin receptors TrkBTK+ and TrkC and their regulation by synaptic dopamine. Developmental Brain Research. 1994;94:133–143. doi: 10.1016/0165-3806(96)00035-1. 10.1016/0165-3806(96)00035-1. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Stephens RM. Neurotrophin signal transduction by the Trk receptor. Journal of Neurobiology. 1994;25:1404–1417. doi: 10.1002/neu.480251108. [DOI] [PubMed] [Google Scholar]
- Kim HG, Wang T, Olafsson P, Lu B. Neurotrophin-3 potentiates neuronal activity and inhibits γ-aminobutyratergic synaptic transmission in cortical neurons. Proceedings of the National Academy of Sciences of the USA. 1994;91:12341–12345. doi: 10.1073/pnas.91.25.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knüsel B, Hefti F. K-252 compounds: modulators of neurotrophin signal transduction. Journal of Neurochemistry. 1992;59:1987–1996. doi: 10.1111/j.1471-4159.1992.tb10085.x. [DOI] [PubMed] [Google Scholar]
- Lamballe F, Smeyne RJ, Barbacid M. Developmental expression of TrkC, the neurotrophin-3 receptor, in the mammalian nervous system. Journal of Neuroscience. 1994;14:14–28. doi: 10.1523/JNEUROSCI.14-01-00014.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamballe F, Tapley P, Barbacid M. TrkC encodes multiple neurotrophin-3 receptors with distinct biological properties and substrate specificities. EMBO Journal. 1993;12:3083–3094. doi: 10.1002/j.1460-2075.1993.tb05977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinekugel X, Medina I, Khalilov I, Ben-Ari Y, Khazipov R. Ca2+ oscillations mediated by the synergistic excitatory actions of GABA-A and NMDA receptors in the neonatal hippocampus. Neuron. 1997;18:243–255. doi: 10.1016/s0896-6273(00)80265-2. [DOI] [PubMed] [Google Scholar]
- Levine ES, Dreyfus CF, Black IB, Plummer MR. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proceedings of the National Academy of Sciences of the USA. 1995;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm D, Castren E, Berzaghi M, Blochl A, Thoenen H. Activity-dependent and hormonal regulation of neurotrophin mRNA levels in the brain - implications for neuronal plasticity. Journal of Neurobiology. 1994;25:1362–1372. doi: 10.1002/neu.480251105. [DOI] [PubMed] [Google Scholar]
- Lindholm D, Castren E, Tsoulfas P, Kolbeck R, Berzaghi da PM, Leingartner A, Heisenberg CP, Tesarollo L, Parada LF, Thoenen H. Neurotrophin-3 induced by tri-iodothyronine in cerebellar granule cells promotes Purkinje cell differentiation. Journal of Cell Biology. 1993;122:443–450. doi: 10.1083/jcb.122.2.443. 10.1083/jcb.122.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou JC, Yang RS, Fu WM. Regulation of quantal secretion by neurotrophic factors at developing motoneurons in Xenopus cell cultures. The Journal of Physiology. 1997;503:129–139. doi: 10.1111/j.1469-7793.1997.129bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohof AM, Ip NY, Poo MM. Potentiation of developing neuromuscular synapses by the neurotrophin NT-3 and BDNF. Nature. 1993;363:350–353. doi: 10.1038/363350a0. 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- LoTurco JJ, Owens DF, Heath MJS, Davis MBE, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. 10.1016/0896-6273(95)90008-X. [DOI] [PubMed] [Google Scholar]
- Meier E, Drejer J, Schousboe A. GABA induces functionally active low-affinity GABA receptors on cultured cerebellar granule cells. Journal of Neurochemistry. 1984;43:1737–1744. doi: 10.1111/j.1471-4159.1984.tb06102.x. [DOI] [PubMed] [Google Scholar]
- Michler A. Involvement of GABA receptors in the regulation of neurite growth in cultured embryonic chick tectum. International Journal of Developmental Neuroscience. 1990;8:463–472. doi: 10.1016/0736-5748(90)90078-g. 10.1016/0736-5748(90)90078-G. [DOI] [PubMed] [Google Scholar]
- Mintz IM, Sabatini BL, Regehr WG. Calcium control of transmitter release at a cerebellar synapse. Neuron. 1995;15:675–688. doi: 10.1016/0896-6273(95)90155-8. 10.1016/0896-6273(95)90155-8. [DOI] [PubMed] [Google Scholar]
- Obrietan K, van den Pol AN. Growth cone calcium rise by GABA. Journal of Comparative Neurology. 1996;372:167–175. doi: 10.1002/(SICI)1096-9861(19960819)372:2<167::AID-CNE1>3.0.CO;2-1. 10.1002/(SICI)1096-9861(19960819)372:2<167::AID-CNE1>3.3.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Obrietan K, van den Pol AN. GABA neurotransmission in the hypothalamus: developmental reversal from Ca2+ elevating to depressing. Journal of Neuroscience. 1995;15:5065–5077. doi: 10.1523/JNEUROSCI.15-07-05065.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Boyce LH, Davis MBE, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. Journal of Neuroscience. 1996;16:6414–6423. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JD, Brien JF. Ontogeny of glutamate and γ-aminobutyric acid in the hippocampus of the guinea pig. Journal of Developmental Physiology. 1992;18:243–252. [PubMed] [Google Scholar]
- Rodriguez-Tebar A, Dechant G, Gotz R, Barde YA. Binding of neurotrophin-3 to its neuronal receptors and interactions with nerve growth factor and brain-derived neurotrophic factor. EMBO Journal. 1992;11:917–922. doi: 10.1002/j.1460-2075.1992.tb05130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano C, Nichols RA, Greengard P. Synapsin I in PC12 cells. II. Evidence for regulation by NGF of phosphorylation on a novel site. Journal of Neuroscience. 1987;7:1300–1306. doi: 10.1523/JNEUROSCI.07-05-01300.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai N, Yamada M, Numakawa T, Ogura A, Hatanaka H. BDNF potentiates spontaneous Ca2+ oscillations in cultured hippocampal neurons. Brain Research. 1997;778:318–328. doi: 10.1016/s0006-8993(97)01052-4. 10.1016/S0006-8993(97)01052-4. [DOI] [PubMed] [Google Scholar]
- Schnell L, Schneider R, Kolbeck R, Barde Y-A, Schwab ME. Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature. 1994;367:170–173. doi: 10.1038/367170a0. 10.1038/367170a0. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Altemus M, Michelson D, Hong SK, Kvetnansky R, Post RM. Stress and antidepressants differentially regulate neurotrophin 3 mRNA expression in the locus coeruleus. Proceedings of the National Academy of Sciences of the USA. 1995;92:8788–8792. doi: 10.1073/pnas.92.19.8788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. The Journal of Physiology. 1987;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Saito H, Matsuki N. Inhibition of GABAA synaptic responses by brain-derived neurotrophic factors (BDNF) in the rat hippocampus. Journal of Neuroscience. 1997;17:2959–2966. doi: 10.1523/JNEUROSCI.17-09-02959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Trombley PQ. Glutamate neurons in hypothalamus regulate excitatory transmission. Journal of Neuroscience. 1993;13:2829–2836. doi: 10.1523/JNEUROSCI.13-07-02829.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Obrietan K, Chen G. Excitatory actions of GABA after neuronal trauma. Journal of Neuroscience. 1996;16:4283–4292. doi: 10.1523/JNEUROSCI.16-13-04283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-H, Poo M-M. Potentiation of developing synapses by postsynaptic release of neurotrophin-4. Neuron. 1997;19:825–835. doi: 10.1016/s0896-6273(00)80964-2. 10.1016/S0896-6273(00)80964-2. [DOI] [PubMed] [Google Scholar]
- Wheeler DB, Randall A, Tsien RW. Roles of N-type and Q-type of Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994;264:107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- Wu LG, Saggau P. Pharmacological identification of two types of presynaptic voltage-dependent calcium channels at CA3-CA1 synapses of the hippocampus. Journal of Neuroscience. 1994;14:5613–5622. doi: 10.1523/JNEUROSCI.14-09-05613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]


